Abstract
The relation between measures of general and central adiposity and individual cardiovascular endpoints remains understudied in older adults. This study investigated the association of measures of body size and composition with incident ischemic stroke or coronary heart disease (1989–2007) in 3,754 community-dwelling US adults aged 65–100 years. Standardized anthropometry and bioelectric impedance measurements were obtained at baseline. Body mass index at age 50 years (BMI50) was calculated on the basis of recalled weight. Although only waist/hip ratio was significantly associated with ischemic stroke in quintile analysis in women, dichotomized body mass index (BMI) (≥30 kg/m2) was the only significant predictor in men. For coronary heart disease, there were significant positive adjusted associations for all adiposity measures, without interaction by sex. This was true for both quintiles and conventional cutpoints for obesity, although BMI-defined overweight (25–29.9 kg/m2) was significant at midlife but not at baseline. Strengths of association for extreme quintiles (quintile 5 vs. quintile 1) were broadly comparable, but the highest effect estimates were for waist/hip ratio (hazard ratio = 1.56, 95% confidence interval: 1.25, 1.94) and BMI50 (hazard ratio = 1.71, 95% confidence interval: 1.37, 2.14), both of which remained significant after adjustment for mediators, BMI, or each other. Whether these differences translate to better risk prediction will require meta-analytical approaches, as will determination of prognostic cutpoints.
Keywords: aging, body composition, body size, coronary disease, stroke
The rising prevalence of obesity represents the dominant public health challenge confronting modern societies (1). Older adults, who are particularly susceptible to dysglycemia and cardiovascular disease, have not been spared from the broader obesity epidemic: Roughly two-thirds meet the criteria for overweight or obesity in the United States (2). Adults aged 65 years or older are also the fastest growing demographic group (3), with major implications for the public health impact of obesity-related disorders.
General expansion of fat mass fosters insulin resistance, dyslipidemia, and hypertension, but the visceral adipose compartment may be the foremost driver of cardiometabolic risk (4–6). At the clinical level, the question of whether anthropometric measures of abdominal adiposity afford cardiovascular risk assessment superior to general measures has generated keen interest (6). Although various longitudinal studies have found measures of central adiposity to be associated with cardiovascular disease independent of general adiposity (7–10), others have reported abdominal and general measures to be prognostically comparable (11, 12).
Most available studies, however, have focused predominantly or exclusively on middle-aged populations. Aging is associated with marked changes in body composition (13, 14), which could alter the relations of anthropometric measures to cardiovascular disease outcomes in older adults. Indeed, the relation between body mass index (BMI) and mortality has been shown to weaken with age (15) but is strong for central measures of adiposity (16, 17). Less is known, however, regarding the relations of anthropometric measures specifically with incident ischemic stroke or coronary heart disease in older persons, or the extent to which recommended cutpoints (18, 19) derived from younger cohorts apply in elderly populations. In particular, few epidemiologic studies have included meaningful numbers of participants >75 years of age. We addressed these questions in a large longitudinal cohort of older adults.
MATERIALS AND METHODS
Study population
The Cardiovascular Health Study is a population-based investigation of risk factors for cardiovascular disease in older adults. As reported in detail previously (20), participants consisted of community-dwelling persons aged 65–100 years identified from Medicare eligibility lists and enrolled at 4 field centers in the United States. An original cohort (n = 5,201) was recruited in 1989–1990, with a supplemental cohort of African Americans (n = 687) recruited in 1992–1993. Participants underwent standardized health evaluations at site clinics using previously described protocols (20, 21).
For the present analyses, we excluded individuals with prevalent cardiovascular disease (coronary heart disease, stroke, transient ischemic attack, peripheral arterial disease, heart failure) and atrial fibrillation at baseline (n = 1,597), as well as those with missing data on body composition measures (n = 286) or prespecified covariates (n = 251), leaving 3,754 eligible subjects.
Measures of body composition
Anthropometry was performed in standardized fashion by trained personnel on participants wearing examination suits after removal of shoes. Standing height was determined with a stadiometer calibrated in centimeters, while body weight was measured with a balance-beam scale calibrated in kilograms. Waist circumference was measured at the level of the umbilicus, and hip circumference was measured at the point of maximal protrusion of the gluteal muscles. BMI at baseline was calculated (weight (kg)/height (m)2). Body mass index at age 50 years (BMI50) (i.e., midlife” BMI) was based on self-reported weight at age 50 (obtained by questionnaire) and measured height at baseline. The waist/hip ratio and waist/height ratio were calculated by dividing waist circumference by hip circumference or height. Bioelectrical impedance was determined at baseline with a TVI-10 Body Composition Analyzer (Danninger Medical, Columbus, Ohio). Resistance was measured at 50 kHz following standardized placement of 4 adhesive electrocardiograph electrodes on the dorsal right hand and foot. Fat-free mass was calculated as 6,710 × (ht2/R) + (3.1 × S) + 3.9, where ht2 is standing height in meters squared, R is resistance in ohms, and S is sex (0 = female, 1 = male) (22). Fat mass was obtained by subtracting fat-free mass from body weight.
Clinical and laboratory covariates
Glucose was measured on fasting serum samples (23) collected in 1989–1990, 1992–1993, 1996–1997, and 2005–2006. An inventory of medication use was compiled at baseline and annually thereafter (24). Prediabetes was defined by fasting blood glucose of 100–125 mg/dL and diabetes by a value of ≥126 mg/dL or hypoglycemic medication. Hypertension was defined by systolic and diastolic blood pressure cut-offs of 140 and 90 mm Hg or by self-report and antihypertensive therapy. Leisure-time physical activity was calculated as a weighted sum of kilocalories consumed in specific physical tasks (25). Other fasting laboratory measurements (23) included creatinine, lipids, insulin, and high-sensitivity C-reactive protein (26).
Cardiovascular outcomes
Follow-up surveillance and ascertainment entailed regular telephone contacts and clinic examinations (27). Examinations were conducted annually from 1989–1990 through 1998–1999 and again in 2005–2006. Telephone interviews were performed semiannually from 1989 to 1999 and biannually from 2000 to 2004. Potential incident events and all deaths were investigated by review of medical records. Following initial review by local physicians at the field centers, final classification was assigned by cardiovascular events and stroke committees from the Cardiovascular Health Study using standardized criteria (complete through June 2007) (20, 27, 28). Strokes were adjudicated by review of medical records or, for nonhospitalized events, physician questionnaires, supplemented by review of imaging studies. Strokes were designated as ischemic, hemorrhagic, or unclassified by previously described criteria (28). Achieved reliability was excellent, with kappa = 0.86 for stroke versus no stroke and kappa = 1.0 for stroke type (28). The primary outcome was ischemic stroke; secondary analyses considered all strokes. The coronary heart disease outcome consisted of nonfatal myocardial infarction and fatal coronary events, as defined previously (20, 21, 27).
Statistical analysis
Correlations between body composition measures were assessed by computing Spearman coefficients. Each measure of body composition was classified by quintiles of its sex-specific distribution, and Cox models were fit to calculate the relative risk of incident events by using the lowest quintile as the reference category. To assess monotonic associations, we tested for trend using a grouped linear variable. Relative risks were similarly evaluated by using partition values recommended for BMI, waist circumference, and waist/hip ratio by scientific and governmental organizations (18, 19). We tested the proportional hazards assumption by using Schoenfeld's goodness-of-fit procedures, which did not reveal meaningful violations.
Models were adjusted for age, sex, and race, as well as for prespecified potential confounders. These included smoking status (never, former, current), physical activity, alcoholic drinks per week (0, <7, 7–13, ≥14), education (less than high school, high-school graduate, college or higher), hormone replacement (women), and serum creatinine (to account for potential confounding of renal disease-associated weight loss on cardiovascular disease outcomes (29)). Subsequent models considered the impact of putative mediators, namely, systolic blood pressure, antihypertensive therapy, diabetes, prediabetes, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, use of aspirin (marker of cardiovascular disease risk), and high-sensitivity C-reactive protein (5). Joint inclusion of body composition measures examined whether 1) central adiposity remained predictive after controlling for general adiposity, both at baseline and midlife; 2) midlife BMI remained predictive after considering BMI and central adiposity in later life; and 3) concomitant assessment of waist circumference and hip circumference and of fat mass and fat-free mass uncovered inverse associations between lean body mass and outcome. To evaluate interactions with sex, age, or race, appropriate cross-product terms were included.
Because weight loss associated with smoking and aging-related health decline can offset benefits potentially associated with lean body habitus, we conducted sensitivity analyses restricted to never smokers or former smokers quitting >5 years earlier; participants with self-reported health status of good, very good, or excellent; and events occurring beyond the initial 3 years of follow-up.
All analyses were performed with STATA, version 10.0, statistical software (StataCorp LP, College Station, Texas).
