Abstract
Over many millions of years of independent evolution, placental, marsupial and monotreme mammals have diverged conspicuously in physiology, life history and reproductive ecology. The differences in life histories are particularly striking. Compared with placentals, marsupials exhibit shorter pregnancy, smaller size of offspring at birth and longer period of lactation in the pouch. Monotremes also exhibit short pregnancy, but incubate embryos in eggs, followed by a long period of post-hatching lactation. Using a large sample of mammalian species, we show that, remarkably, despite their very different life histories, the scaling of production rates is statistically indistinguishable across mammalian lineages. Apparently all mammals are subject to the same fundamental metabolic constraints on productivity, because they share similar body designs, vascular systems and costs of producing new tissue.
Keywords: production, life history, metabolic ecology, placental, marsupial, monotreme
1. Introduction
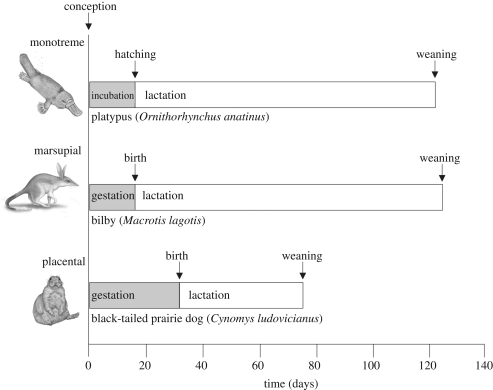
The three lineages of mammals, placentals (eutherians), marsupials (metatherians) and monotremes (prototherians), diverged more than 100 Ma ago [1–3]. They diversified rapidly on different landmasses as the supercontinent Pangaea fragmented and drifted apart [4–9]. As a consequence of their independent evolution, the extant members of these lineages differ conspicuously in physiology, life history and reproductive ecology [10–12]. Metabolic rates of marsupials and monotremes are about 33 and 50 per cent lower, respectively, than placentals [13], and the differences in life history are dramatic (figure 1). Monotremes lay eggs; the stored energy reserves support embryonic development, and then the females supply milk to fuel growth from hatching to independence. Marsupials have a very short period of intrauterine embryonic development, are born at a small size and undeveloped state and then are protected and lactated in a pouch for a long period before they become independent [14]. By contrast, placentals have a more prolonged period of intrauterine embryonic development, are born at larger size and are nourished by lactation until they become independent.
Figure 1.
Schematic of differences in timing of the life history in similar-sized monotremes, marsupials and placentals. Female body mass ca 1500 g.
A common feature of these seemingly divergent life histories is that the mother supplies all of the nutrition to rear offspring to independence [15]. The metabolic rate of the mother fuels the production of biomass, and this limits the ways in which life-history traits can trade off to meet energy and material requirements of offspring at different stages of development. Because the metabolic rate of the mother is highly constrained by body size (cf. [16–20]), many life-history traits correlate closely with adult body size, scaling allometrically following the form
| 1.1 |
where R is the trait of interest, R1 is a taxon- and mass-specific normalization constant, M is adult body size and β is a scaling exponent. Metabolic scaling theory predicts β ≃ 3/4 for whole-organism metabolic rates, β ≃ 1/4 for biological times such as duration of pregnancy and lactation and lifespan and β ≃ −1/4 for most biological rates such as ontogenetic and population growth rates and the rate of offspring production [16].
