Abstract
To assess associations between the timing of hepatitis B virus (HBV) immunization relative to human immunodeficiency virus (HIV) diagnosis and vaccine effectiveness, US Military HIV Natural History Study cohort participants without HBV infection at the time of HIV diagnosis were grouped by vaccination status, retrospectively followed from HIV diagnosis for incident HBV infection, and compared using Cox proportional hazards models. A positive vaccine response was defined as hepatitis B surface antibody level ≥10 IU/L. Of 1,877 participants enrolled between 1989 and 2008, 441 (23%) were vaccinated prior to HIV diagnosis. Eighty percent of those who received vaccine doses only before HIV diagnosis had a positive vaccine response, compared with 66% of those who received doses both before and after HIV and 41% of those who received doses only after HIV (P < 0.01 for both compared with persons vaccinated before HIV only). Compared with the unvaccinated, persons vaccinated only before HIV had reduced risk of HBV infection after HIV diagnosis (hazard ratio = 0.38, 95% confidence interval: 0.20, 0.75). No reduction in HBV infection risk was observed for other vaccination groups. These data suggest that completion of the vaccine series prior to HIV infection may be the optimal strategy for preventing this significant comorbid infection in HIV-infected persons.
Keywords: hepatitis B vaccines, hepatitis B virus, HIV, immunization, vaccination
The burden of hepatitis B virus (HBV) infection in human immunodeficiency virus (HIV)-infected persons is substantial, with as many as 10% of unvaccinated persons being chronically coinfected with HBV (1–4). Compared with persons who are HBV-positive but HIV-negative, coinfected persons generally have increased rates of cirrhosis and liver-related mortality (5–8). Therefore, prevention of HBV infection in persons with HIV is extremely important, and one of the potential means of accomplishing this is vaccination, as recommended by guidelines (9–11).
Preexposure vaccination of adults at high risk of contracting HBV infection was initially recommended in the United States in 1982 (12). Since that time, the Advisory Committee on Immunization Practices has revised US HBV vaccination guidelines to include universal vaccination of infants (13) and, more recently, all persons under 19 years of age (10). Similar vaccination recommendations exist in many other countries (14). Recent data have shown an improvement in HBV vaccination coverage in the United States (15), and it is therefore expected that growing numbers of HIV-infected persons will have a history of receiving HBV immunization prior to HIV infection.
Previous cohort investigations have evaluated the association between HBV vaccination and risk of HBV infection in HIV-infected persons (1, 2, 16). Kellerman et al. (2) reported a reduced risk of acute HBV infection in persons with a history of receiving at least 1 dose of HBV vaccine, but no distinction was made regarding the timing of HBV vaccination in relation to HIV infection. We previously reported that HBV vaccine effectiveness may be low when the vaccine is given after HIV infection, because of a number of factors, including low rates of vaccine response (16). In a cross-sectional study from Taiwan, HIV-infected persons born after implementation of a childhood HBV immunization program had a lower prevalence of HBV infection than those born prior to program initiation (17). However, this study used birth year as a surrogate for receiving HBV vaccination and was conducted in an area of high HBV endemicity where the majority of HBV exposures occur years before HIV infection. In non-HIV-infected persons, immunity to HBV infection following vaccination appears to be long-lived (18), but whether HBV vaccine-induced protection persists following HIV infection has not been thoroughly investigated.
Therefore, to assess the potential impact of HBV immunization of HIV-negative adolescents and high-risk adults upon the risk of HBV infection occurring after HIV diagnosis, we evaluated the associations between the timing of HBV immunization relative to HIV diagnosis, vaccine responses, and risk of HBV infection after HIV diagnosis in the US Military HIV Natural History Study cohort. Records of vaccination prior to HIV diagnosis are available for participants in this study because of their receipt of health care in the military health system.
MATERIALS AND METHODS
Study cohort
The US Military HIV Natural History Study is an ongoing, continuous-enrollment observational cohort of HIV-infected Department of Defense beneficiaries followed at 7 participating military medical centers in the United States. The study has been previously described (16). All adult Department of Defense beneficiaries with a diagnosis of HIV infection followed at a participating site with the ability to provide consent are eligible for participation. Approval for this research was obtained from the institutional review board at each participating site.
Participant selection and definitions
HBV screening became uniform in the US Military HIV Natural History Study in 1989. The median number of HBV screens per participant was 5, and the median interval between HBV screens was 7 months (16). All study participants enrolled between 1989 and May 2008 with a documented date of HIV seropositivity and without HBV infection at the time of HIV diagnosis (defined as nonreactive initial tests for both hepatitis B surface antigen (HBsAg) and total antibody to hepatitis B core antigen (HBcAb) after HIV diagnosis) were included in the current analysis. HBV DNA testing results were not assessed for the current investigation. Similar to previous investigations, vaccination was defined as receipt of at least 1 dose of HBV vaccine (16), and HBV infection was defined as reactive results for 2 of the following 3 tests on 1 occasion: HBsAg, HBcAb, and hepatitis B surface antibody; or reactivity for HBcAb or HBsAg on at least 2 separate occasions (1, 19). Chronic HBV infection was defined as HBsAg reactivity on 2 or more separate occasions at least 6 months apart. Highly active antiretroviral therapy (HAART) was defined as a combination of at least 3 antiretroviral agents (16). The presence of an acquired immunodeficiency syndrome (AIDS)-defining illness was determined using 1993 Centers for Disease Control and Prevention criteria (20), with the exception of an isolated CD4 cell count less than 200 cells/μL.