RESULTS
Baseline characteristics
Compared with the study sample, excluded Cardiovascular Health Study participants were more commonly men (49.4% vs. 38.4%) and African American (18.0% vs. 14.4%); more often reported fair or poor health status (38.3% vs. 18.2%); had higher waist circumference (women, 94.4 (standard deviation (SD), 15.2) vs. 91.3 (SD, 14.1) cm; men, 98.0 (SD, 10.7) vs. 97.4 (SD, 10.1) cm) and lower median physical activity (536 (interquartile range (IQR), 53–1,450) vs. 645 (IQR, 158–1,580) kcal); and exhibited a higher prevalence of hypertension (64.8% vs. 55.4%), diabetes (22.4% vs. 13.2%), and aspirin use (43.7% vs. 28.1%), as well as higher serum creatinine (1.2 (SD, 0.5) vs. 1.0 (SD, 0.3) mg/dL) and high-sensitivity C-reactive protein (2.9 (IQR, 1.5–5.1) vs. 2.4 (IQR, 1.2–4.3) mg/dL).
Baseline characteristics of the study cohort are presented in Tables 1 and 2 by sex-specific quintiles of BMI. There were more African Americans and fewer high-school graduates across increasing quintiles of BMI in women and men, along with decreasing proportions of current smokers, consumers of ≥7 alcoholic beverages per week (in women only), and individuals with self-reported very good or excellent health status. Measures of dysglycemia, hypertension, dyslipidemia, and inflammation were higher with increasing quintiles of BMI in both sexes, while physical activity and current estrogen use were lower.
Table 1.
Distribution of Sociodemographic and Clinical Characteristics Among Women (n = 2,313) in the Cardiovascular Health Study Sample by Quintiles of Body Mass Index at Baseline, United States, 1989–1990 or 1992–1993
| Characteristic | BMI, kg/m2 |
||||||||||||||
| <22.5 |
22.5–24.7 |
24.8–27.0 |
27.1–30.3 |
≥30.4 |
|||||||||||
| Mean (SD) | % | Median (IQR) | Mean (SD) | % | Median (IQR) | Mean (SD) | % | Median (IQR) | Mean (SD) | % | Median (IQR) | Mean (SD) | % | Median (IQR) | |
| Age, years | 73.1 (5.8) | 71.8 (5.1) | 72.0 (5.0) | 71.7 (5.1) | 71.3 (4.7) | ||||||||||
| Black | 5.6 | 10.6 | 10.8 | 18.8 | 27.1 | ||||||||||
| Education | |||||||||||||||
| Less than high school | 19.2 | 24.2 | 21.4 | 27.4 | 36.8 | ||||||||||
| High school graduate | 30.7 | 32.6 | 32.0 | 30.9 | 30.1 | ||||||||||
| College or higher | 50.1 | 43.2 | 46.5 | 41.7 | 33.1 | ||||||||||
| Smoking status | |||||||||||||||
| Never | 56.2 | 51.8 | 58.0 | 57.2 | 60.6 | ||||||||||
| Former | 26.8 | 30.9 | 31.8 | 35.0 | 30.3 | ||||||||||
| Current | 17.1 | 17.3 | 10.2 | 7.8 | 9.1 | ||||||||||
| Alcoholic drinks/week | |||||||||||||||
| 0 | 48.2 | 48.0 | 47.6 | 53.6 | 67.3 | ||||||||||
| <7 | 36.7 | 36.1 | 40.3 | 40.6 | 29.4 | ||||||||||
| 7–13 | 7.1 | 6.7 | 5.6 | 2.8 | 1.7 | ||||||||||
| ≥14 | 8.0 | 9.3 | 6.5 | 3.0 | 1.5 | ||||||||||
| Self-reported health | |||||||||||||||
| Excellent | 18.4 | 19.4 | 16.7 | 14.5 | 9.6 | ||||||||||
| Very good | 31.5 | 28.5 | 29.0 | 28.6 | 20.4 | ||||||||||
| Good | 32.3 | 37.4 | 39.6 | 37.7 | 41.1 | ||||||||||
| Fair | 15.6 | 14.0 | 13.6 | 17.5 | 25.7 | ||||||||||
| Poor | 2.2 | 0.7 | 1.1 | 1.7 | 3.3 | ||||||||||
| Weight change from age 50 years, kg | −2.2 (5.6) | 1.5 (5.7) | 3.5 (6.1) | 6.2 (7.3) | 13.2 (10.9) | ||||||||||
| Physical activity, kcal | 600 (135–1,335) | 540 (120–1,350) | 504 (113–1,240) | 405 (23–1,170) | 263 (0–840) | ||||||||||
| Estrogen use | |||||||||||||||
| Never | 59.6 | 53.1 | 59.5 | 62.0 | 66.0 | ||||||||||
| Past | 24.2 | 29.8 | 25.3 | 25.7 | 26.8 | ||||||||||
| Current | 16.2 | 17.1 | 15.2 | 12.3 | 7.1 | ||||||||||
| Aspirin use >2 days in past 2 weeks | 25.7 | 24.6 | 29.4 | 26.1 | 28.8 | ||||||||||
| Systolic blood pressure, mm Hg | 132.6 (22.2) | 134.2 (21.9) | 135.8 (22.2) | 137.7 (21.2) | 139.2 (20.8) | ||||||||||
| Diastolic blood pressure, mm Hg | 68.2 (10.9) | 68.9 (10.4) | 69.9 (11.2) | 70.6 (11.1) | 72.4 (10.6) | ||||||||||
| Hypertension | 41.7 | 49.8 | 58.2 | 62.6 | 70.6 | ||||||||||
| Use of antihypertensive medication | 27.4 | 34.3 | 38.7 | 45.8 | 53.7 | ||||||||||
| Impaired fasting glucose | 24.0 | 26.4 | 36.4 | 41.0 | 45.7 | ||||||||||
| Diabetes | 4.5 | 6.3 | 10.0 | 13.8 | 23.2 | ||||||||||
| Glucose, mg/dL | 97.4 (20.7) | 99.6 (18.8) | 105.1 (26.7) | 111.5 (42.6) | 121.4 (49.3) | ||||||||||
| Insulin, IU/mL | 10.9 (9.5) | 12.3 (10.6) | 14.8 (18.3) | 18.2 (29.2) | 22.1 (26.7) | ||||||||||
| Total cholesterol, mg/dL | 215.6 (36.0) | 222.2 (37.6) | 223.6 (38.7) | 224.3 (37.7) | 219.9 (38.9) | ||||||||||
| LDL cholesterol, mg/dL | 125.5 (34.5) | 134.0 (37.5) | 136.5 (37.1) | 138.8 (35.6) | 135.9 (35.4) | ||||||||||
| HDL cholesterol, mg/dL | 67.4 (17.8) | 63.1 (15.7) | 60.3 (15.6) | 56.6 (13.3) | 54.3 (12.9) | ||||||||||
| Triglycerides, mg/dL | 100 (82–129) | 110 (86–152) | 123 (96–162) | 129 (10–175) | 135 (102–180) | ||||||||||
| Serum creatinine, mg/dL | 0.89 (0.21) | 0.92 (0.25) | 0.91 (0.20) | 0.92 (0.21) | 0.94 (0.24) | ||||||||||
| hsCRP, mg/L | 1.5 (0.7–2.9) | 1.9 (0.9–3.5) | 2.6 (1.3–2.6) | 3.0 (1.9–4.8) | 3.9 (2.5–8.6) | ||||||||||
Abbreviations: BMI, body mass index; HDL, high density lipoprotein; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; LDL, low density lipoprotein; SD, standard deviation.
Table 2.
