Abstract
The elemental composition of phytoplankton is a fusion of the evolutionary history of the host and plastid, resulting in differences in genetic constraints and selection pressures associated with environmental conditions. The evolutionary inheritance hypothesis predicts similarities in elemental composition within related taxonomic lineages of phytoplankton. To test this hypothesis, we measured the elemental composition (C, N, P, S, K, Mg, Ca, Sr, Fe, Mn, Zn, Cu, Co, Cd and Mo) of 14 phytoplankton species and combined these with published data from 15 more species from both marine and freshwater environments grown under nutrient-replete conditions. The largest differences in the elemental profiles of the species distinguish between the prokaryotic Cyanophyta and primary endosymbiotic events that resulted in the green and red plastid lineages. Smaller differences in trace element stoichiometry within the red and green plastid lineages are consistent with changes in trace elemental stoichiometry owing to the processes associated with secondary endosymbioses and inheritance by descent with modification.
Keywords: phytoplankton, evolution, stoichiometry, endosymbiosis, trace metals, Redfield ratio
1. Introduction
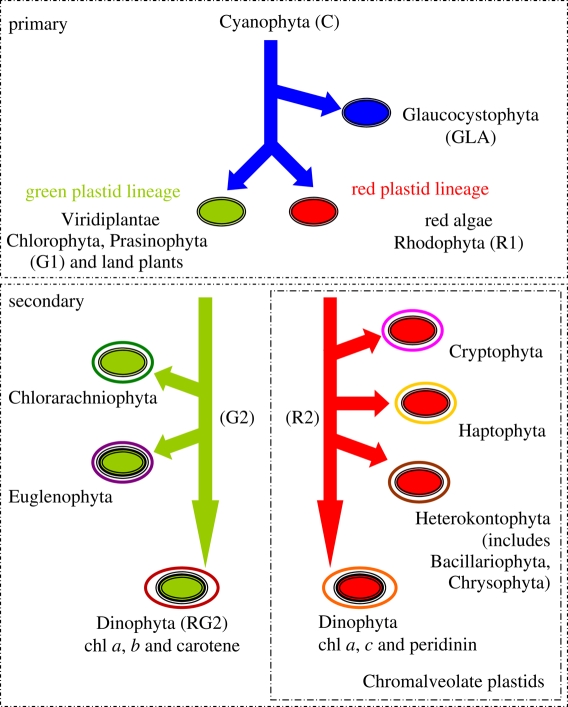
Oxygenic photosynthesis by phytoplankton has significantly contributed to the oxidation state of the ocean and atmosphere [1–3]. Based on inferred atmospheric and oceanic oxygen concentrations, biomolecular and isotopic evidence, phytoplankton may have been present 2.7–2.4 Ga ago ([4–6]; but see [7]). These would probably have been the Cyanophyta, the only extant prokaryotic group of oxygenic phototrophs [6]. Almost a billion years later, organic walled fossils attributable to eukaryotic phytoplankton appeared in the fossil record [6,8,9]. Three lineages of photosynthetic organisms became established as a result of primary endosymbiosis: the green plastid lineage (chlorophyll (chl) b-containing green algae and land plants, Viridiplantae), the red plastid lineage (chl c-containing, Rhodophyta) and the Glaucocystophyta (also known as Glaucophyta) [10–12].
Later, secondary endosymbiotic events occurred in both the green and red plastid lineages. In the green lineage, this gave rise to the Chlorarachniophyceae and Euglenophyceae. In the red plastid lineage, secondary endosymbiosis gave rise to the Cryptophyceae, the Heterokontophyta (including the Bacillariophyceae and Chrysophyceae), the Haptophyceae, the chl c-containing Dinophyceae and several other groups (figure 1). This diverse assemblage of eukaryotic secondary chl c-containing red phytoplankton is collectively referred to as the Chromalveolates [12–14]. In the Dinophyceae, secondary endosymbiotic events also gave rise to chl a and b (e.g. Gymnodinium chlorophorum) and chl a and c with peridinin plastid group (figure 1). As a result of tertiary endosymbiosis, chl a and c with fucoxanthin-containing dinoflagellates arose making them the most diverse and complex of all the phytoplankton groups (e.g. [15,16]). Prior to the Mesozoic, the fossil record indicates that the Cyanophyta and members of the eukaryotic green plastid lineage probably dominated phytoplankton assemblages; by contrast, the red plastid lineage dominates the contemporary ocean [8,11,17]. Coincident with the major evolutionary changes in the phytoplankton are shifts in the oxidation state of the atmosphere and ocean and the availability of macro- and micro-nutrients [18–21].
Figure 1.
The series of endosymbiotic events in the evolution of phytoplankton. A single origin of plastids is hypothesized whereby a single-celled protist acquired and retained a free-living cyanobacterium. Over time, the cyanobacterium endosymbiont was reduced to a plastid and transmitted to subsequent generations. Three lineages arose from a primary engulfment event: the green plastid lineage (chl b-containing green algae and land plants, Viridiplantae), the red plastid lineage (chl c-containing red algae, Rhodophyta) and the Glaucocystophyta. Secondary endosymbiotic events occurred in both the green and red plastid lineages. In the green lineage, this gave rise to the Chlorarachniophyceae and Euglenophyceae. In the red plastid lineage, secondary endosymbiosis gave rise to the Chromalveolates, which include the Cryptophyceae, the Heterokontophyta (such as the Bacillariophyceae and Chrysophyceae), the Haptophyceae, the chl c and peridinin-containing Dinophyceae (but not the chl b-containing dinoflagellates or those that arose as a result of a tertiary endosymbiotic event). Only groups examined in this study are depicted in the figure.
