Abstract
The concept of animal personalities has recently become of major interest as researchers began to wonder why animals within a given population show consistent behaviour across situations and contexts, what led to the evolution of such behavioural inflexibility and what mechanisms might underlie the phenomenon. A recent model explains individual differences in a population as the result of trade-off between present and future reproduction. We tested this model on the two wing morphs, i.e. short-winged (brachypterous) and long-winged (macropterous) specimens of the firebug (Pyrrhocoris apterus). Since it has been already demonstrated that the two wing morphs differ in their life-history strategies, this species is an ideal subject to test whether the specimens with different life-history strategies have different personalities as well. The results show that individuals behave consistently over time and across contexts, meaning observed bugs do have personalities. We also have found that in females, the two wing morphs have different personalities supporting the theoretical predictions, i.e. winged ones, which are supposed to have lower future reproductive value, are braver and more exploratory. We found no difference between the morphs in males. Differences in reproductive investment might explain this discrepancy between the sexes.
Keywords: animal personality, firebug, wing dimorphism, male–female differences, life history
1. Introduction
Animals of the same sex and size in the same population usually differ in their behaviour and the underlying physiology even under standard conditions [1–3]. Moreover, the same set of animals shows the same kind of differences in different situations (e.g. in level of predator avoidance at different foraging sites) and contexts (e.g. boldness in foraging and social interactions). For instance, in the field cricket Gryllus integer Scudder, 1902 (Orthoptera: Gryllidae), more aggressive males who won more fights had shorter latencies to become active when in a novel environment and shorter latencies to emerge from a safe refuge [4]. Besides being more aggressive, these males are also more active in general, and possibly less cautious towards predation risk [4]. Although individuals could adjust their behaviour depending on situations, nevertheless, consistent differences between individuals usually remain [5]. These are frequently characterized as animal personalities [6], temperament [7], behavioural syndromes [5] or coping styles [8].
The acceptance of the phenomenon that animals have personalities is now widespread, but its evolutionary origin is still a mystery, since a more flexible structure of behaviour should provide a selective advantage [3,9,10]. Recently, Wolf et al. [11] have presented a model that offers an adaptive explanation to animal personalities. The model seeks answers to why different personality types (e.g. bold and shy) coexist, why behaviour is not more flexible for a long time and across situations and why the same traits correlate in different taxa. According to this model, density-dependent selection often leads to dimorphic populations where, because of the trade-offs between present and future reproduction, some of the individuals invest more in their future reproduction than others, resulting in the coexistence of different life-history strategies. As a consequence, the individuals with better future prospects are expected to be risk aversive, while those who have less to lose in the future take greater risks in a situation. As these life-history differences between individuals persists over time and across situations and contexts, one can expect consistent individual differences in behaviour that are correlated across situations and contexts, i.e. the emergence of personalities and behavioural syndromes.
To test the model by Wolf et al. [11], one needs a dimorphic population where individuals with different life-history strategies can be readily recognized and then the personality of the different types assessed. Populations of common firebugs (Pyrrhocoris apterus (Linnaeus, 1758) (Heteroptera: Pyrrhocoridae)) offer a good opportunity for such investigations. This bug is one of the most common species of Pyrrhocoridae in Europe, and has two wing morphs: macropterous (long-winged) and brachypterous (short-winged) ones. These morphs coexist in the same population, and several studies have shown that the macropterous individuals have a better ability to disperse, despite their flightlessness, and they delay reproduction, whereas the brachypterous individuals start to reproduce much earlier [12,13]. Consequently, these two forms seem to follow different life-history strategies: they can be considered as two alternative solutions to the dilemma of dispersing or staying [14,15]. The coexistence of disperser and phylopatric morphs in the same populations is rather common in insect species living in a metapopulation structure [16–18], where local catastrophes eradicating local populations make dispersing to be essential for long-term persistence [19]. In order that the two morphs have different personalities, the model by Wolf et al. [11] requires that the individuals belonging to the different morphs should have different residual reproductive values.
