Abstract
The authors examined the association between circulating 25-hydroxyvitamin D3 (25(OH)D3), the best indicator of total vitamin D exposure, and incident, sporadic colorectal adenoma risk in a pooled analysis of primary data from 3 colonoscopy-based case-control studies conducted in Minnesota, North Carolina, and South Carolina between 1991 and 2002. The pooled study included 616 colorectal adenoma cases and 770 polyp-free controls. Multivariable logistic regression was used to estimate the association between circulating 25(OH)D3 and colorectal adenoma risk. Stratified analyses and the likelihood ratio test were used to examine effect modification by various risk factors. In the pooled analysis, higher circulating 25(OH)D3 concentrations were statistically significantly associated with decreased colorectal adenoma risk (highest vs. lowest quartile odds ratio = 0.59, 95% confidence interval: 0.41, 0.84). The observed inverse association was stronger among participants who used nonsteroidal antiinflammatory drugs regularly (highest vs. lowest quartile odds ratio = 0.33, 95% confidence interval: 0.19, 0.56). Inverse associations between 25(OH)D3 and colorectal adenoma did not differ substantially by other risk factors or by adenoma characteristics. These findings support the hypothesis that greater vitamin D exposure may reduce the risk of colorectal adenoma and suggest that it may do so more strongly in combination with antiinflammatory agents.
Keywords: case-control studies, colorectal neoplasms, vitamin D
In 1980, on the basis of ecologic observations of a correlation between sun exposure and reduced colorectal cancer incidence, Garland and Garland (1) hypothesized that vitamin D (collective term for vitamins D2 and D3) status accounted for this inverse association. At that time, the antineoplastic effects of vitamin D were unknown, but, since then, the literature on the biologic basis of a vitamin D–colorectal neoplasms association has evolved considerably.
Recent studies found that normal human colon epithelium expresses the vitamin D receptor and key vitamin D metabolizing enzymes (2–4). In the colon, the active metabolite of vitamin D, 1,25-(OH)2 vitamin D, exerts its antineoplastic effects by genomic (mediated by the vitamin D receptor) and nongenomic mechanisms (5), including regulation of more than 200 vitamin D–responsive genes and rapid activation of intracellular signaling pathways resulting in cell cycle modulation, degradation of bile acids, immune response, and growth factor signaling (6, 7).
Although 90%–95% of vitamin D exposure is from sunlight, most observational studies that investigated the vitamin D–colorectal neoplasms association were limited to assessing only poorly measured dietary vitamin D intake (8–10). The findings from these studies have mostly been null or inconsistent with one another (11–13). However, few studies have investigated an association between circulating vitamin D, 25-hydroxyvitamin D (25(OH)D), and colorectal adenoma (14–20), but from these studies there is an early indication of stronger, more consistent inverse associations that require confirmation in larger studies. Similarly, all 8 studies that assessed associations of 25(OH)D blood levels with incident colorectal cancer found inverse associations (21–28). Despite experimental evidence that various factors may modify the effect of vitamin D, very few data from human studies are available. We investigated the association between circulating 25-hydroxyvitamin D3 (25(OH)D3) and risk of colorectal adenomas and effect modifiers of this association in, to our knowledge, the largest such study to date, a pooled, colonoscopy-based, case-control study of incident, sporadic colorectal adenomas.
MATERIALS AND METHODS
Case-control studies
We pooled data from 3 colonoscopy-based case-control studies of incident, sporadic colorectal adenomas conducted in 3 different US states by the same principal investigator. We used essentially the same participant recruitment and data collection protocols.
The first case-control study (the Cancer Prevention Research Unit (CPRU) study) was conducted as a collaboration between the University of Minnesota and Digestive Healthcare, Professional Association (Minneapolis, Minnesota), a large, multiclinic, private gastroenterology practice. The second case-control study (the Markers of Adenomatous Polyps (MAP) study was conducted in community gastroenterology practices in Winston-Salem and Charlotte, North Carolina, to assess the validity of colonic epithelial cell proliferation as a biomarker of risk for sporadic colorectal adenomas. The third case-control study (MAPII) was identical in design to the first MAP study (MAP) and was conducted at Consultants in Gastroenterology, Professional Association, a large, private practice gastroenterology group in Columbia, South Carolina, to investigate whether the expression patterns of various genes and cell cycle markers in the normal-appearing colorectal mucosa are associated with incident, sporadic adenomas.
Participants for these case-control studies were recruited from among patients with no prior history of colorectal neoplasms who were scheduled to undergo outpatient, elective colonoscopy in the metropolitan areas of Minneapolis, Minnesota (CPRU); Winston-Salem and Charlotte, North Carolina (MAPI); and Columbia, South Carolina (MAPII) (Figure 1). Assessment of initial participant eligibility was identical in the 3 case-control studies. The detailed study protocols for the CPRU (29), MAP (30, 31), and MAPII (32, 33) studies have been published previously; the overall flow of the study participant selection process is shown in Figure 1. We combined data from the MAP and MAPII studies (hereafter referred to as the MAP study) because they were conducted in the same geographic region only a few years apart by using identical participant selection procedures, study questionnaires, and protocols. Because 96% of all study participants were white, we excluded all participants of other races/ethnicities from the pooled analysis.
Figure 1.
Selection of participants studied to assess blood 25-hydroxyvitamin D3 concentrations and incident sporadic colorectal adenoma risk. Row 1: Study and numbers of colonoscopy patients identified. Row 2: initial eligibility criteria included 30–74 years of age; English speaking; willing to participate and able to understand informed consent; no contraindications to endoscopy; and free of known genetic syndromes associated with predisposition to colonic neoplasia and an individual history of ulcerative colitis, Crohn's disease, colorectal adenomas, and cancer (except nonmelanoma skin cancer). CPRU, Cancer Prevention Research Unit; MAP, Markers of Adenomatous Polyps.
Data collection
Before undergoing colonoscopy, all patients completed mailed questionnaires (Figure 1). The questionnaires collected information on demographics, medical history, reproductive history (women only), family history of colon cancer, self-reported anthropometrics, diet (via a semiquantitative Willett 153-item food frequency questionnaire (34)), lifestyle, alcohol and tobacco use, physical activity, and reasons for colonoscopy.
For all studies, preparation for colonoscopy included a 12-hour fast and bowel cleansing with polyethylene glycol. At the clinic visit, venous blood was drawn from each participant and was stored at −70°C. Plasma and serum were separated according to a standardized protocol. The findings on complete colonoscopies were documented on standardized forms to record colon site and in vivo size and shape of any polyps. Upon removal, polyps were examined by one index study pathologist using diagnostic criteria established for the National Polyp Study (35). On the basis of colonoscopy and pathology findings, participants were assigned to one of the following 3 groups: 1) an adenomatous polyp group, 2) a hyperplastic polyp-only group, or 3) a colonoscopy-negative control group. Participants with hyperplastic polyps and with polyps with invasive carcinoma were excluded. The final sample size for this pooled case-control study included 1,386 white participants with measured circulating 25(OH)D, among whom 616 were incident, sporadic colorectal adenoma cases and 770 were colonoscopy controls without hyperplastic polyps.
The protocols of each study were approved by the institutional review boards of the corresponding institutions: the University of Minnesota and each Digestive Healthcare colonoscopy site for the CPRU study, Wake Forest University School of Medicine for the MAP study, and the University of South Carolina for the MAPII study. Informed consent was obtained from each participant.