Taxonomic differences in macro- and trace element to P (or to C or N) ratios in phytoplankton associated with fundamental biochemical differences or unique phenotypic strategies in response to their environment have been revealed from decades of careful field and laboratory work [22–26]. Unfortunately, well-established variability in elemental stoichiometry in response to growth rate and environmental conditions has made it difficult to quantify taxonomic differences in multi-element composition from multiple studies. A statistical analysis of a multi-element study conducted under defined nutrient-replete environmental conditions on 15 eukaryotic marine phytoplankton species revealed significant variability between species and distinctive elemental profiles associated with the green and red plastid lineages [27]. Phytoplankton in the green plastid lineage (products of a primary endosymbiotic event: Chlorophyceae and Prasinophyceae) generally had higher C, N, Fe, Zn and Cu to P ratios than those in the red plastid lineage (products of a secondary engulfment event: Haptophyceae, Bacillariophyceae and Dinophyceae) when grown under identical conditions. Based on these results, the authors hypothesized that the distinctive elemental signatures across phytoplankton hierarchies under nutrient-replete conditions reflected evolutionary histories in response to the different selective forces present at the time of their evolution and radiation, combined with fundamental biochemical and genetic constraints [11,27].
The elemental composition of each individual species is a function of its unique evolutionary history and we expect a large variation in elemental composition between species. The evolutionary inheritance hypothesis predicts that there may be some broad similarities in elemental composition for some elements at higher taxonomic levels based on fundamental genetic differences between the groups, most probably associated with engulfment events. To test the evolutionary inheritance hypothesis, we extended the elemental analysis presented in Quigg et al. [27] with the present study to take in 14 more species including Cyanophyta, Glaucocystophyta and a range of species from the red and green lineages representing both primary and secondary endosymbiotic events (electronic supplementary material, table S1, and figure 1). We quantify the taxonomic difference in elemental stoichiometry on the combined dataset (present study [26,27]). If the elemental composition of different algal groups reflects evolutionary history, then we would predict that the Cyanophyta would have an elemental signature more similar to the taxonomic groups established by a primary endosymbiotic event and most different from the groups established by secondary endosymbiotic events. In general, we would expect relatively less difference in elemental composition within a lineage than between lineages, i.e. members of the green plastid lineage would have an elemental composition more similar to one another than members of the red plastid lineage, but within the lineages, subsequent endosymbiotic events could lead to further divergence in elemental profiles.
2. Material and methods
(a). Culturing and growth conditions
Culturing, sampling and elemental analysis were all performed essentially as described in Quigg et al. [27] and Ho et al. [26]. Ten marine phytoplankton (electronic supplementary material, table S1) were grown in defined nutrient-replete medium under standard conditions (19 ± 1°C, 12 L : 12 D cycle, 250 µ mol quanta m−2 s−1; modified Aquil). Trichodesmium sp. was grown at 26°C, 85 µ mol quanta m−2 s−1 (12 L : 12 D cycle) in YBCII medium [28]. Freshwater cultures were grown in Fraquil [29]. Nutrients (N, P and Si), EDTA, trace metals and vitamin stock solutions were added to Fraquil in concentrations defined for the modified Aquil recipe [26].
(b). Growth rates and cell size
Replicate exponentially growing cultures (n ≥ 3) for each species were maintained in semi-continuous batch cultures. Growth rates for most marine species were followed by measuring daily changes in cell density using a calibrated Coulter Multisizer particle counter. Growth rates of the freshwater species were followed by measuring daily changes in in vivo chl a fluorescence. Synechococcus sp. and Cyanothece sp. were counted daily using a Neubauer haemocytometer. Specific growth rates (μ; d−1) were calculated from the initial (F0) and final (F) relative fluorescence values according to μ = (ln F − ln F0)/(t – t0), where t was time measured in days or using μ = (ln C − ln C0)/(t – t0), where C was the cell concentration (cells ml−1). Trichodesmium growth rates were measured according to Berman-Frank et al. [30].
(c). Sample preparation and analysis
Detailed information on sample preparation and analysis was provided in Quigg et al. [27] and Ho et al. [26]. Cellular C and N concentrations were measured with a CHN elemental analyser while all other elements were measured using sector field high-resolution inductively coupled plasma mass spectrometry (HR-ICP-MS). Phytoplankton were filtered gently (less than 130 kPa) onto acid-cleaned polycarbonate filters and rinsed with chelated 0.45 M NaCl [26] and Milli-Q H2O for marine and freshwater species, respectively. Optima grade HNO3 was used in all HR-ICP-MS sample preparation and analysis. In order to further improve HR-ICP-MS calibration accuracy and precision, both external and internal standard curves were included with each sample run in this study according to Cullen et al. [31]. Concentration differences were less than 3 per cent for most elements between the two standard curves.
(d). Statistical analyses using the combined dataset
To test hypotheses on the evolutionary inheritance of stoichiometry in phytoplankton, we combined the findings from the present study with those in an earlier study (electronic supplementary material, table S1). Patterns in trace element composition were examined using principal component analysis (PCA) on the correlations of log-transformed trace element to phosphorus (P) ratios [32]. We tested for statistical differences in element : P ratios across classes using ANOVA and pairwise t-tests with Holm's sequential correction [32] using log C, N, Fe, Mn, Zn, Cu, Co and Cd to P ratios. To analyse the hierarchical structure in the elemental profiles of the phytoplankton, we calculated the distance between average log (element : P) ratios and used these distances to construct a dendrogram. The squared distance between observations i and j was defined as:
where xki = log(k : P) for observation i and xki were divided by the standard deviation of the observations for all trace elements so that each element ratio contributed equally to the distance. To analyse the distance between groups (Cyanophyta, C; Glaucocystophyta, GLA; primary green plastid lineage, G1; secondary green plastid lineage, G2; primary red plastid lineage, R1; secondary red plastid lineage, R2 and secondary red with green plastid, RG2), we averaged all log (trace element : P) ratios over replicates within groups before computing distances (for a definition of the taxonomic groupings and abbreviations, see electronic supplementary material, table S1). For the detailed statistical approach taken, refer to electronic supplementary material, M1.
3. Results
Phytoplankton were grown under nutrient-replete conditions and had specific growth rates ranging from 0.23 to 1.11 d−1 (electronic supplementary material, table S1). Elemental ratios for each of the 14 species examined and each element measured are summarized in electronic supplementary material, table S2. Elemental quotas were normalized to P rather than to C or N for direct comparison with previous studies. Statistically similar patterns were observed whether we normalized to P or C or N (see below) [33,34]. Small differences in the average elemental profiles relative to previous studies reflect differences in the relative number of species and observations and proportion of species representing the primary and secondary endosymbiotic events within the lineages [26,27].
