Abstract
Telechelic water-soluble HPMA copolymers and HPMA copolymer-doxorubicin (DOX) conjugates have been synthesized by RAFT polymerization mediated by a new bifunctional chain transfer agent (CTA) that contains an enzymatically degradable oligopeptide sequence. Post-polymerization aminolysis followed by chain extension with a bis-maleimide resulted in linear high molecular weight multiblock HPMA copolymer conjugates. These polymers are enzymatically degradable; in addition to releasing the drug (DOX), the degradation of the polymer backbone resulted in products with molecular weights similar to the starting material and below the renal threshold. The new multiblock HPMA copolymers hold potential as new carriers of anticancer drugs.
Keywords: RAFT chain transfer agent, biodegradation, multiblock, HPMA, copolymer, long circulation
Introduction
Copolymers of N-(2-Hydroxypropyl)methacrylamide (HPMA) have been some of the most frequently studied water-soluble carriers of anticancer drugs.1,2 Their biological evaluation both in animal models and clinical trials revealed their biocompatibility.2 The molecular weight of the drug carriers is crucial for effective functioning. The molecular weight threshold limiting glomerular filtration of poly[N-(2-hydroxypropyl)methacrylamide] (polyHPMA) is approximately 45 kDa for intravenous administration; consequently, the molecular weight of polymeric carriers should be less than 30-40 kDa.3 For example, polyHPMA-GFLG-Doxorubicin, PK1, had an average molecular weight of 28 kDa and a hydrodynamic diameter about 5 nm, with an initial half-life of 2.7 h.4 This resulted in short circulation times and limited accumulation in the tumor tissue.5,6 The molecular weight of currently used HPMA copolymer-drug conjugates is suboptimal.5 Increasing the molecular weight of HPMA copolymers resulted in prolonged circulation times and increased the tumor-to-organ ratios due to the EPR (enhanced permeability and retention) effect.7 However, high molecular weight nonbiodegradable HPMA copolymer conjugates may deposit inside the body and cause unexpected side-effects. The lack of polymer biodegradability will likely limit parenteral use of HPMA copolymer conjugates as treatments for life-threatening diseases.6 Biodegradable polyesters such as PLGA (poly(lactic-co-glycolic acid)) are used in drug delivery systems, but they are limited by their hydrophobicity and/or non-specific degradation. HPMA copolymers that can be biodegraded in cells, but stable in blood circulation are needed.
Shiah et al.8 synthesized biodegradable HPMA copolymer - DOX conjugates by slightly cross-linking of copolymer chains via enzymatic cleavable –GFLG– tetrapeptide and tested the conjugates in mice bearing human ovarian xenografts. The results clearly indicated that the higher the molecular weight of the carrier, the higher the accumulation in solid tumor with concomitant increase in therapeutic efficacy. But this method was limited by poor structural control and reproducibility, and steric hindrance for enzyme hydrolysis. To overcome these shortcomings, biodegradable HPMA copolymers with well controlled structures should be designed.
RAFT polymerization has proven to be an extremely well controlled “living” free radical polymerization method for the preparation of polymers with designed molecular weights and narrow molecular weight distribution.9,10 It permits the synthesis of telechelic polymers11 as well as providing easy modification of terminal groups. For example, post-polymerization aminolysis of thiobenzoylthio end groups results in α,ω-dithiol telechelic polymers.12,13 Biodegradable, long-circulating polymer carriers can be prepared by linking 10-40 kDa linear, telechelic polymer segments (synthesized by RAFT polymerization, with molecular weights below the renal threshold) to enzymatically degradable oligopeptide sequences.
Here we report the design and synthesis of novel lysosomally degradable water-soluble multisegment HPMA copolymers via the combination of reversible addition fragmentation chain transfer (RAFT) polymerization and high efficient thiol-ene reaction. The structure and molecular weight of each segment were controlled by RAFT polymerization, and the incorporation of the enzymatically degradable sequences into the linear polymer backbone provides for an easy formation of the enzyme-substrate complex, resulting in fast degradation.
