Abstract
Aims
Left ventricular assist devices (LVADs) are increasingly used as therapeutic options for patients with advanced congestive heart failure (CHF), many of whom suffer from diabetes mellitus (DM). The aim of this study was to evaluate the effect of restoration of normal cardiac output using LVAD support on diabetes control in patients with advanced CHF.
Methods and results
A retrospective chart review of all clinic patients supported with long-term LVADs between July 2008 and July 2009 at Columbia University Medical Center was performed. Patients with DM diagnosed prior to device implantation were included in this analysis. Clinical and laboratory data within 1 month preceding and 6 months following LVAD implantation were collected. Of 43 LVAD patients followed in our clinic during the study period, 15 had a diagnosis of DM. Thirteen of the 15 patients were male, mean age was 63 ± 11 years, and the pre-LVAD left ventricular ejection fraction (LVEF) was 16.5 ± 5.7%. Fasting glucose levels, HbA1c, and daily insulin requirement within 1 month before and an average of 4.0 ± 2.3 months after LVAD placement were 157.7 ± 50.6 vs. 104.1 ± 21.4 mg/dL, 7.7 ± 0.9 vs. 6.0 ± 0.8.%, and 53.3 ± 51.7 vs. 24.2 ± 27.2 IU, respectively (P < 0.05 for all comparisons). Six of the 15 patients were completely free of antidiabetic medications and had blood glucose <126 mg/dL as well as HbA1c <6% after LVAD. Body mass index (BMI) was slightly increased after LVAD (28.7 ± 5.3 vs. 30.2 ± 4.1 kg/m2, P NS).
Conclusion
Restoration of normal cardiac output after LVAD implantation improves diabetic control in patients with advanced CHF. Additional studies are warranted to determine the mechanisms that worsen or possibly induce DM in patients with advanced CHF.
Keywords: Heart failure, LVAD, HbA1c, Insulin resistance
See page 133 for the editorial comment on this article (doi:10.1093/eurjhf/hfq235)
Introduction
The prevalence of insulin resistance (IR) manifest clinically as either metabolic syndrome or frank diabetes mellitus (DM) is increasing in the developed world.1,2 Congestive heart failure (CHF) will eventually affect one in five Americans and is already responsible for the consumption of an extraordinary proportion of health-care resources.3,4 A relationship between IR and CHF has long been recognized and is classically attributed to the higher prevalence of coronary artery disease and ischaemic cardiomyopathy in patients with DM. However, recent evidence suggests that, conversely, CHF itself may cause IR.5
Left ventricular assist devices (LVADs) are increasingly used for the treatment of advanced CHF.6 Left ventricular assist devices are implanted as a bridge to transplantation or for the purpose of destination therapy.7,8 The resulting restoration of normal cardiac output may improve end-organ function, and may favourably affect metabolic pathways in the myocardium and the periphery.9 Accordingly, we investigated the effect of LVAD implantation on diabetes control in patients with advanced CHF and DM.
Methods
Patient population
A retrospective chart review of all patients actively followed at the Columbia University Medical Center LVAD clinic between July 2008 and July 2009 was performed. Patients with a diagnosis of DM prior to LVAD implantation were identified. Patients who required rescue LVAD support not for chronic CHF but for acute cardiogenic shock were excluded from our analysis. Diabetes mellitus was defined as follows: (i) patients treated with oral antidiabetic medication; and/or (ii) patients treated with insulin; and/or (iii) patients with a former diagnosis of diabetes on the basis of a fasting glucose level >126 mg/dL in the setting of diabetic symptoms (i.e. polyuria, polyphagia, and polydypsia). Baseline clinical characteristics, medications, and LVAD data were collected from the electronic medical record, medical charts, and operative notes. Daily insulin dosage, fasting blood glucose level, and HbA1c level were recorded within 1 month prior to LVAD implantation and between 1 and 6 months following implantation.