(a). Major nutrients: C, N, P and S
The average C : N : P : S ratio for the 29 species investigated was 132 : 18 : 1 : 0.99, compared with the canonical Redfield ratio of 106 : 16 : 1 : 1.7 [35] and consistent with earlier studies with phytoplankton grown under identical conditions [26,27], and in other laboratories and in the field (see reviews of Duarte [36] and Geider & La Roche [37]). While the ratios of the major nutrients were variable (electronic supplementary material, table S2), patterns between plastid lineages were observed (electronic supplementary material, figure S1). C : P is significantly higher in G1 relative to GLA, R1, R2 and RG2 while the C : P in G2 did not differ from other groups. N : P is significantly higher in G2 relative to R1 and R2, but does not differ from other groups. The green plastid lineage (G1 and G2) also tends to have higher C : N ratios. Our average S : P ratio of 0.75 (electronic supplementary material, table S2) for the 14 species in the current study (and 0.99 for all 29 species investigated) fell between the range of previous reports: 1.7 in Redfield et al. [35], 0.65 in Payne & Price [38], 0.3 in Fraga [39] and 1.3 in Ho et al. [26].
(b). Major cations: K, Mg, Ca and Sr
Despite the ubiquitous use of cations in cellular processes [39], phytoplankton accumulate them to differing degrees (electronic supplementary material, table S2). High concentrations of Ca : P (94 ± 5 mmol : mol) and Sr : P (107 ± 4 mmol : mol) specifically were observed in the cyanobacterium Trichodesmium sp. Typically, only calcifying phytoplankton such as the coccolithophores Emiliana huxleyi and Gephyrocapsa oceanica accumulate such high concentrations of Ca (e.g. [26,27]) as it is used to construct calcite and aragonite liths. In a study on the growth of the diatom (Bacillariophyceae) Phaeodactylum tricornutum, Hayward [40] found variations in the external concentration of Na, K, Ca and Mg had little effect on the internal concentration of these elements. Thus, differences in the media (modified Aquil, Fraquil and YBCII) cannot be assumed to be primarily responsible for the differences in cation : P ratios among the phytoplankton examined.
(c). Trace elements: Fe, Mn, Zn, Cu, Co, Cd and Mo
The major trace elements (and Sr) were present in 1000-fold lower concentrations than the nutrient elements and other major cations (electronic supplementary material, figure S1 and table S2). Mean concentrations for the 14 species were generally consistent with the range of element : P previously reported for phytoplankton growing under similar conditions [26,27]. All subsequent analysis refers to the combined dataset of 87 observations on 29 species.
The average mmol Fe : Mn : Zn : Cu : Co : Cd to mol P ratio for the whole dataset is 18 : 2.8 : 1.34 : 0.50 : 0.11 : 0.11. Cyanophyta, Glaucocystophyta and the green plastid lineage had the highest Fe : P ratios while the red plastid lineage tended to have lower values (electronic supplementary material, figure S1 and table S2). The Trichodesmium Fe quota reported in this study is relatively high (109 ± 6 mmol Fe : mol P) compared with recent reports (e.g. [41,42]). Relative to other species examined, Cyanophora paradoxa (Glaucocystophyta) and Chlorarachnion reptans (Chlorarachniophyceae) had the greatest Zn quotas (5 ± 0.8 and 4.7 ± 0.11 m mol Zn : mol P, respectively). Cobalt and cadmium quotas were several orders of magnitude higher in Eutreptiella sp. (Euglenophyceae), which also has significantly lower Zn quotas than the other species examined.
(d). Statistical analysis of phylogenetic hierarchies
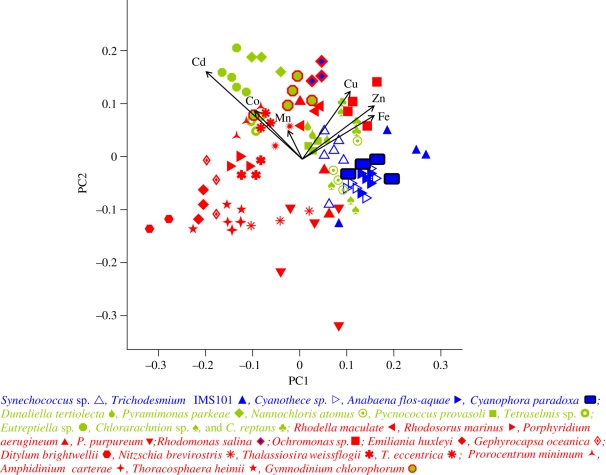
PCA is a visual aid that can be used to identify taxonomic differences and similarities in log trace elemental : P profiles. The first two principal components of PCA account for 76 per cent of the variance in trace metal composition (46% for the first, 30% for the second). The prokaryotic Cyanophyta, the Glaucocystophyta and the green and red plastid lineages are clearly separated along the two principal components (figure 2). The green plastid lineage is closer and exhibits more overlap than the red plastid lineage with the Cyanophyta. There is also some evidence of separation between the primary and secondary endosymbiotic events within the red and green plastid lineages. Phycobilisome-containing groups, the Cyanophyta, the Glaucocystophyta and the Crytophyceae, cluster on the far right end of the first principal component axis.
Figure 2.
PCA of the log trace element profiles on combined dataset. The principal component axes are weighted sums of log trace element : P (mmol : mol). The first and second principal components are: PC1 = 0.460 log Fe : P − 0.105 log Mn : P + 0.468 log Zn : P + 0.318 log Cu : P − 0.282 log Co : P − 0.615 log Cd : P, PC2 = 0.325 log Fe : P + 0.214 log Mn : P + 0.381 log Zn : P + 0.490 log Cu : P + 0.295 log Co : P + 0.614 log Cd : P. Vectors along each of the metal : P axes are shown projected onto the first two principal components. Species examined (symbols centred on location). The green and red colours used in the symbols represent members of the green and red plastid lineages respectively, while blue was used for the Cyanophyta. Along these lines the blue inside the black box was used for Glaucocystophyta (reflecting the cyanobacterial plastid in this group), the blue inside the red diamond was used for the Cryptophyceae (reflecting the phycobilisome based pigments also found in the Cyanophyta) and the green inside the red hexagon was used for the dinoflagellate (secondary red) with a green plastid.
