Abstract
Two population oscillations dominate terrestrial community dynamics in northern Canada. In the boreal forest, the snowshoe hare (Lepus americanus) fluctuates in cycles with an 8–10 year periodicity and in tundra regions lemmings typically fluctuate in cycles with a 3–4 year periodicity. I review 60 years of research that has uncovered many of the causes of these population cycles, outline areas of controversy that remain and suggest key questions to address. Lemmings are keystone herbivores in tundra ecosystems because they are a key food resource for many avian and mammalian predators and are a major consumer of plant production. There remains much controversy over the role of predation, food shortage and social interactions in causing lemming cycles. Predation is well documented as a significant mortality factor limiting numbers. Food shortage is less likely to be a major limiting factor on population growth in lemmings. Social interactions might play a critical role in reducing the rate of population growth as lemming density rises. Snowshoe hares across the boreal forest are a key food for many predators and their cycles have been the subject of large-scale field experiments that have pinpointed predation as the key limiting factor causing these fluctuations. Predators kill hares directly and indirectly stress them by unsuccessful pursuits. Stress reduces the reproductive rate of female hares and is transmitted to their offspring who also suffer reduced reproductive rates. The maternal effects produced by predation risk induce a time lag in the response of hare reproductive rate to density, aiding the cyclic dynamics.
Keywords: lemmings, snowshoe hares, social mortality, predation, food shortage, maternal effects
1. Introduction
Terrestrial ecosystems of northern Canada are broadly divided into boreal forests and tundra. As in most ecosystems, large mammals are the main focus of interest by both the public and many biologists. In the boreal forest, caribou, moose, grizzly bears, black bears and wolves are the main large mammals. On the tundra, caribou, muskoxen, grizzly bears and wolves are a major focus of conservation programmes and native hunters. But these large, charismatic species are not the main players in the ecosystem if biomass or energy flow is used as a measure of relative importance. In the boreal forest of the southwestern Yukon, for example, moose and bears represent only 13.6 per cent of the vertebrate biomass and only 2.6 per cent of the energy flow ([1], p. 6). By contrast, snowshoe hares (Lepus americanus) represent 48 per cent of the biomass and 41 per cent of the average energy flow in this ecosystem. On the tundra, Batzli et al. [2] showed that at Point Barrow brown lemmings used up to 100 times more primary production than caribou, and at Prudhoe Bay where caribou were more common and lemmings less common compared with Barrow, lemmings consumed three to six times more vegetation than caribou. Lemmings are keystone herbivores in many tundra areas.
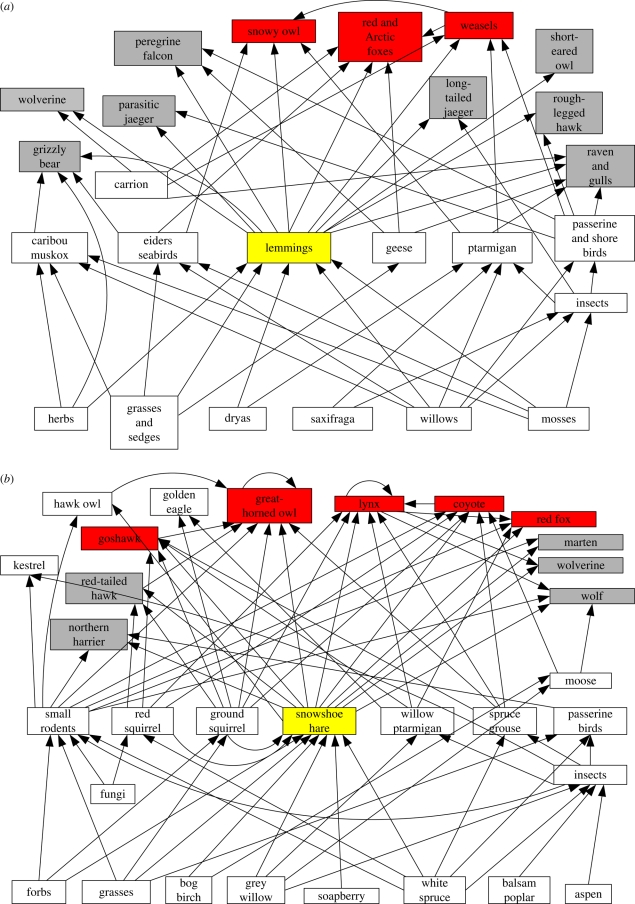
The structure of these two northern ecosystems is most readily seen in the food web for each (figure 1). One of the main jobs of ecologists is to understand the structure of food webs of these types by measuring the strength of the interactions between species and in particular to discover the relative importance of species in the overall food web. The key herbivore species in these systems are lemmings and snowshoe hares, and it is these species that I concentrate on in this review.