Mechanistically, rate of offspring production depends on the rate at which biomass can be produced by the mother. Productivity is constrained by the logistical problems of transporting key materials such as oxygen and nutrients around the body [21]. Because all mammals have similar body plans and energetic costs of producing biomass [22], we expect them to be subject to similar constraints on production. Mass-specific production rate, p, can be estimated as the mean mass of offspring produced per year, normalized by adult body size (or the annual amount of biomass produced per unit of adult body size), so
| 1.2 |
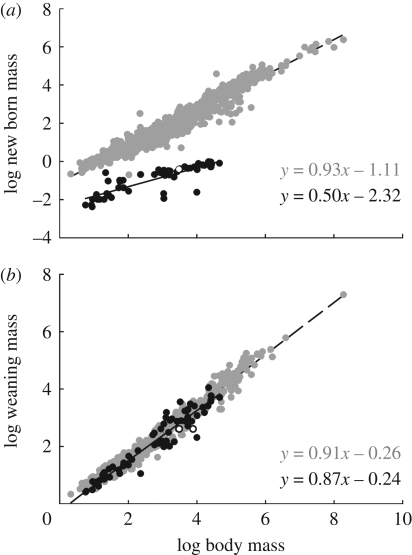
where m is adult body mass, mb is mass at birth, l is litter size and n is the number of litters per year [23]. In placental mammals, mass-specific production rate scales with an exponent between −1/4 and −1/3, similar to mass-specific metabolic rate [23]. There is considerable variation in production rate across different functional and taxonomic groups of eutherian mammals, but most of this variation is in the normalization constant, R1; the exponents are relatively constant across these groups because production rates are highly constrained by body size according to equation (1.1) [23,24]. However, because placentals, marsupials and monotremes give birth to offspring of very different sizes and developmental stages, a better and more standardized measure of production substitutes mass at weaning, mw, the stage at which mothers cease direct investment of energy to offspring growth, for mass at birth. Indeed, figure 2a shows that while size of offspring at birth scales predictably with adult body size in all lineages, newborn placentals are both absolutely larger and scale more steeply with adult body size than marsupials or monotremes. However, figure 2b shows that weaning mass is remarkably predictable across all lineages of mammals. So, mass-specific production measured at weaning is
| 1.3 |
Figure 2.
Bivariate log–log plots of offspring body mass (a) at birth and (b) newly weaned as a function of adult mass in grams. All logs are log-base 10, all mass units are grams and the figure legend is the same for all figures. Filled grey circles, placental; dashed lines, placental OLS; filled black circles, marsupial; solid lines, marsupial OLS; open circles, monotreme.
We address the effects of pre-weaning mortality on this measure of mass-specific production later in the discussion. Because all mammals have similar body plans, vascular systems and costs of producing new tissue, we hypothesize that all mammals should produce biomass at an equivalent mass-specific rate despite the differences between lineages in the size and timing of the life-history stages.
2. Methods
To evaluate these predictions and explore variation in allocation to reproduction across placentals, marsupials and monotremes, we compiled a life-history database for more than 4000 non-volant mammalian species from both primary and secondary published sources (for more details, see electronic supplementary material, table S1). For each species, we recorded neonate mass (g), mass at weaning (g), adult body size (g), gestation time (months), time to weaning (months), age at first reproduction (months), maximum lifespan (months), litter size and litter frequency (per year), where data were available. For the placentals and marsupials, we used ordinary least squares (OLS) regression, fitted to bivariate log–log plots, to quantify allometric scaling of these life-history traits in terms of equation (1.1). For the mass-specific production analyses, we first used OLS regressions to estimate slopes within lineages, and then we used a general linear model (GLM) to test for significant differences between the lineages. For the GLMs between lineages, we controlled for taxonomy by nesting order within lineage because body size is confounded by phylogeny. Within the placental lineage, there are 20 orders represented in our dataset, and in the marsupial lineage, there are 7 orders represented. There were only three monotreme species, and so we show their data in figures, but do not include them in statistical analyses. All statistical analyses were performed in Minitab 15.0.
3. Results
(a). Life-history times
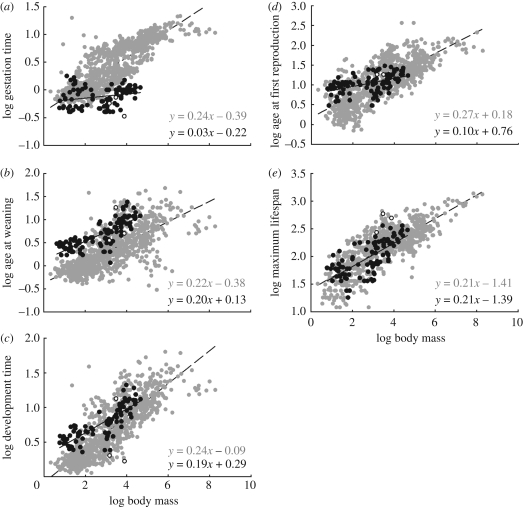
Figure 3a–e shows variation in life-history times as a function of body mass, all plotted on logarithmic axes. Comparing marsupials and placentals: (i) gestation time is short and nearly invariant in marsupials, whereas it is substantially longer and increases significantly with body size in placentals, (ii) time to weaning is substantially longer in marsupials than in placentals, but scales similarly with size, (iii) total development time (the sum of gestation time and time to weaning) is longer in marsupials than in placentals, (iv) age at first reproduction is widely variable and overlaps greatly, but tends to be greater in small marsupials and to scale less steeply with body size than in placentals, and (v) maximum lifespan is similar across lineages and scales very similarly with mass (see table 1 for statistical results). Slopes generally are close to the predicted 1/4, except for gestation time and age of first reproduction in marsupials, which are shallower.