The analysis of variance for each log element : P shows significant differences in elemental ratios at the phylum and species level. Pairwise t-tests with Holm's correction document significant differences in most of the log element : P ratios at the lineage or class level (electronic supplementary material, table S3A), except for Co : P. Some of the clearest taxonomic differences in the elemental ratios are: log Fe : P is lowest in the secondary red plastid lineages; log Zn : P is highest in the Glaucocystophyta and the secondary red plastid lineage; and log Cd : P is lowest in the Cyanophyta and highest in the green plastid lineage. The green plastid lineage had, on average, higher Fe, Cu and Cd to P ratios than the red plastid lineage, while Mn : P is higher in the red relative to the green plastid lineage (figure 2). Cyanophyta and Glaucocystophyta often had trace element ratios more similar to the green relative to the red plastid lineage. Within the green plastid lineage Euteptiella sp. (Euglenophyceae, G2) has considerably higher C : P and N : P, Cd : P and Co : P relative to the Chlorarachnion spp. (Chlorarachniophyceae, G2) examined, which were characterized by higher Fe, Zn and Cu : P (electronic supplementary material, table S2).
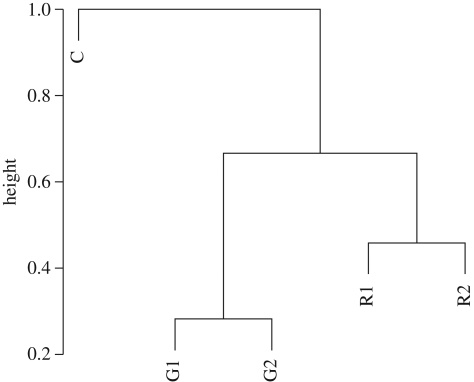
A tree based on the Euclidean distances between average trace elemental profiles illustrates the aggregate elemental differences among the groups owing to their mean log trace element : P (Fe, Mn, Zn, Cu, Cd, Co) stoichiometry (figure 3). We tested the robustness of the tree in several different ways. Resampling the data, omitting one species each time, produced identical trees 87 times (once for each observation; 29 species × 3 observations = 87 trees). Omitting one species from each group (C, G1, G2, R1, R2) at random produced the same tree slightly more than half the time (56/100 trees) and many other topologies for the other samples. Retaining only one species per group produced a large number of different trees with no one topology dominating the samples. A careful examination of the bootstrapped distance matrix (electronic supplementary material, table S3B) (omitting one species at a time) indicates the following distances are significant at the p ≤ 0.05 level: the primary and secondary green plastid lineages are closer to the Cyanophyta than the primary or secondary red plastid lineages; the distance between the primary red plastid lineage and Cyanophyta is larger than the distance between the primary red and green lineages and the primary red and secondary red lineages; and the primary red plastid lineage is closer than the secondary red plastid lineage to Cyanophyta. By contrast, the primary and secondary green lineages are a similar distance from Cyanophyta. The lack of statistical distance between the primary and the secondary green plastid lineage is in part a function of the large variation in elemental stoichiometry between the Euglenophyceae and Chlorarachniophyceae within the secondary green lineage. Note that the Glaucocystophyta (C. paradoxa; GLA) and the dinoflagellate with the green plastid (G. chlorophorum; RG2) cannot be included in the bootstrap analysis because they include only a single species within their groupings.
Figure 3.
Dendrogram based on Euclidean distance between average log trace elemental profiles (vector of log trace element : P) across all the phytoplankton examined. The following groups were examined: Cyanophyta (C), primary green plastid lineage (G1), secondary plastid lineage (G2), primary red plastid lineage (R1) and secondary red plastid lineage (R2). The height is the diameter of the cluster containing observations not yet split, scaled to a maximum of 1.
4. Discussion
Phytoplankton are a polyphyletic group in which primary, secondary and tertiary endosymbiotic engulfment events gave rise to the diversity of species seen today (figure 1). The Cyanophyta are the likely contributor of the ancestral plastid to the green and red plastid lineages and the Glaucocystophyta as a result of primary endosymbiotic engulfment events [10–12,14–16]. The lineages resulting from these primary endosymbiotic events exhibit significant differences in macro- and trace elemental composition (log Fe, Mn, Zn, Cu, Cd to P ratios). Furthermore, there are significant, albeit smaller, differences in the elemental stoichiometry within the green and red plastid lineages (figures 2 and 3). We propose that the larger difference in elemental composition between the major lineages relative to the differences within the lineages supports the hypothesis that there is evolutionary inheritance of micro-element requirements in the phytoplankton.
Element ratios are known to change in response to growth rate and environmental conditions, including irradiance, temperature, CO2, pH, the availability of nutrients in the medium and numerous interactions between trace elemental uptake mechanisms [24,43–46]. We have not attempted and cannot account for all these myriad effects, but we have minimized them by maintaining the experimental species in exponential growth phase in nutrient-enriched media. Under these conditions, we find significant differences in the elemental profiles at the species level and across the lineages, consistent with what is known about their unique evolutionary histories, physiological capacities and adaptations to specific environmental conditions [27]. We interpret the coherent statistically significant differences in the average elemental composition of these species within higher taxonomic groupings, such as the green versus red plastid lineages, to represent a residual phylogenetic signal associated with their common evolutionary history. Distances between average trace elemental profiles (figure 3) between the lineages are consistent with phylogenetic trees based on nucleic and protein sequences (e.g. [12–14,47]). Some of the more obvious elemental differences among the lineages are higher Fe, Zn and Cu to P ratios in the green plastid lineage versus the red plastid lineage (figure 2). It has been hypothesized that these differences in trace element composition between the lineages reflect differences in the acquired plastids, termed the plastid imprint hypothesis [27]. A comparison of trace element stoichiometry of species with similar plastids but different hosts and similar hosts but different endosymbionts permits a coarse assessment of the relative importance of the host relative to the plastid in determining trace element composition. There are three lines of evidence that support the plastid imprint hypothesis: (i) the trace element composition of chl c dinoflagellates with plastids inherited from the red lineage cluster closely with the red plastid lineage while G. chlorophorum, a chl b-containing dinoflagellate, clusters with the green plastid lineage based on trace element stoichiometry [27]; (ii) similarity in the trace element stoichiometry in the secondary red plastid lineages despite differences in host lineages (alveolate host associated with the dinoflagellates versus a pre-chromist-type host associated with the Bacillariophyceae (diatoms), Haptophyceae and Cryptophyceae (figure 1)); and (iii) species with phycobilisomes, the Cyanophyta (prokaryotes, no host), the Glaucocystophyta (a product of primary endosymbiosis) and the Cryptophyceae (a product of secondary endosymbiosis), have similar trace element stoichiometry (high Fe : P and Zn : P) (figure 2). There is some evidence that the host may also contribute, in some cases, to trace element stoichiometry. Within the green plastid lineage, Eutreptiella sp. (Euglenophyceae, G2) has considerably higher Cd : P relative to the Chlorarachnion spp. (Chlorarachniophyceae, G2) (electronic supplementary material, table S2).