Laboratory methods
Serum samples were not available for the CPRU study, and plasma samples were not available for some MAP study participants. Therefore, the 25(OH)D2 and 25(OH)D3 assays were conducted in serum samples for the MAP study and in plasma samples for the CPRU study. To check the comparability of serum and plasma concentrations of total 25(OH)D (D2 + D3) and 25(OH)D3, we analyzed paired serum and plasma samples from 20 participants. For 25(OH)D3, the means were 21.3 (standard deviation, 6.7) in plasma and 23.9 (standard deviation, 8.8) in serum (Spearman's ρ ≥ 0.8, P < 0.001); for total 25(OH)D, the means were 32.4 (standard deviation, 9.2) in plasma and 33.5 (standard deviation, 10.2) in serum (ρ ≥ 0.8, P < 0.001). All laboratory assays for blood 25(OH)D2 and 25(OH)D3 concentrations were performed at the University of Minnesota Medical Center, Fairview, Minnesota, by using a liquid chromatography/tandem mass spectrometry method, as previously described (36). Serum/plasma samples for all subjects were assayed together, ordered randomly, and labeled to mask case-control status and quality control replicates. The average intraassay coefficient of variation for serum/plasma 25(OH)D3 was 3% and for 25(OH)D2 was 80%.
Statistical analysis
The case and control groups were evaluated for comparability with respect to important covariates, including demographics, lifestyle, and other risk factors. Fisher's exact test was used for categorical variables and the t test for continuous variables.
In this paper, analyses for only serum/plasma 25(OH)D3, the primary exposure variable of interest, are presented because of the poor performance of the vitamin D assay in detecting 25(OH)D2 (the intraassay coefficient of variation was 80%). Accordingly, we included 25(OH)D2 measurements only in sensitivity analyses.
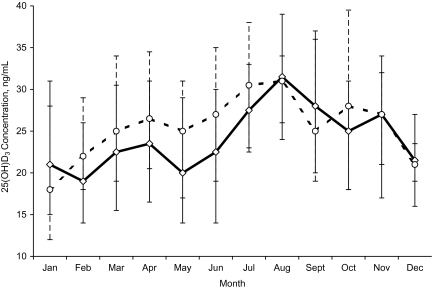
Study-specific quartiles of circulating 25(OH)D3 concentrations were calculated based on the distribution in controls by month of blood draw (Figure 2), as described previously (37). Unconditional logistic regression models were used to assess the association between quartiles of blood vitamin D3 concentrations and risk of colorectal adenoma, with appropriate control for confounding, as described below. In addition, we investigated the association between 25(OH)D3 quartiles and adenoma characteristics (multiplicity, size, location, and pathologic subtype). We also examined associations stratified by potential effect-modifying variables that were dichotomized based on the study-specific median distributions in the controls. In addition, we included the interaction terms in the model and tested the significance of the estimates with the log-likelihood ratio test.
Figure 2.
Circulating 25-hydroxyvitamin D3 (25(OH)D3) concentrations in colonoscopy-negative controls, by month of blood draw, in the Cancer Prevention Research Unit (CPRU) and Markers of Adenomatous Polyps (MAP) studies, United States, 1991–2002. The solid ( ) and dashed (
) and dashed ( ) lines connect median values of 25(OH)D3 by month of blood draw for the CPRU and MAP studies, respectively. Vertical bars: lower 25th and upper 75th percentiles of month-specific 25(OH)D3 values in the CPRU (diamonds) and MAP (circles) studies.
) lines connect median values of 25(OH)D3 by month of blood draw for the CPRU and MAP studies, respectively. Vertical bars: lower 25th and upper 75th percentiles of month-specific 25(OH)D3 values in the CPRU (diamonds) and MAP (circles) studies.
Several criteria were used to assess confounding factors: 1) biologic plausibility, 2) whether the variable of interest was associated with the outcome and exposure, and 3) whether the logistic regression coefficient of the primary exposure variable substantially changed (by >10%) after adding the potential confounding variable to the model. Final covariates included in multivariate-adjusted models were age; sex; study site; body mass index; physical activity; smoking; regular (at least once a week) aspirin or nonsteroidal antiinflammatory drug (NSAID) use; family history of colorectal cancer in a first-degree relative; and dietary intakes of alcohol, calcium, retinol, folate, and red and processed meats.
The results were expressed as odds ratios with corresponding 95% confidence intervals. A test for trend was calculated based on the median of each quartile of blood 25(OH)D3 concentration included in the model as a continuous variable. All statistical tests were 2-sided, and P values of <0.05 were considered statistically significant. All statistical analyses were conducted by using SAS version 9.2 software (SAS Institute, Inc., Cary, North Carolina). Several sensitivity analyses were performed (refer to the Web-only supplement, which is posted on the Journal’s Web site (http://aje.oupjournals.org/)).
RESULTS
Selected characteristics of cases and controls by study are shown in Table 1. Cases and controls did not differ considerably with regard to most risk factors; however, there were more males in the case group than in the control group, and controls were more likely to be younger, to regularly (≥once/week) take an NSAID or aspirin, to have a history of a first-degree relative with colorectal cancer, and to take multivitamins and were less likely to be a current smoker; controls also tended to have lower intakes of red and processed meats and alcohol. Among women, cases were more likely to be postmenopausal; among postmenopausal women, controls were more likely to use hormone replacement therapy. In the CPRU and MAP studies, mean plasma 25(OH)D3 concentrations were slightly statistically nonsignificantly higher in controls than in adenoma cases (Table 1).
Table 1.
Selected Characteristics and Mean Circulating 25(OH)D3 Concentrations in Cases and Controls in 3 Case-Control Studies of Incident, Sporadic Colorectal Adenomas, United States, 1991–2002
| Characteristic | CPRU Study, Minnesota |
MAP Study, North Carolina and South Carolina |
Pooled Analysis |
|||||||||
| Cases (n = 474) |
Controls (n = 563) |
Cases (n = 142) |
Controls (n = 207) |
Cases (n = 616) |
Controls (n = 770) |
|||||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | |
| Age, years | 58.2 (9.7) | 52.7 (11.0)a | 57.5 (8.3) | 55.9 (9.4) | 58.0 (9.4) | 53.6 (10.7)a | ||||||
| Male | 62 | 39a | 56 | 44a | 60 | 39a | ||||||
| College graduate | 30 | 28 | 22 | 31 | 28 | 29 | ||||||
| 25(OH)D3 concentration, ng/mL | 24.0 (9.7) | 24.9 (10.5) | 26.1 (11.6) | 27.3 (11.4) | 24.5 (10.2) | 25.5 (10.8) | ||||||
| Season of blood drawb | ||||||||||||
| Winter | 27 | 23 | 18 | 19 | 25 | 22 | ||||||
| Spring | 27 | 27 | 37 | 38 | 30 | 30 | ||||||
| Summer | 21 | 23 | 24 | 28 | 22 | 24 | ||||||
| Fall | 24 | 27 | 21 | 15 | 23 | 24 | ||||||
| Family history of colorectal cancerc | 13 | 30a | 18 | 30a | 14 | 30a | ||||||
| Regularly take an NSAIDd | 12 | 20a | 23 | 35e | 15 | 24a | ||||||
| Regularly take aspirind | 28 | 31 | 37 | 38 | 30 | 33 | ||||||
| Postmenopausal (women only) | 84 | 68a | 83 | 81 | 83 | 71a | ||||||
| HRT userf | 44 | 67a | 71 | 71 | 51 | 68a | ||||||
| Current smoker | 20 | 16a | 34 | 14a | 23 | 15a | ||||||
| Body mass index, kg/m2 | 27.4 (4.8) | 26.9 (5.0) | 27.8 (6.2) | 27.4 (6.0) | 27.5 (5.1) | 27.1 (5.3) | ||||||
| Physical activity, MET-hours/week | 270.4 (283.1) | 233.4 (218.9)e | 193.8 (139.4) | 183.5 (128.4) | 253.0 (259.5) | 220.2 (200.2)e | ||||||
| Multivitamin supplement user | 23 | 31a | 33 | 39 | 26 | 33a | ||||||
| Vitamin D supplement user | 19 | 25e | 30 | 36 | 22 | 28a | ||||||
| Dietary intake per day | ||||||||||||
| Total energy, kcal | 2,115 (789) | 2,007 (711)e | 2,069 (862) | 1,780 (923)a | 2,104 (806) | 1,946 (780)a | ||||||
| Vitamin D, IUg | 327 (256) | 328 (243) | 349 (270) | 345 (300) | 331 (259) | 332 (259) | ||||||
| Total calcium, mgg | 962 (529) | 987 (523) | 856 (458) | 893 (492) | 937 (515) | 962 (516) | ||||||
| Dietary calcium, mg | 865 (452) | 854 (448) | 712 (349) | 628 (305)e | 830 (435) | 793 (426) | ||||||
| Supplemental calcium, mg | 97 (262) | 132 (320) | 143 (326) | 265 (429)a | 108 (279) | 168 (358)a | ||||||
| Retinol, IUg | 3,039 (2,981) | 3,327 (3,772) | 3,474 (3,512) | 3,752 (4,913) | 3,140 (3,115) | 3,442 (4,112) | ||||||
| Folate, μgg | 400 (232) | 408 (240) | 466 (246) | 471 (279) | 414 (237) | 425 (253) | ||||||
| Red and processed meats, servings | 7.5 (6.3) | 6.6 (5.1)e | 8.4 (8.8) | 6.9 (6.4) | 7.7 (7.0) | 6.6 (5.5) | ||||||
| Dietary fiber, g | 21.9 (9.8) | 21.6 (9.6) | 21.9 (9.8) | 19.2 (11.1)e | 21.9 (9.8) | 20.9 (10.1) | ||||||
| Alcohol, g | 10.0 (16.6) | 6.5 (13.4)a | 7.2 (14.6) | 4.5 (9.6)g | 9.4 (16.2) | 6.0 (12.6)a | ||||||
Abbreviations: CPRU, Cancer Prevention Research Unit; HRT, hormone replacement therapy; MAP, Markers of Adenomatous Polyps; MET, metabolic equivalent task; NSAID, nonsteroidal antiinflammatory drug; 25(OH)D, 25-hydroxyvitamin D3; SD, standard deviation.
