Abstract
Variation in male mating success is often related to rank differences. Males who are unable to monopolize oestrous females alone may engage in coalitions, thus enhancing their mating success. While studies on chimpanzees and dolphins suggest that coalitions are independent of kinship, information from female philopatric species shows the importance of kin support, especially from mothers, on the reproductive success of females. Therefore, one might expect a similar effect on sons in male philopatric species. We evaluate mating success determinants in male bonobos using data from nine male individuals from a wild population. Results reveal a steep, linear male dominance hierarchy and a positive correlation between dominance status and mating success. In addition to rank, the presence of mothers enhances the mating success of sons and reduces the proportion of matings by the highest ranking male. Mothers and sons have high association rates and mothers provide agonistic aid to sons in conflicts with other males. As bonobos are male-philopatric and adult females occupy high dominance status, maternal support extends into adulthood and females have the leverage to intervene in male conflicts. The absence of female support to unrelated males suggests that mothers gain indirect fitness benefits by supporting their sons.
Keywords: dominance hierarchy, maternal effect, mating system, apes, kinship
1. Introduction
To maximize reproductive success, males in multimale–multifemale societies compete for mating opportunities with fertile females [1]. High ranking males are able to restrict other males' access to fertile females and interfere with mating attempts [2]. As a result, mating is skewed, with dominant males accounting for a larger number of matings than their subordinate counterparts (e.g. red deer [3]; soay sheep [4]; primates [5]).
Deviation from the predicted relationship between rank and mating success has been attributed to the influence of social relationships. Males may form alliances and challenge higher-ranking males to displace them from mate guarding [6–8]. As a result, these males may obtain more mating opportunities than expected from their rank position. The relationship between rank and mating success can also be influenced by other factors, such as the number of males in a group [9], female oestrous synchrony [10] and female choice [11].
If social ties have positive effects on fitness, as evidence suggests [12], kin selection theory predicts that they should preferentially form between relatives [13]. Kin relationships between group members are determined by the transfer pattern, and accordingly, social relations can be predicted from the mode of dispersal. Most mammalian species are female philopatric, with males dispersing and females remaining in their natal group. As a result, alliances and cooperation are biased towards female kin (e.g. in cercopithecine primates; [14]) and the closest bonds are between mothers and daughters. In some species, maternal support has been found to enhance the social status and reproductive success of daughters [15]. Examples of such support include agonistic aid in social conflicts and cooperative defence of food sources against other cohorts [16]. In vervet mokeys (Cercopithecus aethiops sabaeus), it was found that the presence of the mother increased the number of a female's surviving offspring and decreased her neonatal mortality [17]. When males disperse, maternal support is limited to immature sons but in some species, maternal support early in life has been found to affect physiological traits [18–20] that may increase fitness after males have been transferred to other groups [21,22].
In social mammals, male philopatry is rare and occurs only sporadically in many orders, such as bats, rodents, carnivores, cetaceans and primates, including humans (overview [23]). Based on the availability of kin for males, male philopatry implies strong social bonds between male group members and between mothers and sons. In male philopatric species such as chimpanzees and spider monkeys, males engage in affiliative and differentiated relationships with other males, which affect dominance status, mating chances and reproductive success [24,25]. Maternal support of adult/subadult sons has been reported in the two Pan species [26,27], but not in muriquis (Brachyteles hypoxanthus; [28]). The reason for this variation in maternal support of sons has never been explored, and the possible effects on the fitness of sons are unknown.
Given the effect of maternal support on the reproductive success of daughters in female philopatric species, one might expect a similar effect on sons in male philopatric species. In the context of mating, agonistic support is expected when access to females is monopolizable [29]. In addition, the leverage of the supporter is decisive for the outcome of a conflict [30,31]. In the majority of mammalian species, in which adult males are dominant over adult females [32], mixed sex alliances and agonistic support from females in the context of mating is rare. If male philopatry is combined with high female social status and male competition for access to fertile females, mother–son bonds may have a direct effect on the reproductive success of males and influence the relationship between male rank and mating.
Bonobos (Pan paniscus) are one of the few primate species that meet these conditions. Bonobos live in communities whose members form temporary parties that vary in size and composition [33]. Males are philopatric and can potentially interact with their mothers, even after reaching adulthood [34,35]. In addition, females are co-dominant to males, and some occupy relatively high ranks [36,37]. In captivity, males form linear hierarchies [37], but mating and reproductive success appear to be independent of rank [38]. In the wild, dominance relations among resident males are often ambiguous and the relationship between rank and mating success is unclear. While some studies have suggested that rank is positively correlated with mating and reproductive success [39], others have not found such a correlation [40,41]. The only study that used molecular genetics methods to determine kin relationships found that high ranking males sired more offspring than other males [42]. These conflicting results about the relationship between rank, mating and reproductive success require explanation.
One reason for the inconsistent relationship between male rank, mating success and paternity might be that males have egalitarian relationships [43].
Mother–son relationships might also influence the relationship between male rank and mating success in that mothers support lower ranking sons and so enhance access to oestrous females. Bonobo mothers and adult sons have high association rates [35], and females support their sons in conflicts with other males [26] and interrupt copulations of third-parties [44]. However, it is not clear if maternal support occurs in the context of mating and how maternal influence affects male mating success.
In the study presented here, we explore (i) the dominance relations between free-ranging male bonobos at LuiKotale, (ii) if male rank and/or maternal presence affect the mating success of males, and (iii) how maternal presence influences the relationship between rank and mating. To answer these questions, we first assessed dominance interactions between the males. Second, we investigated male mating success in relation to rank and the presence/absence of mothers in the party to estimate the impact of these two factors. In addition, we analysed aggressive behaviour in the context of mating and association patterns between mothers, sons and oestrous females.
