Abstract
The purpose of this analysis was to characterize the natural history of weight change in the years prior to death among older persons and to examine how this pattern varies according to longevity and cause of death. Weight trajectories were analyzed by using data from 800 male decedents from the Baltimore Longitudinal Study of Aging (Maryland, 1958–2005) observed beginning an average of 19 years before death. A model including 3 distinct periods of weight change (weight stability/gain, mild weight loss, and accelerated weight loss before death) provided the best fit for all age-at-death groups. Approximately 9 years before death, the rate of weight loss increased to an average of 0.39 kg/year (P < 0.001) for all-cause mortality. For cancer deaths, weight loss accelerated significantly 3 years before death, regardless of age group. For cardiovascular deaths, the best-fitting inflection point increased with age, from 5 years for participants aged 60–69 years to 9–10 years before death for those aged 80 years or older. Results suggest that weight loss in older persons may begin earlier than previously believed. The duration of weight loss for noncancer deaths suggests that even distal changes in energy balance may be linked to risk of death.
Keywords: aged, cachexia, cause of death, cohort studies, mortality, weight loss
Many older individuals experience unintentional weight loss at older ages (1, 2), which is generally considered a marker of precipitous health deterioration and increased mortality risk. Thus, onset and rate of weight loss may be associated with time to death, in addition to chronologic age. Furthermore, because different pathologic conditions have distinct survival trajectories and metabolic effects, the timing and rate of weight loss before death may vary by underlying disease process.
Studies of the association between body weight and mortality have attempted to account for the effect of illness on weight by “temporal separation,” excluding deaths in the first 3–5 years of follow-up (3–5). However, exclusion of early deaths does not substantially change estimated associations between body mass index and mortality (5). In fact, weight loss may start as long as 15–20 years before death (6–8), implying that even distal unintentional weight loss reflects altered physiology linked to risk of death. This possibility is consistent with findings that weight loss in old age and elevated basal metabolic rate predict mortality independent from chronic disease (9–11).
Understanding the timing of weight loss in relation to mortality may shed light on the mechanisms linking age, weight change, chronic conditions, and death. For instance, a relatively uniform period of accelerated weight loss across age groups would suggest a “terminal decline” in weight, similar to that observed for cognitive function (12). Weight loss onset many years before death would indicate that weight loss may precede the clinical development of mortality-related conditions, as has been observed for dementia (13). Alternately, no marked acceleration in weight loss related to time to death could suggest that weight loss before death is driven by aging and age-related conditions not uniquely associated with time to death.
Little research has attempted to identify the typical length, severity, and pattern of weight loss in the period preceding death. A better understanding of weight loss patterns at the end of life would inform research on changes in energy balance in late life and the effects of obesity on health status in older persons. The purpose of this analysis was to characterize weight change in individuals who die at different ages and from different causes.
MATERIALS AND METHODS
Data
Data were drawn from Baltimore Longitudinal Study of Aging participants. A description of the Baltimore Longitudinal Study of Aging sample is available elsewhere (14). Male participants have been continuously recruited since 1958, primarily from the Baltimore, Maryland–Washington, DC, area. Women entered the study in 1978; too few have died to evaluate associations between weight and mortality in women. Assessment intervals varied over the course of the study. Initially, Baltimore Longitudinal Study of Aging assessments occurred every 2 years, but intervals have been revised to test participants younger than age 50 years every 4 years, those aged 50–79 years every 2 years, and those aged 80 years or older every year.
Eligible participants were community-dwelling males who survived to age 60 years (N = 800) and died before December 31, 2005. Data included 6,591 observations of body weight across 800 participants, for an average of 8.3 (standard deviation, 6.1) observations per person beginning an average 19.4 (standard deviation, 11.4) years before death. Mortality data were collected by telephone follow-up, correspondence with participants’ relatives, and the National Death Index. Vital status could be ascertained for 96% of participants. International Classification of Diseases, Ninth Revision, codes obtained from death certificates were available for 703 deaths (87.9%) and were used to classify causes of death as cancer (codes 140–239), cardiovascular (cardiovascular disease (CVD), codes 390–459), or other. Only 7 heart failure deaths were identified; they were included with all cardiovascular deaths. Weight in kilograms was measured on a standard balance scale after participants, wearing a hospital gown, had fasted overnight.