Distribution of Sociodemographic and Clinical Characteristics Among Men (n = 1,441) in the Cardiovascular Health Study Sample by Quintiles of Body Mass Index at Baseline, United States, 1989–1990 or 1992–1993
| Characteristic | BMI, kg/m2 |
||||||||||||||
| <23.4 |
23.4–25.2 |
25.3–26.8 |
26.9–29.1 |
≥29.2 |
|||||||||||
| Mean (SD) | % | Median (IQR) | Mean (SD) | % | Median (IQR) | Mean (SD) | % | Median (IQR) | Mean (SD) | % | Median (IQR) | Mean (SD) | % | Median (IQR) | |
| Age, years | 73.9 (6.1) | 72.7 (5.4) | 72.9 (5.4) | 72.5 (5.2) | 71.7 (5.0) | ||||||||||
| Black | 11.8 | 10.8 | 15.3 | 15.6 | 17.4 | ||||||||||
| Education | |||||||||||||||
| Less than high school | 28.0 | 31.9 | 28.8 | 30.6 | 30.2 | ||||||||||
| High school graduate | 23.2 | 22.2 | 22.6 | 22.9 | 26.7 | ||||||||||
| College or higher | 48.8 | 45.8 | 48.6 | 46.5 | 43.1 | ||||||||||
| Smoking status | |||||||||||||||
| Never | 31.8 | 30.2 | 34.4 | 34.0 | 35.4 | ||||||||||
| Former | 52.3 | 55.2 | 55.2 | 53.8 | 57.6 | ||||||||||
| Current | 15.9 | 14.6 | 10.4 | 12.2 | 6.9 | ||||||||||
| Alcoholic drinks/week | |||||||||||||||
| 0 | 43.6 | 36.5 | 38.5 | 36.1 | 47.2 | ||||||||||
| <7 | 35.3 | 41.7 | 39.2 | 40.6 | 36.8 | ||||||||||
| 7–13 | 9.3 | 9.0 | 10.8 | 9.7 | 5.9 | ||||||||||
| ≥14 | 11.8 | 12.9 | 11.5 | 13.5 | 10.1 | ||||||||||
| Self-reported health | |||||||||||||||
| Excellent | 18.7 | 20.1 | 19.1 | 22.9 | 14.0 | ||||||||||
| Very good | 31.1 | 28.1 | 29.2 | 24.3 | 26.2 | ||||||||||
| Good | 32.9 | 37.2 | 33.0 | 37.2 | 42.7 | ||||||||||
| Fair | 15.6 | 10.8 | 16.0 | 14.9 | 15.0 | ||||||||||
| Poor | 1.7 | 3.8 | 2.8 | 0.7 | 2.1 | ||||||||||
| Weight change from age 50 years, kg | −4.1 (5.3) | −0.7 (6.0) | 0.9 (6.2) | 3.1 (7.6) | 6.6 (10.1) | ||||||||||
| Physical activity, kcal | 1,103 (416–2,501) | 1,080 (415–2,455) | 1,120 (432–2,471) | 1,080 (405–2,700) | 840 (268–1,860) | ||||||||||
| Estrogen use | |||||||||||||||
| Never | —a | — | — | — | — | ||||||||||
| Past | — | — | — | — | — | ||||||||||
| Current | — | — | — | — | — | ||||||||||
| Aspirin use >2 days in past 2 weeks | 28.4 | 29.2 | 30.9 | 30.9 | 30.9 | ||||||||||
| Systolic blood pressure, mm Hg | 134.2 (22.8) | 133.6 (20.7) | 135.3 (19.9) | 135.8 (20.3) | 139.5 (20.2) | ||||||||||
| Diastolic blood pressure, mm Hg | 70.9 (11.3) | 71.4 (11.7) | 73.3 (11.8) | 73.7 (10.7) | 75.6 (10.9) | ||||||||||
| Hypertension | 47.8 | 45.8 | 50.0 | 56.9 | 66.7 | ||||||||||
| Use of antihypertensive medication | 25.6 | 27.8 | 30.9 | 42.0 | 46.2 | ||||||||||
| Impaired fasting glucose | 36.3 | 39.9 | 43.8 | 49.0 | 47.9 | ||||||||||
| Diabetes | 10.0 | 11.1 | 13.2 | 17.0 | 28.5 | ||||||||||
| Glucose, mg/dL | 106.1 (31.8) | 109.5 (35.5) | 107.2 (25.1) | 112.2 (28.6) | 122.1 (41.3) | ||||||||||
| Insulin, IU/mL | 12.7 (24.7) | 14.4 (24.6) | 14.2 (8.7) | 16.1 (9.8) | 22.0 (26.8) | ||||||||||
| Total cholesterol, mg/dL | 193.7 (34.4) | 196.8 (34.5) | 202.9 (36.9) | 201.7 (35.4) | 200.6 (38.5) | ||||||||||
| LDL cholesterol, mg/dL | 118.3 (31.3) | 122.7 (32.0) | 125.9 (33.8) | 126.9 (33.7) | 125.9 (35.9) | ||||||||||
| HDL cholesterol, mg/dL | 53.7 (14.3) | 48.6 (11.3) | 50.8 (14.1) | 46.4 (11.7) | 45.8 (10.6) | ||||||||||
| Triglycerides, mg/dL | 98 (78–129) | 113 (87–149) | 113 (88–155) | 127 (95–180) | 130 (103–172) | ||||||||||
| Serum creatinine, mg/dL | 1.19 (0.25) | 1.18 (0.30) | 1.21 (0.29) | 1.22 (0.26) | 1.20 (0.31) | ||||||||||
| hsCRP, mg/L | 1.6 (0.7–3.1) | 2.0 (1.0–3.7) | 2.0 (1.1–3.7) | 2.3 (1.3–4.1) | 2.8 (1.6–4.8) | ||||||||||
Abbreviations: BMI, body mass index; HDL, high density lipoprotein; hsCRP, high sensitivity C-reactive protein; IQR, intrerquartile range; LDL, low density lipoprotein; SD, standard deviation.
—, not applicable.
Mean values for BMI, waist circumference, and waist/hip ratio in women and men, respectively, were as follows: 26.6 (SD, 5.0) kg/m2 and 26.4 (SD, 3.6) kg/m2; 91.3 (SD, 14.1) cm and 97.4 (SD, 10.1) cm; and 0.89 (SD, 0.09) and 0.96 (SD, 0.06). Baseline BMI was strongly correlated in both women and men with fat mass (r = 0.90 and 0.80), waist circumference (r = 0.79 and 0.85), hip circumference (r = 0.88 and 0.81), and waist/height ratio (r = 0.80 and 0.85); somewhat less so with BMI50 (r = 0.75 and 0.70); and more moderately with fat-free mass (r = 0.51 and 0.48) and waist/hip ratio (r = 0.34 and 0.49) (all P < 0.001). Corresponding correlations for BMI50 with baseline measures of adiposity were attenuated but remained moderately strong for women and men in relation to waist/height ratio (r = 0.64 and 0.61), fat mass (r = 0.62 and 0.52), waist circumference (r = 0.61 and 0.59), and hip circumference (r = 0.63 and 0.57) (all P < 0.001).
Body composition and stroke
During median follow-up of 14 years, there were 490 ischemic strokes (622 total strokes). There was an interaction with sex for waist/hip ratio and ischemic stroke (P < 0.001). Tables 3 and 4 present the hazard ratios for different measures of body composition and ischemic stroke stratified by sex. There were no significant associations for quintiles of any of the adiposity measures in age- and race-adjusted analyses or in analyses adjusting for potential confounders, with the exception of waist/hip ratio in women. After adjustment for potential confounders, there was an approximate 70% increase in the risk of ischemic stroke associated with quintiles 3 and 5, but not quintile 4, compared with quintile 1 (Ptrend = 0.006). Additional adjustment for potential mediators somewhat weakened but did not eliminate these relations (Table 3). Additional adjustment for BMI modestly strengthened the waist/hip ratio estimates. Findings were similar in sensitivity analyses, including in relation to all strokes as the outcome (not shown).
Table 3.