2. Material and methods
(a). Study site and subjects
Data were collected during an ongoing field study at LuiKotale, Salonga National Park, Democratic Republic of Congo [45]. All adult members of the bonobo community were habituated to human presence by the time the study started in mid-2007. During the period of data collection, the community consisted of 33–35 individuals, including five adult and four adolescent males, and 11 adult and up to five adolescent females. We based individual age estimates on physical features such as body size, dentition and (in females) genital swellings [46]. Maternities were determined using genetic maternity assignment (see electronic supplementary material, S1), which revealed that six out of nine males had their mothers in the group. The estimated ages of males ranged between 10 and 30 years. Previous studies have shown that male bonobos start to reproduce at the age of 7 years [47]. All adolescent males in our study community were seen to mate with oestrous females, and three of the four were seen to ejaculate. Therefore, all adult and adolescent males were included in this study.
(b). Behavioural observation
Data were collected from May to August 2007, and December 2007 to July 2009. Parties containing males were followed from the time subjects left the nest in the morning until they constructed night nests in the evening. Party composition was recorded every full hour (total = 2112 h). All occurrences of aggressive interactions and mating behaviours were scored during both party follows and individual male focal follows [48]. Focal follows (total = 470 h of focal time) of the same individual lasted for 10 min and were separated by at least 1 h. Focal individuals were randomly chosen from all males travelling in the same party. At the start and end of these 10 min intervals, identities of all individuals in proximity (within 5 m) to the focal individual were recorded.
(c). Behavioural parameters
Mating refers to sexual interactions between males and females when intromission was achieved. Mating scores included information on the stage of sexual swellings of the female mating partner (see below), the duration of the copulation and indications for ejaculation. Aggression refers to directed agonistic behaviours, including both contact aggression (e.g. hit, pull, bite), and chases without physical contact (e.g. charge). Following definitions used by Wittig & Boesch [49], conflicts were assigned to different contexts, including mate competition, feeding and access to social partners. Submission refers to different forms of retreat, such as flee and jump aside [26].
(i). Dominance interactions
Assessments of dominance relations among male community members were based on dyadic interactions. Individuals showing submissive behaviour in response to a non-aggressive approach or to aggression were classified as subordinate [37]. Multiple unidirectional dominance interactions occurring within 10 min were counted as one single event. Information on dyadic dominance relations among males was used to assess absolute rank and relative rank (see below).
(ii). Oestrous cycle and fertility
‘Oestrous’ refers to the period when female genital swellings are maximally tumescent [50]. Recent analyses indicate that time of ovulation in bonobo females falls within this period (T. Deschner 2008, unpublished data). In our study, genital swellings of all female party members were scored daily. We distinguished four swelling stages ranging from minimal (stage 1) to maximal tumescence (stage 4) based on firmness and skin surface structure [51].
The probability of fertility in Pan depends on a number of life-history variables [52]. Therefore, additional information was used to characterize female reproductive status: pregnant female (counted back from birth of offspring), nulliparous female, female with dependent offspring (neither pregnant nor close to conception and offspring younger than 5 years), female with older offspring (neither pregnant nor close to conception and youngest offspring older than 5 years) and females close to conception (last six to seven cycles before conception, counted back from birth of next offspring).
(d). Data analysis
(i). Rank
Using information on dyadic dominance interactions between males (see above), we carried out hierarchical rank-order analysis with MatMan (v. MfW 1.1; earlier version described in de Vries et al. [53]) for all male dyads and calculated the improved index of linearity (h′) rather than Landau's index, as it allows for the possibility of tied dominance relationships [54]. To indicate a clearly linear hierarchy, the index of linearity should be greater than 0.90 [55]. Since comparing h′ indices between studies is prone to errors [56], we also calculated the steepness of the hierarchy based on the normalized David's score according to de Vries et al. [57].
(ii). Party rank
To analyse copulation rates, we ranked the males in relation to other males present in a given party, and disregarded their dominance status in relation to males not present. The highest ranking individual within a given party (not necessarily the highest ranking male in the community) was assigned a rank value of 1 and the lowest ranking individual was assigned a rank value of 0. Other males were ranked equidistantly between these two males. If a party contained only one male, he was assigned a rank of 1.
(iii). Party association
To control for individual differences in gregariousness, observation time and non-independence of the hourly party scans, we calculated a pairwise affinity index (PAI). For each dyad, we related the observed value of dyadic association calculated by the simple ratio index (SRIobs) to its expected value (SRIexp), derived by randomization (see below), and then standardized the outcome value to a range from −1 (together as little as possible) to +1 (together as much as possible). Positive values indicate association preference and negative values indicate avoidance.
We calculated SRIobs = Pa(AB)/(Pa(A) + Pa(B) − Pa(AB)), with Pa(AB) = number of parties containing both A and B, Pa(A) = number of parties containing A, and Pa(B) = number of parties containing B.
The value of PAI was then derived as
and as
To obtain a randomized party association, we shuffled blocks of consecutive focal party attendance and absence separately for each individual. Such a randomization keeps constant both the total duration and temporal autocorrelation in an individual's party attendance, and the frequency distribution of the duration of its party attendances. The dyadic SRIexp was the mean SRI value of 1000 of such randomizations (for further details, see electronic supplementary material, S2).
(iv). Analysis of male mating success
To analyse what predicts male mating success, we used a generalized linear mixed model (GLMM; [58]). We included the following predictor variables as fixed effects in this model.
— Party rank. When priority of access to oestrous females depends on rank, access of a given male to a female depends on his rank in relation to the ranks of the other males present in the same party. However, owing to the fission–fusion grouping pattern of bonobos, the composition of the party changes frequently. Because of this, the relative rank position of a given male could change depending on the presence of other males in the same party. For instance, the third ranking male in the community will occupy the highest party rank when the highest and the second highest male of the community are absent from this party (for details of calculation, see above).
— Mother presence. To test whether the actual presence of the mother in a party influences her son's access to oestrous females, we included this variable into the model. Note that this does not distinguish between males without a mother in the actual party and males without a mother in the community.