Indicates P < 0.01 for comparison with adenoma cases; by Fisher's exact test for categorical variables and t test for continuous variables.
Winter: December, January, February; spring: March, April, May; summer: June, July, August; fall: September, October, November.
In a first-degree relative.
At least once a week.
Indicates P < 0.05 for comparison with adenoma cases; by Fisher's exact test for categorical variables and t test for continuous variables.
Calculated among postmenopausal women only.
From diet plus supplements.
Among all cases, 31.7% had at least one adenoma located in the right colon, 32.2% had multiple adenomas, and 26.8% had an adenoma that was ≥1 cm in diameter. In 23.2% of cases, the largest or most advanced adenoma was located in the right colon. The largest or most advanced adenoma had a pedunculated shape in 29.5% of cases and was of villous or tubulovillous histology in 28.7% of cases.
Vitamin D deficiency, defined as a 25(OH)D3 blood level of <20 ng/mL, was relatively common in both studies. In the CPRU study, 33.3% of participants were vitamin D deficient, and only 23.0% had sufficient levels of 25(OH)D3 above 32 ng/mL. In the MAP study, 29.8% of participants were vitamin D deficient, and 31.8% were vitamin D sufficient. When we pooled the studies, 32.4% and 25.2% of participants were vitamin D deficient and sufficient, respectively.
In the pooled analysis, higher levels of 25(OH)D3 were associated with a statistically significant decreased risk of colorectal adenoma after multivariable adjustment (highest vs. lowest month- and study-specific quartile odds ratio = 0.59, 95% confidence interval: 0.41, 0.84; Ptrend = 0.01). Similar inverse associations were observed in the separate analyses of the CPRU and the MAP studies. However, the association was stronger in the MAP study (odds ratio = 0.35, 95% confidence interval: 0.17, 0.70; Ptrend = 0.003). In the pooled study, we also analyzed circulating 25(OH)D3 as a continuous variable in the multivariable model (adjusted additionally for season of blood draw) and found a marginally statistically significant inverse association with colorectal adenoma per 10-ng/mL increment in 25(OH)D3 (odds ratio = 0.89, 95% confidence interval: 0.79, 1.00). The inverse association of circulating 25(OH)D3 with adenoma did not differ substantially according to adenoma characteristics (Table 2); however, the sample size was relatively small for these analyses.
Table 2.
Multivariable-adjusted Associations of Circulating 25(OH)D3 Concentrations With Colorectal Adenoma Overall and by Adenoma Characteristics in the CPRU, MAP (Combined MAPI and MAPII), and Pooled Studies, United States, 1991–2002
| Adenoma Characteristic and Quartile of 25(OH)D3 | CPRU Study, Minnesota |
MAP Study, North Carolina and South Carolina |
Pooled Analysis |
|||||||||
| No. of Cases | No. of Controls | ORa | 95% CIa | No. of Cases | No. of Controls | ORa | 95% CIa | No. of Cases | No. of Controls | ORa | 95% CIa | |
| All colorectal adenomas | ||||||||||||
| 1 | 110 | 126 | 1.00 | 44 | 44 | 1.00 | 154 | 170 | 1.00 | |||
| 2 | 130 | 150 | 0.85 | 0.57, 1.27 | 29 | 54 | 0.61 | 0.30, 1.22 | 159 | 204 | 0.77 | 0.55, 1.09 |
| 3 | 132 | 136 | 0.99 | 0.66, 1.48 | 35 | 48 | 0.55 | 0.27, 1.10 | 167 | 184 | 0.85 | 0.60, 1.20 |
| 4 | 102 | 151 | 0.69 | 0.45, 1.06 | 34 | 61 | 0.35 | 0.17, 0.70 | 136 | 212 | 0.59 | 0.41, 0.84 |
| Ptrendb | 0.16 | 0.003 | 0.01 | |||||||||
| Location | ||||||||||||
| Right colonc | ||||||||||||
| 1 | 36 | 126 | 1.00 | 19 | 44 | 1.00 | 55 | 170 | 1.00 | |||
| 2 | 43 | 150 | 0.76 | 0.42, 1.38 | 13 | 54 | 0.81 | 0.32, 2.07 | 56 | 204 | 0.76 | 0.46, 1.24 |
| 3 | 31 | 136 | 0.68 | 0.36, 1.31 | 10 | 48 | 0.34 | 0.12, 0.97 | 41 | 184 | 0.58 | 0.34, 0.98 |
| 4 | 27 | 151 | 0.52 | 0.27, 1.03 | 17 | 61 | 0.44 | 0.18, 1.09 | 44 | 212 | 0.50 | 0.29, 0.85 |
| Ptrendb | 0.06 | 0.04 | 0.007 | |||||||||
| Left colond | ||||||||||||
| 1 | 94 | 126 | 1.00 | 36 | 44 | 1.00 | 130 | 170 | 1.00 | |||
| 2 | 102 | 150 | 0.82 | 0.54, 1.26 | 24 | 54 | 0.57 | 0.27, 1.19 | 126 | 204 | 0.76 | 0.53, 1.08 |
| 3 | 120 | 136 | 1.06 | 0.70, 1.62 | 30 | 48 | 0.58 | 0.28, 1.23 | 150 | 184 | 0.92 | 0.63, 1.32 |
| 4 | 89 | 151 | 0.71 | 0.45, 1.11 | 24 | 61 | 0.30 | 0.14, 0.65 | 113 | 212 | 0.58 | 0.40, 0.85 |
| Ptrendb | 0.30 | 0.003 | 0.02 | |||||||||
| Multiplicity | ||||||||||||
| Multiple adenomas | ||||||||||||
| 1 | 35 | 126 | 1.00 | 17 | 44 | 1.00 | 52 | 170 | 1.00 | |||