Design and statistical methods
Eligible participants were initially classified into one of 4 exclusive groups on the basis of their vaccination history: those who received vaccine only prior to HIV diagnosis (the vaccinated-before (VB) group), those who received vaccine both before and after HIV diagnosis (the vaccinated-before-and-after (VBA) group), those who received vaccine only after HIV diagnosis (the vaccinated-after (VA) group), and those who did not receive vaccine during study follow-up (the not-vaccinated (NV) group). Descriptive statistics for the 4 vaccination groups are summarized in Table 1. Median values and interquartile ranges are presented and were compared using Wilcoxon tests. Differences in proportions were compared with χ2 and Fisher's exact tests. The numbers of events, person-years at risk, and rates of HBV infection (per 100 person-years of follow-up) were calculated overall and for the 4 vaccination groups.
Table 1.
Characteristics of Participants at Human Immunodeficiency Virus Diagnosis and During Follow-up, by Hepatitis B Virus Vaccination Group, US Military HIV Natural History Study, 1989–2008
| Characteristic | No. of Participants | Total (n = 1,877) | Vaccination Group Relative to HIV Diagnosis |
P Valuea | |||
| Before Only (n = 262) | Before and After (n = 179) | After Only (n = 879) | Not Vaccinated (n = 557) | ||||
| Median ageb, years (IQR) | 1,877 | 27.2 (23.3–33.0) | 28.4 (23.2–34.9) | 27.7 (23.5–33.0) | 27.2 (23.5–33.4) | 26.7 (23.1–32.5) | 0.35 |
| HIV diagnosis before 1996, % | 1,877 | 47 | 26 | 25 | 49 | 62 | <0.001 |
| Male gender, % | 1,877 | 88 | 94 | 94 | 87 | 84 | <0.001 |
| Race/ethnicity, % | 1,877 | 0.12 | |||||
| Caucasian | 44 | 45 | 48 | 45 | 39 | ||
| African-American | 43 | 44 | 42 | 41 | 47 | ||
| Other | 13 | 11 | 10 | 14 | 14 | ||
| Branch of serviceb, % | 1,877 | <0.01 | |||||
| Army | 28 | 32 | 21 | 24 | 34 | ||
| Navy | 34 | 37 | 34 | 40 | 24 | ||
| Air Force | 26 | 24 | 30 | 24 | 30 | ||
| Other/civilian | 12 | 7 | 15 | 13 | 11 | ||
| Median body mass indexb,c (IQR) | 974 | 24.8 (22.6–27.1) | 25.4 (23.1–28.0) | 25.1 (23.0–27.2) | 24.8 (22.6–27.0) | 24.4 (22.1–27.0) | 0.04 |
| Median CD4 cell countb, cells/μL (IQR) | 1,703 | 494 (347–651) | 486 (362–647) | 482 (376–636) | 500 (363–648) | 498 (313–659) | 0.62 |
| Median HIV RNA loadb, log10 copies/mL (IQR) | 1,140 | 4.4 (3.7–4.9) | 4.4 (3.6–4.8) | 4.3 (3.7–4.8) | 4.4 (3.7–4.9) | 4.3 (3.6–4.9) | 0.44 |
| AIDS-defining illnessb, % | 1,877 | 1 | 2 | 1 | 1 | 2 | 0.24 |
| Prior sexually transmitted infectionb, % | 1,877 | ||||||
| Any | 22 | 20 | 25 | 21 | 23 | 0.51 | |
| Gonorrhea | 12 | 8 | 14 | 12 | 13 | 0.18 | |
| Chlamydia | 8 | 9 | 10 | 8 | 8 | 0.78 | |
| Herpes | 3 | 5 | 3 | 2 | 3 | 0.16 | |
| Syphilis | 4 | 3 | 4 | 3 | 4 | 0.46 | |
| Positive for antibodies to hepatitis C virusb, % | 1,298 | 2 | 2 | 1 | 2 | 3 | 0.40 |
| Initiation of HAART during follow-up, % | 1,877 | 54 | 52 | 65 | 68 | 30 | < 0.001 |
| At least 3 doses of HBV vaccine, % | 1,320 | ||||||
| Before HIV diagnosis | 62 | 41d | |||||
| Ever | 62 | 90d | 58 | ||||
| Median time from first HBV vaccine dose to HIV diagnosis, months (IQR) | 1,320 | 36.9 (18.6 to 75.2) | 21.6 (5.9 to 58.7)d | −4.7 (−16.0 to –1.5) | |||
| Total person-years of follow-up | 1,877 | 10,202 | 1,129 | 1,035 | 6,112 | 1,926 | |
| Median duration of follow-up, months (IQR) | 1,877 | 52.9 (21.9–92.9) | 33.7 (12.8–77.0) | 61.0 (22.5–103.5) | 71.1 (38.1–117.9) | 31.5 (11.2–62.0) | |
| No. of HBV cases | 1,877 | 186 | 12 | 16 | 80 | 78 | |
| No. of chronic HBV cases | 1,877 | 39 | 2 | 2 | 18 | 17 | |
| Rate of HBV infection per 100 person-years of observation (95% confidence interval) | 1,877 | 1.82 (1.56, 2.09) | 1.06 (0.46, 1.66) | 1.55 (0.79, 2.30) | 1.31 (1.02, 1.60) | 4.05 (3.15, 4.95) | |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HAART, highly active antiretroviral therapy; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IQR, interquartile range.