— Potential confounders. In addition to party rank and maternal presence, we included female swelling state, party composition (number of males and females) and female reproductive status into the model. These variables have been shown to influence male mating success in other studies and hence needed to be controlled for.
As a response variable, we used the number of copulations per male–female dyad during a given party with constant composition. We chose this response because changes in party composition were usually accompanied by changes in one or several of the predictor variables. We included male and female identity as well as the identity of the specific party as random effects. Since the duration that a specific party with a specific composition lasted varied, we included the duration of a specific party as an offset variable (for more details on the GLMM and interactions included, see electronic supplementary material, S3).
(v). Spatial position within parties
Spatial position of an individual within a party was inferred from the proximity scans of the focal males. Given the relevance of a male's position in relation to the position of oestrous females, this position was characterized by the number of oestrous females in proximity (≤5 m) during times when one or more oestrous females were present in the party. To analyse the mother's influence on a male's proximity to oestrous females, we ran a GLMM similar to that for male mating success. We used the number of oestrous females in proximity to a given male as a response variable and a male's party rank and his mother's presence in proximity as predictor variables. We controlled for the number of males and oestrous females in the party by including them as additional predictor variables (for more details on the GLMM and interactions included, see electronic supplementary material, S4).
3. Results
(a). Male dominance rank
All males participated in dominance interactions (n = 431) with other males, and there were no ties or unknown relationships among the 36 male–male dyads (table 1). We found that male bonobos at LuiKotale formed a linear hierarchy (linearity index: h′ = 1, p = 0.0004) and that dyadic dominance relations had a high directional consistency (DC = 0.94). The exception was a dyad of two males (JA and DA) who had undecided dominance relations for a few months. The calculated steepness of the dominance hierarchy was 0.994 (David's score; p = 0.001).
Table 1.
Dominance interactions between male bonobos at LuiKotale: rows represent dominant (winner) and columns subordinate individuals (loser) within a dominance interaction. (IDs of adult males are written in capitals, IDs of sub-adult males in lower-case (linearity index h′ = 1, p = 0.0004, David's score of steepness of a dominance hierarchy = 0.94).)
| CA | TI | JA | DA | BE | ap | pn | em | mx | |
|---|---|---|---|---|---|---|---|---|---|
| CA | 10 | 19 | 7 | 28 | 16 | 15 | 11 | 1 | |
| TI | 0 | 28 | 8 | 18 | 5 | 7 | 5 | 3 | |
| JA | 0 | 0 | 21 | 22 | 23 | 2 | 5 | 3 | |
| DA | 0 | 0 | 12 | 24 | 4 | 14 | 23 | 4 | |
| BE | 0 | 0 | 0 | 0 | 16 | 12 | 19 | 11 | |
| ap | 0 | 0 | 0 | 0 | 0 | 6 | 7 | 12 | |
| pn | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | |
| em | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | |
| mx | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
(b). Maternal presence
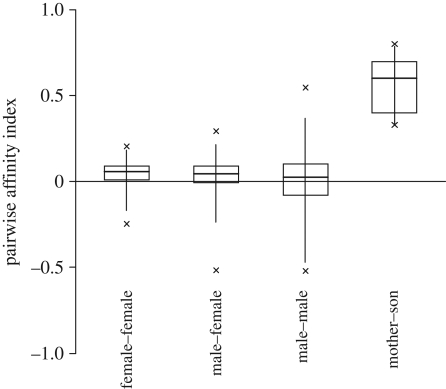
Six out of the nine males had mothers living in the community (table 2). Mothers and sons had higher PAIs than any other female–male dyad (figure 1). Males were travelling together with their mothers between 81 and 92 per cent of their observation time. The one male–male dyad with a PAI within the range of mother–son values was found, through genotyping data, to consist of brothers, whose mother was in the same community. Presence or absence of mothers in the community alone did not predict male dominance status (exact Mann–Whitney U-test: NMopres = 6, NMoabs = 3, U = 8, p = 0.95).
Table 2.
Mating patterns of male bonobos at LuiKotale: data combine observations from focal follows and from all-occurrences-sampling during party follows. (Males with a mother in the community are marked with an asterisk (‘*’) after their code.)
| individual | code | rank | age | total copulations | copulations with oestrous females | observation hours on days with oestrous females |
|---|---|---|---|---|---|---|
| Camillo | CA* | 1 | adult | 199 | 165 | 778 |
| Tito | TI* | 2 | adult | 106 | 73 | 681 |
| Jack | JA | 3 | adult | 106 | 75 | 604 |
| Dante | DA | 4 | adult | 50 | 33 | 629 |
| Ben | BE* | 5 | adult | 135 | 97 | 776 |
| Apollo | ap* | 6 | subadult | 138 | 57 | 532 |
| Pan | pn* | 7 | subadult | 56 | 32 | 520 |
| Emil | em* | 8 | subadult | 65 | 43 | 466 |
| Max | mx | 9 | subadult | 23 | 10 | 402 |
Figure 1.
Pairwise affinity indices for different dyads. Indices are standardized to a range from −1 (together as little as possible) to +1 (together as much as possible) per dyad. Male–female dyads do not include mother–son dyads. Indicated are medians, percentiles, 5% and 95% quartile percentiles, as well as maximum and minimum.
(c). Male mating success
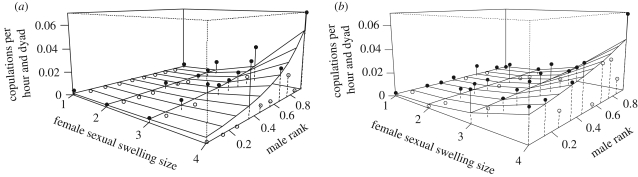
Overall, 878 copulations between females and mature males were recorded. Of these, 336 involved females with maximally tumescent swellings (table 2). Overall, high ranking males had more copulations with females of higher swelling stages, but the mating success of low- and mid-ranking males tended to be higher when their mothers were in the party (figure 2; GLMM, three-way interaction between male party rank, mother presence and female swelling state: p = 0.077; for further details, see electronic supplementary material, S5 and table 1).