Statistical analysis
Analysis was performed by fitting mixed-effects models to all available longitudinal observations using the Proc Mixed procedure in SAS software (SAS Institute, Inc., Cary, North Carolina). Participants were classified by age (years) at death into 4 groups: 60–69, 70–79, 80–89, and 90 or older. Our goal was to identify a data-driven set of clinically useful and interpretable functions that described expected weight loss as death approached. For the entire sample and for each age-at-death group, 5 models developed on the basis of theory and descriptive exploration of trajectories were compared by using the Akaike Information Criterion (AIC) for model fit.
The first model predicted weight by a linear specification of time to death:
where weightti represents weight in kilograms for individual i at time t, β0 represents the intercept (or weight at death, where time to death = 0), β1 represents the expected weight change associated with each 1-year distance from time to death, and b0i and b1i represent random effects in the intercept and the effect of time, respectively, for individual i. Random effects are assumed to be distributed normally with a mean of zero and an unstructured covariance matrix, and the error term, eij, is assumed to be independent and normally distributed with a mean of zero and constant variance.
The second model predicted weight by a quadratic specification of time to death:
A random effect for t2 was examined but not included here because models including this effect did not converge.
The third model predicted weight based on a linear spline specification with a single knot:
This model allows for different slopes for time to death before and after a knot point, k, where (t − k)+ = 0 for t ≤ k.
The fourth model extended this specification to allow for a quadratic effect of time on weight before a knot point, and a linear effect of time on weight after the knot point, as death approaches:
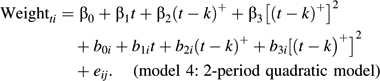 |
The final model allowed for 3 distinct periods of linear weight change across time to death:
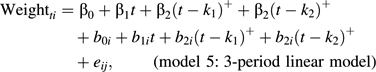 |
where k1 = inflection point 1 and k2 = inflection point 2 describe the times from death at which the slopes of weight loss change.
In models 3–5, knot points up to 25 years before death were examined. Confidence intervals for knot placements were estimated by using a likelihood ratio testing approach. The last integer valued knots that were significantly different from the best-fitting knot at the 0.025 level on either side were included as upper and lower confidence limits.
Sensitivity analyses were conducted to evaluate potential bias due to informative missingness in the last 3 years of life using 4 alternative imputation scenarios. For participants with missing visit information within 3 years of death (n = 426, 53.3%), we imputed a weight for this period under the following scenarios: 1) modest informatively missing weight loss: 4% weight loss since the last visit (consistent with the bottom quartile of observed weight changes in the last 3 years of life); 2) dramatic informatively missing weight loss: 8% weight loss since the last visit (consistent with the bottom decile of weight change); 3) modest informatively missing annual weight loss: 0.7% weight loss/year since the last visit (consistent with the bottom quartile of observed weight change/year in the last 3 years); and 4) dramatic informatively missing annual weight loss: 2.6% weight loss/year since the last visit (consistent with the bottom decile of observed weight change/year). A more detailed description of sensitivity analyses is included in the online Appendix, which is posted on the Journal’s Web site (http://aje.oupjournals.org/).
RESULTS
Table 1 provides causes of death by age group. Among all ages, CVD was the most common, accounting for nearly half of all deaths. With increasing age, the frequency of other deaths increased, from 14.6% of deaths in the age group 60–69 years to 41.2% in the age group 90 years or older.
Table 1.
Causes of Death, by Age Group, for Males From the Baltimore Longitudinal Study of Aging, Maryland,1958–2005
| Age Group, Years | Cancer (ICD-9 Codes 140–239) |
CVD (ICD-9 Codes 390–459) |
Other |
|||
| No. | % | No. | % | No. | % | |
| All ages | 143 | 20.3 | 349 | 49.6 | 211 | 30.0 |
| 60–69 | 23 | 28.1 | 47 | 57.3 | 12 | 14.6 |
| 70–79 | 48 | 24.9 | 94 | 48.7 | 51 | 26.4 |
| 80–89 | 60 | 21.4 | 133 | 47.5 | 87 | 31.1 |
| ≥90 | 12 | 8.1 | 75 | 50.7 | 61 | 41.2 |
Abbreviations: CVD, cardiovascular disease; ICD-9, International Classification of Diseases, Ninth Revision.
