Abstract
Frailty, a poorly measured confounder in older patients, can promote treatment in some situations and discourage it in others. This can create unmeasured confounding and lead to nonuniform treatment effects over the propensity score (PS). The authors compared bias and mean squared error for various PS implementations under PS trimming, thereby excluding persons treated contrary to prediction. Cohort studies were simulated with a binary treatment T as a function of 8 covariates X. Two of the covariates were assumed to be unmeasured strong risk factors for the outcome and present in persons treated contrary to prediction. The outcome Y was simulated as a Poisson function of T and all X’s. In analyses based on measured covariates only, the range of PS's was trimmed asymmetrically according to the percentile of PS in treated patients at the lower end and in untreated patients at the upper end. PS trimming reduced bias due to unmeasured confounders and mean squared error in most scenarios assessed. Treatment effect estimates based on PS range restrictions do not correspond to a causal parameter but may be less biased by such unmeasured confounding. Increasing validity based on PS trimming may be a unique advantage of PS's over conventional outcome models.
Keywords: bias (epidemiology); causal inference; cohort studies; confounding factors (epidemiology); epidemiologic methods; models, statistical; propensity score; research design
Restriction of treatment comparisons to subjects with a common range of covariates (e.g., age) or any summary score of covariates (1) can improve the validity of effect estimates regardless of the analytic technique used. Such restriction provides a pragmatic focus on persons for whom uncertainty regarding the value of treatment is most relevant. In practice, implementation of such restrictions can be complicated and is rarely done outside of propensity score (PS) analyses (2, 3).
PS analyses offer some advantages in the context of nonexperimental treatment comparisons. These include a focus on treatment assignment, improved control of confounding with scarce outcomes, and the ability to easily match cohorts on a large number of covariates (3). PS analyses do not offer any advantages with respect to unmeasured confounders, however (4).
Frailty is a plausible explanation for paradoxical treatment effects observed in the elderly (5). Frailty may reduce the likelihood of a particular treatment if physicians focus on a patient's main medical problem and do not initiate useful therapies for alternative conditions (6). The practitioner may determine that in the presence of competing risks, a new therapy offers little expected benefit (7). Conversely, in patients with short life expectancies, physicians may be more willing to try therapies with potentially serious side effects as a last resort. Thus, if mortality is the outcome of interest, frailty can be a powerful confounder that is difficult to measure and can either increase or decrease the likelihood of treatment. Although we describe the problem using the terminology of pharmacoepidemiology, the issues are more general and the principles should apply broadly to any type of epidemiologic study.
Recent studies have provided examples of strong heterogeneity of treatment effect estimates over the PS that may be explained by confounding due to unmeasured frailty (8, 9). In one study of the effects of thrombolysis on all-cause in-hospital mortality among patients with stroke, mortality was much higher in the 17 stroke patients (out of a total of 212) who received thrombolytic therapy despite having the lowest PS for receiving it (41% mortality), in comparison with the remaining 195 patients (14% mortality) (8). These 17 patients with a very low predicted probability of receiving treatment may have received it because they were very frail—that is, as a treatment of last resort.
In another study that addressed the effects of treatment with biologics on all-cause mortality in patients with rheumatoid arthritis, mortality was much higher in the untreated patients in the highest PS quintile (72/1,000 person-years) than in the remainder of the untreated patients (11/1,000 person-years) (9). Frailty may also explain this difference, if the high-risk untreated patients did not receive the treatment that they might have received given their clinical condition because they were deemed too frail by the treating physician (treatment withheld).
If increases in mortality in a few patients who are treated contrary to prediction are due to unmeasured frailty, then treatment effects over the PS will appear heterogeneous, and excluding some or all of the patients treated contrary to prediction could reduce unmeasured confounding by this frailty under the assumption of uniform effects (10). In theory, if we excluded all patients with unmeasured frailty, the resulting treatment effect estimate would not be biased from unmeasured confounding by frailty. In practice, excluding all such patients will be impossible. Excluding increasing proportions of those treated contrary to prediction, however, would increase internal validity at the price of not being able to describe precisely the population to which the treatment effect estimate would apply (11, 12). In other words, the treatment effect that is estimated would not be a causal parameter even when implementing the PS in a way that should produce a causal estimate (13). If, contrary to this assumption, treatment effect heterogeneity is real and not due to unmeasured confounding, then excluding some patients will affect the generalizability of the results.
Our aim in this simulation study was to compare bias and mean squared error in the treatment effect estimates for varying degrees of asymmetric restriction of the PS distribution under the assumption of the presence of unmeasured frailty that leads to “last resort” treatment, “treatment withheld,” or both. We analyzed the data using a variety of methods to control for confounding using PS's.
MATERIALS AND METHODS
Simulation
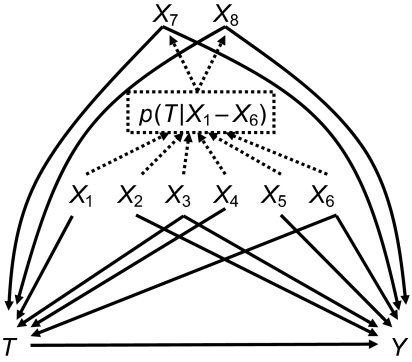
To simulate confounding by frailty in persons who are treated contrary to prediction, we used a 2-step process to define covariates (see Figure 1). We started with 3 dichotomous covariates, X1, X2, and X3, each with a prevalence of 0.2, and 3 continuous covariates, X4, X5, and X6, each with a mean of 0 and unit variance. All covariates were independent of one another. We then calculated the predicted probability of the dichotomous intended treatment T based on these 6 “measured” covariates and the covariate-treatment associations presented in Table 1, using a logistic model:
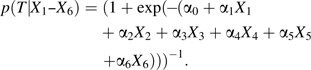 |
(1) |
Figure 1.
Conceptual diagram of a simulation study depicting treatment (T) and outcome (Y) as a function of measured covariates (X1–X6) and unmeasured covariates (X7 and X8). The solid lines represent causal associations, and the dashed lines represent noncausal associations used in the 2-step simulation process to mimic treatment contrary to prediction by measured covariates (X1–X6).
Table 1.