Figure 2.
Influence of male rank within a party, female sexual swelling size and presence of mothers within a party on the mating success of males. The surface represents results from the GLMM controlling for number of males and females, number of oestrous females and female reproductive status. The dots represent averages per swelling state and party rank and do not control for other predictor variables. For clarity, dots above the surface are in black and dots below the surface are open. (a) Mother absent; (b) mother present.
Results of models without the three-way interactions indicated similar patterns. First, males with high party ranks had more copulations, particularly with females of higher swelling stages (GLMM, interaction between party rank and female swelling: p < 0.001; for further details, see electronic supplementary material, S5 and table 2). Second, low- and mid-ranking males had increased mating success when their mothers were present in the party (GLMM, interaction between party rank and mother presence: p < 0.001; for further details, see electronic supplementary material, S5 and table 2).
Comparing the mating rates of males in the presence/absence of their mothers, we found that the highest ranking male in a party achieved 40.8 per cent of all matings with maximally tumescent females in parties without any mother, whereas in parties with all mothers present, the highest ranking male achieved only 25 per cent of all matings with oestrous females (calculated from the observed mating frequencies shown in figure 2).
(d). Aggression in the context of mating
A total of 134 aggressive interactions took place when conflicts arose over access to oestrous females. The majority of these (n = 95, 70% of all mating conflicts) involved two males, 37 involved females and males and two involved two females. In six of the 37 male–female interactions, the aggression involved the oestrous female, while in 30 of the cases, mothers of the mature males trying to mate with the oestrous female were involved. This involvement included mothers intervening in the mating attempts of unrelated males (n = 13) and engaging in agonistic aid when unrelated males tried to interfere with their sons' mating (n = 17).
To explore whether the presence/absence of mothers influenced the motivation of other individuals, both male and female, to direct aggression towards their sons, we compared rates of received aggression for the six males with mothers. Changes in the rates of aggression received in a situation without a mother compared with a situation with mother present were not significant (CA 0.011, TI 0.002, BE −0.041, ap −0.025, pn 0.022, em −0.039, exact Wilcoxon test: T+ = 15, n = 6, p = 0.44).
(e). Spatial position of males in parties containing oestrous females
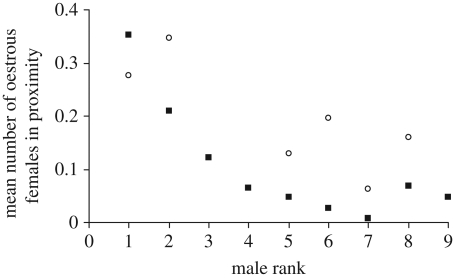
Out of the 2545 proximity scans of males in parties with oestrous females, 1562 were of focal males with a mother in the community. Overall, higher ranking males were more frequently in proximity to oestrous females, but the mean number of oestrous females in proximity to mid- and low-ranking males increased when their mothers were in proximity (GLMM, interaction between party rank and mother in proximity: p < 0.01; for further details, see electronic supplementary material, S6). Except for the highest ranking male in the community, the mean number of oestrous females in proximity was higher when their mothers were also in proximity (figure 3).
Figure 3.
Mean number of oestrous females in proximity of focal males in relation to absence (filled squares) or presence (open circles) of the male's mother. For all but the highest ranking male, the number of oestrous females in proximity was higher when the mother was also in proximity.
4. Discussion
The results of this study show that male bonobos form a stable and linear dominance hierarchy. Overall, male mating success with oestrous females follows the pattern commonly found in other mammalian species, with rank and mating success being positively correlated. Mother–son dyads had high association rates, and the presence of mothers enhanced both their son's proximity to and mating success with oestrous females. However, only low- and mid-ranking males had clearly increased mating success when their mothers were present versus absent (figure 2). While maternal presence did not change the pattern of rank-related mating success (with higher ranking males having more matings), maternal presence significantly enhanced the proportion of matings by low- and medium-ranking males relative to matings by the highest-ranking male (figure 2). It is tempting to speculate that the difference in a son's mating success was because of the agonistic aid and interventions by its mother against other males. However, given the relatively low rate of interventions, agonistic aid by mothers alone is not sufficient to explain the link between mating success and the presence/absence of mothers. One additional mechanism may be that mothers help sons occupy desirable spatial positions within the group, allowing them to interact closely with other females, including those in oestrous (figure 3). Another possibility is that maternal intervention reduces the dominant male's potential to monopolize oestrous females and, by doing so, facilitates female choice.
(a). Male rank
Bonobo society is usually described as egalitarian ([43], but see [59]). However, the data collected in this study revealed a linear dominance hierarchy among resident males, similar in steepness to that reported for despotic societies [60]. Strong dominance hierarchies are indicative of within-group contest competition for mating partners [61,62]. The behavioural data shown above demonstrate that males compete for access to females and that a male's rank has a strong effect on his individual mating success. The alpha male of the community, as well as the highest ranking male in a given party, had the highest mating rates with oestrous females. Given that the copulation rates of the highest ranking male in a party did not obviously depend on the presence or absence of his mother, a large proportion of the observed mating performance seems to reflect dominance status rather than maternal support (figure 2).
Behavioural observations collected in this study neither provide evidence for coercive mating or other aggression by males against females in the context of mating, nor did females avoid mating efforts by dominant males. However, when mothers did not intervene, dominant males were able to restrict the mating behaviour of other males. The finding that high ranking males had priority of access to oestrous females suggests that mating success reflects individual resource-holding potential.
(b). Influence of maternal presence on mating
It has been shown that a high-ranking male's ability to monopolize mating with oestrous females is determined by many factors, including the number of males travelling together [5], synchrony of female oestrous [9], female choice [63], male coalitions [6] and incest avoidance [64]. Our study suggests that maternal support is an additional parameter affecting mating success. When controlling for the number of males and oestrous females in a party, low- and mid-ranking males gained substantially from the presence of, and possibly from interventions by, their mothers: the overall rate of mating with oestrous females increased, and the proportion of mating by the highest ranking male decreased from 40 to 25 per cent (figure 2).
