Abstract
The degree to which animals use public and private sources of information has important implications for research in both evolutionary ecology and cultural evolution. While researchers are increasingly interested in the factors that lead individuals to vary in the manner in which they use different sources of information, to date little is known about how an animal's reproductive state might affect its reliance on social learning. Here, we provide experimental evidence that in foraging ninespine sticklebacks (Pungitius pungitius), gravid females increase their reliance on public information generated by feeding demonstrators in choosing the richer of two prey patches than non-reproductive fish, while, in contrast, reproductive males stop using public information. Subsequent experiments revealed reproductive males to be more efficient asocial foragers, less risk-averse and generally less social than both reproductive females and non-reproductives. These findings are suggestive of adaptive switches in reliance on social and asocial sources of information with reproductive condition, and we discuss the differing costs of reproduction and the proximate mechanisms that may underlie these differences in information use. Our findings have important implications for our understanding of adaptive foraging strategies in animals and for understanding the way information diffuses through populations.
Keywords: social learning, transmission, diffusion, foraging, producer–scrounger, group
1. Introduction
Animals living in changing environments need up-to-date information about their surroundings if they are to avoid danger, maximize foraging success and generally operate efficiently. One way that they can achieve this is by observing others as they interact with the environment, monitoring the success with which they do so in order to gain social information about the location and quality of different resources. While social information can be gathered relatively cheaply compared with private or direct sampling, theoretical analyses suggest that animals should not simply always copy others [1–3]. Information transmission is often imperfect, and conditions vary over space and through time, meaning that blind or obligate copying might give rise to accumulated errors. Accordingly, social information is expected to be associated with reduced reliability relative to asocial learning [1], and animals' reliance on social learning is predicted to be highly selective [1–7]. Such theory predicts that the extent to which animals attend to and use social information should vary, depending upon the environmental conditions and the state of the individual.
A number of recent experimental studies have identified factors that influence the relative reliance of individual animals upon social and asocial sources of information [6]. These include prior experience [8,9], predation risk [10] (but see [11,12]), phenotype of both observers and demonstrators [13], demonstrator pay-off [14] and demonstrator numbers [15]. To date, however, little attention has been paid to the role that reproductive state might play in affecting the relative use of social information. Reproductive state directly influences fitness by determining immediate and future reproductive potential, while the development of eggs and conspicuous breeding ornamentation require the sequestration of energy and resources that can increase conspicuousness to predators [16,17], lead to preferential targeting by predators [18] and reduce the capacity for escape [16,19–21].
The aforementioned theory predicts that social and asocial sources of information will be associated with different costs and benefits [6]. Socially acquired information can potentially be gathered cheaply and remotely, and from the relative safety of cover (e.g. [22]), but doing so may incur opportunity costs and risks potentially receiving outdated or inaccurate information [1]. Privately acquired information can be more accurate; however, individuals must invest time and energy in gathering it, and may expose themselves to predation risk while doing so [23]. Given that reproductive state has been shown to affect the energetic costs and requirements of movement [24,25], as well as susceptibility to predation risk [16,17,19–21], it is reasonable to consider whether the reproductive state of an individual might affect the weighting it gives to sources of public information.
In this study, we explored the hypothesis that reliance on public information in a foraging context would vary significantly among ninespine sticklebacks (Pungitius pungitius) that differed in their reproductive state. Public information use refers to the capacity of animals to estimate the quality of a resource, such as the richness of a food patch, by monitoring the success and failure of other animals interacting with that resource [26]. Ninespine sticklebacks were chosen in part because they have proved a robust and productive model system with which to explore the factors affecting reliance on social and asocial learning [8,9,13–15,22,27]. Previous experiments in our laboratory have established that ninespines will use both public and private information when foraging [8,9,22]. Accordingly, here we measured public information using an established binary choice assay [8,22,28] in which test fish were first allowed to observe from distance equal-sized groups of conspecifics feeding at two prey patches that yielded prey at different rates [8], and subsequently given the opportunity to choose a prey patch after the other fish had been removed. Selection of the socially demonstrated richer patch is indicative of public information use.
Several other factors make ninespine sticklebacks ideal candidates for testing hypotheses about the influence of reproductive state on social learning behaviour. Ninespines exhibit phenotypic changes when entering the breeding phase, which may make them more vulnerable to predation and incur energetic costs compared with non-reproductives. Females produce a sizeable egg mass, leading them to develop a greatly distended abdomen. In the closely related threespine stickleback (Gasterosteus aculeatus), gravidity has been shown to reduce the likelihood of escape from an attacking predator [29] and to lead females to become more vigilant and more likely to inspect predators [30], while in other fish species gravidity can lead to slower general swimming speeds [31] and slower rapid responses, owing to reduction in the contractile properties of the fast muscle fibre [25]. Males develop nuptial pigmentation, consisting of matt black coloration across their undersides and flanks, while their pelvic spines turn a contrasting white; this form of ‘counter-countershading’ may potentially make them more susceptible to detection by visual predators [32]. In threespine sticklebacks, nuptial coloration and the associated courtship behaviour are also associated with increased predation risk [17]. Males establish and defend territories, and, post-mating, provide energetically costly parental care, during which they have very limited opportunities to feed [33–37]. Not all adults enter the breeding phase simultaneously, meaning that within a population during the breeding season there can be found not only reproductive males and females but also non-reproductives of the same size and age that retain their cryptic coloration.
We predicted that the use of public information would vary significantly as a function of stickleback reproductive state. Specifically, we predicted that gravid females, which are more conspicuous to predators and may be more likely to be targeted by them [18,21,29], would rely most heavily upon the comparatively low-risk strategy of utilizing public information in order to minimize predation risk. Gravid females of many species adopt other risk-averse strategies such as fleeing sooner [38], reducing activity [39] and remaining close to cover [29,40] in order to minimize encounter rates with predators. Thus, we anticipate that risk sensitivity would lead gravid females to exploit public information and to pay the associated cost of reduced reliability in foraging information relative to asocial learning.