Parameters Covered in the Simulation Study and Their Valuesa
| Variable | Prevalence | ORTb | Parameter | Equation No(s).c | RRYd | Parameter | Equation No(s).c |
| X1 | 0.2 | 2.0 | α1 | 1, 2 | 1.0 | β1 | 3 |
| X2 | 0.2 | 1.0 | α2 | 1, 2 | 2.0 | β2 | 3 |
| X3 | 0.2 | 0.2 | α3 | 1, 2 | 0.2 | β3 | 3 |
| X4 | Continuous (0, 1) | 1.5 | α4 | 1, 2 | 1.0 | β4 | 3 |
| X5 | Continuous (0, 1) | 1.0 | α5 | 1, 2 | 1.5 | β5 | 3 |
| X6 | Continuous (0, 1) | 0.5 | α6 | 1, 2 | 0.5 | β6 | 3 |
| X7 | 0.01ef | 1, 10 | α7 | 2 | 1, 10 | β7 | 3 |
| X8 | 0.01fg | 1, 0.1 | α8 | 2 | 1, 10 | β8 | 3 |
| T | 0.2, 0.05f | α0 | 2 | 2.0 | βT | 3 | |
| Y | 0.1f (incidence rate in untreated) | β0 | 3 |
Abbreviations: OR, odds ratio; RR, rate ratio.
Parameter values are chosen to represent a study with both prevalent and rare treatment and a low incidence of outcomes over a fixed follow-up time. Covariates are either instruments (X1 and X4), risk factors for the outcome (X2 and X5), or confounders (X3, X6, X7, X8). X7 and X8 are strongly associated with both treatment and outcome but very rare, to mimic few patients treated contrary to prediction. Some parameter values are set to 1 (no association) for the tables separating “last resort” treatment from “treatment withheld.”
Odds ratio for the relation between the covariate and treatment T; parameters are for log(ORT).
For equations, see text.
Rate ratio for the relation between the covariate and the outcome Y; parameters are for log(RRY).
“Last resort” treatment if random uniform number ≤[γ – p(T|X1–X6)]; γ was chosen so that the prevalence of X7 was close to 0.01.
Approximate numbers.
“Treatment withheld” if random uniform number ≤[p(T|X1–X6) − δ]; δ was chosen so that the prevalence of X8 was close to 0.01.
We used this probability of intended treatment to assign 2 additional dichotomous covariates. One, X7, was defined as most likely to be present when the intended treatment was least likely. X7 was set to 1 (present) when a random uniform number was less than or equal to [γ − p(T|X1–X6)] and set to 0 otherwise. Thus, observations with a probability of intended treatment close to 0 would be most likely to have X7 = 1. The second covariate, X8, was likely to be present when the intended treatment was most likely. X8 was set to 1 (present) when a random uniform number was less than or equal to [p(T|X1–X6) − δ], absent otherwise. Thus, observations with a probability of intended treatment close to 1 would be most likely to have X8 = 1. The values for γ and δ were chosen to result in a low prevalence of both X7 and X8 (see Table 1). We assumed a low prevalence for persons treated contrary to prediction because of the empirical examples.
Based on these 8 covariates (i.e., the “measured” covariates X1–X6 and the “unmeasured” covariates X7 and X8), we then recalculated the probability of actual treatment, again using a logistic model:
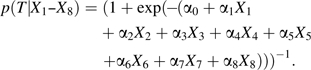 |
(2) |
The actual treatment T was then assigned on the basis of this probability using a random uniform number. Finally, the expected number of disease outcomes Y over a fixed follow-up time interval was derived from all 8 covariates and the treatment T using a log-linear model:
| (3) |
The number of outcomes Y was assigned using a random number from a Poisson distribution based on this expected value. The Poisson outcome and the log-linear outcome model were chosen because the incidence rate ratios obtained are collapsible under exchangeability (14) and therefore allow direct comparisons between the analytic strategies (15).
The range of values covered in the simulation study is presented in Table 1. The 6 measured covariates X1–X6 were associated only with treatment (X1 and X4), associated only with outcome (X2 and X5), or associated with both treatment and outcome (X3 and X6). X7 was strongly positively associated with both actual treatment and outcome (or not), mimicking frailty that leads to “last resort” treatment. X8 was strongly inversely associated with actual treatment and positively with outcome (or not), mimicking frailty that leads to “treatment withheld.” The parameter value for α0 in equation 2 was chosen to result in a prevalence of T of approximately 0.2 or 0.05, the one for β0 in equation 3 for an incidence of approximately 0.1 per observation over a fixed follow-up time in the untreated. For each scenario or parameter constellation, we simulated 1,000 closed cohort studies with n = 10,000.
Analysis
PS estimation and implementation.
We first estimated PSX1–X6 based on the measured covariates X1–X6 using logistic regression. The treatment-outcome incidence rate ratio controlling for confounding by the measured X’s was estimated using log-linear models and 5 different methods to implement PSX1–X6: modeling, stratification assuming uniform effects, stratification not assuming uniform effects, matching, and weighting (4).
We first estimated the rate ratio based on treatment and PSX1–X6 as a continuous covariate, not to encourage this PS implementation but because it is widely used in medical research (16). We then stratified the study population into 5 equal-sized strata of PSX1–X6 based on the overall (marginal) distribution of PSX1–X6. We used 5 strata because that number of strata has been shown to be sufficient to remove most confounding (17) and thus has become a widely used approach in stratifying PS's (16). We estimated the rate ratio based on a model including treatment and 4 indicator variables for PSX1–X6 quintiles 2–5.
In addition to these 2 PS implementations based on the assumption of uniform effects, we analyzed the data using 3 different approaches that do not rely on this assumption. First we combined the 5 PSX1–X6 quintile-specific treatment effect estimates based on the standardized mortality ratio—that is, using weights that reflect the distribution of treated patients over the quintiles as the standard. We then tried to find untreated matches for every treated patient based on the estimated PSX1–X6 (1:1 individual matching). We used 5-digit to 1-digit matching: starting with a very narrow caliper of the PSX1–X6 (±0.000005) to find an untreated match for every treated observation without replacement and gradually increasing the width of the caliper up to ±0.05 if no match could be found (18). Within the matched data set, we estimated the unconfounded treatment effect without taking matching into account. This approach is commonly used and valid, though it is slightly less efficient than taking matching into account (19). Both the standardized mortality ratio method and matching as we implemented it result in an estimate of a causal treatment effect in the treated, in the presence of nonuniform treatment effects (13).
Finally, we analyzed the data using inverse probability of treatment weighting (IPTW). IPTW creates a pseudo-population in which the association between covariates and treatment is removed by weighting each observation by the inverse of the probability of receiving the actual treatment. To end up with a sum of weights close to the size of the original study population, we used stabilized weights—that is, we multiplied the IPTW weights by the marginal prevalence of the treatment actually received (20). We used a (conservative) robust variance estimation. IPTW produces an estimate of a causal treatment effect in the population in the presence of nonuniform treatment effects (13, 20).
PS trimming.