To our knowledge, this is the first report of direct maternal support of sons in agonistic conflicts over access to oestrous females. Studies on chimpanzees and dolphins found that cooperation in the context of mating was restricted to males [7,65]. In bonobos, affiliative relations between males do exist, but coalitions or alliances in the mating context seem to be absent. This may be explained by the association of male philopatry with high female leverage in bonobos (but not in chimpanzees). Females are able to engage in aggressive interactions with males without the high cost of injuries. One could also speculate that the presence of mothers reduces the probability of aggression by high-ranking males against their sons, and thus, affects the distribution of mating among male party members. Our data show that the presence of mothers affects neither male–male competition nor the rate of aggression directed towards sons. It should be noted though, that we did not control for the spatial positions of males. In the absence of their mothers, lower ranking males appear to be more peripheral (M. Surbeck 2007–2009, personal observation) and were not involved in any form of competition. This suggests that males staying in the centre of a party are likely to receive more aggression than males in the periphery. If the mother of a mid- or low-ranking male was in proximity, that male would also have more oestrous females in proximity than he would if his mother were not in proximity (figure 3). This implies that sons with mothers nearby spend more time interacting with oestrous females, leading to more mating opportunities. Mutual attraction between females may also affect the proximity of sons to oestrous females but our data do not allow testing for this. Further data are needed to investigate the mechanisms by which a mother's presence influences her son's mating success, including the role of female choice.
(c). Implications of maternal influence in the context of mating
In order to gain benefits from inclusive fitness, maternal support should not only enhance mating success but also affect the reproductive success of sons. Male bonobos have been reported to mate throughout the oestrous cycle [41], but in our study, mating efforts were clearly more pronounced during periods of maximum swelling size. Within this phase, the probability of fertilization changes, reaching a peak at the end of this phase (T. Deschner 2008, unpublished data). Additional behavioural studies and ongoing genetic analyses of the subjects involved in this study will enable us to test the relationship between maternal support, male rank and paternity.
Depending on the presence or absence of mothers, male mate competition can shift from contest to scramble, reducing disparity between mating rates of different individuals and therefore increasing promiscuity. Direct mate competition between males is also likely to promote sperm competition [66]. Under such conditions, traits coding for male dominance, such as physical strength and aggressive behaviour, may become less important than morphological traits, like ejaculate size [67] and sperm morphology [68], and behavioural strategies in the dynamics of male copulatory behaviour, such as mate order and copulation duration [69]. Sperm competition is rarely measured but usually inferred from testes size which, in turn, is considered as a proxy for ejaculate volume [70]. Still, male philopatric species with large testes compared with body size, such as muriquis, chimpanzees and bonobos, differ in terms of mate competition. In chimpanzees, males form despotic hierarchies and use coercive mating strategies, and high ranking males prevent other males from mating [7]. Male muriquis do not develop dominance hierarchies and are highly tolerant in the context of mating [71]. Bonobos show intermediate traits: although males compete for access to females, maternal influence on sons seems to render male–male competition less effective.
Our study presents a previously undescribed mechanism of maternal support to sons in a male-philopatric primate society. Evidence suggests that social bonds between mothers and adult sons exist in other male-philopatric species, but little is known about the function of these bonds. Strong social bonds between mothers and adult sons have been reported in chimpanzees, orcas and humans [27,72,73], but in all these species, close social relationships also exist between males. In bonobos, males do not engage in same-sex coalitions, and only males with maternal kin enjoy coalitionary support from females in the mating context. This lack of male coalitions is associated with high dominance status in females. If enhanced mating success provides reproductive advantages to sons, maternal support may have important effects on females' fitness.
Acknowledgements
The methods used to collect observational data in the field were in compliance with the requirements and guidelines of the ICCN and adhere to the legal requirements of the host country, the Democratic Republic of Congo.
We thank the Institut Congolaise pour la Conservation de la Nature (ICCN) for granting permission to conduct research at Salonga National Park. Fieldwork at LuiKotale was supported by the Max-Planck Society, the L.S.B. Leakey Foundation, National Geographic Society, the Volkswagen Foundation and private donors. We thank Christophe Boesch for support during various stages of the project, Barbara Fruth for stimulating discussions and help in conducting fieldwork; Grit Schubert for insights into the genetic relationships of the individuals; Lambert Booto, Andrew Fowler, Isaak Schamberg and Wilson Schersten for assistance in the field; and Tobias Deschner, Oliver Schuelke, Kevin Langergraber, Vanessa Van Doren and two anonymous reviewers for helpful comments on the manuscript.