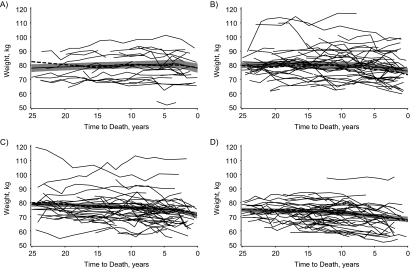
Figure 1 provides weight by time to death for 30 randomly selected participants in each age-at-death group to visualize heterogeneity in individual weight change trajectories. In spite of great variability in weight trajectories across individuals, progressively longer periods of weight decline are detectable with increasing longevity. Among individuals who died in their sixties, weight loss was largely confined to the last 5 years of life; among those who died at age 90 years or older, weight loss was evident at least 10 years before death for many individuals.
Figure 1.
Weight (in kilograms) by time to death for male decedents from the Baltimore Longitudinal Study of Aging, Maryland, 1958–2005. Thin solid lines: weight by time to death for a randomly selected male decedent; thick dashed lines: mean weight by time to death for all male decedents (N = 800); thick solid lines: predicted mean weight from model 5 (refer to the Statistical Analysis portion of the text) with 95% confidence interval (shaded region). Participants who died at A) age 60–69 years, B) age 70–79 years, C) age 80–89 years, D) age ≥90 years.
Table 2 provides the AIC, residual, variance, and covariance parameters for each model in the full sample (N = 800). The AIC decreased across models, with model 5 (3-period linear model) having the lowest AIC. Residual error also decreased across models, with similar residuals found for models 4 and 5. Significant variability in both intercept and slopes was observed in all models.
Table 2.
Model Fit Estimates and Variance Parameters: Alternative Models Predicting Weight, by Time to Death, of 800 Male Decedents From the Baltimore Longitudinal Study of Aging, Maryland, 1958–2005
| Model, Description | Model 1: Linear | Model 2: Quadratic | Model 3: 2-Period Linear | Model 4: 2-Period Quadratic | Model 5: 3-Period Linear |
| AIC | 36,942.4 | 36,756.0 | 36,084.9 | 35,826.1 | 35,783.0 |
| Residual (SE) | 7.61 (0.15) | 7.40 (0.15) | 6.07 (0.12) | 5.40 (0.11) | 5.36 (0.11) |
| Variance in model estimates (SE) | |||||
| A: Intercept | 180.88 (9.89) | 177.44 (9.68) | 181.75 (10.65) | 174.51 (10.95) | 172.56 (10.80) |
| B: Time to death | 0.19 (0.01) | 0.17 (0.01) | 0.38 (0.03) | 0.63 (0.06) | 0.63 (0.06) |
| C: Time >k1 | 0.54 (0.05) | 1.01 (0.12) | 0.98 (0.11) | ||
| D: (Time >k1)2 | 0.0006 (0.00008) | ||||
| E: Time >k2 | 0.57 (0.07) | ||||
| Covariance of A and B | −3.37 (0.31) | −3.11 (0.29) | −4.23 (0.46) | −4.14 (0.65) | −4.10 (0.65) |
| Covariance of A and C | 1.72 (0.55) | 0.15 (0.82) | 0.56 (0.80) | ||
| Covariance of A and D | 0.07 (0.02) | ||||
| Covariance of A and E | 1.53 (0.62) | ||||
| Covariance of B and C | −0.38 (0.04) | −0.57 (0.08) | −0.59 (0.07) | ||
| Covariance of B and D | −0.004 (0.002) | ||||
| Covariance of B and E | −0.07 (0.05) | ||||
| Covariance of C and D | −0.01 (0.00008) | ||||
| Covariance of C and E | −0.35 (0.07) |
Abbreviations: AIC, Akaike Information Criterion; SE, standard error.
Additional analyses tested the fit of each model in subgroups defined by age at death and cause of death. Fixed-effects results are shown for only the best-fit model (Table 3). Model 5 had the best fit (i.e., yielded the lowest AIC) for all age-at-death groups. Model 5 also had the best fit for cancer deaths and other deaths. Although model 4 provided the best fit for cardiovascular deaths in all ages combined, model 5 provided the best fit for cardiovascular deaths within each age-at-death group. Model 5 allows for 3 distinct periods of weight change postulated a priori (using a spline model with 2 knots), including an early period of weight gain, a period of weight stability or moderate decline, and a period of accelerated weight decline before death. In the average model for all ages, the best-fitting inflection points were at 17 and 9 years before death. From 10 to 17 years before death, participants lost an average of 0.12 kg/year, with weight stability before this period (weight change = 0.01 kg/year). During the last 9 years of life, the rate of weight loss was faster, and participants lost an average of 0.39 kg/year.
Table 3.