We tested two mutually exclusive predictions about public information use in reproductive males. On the one hand, the development of nuptial coloration in reproductive males potentially increases their conspicuousness to predators [17,32], while investing effort in identifying breeding territories and potential mates could reduce their capacity to detect threats. As with gravid females, this might lead them to adopt a low-risk but low-reliability strategy of utilizing public information, in which case we anticipate no sex difference among reproductives. On the other hand, successful breeding requires that males compete vigorously with other males to establish a territory, build and maintain a nest, court females and provide energy-intensive parental care following mating, guarding and fanning the nest and fry. Doing so limits opportunities to feed and places great demands upon their energy reserves [33–37]. To the extent that male sticklebacks experience greater variance in reproductive success than do females, which is the most frequent pattern [41,42], there may be considerable fitness benefits associated with achieving greater-than-average foraging success. This may drive males to the high-risk but potentially high-return strategy of greater reliance upon private information, leading to a sex difference among reproductives. Finally, we predicted that we would see no inter-sexual variation in behaviour between non-reproductive sticklebacks.
These predictions were tested in three experiments, which investigated the use of public information (experiment 1), asocial foraging efficiency (experiment 2) and shoaling behaviour (experiment 3) in reproductive and non-reproductive sticklebacks of both sexes. In experiment 1, we found that gravid females and non-reproductives were most likely to select the rich patch first, with the gravid females spending more time there (which may indicate a greater relative preference), while reproductive males showed no evidence of using public information at all. We also saw no sex differences in the behaviour of non-reproductive males and females. Experiments 2 and 3, which were conducted to aid interpretation of these findings, revealed reproductive males to be more efficient asocial foragers, and generally less social in their shoaling behaviour than non-reproductives and gravid females.
2. General methods
Details of the capture and husbandry of the test fish prior to testing are provided in the electronic supplementary material. The experiments outlined below took place from February to April 2009 and February to April 2010.
3. Experiment 1: public information use in reproductive and non-reproductive ninespine sticklebacks
This experiment aimed to determine whether the use of public information differed significantly among reproductive male, gravid female and non-reproductive ninespine sticklebacks. We used an experimental assay adapted from that of Coolen et al. [22], who provide a full description of the test arena. For brevity, therefore, we give only an overview of the arena here. A full description and a figure of the apparatus are provided in electronic supplementary material, figure S1a.
(a). Methods
(i). Apparatus
The assay simulated two prey patches, which yielded prey at a 3 : 1 ratio, henceforth termed ‘rich’ and ‘poor’, respectively. These were located at opposite ends of an experimental arena. Individual focal fish, initially held in the centre of the arena and unable to sample either patch directly, nor see the food at the patches, were allowed to observe two equally sized demonstrator groups feeding at each patch. The demonstrator's prey capture rate conveyed public information to the observing focal fish about the relative quality of each patch. The demonstrators were housed in watertight chambers to ensure that no chemical cues originating from the prey were available to focal fish, such that the focal fish could only base their patch choices upon visual cues received during the demonstration phase [43]. Following the demonstration, subjects were tested in isolation to establish whether they had acquired a preference for the richer patch as a result of this observational experience. We tested four categories of focal fish: reproductive males, gravid females, non-reproductive males and non-reproductive females.
We used non-reproductive conspecifics as demonstrators. These were unsexed and were size-matched to within 3 mm body length of the focal fish and each other. The demonstrators were drawn from a pool of around 80 fish. Though demonstrators were used in multiple trials, no individual was used more than once in any 3-day period. Focal fish were only tested once. We tested only reproductive males showing full nuptial coloration, with black pigmentation covering the entire of the head, flanks and ventral surface. Males showing only partial coloration were not tested. The males had not yet established territories or built nests. We selected gravid females with a full egg mass. Non-reproductive males and females drawn from the same holding tanks as the reproductives were unsexed at the time of testing. Post-testing, they were held individually within mesh cylinders (approx. 20 cm diameter, 25 cm tall, eight cylinders per aquarium, 18°C) for up to four weeks until they entered the reproductive state and could be reliably sexed. We tested 22 reproductive males (12 in 2009 and 10 in 2010) and 23 gravid females (11 in 2009 and 12 in 2010). We only used ‘ripe’ females whose individual egg outlines could be seen through the body wall, indicating that they were ready to spawn in the next few days. We also tested 30 initially unsexed fish, yielding data for 14 males and 12 females (four fish failed to enter reproductive condition). All non-reproductives were tested in 2009. The fish measured 40–45 mm long at the time of testing, and we saw no length differences between sexes or reproductives and non-reproductives (one-way ANOVA: F2,68 = 1.74, p = 0.18).
(ii). Procedure
The demonstrators and focal fish were deprived of food for 24 h before testing in order to ensure that they were motivated to feed. Then, three demonstrators were added to each demonstrator chamber and allowed to settle for 10 min before the focal fish was added to the central holding unit and allowed to settle for a further 10 min. The demonstration phase lasted for 6 min and ran as follows. At the beginning of the first, third and fifth minute of the trial, prey suspended in 1 cm3 of tank water were added to the feeder in the designated rich patch, using a pipette. During the first and third minutes of the trial, the poor patch received no prey. A ‘blank’ consisting of 1 cm3 of tank water was added to the feeder at the same time that the rich feeder received prey. During the fifth minute, the poor feeder also received prey. This ensured that while prey were delivered at a 3 : 1 ratio, the focal fish was unable to select a prey patch simply on the basis of it being the last place it saw fish feeding. In all trials, the demonstrators consumed all of the prey.
After 6 min, the Perspex walls set in runners on the side of the tank (electronic supplementary material, figure S1a(v)) were carefully removed and replaced with entirely opaque black plastic walls. The two demonstrator chambers (electronic supplementary material, figure S1a(iii)) were then removed from the test tank. This took approximately 30 s and reduced the water depth in the main tank to 9 cm. Immediately after this, the opaque black plastic walls were removed and the focal fish was allowed to settle for a further 5 min. Following this period, the holding unit was raised 5 cm using the pulley mechanism, releasing the subject. The base of the holding unit was left suspended beneath the water surface, so as not to disturb the surface and startle the test fish. This commenced the trial, and we recorded the following behaviour patterns for 3 min: the location of the focal fish every 6 s, noting whether it was within either goal zone or the central neutral zone yielding a total of 30 data points, whether or not it was in or within one body length of the cover, the latency to enter either goal zone, the first goal zone it entered and the number of switches made between goal zones.