References
- 1.Trivers R. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 136–179 Chicago, IL: Aldine [Google Scholar]
- 2.Altmann S. A. 1962. A field study of sociobiology of rhesus monkeys, Macaca mulatta. Ann. NY Acad. Sci. 102, 338–435 10.1111/j.1749-6632.1962.tb13650.x (doi:10.1111/j.1749-6632.1962.tb13650.x) [DOI] [PubMed] [Google Scholar]
- 3.Clutton-Brock T. H., Guinness F. E., Albon S. D. 1982. Red deer: behaviour and ecology of two sexes. Chicago, IL: University of Chicago Press [Google Scholar]
- 4.Preston B. T., Stevenson I. R., Pemberton J. M., Wilson K. 2001. Dominant rams lose out by sperm depletion: a waning success in siring counters a ram's high score in competition for ewes. Nature 409, 681–682 10.1038/35055617 (doi:10.1038/35055617) [DOI] [PubMed] [Google Scholar]
- 5.Kutsukake N., Nunn C. L. 2006. Comparative tests of reproductive skew in male primates: the roles of demographic factors and incomplete control. Behav. Ecol. Sociobiol. 60, 695–706 10.1007/s00265-006-0213-1 (doi:10.1007/s00265-006-0213-1) [DOI] [Google Scholar]
- 6.Noë R., Sluijter A. A. 1990. Reproductive tactics of male savanna baboons. Behaviour 113, 117–170 10.1163/156853990X00455 (doi:10.1163/156853990X00455) [DOI] [Google Scholar]
- 7.Watts D. P. 1998. Coalitionary mate guarding by male chimpanzees at Ngogo, Kibale National Park, Uganda. Behav. Ecol. Sociobiol. 44, 43–55 10.1007/s002650050513 (doi:10.1007/s002650050513) [DOI] [Google Scholar]
- 8.Alberts S. C., Watts H. E., Altmann J. 2003. Queuing and queue-jumping: long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Anim. Behav. 65, 821–840 10.1006/anbe.2003.2106 (doi:10.1006/anbe.2003.2106) [DOI] [Google Scholar]
- 9.Bulger J. B. 1993. Dominance, rank and access to estrous females in male savanna baboons. Behaviour 127, 67–103 10.1163/156853993X00434 (doi:10.1163/156853993X00434) [DOI] [Google Scholar]
- 10.Ostner J., Nunn C. L., Schuelke O. 2008. Female reproductive synchrony predicts skewed paternity across primates. Behav. Ecol. 19, 1150–1158 10.1093/beheco/arn093 (doi:10.1093/beheco/arn093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stumpf R. M., Boesch C. 2006. The efficacy of female choice in chimpanzees of the Tai Forest, Cote d'Ivoire. Behav. Ecol. Sociobiol. 60, 749–765 10.1007/s00265-006-0219-8 (doi:10.1007/s00265-006-0219-8) [DOI] [Google Scholar]
- 12.Silk J. B., Alberts S. C., Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231–1234 10.1126/science.1088580 (doi:10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 13.Hamilton W. D. 1964. The genetical evolution of social behvaiour. I. and II. J. Theor. Biol. 7, 1–52 [DOI] [PubMed] [Google Scholar]
- 14.Silk J. B. 2009. Nepotistic cooperation in non-human primate groups. Phil. Trans. R. Soc. B 364, 3243–3254 10.1098/rstb.2009.0118 (doi:10.1098/rstb.2009.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fairbanks L. 2000. Maternal investment throughout the life span in Old World monkeys. In Old world monkeys (eds Whitehead P., Clifford J.), pp. 341–367 Cambridge, UK: Cambridge University Press [Google Scholar]
- 16.Wrangham R. W. 1980. An ecological model of female bonded primate groups. Behaviour 75, 262–300 10.1163/156853980X00447 (doi:10.1163/156853980X00447) [DOI] [Google Scholar]
- 17.Fairbanks L., McGuire M. T. 1986. Age, reproductive value, and dominance-related behavior in vervet monkey females: cross-generational influences on social relationships and reproduction. Anim. Behav. 34, 1710–1721 10.1016/S0003-3472(86)80258-5 (doi:10.1016/S0003-3472(86)80258-5) [DOI] [Google Scholar]
- 18.Hofer H., East M. L. 2003. Behavioral processes and costs of co-existence in female spotted hyenas: a life history perspective. Evol. Ecol. 17, 315–331 10.1023/A:1027352517231 (doi:10.1023/A:1027352517231) [DOI] [Google Scholar]
- 19.Altmann J., Alberts S. C. 2005. Growth rates in a wild primate population: ecological influences and maternal effects. Behav. Ecol. Sociobiol. 57, 490–501 10.1007/s00265-004-0870-x (doi:10.1007/s00265-004-0870-x) [DOI] [Google Scholar]
- 20.Onyango P. O., Gesquiere L. R., Wango E. O., Alberts S. C., Altmann J. 2008. Persistence of maternal effects in baboons: mother's dominance rank at son's conception predicts stress hormone levels in subadult males. Horm. Behav. 54, 319–324 10.1016/j.yhbeh.2008.03.002 (doi:10.1016/j.yhbeh.2008.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul A., Kuester J., Arnemann J. 1992. Maternal rank affects reproductive success of male Barbary macaques (Macaca sylvanus): evidence from DNA fingerprinting. Behav. Ecol. Sociobiol. 30, 337–341 [Google Scholar]
- 22.Royle N. J., Lindstrom J., Metcalfe N. B. 2005. A poor start in life negatively affects dominance status in adulthood independent of body size in green swordtails Xiphophorus helleri. Proc. R. Soc. B 272, 1917–1922 10.1098/rspb.2005.3190 (doi:10.1098/rspb.2005.3190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handley L. J. L., Perrin N. 2007. Advances in our understanding of mammalian sex-biased dispersal. Mol. Ecol. 16, 1559–1578 [DOI] [PubMed] [Google Scholar]
- 24.Strier K. B. 1994. Brotherhoods among Atelins: kinship, affiliation, and competition. Behaviour 130, 151–167 10.1163/156853994X00505 (doi:10.1163/156853994X00505) [DOI] [Google Scholar]