Fixed-effect Coefficients From the Best-fitting Model Predicting Weight by Time to Death for 800 Male Decedents From the Baltimore Longitudinal Study of Aging, Maryland, 1958–2005a
| Age Group, Years | No. | Inflection Points, Years |
Weight at Death |
Weight Loss Rate After Inflection |
Change in Weight Loss Rate Before Inflection 1 |
Change in Weight Loss Rate Before Inflection 2 |
|||||||
| k1 | CIb | k2 | CIb | B0 | P Value | B1 (Time) | P Value | B2 (Time > k1) | P Value | B3 (Time > k2) | P Value | ||
| Average Model for All Age Groups, All Causes of Death (N = 800) | |||||||||||||
| 60–107 | 800 | 9 | 7, 10 | 17 | 16, 18 | 73.517 | <0.001 | 0.390 | <0.001 | −0.270 | <0.001 | −0.109 | 0.019 |
| Model With Varied Knot and Slopes by Age Group, All Causes of Death (N = 800) | |||||||||||||
| 60–69 | 87 | 3 | 1, 6 | 15 | 8, 19 | 77.850 | <0.001 | 1.011 | 0.001 | −1.123 | <0.001 | −0.063 | 0.618 |
| 70–79 | 211 | 7 | 6, 9 | 18 | 17, 21 | 75.781 | <0.001 | 0.566 | <0.001 | −0.463 | 0.002 | −0.250 | 0.012 |
| 80–89 | 320 | 3 | 2, 4 | 16 | 14, 17 | 70.903 | <0.001 | 1.263 | <0.001 | −1.003 | 0.001 | −0.189 | 0.003 |
| ≥90 | 182 | 10 | 9, 12 | 16 | 14, 19 | 68.096 | <0.001 | 0.468 | <0.001 | −0.267 | 0.036 | −0.099 | 0.256 |
| Model With Varied Knot and Slopes by Age Group, Cancer Deaths (N = 143) | |||||||||||||
| 60–79 | 71 | 3 | 2c, 4 | 23 | 21, 23c | 75.496 | <0.001 | 0.874 | 0.032 | −0.955 | 0.024 | −0.099 | 0.439 |
| ≥80 | 72 | 3 | 2c, 5 | 18 | 16, 22 | 72.581 | <0.001 | 1.424 | 0.004 | −1.337 | 0.009 | 0.062 | 0.551 |
| Model With Varied Knot and Slopes by Age Group, Cardiovascular Deaths (N = 349) | |||||||||||||
| 60–69 | 47 | 5 | 5c, 5c | 24 | 20, 25c | 76.043 | <0.001 | 0.547 | 0.017 | −0.597 | 0.020 | 0.017 | 0.969 |
| 70–79 | 94 | 8 | 6, 9 | 20 | 18, 23 | 76.157 | <0.001 | 0.447 | 0.003 | −0.365 | 0.124 | −0.222 | 0.251 |
| 80–89 | 133 | 10 | 8, 12 | 25 | 22, 25d | 71.755 | <0.001 | 0.507 | <0.001 | −0.374 | 0.001 | −0.233 | 0.054 |
| ≥90 | 75 | 9 | 7, 11 | 18 | 14, 20 | 66.061 | <0.001 | 0.797 | <0.001 | −0.577 | 0.002 | −0.123 | 0.460 |
| Model With Varied Knot and Slopes by Age Group, Other Deaths (N = 211) | |||||||||||||
| 60–79 | 63 | 6 | 4, 10 | 17 | 15, 20 | 72.230 | <0.001 | 1.192 | <0.001 | −1.066 | 0.001 | −0.346 | 0.041 |
| 80–89 | 87 | 4 | 4c, 5 | 16 | 14, 18 | 68.992 | <0.001 | 0.870 | 0.028 | −0.469 | 0.290 | −0.292 | 0.044 |
| ≥90 | 61 | 4 | 4c, 7 | 10 | 8, 11 | 65.655 | <0.001 | 0.430 | 0.282 | 0.244 | 0.636 | −0.467 | 0.024 |
Abbreviation: CI, confidence interval.
Model includes no intercept term, so that coefficients can be interpreted directly for each age group. P values represent differences from zero based on 2-sided t test.
Confidence intervals are >95%. Knot placements were estimated by using a likelihood ratio testing approach, with the last integer valued knots that were significantly different from the best-fitting knot at the 0.025 level on either side included as upper and lower confidence limits.
Indicates that the model would not converge at the next value beyond this knot point.
Indicates outer limit of tested knots (25 years before death).