Experimental Section
Materials and methods
N-α-Fmoc protected amino acids, 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and 2-Cl-trityl chloride resin (100–200 mesh, 1.27 mmol/g) were from EMD Biosciences (San Diego, CA). Papain (EC 3.4.22.2, from papaya latex) and cathepsin B (EC 3.4.22.1, from bovine spleen) were from Sigma-Aldrich (St. Louis, MO). Bis-MAL-dPEG3 (bis-[1,13-(3-maleimidopropionyl)amido]-4,7,10-trioxatridecane, CAS# 756525-89-0) was purchased from Quanta Biodesign (Powell, OH). 1-Hydroxybenzotriazole (HOBt) was purchased from AnaSpec (Fremont, CA). N,N′-Diisopropylcarbodiimide (DIC), 2,2,2-trifluoroethanol (TFE) and all other reagents and solvents were from Sigma-Aldrich (St. Louis, MO). Doxorubicin (DOX) was a kind gift from Meiji Seika Kaisha Ltd. Tokyo, Japan. HPMA,14 4-cyanopentanoic acid dithiobenzoate15 were synthesized according to literature. N-Methacryloylglycylphenylalanylleucylglycyl-doxorubicin (MA-GFLG-DOX) was prepared by the reaction N-methacryloylglycylphenylalanylleucylglycine 4-nitrophenyl ester (MA-GFLG-ONp) with doxorubicin hydrochloride in DMF in the presence of diisopropylethylamine according to the described procedure.16
UV-vis spectra were measured on a Varian Cary 400 Bio UV-visible spectrophotometer. Mass spectra were measured on an FTMS mass spectrometer (LTQ-FT, ThermoElectron, Waltham, MA). 1H-NMR spectra were recorded on a Mercury400 spectrometer using DMSO-d6 as the solvent. Polymerization conversion was determined by the measurement of remaining HPMA monomer concentration at different time points using RP-HPLC (Agilent Technologies 1100 series, Zorbax C8 column 4.6×150 mm) with gradient elution from 2 to 90% of Buffer B within 30 min at flow rate of 1.0 mL/min (Buffer A: deionized water (DI H2O) with 0.1% TFA, Buffer B: acetonitrile with 0.1% TFA). The molecular weight and polydispersity index (PDI) of polymers were measured on an ÄKTA FPLC (fast protein liquid chromatography) system (GE Healthcare, formerly Amersham) equipped with miniDAWN TREOS and OptilabEX detectors (Wyatt Technology, Santa Barbara, CA) using a Superose 6 or 12 HR10/30 column with PBS (pH 7.3) as the mobile phase. Narrow polydispersity polyHPMA fractions prepared by size exclusion chromatography, whose molecular weights were characterized by multiangle light scattering, were used as molecular weight standards. The multiblock polymers were fractionated on the same FPLC system using Superose 6 HR16/60 preparative column. PBS was used as the mobile phase. The flow rate was 1 mL/min. the fraction was collected every 10 min. The salt in the fractions was removed by dialysis. The narrow polydispersity polymer fractions were obtained after freeze-drying.
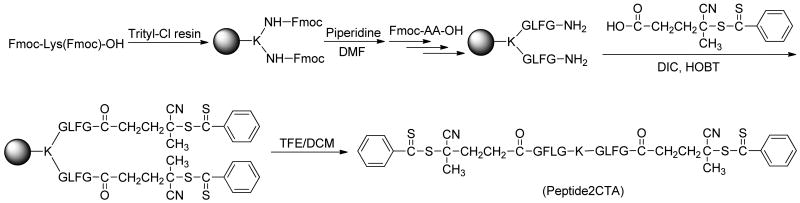
Synthesis of enzyme-sensitive bifunctional CTA, Nα,Nε-bis(4-cyano-4-(phenylcarbonothioylthio)pentanoylglycylphenylalanylleucylglycyl)lysine (Peptide2CTA)
The bifunctional chain transfer agent containing an enzyme-sensitive peptide sequence was synthesized by solid phase peptide synthesis (SPPS) methodology and manual Fmoc/tBu strategy on 2-chlorotrityl chloride resin. HBTU was used as the coupling agent and 20% piperidine in DMF as the deprotection agent for Fmoc protected amino acids (Fmoc-AA-OH). Briefly, Fmoc protected amino acids, Fmoc-Lys(Fmoc)-OH, Fmoc-Gly-OH, Fmoc-Leu-OH, Fmoc-Phe-OH and Fmoc-Gly-OH were coupled sequentially to the beads (0.3 g, 0.11 mmol loading). After deprotection, the chain transfer agent, 4-cyanopentanoic acid dithiobenzoate (3 times excess), was coupled to the terminal glycyl residues using DIC/HOBT (1/1, 3 times excess) in DMF. The peptide was isolated following cleavage from resin by 30 % TFE in DCM for 2 h. Yield 99 mg (62%). The purity of peptide2CTA was measured by HPLC. MS (m/z): [M+H]+ 1417.56467 (cal. C70H88N12O12S4 + H, 1417.56003), [M+2H]2+ 709.28516 (cal. C70H88N12O12S4 + 2H, 709.28365). 1H-NMR (DMSO-d6, δ, ppm): 12.62 (s, 1H, -COOH); 8.27-7.96 (m, 10H, CONH); 7.90 (d, 3H, Ph-H); 7.68 (t, 3H, Ph-H); 7.50 (t, 4H, Ph-H); 7.22 (m, 10H, Ph-H); 4.52 (m, 2H, Phe-CH); 4.25 (m, 2H, Leu-CH); 4.16 (m, 1H, Lys-CH); 3.73-3.55 (m, 8H, Gly-CH2); 3.01 (m, 4H, Phe-CHH, Lys-NH-CH2); 2.74 (m, 2H, Phe-CHH); 2.49-2.33 (m, 8H, CTA-CH2CH2); 1.88 (s, 6H, CTA-CH3); 1.70-1(.27 (m, 12H, Lys-C-CH2CH2CH2-C, Leu-CH2CHMe2); 0.87-0.81 (dd, 12H, Leu-(CH3)2).