To show this, we consider a metapopulation where the two morphs coexist and where a female can produce both morphs. For simplicity, we assume that the two morphs differ only in their migratory tendency: the brachypters never migrate from a patch while the macropters always do so. From their similarity, it follows that their reproductive values just before egg laying (the number of adult descendants gained in the next generation) are the same, W1, if they are in the same patch, i.e. if the macropters have immigrated into an already occupied patch. On the other hand, when a macropter colonizes an empty patch, it gains W0 adult descendants in the next generation. We assume that W0 > W1 because of density-dependent effects. The probability of reaching an empty patch is given by the proportion of empty patches, p (i.e. the macropters choose patches at random), and the probability of surviving migration, q. Then, the reproductive value of brachypters is WB = W1, while that of the macropters before migration, WM, is as follows:
For WM < WB, the following condition must be fulfilled:
An acceptable estimate of p can be 0.01, meaning that a patch exists for 100 years on average, which is reasonable for linden trees, the main habitats for common firebugs [13]. As a brachypter female can have around 150 hatched larvae under ideal laboratory conditions [20], a reasonable value for W0/W1 + 1 would be 50. With these values, WM < WB holds for q < 0.67. Given that brachypters are more fecund than macropters in many cases [18], which contradicts our no-difference assumption above, the condition of WM < WB can hold for even larger values of q. Therefore, we may conclude that macropters have lower residual reproductive value than brachypters. Consequently, based on the model by Wolf et al. [11], one would predict that macropterous morhps would be braver and more exploratory than the brachypterous morphs. Furthermore, as several differences were found between males and females in firebugs (e.g. macropterous females have lower sexual activity [13]) and females invest more in reproduction, we expect that different life-history strategies would have a larger effect on females.
In this paper, we first investigate whether firebug individuals behave consistently over time and across contexts, i.e. whether they have personality. Second, we test the following predictions: (i) macropters are braver and more exploratory than brachypters and (ii) these differences are more expressed in females than in males.
2. Material and methods
(a). Experimental animals
The experimental animals were collected in four batches (to obtain enough of the rarer macropterous forms) from wild populations in Debrecen (47.52° N, 21.62° E; 130 m a.s.l.; subcontinental climate), northeast Hungary, in 2008 and 2010. Each bug was accommodated for a maximum of 8 days in the laboratory before the behavioural observations. The bugs were kept and the behavioural tests were performed in an air-conditioned laboratory that provided buffered conditions (mean air temperature: 23.25°C, range 23.0–24.0°C). Food (sunflower and lime tree seeds) and water were provided ad libitum.
One group of 60 brachypterous female bugs was used to test the consistency of behaviour over time. During these investigations, the firebugs were tested (see below) four times over a period of 5 days (the first two tests were separated by a 1 day break from the second two tests because of logistical reasons). These bugs were kept separately in jars between tests. In the other three groups (84 bugs (macropters: 7♀, 35♂; brachypters: 8♀, 34♂), 80 bugs (macropters: 13♀, 27♂; brachypters: 13♀, 27♂) and 100 bugs (macropters: 30♀, 20♂; brachypters: 30♀, 20♂) collected in the spring, summer and autumn, respectively), we tested our predictions.
(b). Behavioural test and walking path analysis
The tests were carried out in a circular arena of 55 cm in diameter with black wall (46 cm high), where four coloured plugs (made of gum, 0.75 cm high, 1.83 cm in diameter) as novel objects were placed on the floor. The floor of the arena was covered with white filter paper. This paper and the plugs touched by bugs were replaced after each run (they were used only once to avoid uncontrolled olfactory cues). The experimental arena was lit by two 18 W fluorescent-type MASSIVE tubes during the tests.
In the first part of the test, we measured fear responses (emerging from a refuge), while in the second part, we performed an open field test (responses to startling stimulus after arrival to a new environment). At the beginning of the test, the experimental animal was put into the arena in a brown semi-transparent vial (length: 9 cm, diameter: 2.56 cm). The bug was kept in the vial covered with paper (to prevent it from leaving) for a minute to get used to the environment. After the minute had passed, the vial was flicked as a startling stimulus (also to ensure that all bugs were at the end of the vial), laid on its side and the paper removed so that the bug could leave the refuge. Then we waited for the bug to leave the vial for a maximum of 10 min. Both the time when the antennae appeared first and the time when the bug actually came out were registered, but later we only used the time of the first appearance of the antennae (referred to as emergence hereafter) because many bugs (115 out of 264) did not emerge at all from the vial. We consider emergence as a measure of boldness.