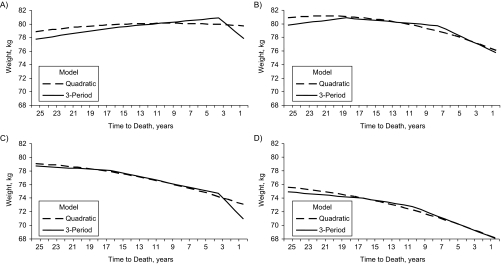
Results from both the quadratic model (model 2) and the 3-period model (model 5) estimated for each age group are directly compared in Figure 2. Model 2 is presented because it most closely simulates hypothesized normal aging processes, provided that disease and/or time to death do not cause departures from gradual weight loss observed with aging. In participants aged 60–69 years at death, results are similar until approximately 5 years before death, when the 3-period model suggests a departure from the quadratic pattern, namely, significantly steeper weight loss (1.01 kg/year) 3 years before death. In participants aged 70–79 years at death, the 3-period model also shows a departure from the quadratic model, with a significant change in the slope of weight decline 18 years before death and again 7 years before death. Similarly, in participants aged 80–89 years at death, the 3-period model shows significant changes in slope at 3 and 16 years before death. Finally, for participants aged 90 years or older at death, the only significant change in slope occurs 10 years before death, but the overall pattern of weight change is similar to that predicted by the quadratic model. It is notable that weight at death decreased with age, from 77.85 kg in those who died at ages 60–69 years to 68.10 kg in those who died at age 90 years or older.
Figure 2.
Predicted weight (in kilograms) by time to death and age at death for male decedents from the Baltimore Longitudinal Study of Aging, Maryland, 1958–2005. Participants who died at A) age 60–69 years (n = 87), B) age 70–79 years (n = 211), C) age 80–89 years (n = 320), D) age ≥90 years (n = 182). Refer to the Statistical Analysis portion of the text for a description of the models.
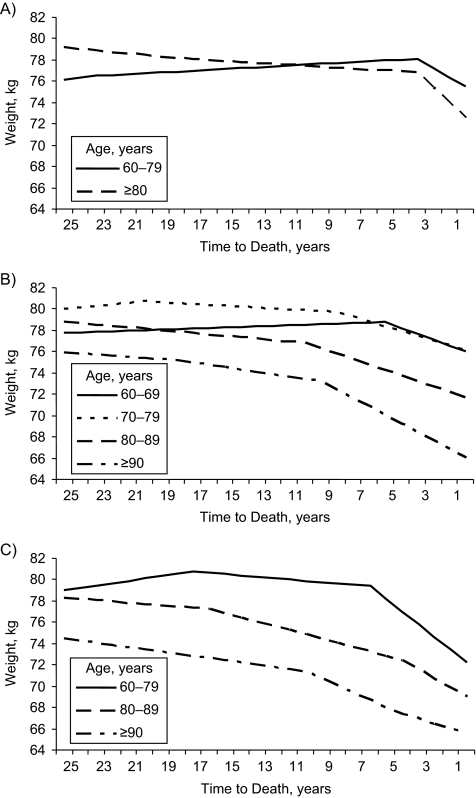
Additional analyses were stratified by cause of death (Figure 3). Age groups were combined when too few deaths were available for model convergence within single age groups. For cancer, weight loss accelerated significantly at 3 years before death, regardless of age group. Participants aged 60–79 years lost an average of 2.62 kg in this period (P = 0.032), and those aged 80 years or older lost an average of 4.27 kg (P = 0.004). For CVD, the best-fitting inflection point increased with age, from 5 years in participants aged 60–69 years to 9–10 years in those aged 80 years or older. For other (noncardiovascular, noncancer) deaths, significant changes in slope were observed at 6 years and 17 years before death in participants aged 60–79 years, such that weight was stable until 6 years before death (weight change = 0.13 kg/year, P = 0.372), followed by a decline of 1.19 kg/year (P < 0.001). In participants aged 80–89 years, weight loss increased to 0.40 kg/year 16 years before death, with a slight but nonsignificant additional inflection observed at 4 years before death. Similarly, in participants aged 90 years or older at death, weight loss increased to 0.67 kg/year 10 years before death, with a slight but nonsignificant additional inflection observed at 4 years before death.
Figure 3.
Predicted weight (in kilograms) by time to death, age at death, and cause of death for male decedents from the Baltimore Longitudinal Study of Aging, Maryland, 1958–2005. Shown are A) cancer deaths (n = 143), B) cardiovascular disease deaths (n = 349), C) other deaths (n = 211).