Polymerization
RAFT polymerization of HPMA mediated by peptide2CTA
HPMA homopolymerizations were performed in methanol at 60 °C using 2,2′-azobisisobutyronitrile (AIBN) as initiator. A typical procedure was as follows: HPMA (200 mg, 1.4 mmol), peptide2CTA (7 mg, 5 μmol) and AIBN (0.45 mg, 2.7 μmol) were dissolved in methanol (1.2 mL). The solution was bubbled with N2 for 30 min, sealed and polymerized at 60 °C for 24 h. After polymerization, the polymer was purified by dissolution-precipitation in methanol-acetone 3 times, then washed with acetone and dried under reduced pressure at room temperature. Yield 145 mg (72.5%) of pink α,ω-di(phenylcarbonothioylthio)-polyHPMA (Mw 30.3 kDa, PDI 1.08).
Kinetic studies
Stock solution was prepared by dissolving HPMA (720 mg, 5 mmol), peptide2CTA (17.7 mg, 12.5 μmol) and AIBN (0.4 mg, 2.4 μmol) in methanol (4 mL) in a vial equipped with a rubber septum and then bubbled with N2 for 30 min. Aliquots were transferred via syringe to six different ampoules; each one was attached to Schlenk-line and had been purged with N2. The ampoules were sealed under N2 atmosphere and then placed in an oil bath preheated to 60 °C. At different time points, the ampoule was taken out and the polymerization quenched in liquid nitrogen. The sample solution was immediately analyzed by HPLC. The monomer conversion was calculated by comparison of the remaining monomer concentration with initial concentration. The molecular weight of the sample was measured by FPLC using Superose 12 HR10/30 column with PBS (pH 7.3) as the mobile phase after precipitation of the polymer solution into cold anhydrous ether.
Measurement of the molecular weight of the polyHPMA by extinction coefficient of peptide2CTA
A series of peptide2CTA solutions in methanol ranging from 6.33 × 10-6 M to 6.33 × 10-5 M were prepared. The absorbance of each sample at 302 nm (λmax PhC=SS) was measured using a 1.0 cm path length quartz cuvette. The molar absorption coefficient (ε, in methanol) of 2.69 × 104 M-1cm-1 was obtained from the slope of the plot Concentration (M) ∼ Absorbance302nm.
Polymers obtained from kinetics study were dissolved in methanol. Similarly, the absorbance of each sample at 302 nm (λmax PhC=SS) was measured using a 1.0 cm path length quartz cuvette. The molecular weight of each polymer was calculated using the extinction coefficient of peptide2CTA.
RAFT copolymerization of HPMA with MA-GFLG-DOX
HPMA copolymerization was performed similarly as the homopolymerization: HPMA (140 mg, 977.7 μmol, 98 mol%), MA-GFLG-DOX (20 mg, 20.3 μmol, 2 mol%), peptide2CTA (5.7 mg, 4 μmol) and AIBN (0.4 mg, 2.4 μmol) were dissolved in methanol (1.0 mL). The solution was bubbled with N2 for 30 min, sealed and polymerized at 50 °C for 24 h. After polymerization, the polymer was purified by dissolution-precipitation in methanol-acetone 3 times, then washed with acetone and dried under reduced pressure at room temperature. Yield 100 mg (62.5%) of α,ω-di(phenylcarbonothioylthio)-HPMA copolymer-DOX conjugate (Mw 27.8 kDa, PDI 1.19).
End group modification by aminolysis
α,ω-Di(phenylcarbonothioylthio)-polyHPMA (100 mg) was dissolved in 800 μL of methanol (bubbled with N2 for 30 min); n-butylamine (100 μL) was added under N2 and the mixture was stirred for 10 min at room temperature. Then, the polymer was purified by dissolution-precipitation in methanol-ether 3 times, washed with ether and dried under reduced pressure at room temperature. Yield 76 mg of α,ω-dithiol-polyHPMA.
Aminolysis of α,ω-di(phenylcarbonothioylthio)-HPMA copolymer-DOX conjugate was performed similarly as α,ω-di(phenylcarbonothioylthio)-polyHPMA.
Preparation of multiblock polyHPMA by chain extension with bis-MAL-dPEG3
α,ω-dithiol-polyHPMA (70 mg, 2.49 μmol, Mn 28.1 kDa, PDI 1.08) and bis-MAL-dPEG3 (1.3 mg, 2.49 μmol) at molar ratio of 1:1 were dissolved in 160 μL MeOH, and the reaction mixture was stirred at room temperature for 24 h. The polymer was purified by dissolution-precipitation in methanol-acetone 3 times, washed with acetone and dried under reduced pressure. Yield 65 mg of polymer.
Chain extension of α,ω-dithiol-HPMA copolymer-DOX conjugate (70 mg, 3.00 μmol, Mn 23.3 kDa, PDI 1.19) with bis-MAL-dPEG3 (1.6 mg, 3.06 μmol) was performed similarly. Yield 60 mg of extended multiblock HPMA copolymer-DOX conjugate.
Enzyme-catalyzed degradation of multiblock polyHPMA
Papain and cathepsin B were used for polymer degradation. The activities of papain and cathepsin B were assessed using the substrate Bz-Phe-Val-Arg-p-nitroanilide (Bz-Phe-Val-Arg-NAp, NAp). The concentration of the enzyme stock solution in buffer (McIlvaine's buffer with 2 mM EDTA, pH 517,18) was measured spectrophotometrically (280 nm, 1 cm cuvette, using ABS1% = 25 and 20 for papain and cathepsin B, respectively). The concentration of Bz-Phe-Val-Arg-NAp stock solution in DMF was measured by UV-vis at 316 nm using extinction coefficient of 1.36 × 104 M-1cm-1 after 20 × dilution with water.