Comparing the 4 vaccination groups.
At HIV diagnosis.
Weight (kg)/height (m)2.
P < 0.001 when the vaccinated-before-and-after group was compared with the vaccinated-before group by χ2 test.
Cox proportional hazards models were used to evaluate the association of vaccination status with HBV infection following HIV diagnosis in 2 different models defined a priori. In model 1, eligible participants with any history of vaccination prior to HIV diagnosis (VB + VBA groups) were compared with those without receipt of vaccination prior to HIV diagnosis (VA + NV groups) without any adjustment for vaccination after HIV diagnosis. In model 2, vaccination status was considered with 3 mutually exclusive time-updated indicators (VB, VBA, and VA), with the reference group being persons who were not vaccinated. In both models, analyses were performed with and without adjustment for other covariates. The multivariate models were also stratified by era of HIV diagnosis (prior to 1996 or 1996–2008) and further adjusted for year of HIV diagnosis. Covariates which could change during follow-up (HIV RNA level, CD4 cell count, history of a sexually transmitted infection or AIDS event, and use of antiretroviral therapy) were considered as time-updated covariates. For all analyses, the censoring date for persons with no HBV infection was the latest of the last recorded study visit or the date of the most recent HBV screening panel. The latest censoring date for this study was July 2008.
To consider the impact of potential vaccination selection bias, propensity score methods (21, 22) were used as sensitivity analyses for both models 1 and 2. Each participant was assigned a propensity score estimated with a logistic regression model assessing the probability of receiving at least 1 dose of HBV vaccine prior to HIV diagnosis given baseline characteristics, including age, gender, race/ethnicity, year of HIV diagnosis, branch of military service, previous sexually transmitted infection and AIDS events, and CD4 cell count. Five equal-sized propensity score subclasses were formed on the basis of rank order of the propensity scores. In each of the 5 propensity score subclasses, the percentage of participants vaccinated prior to HIV diagnosis ranged from 7% to 39%. Cox proportional hazards models were then stratified by propensity score subclass.
For the subset of persons with available data on antibody to hepatitis B surface antigen (anti-HBs) following vaccination, response to HBV vaccine was categorized by anti-HBs status within 1 year after HIV diagnosis for the VB group and within 1 year of the last vaccine dose for the VBA and VA groups. A positive vaccine response was defined as anti-HBs level ≥10 IU/L (23). For this subset of participants, the proportions with HBV infection and chronic HBV infection were compared across the 3 HBV vaccination groups by vaccine response status with χ2 tests and Fisher's exact tests.
To determine HBV vaccination coverage prior to HIV diagnosis, the yearly cross-sectional prevalence of HBV vaccination prior to HIV diagnosis was calculated by determining the proportion of study participants diagnosed with HIV in a particular year with documentation of HBV vaccination prior to HIV diagnosis. Changes in vaccine coverage over time were examined using the Cochran-Armitage test for trend. Results were calculated as hazard ratios and rates with 95% confidence intervals. Significance was defined as P < 0.05, and all P values were 2-sided. All analyses were conducted using SAS, version 9.2 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Of the 1,877 participants who met the inclusion criteria, 441 (23%) had documented receipt of 1 or more doses of HBV vaccine prior to HIV diagnosis (262 in the VB group and 179 in the VBA group), and 1,436 (77%) had no history of HBV vaccination at the time of HIV diagnosis (879 in the VA group and 557 in the NV group) (Table 1). The median age at HIV diagnosis did not vary by vaccination group. The proportions of persons with an HIV diagnosis before 1996 were significantly higher in the VA and NV groups than in the VB group.
Overall, 186 (10%) participants developed HBV infection during 10,202 person-years of observation (median, 4.4 years; interquartile range, 1.8–7.7), resulting in an overall HBV infection rate of 1.82 (95% confidence interval (CI): 1.56, 2.09) per 100 person-years (Table 1). Of the 4 vaccination groups, the highest rate was observed in the NV group (4.05 per 100 person-years; 95% CI: 3.15, 4.95), whereas the lowest rate was observed in the VB group (1.06 per 100 person-years; 95% CI: 0.46, 1.66).
Number of doses and vaccine response
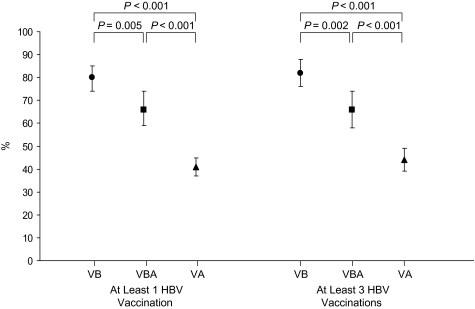
In the VB and VBA groups, the proportions of participants receiving ≥3 doses of vaccine prior to HIV diagnosis were 62% and 41%, respectively (P < 0.001) (Table 1). However, including doses given after HIV diagnosis, 90% of the VBA group ultimately received ≥3 doses of vaccine (P < 0.001 compared with 62% for the VB group). The median time from first vaccine dose to HIV diagnosis was 36.9 months (interquartile range, 18.6–75.2) for the VB group as compared with 21.6 months (interquartile range, 5.9–58.7) for the VBA group (P < 0.001). Vaccine responses were known for 230 (88%), 137 (77%), and 646 (73%) participants from the VB, VBA, and VA groups, respectively. The proportion of those responding to vaccine was significantly higher for the VB group (80% overall and 82% for the subset receiving ≥3 doses) than for any other vaccinated group (Figure 1).
