Abstract
Sibling and twin study designs provide control for confounding factors that are typically unmeasured in traditional cohort studies. Using nationally representative data from the National Longitudinal Study of Adolescent Health collected at 3 visits during 1994–2002, the authors evaluated the longitudinal association between birth weight and later obesity in a traditional cohort study (n = 13,763; ages 11–21 years at baseline), controlling for sex, age, race/ethnicity, and parental education. Among persons with a nonobese mother, high birth weight (>4 kg) participants were more likely than normal birth weight (≥2.5–≤4 kg) participants to become obese later in life (incidence rate ratio = 1.46, 95% confidence interval: 1.28, 1.67). In a matched sibling pair sample (full siblings: n = 513; monozygotic twins: n = 207; dizygotic twins: n = 189), the authors examined longitudinal within-pair differences. Birth weight difference was positively associated with body mass index difference later in life for female monozygotic pairs only (β = 2.67, 95% confidence interval: 0.99, 4.35). Given the null associations observed in the sibling sample, the commonly observed positive association between birth weight and later obesity from cohort analyses may be attributed to confounding by maternal characteristics. Further research is needed to identify specific factors that contribute to the birth weight–obesity relation.
Keywords: birth weight, cohort studies, longitudinal studies, obesity, siblings, twins
Obesity, a complex, multifactorial disease with genetic and environmental etiology (1, 2), continues to be a major and global public health concern. Early-life factors such as intrauterine growth may contribute to the development of obesity (3, 4). Birth weight, an indicator of intrauterine growth, is associated with childhood and adult obesity (5–14). Investigators in most cohort studies report a positive association, while others observe U-shaped or null associations (7–15).
Environmental and genetic factors may underlie the association between birth weight and size later in life (16). One hypothesis suggests that persons who experience an adverse intrauterine environment may metabolically and physiologically adapt to enhance survival early in life (17, 18); however, these adaptations may increase risk of later obesity in an obesogenic postnatal environment. In addition, intrauterine exposure to excess glucose via gestational diabetes and/or overnutrition may increase fetal insulin production and change pancreatic and brain development, resulting in increased birth size and altered postnatal body composition (19–22). Alternatively, a common genotype may influence both birth weight and later obesity (23). The complex independent and joint influences of intrauterine environmental and genetic factors on later size remain poorly understood.
Sibling and twin study designs offer a unique opportunity to better understand the relation between birth weight and obesity, since they provide control for environmental and genetic factors that are typically unmeasured in traditional cohort studies (16, 24–28). In general, full siblings share genetic and maternal environments, in contrast to unrelated persons in cohort studies (16, 24–28). Additionally, twins have identical gestational ages and equally share certain maternal exposures (e.g., maternal age or socioeconomic status). Further, monozygotic twins are genetically identical (16, 24–28). Thus, differences in birth weight within sibling and twin pairs are not likely to be due to these shared influences, but rather to factors influencing the growth of each individual (26). Comparisons within full-sibling and twin pairs can shed light on genetic and environmental influences of the association between birth weight and later size. Findings from twin studies are inconsistent and dominated by data from Caucasian populations (5, 6, 29–34). Thus, there is a great need for additional studies that provide findings in a racially/ethnically diverse sample.
Using longitudinal, nationally representative, prospective data enriched with sibling and twin pairs, we examined: 1) in the full cohort, the association between birth weight and obesity using traditional cohort analyses, hypothesizing that persons of high (versus normal) birth weight would be more likely to become obese in later life; and 2) in the full-sibling and twin pairs sample, the association between birth weight difference and body mass index (BMI; weight (kg)/height (m)2) difference using a within-pairs difference method, hypothesizing that the sibling with higher birth weight would be heavier during adolescence and young adulthood.
MATERIALS AND METHODS
The National Longitudinal Study of Adolescent Health (Add Health) was a prospective cohort study of 20,745 adolescents representative of the US school population in grades 7–12 in 1994–1995 (wave I) who were followed into adulthood. Wave II of Add Health (1996; n = 14,438) included wave I adolescents who had not graduated from high school, even if they had dropped out of high school. In wave III (2001–2002; n = 15,197), all wave I respondents were followed, regardless of wave II participation (ages 18–27 years). Waves II and III included an additional 29 respondents, for a total of 20,774 persons in the full cohort sample. The Add Health data set consists of a core sample plus additional subsamples, including full-sibling pairs and twin pairs, collected for the purpose of genetic analyses. Survey procedures have been described elsewhere and were approved by the institutional review board at the University of North Carolina at Chapel Hill (35).
Height and weight were self-reported in waves I, II, and III and measured in waves II and III during in-home surveys using standardized procedures. We used self-reported data from all waves to maximize the sample size and for comparability across waves. The discrepancy in weight change based on self-report data versus measured data in this data set was relatively minor and was not related to important covariates such as race/ethnicity, weight change efforts, activity, or inactivity, suggesting random differences versus systematic differences (36). As recommended for longitudinal studies in adolescents, we used BMI as the main outcome instead of BMI z score (37). Additionally, as recommended by expert panels and for comparability, obesity was defined consistently across racial/ethnic groups as BMI ≥95th percentile of the age- and sex-specific National Center for Health Statistics/Centers for Disease Control and Prevention growth reference or ≥30 for adolescents and BMI ≥30 for adults (38, 39). For women who were pregnant at the time of measurement, BMI and obesity status were coded as missing. Birth weight was reported by the adolescent's mother (93%) or caregiver (7%) during the in-home wave I parental interview. Covariates included age, sex, parental self-reported maternal obesity status (yes/no), parental self-reported paternal obesity status (yes/no), living with the sibling (for the family sample only (yes/no)), parental education, parental income, and race/ethnicity (by parental and adolescent report).