Statistical analysis
Data were collected using Excel Software (2007 Microsoft Corporation). All data were analysed using the Statistical Program of Social Sciences (SPSS, version 17.0, SPSS, Inc., Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation and compared using the paired t-test. Categorical variables are presented as proportions.
Results
During the study period, 43 patients who had received LVAD support for chronic CHF were followed at the LVAD clinic. Fifteen patients were diagnosed with DM prior to LVAD implantation and included in this analysis. Baseline clinical characteristics and treatment are summarized in Tables 1 and 2, respectively. Thirteen patients were male and mean age was 63 ± 11 years. Heart failure aetiology was ischaemic in 8 of the 15 subjects. All patients had advanced NYHA functional class IIIb or class IV prior to implant. Left ventricular ejection fraction (LVEF) averaged 16 ± 6% and left ventricular end-diastolic diameter was 6.8 ± 1.1 cm. Moderate to severe right ventricular failure was present in 12 of the 15 patients on echocardiographic evaluation. Mitral regurgitation was moderate to severe in nine patients (Table 3).
Table 1.
Baseline characteristics n = 15
| Age | 63.0 ± 11.06 |
| Male sex | 13 (86.7%) |
| Weight (kg) | 87.9 ± 16.0 |
| Height (m) | 1.75 ± 0.1 |
| BMI (kg/m2) | 28.7 ± 5.3 |
| HF aetiology | |
| Ischemic cardiomyopathy | 8 (53.3%) |
| Non-ischaemic cardiomyopathy | 7 (46.7%) |
| LVAD | |
| Bridge to transplant | 9 (60.0%) |
| Destination therapy | 6 (40.0%) |
| LVAD type | |
| Pulsatile | 4 (26.7%) |
| Non-pulsatile | 9 (73.3%) |
BMI, body mass index; HF, heart failure; LVAD, left ventricular assist device.
Table 2.
Medication used before and after left ventricular assist device implantation
| Pre-implantation | Post-implantation | |
|---|---|---|
| Milrinone | 14 (93.3%) | 0 |
| ACE-I | 4 (26.7%) | 6 (40.0%) |
| ARB | 2 (13.3%) | 0 (0%) |
| β-Blocker | 12 (80.0%) | 12 (80.0%) |
| Diuretic | 15 (100%) | 12 (80.0%) |
| Aldosterone blocker | 8 (53.3%) | 5 (33.3%) |
| CCB | 0 | 5 (33.3%) |
| Statin | 7 (46.7%) | 7 (46.7%) |
| Nitrates | 2 (13.3%) | 0 |
| Anti arrhythmic | 7 (46.7%) | 7 (46.7%) |
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker.
Table 3.
Echocardiographic parameters before and after left ventricular assist device implantation
| Pre-implantation | Post-implantation | P-value | |
|---|---|---|---|
| LVEF | 16.5 ± 5.7 | 21.6 ± 2.2 | 0.062 |
| LVEDD | 6.8 ± 1.06 | 5.8 ± 1.1 | 0.001 |
| LVESD | 6.7 ± 1.5 | 5.0 ± 1.6 | 0.001 |
| MOD-SEVERE RV dysfunction | 11 (73.3%) | 12 (83.3%) | 0.6 |
| MOD-SEVERE MR | 9 (60.0%) | 1 (6.7%) | 0.001 |
LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; RV, right ventricular; MR, mitral regurgitation.
Prior to LVAD implantation, all but one patient (93%) received continuous intravenous inotropic support with milrinone (mean dose 0.29 µg/kg/min). All patients were treated with diuretics, 12 (80%) with β-blockers; 6 (40%) with ACE-inhibitors or ARBs, and 8 (53%) with aldosterone blockers. Nine patients (60%) received an LVAD as bridge to transplant, and 6 (40%) as destination therapy. Eleven patients were implanted with a non-pulsatile flow pump (i.e. Heart Mate II). The remaining four received a pulsatile pump (i.e. Heart Mate XVE) (Table 1).