Relations of Quintiles of Body Size and Composition Measures with Incident Ischemic Stroke Among Women in the Cardiovascular Health Study Sample, United States, 1989–2007
| Adipose Measure (Quintile Cutpoints) | Women | |||||
| Person-Years | No. of Strokes | HRa | 95% CI | HRb | 95% CI | |
| Body mass index, kg/m2 | ||||||
| Quintile 1 (<22.5) | 5,684 | 62 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (22.5–24.7) | 6,081 | 56 | 0.91 | 0.63, 1.30 | 0.88 | 0.61, 1.27 |
| Quintile 3 (24.8–27.0) | 5,970 | 73 | 1.18 | 0.84, 1.66 | 1.19 | 0.84, 1.67 |
| Quintile 4 (27.1–30.3) | 6,135 | 56 | 0.89 | 0.62, 1.28 | 0.87 | 0.60, 1.26 |
| Quintile 5 (>30.3) | 5,724 | 62 | 1.09 | 0.76, 1.57 | 1.00 | 0.69, 1.46 |
| Ptrend | 0.71 | 0.99 | ||||
| Body mass index at age 50 years, kg/m2 | ||||||
| Quintile 1 (<21.7) | 6,032 | 63 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (21.7–24.7) | 6,127 | 63 | 0.99 | 0.70, 1.41 | 1.03 | 0.73, 1.47 |
| Quintile 3 (24.8–27.0) | 6,146 | 56 | 0.85 | 0.59, 1.22 | 0.85 | 0.59, 1.22 |
| Quintile 4 (27.1–30.3) | 5,804 | 64 | 1.02 | 0.72, 1.45 | 0.99 | 0.70, 1.41 |
| Quintile 5 (>30.3) | 5,486 | 63 | 1.08 | 0.75, 1.54 | 0.98 | 0.68, 1.42 |
| Ptrend | 0.70 | 0.82 | ||||
| Fat mass, kg | ||||||
| Quintile 1 (<25.6) | 5,513 | 66 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (25.6–30.8) | 5,984 | 62 | 0.98 | 0.69, 1.38 | 0.97 | 0.69, 1.38 |
| Quintile 3 (30.9–35.8) | 6,073 | 64 | 0.95 | 0.67, 1.35 | 0.95 | 0.67, 1.35 |
| Quintile 4 (35.9–42.5) | 6,091 | 65 | 1.02 | 0.72, 1.44 | 0.97 | 0.68, 1.38 |
| Quintile 5 (>42.5) | 5,933 | 52 | 0.86 | 0.59, 1.25 | 0.81 | 0.55, 1.19 |
| Ptrend | 0.56 | 0.36 | ||||
| Fat-free mass, kg | ||||||
| Quintile 1 (<29.1) | 5,722 | 61 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (29.1–31.2) | 5,776 | 65 | 1.13 | 0.79, 1.60 | 1.16 | 0.81, 1.64 |
| Quintile 3 (31.3–33.3) | 6,180 | 61 | 1.03 | 0.72, 1.47 | 1.06 | 0.74, 1.52 |
| Quintile 4 (33.4–36.2) | 6,033 | 58 | 1.04 | 0.72, 1.49 | 1.05 | 0.73, 1.51 |
| Quintile 5 (>36.2) | 5,883 | 64 | 1.16 | 0.81, 1.66 | 1.19 | 0.83, 1.70 |
| Ptrend | 0.59 | 0.56 | ||||
| Waist circumference, cm | ||||||
| Quintile 1 (<79.0) | 6,267 | 65 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (79.0–86.4) | 5,766 | 50 | 0.86 | 0.59, 1.24 | 0.86 | 0.59, 1.25 |
| Quintile 3 (86.5–93.9) | 5,978 | 64 | 1.02 | 0.72, 1.44 | 1.00 | 0.71, 1.42 |
| Quintile 4 (94.0–102.4) | 6,002 | 62 | 0.99 | 0.70, 1.40 | 0.97 | 0.68, 1.39 |
| Quintile 5 (>102.4) | 5,580 | 68 | 1.21 | 0.85, 1.71 | 1.13 | 0.79, 1.61 |
| Ptrend | 0.22 | 0.39 | ||||
| Hip circumference, cm | ||||||
| Quintile 1 (<94.0) | 6,339 | 77 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (94.0–98.4) | 5,141 | 63 | 1.06 | 0.76, 1.49 | 1.06 | 0.76, 1.48 |
| Quintile 3 (98.5–102.9) | 6,310 | 57 | 0.82 | 0.58, 1.16 | 0.84 | 0.59, 1.19 |
| Quintile 4 (103.0–109.9) | 6,247 | 58 | 0.84 | 0.59, 1.18 | 0.83 | 0.59, 1.17 |
| Quintile 5 (>109.9) | 5,556 | 54 | 0.89 | 0.62, 1.27 | 0.83 | 0.58, 1.19 |
| Ptrend | 0.23 | 0.14 | ||||
| Waist/hip ratio | ||||||
| Quintile 1 (<0.81) | 6,390 | 49 | 1.00 | Referent | 1.00c | Referent |
| Quintile 2 (0.81–0.86) | 6,027 | 47 | 0.98 | 0.65, 1.46 | 0.96c | 0.65, 1.44 |
| Quintile 3 (0.87–0.91) | 5,761 | 82 | 1.73 | 1.21, 2.46 | 1.72c | 1.20, 2.45 |
| Quintile 4 (0.92–0.96) | 5,912 | 51 | 1.04 | 0.70, 1.54 | 1.02c | 0.69, 1.52 |
| Quintile 5 (>0.96) | 5,504 | 80 | 1.76 | 1.23, 2.52 | 1.71c | 1.19, 2.46 |
| Ptrend | 0.003 | 0.006 | ||||
| Waist/height ratio | ||||||
| Quintile 1 (<0.49) | 6,102 | 58 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (0.49–0.54) | 6,165 | 50 | 0.84 | 0.57, 1.22 | 0.83 | 0.57, 1.21 |
| Quintile 3 (0.55–0.59) | 5,844 | 62 | 1.07 | 0.75, 1.53 | 1.06 | 0.74, 1.52 |
| Quintile 4 (0.60–0.65) | 6,002 | 70 | 1.17 | 0.83, 1.66 | 1.13 | 0.80, 1.61 |
| Quintile 5 (>0.65) | 5,480 | 69 | 1.25 | 0.88, 1.78 | 1.17 | 0.82, 1.68 |
| Ptrend | 0.06 | 0.14 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Adjusted for age and race (black, nonblack).
Adjusted for age, race (black, nonblack), smoking status (never, former, current), physical activity, alcoholic drinks/week (0, <7, 7–13, ≥14), educational attainment (less than high school, high school graduate, college or higher), serum creatinine, and hormone replacement therapy.
After additional adjustment for potential mediators (systolic blood pressure, antihypertensive medication use, diabetes, prediabetes, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, use of aspirin, and high-sensitivity C-reactive protein), the corresponding effect estimates were as follows: quartile 2 vs. quartile 1 (HR = 0.93, 95% CI: 0.62, 1.40), quartile 3 vs. quartile 1 (HR = 1.61, 95% CI: 1.11, 2.32), quartile 4 vs. quartile 1 (HR = 0.95, 95% CI: 0.64, 1.42), and quartile 5 vs. quartile 1 (HR = 1.53, 95% CI: 1.05, 2.24); Ptrend = 0.057.
Table 4.
Relations of Quintiles of Body Size and Composition Measures with Incident Ischemic Stroke Among Men in the Cardiovascular Health Study Sample, United States, 1989–2007
| Adipose Measure (Quintile Cutpoints) | Men |
|||||
| Person-Years | No. of Strokes | HRa | 95% CI | HRb | 95% CI | |
| Body mass index, kg/m2 | ||||||
| Quintile 1 (<23.4) | 3,109 | 39 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (23.4–25.2) | 3,299 | 36 | 0.93 | 0.59, 1.46 | 0.93 | 0.59, 1.47 |
| Quintile 3 (25.3–26.8) | 3,390 | 35 | 0.87 | 0.55, 1.38 | 0.86 | 0.54, 1.37 |
| Quintile 4 (26.9–29.1) | 3,406 | 31 | 0.80 | 0.50, 1.28 | 0.78 | 0.48, 1.25 |
| Quintile 5 (>29.1) | 3,325 | 40 | 1.11 | 0.71, 1.73 | 1.09 | 0.69, 1.71 |
| Ptrend | 0.91 | 0.99 | ||||
| Body mass index at age 50 years, kg/m2 | ||||||
| Quintile 1 (<23.4) | 3,356 | 34 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (23.4–24.8) | 3,406 | 37 | 1.08 | 0.68, 1.72 | 1.06 | 0.66, 1.69 |
| Quintile 3 (24.9–26.2) | 3,260 | 40 | 1.30 | 0.82, 2.05 | 1.30 | 0.82, 2.06 |
| Quintile 4 (26.3–28.2) | 3,411 | 32 | 0.96 | 0.59, 1.56 | 0.97 | 0.60, 1.58 |
| Quintile 5 (>28.2) | 3,097 | 38 | 1.29 | 0.81, 2.05 | 1.29 | 0.81, 2.07 |
| Ptrend | 0.45 | 0.43 | ||||
| Fat mass, kg | ||||||
| Quintile 1 (<23.1) | 3,137 | 36 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (23.1–27.9) | 3,396 | 35 | 0.99 | 0.62, 1.57 | 0.98 | 0.61, 1.57 |
| Quintile 3 (28.0–32.0) | 3,469 | 34 | 0.95 | 0.59, 1.51 | 0.94 | 0.58, 1.50 |
| Quintile 4 (32.1–37.6) | 3,358 | 33 | 1.02 | 0.63, 1.65 | 0.98 | 0.61, 1.59 |
| Quintile 5 (>37.6) | 3,168 | 43 | 1.44 | 0.92, 2.25 | 1.42 | 0.90, 2.23 |
| Ptrend | 0.14 | 0.18 | ||||
| Fat-free mass, kg | ||||||
| Quintile 1 (<32.6) | 2,931 | 37 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (32.6–36.6) | 3,278 | 35 | 0.92 | 0.58, 1.46 | 0.94 | 0.59, 1.49 |
| Quintile 3 (36.7–39.9) | 3,312 | 35 | 0.90 | 0.57, 1.43 | 0.93 | 0.58, 1.48 |
| Quintile 4 (40.0–43.5) | 3,523 | 41 | 1.09 | 0.69, 1.71 | 1.09 | 0.69, 1.73 |
| Quintile 5 (>43.5) | 3,484 | 33 | 0.87 | 0.54, 1.40 | 0.86 | 0.53, 1.39 |
| Ptrend | 0.86 | 0.80 | ||||
| Waist circumference, cm | ||||||
| Quintile 1 (<89.5) | 3,349 | 42 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (89.5–94.4) | 3,323 | 33 | 0.84 | 0.53, 1.32 | 0.83 | 0.53, 1.31 |
| Quintile 3 (94.5–99.1) | 3,277 | 40 | 1.06 | 0.69, 1.64 | 1.05 | 0.68, 1.62 |
| Quintile 4 (99.2–104.9) | 3,410 | 27 | 0.67 | 0.41, 1.09 | 0.66 | 0.40, 1.07 |
| Quintile 5 (>104.9) | 3,170 | 39 | 1.09 | 0.70, 1.69 | 1.05 | 0.67, 1.64 |
| Ptrend | 0.95 | 0.79 | ||||
| Hip circumference, cm | ||||||
| Quintile 1 (<95.0) | 3,324 | 48 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (95.0–98.9) | 3,780 | 33 | 0.62 | 0.40, 0.97 | 0.60 | 0.38, 0.93 |
| Quintile 3 (99.0–101.9) | 3,010 | 31 | 0.75 | 0.48, 1.18 | 0.72 | 0.46, 1.14 |
| Quintile 4 (102.0–106.4) | 3,325 | 32 | 0.74 | 0.47, 1.16 | 0.72 | 0.46, 1.13 |
| Quintile 5 (>106.4) | 3,090 | 37 | 0.89 | 0.58, 1.37 | 0.86 | 0.56, 1.33 |
| Ptrend | 0.80 | 0.73 | ||||
| Waist/hip ratio | ||||||
| Quintile 1 (<0.92) | 3,432 | 34 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (0.92–0.95) | 3,356 | 47 | 1.47 | 0.95, 2.29 | 1.46 | 0.94, 2.27 |
| Quintile 3 (0.96–0.97) | 3,297 | 29 | 0.89 | 0.54, 1.46 | 0.89 | 0.54, 1.46 |
| Quintile 4 (0.98–1.00) | 3,281 | 39 | 1.27 | 0.80, 2.01 | 1.25 | 0.79, 1.98 |
| Quintile 5 (>1.00) | 3,163 | 32 | 1.08 | 0.67, 1.76 | 1.02 | 0.63, 1.67 |
| Ptrend | 0.98 | 0.82 | ||||
| Waist/height ratio | ||||||
| Quintile 1 (<0.51) | 3,310 | 41 | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 (0.51–0.54) | 3,454 | 35 | 0.81 | 0.51, 1.27 | 0.80 | 0.51, 1.26 |
| Quintile 3 (0.55–0.57) | 3,336 | 39 | 0.93 | 0.60, 1.43 | 0.90 | 0.58, 1.41 |
| Quintile 4 (0.58–0.61) | 3,215 | 28 | 0.70 | 0.44, 1.14 | 0.69 | 0.42, 1.11 |
| Quintile 5 (>0.61) | 3,214 | 38 | 0.97 | 0.63, 1.51 | 0.94 | 0.60, 1.47 |
| Ptrend | 0.73 | 0.60 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Adjusted for age and race (black, nonblack).