| 2 | 35 | 150 | 0.70 | 0.37, 1.30 | 11 | 54 | 0.61 | 0.23, 1.67 | 46 | 204 | 0.69 | 0.41, 1.15 |
| 3 | 41 | 136 | 1.00 | 0.53, 1.87 | 13 | 48 | 0.53 | 0.19, 1.44 | 54 | 184 | 0.85 | 0.51, 1.43 |
| 4 | 29 | 151 | 0.57 | 0.29, 1.13 | 18 | 61 | 0.46 | 0.18, 1.17 | 47 | 212 | 0.55 | 0.32, 0.93 |
| Ptrendb | 0.25 | 0.11 | 0.06 | |||||||||
| Single adenoma | ||||||||||||
| 1 | 75 | 126 | 1.00 | 29 | 44 | 1.00 | 104 | 170 | 1.00 | |||
| 2 | 95 | 150 | 0.97 | 0.63, 1.49 | 18 | 54 | 0.59 | 0.27, 1.32 | 113 | 204 | 0.83 | 0.57, 1.21 |
| 3 | 91 | 136 | 1.04 | 0.66, 1.62 | 22 | 48 | 0.54 | 0.24, 1.18 | 113 | 184 | 0.87 | 0.60, 1.27 |
| 4 | 73 | 151 | 0.75 | 0.47, 1.20 | 16 | 61 | 0.26 | 0.11, 0.60 | 89 | 212 | 0.60 | 0.40, 0.89 |
| Ptrendb | 0.29 | 0.002 | 0.02 | |||||||||
| Sizee | ||||||||||||
| Large adenoma | ||||||||||||
| ≥1 cm | ||||||||||||
| 1 | 33 | 126 | 1.00 | 13 | 44 | 1.00 | 46 | 170 | 1.00 | |||
| 2 | 38 | 150 | 0.87 | 0.48, 1.59 | 9 | 54 | 0.69 | 0.24, 2.00 | 47 | 204 | 0.82 | 0.49, 1.37 |
| 3 | 44 | 136 | 1.26 | 0.69, 2.30 | 10 | 48 | 0.54 | 0.18, 1.62 | 54 | 184 | 0.98 | 0.59, 1.63 |
| 4 | 39 | 151 | 0.99 | 0.53, 1.85 | 10 | 61 | 0.34 | 0.12, 0.99 | 49 | 212 | 0.75 | 0.44, 1.26 |
| Ptrendb | 0.71 | 0.04 | 0.40 | |||||||||
| Small adenoma | ||||||||||||
| <1 cm | ||||||||||||
| 1 | 77 | 126 | 1.00 | 31 | 44 | 1.00 | 108 | 170 | 1.00 | |||
| 2 | 92 | 150 | 0.88 | 0.56, 1.36 | 20 | 54 | 0.63 | 0.29, 1.39 | 112 | 204 | 0.79 | 0.54, 1.15 |
| 3 | 88 | 136 | 0.92 | 0.58, 1.45 | 25 | 48 | 0.61 | 0.29, 1.32 | 113 | 184 | 0.83 | 0.57, 1.22 |
| 4 | 63 | 151 | 0.55 | 0.34, 0.90 | 24 | 61 | 0.36 | 0.17, 0.78 | 87 | 212 | 0.52 | 0.34, 0.77 |
| Ptrendb | 0.03 | 0.01 | 0.003 | |||||||||
| Shape | ||||||||||||
| Pedunculated | ||||||||||||
| 1 | 30 | 126 | 1.00 | 11 | 44 | 1.00 | 41 | 170 | 1.00 | |||
| 2 | 25 | 150 | 0.53 | 0.27, 1.05 | 6 | 54 | 0.44 | 0.12, 1.64 | 31 | 204 | 0.53 | 0.30, 0.96 |
| 3 | 33 | 136 | 1.11 | 0.57, 2.16 | 5 | 48 | 0.38 | 0.10, 1.49 | 38 | 184 | 0.81 | 0.46, 1.44 |
| 4 | 22 | 151 | 0.58 | 0.28, 1.20 | 6 | 61 | 0.28 | 0.07, 1.06 | 28 | 212 | 0.47 | 0.25, 0.86 |
| Ptrendb | 0.50 | 0.65 | 0.06 | |||||||||
| Sessile | ||||||||||||
| 1 | 51 | 126 | 1.00 | 32 | 44 | 1.00 | 83 | 170 | 1.00 | |||
| 2 | 80 | 150 | 1.21 | 0.74, 1.95 | 22 | 54 | 0.69 | 0.32, 1.49 | 102 | 204 | 1.01 | 0.68, 1.50 |
| 3 | 70 | 136 | 1.20 | 0.73, 1.97 | 29 | 48 | 0.66 | 0.31, 1.40 | 99 | 184 | 1.01 | 0.67, 1.51 |
| 4 | 52 | 151 | 0.78 | 0.46, 1.33 | 27 | 61 | 0.38 | 0.18, 0.81 | 79 | 212 | 0.65 | 0.42, 0.99 |
| Ptrendb | 0.31 | 0.01 | 0.05 | |||||||||
| Histologic type | ||||||||||||
| Villous or tubulovillous | ||||||||||||
| 1 | 42 | 126 | 1.00 | 6 | 44 | 1.00 | 48 | 170 | 1.00 | |||
| 2 | 42 | 150 | 0.75 | 0.43, 1.33 | 4 | 54 | 0.43 | 0.08, 2.28 | 46 | 204 | 0.73 | 0.43, 1.24 |
| 3 | 43 | 136 | 0.95 | 0.53, 1.69 | 3 | 48 | 0.23 | 0.04, 1.42 | 46 | 184 | 0.84 | 0.49, 1.44 |
| 4 | 28 | 151 | 0.69 | 0.38, 1.27 | 6 | 61 | 0.36 | 0.08, 1.63 | 44 | 212 | 0.66 | 0.38, 1.14 |
| Ptrendb | 0.39 | 0.20 | 0.22 | |||||||||
| Tubular | ||||||||||||
| 1 | 68 | 126 | 1.00 | 38 | 44 | 1.00 | 106 | 170 | 1.00 | |||
| 2 | 87 | 150 | 0.94 | 0.60, 1.48 | 25 | 54 | 0.63 | 0.31, 1.30 | 112 | 204 | 0.82 | 0.56, 1.18 |
| 3 | 89 | 136 | 1.08 | 0.68, 1.71 | 32 | 48 | 0.62 | 0.30, 1.26 | 121 | 184 | 0.91 | 0.62, 1.33 |
| 4 | 64 | 151 | 0.70 | 0.43, 1.13 | 27 | 61 | 0.32 | 0.16, 0.68 | 91 | 212 | 0.56 | 0.38, 0.84 |
| Ptrendb | 0.22 | 0.004 | 0.01 | |||||||||
| Degree of atypia of the worst adenoma | ||||||||||||
| Mild | ||||||||||||
| 1 | 45 | 126 | 1.00 | 15 | 44 | 1.00 | 60 | 170 | 1.00 | |||
| 2 | 67 | 150 | 1.05 | 0.64, 1.74 | 10 | 54 | 0.85 | 0.31, 2.35 | 77 | 204 | 1.00 | 0.64, 1.54 |
| 3 | 65 | 136 | 1.19 | 0.71, 2.00 | 16 | 48 | 1.01 | 0.38, 2.69 | 81 | 184 | 1.17 | 0.75, 1.83 |
| 4 | 35 | 151 | 0.53 | 0.30, 0.95 | 7 | 61 | 0.24 | 0.07, 0.77 | 42 | 212 | 0.49 | 0.30, 0.80 |
| Ptrendb | 0.06 | 0.04 | 0.02 | |||||||||
| Moderate/severe | ||||||||||||
| 1 | 65 | 126 | 1.00 | 29 | 44 | 1.00 | 94 | 170 | 1.00 | |||
| 2 | 63 | 150 | 0.73 | 0.45, 1.20 | 19 | 54 | 0.51 | 0.23, 1.17 | 82 | 204 | 0.66 | 0.44, 1.00 |
| 3 | 67 | 136 | 0.86 | 0.52, 1.41 | 19 | 48 | 0.32 | 0.14, 0.75 | 86 | 184 | 0.67 | 0.44, 1.02 |
| 4 | 67 | 151 | 0.77 | 0.47, 1.28 | 26 | 61 | 0.34 | 0.16, 0.73 | 93 | 212 | 0.63 | 0.42, 0.95 |
| Ptrendb | 0.50 | 0.005 | 0.05 | |||||||||
Abbreviations: CI, confidence interval; CPRU, Cancer Prevention Research Unit; MAP, Markers of Adenomatous Polyps; 25(OH)D, 25-hydroxyvitamin D3; OR, odds ratio.