Figure 3.
Bivariate log–log plots of life-history timings in months as a function of adult body mass: (a) gestation time, (b) age at weaning; (c) development time; (d) age at first reproduction; (e) maximum lifespan.
Table 1.
Results of regressions of life-history variables and adult body mass.
| trait | lineage | slope | s.e. | intercept | s.e. | d.f. | r2 | p |
|---|---|---|---|---|---|---|---|---|
| newborn mass | placentals | 0.93 | 0.01 | −1.11 | 0.03 | 888 | 0.95 | 0.00 |
| marsupials | 0.50 | 0.04 | −2.32 | 0.14 | 54 | 0.70 | 0.00 | |
| weaning mass | placentals | 0.91 | 0.01 | −0.26 | 0.03 | 391 | 0.97 | 0.00 |
| marsupials | 0.87 | 0.03 | −0.24 | 0.10 | 64 | 0.92 | 0.00 | |
| gestation time | placentals | 0.24 | 0.00 | −0.39 | 0.02 | 1098 | 0.73 | 0.00 |
| marsupials | 0.04 | 0.02 | −0.22 | 0.05 | 94 | 0.06 | 0.02 | |
| age at weaning | placentals | 0.22 | 0.01 | −0.38 | 0.02 | 925 | 0.52 | 0.00 |
| marsupials | 0.20 | 0.01 | 0.13 | 0.04 | 109 | 0.63 | 0.00 | |
| development | placentals | 0.24 | 0.01 | −0.08 | 0.02 | 853 | 0.70 | 0.00 |
| time | marsupials | 0.17 | 0.01 | 0.29 | 0.04 | 83 | 0.66 | 0.00 |
| age at first | placentals | 0.27 | 0.01 | 0.18 | 0.03 | 886 | 0.57 | 0.00 |
| reproduction | marsupials | 0.10 | 0.02 | 0.76 | 0.06 | 101 | 0.22 | 0.00 |
| max. lifespan | placentals | 0.21 | 0.01 | 1.41 | 0.02 | 737 | 0.65 | 0.00 |
| marsupials | 0.21 | 0.02 | 1.39 | 0.07 | 93 | 0.51 | 0.00 | |
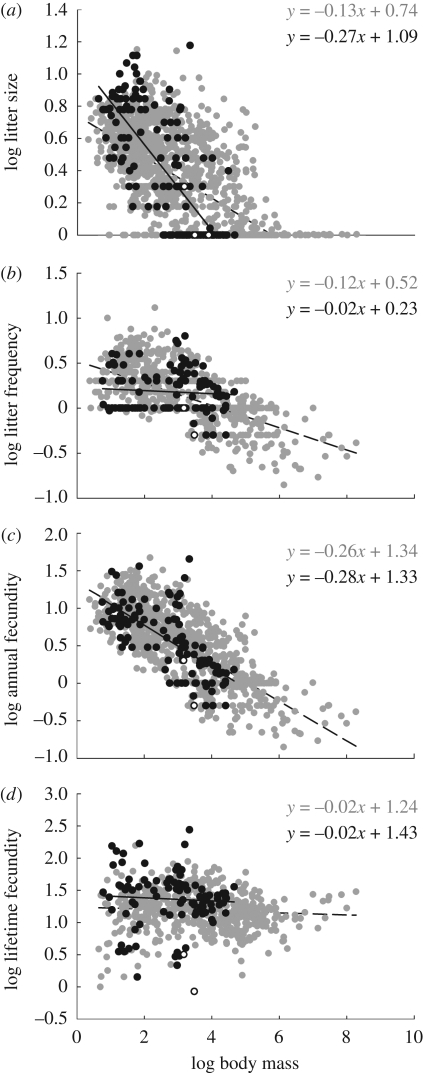
| litter size | placentals | −0.13 | 0.00 | 0.74 | 0.01 | 1432 | 0.41 | 0.00 |
| marsupials | −0.27 | 0.02 | 1.09 | 0.05 | 167 | 0.57 | 0.00 | |
| litters per year | placentals | −0.12 | 0.01 | 0.52 | 0.02 | 869 | 0.39 | 0.00 |
| marsupials | −0.02 | 0.02 | 0.23 | 0.06 | 111 | 0.01 | 0.40 | |
| annual | placentals | −0.26 | 0.01 | 1.34 | 0.03 | 847 | 0.59 | 0.00 |
| fecundity | marsupials | −0.28 | 0.03 | 1.33 | 0.08 | 110 | 0.47 | 0.00 |
| lifetime | placentals | −0.02 | 0.01 | 1.24 | 0.03 | 530 | 0.01 | 0.10 |
| fecundity | marsupials | −0.02 | 0.04 | 1.43 | 0.13 | 93 | 0.00 | 0.57 |
(b). Fecundity
Figure 4a,b shows variation in the different components of fecundity. Litter size (figure 4a) is highly variable and scales more steeply in marsupials than in placentals, and the number of litters per year (figure 4b) overlaps greatly between marsupials and placentals of small body size, but is approximately invariant with size in marsupials and scales negatively in placentals. We combine litter size and the number of litters per year to estimate annual fertility, FY, by rearranging equation (1.2) so that FY = l · n = p · (m/mw). Figure 4c shows that for both placentals and marsupials, annual fecundity scales similarly, and close to −1/4 power. So, despite the very different allocations to litter size and frequency, the number of offspring produced per year is identical across lineages, but the relationship is not tight in either group. Further, lifetime fertility, FL, is annual fertility × adult lifespan, a, or FL = a · p · (m/mw) (where adult lifespan = maximum lifespan − age at sexual maturity). Figure 4d shows that lifetime fertility is statistically invariant with adult body size across the lineages, consistent with Charnov et al. [25]. So, even though the allocations to the components of fertility differ conspicuously between the lineages in terms of litter size and frequency, the overall scaling of both annual fecundity and lifetime fertility is predictable though with limited precision from adult body size.
Figure 4.
Bivariate log–log plots of components of fecundity as a function of adult body mass: (a) litter size, (b) litter frequency, (c) annual fecundity, and (d) lifetime fecundity.
For all variables, values for monotremes tend to overlap those of similar-sized marsupials.
(c). Mass-specific production
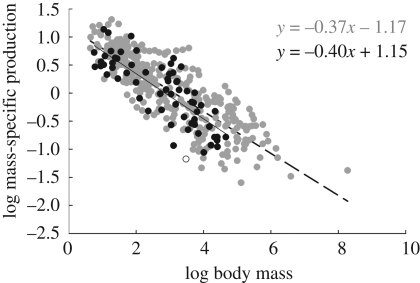
Mass-specific production rate, calculated using equation (1.3), is very similar between marsupials and placentals. This is evidenced both by the extensive overlap in values for the two groups in figure 5 and by the parameters of the fitted regression models (table 2): marsupials β = −0.40 ± 0.08 (95% CI) and placentals β = −0.37 ± 0.02. A GLM testing for differences between the slopes and intercepts, while controlling for lineage and order, shows that the groups collapse onto a single scaling function, where β = −0.37 ± 0.04. To control for the effects of phylogeny at a finer grained taxonomic level, we also ran a model that nested families within order, but the slopes and intercepts were not significantly different from the model controlling solely for order.
Figure 5.
Bivariate log–log plot of mass-specific production as a function of adult body mass. A GLM testing for differences in scaling among lineages finds no significant difference, and gives an overall slope of −0.37. See table 2 for further details.
Table 2.
Results of regressions of mass-specific production and adult body mass. The ‘combined’ rows report the result of GLMs testing for the significance between intercepts and slopes between the lineages. In both models, neither the intercepts nor the slopes were significantly different between the lineages.