The bugs leaving within 10 min were put back into the vial, while bugs remaining in the vial were left in it. In both cases, i.e. when (i) the bug left the vial itself and then was put back or (ii) remained in the vial, the vial was then flicked again (to be in the end of the vial) and the bug was kept in it for another minute, which was followed by yet another flick (to detach the bug from the wall of the vial), after which the bug was placed into the arena by shaking it out from the vial preferably into the centre of the arena. The time when the bug started to move after its placement into the arena (walking latency, another measure of boldness) and the time when it reached the wall (wall time, a measure of exploration) were registered and an observation lasted 10 min. We also recorded how many novel objects were visited (number of novel objects, measuring explorativeness).
A Quickcam S5500 webcam was used to record the movement of the bug at 1 s intervals, until it reached the wall for a maximum of 10 min. From the pictures taken, we reconstructed the path of each bug as a list of x-, y-coordinates. These lists were then used to derive the following variables for each bug: (i) the mean and (ii) the variance of step sizes (the distance between two consecutive positions), and (iii) the mean and (iv) variance of turning angles [21–24].
(c). Statistical analyses
To test the consistency of the behaviour over time, we measured the behavioural variables (see above) four times for each individual in a group of 60 brachypterous females. Then we calculated Kendall's W coefficient of concordance for each measured variable. Values of W significantly differing from random expectation mean that the individuals' rankings based on a given behavioural variable are in agreement among the different times of measurement [25], i.e. the individuals behave consistently over time [26]. We used permutation tests to infer levels of significance [25]. As we did not vary the situations over the tests (i.e. the level of risk was the same across all four repetitions), this procedure is unable to detect behaviour plasticity.
We have also investigated whether the behaviour of firebugs is consistent across contexts by calculating Kendall's W involving all measured behavioural variables. This analysis was performed on the combined dataset of the three larger groups of bugs (see above). To account for the possible effects of grouping, we subtracted the appropriate group median from each individual measurement for those variables where a Kruskal–Wallis test indicated significant difference among the groups (we used median because of the highly skewed distribution of many of the variables). To identify possible associations among the behavioural variables, i.e. possible personality axes, we followed the procedure outlined in [25]. In short, we computed a Spearman rank-correlation matrix among the variables and performed an agglomerative clustering (‘agnes’ function of the R statistical environment with Ward's clustering method) using one minus the absolute value of correlation coefficients as dissimilarity measures. Groups of correlated variables were identified by inspecting the mean overall silhouette values for the given number of groups (M. Maechler, P. Rousseeuw, A. Struyf & M. Hubert 2005, unpublished data; http://cran.r-project.org/web/packages/cluster/citation.html). To assess the robustness of this partition of the variables, we calculated consensus partition from 1000 random permutations of the original data frame [27]. A random permutation is obtained by randomly permutating a randomly chosen column of the original data frame. To test for significant associations, we submit each group of variables to a separate test of concordance. To characterize the bugs with these personality ‘axes’, we calculated a composite rank variable for each group of variables. This composite variable is simply the sum of the individuals' ranks for the variables in the given group [25].
To test the effect of wing morphology and sex on personality traits, we performed general linear model analyses on the composite variables. In these models, the given composite variable was entered as response and the wing morphology (two levels: macropterous and brachypterous) and sex (male, female) were entered as explanatory variables. We also analysed the individual behavioural and path variables. As most of the behavioural variables were highly skewed and often truncated, we dichotomized these variables (using the medians as cut points) and used generalized linear models with binomial error distribution and logit link function (i.e. logistic regressions). Here, the categorized behavioural variable (e.g. ‘slow’ and ‘fast’ emergence) was the dependent variable while wing morphology and sex were entered as explanatory variables.
All statistical analyses were carried out in the R interactive statistical environment [28] with irr [29], cluster (M. Maechler, P. Rousseeuw, A. Struyf & M. Hubert 2005, unpublished data), boot [30,31] and vegan [32] packages.