(iii). Statistical analyses
We used linear mixed models to compare the latency to enter a goal zone, the difference in time allocation to the goal zones, the switching rate between goal zones and the proportion of time spent in cover of the non-reproductive males and females, with reproductive state category as a fixed factor and year of testing as a random factor. Proportional data on time allocation to goal zones and the proportion of trial time spent in cover were normalized by arcsine transformation. The difference in time allocation was calculated by subtracting the proportion of time spent in the poor goal zone from that spent in the rich goal zone. These were seen not to differ significantly between non-reproductive males and females (see below). These data were therefore pooled for a mixed-sex class, ‘non-reproductives’, which were compared against the gravid females and reproductive males in subsequent analyses.
We used binary logistic regression to investigate the first goal zone choice of focal fish. Reproductive state (non-reproductive, gravid female or reproductive male) was included as a fixed factor, along with year of testing. Reverse Helmert contrasts were used to make comparisons between groups. Latency to select a prey patch, switching rate and time in cover were also included in the initial analysis as covariates. However, in sequential iterations of the analysis, non-significant covariates were dropped, resulting in a minimum adequate model that included reproductive state and time in cover only. For each group, the difference in time allocation was also compared to a null expected score of zero using t-tests.
(b). Results
(i). Non-reproductives
We saw no sex differences between male and female non-reproductives in terms of either time allocation (one-way ANOVA: F1,25 = 0.44, p = 0.83), time spent in cover (F1,25 = 0.20, p = 0.65), switching rate between patches (F1,25 = 0.12, p = 0.73) or latency to select a patch (F1,25 = 0.02, p = 0.89). Henceforth, we combine these into the single category of non-reproductives.
(ii). First patch choice
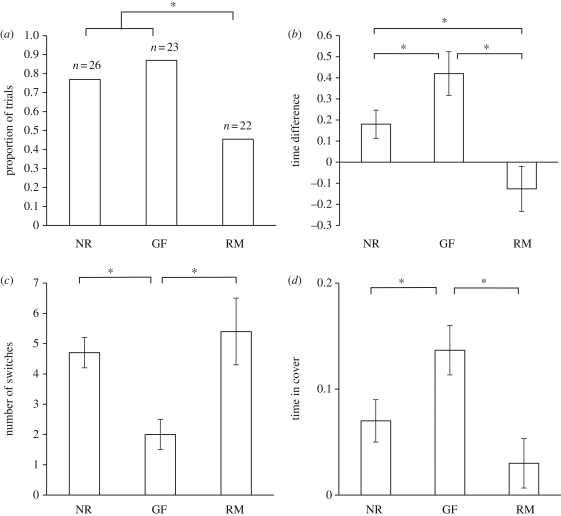
The three reproductive status groups differed significantly in their likelihood of choosing the rich patch first (Binary logistic regression: Z = −9.94, n = 71, p = 0.007; figure 1a). Reverse Helmert contrasts revealed that both the gravid females and the non-reproductives were more likely than the reproductive males to select the rich patch first (t = 9.44, d.f. = 1, p = 0.007 and t = 5.81, d.f. = 1, p = 0.016, respectively). While a greater proportion of gravid females than non-reproductives chose the rich patch first, this difference was not statistically significant (t = 0.79, d.f. = 1, p = 0.33). Binomial tests indicated that gravid females and non-reproductives were significantly more likely to choose the rich patch over the poor patch first (20 out of 23, p < 0.001 and 20 out of 26, p = 0.009, respectively), whereas reproductive males showed no preference for either patch (10 out of 22, p = 0.83). We saw no significant effect of time in cover upon first choice (Z = −1.81, n = 71, p = 0.18). Latency to select a prey patch did not differ significantly between the three groups (analysed separately, linear mixed model, reproductive group: F2,68 = 0.39, p = 0.67; year of testing: F1,68 = 2.47, p = 0.12).
Figure 1.
Experiment 1: investigating public information use. (a) The proportion of fish choosing the rich patch first. Sample sizes are indicated above each bar. (b) Proportional time allocation (time in rich patch−time in poor patch, mean ± s.e.). (c) The number of switches between patches during the trial (mean ± s.e.). (d) Proportional time spent in cover (mean ± s.e.). Based on untransformed data. RM, reproductive males; GF, gravid females; NR, non-reproductives. *p < 0.05.
(iii). Time allocation
Time allocation differed significantly between the three groups (F2,68 = 5.93, p = 0.002; figure 1b), while we saw no effect of year of testing (F1,68 = 1.66, p = 0.20). Tukey post hocs revealed that gravid females spent significantly more time in the rich patch compared with either the reproductive males or non-reproductives (p = 0.004 and p = 0.035), while the non-reproductives spent more time there than did the reproductive males (p = 0.05). t-tests confirmed that both gravid females and non-reproductives spent significantly more time in the rich patch than in the poor patch (t = 4.01, d.f. = 22, p = 0.001 and t = 2.02, d.f. = 25, p = 0.042, respectively) while the reproductive males showed no patch preference (t = −1.18, d.f. = 21, p = 0.24).
(iv). Switching between patches
Switching rate differed significantly between the three groups (F2,68 = 4.99, p = 0.004; figure 1c), but was unaffected by year of testing (F1,68 = 0.85, p = 0.32). Gravid females switched significantly less than either the reproductive males or non-reproductives (Tukey post hoc: p = 0.008 and p = 0.030, respectively), while the latter two groups did not differ significantly (p = 0.80).
(v). Time in cover
Time spent in cover differed between groups (F2,68 = 5.29, p = 0.007; figure 1d), but not between years of testing (F1,68 = 1.12, p = 0.29). Gravid females spent significantly more time in cover than either the reproductive males or non-reproductives (Tukey post hoc: p = 0.007 and p = 0.049, respectively), while again the latter two groups did not differ significantly (p = 0.27).
4. Experiment 2: asocial foraging in reproductive and non-reproductive ninespine sticklebacks
Experiment 1 revealed a significant effect of reproductive state upon the use of social information related to foraging. As a vehicle to interpret these findings better, we went on to explore how reproductive state affected foraging in an asocial context. Experiment 2 set out to determine whether there existed differences between reproductive males, gravid females and non-reproductive sticklebacks in how they behaved while gathering foraging information asocially in a novel environment.