Adjusted for age, race (black, nonblack), smoking status (never, former, current), physical activity, alcoholic drinks/week (0, <7, 7–13, ≥14), educational attainment (less than high school, high school graduate, college or higher), and serum creatinine.
When anthropometric measures were dichotomized according to published cutpoints, no significant associations with ischemic stroke were observed in women. The hazard ratios adjusted for confounders were as follows: 1.00 (95% confidence interval (CI): 0.75, 1.33) for BMI ≥30 kg/m2; 1.10 (95% CI: 0.75, 1.61) for BMI50 ≥30 kg/m2; 1.06 (95% CI: 0.84, 1.33) for waist circumference ≥88 cm; and 1.16 (95% CI: 0.84, 1.59) for waist/hip ratio ≥0.80. By contrast, a significant confounder-adjusted association for BMI, though not for the other measures, was observed in men with hazard ratios of 1.49 (95% CI: 1.02, 2.19) for BMI ≥30 kg/m2; 1.25 (95% CI: 0.78, 1.98) for BMI50 ≥30 kg/m2; 0.97 (95% CI: 0.70, 1.34) for waist circumference ≥102 cm; and 0.87 (95% CI: 0.65, 1.17) for waist/hip ratio ≥0.95. The relation for BMI with ischemic stroke ceased to be significant after adjustment for mediators (hazard ratio = 1.15, 95% CI: 0.76, 1.72).
Body composition and coronary heart disease
Corresponding relations for coronary events (nonfatal myocardial infarction, n = 519; fatal coronary heart disease, n = 326) are shown in Table 5. Because no significant interactions were observed for this outcome, findings are presented for women and men combined. After adjustment for potential confounders, every body composition parameter except for fat-free mass exhibited a significant positive association with incident coronary heart disease. These relations tended to be graded and statistically significant for comparisons of extreme quintiles. Coronary heart disease risk increases ranged from 26% for hip circumference to 71% for BMI50, but with considerable overlap of confidence intervals. Adjustment for putative mediators abolished these relations for BMI, fat mass, waist circumference, and hip circumference and nearly so for waist/height ratio. Yet, significant, if attenuated, associations persisted for extreme quintiles in the case of waist/hip ratio (hazard ratio = 1.36, 95% CI: 1.08, 1.71) and BMI50 (hazard ratio = 1.40, 95% CI: 1.11, 1.76), along with significant overall linear trends. These associations were not meaningfully altered when fasting glucose and insulin, together with hypoglycemic medications, replaced prediabetes and diabetes as covariates. The findings were also not influenced by consideration of incident diabetes, modeled as a time-varying covariate, nor did the effect estimates change substantively in analyses restricted to participants with good or better health status, to never- or remote smokers, or to events beyond 3 years of follow-up.
Table 5.
Relations of Quintiles of Body Size and Composition Measures With Incident Coronary Heart Disease in the Cardiovascular Health Study Sample, United States, 1989–2007
| Adiposity Measurea | Person-Years | No. of CHD Events | HRb | 95% CI | HRc | 95% CI | HRde | 95% CI |
| Body mass index | ||||||||
| Quintile 1 | 8,712 | 152 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 | 9,308 | 168 | 1.11 | 0.89, 1.39 | 1.08 | 0.87, 1.35 | 1.03 | 0.82, 1.28 |
| Quintile 3 | 9,350 | 156 | 1.02 | 0.81, 1.27 | 1.00 | 0.80, 1.25 | 0.90 | 0.72, 1.14 |
| Quintile 4 | 9,449 | 167 | 1.11 | 0.89, 1.39 | 1.06 | 0.85, 1.32 | 0.92 | 0.73, 1.16 |
| Quintile 5 | 8,872 | 202 | 1.53 | 1.23, 1.89 | 1.42 | 1.14, 1.77 | 1.11 | 0.88, 1.40 |
| Ptrend | 0.001 | 0.006 | 0.66 | |||||
| Body mass index at age 50 years | ||||||||
| Quintile 1 | 9,453 | 137 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 | 9,411 | 165 | 1.23 | 0.98, 1.54 | 1.23 | 0.98, 1.55 | 1.19 | 0.95, 1.49 |
| Quintile 3 | 9,231 | 159 | 1.26 | 1.00, 1.58 | 1.25 | 1.00, 1.58 | 1.21 | 0.96, 1.52 |
| Quintile 4 | 9,121 | 182 | 1.41 | 1.13, 1.76 | 1.40 | 1.12, 1.75 | 1.24 | 0.99, 1.55 |
| Quintile 5 | 8,475 | 202 | 1.80 | 1.45, 2.24 | 1.71 | 1.37, 2.14 | 1.40 | 1.11, 1.76 |
| Ptrend | <0.001 | <0.001 | 0.009 | |||||
| Fat mass | ||||||||
| Quintile 1 | 8,537 | 165 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 | 9,324 | 172 | 1.07 | 0.86, 1.32 | 1.06 | 0.86, 1.32 | 1.00 | 0.80, 1.24 |
| Quintile 3 | 9,541 | 161 | 0.96 | 0.77, 1.19 | 0.92 | 0.74, 1.15 | 0.82 | 0.66, 1.03 |
| Quintile 4 | 9,413 | 147 | 0.97 | 0.77, 1.21 | 0.94 | 0.75, 1.18 | 0.79 | 0.62, 1.00 |
| Quintile 5 | 8,875 | 200 | 1.49 | 1.21, 1.85 | 1.40 | 1.13, 1.74 | 1.13 | 0.89, 1.42 |
| Ptrend | 0.003 | 0.02 | 0.88 | |||||
| Fat-free mass | ||||||||
| Quintile 1 | 8,564 | 172 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 | 9,090 | 153 | 0.88 | 0.71, 1.10 | 0.91 | 0.73, 1.14 | 0.91 | 0.73, 1.13 |
| Quintile 3 | 9,353 | 177 | 1.01 | 0.82, 1.24 | 1.04 | 0.85, 1.29 | 1.02 | 0.83, 1.27 |
| Quintile 4 | 9,286 | 188 | 1.14 | 0.93, 1.41 | 1.17 | 0.95, 1.44 | 1.07 | 0.87, 1.33 |
| Quintile 5 | 9,397 | 155 | 0.92 | 0.74, 1.15 | 0.93 | 0.74, 1.15 | 0.83 | 0.66, 1.04 |
| Ptrend | 0.69 | 0.68 | 0.48 | |||||
| Waist circumference | ||||||||
| Quintile 1 | 9,555 | 163 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 | 9,056 | 142 | 0.96 | 0.77, 1.20 | 0.95 | 0.76, 1.20 | 0.90 | 0.72, 1.13 |
| Quintile 3 | 9,275 | 166 | 1.10 | 0.89, 1.36 | 1.08 | 0.87, 1.34 | 0.99 | 0.79, 1.24 |
| Quintile 4 | 9,166 | 178 | 1.19 | 0.96, 1.47 | 1.16 | 0.94, 1.44 | 1.05 | 0.84, 1.31 |
| Quintile 5 | 8,638 | 196 | 1.49 | 1.21, 1.84 | 1.39 | 1.12, 1.72 | 1.11 | 0.88, 1.39 |
| Ptrend | <0.001 | 0.001 | 0.17 | |||||
| Hip circumference | ||||||||
| Quintile 1 | 9,656 | 180 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 | 8,893 | 163 | 0.99 | 0.80, 1.23 | 0.97 | 0.79, 1.20 | 0.92 | 0.74, 1.14 |
| Quintile 3 | 9,121 | 162 | 1.05 | 0.85, 1.31 | 1.06 | 0.85, 1.31 | 0.99 | 0.79, 1.22 |