Full cohort analyses
From the initial 20,774 participants in the full cohort sample, there were 62,322 possible observations across the 3 study waves. We excluded participants who were Native American (n = 156), were severely disabled (n = 383), or had missing data on birth weight (n = 696)), height and weight (n = 505), maternal obesity (n = 2,991), sampling weights (n = 1,850), or covariates (n = 430). The final analytic sample included all available exposure, outcome, and covariate data collected across the 3 study waves, totaling 33,557 observations among 13,763 persons. Comparing the participants included in our analysis with those who were not, there were significant differences by race/ethnicity, parental education, age at baseline, maternal obesity, and BMI at baseline. To assess selection bias, we conducted additional multivariate analyses using inverse probability weighting, finding no evidence of selection bias in our final models (40, 41).
Statistical analyses were conducted using Stata (release 10.0; Stata Corporation, College Station, Texas). To account for the stratified sampling strategy and the clustered sampling design, we used sample weights and survey analysis techniques in all descriptive analyses of the longitudinal cohort. Percentages were calculated for categorical variables, while mean values were calculated for continuous variables. To compare persons with a nonobese mother at baseline to those with an obese mother at baseline, we used a χ2 test and F statistic to test statistical differences.
We pooled data from all 3 waves and used longitudinal, random-effects, Poisson regression models to examine the association between birth weight and obesity among unrelated persons (e.g., between-mother effect). These models adjusted for the correlation between repeated observations in the same subject and had the advantage of handling longitudinal data on subjects with varying numbers of observations, thereby allowing for inclusion of the maximum number of data points (42–44). Each individual contributed 1–3 observations (mean = 2.4). Since we observed a nonlinear relation between birth weight and obesity, we categorized birth weight as low (<2.5 kg), normal (≥2.5–≤4 kg (referent)), or high (>4 kg); alternatively, we split birth weight into 8 categories (<1.8, 1.8–2.2, 2.3–2.6, 2.7–3.1, 3.2–3.5 (referent), 3.6–4.0, 4.1–4.4, and ≥4.5 kg).
Given evidence suggesting that the birth weight–obesity relation may differ by age, maternal obesity, paternal obesity, and sex, we examined effect measure modification using interaction terms and likelihood ratio tests, with α = 0.10 (10, 21, 45, 46). Further, since most research in this area has been predominated by data from Caucasian populations, we explicitly examined whether race/ethnicity modified the association. Finding no such modification, we did not stratify results by race/ethnicity. Only maternal obesity was identified as an effect measure modifier (P = 0.04); thus, final models were stratified by maternal obesity. Potential confounders were included if they changed the main effect coefficient by ≥10% or if they met a conceptual rationale. Multivariate model results were adjusted for race/ethnicity, age, age squared, sex, and parental education.
Sibling sample
To expand the findings of traditional cohort analyses, we analyzed data from the Add Health sibling sample (1,270 full-sibling and twin pairs; 713 same-sex full-sibling, nontwin pairs; 270 same-sex dizygotic twin pairs; and 287 monozygotic twin pairs) to examine whether within-pair birth weight differences influenced within-pair BMI differences in adolescence and young adulthood (e.g., within-pair effect). Twin zygosity was determined by matching 12 molecular genetic markers at wave I or III or by full agreement of self-report measures, including confusability of appearance. Nontwin sibships were classified by self-report. From the initial 1,270 sibling pairs, there were 3,810 possible observations across the 3 study waves. Using the same exclusion criteria as for the full cohort, the analytic sample for the sibling sample consisted of 2,225 observations among 909 sibling pairs. Each pair contributed 1–3 observations (mean = 2.4).
We used longitudinal, random-effects, linear regression modeling to examine whether within-pair birth weight differences predicted within-pair BMI differences over time (equations 1 and 2). Difference measures were calculated between sibling 1 and sibling 2 (e.g., BMIsibling 1 − BMIsibling 2), with siblings randomly ordered. We hypothesized that if birth weight was positively associated with later weight, the sibling who was heavier at birth would also be heavier in adolescence and young adulthood. Therefore, if birth weight difference is positively associated with later BMI difference, we expected the regression coefficient for β1 to be significantly greater than 0 (if the association were inverse, β1 would be significantly less than 0; if there were no association, β1 would not differ significantly from 0). The models for full siblings and for dizygotic and monozygotic twins, respectively, are
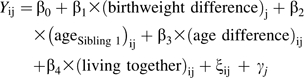 |
(1) |
and
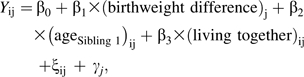 |
(2) |
where Y is the BMI difference between siblings in pair j at wave i, ξij is the error term specific to each pair j at each wave i, and γj is the error term specific to each pair j and constant across wave i.