| trait | lineage | slope | s.e. | intercept | s.e. | d.f. | r2 | p |
|---|---|---|---|---|---|---|---|---|
| mass-specific | placentals | −0.37 | 0.01 | 1.17 | 0.05 | 315 | 0.69 | 0.00 |
| production | marsupials | −0.40 | 0.04 | 1.15 | 0.11 | 62 | 0.66 | 0.00 |
| combined | −0.37 | 0.02 | 1.16 | 0.08 | 378 | 0.79 | 0.00 | |
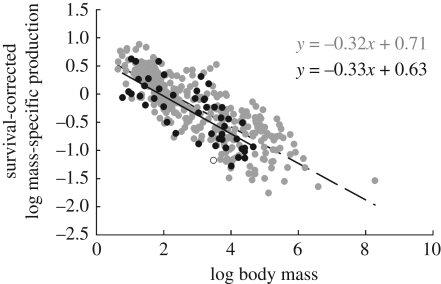
| corrected | placentals | −0.32 | 0.01 | 0.71 | 0.04 | 315 | 0.67 | 0.00 |
| mass-specific | marsupials | −0.33 | 0.04 | 0.63 | 0.13 | 46 | 0.59 | 0.00 |
| production | combined | −0.33 | 0.04 | 0.74 | 0.11 | 362 | 0.76 | 0.00 |
4. Discussion and conclusions
Our data analysis not only quantifies well-known differences in life histories among the lineages but also offers new insights into the variation in life-history strategies within and among these groups. The differences in absolute values and allometric scalings of the various life-history traits among placentals, marsupials and monotremes reflect different ways that these three lineages have adapted to allocate time and energy to growth and development in order to maximize reproductive output. These differences reflect a complex combination of phylogenetic constraints owing to ancient divergence and selection owing to environmental conditions during subsequent evolution. Despite these differences in reproductive strategies, however, female placentals, marsupials and monotremes of the same body size all allocate energy to reproduction at essentially the same rate.
An important life-history parameter not considered in equation (1.3) is pre-weaning survival, which would adjust production values by accounting for offspring that received energy investment from the mother, but died before weaning [25]. However, accurate measures of pre-weaning survival are extremely rare, especially in wild populations. To estimate the effect of pre-weaning survival on mass-specific production, we used a method similar to Charnov et al. [25]. Using a small sample of placental mammals (n = 13), Charnov et al. [25] show that pre-weaning survival rates scale with litter size as sw = 0.7l−0.35 (r2 = 0.69, p < 0.01). We collected available data on marsupial pre-weaning survival (n = 9), and found that pre-weaning survival is approximately invariant with litter size as sw = 0.6l0.03 (r2 = 0.02, p > 0.05), but scales positively with body size, sw = 0.2 m0.1 (r2 = 0.32, p < 0.05) (see appendix A for table of data and sources). The differences in the independent variables in offspring survival here probably follow from the differences in reproductive strategies between placentals and marsupials. For example, pouch-rearing in marsupials probably decreases litter size but increases pre-weaning survival as pre-weaned offspring effectively take on the mothers' mortality, whereas in placentals, pre-weaned offspring are exposed to many additional sources of mortality, resulting in lower survival and so larger litter sizes. Therefore, we estimated pre-weaning survival rates from adult body size data for marsupials and from litter size data for placentals. For each species, we calculated the survival-corrected mass-specific production, p · sw, and plotted this as a function of log body size (figure 6). A GLM showed no significant difference between marsupial and placental production rates, and resulted in an overall scaling slope of −0.33 ± 0.07, which is not significantly different from the uncorrected analyses. Thus, despite the different effects of pre-weaning survival on the ecologies of marsupials and placentals, its effect on production is consistent across lineages, reducing the overall scaling exponent slightly from between −0.37 and −0.40 to −0.33.
Figure 6.
Bivariate log–log plot of survival-corrected mass-specific production as a function of adult body mass. A GLM testing for differences in scaling among lineages finds no significant difference, and gives an overall slope of −0.33, which is not significantly different from the uncorrected result in figure 5. See table 2 for further details.
The relatively tight clustering of data about the regression lines in figures 5 and 6 is predicted by metabolic scaling theory on the grounds that all types of mammal are subject to essentially the same constraint on production rate. However, scaling theory would predict a slope of −1/4, whereas the observed slope is closer to −1/3. This discrepancy occurs because weaning mass is not a constant function of adult mass, but scales sublinearly with an exponent of −0.87 for marsupials and −0.91 for placentals (figure 2b). Why does relative weaning mass scale negatively? We offer three hypotheses for future consideration: (i) Larger mammals differentially allocate resources to adult survivorship at the expense of weaning mass. This should be particularly advantageous in seasonal environments, where it helps adults to survive the winter. (ii) Seasonality should place additional demands on small mammals that breed more than once a year. By producing relatively large offspring, mothers increase the chance their offspring survive a stressful period. (iii) Capital breeding (i.e. fuelling reproduction from stored reserves) is increasingly common in larger mammals. Fitness tradeoffs associated with acquisition, storage and mobilization of stored reserves may favour reducing size of offspring at weaning in larger mammals.