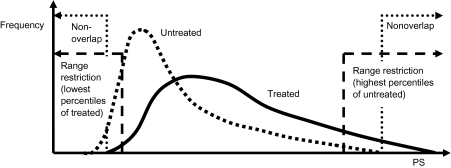
All of the above analyses were first conducted without any restriction of the PS range. We then restricted the analysis to observations within a PS range that was common to both treated and untreated persons—that is, by excluding all patients in the nonoverlapping parts of the PS distribution (see Figure 2). Individual matching on the PS also effectively resulted in a PS range that is common to treated and untreated persons.
Figure 2.
Schematic of asymmetric range restriction. PS, propensity score.
We then applied additional asymmetric PS trimming in order to exclude those patients who were treated most contrary to prediction. We assessed 3 different cutpoints corresponding to the 1st and 99th percentiles, the 2.5th and 97.5th percentiles, and the 5th and 95th percentiles of the PS distribution in the treated and untreated patients, respectively. Stratification into quintiles and matching were performed after trimming.
RESULTS
In Table 2, we present the mean number of observations and mean incidence rates in treated and untreated persons, as well as the corresponding rate ratio from the simulated data sets, according to a combination of the PSX1–X6 percentiles in treated and untreated patients. At the lower end of the PSX1–X6 range (up to the 5th percentile), percentiles are derived from the distribution of PSX1–X6 in the treated. All other percentiles, including those at the high end of the distribution, are derived from the distribution of PSX1–X6 in the untreated. This approach allows us to concentrate on the patients treated contrary to prediction (which would otherwise be swamped by patients treated according to prediction). It also leads to untreated patients below the 0th percentile and treated patients above the 100th percentile.
Table 2.
Mean Incidence Rates and Rate Ratios as a Function of Estimated Asymmetric Propensity Score Percentiles From 1,000 Simulated Data Sets in the Presence of Unmeasured Confounding Due to “Last Resort Treatment” and “Treatment Withheld”
| PS “Percentile”a | Treatment Prevalence = 0.2 |
Treatment Prevalence = 0.05 |
||||||||
| Treated |
Untreated |
RRb | Treated |
Untreated |
RRb | |||||
| No. | IRc | No. | IRc | No. | IRc | No. | IRc | |||
| “Last resort treatment” | ||||||||||
| <0d | 63 | 11 | 261 | 12 | ||||||
| 0–1 | 19 | 133 | 561 | 22 | 6.68 | 4 | 117 | 635 | 24 | 5.51 |
| 1–2.5 | 29 | 188 | 528 | 34 | 5.85 | 7 | 181 | 685 | 38 | 5.07 |
| 2.5–5 | 49 | 219 | 659 | 45 | 5.04 | 12 | 244 | 771 | 53 | 4.75 |
| 5–25 | 18 | 207 | 201 | 52 | 4.58 | 3 | 231 | 117 | 59 | 4.68 |
| 25–50 | 242 | 160 | 2,007 | 67 | 2.40 | 62 | 287 | 2,283 | 81 | 3.56 |
| 50–75 | 451 | 221 | 2,010 | 108 | 2.06 | 112 | 259 | 2,376 | 120 | 2.17 |
| 75–95 | 719 | 360 | 1,608 | 172 | 2.10 | 182 | 399 | 1,901 | 190 | 2.10 |
| 95–97.5 | 163 | 514 | 201 | 256 | 2.06 | 42 | 579 | 238 | 282 | 2.09 |
| 97.5–99 | 129 | 607 | 121 | 301 | 2.10 | 34 | 673 | 143 | 335 | 2.06 |
| 99–100 | 137 | 834 | 80 | 406 | 2.14 | 36 | 949 | 95 | 455 | 2.15 |
| >100d | 5 | 1,679 | 1 | 1,772 | ||||||
| “Treatment withheld” | ||||||||||
| <0d | 91 | 6 | 321 | 10 | ||||||
| 0–1 | 19 | 33 | 718 | 15 | 2.48 | 4 | 39 | 705 | 19 | 2.08 |
| 1–2.5 | 29 | 54 | 601 | 27 | 2.18 | 7 | 66 | 712 | 32 | 2.13 |
| 2.5–5 | 49 | 79 | 683 | 40 | 2.06 | 12 | 96 | 813 | 47 | 2.12 |
| 5–25 | 2 | 99 | 19 | 44 | 2.54 | 1 | 97 | 44 | 48 | 2.30 |
| 25–50 | 239 | 136 | 1,914 | 66 | 2.07 | 57 | 151 | 2,160 | 74 | 2.06 |
| 50–75 | 474 | 222 | 2,014 | 108 | 2.06 | 117 | 242 | 2,378 | 119 | 2.04 |
| 75–95 | 767 | 367 | 1,611 | 183 | 2.01 | 188 | 408 | 1,903 | 233 | 1.76 |
| 95–97.5 | 160 | 591 | 201 | 554 | 1.10 | 41 | 618 | 238 | 526 | 1.20 |
| 97.5–99 | 114 | 830 | 121 | 1,058 | 0.81 | 31 | 777 | 143 | 803 | 1.00 |
| 99–100 | 92 | 1,623 | 80 | 2,348 | 0.71 | 29 | 1,296 | 95 | 1,673 | 0.80 |
| >100d | 2 | 5,662 | 1 | 4,726 | ||||||
| “Last resort treatment” and “treatment withheld” | ||||||||||
| <0d | 61 | 11 | 261 | 12 | ||||||
| 0–1 | 19 | 125 | 550 | 21 | 6.75 | 5 | 103 | 627 | 22 | 5.25 |
| 1–2.5 | 29 | 173 | 516 | 31 | 5.99 | 7 | 159 | 651 | 35 | 4.87 |
| 2.5–5 | 49 | 199 | 650 | 41 | 5.01 | 12 | 219 | 754 | 49 | 4.65 |
| 5–25 | 21 | 200 | 238 | 48 | 4.84 | 3 | 238 | 150 | 55 | 4.75 |
| 25–50 | 248 | 148 | 2,013 | 64 | 2.35 | 65 | 261 | 2,308 | 77 | 3.44 |
| 50–75 | 466 | 212 | 2,013 | 104 | 2.05 | 117 | 241 | 2,375 | 114 | 2.12 |
| 75–95 | 752 | 349 | 1,611 | 173 | 2.03 | 188 | 389 | 1,900 | 222 | 1.76 |
| 95–97.5 | 158 | 562 | 201 | 500 | 1.16 | 41 | 591 | 238 | 500 | 1.21 |
| 97.5–99 | 112 | 778 | 121 | 978 | 0.82 | 31 | 742 | 143 | 760 | 1.01 |
| 99–100 | 91 | 1,518 | 80 | 2,197 | 0.71 | 29 | 1,236 | 95 | 1,592 | 0.80 |
| >100d | 2 | 5,219 | 1 | 4,170 | ||||||
Abbreviations: IR, incidence rate; PS, propensity score; RR, rate ratio.