Complete results from sensitivity analyses evaluating potential bias due to informative missingness are provided in the Web Appendix. Across all alternative scenarios, our main finding was consistent: Weight loss likely begins earlier than previously thought, and the 3-period model (model 5) was supported as the best fitting for the majority of age and cause-of-death subgroups. Additionally, the best-fitting knots identified under alternative missing assumptions were within the confidence intervals from the main analysis for the majority of age and cause-of-death subgroups under all but the most extreme scenario (imputation 4, assuming 2.6% weight loss/year).
Missing data had the greatest potential to affect results in the smallest group examined, which included deaths occurring at ages 60–69 years (n = 87). In all alternative scenarios, the location of the second knot (k2) for this group was outside the confidence intervals estimated in our main results. However, the coefficient on this term was small and nonsignificant in both the main results and all imputation scenarios. We infer that this difference does not substantively affect interpretation of the results. Two imputation scenarios (imputations 2 and 4) also found a difference in the location of the best-fitting first knot (k1) for deaths occurring between ages 80 and 89 years, with the best-fitting knot occurring 9 years before death. Although the best-fitting knot identified in the main analysis occurred at 3 years before death, preliminary analysis also identified a local maxima in model fit 9 years before death, providing some support for this additional knot. With the exception of these 2 changes, large deviations in knot placement were observed under only the most extreme assumption of informative missingness (imputation 4), which assumed that all participants missing data in the last 3 years of life had experienced a 2.6% weight loss per year since their last observation.
DISCUSSION
We observed great heterogeneity in weight change trajectories across participants. A model that describes these trajectories as 3 periods, characterized by weight stability or weight gain followed by mild and then accelerated weight loss before death, was consistently supported by the data. This model provided the best fit across all ages and demonstrated important differences from a quadratic model that postulated more gradual change in weight with age.
Weight loss associated with cancer mortality was confined to the last 3 years before death across all age groups, which may reflect direct consequences of the disease (15). Interestingly, an end-of-life weight decline was also prominent in CVD deaths, with accelerated weight loss beginning on average 8–10 years before death for deaths after age 70 years. Other deaths were associated with extended periods of weight loss of at least 6 years, although noncancer, non-CVD deaths were rare before age 70 years.
Differences in weight change by age group may be due to systematic differences in cause of death between age groups, with cancer mortality more common at younger ages and other causes of death more frequent at older ages. When all ages and causes of death were combined, the average curve was similar to that observed for CVD, the most frequent cause of death in this sample. For participants who died at ages 80–89 years, evidence was found for best-fitting knots at 3, 9, and 16, which roughly correspond to the most predictive knots identified for cancer, CVD, and other deaths. Furthermore, within cause-of-death groups, knot locations were relatively similar across age groups above 70 years.
Alternately, differences in weight change by age group may result from rising instability in energetic equilibrium with age (16, 17). Because the prevalence of disease increases at older ages, individuals may lose homeostatic capacity and become more susceptible to the catabolic effect of chronic morbidity. Among persons who died at ages younger than 90 years, we observed a period of terminal drop in weight before death. Above age 90 years, large declines in physiologic reserve may lead to continuing gradual weight loss, regardless of illness severity. This is consistent with the notion that, in very long-lived individuals, cause of death may not be easily recognizable, reflecting progressive multisystem dysregulation and increased vulnerability to stressors more than any single condition.
Previous research has extensively documented that weight loss, particularly unintentional weight loss, is associated with increased mortality in older persons (2, 8, 18–20). Importantly, research has also shown that the association between body mass index and mortality is stronger based on midlife weight than on old-age weight (21) and reverses between middle age and old age: at younger ages, those with high weight have the highest risk; at older ages, those with low weight have the highest risk (8). These findings suggest that the biologic process that eventually leads to death in older persons is paralleled by progressive weight loss. To our knowledge, this study is the first to examine the duration, pattern, and severity of weight loss prior to death.
This study has several strengths. First is the frequency of weight measurements and the length of follow-up, including an average of 8 observations per person beginning an average of 19 years before death. Second, the sample included a large number of participants older than age 80 years (n = 502), allowing for stratification by age at death and cause of death. Third, an innovative statistical approach was used to characterize the natural history of weight loss in a flexible way that utilized all available data.