The interactions and replacements that occurred when the previously isolated lineages came into contact, such as occurred when the Panamanian land bridge allowed interchange between North and South America approximately 3 Ma ago or when modern humans introduced placental mammals into Australia, have often been attributed to direct superiority of one phylogenetic lineage over another [26,27]. The fact that all lineages produce biomass at equivalent rates suggests that this interpretation may be too simplistic. Instead, the different reproductive physiologies and ecologies of marsupials and placentals may reflect adaptations to different environments. For example, pouch-rearing may be particularly beneficial in the seasonal, arid and generally nutrient-poor environments of much of Australia [28,30].
Despite many millions of years of independent evolution and widely differing allocations to the components of growth and reproduction, some remarkable convergences across the lineages emphasize the central role of metabolic scaling in mammalian life-history evolution. For example, while marsupial (and monotreme) gestation and weaning times differ considerably from placentals (figure 3a,b), the size of offspring at weaning is strikingly consistent across lineages (figure 2b), suggesting that while the underlying allocations of energy to the components of pre-weaning development may be free to tradeoff in various ways, the resulting mass at weaning is under strong stabilizing selection. Similarly, despite slower development times, and ages at first reproduction in marsupials and monotremes than in placentals, the maximum lifespan of all mammals scales similarly with body size. The components of fertility also differ conspicuously among the lineages. In marsupials, litter size is approximately invariant with adult body size, and the number of litters per year decreases as approximately the −1/4 power of adult body size, whereas in placentals, both litter size and frequency decrease at about −1/8 power of body size (figure 4a,b), with the result that annual fecundity scales similarly across the lineages, and lifetime fertility is invariant (figure 4c,d).
The strikingly similar scaling of production in monotremes, marsupials and placentals suggests that the evolutionary pathways that led to the seemingly divergent mammalian physiologies, reproductive ecologies and life histories are metabolically equivalent ways of being a mammal.
Acknowledgements
The authors thank Eric Charnov, Felisa Smith, Stephen Dobson, Oskar Burger members of the Integrative Macroecological Patterns and Process across Scales (IMPPS) working group (NSF Research Collaboration Network grant DEB-0541625, F. A. Smith, S. K. Lyons and S. K. M. Ernest, PIs), members of the Smith and Brown labs and other colleagues for stimulating discussions on the ideas presented here. This paper is IMPPS RCN publication no. 12. M.J.H. was funded by the IMPPS working group and the Rockefeller Foundation, A.D.D. by NSF, R.M.S. was partially supported by IMPPS and JHB by IMPPS, the Howard Hughes Medical Institute and the National Institutes of Health. The artistic images in figure 1 were reproduced from the originals with kind permission from Sharyn Davidson (Cynomys ludovicianus), Joe Trumpey (Ornithorhynchus anatinus) and Bruce Worden (Macrotis lagotis).
Appendix A. Marsupial pre-weaning survival data
| species | pre-weaning survival | source |
|---|---|---|
| Macropus giganteus | 0.83 | [31] |
| Dasyurus hallucatus | 0.34 | [32] |
| Tarsipes rostratus | 0.33 | [33] |
| Dasyurus maculatus | 0.28 | [34] |
| Trichosurus vulpecula | 0.38 | [35] |
| Phasogale tapoatafa | 0.66 | [36] |
| Didelphis virginia | 0.78 | [37] |
| Phascolarctos cinereus | 0.85 | [38] |
| Macropus rufogriseus | 0.65 | [39] |
References