We stratified the models by sibling pair type (full siblings, dizygotic twins, or monozygotic twins) to capture any heterogeneity in the relation between birth weight and later BMI which may be attributed to genetics and/or environment. Given research indicating that sex may differentially influence fetal growth, length of gestation, and body composition among twins (47, 48), we tested effect modification by the sex combination of the pair (using sex pair × birth weight difference interactions), finding effect modification for monozygotic twins only. For comparability across the models, all model results were stratified by sex of the pairs (male-male and female-female).
Potential confounders were identified using a directed acyclic graph (not shown) (49, 50). Only covariates that differed between siblings were included in the final models (age and living with the sibling). Characteristics shared between siblings, such as parental education, parental income, maternal obesity, paternal obesity, and race/ethnicity, were not included. For full sibling models, we used robust cluster commands in Stata to account for household clustering, since multiple sibling pairs per household were included. Sampling weights were not computed for the oversampled persons participating in the sibling sample.
RESULTS
Full cohort sample
Descriptive characteristics of the full cohort at baseline are given in Table 1. At baseline, persons with an obese mother were markedly different from persons with a nonobese mother with respect to birth weight, race/ethnicity, age, parental education, and obesity. Irrespective of maternal obesity status, obesity prevalence increased over the 8-year study period (results not shown).
Table 1.
Characteristics of the Analytic Sample at Baseline, According to Maternal Obesitya, National Longitudinal Study of Adolescent Health (n = 13,763), 1994–1995b
| Characteristic | Mother Nonobese (n = 11,285) | Mother Obese (n = 2,478) |
| Birth weight category, % | ||
| Low (<2.5 kg) | 8.4 (0.5)c | 4.8 (0.5) |
| Normal (2.5–4 kg) | 79.8 (0.6) | 76.3 (1.0) |
| High (>4 kg) | 11.8 (0.5) | 18.9 (0.9) |
| Mean birth weight, kg | 3.3 (0.01) | 3.5 (0.02) |
| Race/ethnicity, % | ||
| White | 70.9 (2.9) | 74.9 (2.6) |
| Black | 14.2 (2.0) | 14.3 (2.0) |
| Hispanic | 11.6 (1.7) | 9.4 (1.4) |
| Asian | 3.2 (0.7) | 1.4 (0.5) |
| Female sex, % | 48.5 (0.7) | 50.6 (1.5) |
| Mean age, years | 15.3 (0.1) | 15.5 (0.1) |
| Parental educationd, % | ||
| Not a high school graduate | 14.6 (1.3) | 11.9 (1.3) |
| High school graduate | 33.3 (1.2) | 31.1 (1.4) |
| Some college | 28.2 (0.8) | 33.7 (1.5) |
| College graduate | 24.0 (1.7) | 23.3 (1.3) |
| Obesitye, % | 7.6 (0.4) | 22.0 (1.2) |
| Mean BMIf | 21.8 (0.1) | 24.5 (0.2) |
Abbreviation: BMI, body mass index.
Maternal obesity was reported during the wave I parental interviews.
All results were weighted for national representation. The standard errors were corrected for multiple stages of cluster sample design and the unequal probability of selection.
Numbers in parentheses, standard error.
Parental education was defined as the highest level of education achieved by either parent.
For all racial/ethnic groups, adolescent obesity (age <20 years) was defined using the 2000 National Center for Health Statistics/Centers for Disease Control and Prevention growth chart (38, 39) age- and sex-specific BMI ≥95th percentile or BMI ≥30, and adult obesity (≥20 years) was defined as BMI ≥30.
Weight (kg)/height (m)2.
Using multivariate, longitudinal, random-effects Poisson regression modeling, we found a significant association between birth weight and obesity in persons with a nonobese mother (Table 2). High birth weight participants with a nonobese mother were more likely to be obese adolescents/young adults than normal birth weight participants (incidence rate ratio = 1.46, 95% confidence interval: 1.28, 1.67).
Table 2.
Association Between Birth Weight and Adolescent/Young Adult Obesity (Longitudinal Cohort), National Longitudinal Study of Adolescent Health, 1994–2002a
| Birth Weight Category, kg | Mother Nonobese (27,497 Observations, 11,285 Individuals) |
Mother Obese (6,060 Observations, 2,478 Individuals) |
||
| IRR | 95% CI | IRR | 95% CI | |
| Low (<2.5) | 1.07 | 0.93, 1.24 | 0.92 | 0.74, 1.14 |
| Normal (2.5–4) | 1.00 | Referent | 1.00 | Referent |
| High (>4) | 1.46 | 1.28, 1.67 | 1.15 | 1.00, 1.34 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
IRRs and 95% CIs were obtained from multivariate longitudinal, random-effects Poisson regression models predicting obesity, accounting for sex, age (years; continuous), age squared, race/ethnicity (white, black, Hispanic, or Asian), and parental education (highest level of education achieved by either parent; less than high school graduate, high school graduate, some college, or college graduate).