Odds ratios with 95% confidence intervals were adjusted for age (continuous), sex, family history of colorectal cancer in a first-degree relative, regular use of aspirin or nonsteroidal antiinflammatory drugs, smoking (current, ever, or never), physical activity (continuous), body mass index (continuous), total red and processed meat intake (continuous), alcohol intake (continuous), calcium intake (continuous), retinol intake (continuous), and folate intake (continuous). Pooled odds ratios were adjusted for study (CPRU, MAP) in addition to the other covariates.
Ptrend values (2-sided) were calculated by including the median of each quartile of blood 25(OH)D3 concentration as a continuous variable in addition to all above-mentioned covariates in the multivariable models.
At least one adenoma in the right colon, which includes the cecum, ascending colon, hepatic flexure, and transverse colon.
At least one adenoma in the left colon, which includes the splenic flexure, descending colon, sigmoid colon, and rectum.
Adenoma size from in vivo comparison of maximum diameter to fully opened endoscope forceps.
We also examined whether the 25(OH)D3–adenoma association was modified by various demographic/lifestyle (Table 3) and dietary (Table 4) risk factors for colorectal neoplasms. The inverse association of circulating 25(OH)D3 with colorectal adenoma was stronger among those who regularly took aspirin or other NSAIDs than among those who reported no aspirin or NSAID use (Pinteraction = 0.04). Higher levels of circulating 25(OH)D3 were associated with statistically significant reductions in adenoma risk for older and more physically active participants, but the interactions were not statistically significant (Table 3). The associations of higher 25(OH)D3 concentration with adenoma did not differ substantially by sex, obesity, dietary soy intake, or, for women, by menopausal status or hormone replacement therapy use (data not shown). There were no substantial differences in the 25(OH)D3–adenoma association according to strata (less than or greater than or equal to the median) of dietary intakes of calcium or retinol (Table 4).
Table 3.
Multivariable-adjusted Associations of Study- and Month-specific Quartiles of Circulating 25(OH)D3 Concentrations With Colorectal Adenoma by Demographic and Lifestyle Characteristics in the Pooled CPRU and MAP Studies, United States, 1991–2002
| Characteristic and Quartile of 25(OH)D3 | No. of Cases | No. of Controls | ORa | 95% CIa | Pinteraction |
| Ageb | 0.35 | ||||
| <Median | |||||
| 1 | 42 | 69 | 1.00 | ||
| 2 | 38 | 96 | 0.58 | 0.32, 1.06 | |
| 3 | 54 | 93 | 0.85 | 0.48, 1.53 | |
| 4 | 44 | 98 | 0.67 | 0.36, 1.22 | |
| Ptrendc | 0.44 | ||||
| ≥Median | |||||
| 1 | 112 | 101 | 1.00 | ||
| 2 | 121 | 108 | 0.84 | 0.55, 1.28 | |
| 3 | 113 | 91 | 0.79 | 0.51, 1.21 | |
| 4 | 91 | 114 | 0.47 | 0.30, 0.73 | |
| Ptrendc | 0.001 | ||||
| Obesed | 0.14 | ||||
| No | |||||
| 1 | 108 | 108 | 1.00 | ||
| 2 | 102 | 151 | 0.60 | 0.40, 0.91 | |
| 3 | 121 | 143 | 0.73 | 0.48, 1.09 | |
| 4 | 116 | 177 | 0.55 | 0.36, 0.82 | |
| Ptrendc | 0.02 | ||||
| Yes | |||||
| 1 | 41 | 58 | 1.00 | ||
| 2 | 54 | 49 | 1.16 | 0.60, 2.23 | |
| 3 | 44 | 36 | 1.10 | 0.54, 2.23 | |
| 4 | 16 | 30 | 0.46 | 0.20, 1.06 | |
| Ptrendc | 0.14 | ||||
| Physical activitye | 0.06 | ||||
| <Median | |||||
| 1 | 87 | 110 | 1.00 | ||
| 2 | 84 | 99 | 1.00 | 0.63, 1.58 | |
| 3 | 76 | 81 | 1.14 | 0.71, 1.84 | |
| 4 | 51 | 93 | 0.55 | 0.33, 0.91 | |
| Ptrendc | 0.06 | ||||
| ≥Median | |||||
| 1 | 67 | 59 | 1.00 | ||
| 2 | 73 | 103 | 0.50 | 0.30, 0.86 | |
| 3 | 90 | 102 | 0.58 | 0.34, 0.97 | |
| 4 | 84 | 118 | 0.52 | 0.30, 0.88 | |
| Ptrendc | 0.05 | ||||
| NSAID or aspirin use | 0.04 | ||||
| <Once/week | |||||
| 1 | 77 | 84 | 1.00 | ||
| 2 | 90 | 111 | 0.86 | 0.54, 1.38 | |
| 3 | 109 | 94 | 1.33 | 0.83, 2.14 | |
| 4 | 86 | 105 | 0.92 | 0.56, 1.50 | |
| Ptrendc | 0.80 | ||||
| ≥Once/week | |||||
| 1 | 77 | 85 | 1.00 | ||
| 2 | 68 | 92 | 0.68 | 0.41, 1.13 | |
| 3 | 56 | 88 | 0.47 | 0.27, 0.79 | |
| 4 | 49 | 106 | 0.33 | 0.19, 0.56 | |
| Ptrendc | 0.0001 |
Abbreviations: CI, confidence interval; CPRU, Cancer Prevention Research Unit; MAP, Markers of Adenomatous Polyps; NSAID, nonsteroidal antiinflammatory drug; 25(OH)D, 25-hydroxyvitamin D3; OR, odds ratio.
Odds ratios with 95% confidence intervals were adjusted for age (continuous), sex, family history of colorectal cancer in a first-degree relative, regular use of aspirin or NSAIDs, study (CPRU vs. MAP), smoking (current, ever, or never), physical activity (continuous), body mass index (continuous), total red and processed meat intake (continuous), alcohol intake (continuous), calcium intake (continuous), retinol intake (continuous), and folate intake (continuous). A stratification variable was not included in the model.
Study-specific cutpoints were calculated on the basis of the median distribution in controls and were defined as follows for age: CPRU: <52 versus ≥52 years; MAP: <55 versus ≥55 years.
Ptrend values (2-sided) were calculated by including the median of each quartile of blood 25(OH) D3 as a continuous variable in addition to all above-mentioned covariates in the multivariable models.
Obesity was defined as a body mass index of ≥30 kg/m2.
Study-specific cutpoints were calculated on the basis of the median distributions in controls and were defined as follows for physical activity: CPRU: <166 versus ≥166 metabolic equivalent task-hours/week; MAP: <165 versus ≥165 metabolic equivalent task-hours/week.
Table 4.