What role do the subsequent secondary engulfment events that produce the secondary red and green lineages play in altering the metallome? The smaller differences in trace element stoichiometry within the red and green plastid lineages (G1–G2 and R1–R2) are consistent with changes in trace elemental stoichiometry, the metallome [48], owing to the processes associated with secondary endosymbioses and inheritance by descent with modification of trace element stoichiometric profiles over time (figure 3 and electronic supplementary material, table S3B). Although the specific mechanisms controlling the evolution of the metallome during the process of engulfment and successful endosymbiotic events are unknown, the potential for gene loss, reorganization and radical changes in gene regulation may become increasingly constrained with each subsequent engulfment event. In the primary engulfment event, the host cyanobacteria lost or transferred the majority of its genes to the host nucleus [12–14,47], and new regulation pathways must be established to control metabolite transport between the endosymbiont and the host [12]. In subsequent secondary engulfment events, a eukaryotic symbiont with its own nuclear and plastid genome must be housed within a new eukaryotic host. After the primary endosymbiotic event, subsequent endosymbiotic events may be more likely to maintain established regulatory pathways, and the associated trace metal metallome, which contribute to successful endosymbiosis, as genes are transferred from the symbiont plastid and nucleus to the host nucleus.
All species require Fe, Mn, Zn, Cu and Co for growth, so differences in trace element stoichiometry probably reflect differences in the relative demand for trace metals in biochemical pathways, transporter capabilities and storage strategies, and not simply the presence or absence of a requirement for a particular metal or metalloenzyme. Cadmium may be an exception. Generally considered a toxin and not required for growth, it has been shown to be used as a metallo-centre in carbonic anhydrase in a diatom [49]. Similarly, nitrogen fixation, present in some species of the Cyanophyta, has a high Fe and Mo requirement owing to the enzyme nitrogenase [30,50,51]. We propose that the combination of gene loss, transfer of plastid genes to the nucleus and the establishment of new regulatory pathways to balance the needs of the plastid in the host [12] under different selection regimes could be responsible for the relatively large differences in the trace metal metallome among the Glaucocystophyta, green and red plastid lineages.
The strong correlation between phylogenetic differences and trace element stoichiometries is consistent with the engulfment events occurring at different times in oceanic environments with different metal availabilities. The Cyanophyta originated in a reduced ocean with low pO2 in surface and deep waters and high bioavailability of Fe, Mn and Co and lower availability of Zn and Cd [20,52–55], which appears to be reflected in a high Fe : P and lower Cd : P in their particulate matter. Shifts in ocean chemistry from an anoxic Fe-rich ocean to a euxinic/sulphidic and then a more oxic ocean over the last two billion years of Earth's history have altered the bioavailability of many trace elements [20,52–55]. The euxinic ocean would probably have had lower Fe, Mn, Co and Zn relative to the anoxic ocean, while the modern oxic ocean has relatively higher bioavailability of Zn and Cu but lower Fe, Mn and Co than past reducing oceans. An analysis of proteomes reveals differences in the abundance of Zn-and Fe-binding domains between prokaryotic and eukaryotic proteomes consistent with eukaryotes evolving in a more oxic environment [54].
If the red and green plastid lineages originated contemporaneously in similar environments, we might expect that the trace elemental profiles would be similar; alternatively, the lineages may have originated in contrasting environments. Some evidence suggests eukaryotes may have originated in shallow coastal oxic zones with terrestrial sources of metals, but their distribution and diversification were impeded by the low availabilities of Cu and Mo in the euxinic ocean [6,19,56]. Based on fossil and molecular evidence, it appears that the green plastid lineage (G1) may have been more ecologically dominant in the Neoproterozoic and Paleozoic (1000–251 Myr) ocean and the red plastid lineage, specifically those lineages produced by a secondary endosymbiotic event, the Bacillariophyceae, Haptophyceae and Dinophyceae (R2), became more ecologically dominant in the Mesozoic and Cenozoic (251 Ma to present) ocean [4,5,8]. If the radiation of the lineages was constrained until the elemental needs and physiological strategies of the groups matched the dominant environmental conditions in the ocean surface layers, then the differences in the trace element stoichiometries may better match, or have been secondarily affected by, the environmental conditions when the lineages held ecological dominance and radiated as opposed to when the lineages first originated.
Changes in environmental conditions continue to have differential effects on phytoplankton community composition. Oceanic species of eukaryotic phytoplankton from the same taxonomic group have adapted to grow at much lower concentrations of Fe, Zn, Mn, Cu and Cd than those associated with coastal and estuarine environments [57,58]. Similar differences may exist in prokaryotic species. A sequence analysis found that a coastal Synechococcus CC9311 has an increased capacity to transport, store or use Fe and Cu compared with an oceanic strain [59]. A potential consequence of phylogenetic differentiation in specific metal requirements is that ongoing environmental change may relegate some taxonomic groups to minor or regional status (e.g. Glaucocystophyta) as the habitats they dominate change from being global to regional.
In summary, there is a great deal of variability in the elemental composition of different phytoplankton species when grown under similar nutrient-replete culture conditions. Although there is variability in elemental composition at the species level, there are similarities in elemental composition in species aggregated into higher taxonomic groupings, which reflect their common evolutionary history. Evolutionary change can occur throughout the history of a lineage, although endosymbiotic events are among the most dramatic and rapid of such changes. The probability of metal profiles of so many different species clustering into the Cyanophyta, Glaucocystophyta and the green and red plastid lineages (and separating on primary and secondary endosymbioses) is low unless much of the trace metal differences were established by the endosymbiotic events (figures 2 and 3). Much more work is required to compile data on the elemental composition of species from diverse taxonomic groups under a variety of environmental conditions to confirm the evolutionary inheritance hypothesis and better understand the evolutionary and physiological controls and ecological consequences of elemental composition in organisms.