All study subjects were diagnosed with DM prior to LVAD implantation. The mean time of DM diagnosis prior to LVAD implantation was 6.0 ± 5.9 years. Four patients had neuropathy, none had retinopathy. Assessment of diabetic nephropathy was limited by the absence of kidney biopsies and by inconsistent documentation of proteinuria. However, renal function significantly improved after implant (1.7 ± 0.5 vs. 1.3 ± 0.3, P < 0.05), suggesting that the degree of intrinsic renal disease was only mild.
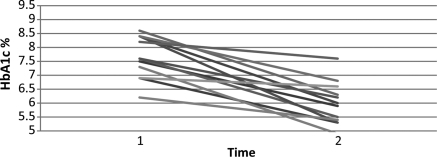
Three patients received both insulin and an oral antidiabetic agent, seven patients received insulin alone, three an oral antidiabetic agent alone, and two were on dietary control only. Mean HbA1c was 7.7 ± 0.9%, and the mean fasting glucose level was 158 ± 51 mg/dL despite a mean insulin dose of 55 IU/day (Table 4). Repeat measurement of these variables 4.0 ± 2.3 months after LVAD implantation revealed significantly improved diabetes control: HbA1c was 6.0 ± 0.9%, and fasting glucose was 104.1 mg/dL (Figure 1). These improvements were seen despite a substantially lower mean insulin requirement of 24 IU/day (Table 4). Six patients (all of whom had been on insulin and/or antidiabetic agents prior to implant) had normal glucose levels as well as HbA1c <6.0, although they were no longer receiving insulin or oral antidiabetic agents. Average BMI had slightly increased at the time of reassessment from 28.7 to 30.2 kg/m2, although this change did not reach statistical significance. Improvement in diabetes control was similar in patients who received a pulsatile vs. a continuous flow LVAD.
Table 4.
Metabolic status
| Pre-implantation | Post-implantation | P-value | |
|---|---|---|---|
| Fasting glucose (mg/dL) | 158 ± 51 | 104 ± 21 | <0.001 |
| HbA1c (%) | 7.7 ± 0.9 | 6.0 ± 0.8 | <0.001 |
| Number of patients with insulin | 10 | 9 | |
| Average insulin daily dose (IU/day) | 55 ± 52 | 24 ± 27 | 0.07 |
| Number of patients taking oral anti-glycaemics | 6 | 4 | |
| Weight (kg) | 88 ± 16 | 93 ± 12 | 0.056 |
| BMI (kg/m2) | 28.7 ± 5.3 | 30.2 ± 4.1 | 0.06 |
| Serum creatinine (mg/dL) | 1.7 ± 0.5 | 1.3 ± 0.3 | 0.034 |
BMI, body mass index.
Figure 1.
HbA1c before and after left ventricular assist device implantation.
Prior to LVAD implantation all patients were in NYHA class IIIb-IV. Three and six months after device implantation all patients were assessed as NYHA class II. Prior to device implantation only one patient, who was not receiving milrinone, completed the 6-min walk test. Six months after device implantation the mean walking distance was 331.8 ± 61.5 m.
Although there was no significant change in LVEF after device implantation, the ventricle was more decompressed and there was less mitral regurgitation (Table 3).
Pre-implant (14 of 15) patients were receiving intravenous milrinone, but none of the patients was on milrinone at follow-up. There was no significant difference in other heart failure medications (Table 2).
Discussion
We examined the effect of LVAD implantation on diabetes control by measuring fasting glucose, HbA1c, and insulin requirements in 15 patients with DM. Left ventricular assist device implantation resulted in a significant reduction in insulin requirements and HbA1c, whereas body weight concurrently increased. The present data provide the first direct evidence that restoration of normal cardiac output with an LVAD improves diabetic control in patients with advanced CHF.