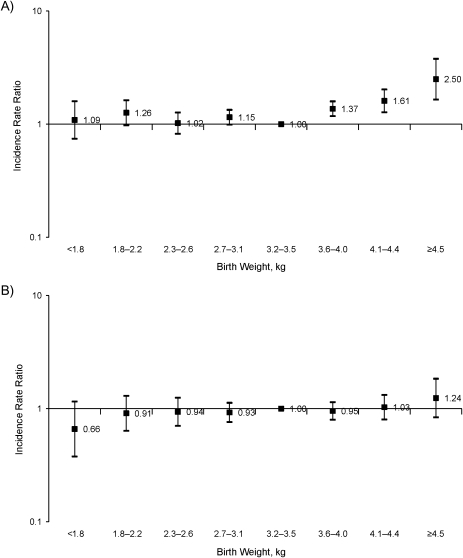
Parallel multivariate Poisson models using additional categories (8) of birth weight similarly showed that persons with a nonobese mother who weighed more than 3.6 kg at birth were significantly more likely to become obese than those weighing 3.2–3.5 kg at birth (Figure 1)—resulting, for example, in a 1.2-unit higher BMI later in life for a birth weight of 4.5 kg (24.1 units) versus a birth weight of 3.2 kg (22.9 units) (results not shown). In contrast, a comparable 1.3-kg difference in birth weight was not associated with later BMI differences for persons with an obese mother.
Figure 1.
Incidence rate ratios for obesity according to birth weight and parental self-reported maternal obesity status (panel A, mother nonobese; panel B, mother obese), National Longitudinal Study of Adolescent Health, 1994–2002. Incidence rate ratios were derived from a longitudinal, random-effects Poisson model with adjustment for age (in years), age squared (in years), sex, race/ethnicity, and parental education. Bars, 95% confidence interval.
Sibling sample
Approximately half of the dizygotic and monozygotic twins were low birth weight, as compared with 5% of full siblings (Table 3). Within sibling pairs, differences in birth weight and BMI were largest for full siblings and smallest for monozygotic twins. Within twin pairs, only 2 persons were high birth weight (monozygotic twins).
Table 3.
Selected Baseline Characteristics of the Family Sample, According to Sibling Type, National Longitudinal Study of Adolescent Health, 1994–1995a
| Characteristic | Full Siblings (473 Pairs, 946 Individuals) | Dizygotic Twins (179 Pairs, 358 Individuals) | Monozygotic Twins (188 Pairs, 376 Individuals) |
| Birth weight measure | |||
| Mean birth weight, kg | 3.4 (0.6)b | 2.6 (0.6) | 2.4 (0.6) |
| Low birth weight (<2.5 kg), % | 5.4 | 41.3 | 53.3 |
| High birth weight (>4 kg), % | 14.0 | 0.0 | 0.5 |
| Mean birth weight difference, kg | 0.5 (0.4) | 0.3 (0.3) | 0.2 (0.2) |
| BMIc measure | |||
| Mean BMI | 22.3 (4.4) | 22.2 (4.1) | 22.0 (4.1) |
| Obesity (BMI ≥30), % | 8.8 | 8.9 | 9.8 |
| Mean BMI difference | 3.4 (3.4) | 3.2 (3.2) | 1.5 (1.7) |
| Mean age, years | 15.6 (1.7) | 15.5 (1.6) | 15.6 (1.5) |
| Female sex, % | 49.3 | 44.1 | 47.9 |
| Total no. of females | 466 | 158 | 180 |
| Race/ethnicity, % | |||
| White | 64.6 | 63.3 | 59.5 |
| Black | 15.0 | 23.0 | 18.4 |
| Hispanic | 15.2 | 11.5 | 16.2 |
| Asian | 5.3 | 2.2 | 6.0 |
Abbreviation: BMI, body mass index.
The analytical sample used for this table included individuals/pairs with complete data at baseline. Individuals/pairs who were missing a BMI value at baseline (40 pairs) were excluded from these descriptive analyses.
Numbers in parentheses, standard deviation.
Weight (kg)/height (m)2.
Using longitudinal, random-effects, linear regression models to predict BMI differences, we observed that among monozygotic females, twin birth weight differences were positively associated with adolescent and young adult BMI differences (β = 2.67, 95% confidence interval: 0.99, 4.35); the twin with a higher birth weight was more likely to have a higher BMI later in life, whereas no such association was observed for full siblings or dizygotic twins (Table 4). Post-hoc power calculations revealed adequate statistical power (>80%) to detect within-pair BMI differences of approximately 1 unit or more (results not shown).
Table 4.
Regression Coefficients (β) for Longitudinal Difference in Body Mass Index per 1-kg Increase in Birth Weight Difference Within Pairs (Family Sample), National Longitudinal Study of Adolescent Health, 1994–2002
| Model | No. of Pairs | Body Mass Indexa Difference |
|
| β | 95% Confidence Interval | ||
| Full siblingsb | |||
| Male-male | 246 | 0.00 | −0.92, 0.92 |
| Female-female | 267 | −0.06 | −1.12, 0.99 |
| Dizygotic twinsc | |||
| Male-male | 104 | 1.28 | −1.03, 3.59 |
| Female-female | 85 | −0.67 | −2.65, 1.31 |
| Monozygotic twinsd | |||
| Male-male | 107 | 0.73 | −0.40, 1.86 |
| Female-female | 100 | 2.67 | 0.99, 4.35 |
Weight (kg)/height (m)2.