(a). Methods
(i). Apparatus
We established a test arena (electronic supplementary material, figure S1b) consisting of a grid of 12 × 16 4 cm wide and 4 cm deep square pits within an opaque plastic container (internal dimensions: 64 × 48 cm). The whole apparatus was constructed from black plastic. The pits were filled with 1 cm of coarse sand. The surface of the water in the test tank was 3 cm above the base of the pits. This meant that the test fish had to pass over the pit to see inside it. The apparatus was designed to represent a structurally complex substrate.
In one corner of the arena, four pits were covered by an 8 cm square plastic base, forming a platform. This held a removable colourless plastic holding unit, measuring 7 × 7 cm and 10 cm tall. The fish was held in this area at the start of the trial. One pit, located four squares from either edge of the grid in the corner directly opposite the platform and holding unit, contained a prey patch of five chironomid larvae. These were added prior to the introduction of these test fish. The whole apparatus was surrounded by plastic screening to minimize outside disturbance and was filmed from above with a tripod-mounted high-definition video camera (Canon HD20). Water and prey were changed after each trial.
We tested 11 gravid females, 10 reproductive males and 10 unsexed non-reproductive focal fish. All fish measured 38–42 mm standard length, and there were no size differences between the three groups (one-way ANOVA: F2,28 = 0.98, p = 0.38). No fish was used more than once.
(ii). Procedure
Subjects were deprived of food for 24 h before the trial began in order to generate motivation to forage. Prior to the start of each trial, prey were added to the designated square. At the beginning of each trial, a single fish was added to the holding unit in the corner of the test arena and allowed to settle for 10 min. Following this, the holding unit was raised and removed, beginning the trial. The arena was filmed for a further 15 min. We recorded the following behaviours: the number and location of all of the grid squares that the fish passed through and the latency of the fish to enter the square containing the prey patch.
(iii). Analysis
We used one-way ANOVAs with Tukey HSD post hoc analyses to compare the following between the three groups: total distance travelled (in grid squares), the total number of different squares entered (out of a total of 188; four squares in the 12 × 16 grid were covered by the starting platform), the proportion of squares entered that were located at the edges of the arena (a measure of thigmotaxis) and the latency to enter the square containing the prey patch.
(b). Results
(i). Space usage
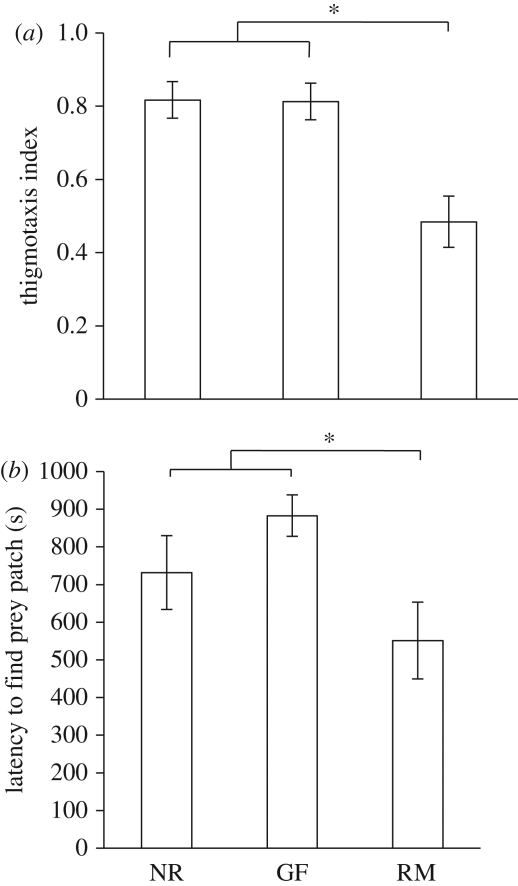
We observed a difference in space use and specifically thigmotaxis (use of space immediately next to the arena walls). Gravid females and non-reproductives performed a significantly greater proportion of their movement in the squares at the edges of the arena (i.e. moving along the walls) than the reproductive males, who were by contrast more likely to explore the inner grid squares of the arena (F2,30 = 11.12, p < 0.001; Tukey post hocs: reproductive males versus gravid females and non-reproductives, p < 0.001 in each case; gravid females versus non-reproductives, p = 0.42; figure 2a).
Figure 2.
Experiment 2: investigating asocial foraging behaviour in a novel arena. (a) The proportion of movement occurring along the arena edge (total movements in edge squares ÷ total movements in all squares, mean ± s.e.), a measure of thigmotaxis. See main text for further details. (b) The latency to find the prey patch (in seconds, mean ± s.e.). Based on untransformed data. RM, reproductive males; GF, gravid females; NR, non-reproductives. *p < 0.05.
(ii). Latency to find food
Reproductive males found the prey patch significantly sooner than either the gravid females or non-reproductives (F2,30 = 4.36, p = 0.002; Tukey post hocs: reproductive males versus gravid females and non-reproductives, p = 0.001 in each case; gravid females versus non-reproductives, p = 0.50; figure 2b).
(iii). Distance travelled
We saw no differences between the three reproductive categories in distance travelled during the trial (mean number of grid squares passed through ±s.e.: gravid females, 386.4 ± 76.0; reproductive males, 339.3 ± 83.4; non-reproductives, 350.6 ± 84.0; one-way ANOVA: F2,30 = 1.04, p = 0.65). We also saw no difference between them in terms of the number of different squares entered, out of a possible total of 188 (mean number of different grid squares entered ± s.e.: gravid females, 91.4 ± 12.0; reproductive males, 108.9 ± 17.5; non-reproductives, 74.8 ± 13.0; one-way ANOVA: F2,30 = 1.04, p = 0.65 and F2,30 = 1.34, p = 0.27).
5. Experiment 3: shoaling behaviour in reproductive and non-reproductive ninespine sticklebacks
Experiment 1 revealed differences among reproductive males, gravid females and non-reproductive ninespine sticklebacks in public-information use, while experiment 2 revealed that reproductive males located food faster and differed in space use from gravid females and non-reproductives. In experiment 3 we explored whether fish from these different reproductive categories differed in their social behaviour outside of a foraging context, by investigating the degree to which they shoaled and their willingness to move away from a group of conspecifics.