Multivariable-adjusted Associations of Study- and Month-specific Quartiles of Circulating 25(OH)D3 Concentrations With Colorectal Adenoma by Dietary Intakes in the Pooled CPRU and MAP Studies, United States, 1991–2002
| Characteristic and Quartile of 25(OH)D3 | No. of Cases | No. of Controls | ORa | 95% CIa | Pinteraction |
| Total calcium intakeb | 0.94 | ||||
| <Median | |||||
| 1 | 99 | 99 | 1.00 | ||
| 2 | 86 | 108 | 0.75 | 0.48, 1.17 | |
| 3 | 82 | 74 | 0.95 | 0.59, 1.54 | |
| 4 | 68 | 93 | 0.58 | 0.35, 0.95 | |
| Ptrendc | 0.08 | ||||
| ≥Median | |||||
| 1 | 54 | 65 | 1.00 | ||
| 2 | 71 | 89 | 0.80 | 0.47, 1.38 | |
| 3 | 84 | 106 | 0.84 | 0.50, 1.42 | |
| 4 | 66 | 116 | 0.62 | 0.36, 1.06 | |
| Ptrendc | 0.10 | ||||
| Total retinol intaked | 0.87 | ||||
| <Median | |||||
| 1 | 91 | 91 | 1.00 | ||
| 2 | 91 | 108 | 0.81 | 0.51, 1.28 | |
| 3 | 82 | 82 | 0.97 | 0.60, 1.56 | |
| 4 | 62 | 93 | 0.59 | 0.35, 0.98 | |
| Ptrendc | 0.10 | ||||
| ≥Median | |||||
| 1 | 62 | 73 | 1.00 | ||
| 2 | 66 | 89 | 0.75 | 0.44, 1.26 | |
| 3 | 84 | 98 | 0.77 | 0.46, 1.30 | |
| 4 | 72 | 116 | 0.59 | 0.35, 1.00 | |
| Ptrendc | 0.07 |
Abbreviations: CI, confidence interval; CPRU, Cancer Prevention Research Unit; MAP, Markers of Adenomatous Polyps; 25(OH)D, 25-hydroxyvitamin D3; OR, odds ratio.
Odds ratios with 95% confidence intervals were adjusted for age (continuous), sex, family history of colorectal cancer in a first-degree relative, regular use of aspirin or nonsteroidal antiinflammatory drugs, study (CPRU vs. MAP), smoking (current, ever, or never), physical activity (continuous), body mass index (continuous), total red and processed meat intake (continuous), alcohol intake (continuous), total (dietary and supplemental) calcium intake (continuous), total retinol intake (continuous), and total folate intake (continuous). A stratification variable was not included in the model.
Study-specific cutpoints were calculated on the basis of median distribution in controls and were defined as follows for total (dietary and supplemental) calcium intake: CPRU: <917 versus ≥917 mg/day; MAP: <763 versus ≥763 mg/day.
Ptrend values (2-sided) were calculated by including the median of each quartile of blood 25(OH)D3 as a continuous variable in addition to all above-mentioned covariates in the multivariable models.
Study-specific cutpoints were calculated on the basis of median distribution in controls and were defined as follows for total (dietary and supplemental) retinol intake: CPRU: <2,245 versus ≥2,245 IU/day; MAP: <2,089 versus ≥2,089 IU/day.
DISCUSSION
In this pooled study, the largest colorectal adenoma case-control study known to date, we observed a substantial, statistically significant inverse association between circulating 25(OH)D3 concentrations and risk of incident, sporadic colorectal adenomas. Our results also suggested that this association may be stronger among those who regularly take aspirin or other NSAIDs but that the association may not substantially differ by other demographic, lifestyle, or dietary risk factors or according to adenoma characteristics.
Proposed mechanisms for vitamin D involve promoting bile acid catabolism, direct effects on the cell cycle, growth factor signaling, and immunomodulation (7, 8). Antineoplastic effects of vitamin D on colon tissue are also supported by recent findings that normal colorectal epithelium expresses the vitamin D receptor and vitamin D metabolizing enzymes (CYP27B1 and CYP24A1) and therefore can locally produce and degrade the active form of vitamin D, 1,25-(OH) vitamin D, from 25(OH)D (2–4).
In epidemiologic studies that have investigated dietary vitamin D intake without considering exposure to ultraviolet B light, the association between vitamin D intake and colorectal neoplasms has been inconsistent (8). This inconsistency may be explained by misclassification of actual vitamin D exposure. All epidemiologic studies that examined circulating vitamin D levels (14–20), except one (17), found inverse associations of 25(OH)D with colorectal adenomas. A recent meta-analysis found that both 25(OH)D and vitamin D intake were inversely associated with incident and recurrent colorectal adenomas (9), but only the finding for 25(OH)D was strong and statistically significant. Similarly, whereas only 16 of 26 studies that assessed dietary vitamin D found inverse associations with colorectal cancer (8, 27), all 8 studies that assessed vitamin D exposure with 25(OH)D found inverse associations (21–28); 2 were statistically significant (25, 26). Consistent with these data, we found that high versus low circulating 25(OH)D3 concentration was statistically significantly, strongly inversely associated with incident, sporadic colorectal adenoma.
Circulating vitamin D level is a better marker of vitamin D exposure than indirect estimates of vitamin D exposure based solely on diet because of its long half-life in the circulation and lack of tight homeostatic regulation of its concentration; 25(OH)D reflects vitamin D supply and usage over a period of time (13). However, using circulating 25(OH)D3 levels as indicative of vitamin D exposure must take into account seasonal variations in vitamin D levels. The previously discussed epidemiologic studies of 25(OH)D and colorectal adenoma sought to minimize potential bias from seasonal variation by either matching the cases and controls on date of blood draw (17) or controlling for month of blood draw in the model (15, 16, 18–20). In our analyses, we used study- and month-specific 25(OH)D3 cutpoints, which in simulation studies were found to be a preferred method for accounting for seasonal variability (37).
Regular aspirin or NSAID use reduces colorectal neoplasm risk (38–40). The major mechanism of the antineoplastic action of antiinflammatory medications is inhibition of the proinflammatory cyclooxygenase-2 pathway. Proinflammatory markers such as tumor necrosis factor-α may interfere with vitamin D signaling through the nuclear factor-kappaB pathway by decreasing the transcription efficiency of vitamin D–responsive genes (41) and down-regulating the human 1α-hydroxylase (CYP27B1) promoter (42). In turn, the active form of vitamin D, 1,25-(OH)2 vitamin D, and/or its analogs may inhibit cyclooxygenase-2 activity (43, 44), modulate arachidonic acid release, decrease prostaglandin E1 and E2 levels (45), and induce 15-prostaglandin dehydrogenase expression (44). To our knowledge, no reported studies of humans have investigated the joint association of vitamin D and NSAIDs on colorectal neoplasm incidence or recurrence. Consistent with synergistic effects of higher 25(OH)D3 and NSAID use on colorectal adenoma risk, we found that the inverse association between circulating 25(OH)D3 and colorectal adenoma was stronger among persons who regularly took aspirin or other NSAIDs (Pinteraction = 0.04), a finding that requires further investigation.
Obese individuals have chronic low-grade inflammation, which is characterized by greater production of proinflammatory cytokines (e.g., tumor necrosis factor-α and interleukin-6) by adipose tissue (46). Furthermore, adipose tissue stores fat-soluble vitamin D3, resulting in lower circulating 25(OH)D3 levels. In addition, for US adults, more frequent physical activity may be associated with decreased inflammation, as indicated by decreased C-reactive protein levels (47, 48). We also found that the 25(OH)D3–colorectal adenoma association was suggestively stronger among nonobese and more physically active participants; however, the interactions were not statistically significant.
Vitamin D and calcium are highly physiologically interrelated, and both agents are thought to be important in colorectal carcinogenesis. They both influence bile-acid metabolism and modulate multiple proteins and genes involved in colorectal carcinogenesis (8). However, few observational epidemiologic studies have investigated whether vitamin D and calcium synergistically modify risk of colorectal adenoma; among them, very few have presented complete data for assessing a potential interaction (14, 18, 49). Results of these studies suggest that calcium and vitamin D may interact to reduce risk of colorectal adenomas, albeit inconclusively. Furthermore, in a meta-analysis of 4 epidemiologic studies (14, 16, 18, 20) that stratified by calcium intake, an inverse 25(OH)D–adenoma association was found with both high (odds ratio = 0.67, 95% confidence interval: 0.46, 0.97) and low (odds ratio = 0.78, 95% confidence interval: 0.54, 1.12) calcium intakes (9). Our findings did not support the hypothesis that the 25(OH)D3–adenoma association differs by calcium intake.