Acknowledgements
We gratefully acknowledge the support and mentorship of Paul Falkowski and Oscar Schofield at the Institute of Marine and Coastal Studies, Rutgers University. This work was supported by a National Science Foundation Biocomplexity grant (OCE-0084032). A.Q. was supported by the Texas Institute of Oceanography and NOAA Award no. NA05NOS4191064. A.J.I. and Z.V.F. were supported by NSERC Canada (and the EPA STAR programme for Z.V.F.). Charlotte Fuller and Kevin Wyman are thanked for assistance with carbon and nitrogen elemental analysis. Paul Field, Susann Perone and Robert Sherrell are thanked for assistance with HR-ICP-MS. We are grateful to François Morel and CEBIC participants at Princeton University for sharing their expertise and time. We thank two anonymous reviewers for constructive comments that greatly improved the final manuscript.
References
- 1.Bekker A., Holland H. D., Wang P.-L., Rumble D., III, Stein H. J., Hannah J. L., Coetzee L. L., Beukes N. J. 2004. Dating the rise of atmospheric oxygen. Nature 427, 117–120 10.1038/nature02260 (doi:10.1038/nature02260) [DOI] [PubMed] [Google Scholar]
- 2.Canfield D. E. 2005. The early history of atmospheric oxygen. Annu. Rev. Earth Planet. Sci. 33, 1–36 10.1146/annurev.earth.33.092203.122711 (doi:10.1146/annurev.earth.33.092203.122711) [DOI] [Google Scholar]
- 3.Holland H. D. 2006. The oxygenation of the atmosphere and oceans. Phil. Trans. R. Soc. B 361, 903–915 10.1098/rstb.2006.1838 (doi:10.1098/rstb.2006.1838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brocks J. J., Logan G. A., Buick R., Summons R. E. 1999. Archean molecular fossils and the early rise of eukaryotes. Science 285, 1033–1036 [DOI] [PubMed] [Google Scholar]
- 5.Summons R. E., Jahnke L. L., Hope J. M., Logan G. A. 1999. 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature 400, 554–557 10.1038/23005 (doi:10.1038/23005) [DOI] [PubMed] [Google Scholar]
- 6.Knoll A. H., Javaux E. J., Hewitt D., Cohen P. 2006. Eukaryotic organisms in Proterozoic oceans. Phil. Trans R. Soc. B 361, 1023–1038 10.1098/rstb.2006.1843 (doi:10.1098/rstb.2006.1843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen B., Fletcher I. R., Brocks J. J., Kilburn M. R. 2008. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455, 1101–1104 10.1038/nature07381 (doi:10.1038/nature07381) [DOI] [PubMed] [Google Scholar]
- 8.Tappan H. 1980. Paleobiology of plant protists. San Francisco, CA: W. H. Freeman [Google Scholar]
- 9.Javaux E. J., Knoll A. H., Walter M. R. 2004. TEM evidence for eukaryotic diversity in mid-Proterozoic oceans. Geobiology 2, 121–132 10.1111/j.1472-4677.2004.00027.x (doi:10.1111/j.1472-4677.2004.00027.x) [DOI] [Google Scholar]
- 10.Delwiche C. F. 1999. Tracing the thread of plastid diversity through the tapestry of life. Am. Nat. 154, S164–S177 10.1086/303291 (doi:10.1086/303291) [DOI] [PubMed] [Google Scholar]
- 11.Falkowski P. G., Katz M. E., Knoll A. H., Quigg A., Raven J. A., Schofield O., Taylor F. J. R. 2004. The evolutionary history of eukaryotic phytoplankton. Science 305, 354–360 10.1126/science.1095964 (doi:10.1126/science.1095964) [DOI] [PubMed] [Google Scholar]
- 12.Hackett J. D., Yoon H. S., Butterfield N. J., Sanderson M. J., Bhattacharya D. 2007. Plastid endosymbiosis: sources and timing of the major events. In Evolution of primary producers in the sea (eds Falkowski P. G., Knoll A. H.), pp. 109–132 Burlington, MA: Elsevier Academic Press [Google Scholar]
- 13.Cavalier-Smith T. 2006. Cell evolution and earth history: stasis and revolution. Phil. Trans. R. Soc. B 361, 969–1006 10.1098/rstb.2006.1842 (doi:10.1098/rstb.2006.1842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keeling P. J. 2009. Chromalveolates and the evolution of plastids by secondary endosymbiosis. J. Eukaryot. Microbiol. 56, 1–8 10.1111/j.1550-7408.2008.00371.x (doi:10.1111/j.1550-7408.2008.00371.x) [DOI] [PubMed] [Google Scholar]
- 15.Yoon H. S., Hackett J. D., Bhattacharya D. 2002. A single origin of the peridinin- and fucoxanthin-containing plastids in dinoflagellates through tertiary endosymbiosis. Proc. Natl Acad. Sci. USA 99, 11 724–11 729 10.1073/pnas.172234799 (doi:10.1073/pnas.172234799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon H. S., Hackett J. D., Pinto G., Bhattacharya D. 2003. The single, ancient origin of chromist plastids. Proc. Natl Acad. Sci. USA 99, 15 507–15 512 10.1073/pnas.242379899 (doi:10.1073/pnas.242379899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz M. E., Finkel Z. V., Grzebyk D., Knoll A. H., Falkowski P. G. 2004. Evolutionary trajectories and biogeochemical impacts of marine eukaryotic phytoplankton. Annu. Rev. Ecol. Evol. Syst. 35, 523–556 10.1146/annurev.ecolsys.35.112202.130137 (doi:10.1146/annurev.ecolsys.35.112202.130137) [DOI] [Google Scholar]
- 18.Bjerrum C. J., Canfield D. E. 2002. Ocean productivity before about 1.9 Gyr ago limited by phosphorus adsorption onto iron oxides. Nature 417, 159–162 10.1038/417159a (doi:10.1038/417159a) [DOI] [PubMed] [Google Scholar]