During the measurement, enzyme stock solution was pre-incubated in buffer containing 5 mM GSH at 37 °C for 5 min, then Bz-Phe-Val-Arg-NAp stock solution was added. For papain, the final concentrations in the incubation mixture were 1.7 × 10-7 M enzyme, 3.69 × 10-4 M p-nitroanilide. The release of p-nitroaniline was measured at 410 nm (using extinction coefficient 8480 M-1cm-1). At 10 min, the cleavage was 7.3% of NAp, Δ[NAp]/[Enzyme]/min = 16.6/min. For cathepsin B, the final concentrations in the incubation mixture were 6.52 × 10-7 M enzyme, 3.34 × 10-4 M p-nitroanilide. The release of p-nitroaniline was measured at 410 nm (using extinction coefficient 8480 M-1cm-1). At 10 min, the cleavage was 7.6% of NAp, Δ[NAp]/[Enzyme]/min = 5.75/min.
The degradation of multiblock polyHPMA obtained from thiol-ene reactions was performed in McIlvaine's buffer (50 mM citrate/0.1 M phosphate) at pH 5.0, 37 °C, using papain or cathepsin B. Papain (0.45 mg/mL, 0.1 mL) (or cathepsin B, 0.35 mg/mL, 0.1 mL) was mixed with 0.8 mL of buffer containing 5 mM GSH and the mixture was pre-incubated for 5 min at 37 °C. Multiblock polyHPMA (260.8 kDa fraction) or multiblock HPMA copolymer-DOX conjugate (227.7 kDa fraction) in 0.1 mL of buffer containing 4 mg of the polymer was added and incubated at 37 °C. At scheduled time intervals, 0.1 mL sample was withdrawn and mixed with 0.4 mL of PBS (pH 7.3) containing 0.05 mL of 1 M sodium iodoacetate. The sample was analyzed by SEC using an ÄKTA FPLC system equipped with miniDAWN TREOS and OptilabEX detectors using a Superose 6 HR/10/30 column with PBS (pH 7.3) as mobile phase (Figure S8 and S9). For multiblock HPMA copolymer-DOX conjugate, the release of free DOX was also analyzed by HPLC.
Results and Discussion
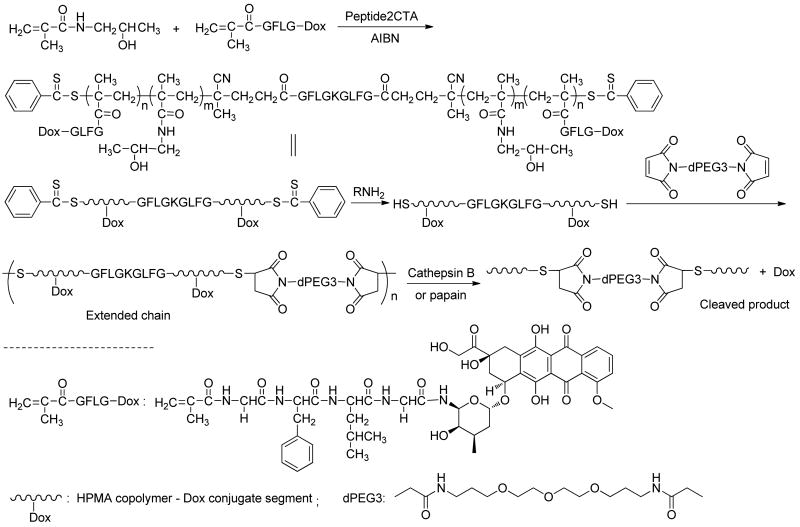
To synthesize long-circulating high molecular weight HPMA copolymer conjugates which are biocompatible and can be eliminated from the organism, biodegradable bonds should be incorporated into the polymer backbone. A new bifunctional RAFT chain transfer agent, Nα,Nε-bis(4-cyano-4-(phenylcarbonothioylthio)pentanoyl glycylphenylalanylleucylglycyl)lysine (Peptide2CTA) was designed. Using Fmoc-Lys(Fmoc)-OH, two copies of lysosomally degradable GFLG sequences19 were introduced at the same time by solid phase peptide synthesis. Both ends of GFLG sequences were capped with 4-cyano-4-(phenylcarbonothioylthio)pentanoate (Scheme 1 and Supporting Information). The long spacer from solid phase peptide synthesis beads can reduce the steric hindrance, speed up the coupling of dithiobenzoate chain transfer agent and reduce the aminolysis of dithiobenzoate groups. Two copies of GFLG peptide may also increase the degradation rate during enzyme degradation process. Different coupling conditions were tested. DIC/HOBt (1/1) coupling method avoided formation of by-products and gave good yields. In the coupling reaction, 3 times excess of 4-cyano-4-(phenylcarbonothioylthio)pentanoate and coupling agents were used.
Scheme 1.
Synthesis of bifunctional chain transfer agent containing an enzyme sensitive peptide sequence.