Asymmetric PS percentiles according to PS distribution in treated and untreated persons separately (to allow closer assessment of persons treated contrary to prediction). Percentiles between 0 and 5 are derived from the PS distribution in the treated patients; all other percentiles are derived from the PS distribution in the untreated patients.
Controlled for measured covariates based on PS stratification; inconsistencies with the incidence rates in the treated and untreated are due to rounding.
Per 1,000 person-years (repeated events).
Untreated patients in the <0 asymmetric PS percentile stratum are those with a lower PS than the lowest one observed in the treated patients (nonoverlap). Treated patients in the >100 asymmetric PS percentile stratum are those with a higher PS than the highest one observed in the untreated patients (nonoverlap).
The first set of rows in Table 2 is based on the “last resort treatment” hypothesis, in which very sick patients receive a treatment contrary to the prediction of no treatment; it mimics the results presented in Table 2 of the Kurth et al. paper (8). Added to the monotonic decrease of incidence rates in both treated and untreated persons with decreasing PSX1–X6, the presence of the unmeasured covariate X7 leads to “abnormally” high incidence rates in the treated with low PSX1–X6. Because we know that the true rate ratio is 2.0, the higher rate ratios are confounded by X7. There is some residual confounding despite stratification on narrow PSX1–X6 strata, even at the high end of PSX1–X6 (e.g., for the 99th–100th percentile, rate ratio (RR) = 2.14). The maximum rate ratio in any stratum is less extreme than the most extreme stratum-specific rate ratio reported by Kurth et al. (8) (RR = 13).
The second set of rows in Table 2 is based on the “treatment withheld” hypothesis, in which a very frail patient does not receive a treatment as expected because of severe disability and/or multiple concurrent medical conditions. It mimics the results presented by Lunt et al. (9) in their Table 4. Added to the monotonic increase of incidence rates in both treated and untreated persons with increasing PSX1–X6, the presence of the unmeasured covariate X8 leads to “abnormally” high incidence rates in the untreated with high PSX1–X6. This pattern is more difficult to detect because the increase of incidence rates in the untreated over PSX1–X6 remains monotonic. High incidence rates in the untreated patients lead to enough confounding to reverse the direction of the association, resulting in apparently “protective” effect estimates confounded by X8. The minimum rate ratio in any stratum is less extreme than the most extreme stratum-specific rate ratio reported by Lunt et al. (9) (RR = 0.24).
Table 4.
Mean Rate Ratios, Empirical Variance, and Percent Coverage of 95% Confidence Intervals From 1,000 Simulated Data Sets in the Presence of Unmeasured Confounding Due to “Treatment Withheld” Without or With Asymmetric Trimming of the Propensity Score According to Analytic Strategy (True RR = 2.0)
| PS Implementation and Trimming Range | Treatment Prevalence = 0.2 |
Treatment Prevalence = 0.05 |
||||||
| RR | Variancea | MSEb | Coveragec | RR | Variancea | MSEb | Coveragec | |
| Crude | 2.85 | 0.007 | 0.130 | 0.1 | 2.93 | 0.016 | 0.157 | 4.4 |
| True outcome model | 2.00 | 0.003 | 0.003 | 94.0 | 2.00 | 0.007 | 0.007 | 94.1 |
| Outcome model (X1–X6)d | 1.30 | 0.006 | 0.193 | 0.0 | 1.34 | 0.013 | 0.182 | 1.1 |
| PS analysesd | ||||||||
| PS continuous | ||||||||
| Unrestricted | 1.31 | 0.007 | 0.191 | 0.0 | 1.38 | 0.023 | 0.171 | 5.9 |
| Restricted | ||||||||
| 0–100 | 1.31 | 0.007 | 0.190 | 0.0 | 1.41 | 0.019 | 0.146 | 5.9 |
| 1–99 | 1.53 | 0.006 | 0.078 | 1.7 | 1.50 | 0.014 | 0.099 | 14.0 |
| 2.5–97.5 | 1.75 | 0.006 | 0.004 | 38.5 | 1.63 | 0.014 | 0.059 | 39.3 |
| 5–95 | 1.95 | 0.005 | 0.006 | 91.0 | 1.75 | 0.014 | 0.033 | 71.9 |
| PS quintiles (Mantel-Haenszel) | ||||||||
| Unrestricted | 1.52 | 0.008 | 0.086 | 2.1 | 1.61 | 0.016 | 0.066 | 23.9 |
| Restricted | ||||||||
| 0–100 | 1.50 | 0.007 | 0.092 | 1.5 | 1.58 | 0.015 | 0.073 | 19.6 |
| 1–99 | 1.65 | 0.006 | 0.045 | 11.7 | 1.63 | 0.012 | 0.056 | 33.8 |
| 2.5–97.5 | 1.82 | 0.005 | 0.015 | 59.7 | 1.71 | 0.013 | 0.040 | 56.6 |
| 5–95 | 1.98 | 0.005 | 0.005 | 91.8 | 1.80 | 0.014 | 0.027 | 77.5 |
| PS quintiles (standardized mortality ratio) | ||||||||
| Unrestricted | 1.46 | 0.009 | 0.109 | 1.3 | 1.60 | 0.016 | 0.070 | 22.4 |
| Restricted | ||||||||
| 0–100 | 1.45 | 0.008 | 0.116 | 1.1 | 1.57 | 0.015 | 0.077 | 19.0 |
| 1–99 | 1.59 | 0.006 | 0.060 | 6.7 | 1.62 | 0.013 | 0.059 | 30.9 |
| 2.5–97.5 | 1.78 | 0.006 | 0.020 | 49.4 | 1.70 | 0.013 | 0.042 | 53.6 |
| 5–95 | 1.97 | 0.005 | 0.006 | 91.3 | 1.79 | 0.014 | 0.028 | 76.7 |
| PS matching | ||||||||
| Unrestricted | 1.22 | 0.008 | 0.255 | 0.0 | 1.28 | 0.033 | 0.250 | 9.5 |
| Restricted | ||||||||
| 0–100 | 1.22 | 0.008 | 0.254 | 0.0 | 1.28 | 0.033 | 0.249 | 9.4 |
| 1–99 | 1.45 | 0.008 | 0.113 | 1.4 | 1.49 | 0.030 | 0.127 | 33.3 |
| 2.5–97.5 | 1.69 | 0.008 | 0.037 | 38.5 | 1.63 | 0.031 | 0.080 | 60.7 |
| 5–95 | 1.94 | 0.008 | 0.009 | 89.2 | 1.77 | 0.034 | 0.054 | 81.7 |
| Inverse probability of treatment weighting | ||||||||
| Unrestricted | 1.31 | 0.007 | 0.192 | 0.0 | 1.57 | 0.014 | 0.076 | 50.2 |
| Restricted | ||||||||
| 0–100 | 1.29 | 0.007 | 0.203 | 0.0 | 1.52 | 0.013 | 0.093 | 38.6 |
| 1–99 | 1.62 | 0.005 | 0.052 | 19.3 | 1.69 | 0.013 | 0.045 | 70.4 |
| 2.5–97.5 | 1.82 | 0.005 | 0.014 | 75.2 | 1.77 | 0.013 | 0.029 | 83.2 |
| 5–95 | 1.99 | 0.005 | 0.005 | 94.5 | 1.85 | 0.014 | 0.022 | 91.1 |
Abbreviations: MSE, mean squared error; PS, propensity score; RR, rate ratio.