- 1.Cifelli R. L. 2001. Early mammalian radiations. J. Paleontol. 75, 1214–1226 (doi:10.1666/0022-3360(2001)075<1214:EMR>2.0.CO;2) [DOI] [Google Scholar]
- 2.Luo Z.-X. 2007. Transformation and diversification in early mammal evolution. Nature 450, 1011–1019 10.1038/nature06277 (doi:10.1038/nature06277) [DOI] [PubMed] [Google Scholar]
- 3.Kemp T. S. 2005. The origin and evolution of mammals. Oxford, UK: Oxford University Press [Google Scholar]
- 4.Asher R. J., Horovitz I., Sánchez-Villagra M. R. 2004. First combined cladistic analysis of marsupial mammal interrelationships. Mol. Phylogenet. Evol. 33, 240–250 10.1016/j.ympev.2004.05.004 (doi:10.1016/j.ympev.2004.05.004) [DOI] [PubMed] [Google Scholar]
- 5.Cifelli R. L., Davis B. M. 2003. Marsupial origins. Science 302, 1899–1900 10.1126/science.1092272 (doi:10.1126/science.1092272) [DOI] [PubMed] [Google Scholar]
- 6.Kumar S., Hedges S. B. 1998. A molecular timescale for vertebrate evolution. Nature 392, 917–920 [DOI] [PubMed] [Google Scholar]
- 7.Luo Z.-X., Ji Q., Wible J. R., Yuan X. 2003. An early Cretaceous tribosphenic mammal and metatherian evolution. Science 302, 1934–1940 10.1126/science.1090718 (doi:10.1126/science.1090718) [DOI] [PubMed] [Google Scholar]
- 8.van Rheede T., Bastiaans T., Boone D. N., Hedges S. B., de Jong W. W., Madsen O. 2006. The platypus is in its place: nuclear genes and indels confirm the sister group relation of monotremes and therians. Mol. Biol. Evol. 23, 587–597 10.1093/molbev/msj064 (doi:10.1093/molbev/msj064) [DOI] [PubMed] [Google Scholar]
- 9.Woodburne M. O., Rich T. H., Springer M. S. 2003. The evolution of tribospheny and the antiquity of mammalian clades. Mol. Phylogenet. Evol. 28, 360–385 10.1016/S1055-7903(03)00113-1 (doi:10.1016/S1055-7903(03)00113-1) [DOI] [PubMed] [Google Scholar]
- 10.Clutton-Brock T. H., Harvey P. H. 1978. Mammals, resources and reproductive strategies. Nature 273, 191–195 10.1038/273191a0 (doi:10.1038/273191a0) [DOI] [PubMed] [Google Scholar]
- 11.Hayssen V., Lacy R. C., Parker P. J. 1985. Metatherian reproduction: transitional or transcending? Am. Nat. 126, 617. 10.1086/284443 (doi:10.1086/284443) [DOI] [Google Scholar]
- 12.May R. M., Rubenstein D. I. 1985. Reproductive strategies. In Reproduction in mammals, vol. 4 (eds Austin C. R., Short R. V.), pp. 1–23 Cambridge, UK: Cambridge University Press [Google Scholar]
- 13.Dawson T. J., Grant T. R., Fanning D. 1979. Standard metabolism of monotremes and the evolution of homeothermy. Aust. J. Zool. 27, 511–515 10.1071/ZO9790511 (doi:10.1071/ZO9790511) [DOI] [Google Scholar]
- 14.Tyndale-Biscoe H., Renfree M. 1987. Reproductive physiology of marsupials. Cambridge, UK: Cambridge University Press [Google Scholar]
- 15.Charnov E. L. 1993. Life history invariants: some explorations of symmetry in evolutionary ecology. Oxford, UK: Oxford University Press [Google Scholar]
- 16.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 10.1890/03-9000 (doi:10.1890/03-9000) [DOI] [Google Scholar]
- 17.Calder W. A. 1984. Size, function, and life history. Cambridge, UK: Harvard University Press [Google Scholar]
- 18.McNab B. K. 2002. The physiological ecology of vertebrates. Ithaca, NY: Cornell University Press [Google Scholar]
- 19.Peters R. H. 1983. The ecological implications of body size. Cambridge, UK: Cambridge University Press [Google Scholar]
- 20.Schmidt-Nielson K. 1984. Scaling: why is animal size so important? Cambridge, UK: Cambridge University Press [Google Scholar]
- 21.West G. B., Brown J. H., Enquist B. J. 1997. A general model for the origin of allometric scaling laws in biology. Science 276, 122–126 10.1126/science.276.5309.122 (doi:10.1126/science.276.5309.122) [DOI] [PubMed] [Google Scholar]