This study also has limitations. First, the sample included mostly white males (97%), who survived to an average age of 82 years and are likely to be healthier than the general population. Second, 53.3% of participants were missing data in the last 3 years of life. Sensitivity analyses suggest that our main findings (i.e., that weight loss begins earlier than previously thought and is well characterized by a 3-period model of weight change prior to death) are likely to be robust to missing data. However, a lack of data in the last 3 years of life could lead to an underestimate of weight loss in this critical period preceding death. Fourth, analysis did not differentiate between intentional and unintentional weight loss. Fifth, because the visit schedule varied by age, estimates of weight change after age 80 years are likely to be more precise than those at earlier ages. In particular, because of a relatively small sample size and less frequent observations, estimates for those who died before age 70 years are less stable than those for older age groups.
A final limitation is that our approach was largely descriptive, with model selection relying on fit statistics. AICs are meant as guides that reflect the relative support for each model presented, not necessarily to choose the single “best” or “final” model. In some cases, 2 or more models provided similar fit. For instance, in many cases, although model 5 yielded the lowest AIC, model 4 (the 2-period quadratic model) provided similar fit. Furthermore, although we tested 5 models, the potential set of functions relating expected weight loss to time to death is infinite. Our approach was based on the premise that “all models are wrong, but some are useful” (22, p. 424). Each model examined here has a theoretical basis, and different models may be preferred in different contexts.
Our findings have important theoretical and practical implications. Results document that weight loss accelerates in the period before death and that, for some causes of death, this acceleration appears to occur several years before death. Thus, the period of weight loss associated with chronic conditions resulting in mortality may be longer than previously believed and may precede clinical development of disease. In all age groups combined, weight loss accelerated an average of 9 years before death, suggesting that the usual practice of excluding deaths within a 5-year period is unlikely to account for bias due to weight loss in the association between weight and mortality. Therefore, existing research may have underestimated the positive effects of remaining at normal weight at older ages. With advancing age, the normal-weight group increasingly includes participants who have lost weight and thus have shorter life expectancies than those who have always been at normal weight.
Additionally, our finding that progressive weight loss occurs for a variety of causes of death reinforces research suggesting that weight loss may be an important prognostic indicator in older persons. Although wasting associated with cancer is confined to a short period, weight loss associated with both CVD and other causes began 5 or more years before death. This finding is consistent with research demonstrating an association between weight loss and both cardiovascular and noncardiovascular mortality (23–26). Weight loss may be a useful marker of physical decline and mortality risk in older persons, and regular assessments of weight may serve as an indicator of health status and mortality risk.
Supplementary Material
Acknowledgments
Author affiliations: Department of Epidemiology and Preventive Medicine, University of Maryland School of Medicine, Baltimore, Maryland (Dawn E. Alley); Clinical Research Branch, National Institute on Aging, Baltimore, Maryland (E. Jeffrey Metter, Eleanor M. Simonsick, Dan L. Longo, Luigi Ferrucci); Center of Biostatistics, University of Mississippi Medical Center, Jackson, Mississippi (Michael E. Griswold); and Laboratory of Epidemiology, Demography, and Biometry, National Institute on Aging, Bethesda, Maryland (Tamara B. Harris).
This work was supported by the National Institute on Aging Intramural Research Program; the Robert Wood Johnson Foundation Health and Society Scholars Program; and the Organized Research Center on Aging at the University of Maryland, Baltimore.
Conflict of interest: none declared.