Figure 1.
Percentage of hepatitis B virus (HBV)-vaccinated participants with a vaccine response of hepatitis B surface antibody level ≥10 IU/L, by vaccination group, overall (left panel; number of participants by group: VB, n = 230; VBA, n = 137; VA, n = 646) and for the subset of persons receiving 3 or more doses of vaccine (right panel; number of participants by group: VB, n = 150; VBA, n = 123; VA, n = 386), US Military HIV Natural History Study, 1989–2008. VA, vaccinated after (vaccinated only after human immunodeficiency virus (HIV) diagnosis); VB, vaccinated before (vaccinated only before HIV diagnosis); VBA, vaccinated before and after (vaccinated before and after HIV diagnosis). Bars, 95% confidence interval.
HBV events by group and vaccine response
Of the 186 HBV infections, 39 (21%) were subsequently classified as chronic infections (2/12 (17%) in the VB group, 2/16 (13%) in the VBA group, 18/80 (23%) in the VA group, and 17/78 (22%) in the NV group (P = 0.89)). Of the 538 vaccine responders in the VB, VBA, and VA groups, 29 (5%) developed HBV, as compared with 66 (14%) of the 475 nonresponders (P < 0.001) (Table 2). Of participants with a positive response, only 1 (0.2%) developed chronic HBV infection, as compared with 20 (4%) nonresponders (P < 0.001). The person developing chronic HBV after having a documented response to vaccination was an African-American male who received 2 HBV vaccine injections, both prior to HIV infection. His last HBV vaccination was in 1990; he was diagnosed with HIV infection 47 months later at age 33.5 years, and was positive for anti-HBs but negative for HBsAg and HBcAb. Twenty-seven months after HIV diagnosis, he tested positive for HBsAg and later met criteria for chronic HBV. His CD4 cell count prior to HBV diagnosis was 279 cells/μL; no HIV RNA level was available.
Table 2.
Distribution of Hepatitis B Virus Infections by Vaccine Response and Vaccination Group Among Persons Tested for Antibody to Hepatitis B Surface Antigen, US Military HIV Natural History Study, 1989–2008
| HBV Infection Group | HBV Vaccination Groupa | Anti-HBs Level < 10 IU/L |
Anti-HBs Level ≥ 10 IU/L |
P Valueb | ||||
| No. of Participants | No. With HBV Infection | % With HBV Infection | No. of Participants | No. With HBV Infection | % With HBV Infection | |||
| All HBV infections | Before only | 47 | 6 | 12.8 | 183 | 6 | 3.3 | 0.009 |
| Before and after | 46 | 7 | 15.2 | 91 | 7 | 7.7 | 0.17 | |
| After only | 382 | 53 | 13.9 | 264 | 16 | 6.1 | 0.002 | |
| Total | 475 | 66 | 13.9 | 538 | 29 | 5.4 | <0.001 | |
| Chronic HBV infections | Total | 475 | 20 | 4.2 | 538 | 1 | 0.2 | <0.001c |
Abbreviations: anti-HBs, antibody to hepatitis B surface antigen; HBV, hepatitis B virus; HIV, human immunodeficiency virus.
Relative to HIV diagnosis.
Determined by χ2 test unless otherwise noted.
Determined by Fisher's exact test.
Risk of HBV infection
Comparing the combined VB and VBA groups with the combined NV and VA groups, HBV vaccination prior to HIV diagnosis was associated with reduced risk of HBV following HIV diagnosis (unadjusted hazard ratio (HR) = 0.65, 95% CI: 0.43, 0.97), although the association did not reach statistical significance in multivariate model 1 (HR = 0.68, 95% CI: 0.45, 1.04) (Table 3). However, from unadjusted and multivariate analyses with time-updated vaccination categories, only vaccination before HIV diagnosis was associated with reduced risk of HBV infection following HIV diagnosis in comparison with no vaccination. In multivariate model 2, persons vaccinated only before HIV diagnosis had a 62% reduced risk of HBV infection following HIV diagnosis (HR = 0.38, 95% CI: 0.20, 0.75). Neither those vaccinated before and after HIV diagnosis (HR = 1.08, 95% CI: 0.61, 1.92) nor those vaccinated only after HIV diagnosis (HR = 0.87, 95% CI: 0.62, 1.23) had reduced risk of HBV infection. HIV RNA load was not included in either multivariate model 1 or multivariate model 2, since 18% of our study population was censored prior to 1996, when testing for HIV RNA became widely available.
Table 3.