- 19.Anbar A. D., Knoll A. H. 2002. Proterozoic ocean chemistry and evolution: a bioinorganic bridge? Science 297, 1137–1142 10.1126/science.1069651 (doi:10.1126/science.1069651) [DOI] [PubMed] [Google Scholar]
- 20.Saito M. A., Sigman D. M., Morel F. M. M. 2003. The bioinorganic chemistry of the ancient ocean: the co-evolution of cyanobacterial metal requirements and the biogeochemical cycles at the Archean-Proterozoic boundary. Inorg. Chim. Acta 356, 308–318 10.1016/S0020-1693(03)00442-0 (doi:10.1016/S0020-1693(03)00442-0) [DOI] [Google Scholar]
- 21.Martin R. E., Quigg A., Podkovyrov V. 2008. The evolution of ocean stoichiometry and diversification of the marine biosphere. Palaeogeogr. Palaeoclim. Palaeoecol. 258, 277–291 10.1016/j.palaeo.2007.11.003 (doi:10.1016/j.palaeo.2007.11.003) [DOI] [Google Scholar]
- 22.Martin J. H., Knauer G. A. 1973. The elemental composition of plankton. Geochim. Cosmochim. Acta 37, 1639–1653 10.1016/0016-7037(73)90154-3 (doi:10.1016/0016-7037(73)90154-3) [DOI] [Google Scholar]
- 23.Bruland K. W., Donat J. R., Hutchins D. A. 1991. Interactive influences of bioactive trace metals on biological production in oceanic waters. Limnol. Oceanogr. 38, 1555–1577 [Google Scholar]
- 24.Sunda W. G., Huntsman S. A. 1996. Antagonisms between cadmium and zinc toxicity and manganese limitation in a coastal diatom. Limnol. Oceanogr. 41, 373–387 10.4319/lo.1996.41.3.0373 (doi:10.4319/lo.1996.41.3.0373) [DOI] [Google Scholar]
- 25.Sunda W. G., Huntsman S. A. 1998. Processes regulating cellular metal accumulation and physiological effects: phytoplankton as model systems. Sci. Total Environ. 219, 165–181 [Google Scholar]
- 26.Ho T.-Y., Quigg A., Finkel Z. V., Milligan A., Wyman K., Falkowski P. G., Morel F. M. M. 2003. On the elemental composition of some marine phytoplankton. J. Phycol. 39, 1–15 [Google Scholar]
- 27.Quigg A., Finkel Z. V., Irwin A. J., Rosenthal Y., Ho T.-Y., Reinfelder J. R., Schofield O., Morel F. M. M., Falkowski P. G. 2003. The evolutionary inheritance of elemental stoichiometry in marine phytoplankton. Nature 425, 291–294 10.1038/nature01953 (doi:10.1038/nature01953) [DOI] [PubMed] [Google Scholar]
- 28.Chen Y. B., Zehr J. P., Mellon M. 1996. Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp IMS101 in defined media: evidence for a circadian rhythm. J. Phycol. 32, 916–923 10.1111/j.0022-3646.1996.00916.x (doi:10.1111/j.0022-3646.1996.00916.x) [DOI] [Google Scholar]
- 29.Morel F. M. M., Westall J. C., Rueter J. G., Jr, Chaplick J. P. 1975. Description of algal growth media AQUIL and FRAQUIL. Cambridge, MA: R.M. Parsons Laboratory for Water Resources and Hydrodynamics, Massachusetts Institute of Technology [Google Scholar]
- 30.Berman-Frank I., Quigg A., Finkel Z. V., Irwin A. J., Haramaty L. 2007. Cyanobacterial strategy of nitrogen-fixation influences diazotroph dependence on iron resources. Limnol. Oceanogr. 52, 2260–2269 [Google Scholar]
- 31.Cullen J. T., Field P. S., Sherrell R. M. 2001. The determination of trace metals in filtered suspended marine particulate material by sector field HR-ICP-MS. J. Anal. Atom. Spectrom. 16, 1307–1312 10.1039/b104398f (doi:10.1039/b104398f) [DOI] [Google Scholar]
- 32.R Development Core Team 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; ISBN 3-900051-07-0. See http://www.R-project.org [Google Scholar]
- 33.Finkel Z. V., Quigg A., Raven J. A., Reinfelder J., Schofield O. E., Falkowski P. G. 2006. Irradiance-induced changes in the elemental stoichiometry of marine phytoplankton. Limnol. Oceanogr. 51, 2690–2701 10.4319/lo.2006.51.6.2690 (doi:10.4319/lo.2006.51.6.2690) [DOI] [Google Scholar]
- 34.Finkel Z. V., Quigg A., Chiampi R., Schofield O., Falkowski P. G. 2007. Phylogenetic diversity in Cd : P regulation by marine phytoplankton. Limnol. Oceanogr. 52, 1131–1138 10.4319/lo.2007.52.3.1131 (doi:10.4319/lo.2007.52.3.1131) [DOI] [Google Scholar]
- 35.Redfield A. C., Ketchum B. H., Richards F. A. 1963. The influence of organisms on the composition of sea-water. In The Sea (ed. Hill M. N.), pp. 26–77 New York, NY: Interscience Publication [Google Scholar]
- 36.Duarte C. M. 1992. Nutrient concentration of aquatic plants: patterns across species. Limnol. Oceanogr. 37, 882–889 10.4319/lo.1992.37.4.0882 (doi:10.4319/lo.1992.37.4.0882) [DOI] [Google Scholar]
- 37.Geider R. J., La Roche J. 2002. Redfield revisited: variability of C : N : P in marine microalgae and its biochemical basis. Eur. J. Phycol. 37, 1–17 10.1017/S0967026201003456 (doi:10.1017/S0967026201003456) [DOI] [Google Scholar]
- 38.Payne C. D., Price N. M. 1999. Effects of cadmium toxicity on growth and elemental composition of marine phytoplankton. J. Phycol. 35, 293–302 10.1046/j.1529-8817.1999.3520293.x (doi:10.1046/j.1529-8817.1999.3520293.x) [DOI] [Google Scholar]
- 39.Fraga F. 2001. Phytoplanktonic biomass synthesis: application to deviations from Redfield stoichiometry. Sci. Mar. 65, 153–169 [Google Scholar]