Figure 1.
Food webs for the terrestrial ecosystems of northern Canada. Only the major items in the diet are connected, and energy flows in the directions of the arrows. (a) The food web for Herschel Island, North Yukon. Major lemming predators are coded red, minor predators are grey. (b) The food web for the boreal forest of the southwestern Yukon near Kluane Lake. Major predators of snowshoe hares are coded red, minor predators grey.
Population fluctuations in both lemmings and snowshoe hares have been known for more than 100 years [3,4], but ecological research on these fluctuations began only in the 1930s. A large literature has accumulated on these fluctuations (often referred to as ‘cycles’) with wildly conflicting views about the mechanisms that might be behind these population changes [5–10]. Three basic models are used to explain small mammal population fluctuations: the bottom-up model in which food supplies are paramount [11], the top-down model in which predation or disease dominates [12,13], and the social behaviour model in which social interactions involving territoriality or possibly infanticide are key [14]. I evaluate here which of these three models best explains lemming and snowshoe hare population fluctuations in two study sites in northern Canada. The same mechanism could be causing both cycles, but because generation time differs in lemmings and hares, cycle length will differ [15].
2. Material and methods
(a). Study areas
The boreal forest study site was located in the southwestern Yukon Territory near Kluane Lake by the Alaska Highway within the Shakwak Trench system (61°01′ N, 138°24′ W), and lies within the St Elias Mountains' rain shadow. Mean annual precipitation is ca 280 mm and includes an average annual snowfall of approximately 100 cm. The tree community is dominated by white spruce (Picea glauca) interspersed with trembling aspen (Populus tremuloides Michx.) and balsam poplar (Populus balsamifera L.). The upper shrub layer is composed of willow (Salix spp), soapberry (Shepherdia canadensis (L.) Nutt.) and dwarf birch (Betula glandulosa Michx.), whereas the ground layers are composed of dwarf shrubs and herbaceous plants described in detail in Turkington et al. [16,17].
The tundra study site was located on Herschel Island, Yukon, which lies 5 km off the north Yukon shore (69°34.2′ N, 138°54.1′ W). Mean annual precipitation from the closest weather station at Shingle Point is ca 161 mm and includes an average annual snowfall of approximately 78 cm [18]. Herschel Island is dominated by two vegetation types. Much of the higher ground is covered by a tussock tundra community composed of Eriophorum vaginatum, Salix pulchra and an assortment of forb, moss and lichen species. On the previously disturbed/younger surfaces, which comprise approximately half of the island, common plant species are Dryas integrifolia, Poa arcticus, Salix arctica, Lupinus arcticus, other forbs, lichen and mosses.
(b). Trapping methods
All our population data were gathered by live trapping, mark-and-release methods. For lemmings, we used Longworth live traps and our trapping methods are described in detail in Wilson et al. [19] with the exception that we used a 16 × 16 grid size with 30 m spacing of live traps. For snowshoe hares we used cage traps on a 20 × 20 grid with 30 m spacing, with all the trapping methods described in Hodges et al. [20].
Mark–recapture population estimates for all species were calculated from the maximum-likelihood spatial estimator in Efford's Density 4.4 program [21,22] to provide absolute density estimates for each capture session except when there were fewer than seven individuals caught, when I used the minimum number known alive with Poisson confidence limits. Both lemmings and snowshoe hares are highly trappable, and hence our population data are as precise as possible with open populations in continuous habitats.