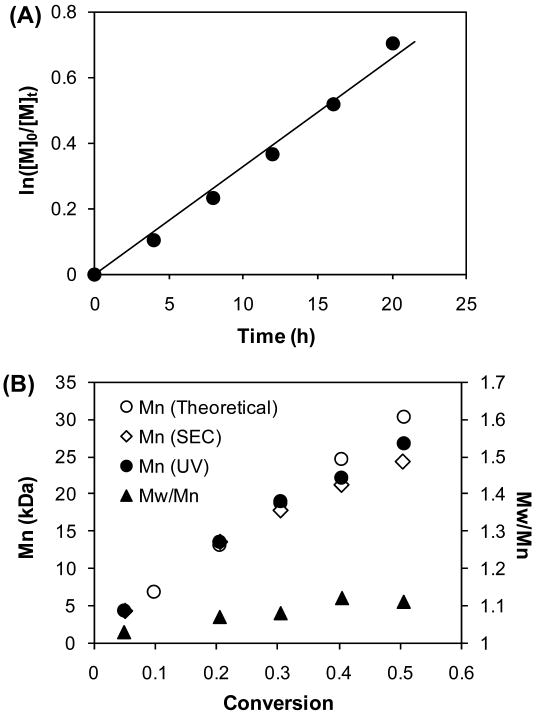
To evaluate the efficiency of the new Peptide2CTA, HPMA polymerization in methanol at 60 °C was studied using 2,2′-azobisisobutyronitrile (AIBN) as the initiator. The molecular weight of the polymers, measured by size exclusion chromatography (SEC) and UV-Vis (dithiobenzoyl end group determination at 302 nm), fit well with theoretical values, which demonstrated the telechelic nature of the polymer and that the majority of the polymers retained the phenylcarbonothioylthio group at both ends. Linearity of the pseudo-first-order kinetics plot (Figure 1A) and the evolution of Mn and Mw/Mn with conversion (Figure 1B) indicated that HPMA polymerization was well controlled over molecular weight and polydispersity. The Mn increased in a linear fashion with conversion up to about 40%, and was in agreement with theoretical values.
Figure 1.
RAFT polymerization of HPMA mediated by Peptide2CTA at 60 °C in methanol. [HPMA]0/[CTA]0 = 400, [HPMA]0 = 1 M; [CTA]0/[AIBN]0 = 5. (A) Kinetics plot; (B) Evolution of Mn and polydispersity index versus conversion.
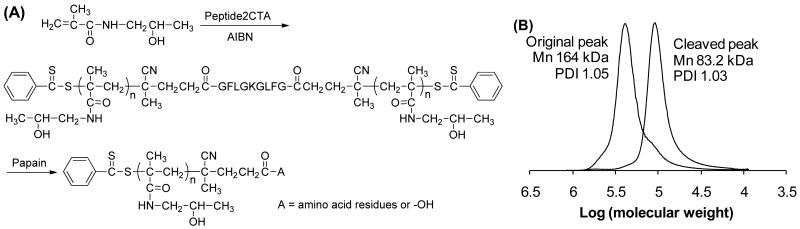
During RAFT polymerization (Figure 2A) the HPMA monomers incorporated at both dithiobenzoate groups of the Peptide2CTA with identical efficiency. When the final polymer was incubated with papain, a thiol proteinase with similar specificity as lysosomal proteinases,20,21 the molecular weight decreased to half of the original value (Figure 2B).
Figure 2.
(A) Polymerization of HPMA in methanol at 60 °C for 24 h mediated by Peptide2CTA and AIBN. [HPMA]0/[CTA]0 = 2000, [HPMA]0 = 1 M; [CTA]0/[AIBN]0 = 2.5. (B) Following incubation of the polyHPMA with papain, the molecular weight decreased to half of the original value.
Telechelic polymers prepared by RAFT polymerization have been used for attachment of biologically active compounds, synthesis of block copolymers, and modification of biomaterial surfaces. Alkyne-azide click reaction or thiol-ene reaction have been used for the synthesis of multisegment copolymers. Matyjaszewski and coworkers prepared multiblock copolymers by Cu+-catalyzed azide-alkyne cycloaddition of α,ω-diazido-terminated triblock and pentablock copolymers with propargyl ether.22 The highly efficient thiol-ene reactions23 do not need a metal catalyst, are compatible with water, and can