The association of CHF and DM is well established.10 Prior studies have not only demonstrated that IR may lead to CHF,10–12 but also that CHF itself can cause IR and that the severity of IR has an adverse prognostic impact.13 Nikolaidis et al.5 described the progressive development of IR as a function of disease severity in a dog model of systolic CHF. In their study, heart failure was induced in healthy dogs and led to the development of IR. Swan et al.14 demonstrated that IR, characterized by both fasting and stimulated hyperinsulinaemia, is frequent in CHF patients whether or not concomitant DM is present. More recently, Azadjai et al.15 showed that IR is highly prevalent among non-diabetic CHF patients and is associated with decreased exercise capacity. Conceptually in line with these experiments, we demonstrate for the first time that improvement of CHF after LVAD implantation is associated with an improvement in diabetes control. It is of particular note that a subset of patients classified as diabetic prior to implant and with suboptimal diabetic control despite pharmacotherapy had normal glucose and HbA1c values only a few months after implant and without pharmacotherapy. This finding, if validated in a larger cohort and accounting for dietary intake, would strongly underscore the existence of a unique diabetes phenotype induced by cardiovascular decompensation and likely completely reversible. Which mechanisms are responsible for this reversal is currently unclear, although it is likely that improved cardiac output resulting in increased blood flow to peripheral and cardiac muscle16 as well as improved physical activity plays a significant role.
Physical activity typically improves after LVAD implantation.6,7 The resultant reduction in body weight may, in turn, favourably impact IR. In our cohort, although there was a clear improvement in physical activity, there was no reduction in patient's weights between baseline and follow-up. Rather, there was a consistent trend towards weight gain. Thus, the improved IR in our patients cannot be ascribed to weight loss due to increased physical activity. Prospective studies that measure physical activity and, more importantly, body composition to assess changes in fat vs. muscle mass after LVAD implantation are needed to mechanistically address the individual contributions of these factors to the observed changes in IR.
While our retrospective data clearly provide proof of concept for decreased IR and possibly its reversal in CHF, it does not provide insights into other candidate mechanisms likely contributing to our observation, such as changes in neurohormonal activation and inflammation.
Neurohormonal activation
Peripheral vasoconstriction resulting in decreased tissue perfusion is a hallmark of the syndrome CHF and is mediated by increased levels of circulating neurohormones, such as noradrenaline, endothelin, and angiotensin II. Several studies have demonstrated that neurohormonal suppression with ACE-Is or ARBs reduces the risk of developing DM by more than 25% in CHF patients.17 Although ACE-I, ARB, and β-blocker use was similar before and after LVAD implantation and plasma neurohormones were not measured, it is conceivable that restoration of normal cardiac output after LVAD implantation had a favourable impact on neurohormonal activity in our patient cohort and thereby improved IR. The same may hold true for other mechanical interventions that increase cardiac output and reduce neurohormonal activation, such as cardiac resynchronization therapy.18
Inflammation
Symptomatic CHF is associated with an elevation of pro-oxidant and pro-inflammatory circulating cytokines, such as tumour necrosis factor-α (TNF-α), interleukin-1β, and interleukin-6 (IL-6).19,20 Circulating levels of IL-6 have been correlated with adiposity and type 2 DM21–23 as well as with the induction of IR in hepatic cells.24 Tumour necrosis factor-α, a major marker of inflammation in CHF can also cause IR.25,26 Unfortunately, plasma cytokines were not measured before or after LVAD implantation in our study.
Lastly, although we consider mechanically induced reversal of the CHF syndrome with an LVAD the principal intervention causing the observed improvement of diabetic control, it is of note that 14 of our 15 patients were receiving intravenous milrinone at baseline, but not at follow-up. Milrinone has been shown to increase IR in various animal models.27,28 Although there is no data in human CHF, it remains conceivable that milrinone may either induce IR through a direct pharmacological effect or alternatively may improve IR through indirect effects, such as an improvement in tissue perfusion and congestion. Either way, the discontinuation of milrinone in all but one patient receiving LVADs in this study must be taken into consideration when interpreting our data.