(c). Changes in lemming numbers
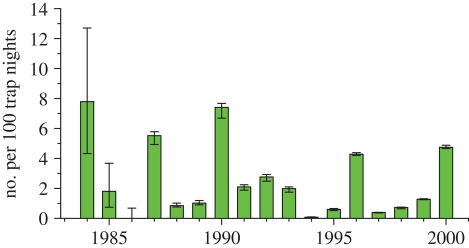
Two lemming species occupy the tundra regions of northern Canada, but they are habitat segregated. The collared lemming (Dicrostonyx spp.) occupies dryer habitats dominated by Dryas, while the brown lemming (Lemmus trimucronatus) occupies wetter habitats dominated by grasses, sedges and mosses [23]. There are relatively few long-term data series for lemming populations in Canada and in this respect Canada has lagged behind the Scandinavian countries (e.g. [24]). Figure 2 shows changes in lemming and vole numbers for the central Arctic region from 1984 to 2000, and illustrates typical cycles in density. Gruyer et al. [25] have reported similar cyclic dynamics in lemming on Bylot Island in the eastern Canadian Arctic with a time series of 13 years. A detailed analysis of these changes in density has produced four generalizations:
— Most cyclic peaks and troughs occur at the same time in brown and collared lemmings—there is interspecific synchrony [26].
— Populations over large geographical regions tend to be in phase so that all tend to peak in the same year—there is geographical synchrony [27].
— Population growth occurs most rapidly in the winter period rather than in the summer [28].
— Some lemming populations do not cycle (particularly in the western Arctic) [29].
Figure 2.
Indices of lemming abundance in the central Canadian Arctic from 1984–2000 from snap trapping lines. Also shown are 95% confidence limits. Both brown and collared lemmings are included in these data, which show clear cyclic fluctuations with a 3–4 year periodicity. This is one of the longest time series currently available for the Canadian Arctic. These data are discussed in more detail in Krebs et al. [27].
What causes lemming population fluctuations? Since there is geographical synchrony, immigration and emigration cannot be a regional explanation, although they may be involved in local areas. Both reproductive rates and mortality rates change to produce the observed density fluctuations, and the key is to determine what might produce delayed density dependence in both these processes. Reproduction is increased by both winter breeding and an earlier age at sexual maturity and could be reduced by food shortage [30]. Mortality can be increased by predation losses and infanticide [31]. All of these processes tend to be delayed density dependent and we seem to have two options. The first is to suggest that all of these processes play a part in generating a density cycle, and that different population cycles will have different sets of limiting factors. The second is to suggest that one or a few of these processes are necessary to generate a population cycle, and that some factors are sufficient to generate a cycle, but are not necessary.
One way out of this dilemma is to model a population and simulate the consequences of a set of assumptions about specific processes. This approach has proved less useful than one might hope because all the existing models contain parameters that cannot be measured or have not been quantified adequately. Whereas 50 years ago, almost no population models would produce cyclic fluctuations, now there are so many that they are of little help in solving the problem of exactly what is essential. This is not the place to evaluate the role of modelling in understanding cyclic fluctuations, and Lambin et al. [8] have reviewed this important issue.
Another way out of this dilemma is to conduct experimental manipulations in the field. Unfortunately, very few field experiments have been carried out on lemming populations. Two attempts have been made to reduce predation on lemming populations by fencing out predators. Reid et al. [31] fenced 11 ha of tundra with a perimeter fence and an aerial mesh of fishing line, and found the lemming population inside the fence survived better and had higher recruitment than in unfenced controls. Thus, summer population growth in this area was clearly limited by predation losses. Wilson et al. [19] repeated the predator fence experiment and confirmed the conclusion that predation in summer limited population growth, particularly in the decline phase of the cycle. But the key question of what happens in winter was left unanswered. Both least weasels (Mustela nivalis) and ermine (Mustela erminea) are important lemming predators that can operate under the snow. No data were available on how effective predator exclosures are in winter. Wilson et al. [19] observed a nearly equal population decline on control grids and the fenced grid over the winter. A major reason for population decline was the lack of reproduction during the autumn and winter of the decline. Wilson's observations could be good evidence that predation in winter is ineffective in determining the rate of population decline and other mortality factors were involved (if the fence was not breached by predators). Or if the fence was not operating to exclude predators over winter, an equal decline inside and outside the fence would be expected if predators control population decline over the winter period in the absence of winter reproduction. Again and again we find that the winter dynamics of lemmings is a key to understanding their fluctuations, and we have very little data on that season [32].