3. Results
(a). Consistency of behaviour over time
We found that firebugs behave consistently over time as all but two W calculated for the behavioural and path variables were significantly different from random expectation (emergence: W = 0.386, p = 0.003; walking latency: W = 0.481, p < 0.001; wall time: W = 0.402, p < 0.001; number of novel objects: W = 0.283, p = 0.236; mean turning angle: W = 0.271, p = 0.322; variance of turning angle: W = 0.394, p = 0.002; mean step size: W = 0.443, p < 0.001; variance of step size: W = 0.495, p < 0.001).
(b). Consistency of behaviour across contexts
Firebugs responded similarly in the different contexts as shown by the significant Kendall's coefficient of concordance (W = 0.198, p < 0.001). This means that the bugs were similarly ranked by all variables.
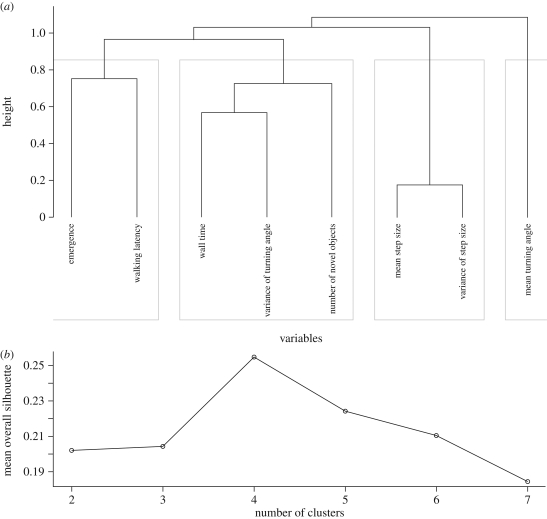
On the basis of the Spearman correlation matrix and the cluster analysis, our variables can be divided into the following groups (figure 1). The first group consists of emergence and walking latency, so it might be considered as a kind of shy–bold axis of personality. Kendall's W calculated for this group of variables is highly significant (W = 0.625, p = 0.003). The second group identified can be considered as the exploratory axis of bugs' personality since it contains the variables of wall time, variance of turning angle and number of novel objects visited. Kendall's W calculated for this group is also high and significant (W = 0.594, p = 0.003). The third group consists of mean step size and variance of step size (Kendall's W = 0.920, p = 0.003). Finally, mean turning angle appears not to correlate with any of the other variables. The interpretation of these last variables is less clear. The three composite variables and the mean turning angle are not significantly consistent (Kendall's W = 0.254, p = 0.429). The separate analyses of the composite rank variables show that macropterous female bugs are bolder (they have a lower rank) than the rest of the population (sex × wing morph interaction; F1,258 = 4.666, p = 0.032). No significant differences were found between sexes and morphs with respect to the other two composite variables and the mean turning angle.
Figure 1.
(a) The dendrogram shows the relationship between the investigated variables according to the cluster analysis (agglomerative coefficient is 0.448). ‘Height’ gives similarity based on the absolute values of the Spearman correlation matrix among the variables (see text for details). Groupings indicate possible personality axes. This partitioning is robust against small random changes in the dataset as the consensus partitioning based on 1000 random perturbations gives the same result. (b) Silhouette plot to identify possible groupings of the variables.