be performed under various experimental conditions. Consequently, this reaction has been used for the synthesis of block polymers,24 dendrimers,25 functional polymers26 and bimolecules modified polymers.27 For example, Li et al. prepared telechelic and modular block copolymers from RAFT polymers by post-polymerization aminolysis and thiol-ene or Diels-Alder reactions.28, 29
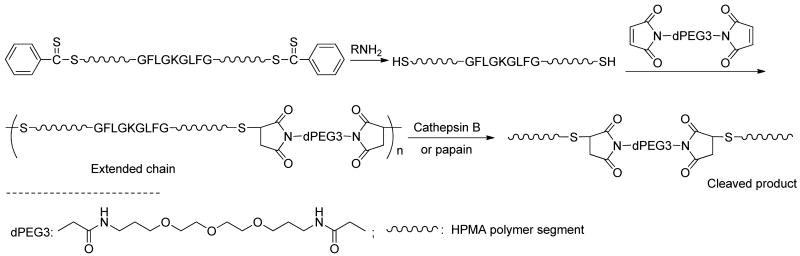
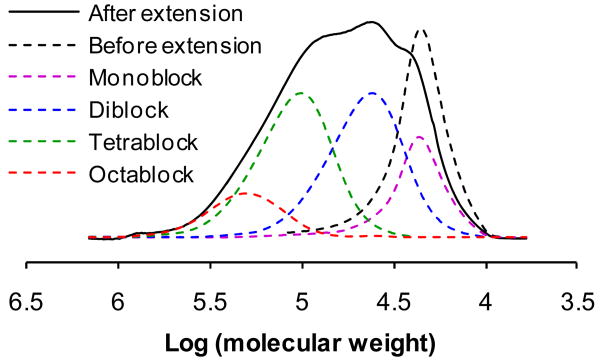
The thiol-ene chain extension of α,ω-dithiol-polyHPMA was evaluated. The dithiobenzoate groups of polyHPMA (Mw 30.3 kDa, PDI 1.08) were converted into thiol groups by aminolysis with n-butylamine in methanol under N2. The telechelic α,ω-dithiol-polyHPMA was chain extended by reaction with equimolar amount of bis-[1,13-(3-maleimidopropionyl)amido]-4,7,10-trioxatridecane (Bis-MAL-dPEG3) in methanol at room temperature for 24 h. The product was fractionated by SEC using preparative Sepharose 6 column and PBS as mobile phase (Scheme 2; Figure 3). Using the peptide2CTA and combination of RAFT polymerization with thiol-ene reaction, enzymatic degradable multiblock high molecular weight HPMA copolymer were prepared. However, the chain extension reaction needs further optimization. Optimization is under way, including aminolysis in presence of ene compounds, using protected thiol functionalized initiators, using templates for chain extension, etc.
Scheme 2.
Synthesis of multiblock HPMA polymer by reaction of telechelic α,ω-dithiol-polyHPMA with bismaleimide and degradation by incubation with cathepsin B or papain.
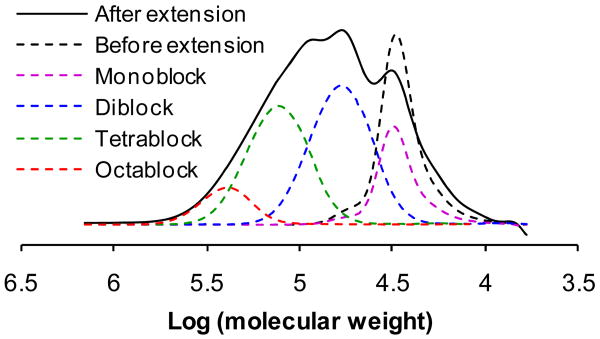
Figure 3.
FPLC profile of chain extended multiblock polyHPMA. The dashed traces are the FPLC profiles of fractions obtained by SEC using a Superose 6 HR16/60 column. The legend indicates the main component. The multiblock polyHPMA contains about 17% monoblock, 40% diblock, and 34% tetrablock and 9% octablock and other multiblock polymers.
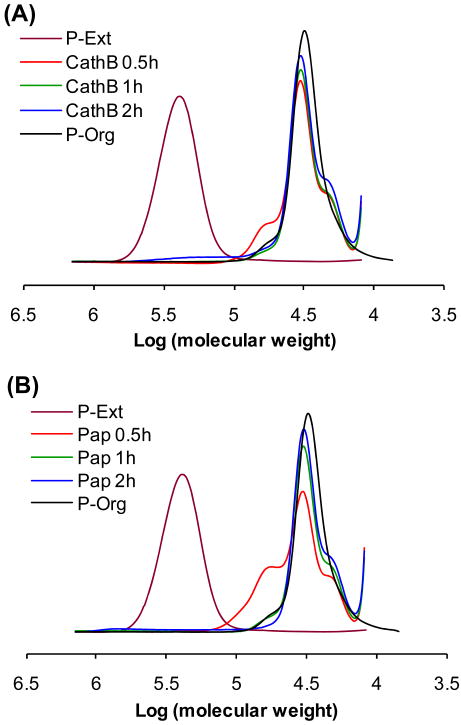
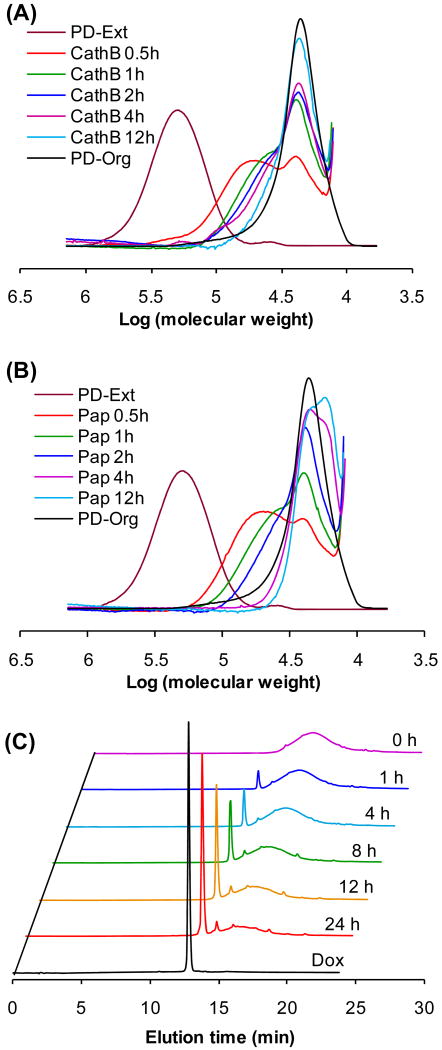
To prove the backbone biodegradability, the multiblock polyHPMA fraction (Mw = 260.8 kDa) was incubated with cathepsin B and papain. Indeed, within 2 h of incubation, the polymer degraded to short segments with molecular weight 30 kDa, similar to the initial telechelic α,ω-dithiol-polyHPMA (Scheme 2; Figure 4).