Winter reproduction in lemmings must operate under the standard physiological constraints on small mammal reproduction with respect to temperature limitations and food restrictions [33]. On Herschel Island, mean ground temperatures in mid-winter reach −18°C to −22°C under the snow (C. Krebs 2010, unpublished data). Winter litter sizes are low in lemmings [30], but there are no detailed data that permit us to determine the temperature threshold at which winter reproduction becomes impossible, assuming that food is not limiting in winter (yet another problem). There is unequivocal evidence that lemmings breed under the snow in winter and are able to increase in numbers, but exactly how this is achieved remains to be determined [30].
Except for laboratory studies, we have virtually no information on the severity of infanticide mortality in lemmings. Mallory & Brooks [34] showed that female collared lemmings committed infanticide in the laboratory, while male lemmings did not. The remaining question is how often infanticide operates in the field, a topic on which we have no data. Millar [35] reviewed nest mortality in small mammals, which he found ranged from 30 to 96 per cent in the first three weeks of life, but he concluded that these losses could rarely be partitioned as to causes of death. Infanticide in the field is very difficult to detect. The sole estimate of infanticide losses in field populations of collared lemmings from Reid et al. [31] ranged from 0 to 5 per cent for a low-density population in which most losses of nestlings (43–70% lost) were due to predation. Whether a similar result would occur at higher lemming densities is not known.
Bottom-up or food limitation in lemmings has been postulated since the early work on the brown lemming at Point Barrow, Alaska [36,37]. Mathematical models of food limitation have been constructed [38], but parameter values for these and other food limitation models are lacking for most tundra ecosystems. The controversy continues to oscillate between food limitation and predation limitation [11,39], and will not be resolved without more field experiments. Food quality and herbivore condition are two key items often missing in evaluation of potential food limitation in herbivores.
Interest in determining the factors affecting the rate of population change in lemmings has overshadowed other questions about the role of food supplies in setting the carrying capacity for lemmings. We do not yet know what particular aspect of plant productivity sets the upper limit for density in lemmings. A particularly strong contrast occurs between the brown lemming on the Arctic coastal plain of Alaska, where densities reach 200 ha−1 or more [40], and brown lemmings in the eastern Canadian Arctic that reach only 3 ha−1 [25]. On Herschel Island, brown lemmings reached a peak density of 59 ha−1 on one study site (C. J. Krebs 2010, unpublished data). G. Batzli (personal communication) has suggested that the peak density of the brown lemming should be directly related to the density of its main food plant, the grass Deschampia flexuosa. Collared lemming populations do not have as wide a variance in peak density as the brown lemming. But there is still substantial variation that needs explanation. On Bylot Island, collared lemmings reach only 1 ha−1 [25] while at Herschel Island they peak at 7 ha−1 (C. J. Krebs, unpublished data), and in east Greenland, Gilg et al. [28] report densities varying from 5–8 ha−1 at the peak. A starting hypothesis could be that the abundance of Dryas spp. sets an upper limit to density for collared lemmings. Hypotheses of these types could also be useful to explain spatial differences in lemming density. Winter weather can clearly affect the potential availability of food for lemmings under the snow [24], but data are needed to test these ideas.
There is much interest in how climate change in the Arctic will affect lemming cycles [24,28,41]. The main effects predicted are a shortening of the snow season and more rain events during the winter season, both of which have the potential to increase overall mortality and decrease winter reproduction. An experimental approach to this issue has been carried out during the International Polar Year by Don Reid on Herschel Island and Gilles Gauthier on Bylot Island by erecting snow fence to increase snow depth and the length of winter snow cover. These results are now in preparation (2010–2011) and will be eagerly awaited as a further step to increasing our understanding of how snow quantity and quality affect lemming density changes.