Random-effects longitudinal linear regression model adjusting for the age difference between siblings (age of index sibling minus age of other sibling) and shared household environment (living with the sibling (yes/no)). The cluster command adjusted for multiple sibling pairs within the same household.
Random-effects longitudinal linear regression model adjusting for age and shared household environment (living with the sibling (yes/no)).
Random-effects longitudinal linear regression model adjusting for age and shared household environment (living with the sibling (yes/no)).
DISCUSSION
Given mounting evidence suggesting that birth weight is associated with adult size and disease, a comprehensive understanding of whether the association between birth weight and adult size reflects intrauterine environmental factors or common genetic factors is needed (16). However, in most studies examining the association between birth weight and obesity, investigators use traditional cohort data, which may not be adequately suited to understanding which factors contribute to the association. Sibling and twin study designs allow within-pair analytic approaches that eliminate much of the influence of factors shared by both siblings, such as maternal exposures (e.g., maternal age), genetics, and the postnatal environment (e.g., parental feeding styles and socioeconomic status) (16, 24–28). Taking advantage of a nationally representative, prospective data set, we observed that maternal obesity modified the relation between birth weight and later obesity. Persons who were high birth weight (versus normal birth weight) and had a nonobese mother were significantly more likely to be obese later in life. We observed a similar (but not statistically significant) trend for persons with an obese mother. Notably, we found no evidence of modification by race/ethnicity, which suggests that findings from predominantly Caucasian populations may be generalizable to all US racial/ethnic groups. In the sibling sample, we observed a positive association of birth weight difference with later BMI difference in monozygotic, female twins only, despite adequate power to detect within-pair differences of ≥1 BMI unit; this suggests that null findings in all other sibling configurations may be due to inadequate power to detect small within-pair BMI differences.
Similarly to our study, investigators in a majority of cohort studies have reported a positive association between birth weight and obesity later in life (7–14); however, only 1 other study found effect-measure modification by maternal size (10). Given the strong link between maternal obesity, infant birth weight, and child obesity, we hypothesized that the relation between birth weight and later obesity may be modified by common genetic factors and/or an obesogenic environment related to both birth weight and later obesity. If these genetic factors and/or behaviors were passed from mother to child, the association between birth weight and later obesity might be modified by maternal obesity status. We found an association between birth weight and later obesity in offspring of nonobese mothers only, suggesting that genetic factors and/or an obesogenic postnatal environment may be stronger determinants of obesity than intrauterine factors.
Twin studies examining the relation between birth weight and later size have yielded inconsistent findings: Some have found positive associations, some null associations (5, 6, 29–34). Discrepant findings may reflect differences in inclusion criteria (e.g., monozygotic twins only and/or combining twins of mixed sex (29–32, 34)). Our findings suggest that the intrauterine environment unique to each fetus contributes to later size differences only in female monozygotic twin pairs. However, we cannot exclude the possibility that the intrauterine environment does contribute to later size differences across other sibling configurations, since we did not have sufficient power to detect within-pair differences of <1 BMI unit, which would not be captured if the intrauterine environment contributes to very small differences in later size. Intrauterine factors influencing birth weight between and within twins include blood and nutrient supply, umbilical cord insertion, placentation, and chorionicity (26, 51, 52). However, it is not possible to entirely preclude the influence of genetic factors even among monozygotic twins, since they may also differ according to a number of genetic factors, including the number of chromosomes present, as well as epigenetic modifications and DNA mutations (53). Alternatively, if the observed null associations are accurate, the commonly observed positive association between birth weight and obesity may be confounded by unmeasured, shared factors (e.g., maternal characteristics), accounted for with the within-pairs difference method in full siblings, dizygotic twins, and male-male monozygotic twins.
Comparing the results from the traditional cohort analyses to those from the within-sibling/twin pair analyses sheds light on which factors contribute to the birth weight–obesity association. If findings for the full cohort and dizygotic twins and siblings are both positive, whereas findings for monozygotic twins are attenuated, this suggests that factors shared between monozygotic twins influence later body size (e.g., genetic factors). Conversely, if findings from the full cohort are positive, whereas null findings are observed in the within-pair sibling/twin sample, this suggests that any factors shared between full sibling and twin pairs underlie the association between birth weight and obesity (e.g., socioeconomic status). If the observed null findings in monozygotic male twins are accurate, the positive findings observed in the full cohort may be attributable to confounding by maternal characteristics or influenced by genetic factors. Alternatively, null findings may result from inadequate power to detect small within-pair differences, which could be the case if within-pair birth weight differences contribute very little to within-pair BMI differences later in life.