Retinol may antagonize the actions of vitamin D by competing for the same substrate, the retinoid X receptor (50–52); thus, high dietary intake of retinol may diminish protective effects of vitamin D. At least one observational study, the Nurses’ Health Study (n = 48,115) (49), found that risk of colorectal adenoma was lowest for persons with high vitamin D/low retinol intake compared with those with low vitamin D/high retinol intake (relative risk = 0.55, 95% confidence interval: 0.28, 1.10; Pinteraction = 0.02). However, consistent with another report (53), we found no evidence for a vitamin D–retinol interaction.
Of the 2 forms of 25(OH)D measured in blood (D2 and D3), we used 25(OH)D3 as the primary measure of vitamin D status in our analysis. The main reason was the poor intraassay reproducibility for 25(OH)D2. Although 25(OH)D2 contributes to total circulating 25(OH)D, we expect this contribution to be minimal. In our data, the vast majority of participants had undetectable or very low levels of 25(OH)D2. Unlike vitamin D3, vitamin D2 cannot be synthesized by humans and is present mostly in fungus-/yeast-derived products. In addition, compared with vitamin D3, vitamin D2 may have lower bioefficacy, which may be due to lower binding affinities of vitamin D2 and its metabolites to the vitamin D receptor, vitamin D binding protein, and CYP27A1 enzyme (54); however, more research is needed to understand the biologic differences between the 2 vitamin D forms. Our additional analyses of total 25(OH)D were consistent with those reported for 25(OH)D3. Therefore, the potential misclassification of participants’ vitamin D status due to excluding circulating 25(OH)D2 concentrations appears negligible.
This study had some limitations. Because the study population included only older whites who underwent colonoscopy, results from this analysis may not be representative of the general population. Colonoscopy controls in the CPRU study (which was conducted between 1991 and 1994, before the use of colonoscopies for routine screening purposes) represent a highly selected group of participants, a substantial proportion of whom were either symptomatic or had known risk factors such as positive family history of colorectal cancer. The proportion of symptomatic and/or high-risk individuals was lower in the MAP study, in which still only one-quarter of the participants underwent colonoscopy for routine screening. Because most participants in our study had an indication for undergoing a colonoscopy, vitamin D status and exposure to other risk factors may have been similar between cases and controls, resulting in attenuation of the results toward the null. Consistent with this expectation are the results of our analyses stratified by reason for colonoscopy, which showed that the 25(OH)D3–colorectal adenoma association was stronger among participants who were asymptomatic or underwent routine screening colonoscopy (data shown in the Web-only supplement). Although this pooled study had 616 colorectal adenoma cases and 770 controls, the sample size for some subgroup analyses was still small.
One of the main strengths of this study was the use of circulating 25(OH)D3 levels instead of questionnaire-based measures of dietary vitamin D, thereby reducing misclassification of true vitamin D exposure. In addition, verifying the adenoma- and hyperplastic polyp–free status of controls by colonoscopy minimized outcome misclassification, and collecting detailed information on multiple covariates before case-control status was ascertained helped reduce both recall bias and unmeasured confounding. It is also important to note that, to our knowledge, this case-control study of incident colorectal adenomas is the largest reported to date.
In conclusion, our findings strongly support the hypothesis that higher vitamin D3 exposures may reduce risk of incident, sporadic colorectal adenoma. Our findings also suggest that vitamin D exposures may act synergistically with antiinflammatory agents to more markedly reduce risk of colorectal neoplasms.
Supplementary Material
Acknowledgments
Author affiliations: Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia (Veronika Fedirko, Roberd M. Bostick, Michael Goodman, W. Dana Flanders); Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Emory University, Atlanta, Georgia (W. Dana Flanders); Winship Cancer Institute, Emory University, Atlanta, Georgia (Veronika Fedirko, Roberd M. Bostick, Michael Goodman); and Department of Laboratory Medicine and Pathology, University of Minnesota Medical School, Minneapolis, Minnesota (Myron D. Gross).
This research was funded by the National Cancer Institute, National Institutes of Health (grants P01 CA50305, R01 CA66539, and R01 CA116795); Fullerton Foundation; Emory Winship Cancer Institute; Georgia Cancer Coalition Distinguished Scholar award (to R.M.B.); and the Franklin Foundation.
The authors thank Dr. Vin Tangpricha for critically reading the manuscript and providing valuable comments.
Conflict of interest: none declared.
Glossary
Abbreviations
- CPRU
Cancer Prevention Research Unit
- MAP
Markers of Adenomatous Polyps
- NSAID
nonsteroidal antiinflammatory drug
- 25(OH)D
25-hydroxyvitamin D
References
- 1.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9(3):227–231. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 2.Matusiak D, Murillo G, Carroll RE, et al. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1{alpha}-hydroxylase in normal and malignant human colon. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2370–2376. doi: 10.1158/1055-9965.EPI-05-0257. [DOI] [PubMed] [Google Scholar]
- 3.Tangpricha V, Flanagan JN, Whitlatch LW, et al. 25-hydroxyvitamin D-1alpha-hydroxylase in normal and malignant colon tissue. Lancet. 2001;357(9269):1673–1674. doi: 10.1016/S0140-6736(00)04831-5. [DOI] [PubMed] [Google Scholar]
- 4.Matusiak D, Benya RV. CYP27A1 and CYP24 expression as a function of malignant transformation in the colon. J Histochem Cytochem. 2007;55(12):1257–1264. doi: 10.1369/jhc.7A7286.2007. [DOI] [PubMed] [Google Scholar]
- 5.Ball GFM. Vitamins: Their Role in the Human Body. Ames, IA: Blackwell Science; 2004. [Google Scholar]
- 6.Ebert R, Schütze N, Adamski J, et al. Vitamin D signaling is modulated on multiple levels in health and disease. Mol Cell Endocrinol. 2006;248(1-2):149–159. doi: 10.1016/j.mce.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 7.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3(8):601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 8.Bostick RM, Goodman M, Sidelnikov E. Calcium and vitamin D. In: Potter JD, Lindor NM, editors. Genetics of Colorectal Cancer. New York, NY: Springer Science + Business Media, LLC; 2009. pp. 277–296. [Google Scholar]
- 9.Wei MY, Garland CF, Gorham ED, et al. Vitamin D and prevention of colorectal adenoma: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2958–2969. doi: 10.1158/1055-9965.EPI-08-0402. [DOI] [PubMed] [Google Scholar]
- 10.Gorham ED, Garland CF, Garland FC, et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol. 2005;97(1-2):179–194. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 12.Hanley DA, Davison KS. Vitamin D insufficiency in North America. J Nutr. 2005;135(2):332–337. doi: 10.1093/jn/135.2.332. [DOI] [PubMed] [Google Scholar]
- 13.Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr. 2008;88(2):500S–506S. doi: 10.1093/ajcn/88.2.500S. [DOI] [PubMed] [Google Scholar]
- 14.Levine AJ, Harper JM, Ervin CM, et al. Serum 25-hydroxyvitamin D, dietary calcium intake, and distal colorectal adenoma risk. Nutr Cancer. 2001;39(1):35–41. doi: 10.1207/S15327914nc391_5. [DOI] [PubMed] [Google Scholar]
- 15.Peters U, Hayes RB, Chatterjee N, et al. Circulating vitamin D metabolites, polymorphism in vitamin D receptor, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2004;13(4):546–552. [PubMed] [Google Scholar]
- 16.Peters U, McGlynn KA, Chatterjee N, et al. Vitamin D, calcium, and vitamin D receptor polymorphism in colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2001;10(12):1267–1274. [PubMed] [Google Scholar]