Glossary
Abbreviations
- AIC
Akaike Information Criterion
- CVD
cardiovascular disease
References
- 1.Wannamethee SG, Shaper AG, Whincup PH, et al. Characteristics of older men who lose weight intentionally or unintentionally. Am J Epidemiol. 2000;151(7):667–675. doi: 10.1093/oxfordjournals.aje.a010261. [DOI] [PubMed] [Google Scholar]
- 2.Newman AB, Yanez D, Harris T, et al. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49(10):1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 3.Freedman DM, Ron E, Ballard-Barbash R, et al. Body mass index and all-cause mortality in a nationwide US cohort. Int J Obes (Lond) 2006;30(5):822–829. doi: 10.1038/sj.ijo.0803193. [DOI] [PubMed] [Google Scholar]
- 4.Andres R, Muller DC, Sorkin JD. Long-term effects of change in body weight on all-cause mortality. A review. Ann Intern Med. 1993;119(7 pt 2):737–743. doi: 10.7326/0003-4819-119-7_part_2-199310011-00022. [DOI] [PubMed] [Google Scholar]
- 5.Allison DB, Faith MS, Heo M, et al. Meta-analysis of the effect of excluding early deaths on the estimated relationship between body mass index and mortality. Obes Res. 1999;7(4):342–354. doi: 10.1002/j.1550-8528.1999.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg JA. Biases in the mortality risk versus body mass index relationship in the NHANES-1 Epidemiologic Follow-up Study. Int J Obes Relat Metab Disord. 2001;25(7):1071–1078. doi: 10.1038/sj.ijo.0801648. [DOI] [PubMed] [Google Scholar]
- 7.Dyer AR, Stamler J, Greenland P. Associations of weight change and weight variability with cardiovascular and all-cause mortality in the Chicago Western Electric Company Study. Am J Epidemiol. 2000;152(4):324–333. doi: 10.1093/aje/152.4.324. [DOI] [PubMed] [Google Scholar]
- 8.Losonczy KG, Harris TB, Cornoni-Huntley J, et al. Does weight loss from middle age to old age explain the inverse weight mortality relation in old age? Am J Epidemiol. 1995;141(4):312–321. doi: 10.1093/aje/141.4.312. [DOI] [PubMed] [Google Scholar]
- 9.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115(6):429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349(9058):1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 11.Ruggiero C, Metter EJ, Melenovsky V, et al. High basal metabolic rate is a risk factor for mortality: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2008;63(7):698–706. doi: 10.1093/gerona/63.7.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson RS, Beckett LA, Bienias JL, et al. Terminal decline in cognitive function. Neurology. 2003;60(11):1782–1787. doi: 10.1212/01.wnl.0000068019.60901.c1. [DOI] [PubMed] [Google Scholar]
- 13.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62(1):55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 14.Shock NW, Yiengst MJ. Age changes in basal respiratory measurements and metabolism in males. J Gerontol. 1955;10(1):31–40. doi: 10.1093/geronj/10.1.31. [DOI] [PubMed] [Google Scholar]
- 15.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2(11):862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 16.Wilson MM, Morley JE. Invited review: aging and energy balance. J Appl Physiol. 2003;95(4):1728–1736. doi: 10.1152/japplphysiol.00313.2003. [DOI] [PubMed] [Google Scholar]
- 17.Walston J. Frailty—the search for underlying causes. Sci Aging Knowledge Environ. 2004 doi: 10.1126/sageke.2004.4.pe4. 2004(4):pe4. (doi:10.1126/sageke.2004.4.pe4) [DOI] [PubMed] [Google Scholar]
- 18.Deeg DJ, Miles TP, Van Zonneveld RJ, et al. Weight change, survival time and cause of death in Dutch elderly. Arch Gerontol Geriatr. 1990;10(1):97–111. doi: 10.1016/0167-4943(90)90048-b. [DOI] [PubMed] [Google Scholar]
- 19.Allison DB, Zannolli R, Faith MS, et al. Weight loss increases and fat loss decreases all-cause mortality rate: results from two independent cohort studies. Int J Obes Relat Metab Disord. 1999;23(6):603–611. doi: 10.1038/sj.ijo.0800875. [DOI] [PubMed] [Google Scholar]
- 20.Locher JL, Roth DL, Ritchie CS, et al. Body mass index, weight loss, and mortality in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62(12):1389–1392. doi: 10.1093/gerona/62.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 22.Box GEP, Draper NR, editors. Empirical Model-Building and Response Surfaces. Hoboken, NJ: John Wiley & Sons; 1987. [Google Scholar]
- 23.Nilsson PM, Nilsson JA, Hedblad B, et al. The enigma of increased non-cancer mortality after weight loss in healthy men who are overweight or obese. J Intern Med. 2002;252(1):70–78. doi: 10.1046/j.1365-2796.2002.01010.x. [DOI] [PubMed] [Google Scholar]
- 24.Peters ET, Seidell JC, Menotti A, et al. Changes in body weight in relation to mortality in 6441 European middle-aged men: the Seven Countries Study. Int J Obes Relat Metab Disord. 1995;19(12):862–868. [PubMed] [Google Scholar]
- 25.Drøyvold WB, Lund Nilsen TI, Lydersen S, et al. Weight change and mortality: the Nord-Trøndelag Health Study. J Intern Med. 2005;257(4):338–345. doi: 10.1111/j.1365-2796.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 26.French SA, Folsom AR, Jeffery RW, et al. Prospective study of intentionality of weight loss and mortality in older women: the Iowa Women's Health Study. Am J Epidemiol. 1999;149(6):504–514. doi: 10.1093/oxfordjournals.aje.a009844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.