Variance of treatment effect estimates [log(RR)] over 1,000 simulated data sets.
Mean of [log(RR) – log(2.0)]2 over 1,000 simulated data sets.
Percentage of simulated studies in which the 95% confidence interval includes the true value (RR = 2.0).
Outcome and PS models including all measured covariates X1–X6 but not including unmeasured covariate X7.
The bottom set of rows in Table 2 combines the “last resort treatment” with the “treatment withheld” hypothesis. These simulations show both overestimated rate ratios in the lowest PSX1–X6 strata and underestimated rate ratios in the highest PSX1–X6 strata.
In Table 3, we present the treatment effect estimates obtained with various restrictions of the PSX1–X6 under the “last resort treatment” hypothesis. Note that the true rate ratio equals 2.0 and that all PS analyses presented rely exclusively on control for the measured covariates X1–X6. The main confounding is due to measured covariates (crude RR = 3.52 vs. RR = 2.13 based on the outcome model for a treatment prevalence of 0.2). There is, however, some uncontrolled confounding due to the unmeasured X7. The confounding by X7 is not strong despite its strong associations with both treatment and outcome because the prevalence of X7 = 1 is only 0.01 (Table 1).
Table 3.
Mean Rate Ratios, Empirical Variance, and Percent Coverage of 95% Confidence Intervals From 1,000 Simulated Data Sets in the Presence of Unmeasured Confounding Due to “Last Resort Treatment” Without or With Asymmetric Trimming of the Propensity Score According to Analytic Strategy (True RR = 2.0)
| PS Implementation and Trimming Range | Treatment Prevalence = 0.2 |
Treatment Prevalence = 0.05 |
||||||
| RR | Variancea | MSEb | Coveragec | RR | Variancea | MSEb | Coveragec | |
| Crude | 3.52 | 0.003 | 0.320 | 0.0 | 3.69 | 0.009 | 0.378 | 0.0 |
| True outcome model | 2.01 | 0.003 | 0.003 | 95.0 | 2.01 | 0.007 | 0.007 | 94.1 |
| Outcome model (X1–X6)d | 2.13 | 0.003 | 0.007 | 79.6 | 2.20 | 0.008 | 0.016 | 76.3 |
| PS analysesd | ||||||||
| PS continuous | ||||||||
| Unrestricted | 2.15 | 0.004 | 0.009 | 74.0 | 2.25 | 0.009 | 0.022 | 69.2 |
| Restricted | ||||||||
| 0–100 | 2.15 | 0.004 | 0.009 | 74.3 | 2.25 | 0.009 | 0.022 | 68.1 |
| 1–99 | 2.13 | 0.004 | 0.008 | 80.2 | 2.21 | 0.010 | 0.018 | 76.9 |
| 2.5–97.5 | 2.11 | 0.004 | 0.007 | 85.7 | 2.21 | 0.011 | 0.020 | 77.0 |
| 5–95 | 2.07 | 0.005 | 0.006 | 90.9 | 2.21 | 0.013 | 0.021 | 82.3 |
| PS quintiles (Mantel-Haenszel) | ||||||||
| Unrestricted | 2.28 | 0.003 | 0.020 | 35.8 | 2.42 | 0.008 | 0.043 | 36.0 |
| Restricted | ||||||||
| 0–100 | 2.27 | 0.003 | 0.019 | 39.2 | 2.40 | 0.008 | 0.040 | 39.0 |
| 1–99 | 2.19 | 0.004 | 0.011 | 66.5 | 2.30 | 0.009 | 0.027 | 62.0 |
| 2.5–97.5 | 2.14 | 0.004 | 0.009 | 78.0 | 2.27 | 0.010 | 0.025 | 70.7 |
| 5–95 | 2.09 | 0.005 | 0.007 | 88.1 | 2.24 | 0.012 | 0.024 | 77.8 |
| PS quintiles (standardized mortality ratio) | ||||||||
| Unrestricted | 2.26 | 0.004 | 0.019 | 42.3 | 2.40 | 0.008 | 0.040 | 38.8 |
| Restricted | ||||||||
| 0–100 | 2.25 | 0.004 | 0.017 | 47.4 | 2.38 | 0.008 | 0.037 | 41.8 |
| 1–99 | 2.16 | 0.004 | 0.009 | 75.2 | 2.28 | 0.009 | 0.025 | 65.6 |
| 2.5–97.5 | 2.12 | 0.004 | 0.007 | 84.3 | 2.25 | 0.010 | 0.023 | 73.1 |
| 5–95 | 2.08 | 0.005 | 0.006 | 90.4 | 2.22 | 0.012 | 0.022 | 79.8 |
| PS matching | ||||||||
| Unrestricted | 2.08 | 0.005 | 0.006 | 91.7 | 2.18 | 0.018 | 0.024 | 90.3 |
| Restricted | ||||||||
| 0–100 | 2.08 | 0.005 | 0.006 | 91.8 | 2.18 | 0.018 | 0.024 | 90.4 |
| 1–99 | 2.08 | 0.006 | 0.007 | 91.3 | 2.20 | 0.022 | 0.029 | 90.3 |
| 2.5–97.5 | 2.08 | 0.007 | 0.008 | 93.5 | 2.22 | 0.025 | 0.034 | 90.1 |
| 5–95 | 2.06 | 0.008 | 0.008 | 93.7 | 2.23 | 0.030 | 0.039 | 91.2 |
| Inverse probability of treatment weighting | ||||||||
| Unrestricted | 2.26 | 0.005 | 0.019 | 64.3 | 2.50 | 0.019 | 0.065 | 66.7 |
| Restricted | ||||||||
| 0–100 | 2.24 | 0.005 | 0.017 | 68.4 | 2.44 | 0.018 | 0.055 | 74.3 |
| 1–99 | 2.25 | 0.005 | 0.018 | 65.8 | 2.48 | 0.018 | 0.060 | 68.6 |
| 2.5–97.5 | 2.21 | 0.005 | 0.014 | 75.4 | 2.46 | 0.018 | 0.057 | 72.0 |
| 5–95 | 2.12 | 0.005 | 0.009 | 88.0 | 2.40 | 0.020 | 0.050 | 76.3 |
Abbreviations: MSE, mean squared error; PS, propensity score; RR, rate ratio.