(d). Changes in snowshoe hare numbers
For more than 60 years, snowshoe hare population cycles have been the staple of ecology textbooks to illustrate population fluctuations because of the long-term data provided by the Canada lynx (Lynx canadensis) fur returns provided by the Hudson's Bay Company since the seventeenth century [42]. The snowshoe hare is a central herbivore in the food web of the boreal forest (figure 1b), and while textbooks tend to discuss the ‘lynx–hare’ cycle as a predator–prey oscillation, many more predators and alternative prey species are involved in the actual dynamics.
Extensive studies on snowshoe hare populations have been carried out at two main locations: by Lloyd Keith and his students in central Alberta [43] and by our group at Kluane Lake in the southwestern Yukon [1]. Keith [43] proposed a combined bottom-up–top-down explanation for the hare cycle in which food shortage slowed population growth during the late increase phase and then predation delivered the coup de grâce to cause the decline phase of the cycle. We tested this model experimentally by feeding snowshoe hares over one 10 year cycle from 1976 to 1985 and found that food addition did not alter cyclic dynamics except to raise the local carrying capacity. Fed populations declined at the same time and at the same rate as control populations [44]. We began in 1986 a second set of experiments manipulating both food and predation, and found that most hares (greater than 90%) died from predation and virtually none from starvation [20,45]. Predation mortality was clearly the dominant process driving hare numbers in the Kluane region.
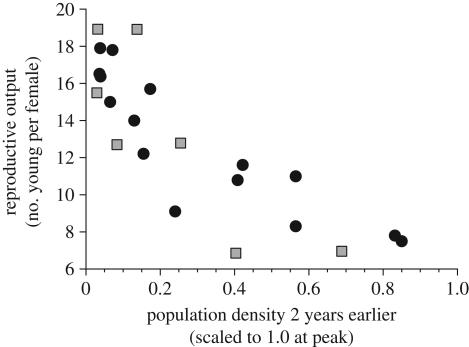
But the problem was that our large-scale experiments identified a joint food supply–predation manipulation as the major effect on hare numbers [46]. How was this possible when our studies showed no signs of food shortage even at the peak of the hare cycle and food-addition experiments failed to affect the rate of population collapse [44]? Moreover, Cary & Keith [47] had identified a strong pattern of change in reproductive output over the hare cycle, a change we also found at Kluane (figure 3). Reproductive output was delayed density dependent with a two year time lag. Boonstra et al. [48] suggested that this reproductive decay could be a response to chronic stress caused by the sublethal effects of predation risk. Sheriff et al. [49] confirmed this hypothesis and showed experimentally that stress effects from predation risk were maternally inherited in the offspring of stressed females, thus explaining the time lag illustrated in figure 3. This research has confirmed that the hare cycle is caused top-down by predation, which by direct effects on hare mortality and indirect effects on hare reproduction drives numbers up and down.
Figure 3.
Reproductive output per adult female snowshoe hare in relation to population density two years earlier for study sites in central Alberta (data from Cary & Keith [47]) and southwestern Yukon. Reproductive output lags 2 years behind changes in population density. Filled circles, Alberta; filled squares, Yukon.
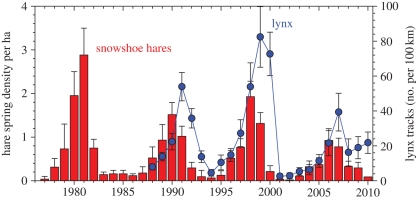
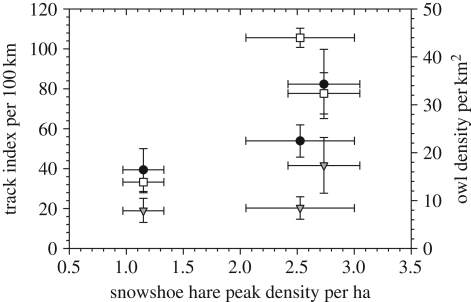
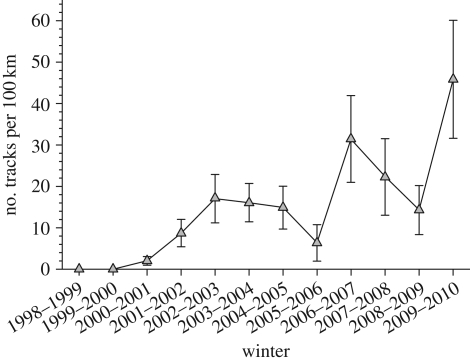
If the hare cycle is largely driven by predation, it should be sensitive to changes in predator diversity and abundance. Our data from the southwestern Yukon show a long-term pattern towards declining peak densities (figure 4). Anecdotal data from local residents at Kluane suggest that the hare peak of the 1960s was very high, and it is clear that there is large variation in the absolute density reached at peak numbers. Our data are limited to three cycles from 1986 to 2010, and figure 5 shows clear trends in predator indices that are related to maximum hare numbers for these three hare cycles. Lynx and great-horned owls (Bubo virginianus) show the strongest relationship and coyotes a much weaker trend. Sheriff et al. [10] have shown that the length of the low phase of the hare cycle is related to predator densities at the peak, which is presumably an index of the amount of predation risk to which hares are exposed. Since there seems to be general agreement that predators are limited in density by the amount of prey available, figure 5 reflects this generalization that all these specialist predators are food-limited [50].