- 40.Hayward J. 1970. Studies on the growth of Phaeodactylum tricornutum. VI. The relationship to sodium, potassium, calcium and magnesium. J. Mar. Biol. Assoc. UK 50, 293–299 [Google Scholar]
- 41.Sañudo-Wilhelmy S. A., et al. 2001. Phosphorus limitation of nitrogen fixation by Trichodesmium in the central Atlantic Ocean. Nature 411, 66–69 10.1038/35075041 (doi:10.1038/35075041) [DOI] [PubMed] [Google Scholar]
- 42.Tovar-Sanchez A., Sañudo-Wilhelmy S. A., Kustka A. B., Agustí S., Dachs J., Hutchins D. A., Capone D. G., Duarte C. M. 2006. Effects of dust deposition and river discharges on trace metal composition of Trichodesmium spp. in the tropical and subtropical North Atlantic Ocean. Limnol. Oceangr. 51, 1755–1761 10.4319/lo.2006.51.4.1755 (doi:10.4319/lo.2006.51.4.1755) [DOI] [Google Scholar]
- 43.Cullen J. T., Lane T. W., Morel F., Sherrell R. M. 1999. Modulation of cadmium uptake in phytoplankton by seawater CO2 concentration. Nature 402, 165–167 [Google Scholar]
- 44.Strzepek R. F., Price N. M. 2000. Influence of irradiance and temperature on the iron content of the marine diatom Thalassiosira weissflogii (Bacillariophyceae). Mar. Ecol. Prog. Ser. 206, 107–117 10.3354/meps206107 (doi:10.3354/meps206107) [DOI] [Google Scholar]
- 45.Finkel Z. V., Sebbo J., Feist-Burkhardt S., Irwin A. J., Katz M. E., Schofield O., Young J. R., Falkowski P. G. 2007. A universal driver of macroevolutionary change in the size of marine phytoplankton over the Cenozoic. Proc. Natl Acad. Sci. USA 104, 20 416–20 420 10.1073/pnas.0709381104 (doi:10.1073/pnas.0709381104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hutchins D. A., Fu F.-X., Zhang Y., Warner M. E. I., Feng Y., Portune K., Bernhardt P. W., Mulholland M. R. 2007. CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates, and elemental ratios: implications for past, present, and future ocean biogeochemistry. Limnol. Oceanogr. 52, 1293–1304 [Google Scholar]
- 47.Baldauf S. L., Roger A. J., Wenk-Siefert I., Doolittle W. F. 2000. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science 290, 972–977 10.1126/science.290.5493.972 (doi:10.1126/science.290.5493.972) [DOI] [PubMed] [Google Scholar]
- 48.Mounicou S., Szpunar J., Lobinski R. 2009. Metallomics: the concept and methodology. Chem. Soc. Rev. 38, 1119–1138 10.1039/b713633c (doi:10.1039/b713633c) [DOI] [PubMed] [Google Scholar]
- 49.Lane T., Saito M. A., George G. N., Pickering I. J., Prince R. C., Morel F. F. M. 2005. Isolation and preliminary characterization of a cadmium carbonic anhydrase from a marine diatom. Nature 435, 42. 10.1038/435042a (doi:10.1038/435042a) [DOI] [PubMed] [Google Scholar]
- 50.Raven J. A. 1988. The iron and molybdenum use efficiencies of plant growth with different energy, carbon and nitrogen sources. New Phytol. 109, 279–287 10.1111/j.1469-8137.1988.tb04196.x (doi:10.1111/j.1469-8137.1988.tb04196.x) [DOI] [Google Scholar]
- 51.Raven J. A., Evans M. C. W., Korb R. E. 1999. The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynth. Res. 60, 111–149 10.1023/A:1006282714942 (doi:10.1023/A:1006282714942) [DOI] [Google Scholar]
- 52.Williams R. J. P., Fraústo Da Silva J. J. R. 1996. The natural selection of the chemical elements. Bath, UK: Bath Press Ltd [Google Scholar]
- 53.Whitfield M. 2001. Interactions between phytoplankton and trace metals in the ocean. Adv. Mar. Biol. 41, 3–128 [Google Scholar]
- 54.Dupont C. L., Yang S., Palenik B., Bourne P. 2006. Modern proteomes contain putative imprints of ancient shifts in trace metal geochemistry. Proc. Natl Acad. Sci. USA 103, 17 822–17 827 10.1073/pnas.0605798103 (doi:10.1073/pnas.0605798103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zerkle A. L., House C. H., Cox R. P., Canfield D. E. 2006. Metal limitation of cyanobacterial N2 fixation and implications for the Precambrian nitrogen cycle. Geobiology 4, 285–297 10.1111/j.1472-4669.2006.00082.x (doi:10.1111/j.1472-4669.2006.00082.x) [DOI] [Google Scholar]
- 56.Javaux E. J., Knoll A. H., Walter M. R. 2001. Morphological and ecological complexity in early eukaryotic ecosystems. Nature 412, 66–69 10.1038/35083562 (doi:10.1038/35083562) [DOI] [PubMed] [Google Scholar]
- 57.Brand L. E., Sunda W. G., Guillard R. R. L. 1983. Limitation of marine phytoplankton reproductive rates by zinc, manganese, and iron. Limnol. Oceanogr. 28, 1182–1198 10.4319/lo.1983.28.6.1182 (doi:10.4319/lo.1983.28.6.1182) [DOI] [Google Scholar]
- 58.Brand L. E., Sunda W. G., Guillard R. R. L. 1986. Reduction of marine phytoplankton reproduction rates by copper and cadmium. J. Exp. Mar. Biol. Ecol. 96, 225–250 10.1016/0022-0981(86)90205-4 (doi:10.1016/0022-0981(86)90205-4) [DOI] [Google Scholar]
- 59.Palenik B., et al. 2006. The genome of Synechococcus CC9311: insights into adaptation to a coastal environment. Proc. Natl Acad. Sci. USA 103, 13 555–13 559 10.1073/pnas.0602963103 (doi:10.1073/pnas.0602963103) [DOI] [PMC free article] [PubMed] [Google Scholar]