Variance of treatment effect estimates [log(RR)] over 1,000 simulated data sets.
Mean of [log(RR) – log(2.0)]2 over 1,000 simulated data sets.
Percentage of simulated studies in which the 95% confidence interval includes the true value (RR = 2.0).
Outcome and PS models including all measured covariates X1–X6 but not including unmeasured covariate X7.
Bias due to the unmeasured confounder X7 is reduced by asymmetric PS trimming in most implementations of the PS (the rate ratio moves closer to the true value of 2.0 and the mean squared error gets smaller). The exception is PS matching, where bias is constant (p(T = 1) = 0.2) or increases with increasing range restrictions (p(T = 1) = 0.05). PS matching provides the least bias without restriction, however, and remains among the least biased implementations with a 5–95 range restriction. With a lower prevalence of the treatment (p(T = 1) = 0.05), IPTW becomes most biased without range restriction. A lower prevalence of treatment leads to more extreme weights in the patients who receive treatment contrary to prediction. Given the increase in variance and the bias reduction following increasing trimming, the effect on the coverage of the 95% confidence interval is very pronounced for most implementations.
In Table 4, we present the treatment effect estimates obtained with various restrictions of the PSX1–X6 under the “treatment withheld” hypothesis. The unmeasured confounding due to X8 is stronger than the one by X7. It leads to a rate ratio of 1.3 based on control for measured covariates. Consequently, the effects of trimming are more pronounced in this setting, monotonic, and similar for all PS implementations. The effects of unmeasured confounding due to X8 are most pronounced for PS matching with an unrestricted rate ratio of 1.2 (p(T = 1) = 0.2).
When combining the 2 hypotheses (Table 5), asymmetric PS trimming again leads to reduction of bias caused by the unmeasured confounders with all PS implementations. Interestingly enough, increasing restrictions lead to increasing reduction of bias with all implementations except IPTW. With IPTW, there is a reduction of bias with restriction up to the 2.5–97.5 level, but further restriction to the 5–95 level increases rather than decreases bias.
Table 5.
Mean Rate Ratios, Empirical Variance, and Percent Coverage of 95% Confidence Intervals From 1,000 Simulated Data Sets in the Presence of Unmeasured Confounding Due to “Last Resort Treatment” and “Treatment Withheld” Without or With Asymmetric Trimming of the Propensity Score According to Analytic Strategy (True RR = 2.0)
| PS Implementation and Trimming Range | Treatment Prevalence = 0.2 |
Treatment Prevalence = 0.05 |
||||||
| RR | Variancea | MSEb | Coveragec | RR | Variancea | MSEb | Coveragec | |
| Crude | 2.86 | 0.006 | 0.131 | 0.0 | 2.98 | 0.015 | 0.169 | 3.6 |
| True outcome model | 2.00 | 0.003 | 0.003 | 95.5 | 1.99 | 0.007 | 0.007 | 93.7 |
| Outcome model (X1–X6)d | 1.39 | 0.006 | 0.142 | 0.0 | 1.45 | 0.014 | 0.124 | 5.0 |
| PS analysesd | ||||||||
| PS continuous | ||||||||
| Unrestricted | 1.38 | 0.007 | 0.149 | 0.0 | 1.46 | 0.022 | 0.128 | 10.6 |
| Restricted | ||||||||
| 0–100 | 1.38 | 0.007 | 0.149 | 0.0 | 1.49 | 0.018 | 0.109 | 12.0 |
| 1–99 | 1.63 | 0.006 | 0.049 | 9.6 | 1.62 | 0.015 | 0.061 | 33.4 |
| 2.5–97.5 | 1.85 | 0.006 | 0.012 | 69.3 | 1.77 | 0.015 | 0.032 | 66.2 |
| 5–95 | 2.01 | 0.005 | 0.005 | 93.6 | 1.90 | 0.015 | 0.018 | 85.9 |
| PS quintiles (Mantel-Haenszel) | ||||||||
| Unrestricted | 1.60 | 0.007 | 0.057 | 6.3 | 1.72 | 0.016 | 0.041 | 44.7 |
| Restricted | ||||||||
| 0–100 | 1.59 | 0.007 | 0.062 | 4.8 | 1.69 | 0.015 | 0.046 | 40.8 |
| 1–99 | 1.75 | 0.006 | 0.024 | 35.2 | 1.77 | 0.014 | 0.031 | 61.6 |
| 2.5–97.5 | 1.92 | 0.005 | 0.007 | 83.3 | 1.86 | 0.014 | 0.021 | 78.8 |
| 5–95 | 2.04 | 0.005 | 0.005 | 92.3 | 1.95 | 0.015 | 0.016 | 88.1 |
| PS quintiles (standardized mortality ratio) | ||||||||
| Unrestricted | 1.53 | 0.008 | 0.084 | 3.1 | 1.69 | 0.016 | 0.047 | 39.4 |
| Restricted | ||||||||
| 0–100 | 1.51 | 0.008 | 0.089 | 2.1 | 1.67 | 0.015 | 0.052 | 34.5 |
| 1–99 | 1.67 | 0.006 | 0.039 | 17.3 | 1.74 | 0.014 | 0.035 | 57.2 |
| 2.5–97.5 | 1.87 | 0.006 | 0.011 | 73.2 | 1.83 | 0.014 | 0.023 | 76.0 |
| 5–95 | 2.02 | 0.005 | 0.005 | 93.4 | 1.93 | 0.015 | 0.016 | 88.0 |
| PS matching | ||||||||
| Unrestricted | 1.28 | 0.009 | 0.215 | 0.0 | 1.35 | 0.036 | 0.202 | 15.0 |
| Restricted | ||||||||
| 0–100 | 1.28 | 0.009 | 0.214 | 0.0 | 1.35 | 0.035 | 0.201 | 15.0 |
| 1–99 | 1.53 | 0.008 | 0.084 | 5.1 | 1.59 | 0.035 | 0.096 | 48.3 |
| 2.5–97.5 | 1.78 | 0.008 | 0.023 | 59.3 | 1.76 | 0.037 | 0.059 | 60.7 |
| 5–95 | 1.99 | 0.008 | 0.008 | 93.2 | 1.90 | 0.040 | 0.045 | 81.7 |
| Inverse probability of treatment weighting | ||||||||
| Unrestricted | 1.49 | 0.008 | 0.096 | 8.9 | 1.90 | 0.019 | 0.023 | 92.5 |
| Restricted | ||||||||
| 0–100 | 1.48 | 0.008 | 0.101 | 6.7 | 1.86 | 0.019 | 0.026 | 89.9 |
| 1–99 | 1.83 | 0.006 | 0.014 | 79.5 | 2.05 | 0.019 | 0.019 | 94.0 |
| 2.5–97.5 | 2.02 | 0.005 | 0.005 | 96.4 | 2.13 | 0.019 | 0.022 | 92.9 |
| 5–95 | 2.11 | 0.005 | 0.008 | 90.2 | 2.18 | 0.020 | 0.026 | 90.7 |
Abbreviations: MSE, mean squared error; PS, propensity score; RR, rate ratio.