Figure 4.
Snowshoe hare spring density at Kluane Lake, Yukon, 1977–2010, and an index of lynx numbers from winter snow tracking, 1988–2010. Lynx data from winters are plotted over the year ending each winter. Estimates with 95% confidence limits are given.
Figure 5.
Relationship of peak predator numbers for lynx, coyotes and great-horned owls to peak snowshoe hare numbers for three cycles from 1985 to 2010. Estimates with 95% confidence limits. Peak predator numbers occur 1–2 years after peak hare numbers. For the 1990 peak, autumn hare density was 2.53 ha−1, for 1998 2.73 ha−1 and for 2006 1.15 ha−1. Filled circles, lynx; filled triangles, coyote; open squares, great-horned owl.
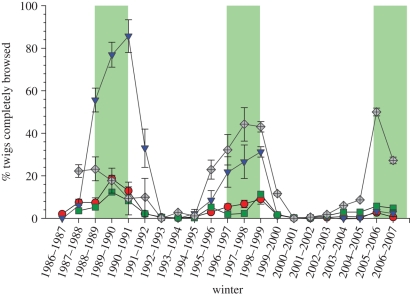
Snowshoe hares have a strong impact on shrub growth in the Kluane region, so that while winter food supplies do not limit hare density, hares reduce shrub biomass [51]. This effect has resulted in a spurt of growth in dwarf birch (B. glandulosa) in the Kluane region after the weak hare peak of 2006. Figure 6 shows the declining rate of browsing on willow and birch over three cycles. Dwarf birch is the snowshoe hare's favourite winter food in this area [51]. During the relatively low peak of 2006, few twigs of dwarf birch were completely browsed but many were partly browsed.
Figure 6.
Percentage of willow (Salix glauca) and dwarf birch (B. glandulosa) 5 mm diameter twigs completely browsed and partly browsed in relation to the hare cycle, Kluane, Yukon. Green bars delimit the peak hare density for each cycle. An average of 600 individually tagged willow twigs and 200 birch twigs were sampled each year. Hares do not browse twigs above about 5 mm diameter, Filled circles, willow total; filled triangles, dwarf birch total; filled squares, willow partial; filled diamonds, dwarf birch partial.
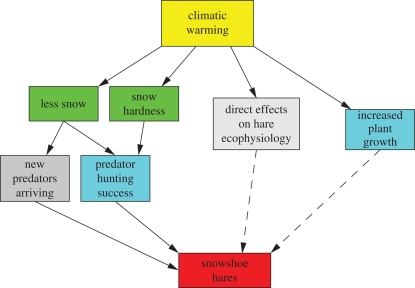
Climate change could affect hare population fluctuations in several ways (figure 7). By increasing plant growth, it could increase the general carrying capacity of hare habitats, at least in the short term. This effect would have minimal impact on boreal forest community structure. It is possible that climate change will select for hares with different ecophysiological traits, such as timing of moult. Such variation already exists from south to north within the range of snowshoe hares, and again would not seem to have much overall impact on boreal forest community structure. The most likely larger impacts could come from changes in the winter snow regime. More snow or less snow over winter could increase the hunting efficiency of predators such as coyotes (Canis latrans) and lynx [52–55]. Improving either the functional or the numerical response of predators would reduce the time lag of response to an increase in hare numbers, and consequently shorten the cycle length as well as reduce the amplitude of the fluctuations.