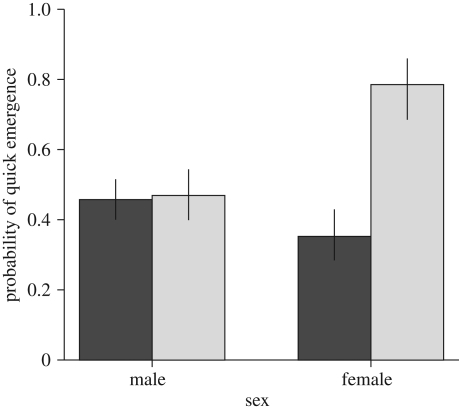
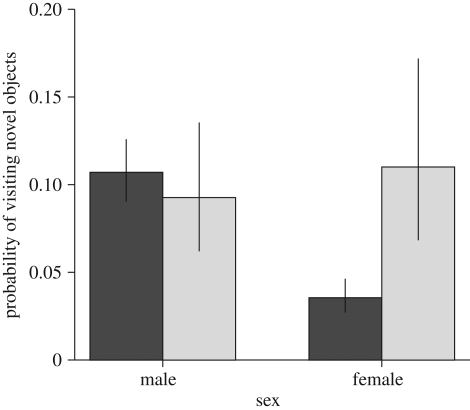
Analysing each measured variable separately showed a similar picture. Macropterous females emerged sooner from the vial (sex × wing morph interaction, χ21 = 6.32, p = 0.012; figure 2) than the rest of the population, while brachypterous females visited fewer novel objects (sex × wing morph interaction, χ21 = 8.28, p = 0.004; figure 3) than the rest of the population. We found no significant effect of sex and wing morph on the other behavioural and path variables (results are not shown).
Figure 2.
Macropterous females emerged quicker from the vial so they are bolder (expected probabilities, ±s.e., from binomial glm, sex × wing morph interaction, χ21 = 6.32, p = 0.012, response variable was dichotomized because of its highly skewed distribution). Wing: black bars, no; grey bars, yes.
Figure 3.
Brachypterous females visited fewer novel objects (expected probabilities, ±s.e., from binomial glm, sex × wing morph interaction, χ21 = 8.28, p = 0.004). Wing: black bars, no; grey bars, yes.
4. Discussion
Our results show that there are personality differences between the individuals of the firebug as we found that they behave consistently over time and across context. According to the cluster analysis of the behavioural variables, two axes of firebug personality can be identified: boldness and explorativeness. We also found that personality of the macropterous and brachypterous females differs; macropterous females are bolder and more explorative than the brachypterous ones. This latter result supports the model of Wolf et al. [11], which offers an explanation for the presence of individual differences in a population, namely that individuals choose different strategies to find the balance between present and future reproduction. In the case of firebug, it is known that there are differences in the behaviour of brachypterous and macropterous individuals, e.g. in the higher walking activity [14] and lowered mating propensity of macropterous individuals [13]. Furthermore, reproduction is delayed in the macropterous form [33], which is a common phenomenon in wing-polymorphic insects [34]. All of these indicate a difference in the life-history strategies of the two wing morphs despite the evolutionary loss of the flight capability in macropters [12]. Specifically, the lower sexual activity of macropterous individuals was found only in females and not in males [13]. This is in accord with our results on the bolder and more explorative behaviour of macropterous females compared with brachypterous ones (figures 2 and 3) [12,13]. The finding that individuals of higher dispersal tendency are bolder and more exploratory indicates the importance of these personality traits in dispersal. Similarly, Cote et al. [35] reported personality being a good predictor of dispersal tendency. The sex differences found can be attributed to the differences in investment into reproduction by the two sexes: as females invest more into reproduction, one would expect a stronger trade-off between current and future reproduction, which in turn can result in a larger difference in personality between morphs in females than in males.
Note that we studied specimens from wild populations, thereby our results validate those of Socha and co-workers [12–15] who found morph differences in firebug behaviour in captive populations maintained for many generations under laboratory conditions.
Further exciting research could investigate why it is only females among which behavioural differences can be detected, to what extent human disturbance alters a given population, whether ‘city dweller’ bugs that have to endure more environmental stress have different personalities compared with the specimens that live in an undisturbed environment (e.g. in a forest or a park) and to what extent the ratio of macropterous and brachypterous morphs is variable and whether it can be related to the environmental effects that influence the population. Such studies would bring us closer to the solution of an important issue of evolution, the coexistence of different morphs in terms of both life history and personality.
Acknowledgements
We are indebted to K. Bertók, V. Bókony, L. Garamszegi, J. Tökölyi and two anonymous reviewers for their useful comments and suggestions that helped to improve the manuscript. We would like to thank Miklós Bán for providing a lot of technical help. A.T. was supported in part by a Marie Curie Intra European Fellowship within the 7th European Community Framework Programme. Our work was partially supported by the Hungarian Scientific Research Fund (OTKA, no. K75696) and by the TÁMOP 4.2.1./B-09/1/KONV-2010-0007 project. The project is implemented through the New Hungary Development Plan, co-financed by the European Social Fund and the European Regional Development Fund.