(a). Methods
(i). Apparatus
We established a binary choice test tank measuring 90 cm long and 30 cm wide, with a water depth of 20 cm. The tank contained a 2 cm deep layer of coarse sand. We created a 5 cm wide stimulus chamber at either end using perforated, colourless plastic screening. On the back wall of the tank, we marked 20 vertical lines at 4 cm intervals. Each interval was approximately equal to one fish body length. The sides and rear of the test tank were covered with black plastic screening. A high-definition camera (Canon HG20) was positioned facing through a 20 cm square hole within a further black plastic screen around 80 cm in front of the test tank. One of the stimulus compartments was left empty, while to the other we added five non-reproductive ninespines measuring 39–42 mm standard length. These were termed the stimulus shoal. They were allowed to settle for 10 min before the focal fish was added to the tank, and were changed after every trial. They were drawn from a pool of approximately 80 stimulus fish. No stimulus fish was used twice in the same 48 h period. We tested 14 gravid females, 15 reproductive males and 15 unsexed non-reproductive focal fish. No focal fish was used more than once. All measured 38–42 mm standard length, and there were no size differences between the three groups (one-way ANOVA: F2,43 = 0.48, p = 0.61).
(ii). Procedure
The focal fish was added to the centre of the test tank 10 min after the stimulus shoal was added and was allowed to move freely and acclimatize for a further 10 min before the trial began. After this, we recorded the behaviour of the focal fish for a further 10 min. We point-sampled the location of the fish at 20 s intervals (n = 30 measures per trial) and noted whether or not the fish was within two body lengths of either stimulus chamber (corresponding to the inter-individual shoaling distance seen in free-moving shoals [44], and also its distance from the stimulus shoal to the nearest body length).
(iii). Statistical analyses
We compared the time spent within two body lengths of the stimulus chambers and the total distance in body lengths from the stimulus shoal for gravid females, reproductive males and non-reproductives. As data were not normally distributed, non-parametric statistical analyses were performed.
(b). Results
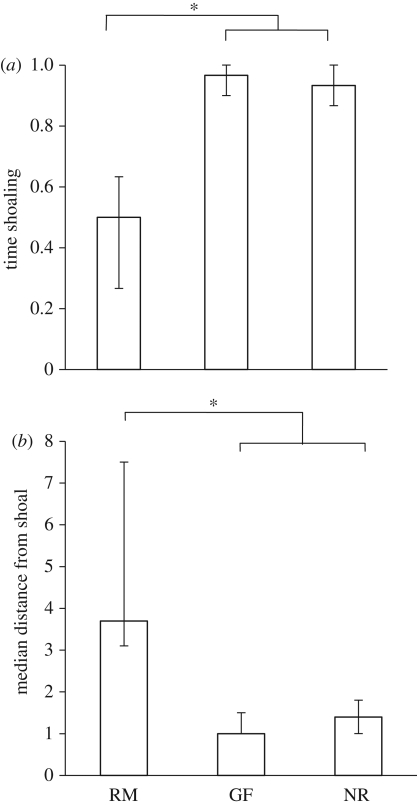
Fish in each category spent significantly more time within two body lengths of the chamber containing the stimulus shoal than they did within two body lengths of the empty control (Wilcoxon signed-rank tests: reproductive males: n = 15, Z = −2.85, p = 0.004; gravid females: n = 14, Z = −3.62, p < 0.001; non-reproductives: n = 15, Z = −2.98, p = 0.003).
However, we also saw that reproductive males spent less time within two body lengths of the stimulus shoal than either the gravid females or the non-reproductives (Kruskall–Wallis: X2 = 21.50, d.f. = 2, p < 0.001; Mann–Whitney, applied as post hoc tests: reproductive males versus gravid females: Z = −4.01, n = 15 and 14, p < 0.001; reproductive males versus non-reproductives: Z = −3.70, n = 15 and 15, p < 0.001; gravid females versus non-reproductives: Z = −1.21, n = 14 and 15, p = 0.32; figure 3a).
Figure 3.
Experiment 3: investigating shoaling behaviour. (a) The proportion of time (median ± quartiles) spent shoaling (time within two body lengths of shoal − time within two body lengths of empty chamber). (b) The median distance (±quartiles) from the shoal in body lengths. Based on untransformed data. RM, reproductive males; GF, gravid females; NR, non-reproductives. *p < 0.05.
Males also tended to remain further from the shoal than either the gravid females or the non-reproductives (Kruskall–Wallis: X2 = 18.30, d.f. = 2, p < 0.001; Mann–Whitney, applied as post hoc tests: reproductive males versus gravid females: Z = −3.89, n = 15 and 14, p < 0.001; reproductive males versus non-reproductives: Z = −3.41, n = 15 and 15, p = 0.001; gravid females versus non-reproductives: Z = −1.03, n = 14 and 15, p = 0.33; figure 3b).
6. Discussion
The findings of this study provide compelling evidence that the reproductive state of ninespine sticklebacks influences the ways in which they gather and use information, in a sex-specific manner. As no sex-specific variation was seen in the behaviour of non-reproductive fish, our findings imply that it is reproductive state, rather than sex per se, that underlies the observed sex-specific differences in attending to the behaviour of others.
Experiment 1 revealed significant differences in how reproductive state affects use of public information in a foraging context. Both gravid females and non-reproductives disproportionately selected the demonstrated rich patch first, indicating a reliance on public information, while reproductive males were equally likely to visit either patch first. Moreover, gravid females spent more time in the rich patch than did the non-reproductives, with both groups spending more time there than the reproductive males. While both the gravid females and non-reproductives displayed an overall preference for the rich patch over the poor patch, the reproductive males exhibited no such preference. Further, the gravid females switched between patches less frequently and spent more time in cover than either the non-reproductives or the reproductive males. These observations are consistent with our hypothesis that gravid females would show more risk-averse behaviour than non-reproductive females.
The differences in public information use observed in experiment 1 are unlikely to be attributable to differences in activity since, when measured directly in experiment 2, this was not seen to differ significantly between groups. Experiment 2 established that reproductive males found food sooner than other fish when foraging alone in a novel environment. Reproductive males were also less thigmotaxic than either the gravid females or non-reproductives, performing a greater proportion of movement in the inner grid squares (away from the arena edges) than fish in other conditions, and this probably contributed to their foraging success. Thigmotaxis is commonly associated with risk aversion [45,46]. Experiment 3 revealed that reproductive males spent less time shoaling and remained a greater distance from a stimulus shoal compared with either gravid females or non-reproductives, which is indicative of a lower degree of sociality.