Figure 4.
FPLC profiles of initial telechelic α,ω-dithiol-polyHPMA (P-Org), extended multiblock polyHPMA fraction (P-Ext) and degradation products after incubation with (A) cathepsin B and (B) papain at different time intervals.
A similar approach was used for synthesizing a copolymer of HPMA and a drug containing comonomer, MA-GFLG-DOX. The HPMA copolymer-DOX conjugate was synthesized by RAFT copolymerization of HPMA and MA-GFLG-DOX (98/2 mol/mol) in methanol using Peptide2CTA as the chain transfer agent and AIBN as the initiator (Scheme 3). HPMA copolymer-DOX conjugate has a Mw 27.8 kDa and PDI 1.19; content of doxorubicin, measured by UV-vis spectrometry at 488 nm, in water using ε488 nm=11,000 M-1cm-1, was 7 wt%.
Scheme 3.
Synthesis of multiblock HPMA copolymer – DOX conjugate and its degradation by incubation with cathepsin B or papain.
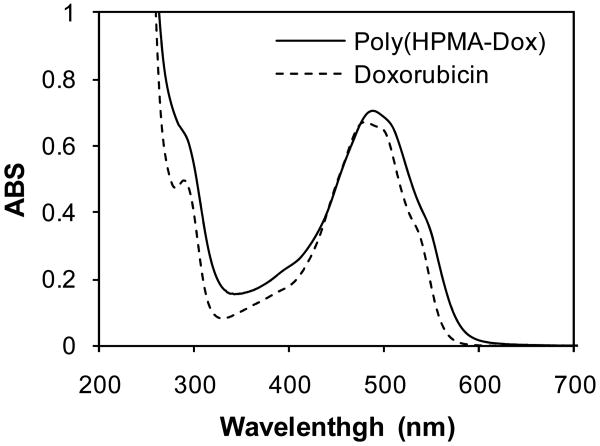
The dithiobenzoyl end groups were aminolyzed to thiol groups in 10 min. The short incubation time with n-butylamine was chosen to avoid structural changes to DOX at high pH. Compared with free DOX, the UV profile of α,ω-dithiol-HPMA copolymer–DOX conjugate after aminolysis (Figure 5) shows that no structural changes in DOX occurred.
Figure 5.
UV-vis spectra of α,ω-dithiol-HPMA copolymer–DOX conjugate at a concentration of 0.5 mg/mL and free doxorubicin.
The α,ω-dithiol-HPMA copolymer-DOX conjugate was extended by reaction with equimolar Bis-MAL-dPEG3 in methanol at room temperature for 24 h. The product was fractionated by SEC using preparative Sepharose 6 column and PBS as mobile phase similar to that of α,ω-dithiol-HPMA homopolymer (Figure 6).
Figure 6.
FPLC profile of chain extended multiblock HPMA copolymer-DOX conjugate. The dashed traces are the FPLC profiles of fractions obtained by SEC using a Superose 6 HR16/60 column. The legend indicates the main component. The multiblock copolymer contains about 17% monoblock, 36% diblock, and 36% tetrablock and 11 % octablock and other multiblock copolymers.
The backbone degradability and release of free doxorubicin were measured during incubation of multiblock HPMA copolymer-DOX conjugate (fraction of Mw 227.7 kDa) with cathepsin B and papain. The backbone degradation was monitored by SEC, and the release of DOX by HPLC. Most of the multiblock long chains were cleaved within 30 min; the SEC profiles of cleaved products, after 4 h of incubation, were similar to initial copolymer (Figure 7A, 7B). The amount of released DOX increased with time (Figure 7C, peak 12.8 min); concomitantly, the intensity of polymer peak (ca. 13-18 min) decreased. The identical elution time of the DOX standard with the peaks of released DOX indicates that aminolysis and chain extension reaction did not significantly affect the structure of DOX.
Figure 7.
FPLC profiles of initial telechelic HPMA copolymer-DOX conjugate (PD-Org), extended multiblock HPMA copolymer-DOX conjugate (PD-Ext), and degradation products after incubation with (A) cathepsin B and (B) papain for different time intervals. (C) HPLC profiles of multiblock HPMA copolymer-DOX conjugate following incubation with cathepsin B for different time intervals; bottom trace – DOX standard.
Conclusions
In conclusion, polyHPMA and HPMA copolymer-DOX conjugates have been synthesized by RAFT polymerization mediated by a new bifunctional CTA that contains an enzymatically degradable oligopeptide sequence and produces telechelic polymers. Post-polymerization aminolysis followed by chain extension with a bis-maleimide resulted in linear high molecular weight multiblock HPMA copolymers. These polymers are enzymatically degradable and the degradation products have molecular weight distributions below the renal threshold. The new multiblock HPMA copolymers hold potential as new carriers of anticancer drugs.