- 17.Platz EA, Hankinson SE, Hollis BW, et al. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and adenomatous polyps of the distal colorectum. Cancer Epidemiol Biomarkers Prev. 2000;9(10):1059–1065. [PubMed] [Google Scholar]
- 18.Grau MV, Baron JA, Sandler RS, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95(23):1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs ET, Alberts DS, Benuzillo J, et al. Serum 25(OH)D levels, dietary intake of vitamin D, and colorectal adenoma recurrence. J Steroid Biochem Mol Biol. 2007;103(3–5):752–756. doi: 10.1016/j.jsbmb.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller EA, Keku TO, Satia JA, et al. Calcium, dietary, and lifestyle factors in the prevention of colorectal adenomas. Cancer. 2007;109(3):510–517. doi: 10.1002/cncr.22453. [DOI] [PubMed] [Google Scholar]
- 21.Garland CF, Comstock GW, Garland FC, et al. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2(8673):1176–1178. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 22.Braun MM, Helzlsouer KJ, Hollis BW, et al. Colon cancer and serum vitamin D metabolite levels 10–17 years prior to diagnosis. Am J Epidemiol. 1995;142(6):608–611. doi: 10.1093/oxfordjournals.aje.a117682. [DOI] [PubMed] [Google Scholar]
- 23.Feskanich D, Ma J, Fuchs CS, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1502–1508. [PubMed] [Google Scholar]
- 24.Tangrea J, Helzlsouer K, Pietinen P, et al. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control. 1997;8(4):615–625. doi: 10.1023/a:1018450531136. [DOI] [PubMed] [Google Scholar]
- 25.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(7):684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 26.Woolcott CG, Wilkens LR, Nomura AM, et al. Plasma 25-hydroxyvitamin D levels and the risk of colorectal cancer: the Multiethnic Cohort Study. Cancer Epidemiol Biomarkers Prev. 2010;19(1):130–134. doi: 10.1158/1055-9965.EPI-09-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenab M, Bueno-de-Mesquita HB, Ferrari P, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. BMJ. 2010;340 doi: 10.1136/bmj.b5500. b5500. (doi: 10.1136/bmj.b5500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otani T, Iwasaki M, Sasazuki S, et al. Plasma vitamin D and risk of colorectal cancer: the Japan Public Health Center-Based Prospective Study. Br J Cancer. 2007;97(3):446–451. doi: 10.1038/sj.bjc.6603892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potter JD, Bigler J, Fosdick L, et al. Colorectal adenomatous and hyperplastic polyps: smoking and N-acetyltransferase 2 polymorphisms. Cancer Epidemiol Biomarkers Prev. 1999;8(1):69–75. [PubMed] [Google Scholar]
- 30.Boyapati SM, Bostick RM, McGlynn KA, et al. Calcium, vitamin D, and risk for colorectal adenoma: dependency on vitamin D receptor BsmI polymorphism and nonsteroidal anti-inflammatory drug use? Cancer Epidemiol Biomarkers Prev. 2003;12(7):631–637. [PubMed] [Google Scholar]
- 31.Gong YL, Xie DW, Deng ZL, et al. Vitamin D receptor gene Tru9I polymorphism and risk for incidental sporadic colorectal adenomas. World J Gastroenterol. 2005;11(31):4794–4799. doi: 10.3748/wjg.v11.i31.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniel CR, Bostick RM, Flanders WD, et al. TGF-alpha expression as a potential biomarker of risk within the normal-appearing colorectal mucosa of patients with and without incident sporadic adenoma. Cancer Epidemiol Biomarkers Prev. 2009;18(1):65–73. doi: 10.1158/1055-9965.EPI-08-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sidelnikov E, Bostick RM, Flanders WD, et al. MutL-homolog 1 expression and risk of incident, sporadic colorectal adenoma: search for prospective biomarkers of risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1599–1609. doi: 10.1158/1055-9965.EPI-08-0800. [DOI] [PubMed] [Google Scholar]
- 34.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien MJ, Winawer SJ, Zauber AG, et al. The National Polyp Study: patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990;98(2):371–379. [PubMed] [Google Scholar]
- 36.Saenger AK, Laha TJ, Bremner DE, et al. Quantification of serum 25-hydroxyvitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006;125(6):914–920. doi: 10.1309/J32U-F7GT-QPWN-25AP. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Jacobs EJ, McCullough ML, et al. Comparing methods for accounting for seasonal variability in a biomarker when only a single sample is available: insights from simulations based on serum 25-hydroxyvitamin D. Am J Epidemiol. 2009;170(1):88–94. doi: 10.1093/aje/kwp086. [DOI] [PubMed] [Google Scholar]
- 38.Chan AT, Giovannucci EL, Meyerhardt JA, et al. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294(8):914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asano TK, McLeod RS. Non steroidal anti-inflammatory drugs (NSAID) and aspirin for preventing colorectal adenomas and carcinomas. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD004079.pub2. (2):CD004079. (doi: 10.1002/14651858.CD004079.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandler RS, Galanko JC, Murray SC, et al. Aspirin and nonsteroidal anti-inflammatory agents and risk for colorectal adenomas. Gastroenterology. 1998;114(3):441–447. doi: 10.1016/s0016-5085(98)70526-8. [DOI] [PubMed] [Google Scholar]
- 41.Lu X, Farmer P, Rubin J, et al. Integration of the NfkappaB p65 subunit into the vitamin D receptor transcriptional complex: identification of p65 domains that inhibit 1,25-dihydroxyvitamin D3-stimulated transcription. J Cell Biochem. 2004;92(4):833–848. doi: 10.1002/jcb.20143. [DOI] [PubMed] [Google Scholar]
- 42.Ebert R, Jovanovic M, Ulmer M, et al. Down-regulation by nuclear factor kappaB of human 25-hydroxyvitamin D3 1alpha-hydroxylase promoter. Mol Endocrinol. 2004;18(10):2440–2450. doi: 10.1210/me.2002-0441. [DOI] [PubMed] [Google Scholar]
- 43.Aparna R, Subhashini J, Roy KR, et al. Selective inhibition of cyclooxygenase-2 (COX-2) by 1alpha,25-dihydroxy-16-ene-23-yne-vitamin D3, a less calcemic vitamin D analog. J Cell Biochem. 2008;104(5):1832–1842. doi: 10.1002/jcb.21749. [DOI] [PubMed] [Google Scholar]
- 44.Moreno J, Krishnan AV, Swami S, et al. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65(17):7917–7925. doi: 10.1158/0008-5472.CAN-05-1435. [DOI] [PubMed] [Google Scholar]
- 45.Boyan BD, Sylvia VL, Dean DD, et al. Differential regulation of growth plate chondrocytes by 1alpha,25-(OH)2D3 and 24R,25-(OH)2D3 involves cell-maturation-specific membrane-receptor-activated phospholipid metabolism. Crit Rev Oral Biol Med. 2002;13(2):143–154. doi: 10.1177/154411130201300205. [DOI] [PubMed] [Google Scholar]
- 46.Bulló M, García-Lorda P, Megias I, et al. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;11(4):525–531. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 47.Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13(5):561–568. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002;162(11):1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 49.Oh K, Willett WC, Wu K, et al. Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol. 2007;165(10):1178–1186. doi: 10.1093/aje/kwm026. [DOI] [PubMed] [Google Scholar]
- 50.Segaert S, Garmyn M, Degreef H, et al. Retinoic acid modulates the anti-proliferative effect of 1,25-dihydroxyvitamin D3 in cultured human epidermal keratinocytes. J Invest Dermatol. 1997;109(1):46–54. doi: 10.1111/1523-1747.ep12276488. [DOI] [PubMed] [Google Scholar]
- 51.Oberg F, Botling J, Nilsson K. Functional antagonism between vitamin D3 and retinoic acid in the regulation of CD14 and CD23 expression during monocytic differentiation of U-937 cells. J Immunol. 1993;150(8 pt 1):3487–3495. [PubMed] [Google Scholar]
- 52.Rohde CM, DeLuca HF. All- trans retinoic acid antagonizes the action of calciferol and its active metabolite, 1,25-dihydroxycholecalciferol, in rats. J Nutr. 2005;135(7):1647–1652. doi: 10.1093/jn/135.7.1647. [DOI] [PubMed] [Google Scholar]
- 53.Wu K, Feskanich D, Fuchs CS, et al. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99(14):1120–1129. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 54.Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84(4):694–697. doi: 10.1093/ajcn/84.4.694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.