- 25.Mitani J. C. 2009. Cooperation and competition in chimpanzees: current understanding and future challenges. Evol. Anthropol. 18, 215–227 10.1002/evan.20229 (doi:10.1002/evan.20229) [DOI] [Google Scholar]
- 26.Furuichi T. 1997. Agonistic interactions and matrifocal dominance rank of wild bonobos (Pan paniscus) at Wamba, Zaire. Int. J. Primatol. 18, 855–875 10.1023/A:1026327627943 (doi:10.1023/A:1026327627943) [DOI] [Google Scholar]
- 27.Boesch C. 2009. The real chimpanzee: sex strategies in the forest. Cambridge, UK: Cambridge University Press [Google Scholar]
- 28.Tolentino K., Roper J., Passos F. C., Strier K. B. 2008. Mother-offspring associations in northern muriquis (Brachyteles hypoxanthus). Am. J. Primatol. 70, 301–305 10.1002/ajp.20488 (doi:10.1002/ajp.20488) [DOI] [PubMed] [Google Scholar]
- 29.Van Hoof J. A. R. A. M., Van Schaik C. P. 1992. Cooperation in competition: the ecology of primate bonds. In Coalitions and alliances in humans and other animals (eds Harcourt A. H., de Waal F. B. M.), pp. 357–390 Oxford, UK: Oxford University Press [Google Scholar]
- 30.Van Schaik C., Pandit S., Vogel E. 2006. Toward a general model for male-male coalitions in primate groups. In Cooperation in primates and humans (eds Kappeler P. M., Van Schaik C.), pp. 151–172 Heidelberg, Germany: Springer [Google Scholar]
- 31.Bissonnette A., de Vries H., Van Schaik C. P. 2009. Coalitions in male Barbary macaques, Macaca sylvanus: strength, success and rules of thumb. Anim. Behav. 78, 329–335 10.1016/j.anbehav.2009.05.010 (doi:10.1016/j.anbehav.2009.05.010) [DOI] [Google Scholar]
- 32.Kappeler P. M. 1993. Female dominance in primates and other mammals. In Perspectives in ethology, vol. 10 (eds Bateson P. P. G., Klopfer P. H., Thompson N. S.), pp. 143–158 New York, NY: Plenum [Google Scholar]
- 33.Kuroda S. 1979. Grouping of the pygmy chimpanzees. Primates 20, 161–183 10.1007/BF02373371 (doi:10.1007/BF02373371) [DOI] [Google Scholar]
- 34.Ihobe H. 1992. Male–male relationships among wild bonobos (Pan paniscus) at Wamba, Republic of Zaire. Primates 33, 163–179 10.1007/BF02382747 (doi:10.1007/BF02382747) [DOI] [Google Scholar]
- 35.Hohmann G., Gerloff U., Tautz D., Fruth B. 1999. Social bonds and genetic ties: kinship association and affiliation in a community of bonobos (Pan paniscus). Behaviour 136, 1219–1235 10.1163/156853999501739 (doi:10.1163/156853999501739) [DOI] [Google Scholar]
- 36.Kuroda S. 1980. Social behavior of the pygmy chimpanzees Pan paniscus. Primates 21, 181–197 [Google Scholar]
- 37.Vervaecke H., de Vries H., Van Elsacker L. 2000. Dominance and its behavioral measures in a captive group of bonobos (Pan paniscus). Int. J. Primatol. 21, 47–68 10.1023/A:1005471512788 (doi:10.1023/A:1005471512788) [DOI] [Google Scholar]
- 38.Marvan R., Stevens J. M. G., Roeder A. D., Mazura I., Bruford M. W., de Ruiter J. R. 2006. Male dominance rank, mating and reproductive success in captive bonobos (Pan paniscus). Folia Primatol. 77, 364–376 10.1159/000093702 (doi:10.1159/000093702) [DOI] [PubMed] [Google Scholar]
- 39.Kano T. 1996. Male rank order and copulation rate in a unit-group of bonobos at Wamba, Zaire. In Great ape societies (eds McGrew W., Nishida T., Marchant L.), pp. 146–155 Cambridge, UK: Cambridge University Press [Google Scholar]
- 40.Takahata Y., Ihobe H., Idani G. 1996. Comparing copulations of chimpanzees and bonobos: do females exhibit proceptivity or receptivity? In Great ape societies (eds McGrew W., Nishida T., Marchant L.), pp. 146–155 Cambridge, UK: Cambridge University Press [Google Scholar]
- 41.Furuichi T., Hashimoto C. 2004. Sex differences in copulation attempts in wild bonobos at Wamba. Primates 45, 59–62 10.1007/s10329-003-0055-7 (doi:10.1007/s10329-003-0055-7) [DOI] [PubMed] [Google Scholar]
- 42.Gerloff U., Hartung B., Fruth B., Hohmann G., Tautz D. 1999. Intracommunity relationships, dispersal pattern and paternity success in a wild living community of bonobos (Pan paniscus) determined from DNA analysis of faecal samples. Proc. R. Soc. Lond. B 266, 1189–1195 10.1098/rspb.1999.0762 (doi:10.1098/rspb.1999.0762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Waal F. B. M. 1997. Bonobo, the forgotten ape. Berkeley, CA: University of California Press [Google Scholar]
- 44.Hohmann G., Fruth B. 2003. Intra- and inter-sexual aggression by bonobos in the context of mating. Behaviour 140, 1389–1413 10.1163/156853903771980648 (doi:10.1163/156853903771980648) [DOI] [Google Scholar]
- 45.Hohmann G., Fruth B. 2003. Lui Kotal: a new site for field research on bonobos in the Salonga National Park. Pan Afr. News 10, 25–27 [Google Scholar]
- 46.Furuichi T., et al. 1998. Population dynamics of wild bonobos (Pan paniscus) at Wamba. Int. J. Primatol. 19, 1029–1044 10.1023/A:1020326304074 (doi:10.1023/A:1020326304074) [DOI] [Google Scholar]
- 47.Thompson-Handler N. 1990. The pygmy chimpanzee: sociosexual behavior, reproductive biology and life history. New Haven, CT: Yale University Press [Google Scholar]
- 48.Altmann J. 1974. Observational study of behavior–sampling methods. Behaviour 49, 227–267 10.1163/156853974X00534 (doi:10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 49.Wittig R. M., Boesch C. 2003. ‘Decision-making’ in conflicts of wild chimpanzees (Pan troglodytes): an extension of the relational model. Behav. Ecol. Sociobiol. 54, 401–502 [Google Scholar]