In conclusion, restoring cardiac output and decreasing CHF severity with LVAD treatment is associated with a marked improvement of glucose control in diabetic patients with advanced CHF. Little attention has been paid to date to the metabolic effects of LVAD implantation, although they likely play an important role in at least two major issues of LVAD therapy: (i) Infections, as they are more frequent in patients with poorly controlled DM29 and (ii) LV recovery, as it may be hampered by abnormal fuel metabolism in the setting of IR. Our study is clearly limited by its retrospective nature, the absence of serial measures of neurohormonal or inflammatory activation, as well as by the lack of a control group. Nevertheless, the described improvements in diabetes control occurred in each and every one of the 15 patients, clearly suggesting that reversal of CHF by restoring cardiac output with an LVAD may be sufficient to antagonize CHF-induced IR. Prospective mechanistic studies that investigate the cardiac and systemic effects of LVAD support on glucose metabolism and on the development or progression of diabetic macro- and micro-vascular complications in CHF patients are needed. Such studies may significantly improve our understanding not only of LVAD therapy, but also of CHF and DM in general.
Funding
This study was supported by NIH grant R01 HL 45095 (Dr Ulrich Jorde).
Conflict of interest: none declared.
References
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. doi:10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Ioannou GN, Bryson CL, Boyko EJ. Prevalence and trends of insulin resistance, impaired fasting glucose, and diabetes. J Diabetes Complications. 2007;21:363–370. doi: 10.1016/j.jdiacomp.2006.07.005. doi:10.1016/j.jdiacomp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 3.From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, Rodeheffer RJ, Roger VL. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119:591–599. doi: 10.1016/j.amjmed.2006.05.024. doi:10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. doi:10.1161/01.CIR.0000039105.49749.6F. [DOI] [PubMed] [Google Scholar]
- 5.Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovas Res. 2004;61:297–306. doi: 10.1016/j.cardiores.2003.11.027. doi:10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. doi:10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 7.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. doi:10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 8.Kirklin JK, Holman WL. Mechanical circulatory support therapy as a bridge to transplant or recovery (new advances) Curr Opin Cardiol. 2006;21:120–126. doi: 10.1097/01.hco.0000210308.64360.8d. doi:10.1097/01.hco.0000210308.64360.8d. [DOI] [PubMed] [Google Scholar]
- 9.Razeghi P, Sharma S, Ying J, Li YP, Stepkowski S, Reid MB, Taegtmeyer H. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation. 2003;108:2536–2541. doi: 10.1161/01.CIR.0000096481.45105.13. doi:10.1161/01.CIR.0000096481.45105.13. [DOI] [PubMed] [Google Scholar]
- 10.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. doi:10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnlov J, Lind L, Zethelius B, Andren B, Hales CN, Vessby B, Lithell H. Several factors associated with the insulin resistance syndrome are predictors of left ventricular systolic dysfunction in a male population after 20 years of follow-up. Am Heart J. 2001;142:720–724. doi: 10.1067/mhj.2001.116957. doi:10.1067/mhj.2001.116957. [DOI] [PubMed] [Google Scholar]