References
- 1.Clark A. B., Ehlinger T. J. 1987. Pattern and adaptation in individual behavioural differences. In Perspectives in ethology (eds Bateson P. P. G., Klopfer P. H.), pp. 1–47, New York, NY: Plenum Press [Google Scholar]
- 2.Magurran A. E. 1993. Individual differences and alternative behaviours. In Behaviour of teleost fishes (ed. Pitcher T. J.), pp. 441–477, 2nd edn. New York, NY: Chapman & Hall [Google Scholar]
- 3.Wilson D. S. 1998. Adaptive individual differences within single populations. Phil. Trans. R. Soc. Lond. B 353, 199–205 10.1098/rstb.1998.0202 (doi:10.1098/rstb.1998.0202) [DOI] [Google Scholar]
- 4.Kortet R., Hedrick A. 2007. A behavioural syndrome in the field cricket Gryllus integer: intrasexual aggression is correlated with activity in a novel environment. Biol. J. Linn. Soc. 91, 475–482 10.1111/j.1095-8312.2007.00812.x (doi:10.1111/j.1095-8312.2007.00812.x) [DOI] [Google Scholar]
- 5.Sih A., Bell A., Johnson J. C. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 6.Gosling S. D. 2001. From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127, 45–86 10.1037/0033-2909.127.1.45 (doi:10.1037/0033-2909.127.1.45) [DOI] [PubMed] [Google Scholar]
- 7.Reale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 8.Koolhaas J. M., Korte S. M., De Boer S. F., Van Der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A. W., Blokhuis H. J. 1999. Coping styles in animals: current status in behaviour and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935 10.1016/S0149-7634(99)00026-3 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- 9.Coleman K., Wilson D. S. 1998. Shyness and boldness in pumpkinseed sunfish: individual differences are context-specific. Anim. Behav. 56, 927–936 10.1006/anbe.1998.0852 (doi:10.1006/anbe.1998.0852) [DOI] [PubMed] [Google Scholar]
- 10.Dall S. R. X., Houston A. I., McNamara J. M. 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- 11.Wolf M., van Doorn G. S., Leimar O., Weissing F. J. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584 10.1038/nature05835 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 12.Socha R., Zemek R. 2003. Wing morph-related differences in the walking pattern and dispersal in a flightless bug, Pyrrhocoris apterus (Heteroptera). Oikos 100, 35–42 10.1034/j.1600-0706.2003.12100.x (doi:10.1034/j.1600-0706.2003.12100.x) [DOI] [Google Scholar]
- 13.Socha R., Zemek R. 2004. Mating behaviour and wing morph-related differences in the sexual activity of a flightless bug, Pyrrhocoris apterus (L.) (Heteroptera). Ethol. Ecol. Evol. 16, 217–229 [Google Scholar]
- 14.Socha R., Zemek R. 2000. Locomotor activity in adult Pyrrhocoris apterus (Heteroptera) in relation to sex, physiological status and wing dimorphism. Physiol. Entomol. 25, 383–389 10.1111/j.1365-3032.2000.00209.x (doi:10.1111/j.1365-3032.2000.00209.x) [DOI] [Google Scholar]
- 15.Socha R., Zemek R. 2000. Wing movement behavior in long-and short-winged morphs of the flightless bug Pyrrhocoris apterus L. (Heteroptera: Pyrrhocoridae). J. Insect Behav. 13, 741–750 10.1023/A:1007800212347 (doi:10.1023/A:1007800212347) [DOI] [Google Scholar]
- 16.Roff D. A. 1986. The evolution of wing dimorphism in insects. Evolution 40, 1009–1020 10.2307/2408759 (doi:10.2307/2408759) [DOI] [PubMed] [Google Scholar]
- 17.Roff D. A. 1994. Habitat persistence and the evolution of wing dimorphism in insects. Am. Nat. 144, 772–798 10.1086/285706 (doi:10.1086/285706) [DOI] [Google Scholar]