Most research in this area has 1) been conducted in racially/ethnically homogenous populations and 2) examined the association between birth weight and size at only a single point in time, thus precluding the generalizability of results to larger populations. The strengths of our study include our use of a large, nationally representative cohort followed over an 8-year time span, providing valuable information regarding the potential influence of modification by maternal obesity, paternal obesity, sex, age, and race/ethnicity, as well as comparative findings from the sibling and twin samples. Our within-pairs difference approach holds all factors related to the mother and pregnancy constant, while our cohort approach provides information on unrelated persons, similar to a between-pair coefficient. Our findings shed light on the complex nature by which environmental and genetic factors influence the relation between birth weight and obesity.
Despite these strengths, our study had some limitations. First, results from a unique sample of twin pairs may not be generalizable to singleton births, particularly those involving term gestations. Generally, twins have lower birth weights than singletons, which largely reflects a shorter gestation period and intrauterine growth restriction (54). Additionally, since development of fat mass largely occurs later in pregnancy, twins may have decreased fat mass in comparison with full-term singleton babies (55). These differences in body composition may modify the association between birth weight and later size, which we were not able to capture using BMI. While other measures may more accurately capture adiposity, BMI is recommended for large epidemiologic studies and adequately correlates with total body fat (56). A second limitation is that while we used a sibling and twin-pair design to reduce effects of shared environmental and genetic factors, the results may have been affected by residual confounding (e.g., birth weight differences may result in a maternal choice to differentially feed offspring). Unfortunately, Add Health investigators did not collect information about early-life factors, such as maternal care-giving practices or other environmental/antenatal factors that differed between the siblings in early life. Furthermore, our results may have been subject to measurement error arising from parental recall of birth weight and respondent self-report of height and weight. However, parental recall of birth weight has been shown to be relatively accurate, and discrepancies between self-reported and measured height/weight in Add Health are relatively minor (57–61). Nonetheless, nondifferential reporting errors regarding birth weight, height, and weight would tend to attenuate the association between birth weight and later size and may underlie null findings in the sibling sample. Alternatively, while our sibling sample was larger than samples in other sibling studies, it was still relatively small, with adequate statistical power to detect only within-pair differences of ≥1 BMI unit (30–32).
In summary, our cohort study findings suggest that high birth weight is positively associated with later obesity. Findings from our sibling and twin study suggest that unmeasured, shared factors, such as maternal characteristics, may be responsible for the commonly observed positive association between birth weight and later obesity. While there is growing evidence that early-life factors may influence later health outcomes, a better understanding of the pathways underlying this relation is needed.
Acknowledgments
Author affiliation: Department of Nutrition, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Natalie S. The, Penny Gordon-Larsen, Linda S. Adair).
This work was supported by the National Institute of Child Health and Human Development (grant 1R01HD057194).
Conflict of interest: none declared.
Glossary
Abbreviations
- Add Health
National Longitudinal Study of Adolescent Health
- BMI
body mass index
References
- 1.Dietz WH, Gortmaker SL. Preventing obesity in children and adolescents. Annu Rev Public Health. 2001;22:337–353. doi: 10.1146/annurev.publhealth.22.1.337. [DOI] [PubMed] [Google Scholar]
- 2.Loktionov A. Common gene polymorphisms and nutrition: emerging links with pathogenesis of multifactorial chronic diseases (review) J Nutr Biochem. 2003;14(8):426–451. doi: 10.1016/s0955-2863(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 3.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59(5):955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- 4.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 5.Loos RJ, Beunen G, Fagard R, et al. Birth weight and body composition in young adult men—a prospective twin study. Int J Obes Relat Metab Disord. 2001;25(10):1537–1545. doi: 10.1038/sj.ijo.0801743. [DOI] [PubMed] [Google Scholar]
- 6.Loos RJ, Beunen G, Fagard R, et al. Birth weight and body composition in young women: a prospective twin study. Am J Clin Nutr. 2002;75(4):676–682. doi: 10.1093/ajcn/75.4.676. [DOI] [PubMed] [Google Scholar]