Unadjusted and Multivariate Risks of Hepatitis B Virus (HBV) Infection According to Timing of HBV Vaccination and Time of Human Immunodeficiency Virus (HIV) Diagnosis, US Military HIV Natural History Study, 1989–2008
| Characteristic | Unadjusted Model |
Multivariate Model 1a |
Multivariate Model 2a |
||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age at HIV diagnosis, per 10-year increase | 0.75 | 0.61, 0.93 | 0.01 | 0.82 | 0.66, 1.02 | 0.08 | 0.83 | 0.67, 1.03 | 0.10 |
| Gender | |||||||||
| Female | 1 | Referent | 1 | Referent | 1 | Referent | |||
| Male | 7.12 | 2.64, 19.16 | <0.001 | 7.97 | 2.95, 21.52 | <0.001 | 8.04 | 2.98, 21.72 | <0.001 |
| Race/ethnicity | |||||||||
| Caucasian | 1 | Referent | 1 | Referent | 1 | Referent | |||
| African-American | 1.37 | 1.01, 1.85 | 0.04 | 1.38 | 1.01, 1.89 | 0.04 | 1.39 | 1.02, 1.90 | 0.04 |
| Other | 0.73 | 0.41, 1.29 | 0.28 | 0.74 | 0.42, 1.33 | 0.31 | 0.76 | 0.43, 1.35 | 0.35 |
| Branch of service | |||||||||
| Army | 1 | Referent | |||||||
| Navy | 1.21 | 0.84, 1.74 | 0.30 | ||||||
| Air Force | 1.07 | 0.72, 1.60 | 0.74 | ||||||
| Other/civilian | 0.89 | 0.51, 1.57 | 0.69 | ||||||
| Body mass indexb, per 1-unit increase | 0.95 | 0.89, 1.01 | 0.10 | ||||||
| CD4 cell countc, per 100-cells/μL increase | 0.95 | 0.90, 1.01 | 0.09 | 0.98 | 0.92, 1.04 | 0.45 | 0.98 | 0.92, 1.04 | 0.48 |
| HIV RNA loadc, per 0.5-log10 copies/mL increase | 1.73 | 1.28, 2.35 | <0.001 | ||||||
| AIDS-defining illnessc | |||||||||
| No | 1 | Referent | |||||||
| Yes | 0.64 | 0.30, 1.36 | 0.35 | ||||||
| Prior sexually transmitted infectionc | |||||||||
| None | 1 | Referent | |||||||
| Any | 1.34 | 1.00, 1.80 | 0.05 | ||||||
| Gonorrhea | 1.20 | 0.84, 1.73 | 0.32 | ||||||
| Chlamydia | 1.39 | 0.93, 2.08 | 0.11 | ||||||
| Herpes | 0.82 | 0.45, 1.47 | 0.50 | ||||||
| Syphilis | 1.73 | 1.14, 2.60 | 0.01 | 1.47d | 0.96, 2.24 | 0.07 | 1.45d | 0.95, 2.21 | 0.09 |
| Positive for antibodies to hepatitis C virusc | |||||||||
| No | 1 | Referent | |||||||
| Yes | 0.74 | 0.24, 2.33 | 0.61 | ||||||
| HAART usec | |||||||||
| None | 1 | Referent | 1 | Referent | 1 | Referent | |||
| HAART | 0.27 | 0.18, 0.41 | <0.001 | 0.38 | 0.24, 0.60 | <0.001 | 0.38 | 0.24, 0.61 | <0.001 |
| HBV vaccination before HIV diagnosis | |||||||||
| Yes (VB + VBA) | 0.65 | 0.43, 0.97 | 0.03 | 0.68 | 0.45, 1.04 | 0.08 | |||
| No (VA + NV) | 1 | Referent | 1 | Referent | |||||
| HBV vaccination groupc,e | |||||||||
| VB | 0.50 | 0.27, 0.93 | 0.03 | 0.38 | 0.20, 0.75 | 0.005 | |||
| VBA | 1.28 | 0.73, 2.27 | 0.39 | 1.08 | 0.61, 1.92 | 0.80 | |||
| VA | 0.91 | 0.65, 1.28 | 0.59 | 0.87 | 0.62, 1.23 | 0.44 | |||
| NV | 1 | Referent | 1 | Referent | |||||
Abbreviations: AIDS, acquired immunodeficiency syndrome; CI, confidence interval; HAART, highly active antiretroviral therapy; HBV, hepatitis B virus; HIV, human immunodeficiency virus; HR, hazard ratio; NV, not vaccinated; VA, vaccinated after; VB, vaccinated before; VBA, vaccinated before and after.
In the final multivariate models, results were stratified by era of HIV diagnosis and further adjusted for year of HIV diagnosis.
Weight (kg)/height (m)2.
Time-updated covariate.
Compared with not having syphilis.
Relative to HIV diagnosis.
Because of the significant difference in the number of vaccine doses prior to HIV diagnosis between the VB and VBA groups, we evaluated risk of HBV infection by number of vaccine doses in these 2 groups combined. In this subset, risks of HBV infection following HIV diagnosis were similar among those who received ≥3 doses of HBV vaccine prior to HIV diagnosis and those who received <3 doses (HR = 0.75, 95% CI: 0.35, 1.58). However, the statistical power of this comparison was limited, since there were only 28 HBV infections in these 441 participants.
Using propensity scoring methods for multivariate model 2, results were similar. Vaccination before HIV diagnosis (HR = 0.38, 95% CI: 0.19, 0.75) was again associated with reduced HBV risk, while vaccination both before and after HIV diagnosis (HR = 1.09, 95% CI: 0.61, 1.96) and vaccination only after HIV diagnosis (HR = 0.86, 95% CI: 0.61, 1.23) produced no reduction in HBV infection risk as compared with no vaccination. To remove potential confounding from HAART use, an additional sensitivity analysis was performed with censoring at January 1, 1996, when HAART became available. The results from multivariate model 2 were again similar: Vaccination only before HIV diagnosis (HR = 0.35, 95% CI: 0.13, 0.96) was associated with reduced HBV infection risk, while vaccination both before and after HIV diagnosis (HR = 0.25, 95% CI: 0.03, 1.81) and only after HIV diagnosis (HR = 0.84, 95% CI: 0.54, 1.31) were not.