| Quintile 4 | 9,549 | 154 | 0.96 | 0.78, 1.19 | 0.94 | 0.75, 1.17 | 0.84 | 0.68, 1.06 |
| Quintile 5 | 8,471 | 186 | 1.35 | 1.10, 1.66 | 1.26 | 1.02, 1.55 | 1.02 | 0.82, 1.27 |
| Ptrend | 0.02 | 0.08 | 0.89 | |||||
| Waist/hip ratio | ||||||||
| Quintile 1 | 9,719 | 139 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 | 9,378 | 162 | 1.21 | 0.96, 1.51 | 1.19 | 0.95, 1.50 | 1.12 | 0.89, 1.41 |
| Quintile 3 | 8,904 | 174 | 1.34 | 1.07, 1.67 | 1.31 | 1.04, 1.63 | 1.16 | 0.92, 1.46 |
| Quintile 4 | 9,106 | 175 | 1.36 | 1.09, 1.70 | 1.33 | 1.06, 1.66 | 1.19 | 0.94, 1.49 |
| Quintile 5 | 8,584 | 195 | 1.64 | 1.32, 2.04 | 1.56 | 1.25, 1.94 | 1.36 | 1.08, 1.71 |
| Ptrend | <0.001 | <0.001 | 0.010 | |||||
| Waist/height ratio | ||||||||
| Quintile 1 | 9,358 | 144 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Quintile 2 | 9,560 | 144 | 0.97 | 0.77, 1.22 | 0.95 | 0.75, 1.20 | 0.88 | 0.70, 1.12 |
| Quintile 3 | 9,137 | 179 | 1.27 | 1.02, 1.59 | 1.25 | 1.00, 1.55 | 1.16 | 0.93, 1.45 |
| Quintile 4 | 9,143 | 172 | 1.22 | 0.98, 1.53 | 1.16 | 0.93, 1.45 | 1.01 | 0.80, 1.28 |
| Quintile 5 | 8,483 | 206 | 1.63 | 1.32, 2.02 | 1.51 | 1.22, 1.88 | 1.21 | 0.96, 1.52 |
| Ptrend | <0.001 | <0.001 | 0.040 | |||||
Abbreviations: CHD, coronary heart disease; CI, confidence interval; HR, hazard ratio.
Adjusted for age, sex, and race (black, nonblack).
Adjusted for age, sex, race (black, nonblack), smoking status (never, former, current), physical activity, alcoholic drinks/week (0, <7, 7–13, ≥14), educational attainment (less than high school, high school graduate, college or higher), serum creatinine, and hormone replacement therapy (women).
Adjusted for age, sex, race (black, nonblack), smoking status (never, former, current), physical activity, alcoholic drinks/week (0, <7, 7–13, ≥14), educational attainment (less than high school, high school graduate, college or higher), serum creatinine, hormone replacement therapy (women), systolic blood pressure, antihypertensive medication use, diabetes, prediabetes, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, use of aspirin, and high-sensitivity C-reactive protein.
Models with and without inclusion of high-sensitivity C-reactive protein yielded similar effect estimates across measures and are therefore not presented separately.
When conventional dichotomous cutpoints were examined, BMI, BMI50, waist circumference, and waist/hip ratio were each significantly associated with coronary heart disease after adjustment for confounders, although only BMI and waist/hip ratio remained significant after adjustment for potential mediators (Table 6). Similarly, significant graded relations were observed after adjustment for confounders when BMI and BMI50 were further subcategorized. The overweight category (25–29.9 kg/m2) was significant only for BMI50, however, and only BMI50 retained significance following adjustment for mediators (Table 6).
Table 6.
Relations of Anthropometric Measures by Recommended Cutpoints and Incident Coronary Heart Disease in the Cardiovascular Health Study Sample, United States, 1989–2007
| Adiposity Measure | Person-Years | No. of CHD Events | HRa | 95% CI | HRb | 95% CI | HRc | 95% CI |
| Binary categories | ||||||||
| Body mass index, kg/m2 | ||||||||
| <30 | 37,144 | 654 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| ≥30 | 8,546 | 191 | 1.51 | 1.28, 1.78 | 1.44 | 1.22, 1.71 | 1.22 | 1.03, 1.46 |
| Body mass index at age 50 years, kg/m2 | ||||||||
| <30 | 41,632 | 745 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| ≥30 | 4,058 | 100 | 1.42 | 1.15, 1.75 | 1.37 | 1.11, 1.70 | 1.18 | 0.95, 1.47 |
| Waist circumference, cm | ||||||||
| <88 (W), <102 (M) | 23,959 | 425 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| ≥88 (W), ≥102 (M) | 21,732 | 420 | 1.34 | 1.16, 1.54 | 1.28 | 1.11, 1.47 | 1.13 | 0.97, 1.31 |
| Waist/hip ratio | ||||||||
| <0.80 (W), <0.95 (M) | 11,968 | 186 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| ≥0.80 (W), ≥0.95 (M) | 33,723 | 659 | 1.49 | 1.26, 1.77 | 1.43 | 1.21, 1.70 | 1.32 | 1.11, 1.57 |
| Multiple categories | ||||||||
| Body mass index, kg/m2 | ||||||||
| <25.0 | 18,030 | 305 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 25–29.9 | 19,114 | 349 | 1.06 | 0.91, 1.24 | 1.03 | 0.90, 1.20 | 0.95 | 0.81, 1.11 |
| 30.0–34.9 | 6,471 | 146 | 1.50 | 1.23, 1.83 | 1.42 | 1.16, 1.73 | 1.14 | 0.92, 1.42 |
| ≥35 | 2,075 | 45 | 1.79 | 1.30, 2.47 | 1.70 | 1.23, 2.35 | 1.37 | 0.98, 1.90 |
| Ptrend | <0.001 | <0.001 | 0.10 | |||||
| Body mass index at age 50 years, kg/m2 | ||||||||
| <25.0 | 24,349 | 370 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 25–29.9 | 17,283 | 375 | 1.37 | 1.18, 1.58 | 1.34 | 1.15, 1.55 | 1.22 | 1.05, 1.42 |
| 30.0–34.9 | 3,374 | 79 | 1.56 | 1.22, 1.99 | 1.53 | 1.19, 1.96 | 1.29 | 1.00, 1.67 |
| ≥35 | 685 | 21 | 2.16 | 1.39, 3.37 | 1.93 | 1.24, 3.02 | 1.51 | 0.96, 2.38 |
| Ptrend | <0.001 | <0.001 | 0.003 | |||||
Abbreviations: CHD, coronary heart disease; CI, confidence interval; HR, hazard ratio; M, men; W, women.
Adjusted for age, sex, and race (black, nonblack).
Adjusted for age, sex, race (black, nonblack), smoking status (never, former, current), physical activity, alcoholic drinks/week (0, <7, 7–13, ≥14), educational attainment (less than high school, high school graduate, college or higher), serum creatinine, and hormone replacement therapy (women).
Adjusted for age, sex, race (black, nonblack), smoking status (never, former, current), physical activity, alcoholic drinks/week (0, <7, 7–13, ≥14), educational attainment (less than high school, high school graduate, college or higher), serum creatinine, hormone replacement therapy (women), systolic blood pressure, antihypertensive medication use, diabetes, prediabetes, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, use of aspirin, and high-sensitivity C-reactive protein.