The behaviour of gravid females (which spent more time in the demonstrated rich patch, more time in cover and which were less likely to switch between patches compared with non-reproductives) is consistent with risk-minimizing foraging behaviour. Gravidity can be costly in terms of increased predation risk through greater conspicuousness, reduced capacity for escape and preferential targeting by predators [16]. Consequently, gravid females of many species adjust their behaviour to reduce this risk (e.g. [29,38–40]). This may reduce foraging opportunities at a time when metabolic demands are high. The requirement to continue foraging while minimizing predation risk should favour increased reliance on social information, as was seen in this study in terms of relative time preference for the rich prey patch. Gravidity may also impose energetic costs owing to higher body mass and greater hydrodynamic drag [16], which could conceivably account for, for example, the lower switching rate seen in this study. However, the absence of overall activity differences between fish categories in our second experiment renders this counter-explanation unlikely.
Conversely, we saw no evidence that reproductive males used public information in our first experiment, while our second and third experiments suggested, respectively, that they were more efficient at foraging alone and that they were generally less attracted to conspecifics than fish in other conditions. Reduced shoaling tendency and reduced thigmotaxis are both potentially risky, since individuals moving alone or in dispersed groups away from cover are potentially more vulnerable to predation [47]. Evidence from other vertebrates suggests that heightened levels of circulating testosterone, associated with the onset of the reproductive phase, can reduce the sensitivity of males to risk, and reduce their responses to predator cues [48,49]. Such behaviour may be adaptive if risk-prone males have greater access to females and therefore greater reproductive success compared with risk-averse males [50]. In sticklebacks, reproductive males stop shoaling in order to establish and defend territories, which may explain the findings of our third experiment (we used males exhibiting nuptial colouring, but which had yet to establish territories).
Plausibly, the same physiological changes that lead reproductive males to cease shoaling may also reduce their attention to the behaviour of conspecifics in a foraging context. This may explain our finding in the first experiment that reproductive males were the only category of fish that did not exploit the public information about patch quality available from the demonstrators. Following mating, males provide parental care, which limits opportunities to feed and places great demands upon their energy reserves [33–37]. Conceivably, this may cause them to rely upon costly but accurate private information to a greater extent. Behavioural changes that cause them to forage more efficiently and to become less social, freeing them from conforming to the behaviour of conspecifics, could allow reproductive males to maximize their food intake immediately prior to establishing a territory and raising a brood, but at the price of increased predation risk. Doing so might be adaptive if low energy levels impact on male–male competition or female choice, or if the cost of failing to find enough food is either starvation or the forced abandonment of the brood. While, in theory, the failure of reproductive males in our study to attend to public information may be a non-adaptive side-effect of increased circulating testosterone (but which may have other fitness benefits, such as enhanced access to females), the enhanced foraging success of these fish in experiment 2 leads us to favour the alternative explanation that reproductive males pursue an adaptive private sampling foraging strategy, which functions to maximize food intake prior to parental care. While the proximate mechanisms underlying the observed differences in the behaviour of reproductive males and females seen in this study remain to be elucidated, the bidirectional influence of reproductive state on the foraging behaviour of this species is clear, and provides a striking indication of the hitherto neglected significance of reproductive state on information use in animals.
Useful further work might focus upon integrating reproductive state into experimental and theoretical analyses of the evolution of social learning strategies, which determine both when and whom individuals should copy as a function of the costs and pay-offs to the copying individual [1,3,7]. Experimental studies have shown that several species are capable of gathering and discriminating between public and private information, and of basing behavioural responses upon these adaptively under the influence of previous experience and the external environment [6,8–10,51]. Future studies could build upon the findings of the current study (i.e. that reliance upon public information varies between the sexes as a function of reproductive state) by making explicit predictions as to how attention to and use of socially transmitted information should change throughout the reproductive cycle. Over and above these ecological implications, reproductive state could be worthy of attention for its importance in explaining within-sample variation in experimental studies of social learning in general. Finally, by determining which individuals generate and which exploit social information within populations, reproductive state potentially has important implications for understanding and predicting rates and patterns of diffusion of socially learned behaviour and preferences through animal populations.
Acknowledgements
Funding to K.N.L. from NERC (NE/D010365/1) and ERC (Advanced Grant, EVOCULTURE 232823). We thank Rob Boyd, Innes Cuthill, Luke Rendell, Ashley Ward and three anonymous referees for their helpful comments. Our research adhered to the Association for the Study of Animal Behaviour's guidelines for using animals in research.