Vaccination coverage
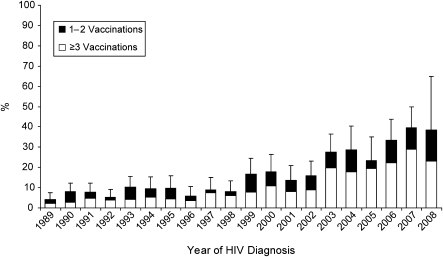
Figure 2 depicts the proportions of participants with documented receipt of HBV vaccine prior to HIV diagnosis by year. The proportion of persons receiving at least 1 dose and the subset receiving at least 3 doses both increased significantly from 1989 to 2008 (for both, P for trend < 0.0001). In 2007 (the most recent calendar year with complete data), 40% of those diagnosed with HIV and concurrently negative for HBV infection received ≥1 dose of HBV vaccine prior to HIV diagnosis, and 30% received ≥3 doses.
Figure 2.
Percentage of participants with hepatitis B virus (HBV) vaccination prior to human immunodeficiency virus (HIV) diagnosis, by year, for those known to be serologically negative for HBV infection at the time of HIV diagnosis (by χ2 test for trend, P < 0.0001 for both ≥1 vaccination and ≥3 vaccinations). The median number of participants diagnosed per year was 91 (interquartile range, 80–109). Bars, 95% confidence interval.
DISCUSSION
Although uncertainty exists regarding the optimal prevention approach for HBV among persons with HIV infection (1), we found that HBV vaccination prior to HIV diagnosis was associated with a 62% reduced risk of HBV infection following HIV diagnosis in comparison with no HBV vaccination. Other factors associated with HBV infection were similar to those reported previously (1). However, our results also suggest that completion of the 3-dose series prior to HIV infection is an important determinant of vaccine-associated protection, since the only group with significantly reduced HBV infection risk (the VB group) was also the group with the highest percentages of both positive vaccine responses and vaccine series completion prior to HIV infection. Therefore, these results suggest that the timing of HBV vaccination relative to HIV diagnosis is an important determinant of vaccine effectiveness, and that vaccination of HIV-negative persons may be a key component of HBV prevention in those with HIV.
Neither persons vaccinated before and after HIV diagnosis (the VBA group) nor persons vaccinated only after HIV diagnosis (the VA group) demonstrated reduced risk of HBV infection following HIV diagnosis. While the VBA group was similar with regard to demographic characteristics and date of HIV diagnosis to those who were vaccinated only before HIV diagnosis (the VB group), persons in the VBA group had a shorter time from first HBV vaccination to HIV infection than the VB group. This may partially explain why significantly fewer persons in the VBA group were able to complete the full 3-dose vaccine series prior to HIV infection. Ultimately, 90% of the participants in the VBA group did complete the vaccination series by receiving additional doses after HIV diagnosis; yet despite having this high rate of vaccine series completion, the vaccine response rate was only 66% for the VBA group. Because the VBA group was relatively small, we also cannot rule out the possibility that the current analysis lacked power to detect the protection these persons may have received from vaccination. The VA group had the lowest rate of vaccine response, although a previous analysis showed that persons vaccinated after HIV diagnosis who develop a vaccine response appear to have reduced risk of infection (18).
Previous trials have demonstrated the association between HBV vaccine response and reduced risk of HBV infection, as well as improved response rates to HBV vaccine with receipt of at least 3 doses in HIV-infected and uninfected adults (11, 23–25). In the present study, the highest response rates were seen in persons receiving all vaccinations before HIV diagnosis (the VB group), while the lowest response rates were seen in those receiving vaccination only after HIV diagnosis (the VA group). Persons who were infected with HIV while completing the vaccine series (the VBA group) had intermediate response rates. These response rate differences are even more striking when considering the differential rate of vaccine series completion and the fact that we were only able to assess vaccine responses for the VB group at the time of HIV diagnosis (after responses may have waned for some), as opposed to within 1 year of last vaccination, as was done for the VBA and VA groups. Therefore, HIV-associated immunodeficiency appears to significantly reduce the likelihood of responding adequately to HBV vaccine and obtaining subsequent clinical protection, even if HIV infection occurs prior to administration of the third dose. It is unclear whether additional or higher doses of the vaccine provided after HIV infection could overcome this detrimental effect. The importance of the timing of the third dose of vaccine in relation to HIV diagnosis is not surprising given that in HIV-negative persons only the third dose elicits an anamnestic response when using a typical immunization schedule with doses at 0, 1, and 6 months. The anamnestic response produces high antibody titers and is therefore the key determinant of efficacy and the duration of protection (26).
The most recent data available for the United States suggest that coverage rates for hepatitis B vaccination continue to increase, including rates for persons classified as high-risk adolescents and adults (15). In recent publications, an estimated 32% of US adults aged 18–49 years classified as being at high risk of HBV infection reported having ever received at least 3 doses of hepatitis B vaccine (45% had ever received at least 1 dose) (15, 27). The results of the present study also show significantly increased HBV vaccination coverage of US military members prior to HIV diagnosis from 1989 through 2008. While vaccination coverage is increasing, the observed coverage of 30%–40% in the general and military populations in the United States in recent years highlights the continued challenges involved in identifying and providing vaccine to HIV-negative adults at high risk of HBV infection. However, with high vaccination coverage rates of infants and children and with policies which provide hepatitis B immunization for all new US military recruits since 2002, substantial increases in HBV immunity in the general US adult population and the US military are expected (28).