- 22.Hou C., Zuo W., Moses M. E., Woodruff W. H., Brown J. H., West G. B. 2008. Energy uptake and allocation during ontogeny. Science 322, 736–739 10.1126/science.1162302 (doi:10.1126/science.1162302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sibly R. M., Brown J. H. 2007. Effects of body size and lifestyle on evolution of mammal life histories. Proc. Natl Acad. Sci. USA 104, 17 707–17 712 10.1073/pnas.0707725104 (doi:10.1073/pnas.0707725104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sibly R. M., Brown J. H. 2009. Mammal reproductive strategies driven by offspring mortality-size relationships. Am. Nat. 173, E185–E199 10.1086/598680 (doi:10.1086/598680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charnov E. L., Warne R., Moses M. 2007. Lifetime reproductive effort. Am. Nat. 170, E129–E142 10.1086/522840 (doi:10.1086/522840) [DOI] [PubMed] [Google Scholar]
- 26.Bakker R. T. 1971. Dinosaur physiology and the origin of mammals. Evolution 25, 636–658 10.2307/2406945 (doi:10.2307/2406945) [DOI] [PubMed] [Google Scholar]
- 27.Simpson G. G. 1980. Splendid isolation: the curious history of South American mammals. New Haven, CT: Yale University Press [Google Scholar]
- 28.Dickman C., Woodford Ganf R. 2006. A fragile balance: the extraordinary story of Australian marsupials. Chicago, IL: University of Chicago Press [Google Scholar]
- 29.Low B. S. 1978. Environmental uncertainty and the parental strategies of marsupials and placentals. Am. Nat. 112, 197–213 10.1086/283260 (doi:10.1086/283260) [DOI] [Google Scholar]
- 30.Russell E. M. 1982. Parental investment and desertion of young in marsupials. Am. Nat. 119, 744–748 10.1086/283950 (doi:10.1086/283950) [DOI] [Google Scholar]
- 31.Poole W. E. 1975. Reproduction in the two species of grey kangaroos, Macropus Giganteus (Shaw) and M. Fuliginosus (Desmarest). II. Gestation, parturition and pouch life. Aust. J. Zool. 23, 333–353 10.1071/ZO9750333 (doi:10.1071/ZO9750333) [DOI] [PubMed] [Google Scholar]
- 32.Oakwood M. 2000. Reproduction and demography of the northern quoll, Dasyurus hallucatus, in the lowland savanna of northern Australia. Aust. J. Zool. 48, 519–539 10.1071/ZO00028 (doi:10.1071/ZO00028) [DOI] [Google Scholar]
- 33.Wooller R. D., Richardson K. C., Garavanta C. A. M., Saffer V. M., Bryant K. A. 2000. Opportunistic breeding in the polyandrous honey possum, Tarsipes rostratus. Aust. J. Zool. 48, 669–680 10.1071/ZO00071 (doi:10.1071/ZO00071) [DOI] [Google Scholar]
- 34.Belcher C. A. 2003. Demographics of tiger quoll (Dasyurus maculatus maculatus) populations in south-eastern Australia. Aust. J. Zool. 51, 611–626 10.1071/ZO02051 (doi:10.1071/ZO02051) [DOI] [Google Scholar]
- 35.Crawley M. C. 1973. A live-trapping of Australian brush-tailed possums, Trichosurus vulpecula (Kerr), in the orongorongo Valley, Wellington, New Zealand. Aust. J. Zool. 21, 75–90 10.1071/ZO9730075 (doi:10.1071/ZO9730075) [DOI] [Google Scholar]
- 36.Soderquist T. R. 1993. Maternal strategies of Phascogale tapoatafa (Marsupialia, Dasyuridae). 1. Breeding seasonality and maternal investment. Aust. J. Zool. 41, 549–566 10.1071/ZO9930549 (doi:10.1071/ZO9930549) [DOI] [Google Scholar]
- 37.Hossler R. J., McAninch J. B., Harder J. D. 1994. Maternal denning behavior and survival of juveniles in opossums in southeastern New York. J. Mammal. 75, 60–70 10.2307/1382236 (doi:10.2307/1382236) [DOI] [Google Scholar]
- 38.Tobey J. R., Andrus C. H., Doyle L., Thompson V. D., Bercovitch F. B. 2006. Maternal effort and joey growth in koalas (Phascolarctos cinereus). J. Zool. 268, 423–431 10.1111/j.1469-7998.2005.00041.x (doi:10.1111/j.1469-7998.2005.00041.x) [DOI] [Google Scholar]
- 39.Higginbottom K., Johnson C. N. 2000. Partial seasonality of breeding in red-necked wallabies (Macropus rufogriseus banksianus). J. Zool. 251, 71–77 10.1111/j.1469-7998.2000.tb00594.x (doi:10.1111/j.1469-7998.2000.tb00594.x) [DOI] [Google Scholar]