- 12.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–341. doi: 10.1001/jama.294.3.334. doi:10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 13.Doehner W, Rauchhaus M, Ponikowski P, Godsland IF, von Haehling S, Okonko DO, Leyva F, Proudler AJ, Coats AJ, Anker SD. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J Am Coll Cardiol. 2005;46:1019–1026. doi: 10.1016/j.jacc.2005.02.093. doi:10.1016/j.jacc.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 14.Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, Stevenson JC, Coats AJ. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30:527–532. doi: 10.1016/s0735-1097(97)00185-x. doi:10.1016/S0735-1097(97)00185-X. [DOI] [PubMed] [Google Scholar]
- 15.AlZadjali MA, Godfrey V, Khan F, Choy A, Doney AS, Wong AK, Petrie JR, Struthers AD, Lang CC. Insulin resistance is highly prevalent and is associated with reduced exercise tolerance in nondiabetic patients with heart failure. J Am Coll Cardiol. 2009;53:747–753. doi: 10.1016/j.jacc.2008.08.081. doi:10.1016/j.jacc.2008.08.081. [DOI] [PubMed] [Google Scholar]
- 16.Tuzun E, Narin C, Gregoric ID, Cohn WE, Frazier OH. Ventricular assist device outflow-graft site: effect on myocardial blood flow. J Surg Res. Published online ahead of print 7 April 2010. [DOI] [PubMed]
- 17.Andraws R, Brown DL. Effect of inhibition of the renin-angiotensin system on development of type 2 diabetes mellitus (meta-analysis of randomized trials) Am J Cardiol. 2007;99:1006–1012. doi: 10.1016/j.amjcard.2006.10.068. doi:10.1016/j.amjcard.2006.10.068. [DOI] [PubMed] [Google Scholar]
- 18.Fantoni C, Regoli F, Ghanem A, Raffa S, Klersy C, Sorgente A, Faletra F, Baravelli M, Inglese L, Salerno-Uriarte JA, Klein HU, Moccetti T, Auricchio A. Long-term outcome in diabetic heart failure patients treated with cardiac resynchronization therapy. Eur J Heart Fail. 2008;10:298–307. doi: 10.1016/j.ejheart.2008.01.006. doi:10.1016/j.ejheart.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Bozkurt B, Kribbs SB, Clubb FJ, Jr, Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–1391. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 20.Yang LL, Gros R, Kabir MG, Sadi A, Gotlieb AI, Husain M, Stewart DJ. Conditional cardiac overexpression of endothelin-1 induces inflammation and dilated cardiomyopathy in mice. Circulation. 2004;109:255–261. doi: 10.1161/01.CIR.0000105701.98663.D4. doi:10.1161/01.CIR.0000105701.98663.D4. [DOI] [PubMed] [Google Scholar]
- 21.Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87:2084–2089. doi: 10.1210/jcem.87.5.8450. doi:10.1210/jc.87.5.2084. [DOI] [PubMed] [Google Scholar]
- 22.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 23.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. doi:10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 24.Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003;278:13740–13746. doi: 10.1074/jbc.M210689200. doi:10.1074/jbc.M210689200. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–625. doi: 10.1046/j.1365-2796.1999.00490.x. doi:10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil GS. Mechanisms of TNF-alpha-induced insulin resistance. Exp Clin Endocrinol Diabetes. 1999;107:119–125. doi: 10.1055/s-0029-1212086. doi:10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 27.Cheung P, Yang G, Boden G. Milrinone, a selective phosphodiesterase 3 inhibitor, stimulates lipolysis, endogenous glucose production, and insulin secretion. Metabolism. 2003;52:1496–1500. doi: 10.1016/s0026-0495(03)00271-3. doi:10.1016/S0026-0495(03)00271-3. [DOI] [PubMed] [Google Scholar]
- 28.Degerman E, Manganiello V, Holst JJ, Ahren B. Milrinone efficiently potentiates insulin secretion induced by orally but not intravenously administered glucose in C57BL6J mice. Eur J Pharmacol. 2004;498:319–323. doi: 10.1016/j.ejphar.2004.07.096. doi:10.1016/j.ejphar.2004.07.096. [DOI] [PubMed] [Google Scholar]
- 29.Carson JL, Scholz PM, Chen AY, Peterson ED, Gold J, Schneider SH. Diabetes mellitus increases short-term mortality and morbidity in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol. 2002;40:418–423. doi: 10.1016/s0735-1097(02)01969-1. doi:10.1016/S0735-1097(02)01969-1. [DOI] [PubMed] [Google Scholar]