Figure 7.
Possible paths by which climatic warming might influence the snowshoe hare cycle. Dashed lines indicate paths that are less certain to be significant.
It is far from clear why the hare cycle of 2006 reached a peak at such a low density. Two possible explanations for low peak hare densities can be suggested.
— There is a predator subsidy of animals moving into the area from adjacent populations that are out of phase with the Kluane region. Lynx movements of up to 800 km have been recorded after hare numbers collapse [56]. Synchrony in population fluctuations may be enforced by climatic factors [54,55], and local synchrony could be broken down by climate change. But there is no clear evidence of predator subsidy in the predator indices from snow tracking (figure 4). Rates of increase for both lynx and coyote tracks were lower in the most recent cycle than they had been in the previous two cycles.
— Additional predators have colonized the food web. The major addition to the Kluane food web in recent years has been the marten (Martes americana), which has increased dramatically since 2000 (figure 8). Marten are generalist predators and feed on small mammals as well as squirrels and hares. Most studies of marten diet in southern areas have found relatively few snowshoe hares in the diet, but Poole & Graf [57] found that hares could comprise up to 64 per cent of the winter diet of marten in the Northwest Territories when hares were common. We have not carried out studies of marten in the Kluane region and consequently do not know how serious a predator they might be of juvenile and adult snowshoe hares or whether their predation is additive to that of other predators like lynx and coyote.
Figure 8.
Index of population density of marten (M. americana) from snow tracking, winter 1998–2010, Kluane Lake, Yukon. A few tracks were counted in 1993–1995 but marten were almost unknown in the Kluane area before 2000 and most winters from 1988 to 2000 had zero counts.
These alternatives can be evaluated only by a large-scale experimental study or by a long-term monitoring programme.
3. Discussion
Analysing fluctuating populations has been controversial because there are several approaches that ecologists have adopted. At the simplest level, any factor that operates in a delayed density-dependent manner will have the potential for generating cyclic dynamics. But the problem is that many factors that affect small mammals have a time delay built into them, and searching for delayed density-dependent factors typically produces a long list of candidate processes. Many small mammal ecologists have tried to cut this Gordian knot by doing field experiments, but the design of field experiments is itself often controversial [8]. In terms of dynamics, the distinction between determining (i) what factors determine the peak density (‘carrying capacity’) for cyclic populations, and (ii) what factors determine the rate of change in numbers is a useful one for designing field studies [58].
Our research on both lemmings and snowshoe hares has changed over the years from a focus on single species to a focus on the community level of organization and the species interactions that are critical for food web structure. In both tundra and boreal forest ecosystems, climate change has the potential to disrupt food webs. Current ecological theory is unable to make credible predictions about the consequences of climate change, and ecologists will be forced to maintain long-term adaptive monitoring programmes [59] to test hypotheses that emerge from such data collection programmes.
Lemming population dynamics has been enmeshed in endless controversy around small rodent population changes in general, well illustrated by the recent exchange between Oksanen et al. [11] and Gauthier et al. [39]. These controversies will never be sorted out until a clear experimental protocol is established for specific hypotheses with predictions that can be tested in field populations on both sides of the Atlantic. Until we have more experiments like those carried out by Graham & Lambin [60] (but see the critique by Korpimäki et al. [61]), we will have little resolution and much unnecessary controversy.
The most critical gaps in our knowledge of population dynamics for both species of lemmings at the present time are as follows:
— Limited studies of winter ecology including survival, movements and the extent of autumn, winter and spring breeding under the snow.
— Information on social aggression and the extent of infanticide in field populations at different densities.
— A quantitative analysis of stress in female lemmings and their offspring to test the Boonstra–Sheriff model for the collapse of reproductive rates in declining populations.
— Hypotheses to explain the variation in peak densities in different ecoregions and in different cyclic peaks.
— Virtually no reliable data on least weasel or ermine population densities, survival, movements and reproduction in relation to lemming numbers.
— Detailed data on offtake by all lemming predators so that a detailed quantitative food web model can be constructed.