Variance of treatment effect estimates [log(RR)] over 1,000 simulated data sets.
Mean of [log(RR) – log(2.0)]2 over 1,000 simulated data sets.
Percentage of simulated studies in which the 95% confidence interval includes the true value (RR = 2.0).
Outcome and PS models including all measured covariates X1–X6 but not including unmeasured covariate X7.
DISCUSSION
We simulated data sets to mimic treatment effect heterogeneity in 2 separate published clinical studies under the assumption that such heterogeneity is due to unmeasured confounding by patient frailty. Our simulation study shows that under this assumption, increasing asymmetric PS trimming can increase the validity of the treatment effect estimates. This increase in validity was observed with most of the different PS implementations and over all of the scenarios assessed in the simulations.
How can we detect unmeasured confounding by frailty? Sensitivity of treatment effects to the approach of estimation, especially very different results from untrimmed IPTW, raised caution in the examples cited (8, 9). “Last resort treatment” and “treatment withheld” will lead to apparent heterogeneity of treatment effect estimates in the opposite ends of the overlapping PS distribution. This heterogeneity could easily be missed by stratifying the data into broad PS categories, such as quintiles. The heterogeneity becomes apparent, however, if one stratifies the data finely by PS at both ends of the PS distribution. Disadvantages of stratifying by broad percentile categories such as quintiles have been pointed out in other settings (21). Combining the lower percentiles from the treated patients and the higher percentiles from the untreated patients into a single “percentile” is an idea proposed previously by Stürmer et al. (10). Although some variability will occur by chance, trends such as those reported (8, 9) should raise caution.
We are aware of few published implementations of PS range restrictions (8, 22, 23). Here we assessed the performance of asymmetric PS trimming (10) when the treatment effect is homogeneous. We observed some differences between different methods of using the PS to control confounding. PS matching was least affected by bias due to unmeasured frailty that led to “last resort treatment.” This result can be explained by the fact that the treatment effect in the treated patients estimated by PS matching is based on few matched sets with very low PS's. Estimating the treatment effect in the treated patients thus guards against major bias due to “last resort” treatment, even without trimming. Without trimming, however, estimating the treatment effect in the treated is more susceptible to bias due to “treatment withheld.” In the scenario with both “last resort treatment” and “treatment withheld,” trimming IPTW provided the least correction of uncontrolled confounding and increasing trimming did not monotonically lead to reduced bias. This result is in contrast to all other PS methods and scenarios that we assessed.
It was difficult to mimic the results presented by Kurth et al. (8) and Lunt et al. (9) in simulations. The difficulty relates to the hypothesized phenomena being concentrated within a few people in the extremes of the PS distribution. With our 2-stage process, which first calculates the probability of intended treatment given the measured covariates to assign our frailty covariates and then assumes strong associations between the frailty covariates and both actual treatment (odds ratio = 10) and outcome (RR = 10), we came close. Nevertheless, the treatment effect heterogeneity in our simulation study is still less extreme than the heterogeneity observed in published clinical studies (8, 9). Owing to this concentration of the effect in the extremities of the PS distribution, other methods that have been proposed to deal with unmeasured confounders in pharmacoepidemiology (e.g., 24–28) are likely to not perform well in this setting, even if a measure for frailty were available.
Asymmetric trimming does not always lead to bias reduction with IPTW. With unmeasured confounding at both ends of the PS distribution (Table 5), the 5–95 trimmed estimate is slightly more biased than the 2.5–97.5 trimmed estimate. We do not have an explanation for this observation, and we encourage more research into the behavior of IPTW in the presence of unmeasured confounders such as frailty and the value of trimming the population to reduce such bias. Reestimation of PS within the trimmed population slightly alleviated but did not suppress this problem (e.g., RR = 2.06 for 5–95 trimming vs. RR = 2.11 for p(T = 1) = 0.2), while results for all other scenarios assessed were virtually identical.
Because IPTW estimates the treatment effect in the whole population, it should only be implemented if everyone in the study has the potential to be treated. Cole and Hernán (29) have proposed truncation of IPTW to improve the trade-off between variance and residual confounding by measured covariates. Thus, truncation is not intended to reduce bias due to unmeasured confounders, nor does it result in bias reduction in this setting (data not shown). Trimming by the absolute value of the PS has recently been proposed to address lack of PS overlap and to reduce the variance of an IPTW estimator of the average treatment effect (30). Crump et al. (30) proposed a selection method that maximizes precision and concluded that a simple rule of thumb excluding all observations outside a PS range of 0.1–0.9 is a good approximation to maximize precision.
Real treatment effect heterogeneity is another plausible explanation for nonuniform treatment effects. Treatment may be more beneficial and less harmful in patients most likely to be treated because they have the strongest indications and the fewest contraindications, and vice versa. If treatment effect heterogeneity is real rather than caused by unmeasured confounders, then methods of PS implementation that do not assume uniform treatment effects should be used and trimming will limit generalizability. Separate analyses after trimming may provide a more meaningful estimate in the patients where there is equipoise, however, and highlight the difficulties in reliably estimating the treatment effect in patients with reduced equipoise. In the study by Kurth et al. (8), thrombolysis may have been the cause of the increased mortality observed in a subgroup of patients defined by their low probability of receiving thrombolysis. In contrast, it is not plausible that treatment with biologics would prevent 75% of all deaths in a subgroup of patients defined by a high probability of receiving biologics in the study by Lunt et al. (9).
Because the data cannot distinguish bias caused by unmeasured confounders from real treatment effect heterogeneity, the validity benefit of any range restriction is arguable. Any data excluded from primary analyses by trimming should be presented for readers to evaluate, along the lines of our Table 2. Such a table will illuminate the nature of the population to which the treatment effect estimate based on PS range restrictions applies. After range restriction, these estimates will not be causal in the sense that they do not apply to any clearly defined population, such as all treated patients, anymore. Thus, there will be a trade-off between validity and estimating the treatment effect in a clearly defined population.