References
- 1.Boyd R., Richerson P. J. 1985. Culture and the evolutionary process. Chicago, IL: University of Chicago Press [Google Scholar]
- 2.Henrich J., McElreath R. 2003. The evolution of cultural evolution. Evol. Anthropol. 12, 123–135 10.1002/evan.10110 (doi:10.1002/evan.10110) [DOI] [Google Scholar]
- 3.Laland K. N. 2004. Social learning strategies. Learn. Behav. 32, 4–14 [DOI] [PubMed] [Google Scholar]
- 4.Feldman M., Aoki K., Kumm J. 1996. Individual versus social learning: evolutionary analysis in a fluctuating environment. Anthropol. Sci. 104, 209–231 [Google Scholar]
- 5.Giraldeau L.-A., Valone T. J., Templeton J. J. 2002. Potential disadvantages of using socially acquired information. Phil. Trans. R. Soc. Lond. B 357, 1559–1566 10.1098/rstb.2002.1065 (doi:10.1098/rstb.2002.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kendal R. L., Coolen I., Van Bergen Y., Laland K. N. 2005. Tradeoffs in the adaptive use of social and asocial learning. Adv. Stud. Behav. 35, 333–379 10.1016/S0065-3454(05)35008-X (doi:10.1016/S0065-3454(05)35008-X) [DOI] [Google Scholar]
- 7.Rendell L., et al. 2010. Why copy others? Insights from the social learning strategies tournament. Science 328, 208–213 10.1126/science.1184719 (doi:10.1126/science.1184719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Bergen Y., Coolen I., Laland K. N. 2004. Nine-spined sticklebacks exploit the most reliable source when public and private information conflict. Proc. R. Soc. Lond. B 271, 957–962 10.1098/rspb.2004.2684 (doi:10.1098/rspb.2004.2684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendal J. R., Rendell L., Pike T. W., Laland K. N. 2009. Nine-spined sticklebacks deploy a hill-climbing social learning strategy. Behav. Ecol. 20, 238–244 10.1093/beheco/arp016 (doi:10.1093/beheco/arp016) [DOI] [Google Scholar]
- 10.Webster M. M., Laland K. N. 2008. Social learning strategies and predation risk: minnows copy only when using private information would be costly. Proc. R. Soc. B 275, 2869–2876 10.1098/rspb.2008.0817 (doi:10.1098/rspb.2008.0817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briggs S., Godin J.-G. J., Dugatkin L. A. 1996. Mate choice copying under predation risk in the Trinidadian guppy (Poecilia reticulata). Behav. Ecol. 7, 151–157 10.1093/beheco/7.2.151 (doi:10.1093/beheco/7.2.151) [DOI] [Google Scholar]
- 12.Galef B. G., Whiskin E. E. 2006. Increased reliance on socially acquired information while foraging in risky situations? Anim. Behav. 72, 1169–1176 10.1016/j.anbehav.2006.05.003 (doi:10.1016/j.anbehav.2006.05.003) [DOI] [Google Scholar]
- 13.Duffy G. A., Pike T. W., Laland K. N. 2009. Size-dependent directed social learning in nine-spined sticklebacks. Anim. Behav. 78, 371–375 10.1016/j.anbehav.2009.05.015 (doi:10.1016/j.anbehav.2009.05.015) [DOI] [Google Scholar]
- 14.Pike T. W., Kendal J. R., Rendell L., Laland K. N. 2010. Learning by proportional observation in a species of fish. Behav. Ecol. 21, 570–575 [Google Scholar]
- 15.Pike T. W., Laland K. N. 2010. Conformist learning in nine-spined sticklebacks' foraging decisions. Biol. Lett. 6, 466–468. (doi:10.1098/rsbl.2009.1014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnhagen C. 1991. Predation risk as a cost of reproduction. Trends Ecol. Evol. 6, 183–185 10.1016/0169-5347(91)90210-O (doi:10.1016/0169-5347(91)90210-O) [DOI] [PubMed] [Google Scholar]
- 17.Candolin U. 1997. Predation risk affects courtship and attractiveness of competing threespine stickleback males. Behav. Ecol. Sociobiol. 41, 81–87 10.1007/s002650050367 (doi:10.1007/s002650050367) [DOI] [Google Scholar]
- 18.Trexler .J. C., Tempe R. C., Travis J. 1994. Size-selective predation of sailfin mollies by two species of heron. Oikos 69, 250–258 10.2307/3546145 (doi:10.2307/3546145) [DOI] [Google Scholar]
- 19.Cooper W. E., Vitt L. J., Hedges R., Huey R. B. 1990. Locomotor impairment and defense in gravid lizards (Eumeces laticeps)—behavioral shift in activity may offset costs of reproduction in an active forager. Behav. Ecol. Sociobiol. 27, 153–157 [Google Scholar]
- 20.Roelke D. L., Sogard S. M. 1993. Gender-based differences in habitat selection and activity level in the northern pipefish (Syngnathus fuscus). Copeia 2, 528–532 [Google Scholar]
- 21.Hoogland J. L., Cannon K. E., DeBarbieri L. M., Manno T. G. 2006. Selective predation on Utah prairie dogs. Am. Nat. 168, 546–552 [DOI] [PubMed] [Google Scholar]
- 22.Coolen I., Van Bergen Y., Day R. L., Laland K. N. 2003. Species difference in adaptive use of public information in sticklebacks. Proc. R. Soc. Lond. B 270, 2413–2419 10.1098/rspb.2003.2525 (doi:10.1098/rspb.2003.2525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coolen I., Giraldeau L. A. 2003. Incompatibility between antipredatory vigilance and scrounger tactic in nutmeg mannikins, Lonchura punctulata. Anim. Behav. 66, 657–664 10.1006/anbe.2003.2236 (doi:10.1006/anbe.2003.2236) [DOI] [Google Scholar]
- 24.Shaffer L. R., Formanowicz D. R. 1996. A cost of viviparity and parental care in scorpions: reduced sprint speed and behavioural compensation. Anim. Behav. 51, 1017–1023 10.1006/anbe.1996.0104 (doi:10.1006/anbe.1996.0104) [DOI] [Google Scholar]
- 25.James R. S., Johnston I. A. 1998. Influence of spawning on swimming performance and muscle contractile properties in the short-horn sculpin. J. Fish Biol. 53, 485–501 10.1111/j.1095-8649.1998.tb00997.x (doi:10.1111/j.1095-8649.1998.tb00997.x) [DOI] [Google Scholar]
- 26.Valone T. J. 1989. Group foraging, public information and patch estimation. Oikos 56, 357–363 10.2307/3565621 (doi:10.2307/3565621) [DOI] [Google Scholar]
- 27.Coolen I., Ward A., Hart P., Laland K. N. 2005. Foraging nine-spined sticklebacks prefer to rely on public information over simpler social cues. Behav. Ecol. 16, 865–870 10.1093/beheco/ari064 (doi:10.1093/beheco/ari064) [DOI] [Google Scholar]