- 7.Fall CH, Osmond C, Barker DJ, et al. Fetal and infant growth and cardiovascular risk factors in women. BMJ. 1995;310(6977):428–432. doi: 10.1136/bmj.310.6977.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ. 2001;323(7325):1331–1335. doi: 10.1136/bmj.323.7325.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curhan GC, Willett WC, Rimm EB, et al. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94(12):3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 10.Curhan GC, Chertow GM, Willett WC, et al. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94(6):1310–1315. doi: 10.1161/01.cir.94.6.1310. [DOI] [PubMed] [Google Scholar]
- 11.Sørensen HT, Sabroe S, Rothman KJ, et al. Relation between weight and length at birth and body mass index in young adulthood: cohort study. BMJ. 1997;315(7116):1137. doi: 10.1136/bmj.315.7116.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson J, Forsén T, Tuomilehto J, et al. Size at birth, childhood growth and obesity in adult life. Int J Obes Relat Metab Disord. 2001;25(5):735–740. doi: 10.1038/sj.ijo.0801602. [DOI] [PubMed] [Google Scholar]
- 13.Seidman DS, Laor A, Gale R, et al. A longitudinal study of birth weight and being overweight in late adolescence. Am J Dis Child. 1991;145(7):782–785. [PubMed] [Google Scholar]
- 14.Braddon FE, Rodgers B, Wadsworth ME, et al. Onset of obesity in a 36 year birth cohort study. Br Med J (Clin Res Ed) 1986;293(6542):299–303. doi: 10.1136/bmj.293.6542.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stettler N, Tershakovec AM, Zemel BS, et al. Early risk factors for increased adiposity: a cohort study of African American subjects followed from birth to young adulthood. Am J Clin Nutr. 2000;72(2):378–383. doi: 10.1093/ajcn/72.2.378. [DOI] [PubMed] [Google Scholar]
- 16.Ijzerman RG, Boomsma DI, Stehouwer CD. Intrauterine environmental and genetic influences on the association between birthweight and cardiovascular risk factors: studies in twins as a means of testing the fetal origins hypothesis. Paediatr Perinat Epidemiol. 2005;19(suppl 1):10–14. doi: 10.1111/j.1365-3016.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Twinn DS, Ozanne SE. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav. 2006;88(3):234–243. doi: 10.1016/j.physbeh.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 18.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 19.Aalinkeel R, Srinivasan M, Song F, et al. Programming into adulthood of islet adaptations induced by early nutritional intervention in the rat. Am J Physiol Endocrinol Metab. 2001;281(3):E640–E648. doi: 10.1152/ajpendo.2001.281.3.E640. [DOI] [PubMed] [Google Scholar]
- 20.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29(12):1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 21.Silverman BL, Rizzo T, Green OC, et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40(suppl 2):121–125. doi: 10.2337/diab.40.2.s121. [DOI] [PubMed] [Google Scholar]
- 22.Silverman BL, Rizzo TA, Cho NH, et al. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. Long-term effects of the intrauterine environment. 1998;21(suppl 2):B142–B149. [PubMed] [Google Scholar]
- 23.Hattersley AT, Tooke JE. The fetal insulin hypothesis: an alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet. 1999;353(9166):1789–1792. doi: 10.1016/S0140-6736(98)07546-1. [DOI] [PubMed] [Google Scholar]
- 24.Nelson MC, Gordon-Larsen P, Adair LS. Are adolescents who were breast-fed less likely to be overweight? Analyses of sibling pairs to reduce confounding. Epidemiology. 2005;16(2):247–253. doi: 10.1097/01.ede.0000152900.81355.00. [DOI] [PubMed] [Google Scholar]
- 25.Morley R, Dwyer T. Studies of twins: what can they tell us about the fetal origins of adult disease? Paediatr Perinat Epidemiol. 2005;19(suppl 1):2–7. doi: 10.1111/j.1365-3016.2005.00608.x. [DOI] [PubMed] [Google Scholar]
- 26.Morley R, Dwyer T, Carlin JB. Studies of twins: can they shed light on the fetal origins of adult disease hypothesis? Twin Res. 2003;6(6):520–525. doi: 10.1375/136905203322686527. [DOI] [PubMed] [Google Scholar]
- 27.Dwyer T, Blizzard L. A discussion of some statistical methods for separating within-pair associations from associations among all twins in research on fetal origins of disease. Paediatr Perinat Epidemiol. 2005;19(suppl 1):48–53. doi: 10.1111/j.1365-3016.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 28.Morley R. Fetal origins of adult disease. Semin Fetal Neonatal Med. 2006;11(2):73–78. doi: 10.1016/j.siny.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Allison DB, Paultre F, Heymsfield SB, et al. Is the intra-uterine period really a critical period for the development of adiposity? Int J Obes Relat Metab Disord. 1995;19(6):397–402. [PubMed] [Google Scholar]
- 30.Pietiläinen KH, Rissanen A, Laamanen M, et al. Growth patterns in young adult monozygotic twin pairs discordant and concordant for obesity. Twin Res. 2004;7(5):421–429. doi: 10.1375/1369052042335368. [DOI] [PubMed] [Google Scholar]
- 31.Keet MP, Jaroszewicz AM, Lombard CJ. Follow-up study of physical growth of monozygous twins with discordant within-pair birth weights. Pediatrics. 1986;77(3):336–344. [PubMed] [Google Scholar]
- 32.Baird J, Osmond C, MacGregor A, et al. Testing the fetal origins hypothesis in twins: the Birmingham Twin Study. Diabetologia. 2001;44(1):33–39. doi: 10.1007/s001250051577. [DOI] [PubMed] [Google Scholar]
- 33.Johansson M, Rasmussen F. Birthweight and body mass index in young adulthood: the Swedish young male twins study. Twin Res. 2001;4(5):400–405. doi: 10.1375/1369052012588. [DOI] [PubMed] [Google Scholar]