- 50.Dixson A. F. 1998. Primate sexuality. Oxford, UK: Oxford University Press [Google Scholar]
- 51.Hohmann G., Fruth B. 2000. Use and function of genital contacts among female bonobos. Anim. Behav. 60, 107–120 10.1006/anbe.2000.1451 (doi:10.1006/anbe.2000.1451) [DOI] [PubMed] [Google Scholar]
- 52.Wrangham R. W. 2002. The cost of sexual attraction: is there a trade-off in female Pan between sex appeal and received coercion? In Behavioral diversity of chimpanzees and bonobos (eds Boesch C., Hohmann G., Marchant L. F.), pp. 204–215 Cambridge, UK: Cambridge University Press [Google Scholar]
- 53.de Vries H., Netto W. J., Hanegraaf P. L. H. 1993. MatMan: a program for the analysis of sociometric matrices and behavioural transition matrices. Behaviour 125, 157–175 10.1163/156853993X00218 (doi:10.1163/156853993X00218) [DOI] [Google Scholar]
- 54.de Vries H. 1995. An improved test of linearity in dominance hierarchies containing unknown or tied relationships. Anim. Behav. 50, 1375–1389 [Google Scholar]
- 55.Martin P., Bateson P. 1993. Measuring behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- 56.Koenig A., Borries C. 2006. The predictive power of socioecological models: a reconsideration of resource characteristics, agonism, and dominance hierarchies. In Feeding ecology in apes and other primates (eds Hohmann G., Robbins M., Boesch C.), pp. 263–284 Cambridge, UK: Cambridge University Press [Google Scholar]
- 57.de Vries H., Stevens J. M. G., Vervaecke H. 2006. Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 71, 585–592 [Google Scholar]
- 58.Baayen R. 2008. Analyzing linguistic data. Cambridge, UK: Cambridge University Press [Google Scholar]
- 59.Jaeggi A. V., Stevens J., Van Schaik C. 2010. Tolerant food sharing and reciprocity is precluded by despotism among bonobos but not chimpanzees. Am. J. Phys. Anthropol. 143, 41–51 10.1002/ajpa.21288 (doi:10.1002/ajpa.21288) [DOI] [PubMed] [Google Scholar]
- 60.Van Schaik C. 1989. The ecology of social relationships amongst female primates. In Comparative socioecology. The behavioural ecology of humans and other mammals (eds Standen V., Foley R. A.), pp. 195–218 Oxford, UK: Blackwell [Google Scholar]
- 61.Vehrencamp S. L. 1983. A model for the evolution of despotic versus egalitarian societies. Anim. Behav. 31, 667–682 10.1016/S0003-3472(83)80222-X (doi:10.1016/S0003-3472(83)80222-X) [DOI] [Google Scholar]
- 62.Bercovitch F. B. 1991. Social stratification, social strategies, and reproductive success in primates. Ethol. Sociobiol. 12, 315–333 10.1016/0162-3095(91)90023-J (doi:10.1016/0162-3095(91)90023-J) [DOI] [Google Scholar]
- 63.Hayakawa S. 2007. Female defensibility in small troops of Japanese macaques vis-a-vis nontroop males and copulation on the periphery of the troop. Int. J. Primatol. 28, 73–96 10.1007/s10764-006-9109-1 (doi:10.1007/s10764-006-9109-1) [DOI] [Google Scholar]
- 64.Muniz L., Perry S., Manson J. H., Gilkenson H., Gros-Louis J., Vigilant L. 2006. Father-daughter inbreeding avoidance in a wild primate population. Curr. Biol. 16, R156–R157 10.1016/j.cub.2006.02.055 (doi:10.1016/j.cub.2006.02.055) [DOI] [PubMed] [Google Scholar]
- 65.Möller L. M., Beheregaray L. B., Harcourt R. G., Krützen M. 2001. Alliance membership and kinship in wild male bottlenose dolphins (Tursiops aduncus) of southeastern Australia. Proc. R. Soc. Lond. B 268, 1941–1947 10.1098/rspb.2001.1756 (doi:10.1098/rspb.2001.1756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parker G. A. 1990. Sperm competition games: raffles and roles. Proc. R. Soc. Lond. B 242, 120–126 10.1098/rspb.1990.0114 (doi:10.1098/rspb.1990.0114) [DOI] [Google Scholar]
- 67.Møller A. P. 1989. Ejaculate quality, testes size and sperm production in mammals. Funct. Ecol. 3, 91–96 10.2307/2389679 (doi:10.2307/2389679) [DOI] [Google Scholar]
- 68.Anderson M. J., Dixson A. F. 2002. Sperm competition: motility and the midpiece in primates. Nature 416, 496–496 10.1038/416496a (doi:10.1038/416496a) [DOI] [PubMed] [Google Scholar]
- 69.Preston B. T., Stockley P. 2006. The prospect of sexual competition stimulates premature and repeated ejaculation in a mammal. Curr. Biol. 16, R239–R241 10.1016/j.cub.2006.03.018 (doi:10.1016/j.cub.2006.03.018) [DOI] [PubMed] [Google Scholar]
- 70.Harcourt A. H., Purvis A., Liles L. 1995. Sperm competition: mating system, not breeding-season, affects testes size of primates. Funct. Ecol. 9, 468–476 10.2307/2390011 (doi:10.2307/2390011) [DOI] [Google Scholar]
- 71.Strier K. B. 1992. Causes and consequences of nonaggression in woolly spider monkeys. In Aggression and peacefulness in humans and other primates (eds Silverberg J., Gray J. P.), pp. 100–116 New York, NY: Oxford University Press [Google Scholar]
- 72.Rodseth L., Smuts B. B., Harrigan A. M., Wrangham R. W. 1991. On the human community as a primate society: reply to comments. Curr. Anthropol. 32, 429–433 10.1086/203977 (doi:10.1086/203977) [DOI] [Google Scholar]
- 73.Baird R. W., Whitehead H. 2000. Social organization of mammal-eating killer whales: group stability and dispersal patterns. Can. J. Zool. 78, 2096–2105 10.1139/cjz-78-12-2096 (doi:10.1139/cjz-78-12-2096) [DOI] [Google Scholar]