Joint analyses
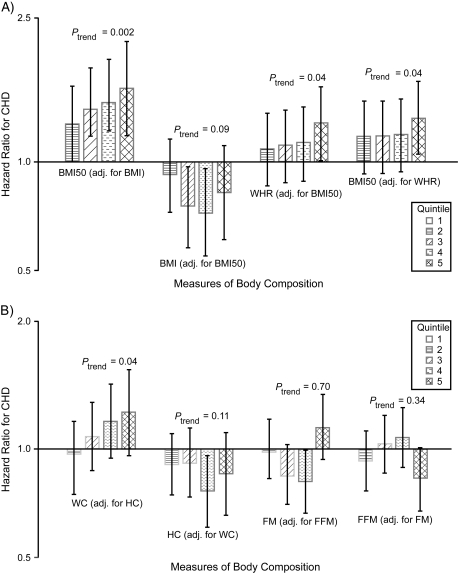
When body composition measures were entered together in multivariable models (Figure 1), a significant relation of midlife BMI with incident coronary heart disease persisted after adjustment for baseline BMI, as well as for potential confounders and mediators. Likewise, the association between waist/hip ratio and coronary heart disease detailed in Table 5 did not change meaningfully after accounting concurrently for baseline BMI (not shown). When the same model was adjusted for midlife BMI instead of baseline BMI, significant adjusted associations with coronary heart disease were observed for both waist/hip ratio and midlife BMI (Figure 1A). There were no significant interactions between waist/hip ratio and BMI or BMI50.
Figure 1.
Joint evaluation of selected measures of body composition in Cardiovascular Health Study participants in relation to incident coronary heart disease, United States, 1989–2007 (A and B). All models were adjusted for the indicated adiposity measure plus age, sex, race (black, nonblack), smoking status (never, former, current), physical activity, alcoholic drinks/week (0, <7, 7–13, ≥14), educational attainment (less than high school, high-school graduate, college or higher), serum creatinine, hormone replacement therapy (women), systolic blood pressure, antihypertensive medication use, diabetes, prediabetes, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, use of aspirin, and high-sensitivity C-reactive protein. Adj., adjusted; BMI, body mass index; BMI50, body mass index at age 50 years; CHD, coronary heart disease; FFM, fat-free mass; FM, fat mass; HC, hip circumference; WC, waist circumference; WHR, waist/hip ratio.
Joint inclusion of waist circumference and hip circumference in the full model (confounders plus mediators) revealed a significantly positive linear trend for waist circumference and an inverse trend for hip circumference that was not statistically significant (Figure 1B). In turn, joint consideration of fat mass and fat-free mass showed no significant association for fat mass. There was, however, a suggestion of an inverse association in the comparison of extreme fat-free mass quintiles after adjustment for potential confounders and mediators, but this fell short of significance (quintile 5 vs. quintile 1: hazard ratio = 0.80, 95% CI: 0.63, 1.01) (Figure 1B).
DISCUSSION
Prior longitudinal studies of older subjects have often failed to find a relation between measures of central adiposity and coronary heart disease (30–32) or stroke (33), particularly after adjustment for general adiposity. Subgroup analyses in cohorts including middle-aged and older subjects, however, have shown stronger adjusted relations with coronary heart disease for waist/hip ratio than BMI in those aged ≥65 years, with the waist/hip ratio associations persisting after adjustment for BMI (8, 34). Although another report on male and female cohorts documented that both waist/height ratio and BMI were significantly associated with cardiovascular disease events after adjustment for confounders in the subgroup aged >60 years (11), a separate study of women aged 60–79 years reported significant adjusted associations with coronary heart disease for different measures of central adiposity but not for BMI (35). The present analyses extend available findings by demonstrating, for the first time in a cohort aged 65–100 years, that different measures of body composition have broadly comparable significant relations with coronary heart disease, but that the association for waist/hip ratio is largely independent of a wide array of proposed mediators, including high-sensitivity C-reactive protein. Moreover, the association of waist/hip ratio with coronary heart disease was independent of baseline BMI, and even BMI at midlife, which exhibited a stronger association with incident coronary heart disease in quintiles analysis than any baseline adiposity measure obtained later in life. In the case of ischemic stroke, however, the waist/hip ratio emerged as the sole independent predictor in quintiles analysis in women, but use of binary cutpoints showed only baseline BMI to have a significant association in men.
Our findings also show that binary cutpoints recommended by scientific and government agencies for BMI, waist circumference, and waist/hip ratio based on studies in middle-aged and young adults (18, 19) exhibit significant associations with coronary heart disease (and, for BMI, with stroke in men) when applied to an elderly population. Moreover, those for BMI and waist/hip ratio with coronary heart disease were even significant after adjustment for mediators. The applicability of these partition values to older adults has been questioned in light of the well-documented physical changes that accompany aging (6, 36). Such changes include a loss of height, which would tend to overestimate BMI for a constant body weight in older persons (36). Aging is also characterized by an increase in fat mass, however, such that adipose content would be higher for any given BMI in older individuals, offsetting the previous overestimation (36). These opposing influences might help to explain the significant associations with coronary heart disease observed for obesity (BMI ≥30 kg/m2) and, more strongly, severe obesity (BMI ≥35 kg/m2). Of note, however, heightened risk of coronary heart disease was not similarly observed for the overweight group (BMI 25–29.9 kg/m2) at baseline, as opposed to midlife, suggesting that the “overweight” category may not be suitable for prognostication in older adults. Determination of optimal partition values for older adults will require additional work, ideally involving meta-analytical techniques assessing larger numbers of subjects.
Although our analyses showed overlapping confidence intervals for confounder-adjusted associations of upper quintiles for various parameters with coronary heart disease, the waist/hip ratio did exhibit the highest effect estimate. Moreover, the waist/hip ratio was the only baseline measure for which a significant relation persisted after accounting for mediators, and its association was independent of both BMI and BMI50. That the waist/hip ratio might be a better measure of adiposity-related cardiovascular disease risk than BMI, waist circumference, hip circumference, directly measured fat mass, and even the waist/height ratio can be proposed on the basis of the distinct physiologic characteristics documented for different fat depots. Visceral fat has a lower threshold for lipolysis than subcutaneous fat, and released free-fatty acids have direct access to the liver, which could accentuate their metabolic consequences (6). Expansion of visceral fat also alters production of bioactive peptides with myriad local and systemic effects (4). By contrast, subcutaneous fat appears to act as a sink for free-fatty acids (6), and higher subcutaneous fat (37), particularly in the lower limbs (38), has been associated with metabolic benefits in older persons.
Furthermore, because waist circumference is a direct measure of abdominal fat, while hip circumference reflects subcutaneous fat and muscle of the thighs and buttocks, the waist/hip ratio might serve as a superior composite metric subsuming the harmful effects of visceral fat and the beneficial properties of subcutaneous fat (6). Indeed, prospective studies in predominantly middle-aged cohorts have found not only positive independent associations of waist circumference with cardiovascular disease but also inverse associations for hip circumference when both measures are considered jointly (8, 9). These associations might be expected to strengthen in older adults owing to aging-associated central fat redistribution and skeletal muscle loss (13, 14, 39).
Our joint models suggested an inverse association between hip circumference and coronary heart disease concurrent with a positive association with waist circumference, but this was not statistically significant. Interestingly, adjustment of fat-free mass for fat mass uncovered a near-significant inverse association with coronary heart disease for extreme quintiles, consistent with a possible salutary effect for higher skeletal muscle mass after accounting for adipose mass. These observations may explain why the association of waist/hip ratio with coronary heart disease was least likely to be abolished after adjustment for the chief proposed mediators of the adverse effects associated with adiposity—and by BMI itself—insofar as this measure subsumes information not only relating to visceral and subcutaneous fat distribution but also to gluteofemoral muscle wasting. Whether the mediator-independent associations with coronary heart disease detailed here for waist/hip ratio quintiles confer an advantage in risk prediction or could better serve to target interventions in older adults should be explored in systematic reviews compiling larger numbers of events.
Our study also found increased midlife BMI to be a significant predictor of future coronary heart disease risk, with higher effect estimates for quintiles or standard categories than BMI in later life. Previous work in older men showed that recalled midlife weight was only moderately correlated (r ∼ 0.75) with actual measurement, with a tendency to weight underestimation particularly when midlife weight was in the overweight/obese range (40). Such findings suggest that the associations for recalled BMI50 documented here might have been stronger had direct measurements been available. Hence, the prominent relation seen for recalled BMI50 may be consistent with prior reports demonstrating weakening of the relation between BMI and adverse outcomes with age (15, 34). This may reflect BMI's greater accuracy as a surrogate of visceral adiposity in middle-aged than older adults, in whom lower BMI may also signal muscle wasting (6). Another potential explanation, however, is that use of self-reported weight at age 50 years but measured height at baseline might have overestimated midlife BMI in participants experiencing loss of vertebral height, which itself could heighten cardiovascular disease risk (41). Nevertheless, this effect was likely minor, because the BMI50–coronary heart disease association was not stronger in women, who experience greater aging-associated height loss than men do (42).
This study has various strengths, including its focus on noninstitutionalized older adults through age 100 years; its inclusion of both women and men; standardized measurement, and not self-report, of baseline anthropometry; evaluation of directly measured body composition; availability of self-reported midlife weight; a large number of “hard” cardiovascular disease endpoints; long-term follow-up to minimize the effect of reverse causality; broad assessment of potential confounders and, particularly, mediators; and use of sensitivity analyses to explore potential biases.