- 34.Pietiläinen KH, Kaprio J, Räsänen M, et al. Genetic and environmental influences on the tracking of body size from birth to early adulthood. Obes Res. 2002;10(9):875–884. doi: 10.1038/oby.2002.120. [DOI] [PubMed] [Google Scholar]
- 35.Popkin BM, Udry JR. Adolescent obesity increases significantly in second and third generation U.S. immigrants: the National Longitudinal Study of Adolescent Health. J Nutr. 1998;128(4):701–706. doi: 10.1093/jn/128.4.701. [DOI] [PubMed] [Google Scholar]
- 36.Field AE, Aneja P, Rosner B. The validity of self-reported weight change among adolescents and young adults. Obesity (Silver Spring) 2007;15(9):2357–2364. doi: 10.1038/oby.2007.279. [DOI] [PubMed] [Google Scholar]
- 37.Berkey CS, Colditz GA. Adiposity in adolescents: change in actual BMI works better than change in BMI z score for longitudinal studies. Ann Epidemiol. 2007;17(1):44–50. doi: 10.1016/j.annepidem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Himes JH, Dietz WH. Guidelines for overweight in adolescent preventive services: recommendations from an expert committee. The Expert Committee on Clinical Guidelines for Overweight in Adolescent Preventive Services. Am J Clin Nutr. 1994;59(2):307–316. doi: 10.1093/ajcn/59.2.307. [DOI] [PubMed] [Google Scholar]
- 39.Must A, Anderson SE. Body mass index in children and adolescents: considerations for population-based applications. Int J Obes (Lond) 2006;30(4):590–594. doi: 10.1038/sj.ijo.0803300. [DOI] [PubMed] [Google Scholar]
- 40.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald J, Gottschalk P, Moffitt R. An analysis of sample attrition in panel data: the Michigan Panel Study of Income Dynamics. J Hum Resour. 1998;33(2):251–299. [Google Scholar]
- 42.Baltagi BH. Econometric Analysis of Panel Data. New York, NY: John Wiley & Sons, Inc; 2001. [Google Scholar]
- 43.Greene WH. Econometric Analysis. Upper Saddle River, NJ: Prentice Hall, Inc; 2003. [Google Scholar]
- 44.Hsiao C. Analysis of Panel Data. New York, NY: Cambridge University Press; 2003. [Google Scholar]
- 45.O'Callaghan MJ, Williams GM, Andersen MJ, et al. Prediction of obesity in children at 5 years: a cohort study. J Paediatr Child Health. 1997;33(4):311–316. doi: 10.1111/j.1440-1754.1997.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 46.Hindmarsh PC, Geary MP, Rodeck CH, et al. Intrauterine growth and its relationship to size and shape at birth. Pediatr Res. 2002;52(2):263–268. doi: 10.1203/00006450-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 47.Luke B, Hediger M, Min SJ, et al. Gender mix in twins and fetal growth, length of gestation and adult cancer risk. Paediatr Perinat Epidemiol. 2005;19(suppl 1):41–47. doi: 10.1111/j.1365-3016.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- 48.Derom R, Derom C, Loos RJ, et al. Gender mix: does it modify birthweight–outcome association? Paediatr Perinat Epidemiol. 2005;19(suppl 1):37–40. doi: 10.1111/j.1365-3016.2005.00613.x. [DOI] [PubMed] [Google Scholar]
- 49.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 50.Hernán MA, Hernández-Díaz S, Werler MM, et al. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155(2):176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 51.Chescheir NC. Twin-to-twin transfusion syndrome: a model for the fetal origins of adult health. Paediatr Perinat Epidemiol. 2005;19(suppl 1):32–36. doi: 10.1111/j.1365-3016.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 52.Loos RJ, Derom C, Derom R, et al. Determinants of birthweight and intrauterine growth in liveborn twins. Paediatr Perinat Epidemiol. 2005;19(suppl 1):15–22. doi: 10.1111/j.1365-3016.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 53.Gringras P, Chen W. Mechanisms for differences in monozygous twins. Early Hum Dev. 2001;64(2):105–117. doi: 10.1016/s0378-3782(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 54.Demarini S, Koo WW, Hockman EM. Bone, lean and fat mass of newborn twins versus singletons. Acta Paediatr. 2006;95(5):594–599. doi: 10.1080/08035250500462091. [DOI] [PubMed] [Google Scholar]
- 55.Enzi G, Zanardo V, Caretta F, et al. Intrauterine growth and adipose tissue development. Am J Clin Nutr. 1981;34(9):1785–1790. doi: 10.1093/ajcn/34.9.1785. [DOI] [PubMed] [Google Scholar]
- 56.Hall DM, Cole TJ. What use is the BMI? Arch Dis Child. 2006;91(4):283–286. doi: 10.1136/adc.2005.077339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Sullivan JJ, Pearce MS, Parker L. Parental recall of birth weight: how accurate is it? Arch Dis Child. 2000;82(3):202–203. doi: 10.1136/adc.82.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCormick MC, Brooks-Gunn J. Concurrent child health status and maternal recall of events in infancy. Pediatrics. 1999;104(5):1176–1181. [PubMed] [Google Scholar]
- 59.Pless CE, Pless IB. How well they remember. The accuracy of parent reports. Arch Pediatr Adolesc Med. 1995;149(5):553–558. doi: 10.1001/archpedi.1995.02170180083016. [DOI] [PubMed] [Google Scholar]
- 60.Seidman DS, Slater PE, Ever-Hadani P, et al. Accuracy of mothers’ recall of birthweight and gestational age. Br J Obstet Gynaecol. 1987;94(8):731–735. doi: 10.1111/j.1471-0528.1987.tb03717.x. [DOI] [PubMed] [Google Scholar]
- 61.Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106(1):52–58. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]