As a cohort investigation, the current study has limitations. First, the HIV cohort analyzed in this study is different from other large HIV cohorts in some respects, including enrollment early after HIV infection due to routine military HIV screening, open access to care and medications in the military health system, and virtually no intravenous drug use (29). However, these characteristics should only improve delivery of HBV vaccine, and receipt of medical care in the military health system allows for improved capture of HBV vaccination dates. In addition, HBV vaccination was not randomized, and there may have been an indication bias for vaccination. However, we adjusted for factors found to be distributed significantly differently between vaccination groups, and we used propensity score methods as sensitivity analyses, which found similar results. Because most persons in our cohort who received HAART received treatment with HBV-active agents, we were unable to fully assess the independent effects of different HAART regimens on HBV risk (1). HIV RNA levels, which are known to predict vaccine responses (23, 30), were unknown for many participants, and the vaccine dose was also unknown, although these factors would only have affected vaccine responses for the VA group. Finally, 2 of the vaccination groups (the VB and VBA groups) were smaller and had fewer events.
The current study demonstrates the impact of HIV infection upon HBV vaccine-induced protection, and it suggests that an effective vaccination approach among persons with HIV is completing the HBV vaccination series before HIV infection ever occurs. If efforts are successful, the anticipated increase of population immunity against HBV due to current vaccination practices may substantially decrease HBV prevalence in HIV-infected adults in the United States in the coming years. However, if low rates of vaccination coverage persist, especially in high-risk groups, HBV infection is likely to continue to pose a significant threat to persons with HIV infection. Those not completing the vaccine series prior to HIV diagnosis may still benefit from vaccination, but more effective vaccines or vaccination strategies or other HBV prevention strategies are needed for such persons.
Acknowledgments
Author affiliations: Infectious Disease Clinical Research Program, Uniformed Services University, Bethesda, Maryland (Michael L. Landrum, Katherine Huppler Hullsiek, Nancy F. Crum-Cianflone, Anuradha Ganesan, Amy C. Weintrob, Robert J. O'Connell, Brian K. Agan); San Antonio Military Medical Center, Fort Sam Houston, Texas (Michael L. Landrum); Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota (Katherine Huppler Hullsiek); Naval Health Research Center, San Diego, California (Helen M. Chun); Naval Medical Center, San Diego, California (Nancy F. Crum-Cianflone); National Naval Medical Center, Bethesda, Maryland (Anuradha Ganesan); Walter Reed Army Medical Center, Washington, DC (Amy C. Weintrob); Naval Medical Center, Portsmouth, Virginia (R. Vincent Barthel); and Walter Reed Army Institute of Research, Silver Spring, Maryland (Robert J. O'Connell).
Support for this work (grant IDCRP-000-27) was provided by the Infectious Disease Clinical Research Program (IDCRP; www.idcrp.org), a Department of Defense program executed through the Uniformed Services University of the Health Sciences. The staff of the IDCRP provided salary support to some of the investigators (M. L. L., K. H. H., N. F. C., A. G., A. C. W., and B. K. A.). This project was funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases under Inter-Agency Agreement Y1-AI-5072.
The authors thank William Bradley for his expertise and assistance with the US Military HIV Natural History Study database. The authors also express their gratitude to the current members of the IDCRP HIV Working Group, to the long line of military HIV researchers who have supported the US Military HIV Natural History Study, and to the research coordinators and support staff for their countless hours of work.
These data were presented in part at the 16th Conference on Retroviruses and Opportunistic Infections, Montreal, Quebec, Canada, February 8–11, 2009.
The staff of the IDCRP reviewed the study design and collected the data. The analyses and conclusions and the decision to submit the manuscript for publication were the independent work and decision of the authors. The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the National Institutes of Health, the Department of Health and Human Services, the Department of Defense, or the Department of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US government.
Conflict of interest: none declared.