- 28.Webster M. M., Hart P. J. B. 2006. Subhabitat selection by foraging threespine stickleback (Gasterosteus aculeatus): previous experience and social conformity. Behav. Ecol. Sociobiol. 60, 77–86 10.1007/s00265-005-0143-3 (doi:10.1007/s00265-005-0143-3) [DOI] [Google Scholar]
- 29.Rodewald A. D., Foster S. D. 1998. Effects of gravidity on habitat use and antipredator behaviour in three-spined sticklebacks. J. Fish Biol. 52, 973–984 10.1111/j.1095-8649.1998.tb00597.x (doi:10.1111/j.1095-8649.1998.tb00597.x) [DOI] [Google Scholar]
- 30.Frommen J. G., Mehlis M., Bakker T. C. M. 2009. Predator-inspection behaviour in female three-spined sticklebacks Gasterosteus aculeatus is associated with status of gravidity. J. Fish Biol. 75, 2143–2153 10.1111/j.1095-8649.2009.02408.x (doi:10.1111/j.1095-8649.2009.02408.x) [DOI] [PubMed] [Google Scholar]
- 31.Evans J. P., Gasparini C., Pilastro A. 2007. Female guppies shorten brood retention in response to predator cues. Behav. Ecol. Sociobiol. 61, 719–727 10.1007/s00265-006-0302-1 (doi:10.1007/s00265-006-0302-1) [DOI] [Google Scholar]
- 32.Ruxton G. D., Speed M. P., Kelly D. J. 2004. What, if anything, is the adaptive function of countershading? Anim. Behav. 68, 445–451 10.1016/j.anbehav.2003.12.009 (doi:10.1016/j.anbehav.2003.12.009) [DOI] [Google Scholar]
- 33.Stanley B. G., Wootton R. J. 1986. Effects of ration and male density on the territoriality and nest building of male three-spined sticklebacks (Gasterosteus aculeatus L). Anim. Behav. 34, 527–535 10.1016/S0003-3472(86)80121-X (doi:10.1016/S0003-3472(86)80121-X) [DOI] [Google Scholar]
- 34.Chellappa S., Huntingford F. A. 1989. Depletion of energy reserves during reproductive aggression in male 3-spined stickleback, Gasterosteus aculeatus L. J. Fish Biol. 35, 315–316 10.1111/j.1095-8649.1989.tb02982.x (doi:10.1111/j.1095-8649.1989.tb02982.x) [DOI] [Google Scholar]
- 35.Fitzgerald G. J., Guderley H., Picard P. 1989. Hidden reproductive costs in the threespine stickleback. Exp. Biol. 48, 295–300 [PubMed] [Google Scholar]
- 36.Dufresne F., FitzGerald G. J., Lachance S. 1990. Age and size-related differences in reproductive success and reproductive costs in threespine stickleback (Gasterosteus aculeatus). Behav. Ecol. 1, 140–147 10.1093/beheco/1.2.140 (doi:10.1093/beheco/1.2.140) [DOI] [Google Scholar]
- 37.Smith C., Wootton R. J. 1999. Parental energy expenditure of the male three-spined stickleback. J. Fish Biol. 54, 1132–1136 10.1111/j.1095-8649.1999.tb00866.x (doi:10.1111/j.1095-8649.1999.tb00866.x) [DOI] [Google Scholar]
- 38.Brown G. P., Shine R. 2004. Maternal nest-site choice and offspring fitness in a tropical snake (Tropidonophis mairii, Colubridae). Ecology 85, 1627–1634 10.1890/03-0107 (doi:10.1890/03-0107) [DOI] [Google Scholar]
- 39.Schwartzkopf L., Shine R. 1992. Costs of reproduction in lizards: escape tactics and susceptibility to predation. Behav. Ecol. Sociobiol. 31, 17–25 [Google Scholar]
- 40.Charland M. B., Gregory P. T. 1995. Movements and habitat use in gravid and non-gravid female garter snakes (Colubridae: Thamnophis). J. Zool. 236, 543–561 10.1111/j.1469-7998.1995.tb02731.x (doi:10.1111/j.1469-7998.1995.tb02731.x) [DOI] [Google Scholar]
- 41.Trivers R. L. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man, 1871–1971 (ed. Campbell B.), pp. 136–179 New Brunswick, NJ: Transaction Publishers [Google Scholar]
- 42.Clutton-Brock T. H. (ed.) 1988. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago, IL: University of Chicago Press [Google Scholar]
- 43.Webster M. M., Atton N., Ward A. J. W., Hart P. J. B. 2007. Turbidity and foraging rate in threespine sticklebacks: the importance of visual and chemical prey cues. Behaviour 144, 1347–1360 10.1163/156853907782418222 (doi:10.1163/156853907782418222) [DOI] [Google Scholar]
- 44.Webster M. M., Goldsmith J., Ward A. J. W., Hart P. J. B. 2007. Habitat-specific chemical cues influence association preferences and shoal cohesion in fish. Behav. Ecol. Sociobiol. 62, 273–280 10.1007/s00265-007-0462-7 (doi:10.1007/s00265-007-0462-7) [DOI] [Google Scholar]
- 45.Uryu Y., Iwasaki K., Hinoue M. 1996. Laboratory experiments on behaviour and movement of a freshwater mussel, Limnoperna fortunei (Dunker). J. Mollusc. Stud. 62, 327–341 10.1093/mollus/62.3.327 (doi:10.1093/mollus/62.3.327) [DOI] [Google Scholar]
- 46.Mashoodh R., Sinal C. J., Perrot-Sinal T. S. 2009. Predation threat exerts specific effects on rat maternal behaviour and anxiety-related behaviour of male and female offspring. Physiol. Behav. 96, 693–702 10.1016/j.physbeh.2009.01.001 (doi:10.1016/j.physbeh.2009.01.001) [DOI] [PubMed] [Google Scholar]
- 47.Krause J., Ruxton G. D. 2002. Living in groups. Oxford, UK: Oxford University Press [Google Scholar]
- 48.Wingfield J. C., Lynn S. E., Soma K. K. 2001. Avoiding the ‘costs’ of testosterone: ecological bases of hormone–behaviour interactions. Brain Behav. Evol. 57, 239–251 10.1159/000047243 (doi:10.1159/000047243) [DOI] [PubMed] [Google Scholar]
- 49.Kambo J. S., Galea L. A. M. 2006. Activational levels of androgens influence risk assessment behaviour but do not influence stress-induced suppression in hippocampal cell proliferation in adult male rats. Behav. Brain Res. 175, 263–270 10.1016/j.bbr.2006.08.032 (doi:10.1016/j.bbr.2006.08.032) [DOI] [PubMed] [Google Scholar]
- 50.Kavaliers M., Choleris E., Colwell D. D. 2001. Brief exposure to female odors ‘emboldens’ male mice by reducing predator-induced behavioural and hormonal responses. Horm. Behav. 40, 497–509 10.1006/hbeh.2001.1714 (doi:10.1006/hbeh.2001.1714) [DOI] [PubMed] [Google Scholar]
- 51.Galef B. G., Dudley K. E., Whiskin E. E. 2008. Social learning of food preferences in ‘dissatisfied’ and ‘uncertain’ Norway rats. Anim. Behav. 75, 631–637 10.1016/j.anbehav.2007.06.024 (doi:10.1016/j.anbehav.2007.06.024) [DOI] [Google Scholar]