Supplementary Material
Acknowledgments
The research was supported in part by NIH grants GM69847, CA51578, and CA13283. We thank Michael Jacobsen for proofreading.
Footnotes
Supporting Information Available: MS, 1H-NMR, 13C-NMR and HPLC profiles for Peptide2CTA. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kopeček J. Polim Med. 1977;7:191–221. [PubMed] [Google Scholar]
- 2.Kopečková P, Kopeček J. Adv Drug Delivery Rev. 2010;62:122–149. doi: 10.1016/j.addr.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seymour WL, Duncan R, Strohalm J, Kopeček J. J Biomed Mater Res. 1987;21:1341–1358. doi: 10.1002/jbm.820211106. [DOI] [PubMed] [Google Scholar]
- 4.Lammers T. Adv Drug Delivery Rev. 2010;62:203–230. doi: 10.1016/j.addr.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Lammers T, Kühnlein R, Kissel M, Šubr V, Etrych T, Pola R, Pechar M, Ulbrich K, Storm G, Huber P, Peschke P. J Controlled Release. 2005;110:103–118. doi: 10.1016/j.jconrel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Duncan R, Vicent MJ. Adv Drug Delivery Rev. 2010;62:272–282. doi: 10.1016/j.addr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Maeda H. Bioconjugate Chem. 2010;21:797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 8.Shiah J, Dvořák M, Kopečková P, Sun Y, Peterson C, Kopeček J. Eur J Cancer. 2001;37:131–139. doi: 10.1016/s0959-8049(00)00374-9. [DOI] [PubMed] [Google Scholar]
- 9.Moad G, Rizzardo E, Thang SH. Acc Chem Res. 2008;41:1133–1142. doi: 10.1021/ar800075n. [DOI] [PubMed] [Google Scholar]
- 10.Lowe AB, McCormick CL. Prog Polym Sci. 2007;32:283–351. [Google Scholar]
- 11.Whittaker MR, Goh YK, Gemici H, Legge TM, Perrier S, Monteiro MJ. Macromolecules. 2006;39:9028–9034. [Google Scholar]
- 12.Patton DL, Mullings M, Fulghum T, Advincula RC. Macromolecules. 2005;38:8597–8602. [Google Scholar]
- 13.Qiu XP, Winnik FM. Macromol Rapid Commun. 2006;27:1648–1653. [Google Scholar]
- 14.Kopeček J, Bažilová H. Eur Polym J. 1973;9:7–14. [Google Scholar]
- 15.Mitsukami Y, Donovan MS, Lowe AB, McCormick CL. Macromolecules. 2001;34:2248–2256. [Google Scholar]
- 16.Ulbrich K, Šubr V, Strohalm J, Plocová D, Jelínková M, Říhová B. J Controlled Release. 2000;64:63–79. doi: 10.1016/s0168-3659(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 17.Dvořák M, Kopečková P, Kopeček J. J Controlled Release. 1999;60:321–332. doi: 10.1016/s0168-3659(99)00087-5. [DOI] [PubMed] [Google Scholar]
- 18.Ji J, Rosenzweig N, Griffin C, Rosenzweig Z. Anal Chem. 2000;72:3497–3503. doi: 10.1021/ac000080p. [DOI] [PubMed] [Google Scholar]
- 19.Rejmanová P, Pohl J, Baudyš M, Kostka V, Kopeček J. Makromol Chem. 1983;184:2009–2020. [Google Scholar]
- 20.Ulbrich K, Zacharieva EI, Obereigner B, Kopeček J. Biomaterials. 1980;1:199–204. doi: 10.1016/0142-9612(80)90017-4. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Jacobsen MT, Pan H, Kopeček J. Macromol Biosci. 2010;10:445–454. doi: 10.1002/mabi.200900295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golas PL, Tsarevsky NV, Sumerlin BS, Walker LM, Matyjaszewski K. Austr J Chem. 2007;60:400–404. [Google Scholar]
- 23.Hoyle CE, Lowe AB, Bowman CN. Chem Soc Rev. 2010;39:1355–1387. doi: 10.1039/b901979k. [DOI] [PubMed] [Google Scholar]
- 24.Boyer C, Liu J, Bulmus V, Davis TP. Australian J Chem. 2009;62:830–847. [Google Scholar]
- 25.Killops KL, Campos LM, Hawker CJ. J Am Chem Soc. 2008;130:5062–5064. doi: 10.1021/ja8006325. [DOI] [PubMed] [Google Scholar]
- 26.Boyer C, Granville A, Davis TP, Bulmus V. J Polym Sci Part A: Polym Chem. 2009;47:3773–3794. [Google Scholar]
- 27.Boyer C, Bulmus V, Davis TP. Macromol Rapid Comm. 2009;30:493–497. doi: 10.1002/marc.200800708. [DOI] [PubMed] [Google Scholar]
- 28.Li M, De P, Gondi SR, Sumerlin BS. J Polym Sci Part A: Polym Chem. 2008;46:5093–5100. [Google Scholar]
- 29.Li M, De P, Li H, Sumerlin BS. Polymer Chemistry. 2010;1:854–859. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.