Glossary
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- anti-HBs
antibody to hepatitis B surface antigen
- CI
confidence interval
- HAART
highly active antiretroviral therapy
- HBcAb
antibody to hepatitis B core antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HIV
human immunodeficiency virus
- HR
hazard ratio
- NV
not vaccinated
- VA
vaccinated after
- VB
vaccinated before
- VBA
vaccinated before and after
References
- 1.Chun HM, Fieberg AM, Hullsiek KH, et al. Epidemiology of hepatitis B virus infection in a US cohort of HIV-infected individuals during the past 20 years. Clin Infect Dis. 2010;50(3):426–436. doi: 10.1086/649885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kellerman SE, Hanson DL, McNaghten AD, et al. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis. 2003;188(4):571–577. doi: 10.1086/377135. [DOI] [PubMed] [Google Scholar]
- 3.Thio CL. Hepatitis B in the human immunodeficiency virus-infected patient: epidemiology, natural history, and treatment. Semin Liver Dis. 2003;23(2):125–136. doi: 10.1055/s-2003-39951. [DOI] [PubMed] [Google Scholar]
- 4.Homann C, Krogsgaard K, Pedersen C, et al. High incidence of hepatitis B infection and evolution of chronic hepatitis B infection in patients with advanced HIV infection. J Acquir Immune Defic Syndr. 1991;4(4):416–420. [PubMed] [Google Scholar]
- 5.Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29(4):1306–1310. doi: 10.1002/hep.510290447. [DOI] [PubMed] [Google Scholar]
- 6.Thio CL, Seaberg EC, Skolasky R, Jr., et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360(9349):1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 7.Soriano V, Puoti M, Peters M, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-hepatitis B virus international panel. AIDS. 2008;22(12):1399–1410. doi: 10.1097/QAD.0b013e3282f8b46f. [DOI] [PubMed] [Google Scholar]
- 8.Tedaldi E, Peters L, Neuhaus J, et al. Opportunistic disease and mortality in patients coinfected with hepatitis B or C virus in the Strategic Management of Antiretroviral Therapy (SMART) Study. Clin Infect Dis. 2008;47(11):1468–1475. doi: 10.1086/593102. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan JE, Benson C, Holmes KH, et al. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207. [PubMed] [Google Scholar]
- 10.Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54(RR-16):1–31. [PubMed] [Google Scholar]
- 11.Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part II: immunization of adults. MMWR Recomm Rep. 2006;55(RR-16):1–33. [PubMed] [Google Scholar]
- 12.Recommendation of the Immunization Practices Advisory Committee (ACIP) Inactivated hepatitis B virus vaccine. MMWR Morb Mortal Wkly Rep. 1982;31(24):317–322. 327–328. [PubMed] [Google Scholar]
- 13.Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP) MMWR Recomm Rep. 1991;40(RR-13):1–25. [PubMed] [Google Scholar]
- 14.Department of Immunization, Vaccines and Biologicals, World Health Organization. WHO Vaccine-Preventable Diseases: Monitoring System—2008 Global Summary. Geneva, Switzerland: World Health Organization; 2008. ( http://whqlibdoc.who.int/hq/2008/WHO_IVB_2008_eng.pdf). (Accessed May 27, 2009) [Google Scholar]
- 15.Hepatitis B vaccination coverage among adults—United States, 2004. MMWR Morb Mortal Wkly Rep. 2006;55(18):509–511. [PubMed] [Google Scholar]
- 16.Landrum ML, Hullsiek KH, Ganesan A, et al. Hepatitis B vaccination and risk of hepatitis B infection in HIV-infected individuals. AIDS. 2010;24(4):545–555. doi: 10.1097/QAD.0b013e32832cd99e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun HY, Ko WC, Tsai JJ, et al. Seroprevalence of chronic hepatitis B virus infection among Taiwanese human immunodeficiency virus type 1-positive persons in the era of nationwide hepatitis B vaccination. Am J Gastroenterol. 2009;104(4):877–884. doi: 10.1038/ajg.2008.159. [DOI] [PubMed] [Google Scholar]
- 18.McMahon BJ, Bruden DL, Petersen KM, et al. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med. 2005;142(5):333–341. doi: 10.7326/0003-4819-142-5-200503010-00008. [DOI] [PubMed] [Google Scholar]
- 19.Landrum ML, Fieberg AM, Chun HM, et al. The effect of human immunodeficiency virus on hepatitis B virus serologic status in co-infected adults. PLoS One. 2010;5(1):e8687. doi: 10.1371/journal.pone.0008687. (doi: 10.1371/journal.pone.0008687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castro KG, Ward JW, Slutsker L, et al. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 21.Hullsiek KH, Louis TA. Propensity score modeling strategies for the causal analysis of observational data. Biostatistics. 2002;3(2):179–193. doi: 10.1093/biostatistics/3.2.179. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 23.Landrum ML, Huppler Hullsiek K, Ganesan A, et al. Hepatitis B vaccine responses in a large US military cohort of HIV-infected individuals: another benefit of HAART in those with preserved CD4 count. Vaccine. 2009;27(34):4731–4738. doi: 10.1016/j.vaccine.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hadler SC, Francis DP, Maynard JE, et al. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N Engl J Med. 1986;315(4):209–214. doi: 10.1056/NEJM198607243150401. [DOI] [PubMed] [Google Scholar]
- 25.Szmuness W, Stevens CE, Harley EJ, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303(15):833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- 26.Jilg W, Schmidt M, Deinhardt F. Vaccination against hepatitis B: comparison of three different vaccination schedules. J Infect Dis. 1989;160(5):766–769. doi: 10.1093/infdis/160.5.766. [DOI] [PubMed] [Google Scholar]
- 27.Schiller JS, Euler GL. Vaccination Coverage Estimates From the National Health Interview Survey: United States, 2008. Hyattsville, MD: National Center for Health Statistics; 2009. ( http://www.cdc.gov/nchs/data/hestat/vaccine_coverage/vaccine_coverage.pdf). (Accessed March 9, 2010) [Google Scholar]
- 28.Scott PT, Niebuhr DW, McGready JB, et al. Hepatitis B immunity in United States military recruits. J Infect Dis. 2005;191(11):1835–1841. doi: 10.1086/429965. [DOI] [PubMed] [Google Scholar]
- 29.Brodine SK, Starkey MJ, Shaffer RA, et al. Diverse HIV-1 subtypes and clinical, laboratory and behavioral factors in a recently infected US military cohort. AIDS. 2003;17(17):2521–2527. doi: 10.1097/00002030-200311210-00016. [DOI] [PubMed] [Google Scholar]
- 30.Overton ET, Sungkanuparph S, Powderly WG, et al. Undetectable HIV plasma RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis. 2005;41(7):1045–1048. doi: 10.1086/433180. [DOI] [PubMed] [Google Scholar]