- 18.Roff D. A., Fairbairn D. J. 1991. Wing dimorphisms and the evolution of migratory polymorphisms among the insecta. Am. Zool. 31, 243–251 10.1093/icb/31.1.243 (doi:10.1093/icb/31.1.243) [DOI] [Google Scholar]
- 19.Jansen V. A. A., Sigmund K. 1998. Shaken not stirred: on permanence in ecological communities. Theor. Popul. Biol. 54, 195–201 10.1006/tpbi.1998.1384 (doi:10.1006/tpbi.1998.1384) [DOI] [PubMed] [Google Scholar]
- 20.Socha R. 2008. Wing morph- and age-related differences in fertilization success of adult males of a flightless bug, Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). Eur. J. Entomol. 105, 93–98 [Google Scholar]
- 21.Bovet P., Benhamou S. 1988. Spatial analysis of animals' movements using a correlated random walk model. J. Theor. Biol. 131, 419–433 10.1016/S0022-5193(88)80038-9 (doi:10.1016/S0022-5193(88)80038-9) [DOI] [Google Scholar]
- 22.Bell W. J. 1991. Searching behaviour: the behavioural ecology of finding resources New York, NY: Chapman & Hall [Google Scholar]
- 23.Wratten W. D. 1994. Video techniques in animal ecology and behaviour. London, UK: Chapman & Hall [Google Scholar]
- 24.Clobert J., Danchin R., Dhondt A. A., Nichols J. D. 2001. Dispersal. New York, NY: Oxford University Press [Google Scholar]
- 25.Legendre P. 2005. Species associations: Kendall coefficient of concordance revisited. J. Agric. Biol. Environ. Stat. 10, 226–245 10.1198/108571105X46642 (doi:10.1198/108571105X46642) [DOI] [Google Scholar]
- 26.Briffa M., Rundle S. D., Fryer A. 2008. Comparing the strength of behavioural plasticity and consistency across situations: animal personalities in the hermit crab. Pagurus bernhardus. Proc. R. Soc. B 275, 1305–1311 10.1098/rspb.2008.0025 (doi:10.1098/rspb.2008.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornik K. 2005. A CLUE for Cluster ensembles. J. Stat. Softw. 14 See http://www.jstatsoft.org/v14/i12/ [Google Scholar]
- 28.R Development Core Team 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 29.Gamer M., Lemon J., Fellows I.Irr: various coefficients of interrater reliability and agreement. 2007. R package v. 0.70. See http://www.r-project.org .
- 30.Davison A. C., Hinkley D. V. 1997. Bootstrap methods and their applications. Cambridge, UK: Cambridge University Press [Google Scholar]
- 31.Canty A., Ripley B.Boot: Bootstrap R (S-Plus) functions. 2008. R package, v. 1.2-33. See http://vegan.r-forge.r-project.org/ http://cran.r-project.org/
- 32.Oksanen J., Kindt R., Legendre P., O'Hara B., Simpson G. L., Sólymos P., Henry M., Stevens H., Wagner H. 2009. Vegan: community ecology package. R package v. 1.15-3. See http://vegan.r-forge.r-project.org/, http://cran.r-project.org/
- 33.Socha R., Šula J. 2006. Flight muscles polymorphism in a flightless bug, Pyrrhocoris apterus (L.): developmental pattern, biochemical profile and endocrine control. J. Insect Physiol. 52, 231–239 10.1016/j.jinsphys.2005.10.009 (doi:10.1016/j.jinsphys.2005.10.009) [DOI] [PubMed] [Google Scholar]
- 34.Zera A. J., Denno R. F. 1997. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 42, 207–230 10.1146/annurev.ento.42.1.207 (doi:10.1146/annurev.ento.42.1.207) [DOI] [PubMed] [Google Scholar]
- 35.Cote J., Fogarty S., Weinersmith K., Bordin T., Sih A. 2010. Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc. R. Soc. B 277, 1571–1579 10.1098/rspb.2009.2128 (doi:10.1098/rspb.2009.2128) [DOI] [PMC free article] [PubMed] [Google Scholar]