There are likewise several limitations. First, because prevalent cardiovascular disease and comorbidities can confound the association of adiposity measures with outcome (29), the present findings are generalizable only to healthier older adults free of clinically overt cardiovascular disease. Second, the number of ischemic strokes in each sex stratum was modest, particularly in men; the inconsistent sex-specific findings observed for waist/hip ratio and BMI must be interpreted with caution and will require investigation in larger (meta-analytical) samples. Third, formal assessment of reproducibility of anthropometric measurements was not undertaken. Fourth, while bioelectric impedance generally provides valid estimates of body composition in older persons (43), the technique may be less accurate in the setting of abnormal hydration status. Exclusion of prevalent heart failure and adjustment for serum creatinine and antihypertensive medications, however, would tend to minimize associated measurement errors. Finally, we did not have measures of visceral and hepatic adiposity, lower extremity muscle mass, or intermyocellular adiposity, which could allow more apt investigation of aging-related changes in regional fat content and skeletal muscle mass and their cardiometabolic consequences.
Acknowledgments
Author affiliations: Department of Medicine, Weill Medical College of Cornell University, New York, New York (Jorge R. Kizer); Department of Public Health, Weill Medical College of Cornell University, New York, New York (Jorge R. Kizer); Department of Biostatistics, University of Washington, Seattle, Washington (Mary L. Biggs); Division of Nephrology, Department of Medicine, University of California San Diego, San Diego, California (Joachim H. Ix); Veterans Affairs San Diego Healthcare System, San Diego, California (Joachim H. Ix); Division of General Medicine and Primary Care, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts (Kenneth J. Mukamal); Department of Medicine, The Johns Hopkins University, Baltimore, Maryland (Susan J. Zieman); Division of Nephrology, Department of Medicine, University of Washington, Seattle, Washington (Ian H. de Boer); Division of Cardiovascular Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Dariush Mozaffarian); Division of Endocrinology, Emory University School of Medicine, Atlanta, Georgia (Joshua I. Barzilay); Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania (Elsa S. Strotmeyer, Lewis H. Kuller); Taub Institute for Research in Alzheimer's Disease and the Aging Brain, Columbia University Medical Center, New York, New York (Jose A. Luchsinger); Department of Medicine, Columbia University Medical Center, New York, New York (Jose A. Luchsinger); Department of Neurology, Columbia University Medical Center, New York, New York (Mitchell S. V. Elkind); Department of Neurology, University of Washington, Seattle, Washington (W. T. Longstreth, Jr.); Department of Epidemiology, University of Washington, Seattle, Washington (W. T. Longstreth, Jr.); Cardiovascular Health Research Unit, Department of Medicine, University of Washington, Seattle, Washington (David S. Siscovick); and Cardiovascular Health Research Unit, Department of Epidemiology, University of Washington, Seattle, Washington (David S. Siscovick).
The Cardiovascular Health Study was supported by contracts N01 HC-85079 through N01 HC-85086, N01 HC-35129, N01 HC-15103, N01 HC-55222, N01 HC-75150, and N01 HC-45133 and by grant U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke (http://www.chs-nhlbi.org/pi.htm). Additional support was provided by R01 HL-094555 from the National Heart, Lung, and Blood Institute and by award AG-023629 from the National Institute on Aging.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- BMI50
body mass index at age 50 years
- CI
confidence interval
- IQR
interquartile range
- SD
standard deviation
References
- 1.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356(23):2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Trends in aging—United States and worldwide MMWR Morb Mortal Wkly Rep. 2003;52(6):101–104. 106. [PubMed] [Google Scholar]
- 4.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21(12):1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 5.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 6.Snijder MB, van Dam RM, Visser M, et al. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35(1):83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 7.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280(21):1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 8.Canoy D, Boekholdt SM, Wareham N, et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation. 2007;116(25):2933–2943. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Rexrode KM, van Dam RM, et al. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117(13):1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 10.Walker SP, Rimm EB, Ascherio A, et al. Body size and fat distribution as predictors of stroke among US men. Am J Epidemiol. 1996;144(12):1143–1150. doi: 10.1093/oxfordjournals.aje.a008892. [DOI] [PubMed] [Google Scholar]
- 11.Gelber RP, Gaziano JM, Orav EJ, et al. Measures of obesity and cardiovascular risk among men and women. J Am Coll Cardiol. 2008;52(8):605–615. doi: 10.1016/j.jacc.2008.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Shu XO, Gao YT, et al. General and abdominal adiposity and risk of stroke in Chinese women. Stroke. 2009;40(4):1098–1104. doi: 10.1161/STROKEAHA.108.539692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26(2):372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 15.Stevens J, Cai J, Pamuk ER, et al. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338(1):1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 16.Visscher TL, Seidell JC, Molarius A, et al. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam Study. Int J Obes Relat Metab Disord. 2001;25(11):1730–1735. doi: 10.1038/sj.ijo.0801787. [DOI] [PubMed] [Google Scholar]
- 17.Price GM, Uauy R, Breeze E, et al. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84(2):449–460. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]
- 18.Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158(17):1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 19.US Department of Agriculture. Dietary Guidelines for Americans. Washington, DC: US Department of Agriculture; 1990. (Publication no. 261-495/20124) [Google Scholar]
- 20.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 21.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 22.Deurenberg P, van der Kooij K, Evers P, et al. Assessment of body composition by bioelectrical impedance in a population aged greater than 60 y. Am J Clin Nutr. 1990;51(1):3–6. doi: 10.1093/ajcn/51.1.3. [DOI] [PubMed] [Google Scholar]
- 23.Cushman M, Cornell ES, Howard PR, et al. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41(2):264–270. [PubMed] [Google Scholar]
- 24.Psaty BM, Lee M, Savage PJ, et al. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45(6):683–692. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 25.Taylor HL, Jacobs DR, Jr, Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 26.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43(1):52–58. [PubMed] [Google Scholar]
- 27.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 28.Longstreth WT, Jr, Bernick C, Fitzpatrick A, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001;56(3):368–375. doi: 10.1212/wnl.56.3.368. [DOI] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Horwich TB, Oreopoulos A, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10(4):433–442. doi: 10.1097/MCO.0b013e3281a30594. [DOI] [PubMed] [Google Scholar]
- 30.Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes Relat Metab Disord. 2001;25(7):1047–1056. doi: 10.1038/sj.ijo.0801615. [DOI] [PubMed] [Google Scholar]
- 31.Dey DK, Lissner L. Obesity in 70-year-old subjects as a risk factor for 15-year coronary heart disease incidence. Obes Res. 2003;11(7):817–827. doi: 10.1038/oby.2003.113. [DOI] [PubMed] [Google Scholar]
- 32.Nicklas BJ, Penninx BW, Cesari M, et al. Association of visceral adipose tissue with incident myocardial infarction in older men and women: the Health, Aging and Body Composition Study. Am J Epidemiol. 2004;160(8):741–749. doi: 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 33.Dey DK, Rothenberg E, Sundh V, et al. Waist circumference, body mass index, and risk for stroke in older people: a 15 year longitudinal population study of 70-year-olds. J Am Geriatr Soc. 2002;50(9):1510–1518. doi: 10.1046/j.1532-5415.2002.50406.x. [DOI] [PubMed] [Google Scholar]
- 34.Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141(12):1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 35.Taylor AE, Ebrahim S, Ben-Shlomo Y, et al. Comparison of the associations of body mass index and measures of central adiposity and fat mass with coronary heart disease, diabetes, and all-cause mortality: a study using data from 4 UK cohorts. Am J Clin Nutr. 2010;91(3):547–556. doi: 10.3945/ajcn.2009.28757. [DOI] [PubMed] [Google Scholar]
- 36.Villareal DT, Apovian CM, Kushner RF, et al. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82(5):923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 37.Tankó LB, Bagger YZ, Alexandersen P, et al. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107(12):1626–1631. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- 38.Gavi S, Feiner JJ, Melendez MM, et al. Limb fat to trunk fat ratio in elderly persons is a strong determinant of insulin resistance and adiponectin levels. J Gerontol A Biol Sci Med Sci. 2007;62(9):997–1001. doi: 10.1093/gerona/62.9.997. [DOI] [PubMed] [Google Scholar]
- 39.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115(6):429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Bayomi DJ, Tate RB. Ability and accuracy of long-term weight recall by elderly males: the Manitoba follow-up study. Ann Epidemiol. 2008;18(1):36–42. doi: 10.1016/j.annepidem.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Tankó LB, Christiansen C, Cox DA, et al. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20(11):1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 42.Sorkin JD, Muller DC, Andres R. Longitudinal change in height of men and women: implications for interpretation of the body mass index: the Baltimore Longitudinal Study of Aging. Am J Epidemiol. 1999;150(9):969–977. doi: 10.1093/oxfordjournals.aje.a010106. [DOI] [PubMed] [Google Scholar]
- 43.Svendsen OL, Haarbo J, Heitmann BL, et al. Measurement of body fat in elderly subjects by dual-energy x-ray absorptiometry, bioelectrical impedance, and anthropometry. Am J Clin Nutr. 1991;53(5):1117–1123. doi: 10.1093/ajcn/53.5.1117. [DOI] [PubMed] [Google Scholar]