The conclusions of all simulation studies are limited to the scenarios assessed. We explicitly simulated our data sets so that the treatment effect estimate based on restricting the range to the 5th–95th percentiles was not unbiased, to avoid the false impression that this arbitrary cutpoint will eliminate bias caused by unmeasured confounders. Misspecification of the PS model in addition to the one due to the unmeasured confounders in persons treated contrary to prediction (e.g., by additional unmeasured confounders or misspecification of measured covariates) could affect the performance of trimming. The interpretation of results following asymmetric PS trimming will depend on the observed change in treatment effect estimates over increasing PS range restrictions.
We conclude that one plausible explanation for the very heterogeneous treatment effects on mortality presented by Kurth et al. (8) and Lunt et al. (9), unmeasured confounding by frailty, can be reduced by increasing asymmetric trimming of the PS. Under this assumption, trimming enhances validity. The ability to trim outliers based on a single variable that summarizes confounding from other, measured variables is an important advantage of PS's compared with conventional outcome models.
Because real treatment effect heterogeneity cannot be excluded as an alternative explanation, asymmetric PS trimming should be used judiciously, perhaps as a sensitivity analysis. To inform interpretation, investigators should present data on the outcomes in treated and untreated patients finely stratified according to the PS distribution for treated patients at the lower end and the PS distribution for untreated patients at the higher end.
Acknowledgments
Author affiliations: Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts (Til Stürmer, Kenneth J. Rothman, Jerry Avorn, Robert J. Glynn); Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Til Stürmer); Department of Biostatistics, Harvard School of Public Health, Boston, Massachusetts (Robert J. Glynn); and RTI Health Solutions, Research Triangle Park, North Carolina (Kenneth J. Rothman).
This study was funded by grants RO1 AG023178 and RO1 AG018833 from the National Institute on Aging.
Conflict of interest: none declared.
Glossary
Abbreviations
- IPTW
inverse probability of treatment weighting
- PS
propensity score
- RR
rate ratio
References
- 1.Miettinen OS. Stratification by a multivariate confounder score. Am J Epidemiol. 1976;104(6):609–620. doi: 10.1093/oxfordjournals.aje.a112339. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 3.Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98(3):253–259. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stürmer T, Schneeweiss S, Brookhart MA, et al. Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol. 2005;161(9):891–898. doi: 10.1093/aje/kwi106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glynn RJ, Knight EL, Levin R, et al. Paradoxical relations of drug treatment with mortality in older persons. Epidemiology. 2001;12(6):682–689. doi: 10.1097/00001648-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338(21):1516–1520. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- 7.Welch HG, Albertsen PC, Nease RF, et al. Estimating treatment benefits for the elderly: the effect of competing risks. Ann Intern Med. 1996;124(6):577–584. doi: 10.7326/0003-4819-124-6-199603150-00007. [DOI] [PubMed] [Google Scholar]
- 8.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163(3):262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 9.Lunt M, Solomon D, Rothman K, et al. Different methods of balancing covariates leading to different effect estimates in the presence of effect modification. Am J Epidemiol. 2009;169(7):909–917. doi: 10.1093/aje/kwn391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stürmer T, Schneeweiss S, Rothman KJ, et al. When patients are treated contrary to prediction—implications for use of propensity scores in extreme cases [abstract] Pharmacoepidemiol Drug Saf. 2007;16(suppl 2) S3. [Google Scholar]
- 11.Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health. 2005;95(suppl 1):S144–S150. doi: 10.2105/AJPH.2004.059204. [DOI] [PubMed] [Google Scholar]
- 12.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578–586. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stürmer T, Rothman KJ, Glynn RJ. Insights into different results from different causal contrasts in the presence of effect-measure modification. Pharmacoepidemiol Drug Saf. 2006;15(10):698–709. doi: 10.1002/pds.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenland S, Robins JM. Identifiability, exchangeability, and epidemiological confounding. Int J Epidemiol. 1986;15(3):413–419. doi: 10.1093/ije/15.3.413. [DOI] [PubMed] [Google Scholar]
- 15.Gail MH, Wieand S, Piantadosi S. Biased estimates of treatment effect in randomized experiments with nonlinear regressions and omitted covariates. Biometrika. 1984;71(3):431–444. [Google Scholar]
- 16.Stürmer T, Joshi M, Glynn RJ, et al. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59(5):437–447. doi: 10.1016/j.jclinepi.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cochran WG. The effectiveness of adjustment by subclassification in removing bias in observational studies. Biometrics. 1968;24(2):295–313. [PubMed] [Google Scholar]
- 18.Parsons LS. SUGI 26 Proceedings. Cary, NC: SAS Institute Inc; 2001. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. (Paper 214-26) ( http://www2.sas.com/proceedings/sugi26/p214-26.pdf). (Accessed May 25, 2010) [Google Scholar]
- 19.Hill J. Discussion of research using propensity-score matching: comments on ‘A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003’ by Peter Austin, Statistics in Medicine. Stat Med. 2008;27(12):2055–2061. doi: 10.1002/sim.3245. [DOI] [PubMed] [Google Scholar]
- 20.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6(4):356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Smith JA, Todd PE. Does matching overcome LaLonde's critique of nonexperimental estimators? J Econom. 2005;125(1):305–353. [Google Scholar]
- 23.Walker AM, Koro CE, Landon J. Coronary heart disease outcomes in patients receiving antidiabetic agents in the PharMetrics database 2000–2007. Pharmacoepidemiol Drug Saf. 2008;17(8):760–768. doi: 10.1002/pds.1598. [DOI] [PubMed] [Google Scholar]
- 24.Schneeweiss S, Glynn RJ, Tsai EH, et al. Adjusting for unmeasured confounders in pharmacoepidemiologic claims data using external information: the example of COX2 inhibitors and myocardial infarction. Epidemiology. 2005;16(1):17–24. doi: 10.1097/01.ede.0000147164.11879.b5. [DOI] [PubMed] [Google Scholar]
- 25.Stürmer T, Schneeweiss S, Avorn J, et al. Adjusting effect estimates for unmeasured confounding with validation data using propensity score calibration. Am J Epidemiol. 2005;162(3):279–289. doi: 10.1093/aje/kwi192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stürmer T, Schneeweiss S, Rothman KJ, et al. Performance of propensity score calibration—a simulation study. Am J Epidemiol. 2007;165(10):1110–1118. doi: 10.1093/aje/kwm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stürmer T, Kaufman JS, Brookhart MA, et al. Control of unmeasured confounding [abstract] Pharmacoepidemiol Drug Saf. 2006;15(suppl 1) S11. [Google Scholar]
- 28.Brookhart MA, Wang PS, Solomon DH, et al. Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology. 2006;17(3):268–275. doi: 10.1097/01.ede.0000193606.58671.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crump RK, Hotz VJ, Imbens GW, et al. Dealing with limited overlap in estimation of average treatment effects. Biometrika. 2009;96(1):187–199. [Google Scholar]