Snowshoe hare population studies are at a more mature level of understanding because of the extensive long-term experimental studies of Lloyd Keith and his students in Alberta and of our group at Kluane Lake. Nevertheless, several gaps in our knowledge await further investigation:
— A quantitative longitudinal analysis of stress in individual female hares and their offspring to test the Boonstra–Sheriff model for declining and low-phase populations.
— Data on the hunting success of different predator species in relation to snow conditions and hare density in order to anticipate potential climate change effects.
— Hypotheses to explain the variation in peak densities in different regions.
— A detailed analysis of the extent of synchrony in hare cycles across Canada and the reasons for lack of synchrony.
The boreal forest and tundra ecosystems of northern Canada represent two of the least human-affected ecosystems on Earth where the influences of climate change can be dissociated from other human alternations associated with forestry, agriculture and urban environments. As such, they are deserving of a long-term commitment to improving our ecological understanding of the polar regions of the world.
Acknowledgements
I thank all those who have worked to gather these data on lemmings and snowshoe hares, especially Rudy Boonstra, Stan Boutin, the late Jamie Smith, Tony Sinclair, Mark O'Donoghue, Don Reid, Liz Hofer, Alice Kenney, Scott Gilbert, Douglas Morris, Deb Wilson and many research assistants for assistance in fieldwork. Research funding was provided by the Natural Science and Engineering Research Council of Canada, the International Polar Year and the EJLB Foundation. The facilities of the Kluane Lake Research Station of the Arctic Institute of North America were essential to the long-term research programme on snowshoe hares, and we thank Andy and Carole Williams for their assistance. Richard Gordon and the staff at Herschel Island Territorial Park provided facilities for our lemming work on Herschel Island.
Footnotes
Invited review by the Canadian Society for Ecology and Evolution biannual award winner.
References
- 1.Krebs C. J., Boutin S., Boonstra R. 2001. Ecosystem dynamics of the boreal forest: the Kluane project. New York, NY: Oxford University Press [Google Scholar]
- 2.Batzli G. O., White R. G., Maclean S. F., Pitelka F. A., Collier B. D. 1980. The herbivore-based trophic system. In An arctic ecosystem (eds Brown J., Miller P. C., Tiezen L. L., Bunnell F. L.), pp. 335–410, Stroudsburg, PA: Dowden, Hutchinson, and Ross [Google Scholar]
- 3.Elton C. S. 1924. Periodic fluctuations in the numbers of animals: their causes and effects. Br. J. Exp. Biol. 2, 119–163 [Google Scholar]
- 4.Chitty D., Elton C. 1937. Canadian Arctic wild life enquiry, 1935–36. J. Anim. Ecol. 6, 368–385 10.2307/1193 (doi:10.2307/1193) [DOI] [Google Scholar]
- 5.Chitty D. 1960. Population processes in the vole and their relevance to general theory. Can. J. Zool. 38, 99–113 10.1139/z60-011 (doi:10.1139/z60-011) [DOI] [Google Scholar]
- 6.Norrdahl K. 1995. Population cycles in northern small mammals. Biol. Rev. 70, 621–637 10.1111/j.1469-185X.1995.tb01654.x (doi:10.1111/j.1469-185X.1995.tb01654.x) [DOI] [PubMed] [Google Scholar]
- 7.Stenseth N. C. 1999. Population cycles in voles and lemmings: density dependence and phase dependence in a stochastic world. Oikos 87, 427–461 10.2307/3546809 (doi:10.2307/3546809) [DOI] [Google Scholar]
- 8.Lambin X., Krebs C. J., Moss R., Yoccoz N. G. 2002. Population cycles: inferences from experimental, modeling, and time series approaches. In Population cycles: the case for trophic interactions (ed. Berryman A.), pp. 155–176 New York, NY: Oxford University Press [Google Scholar]
- 9.Korpimäki E., Brown P. R., Jacob J., Pech R. P. 2004. The puzzles of population cycles and outbreaks of small mammals solved? Bioscience 54, 1071–1079 10.1641/0006-3568(2004)054[1071:TPOPCA]2.0.CO;2 (doi:10.1641/0006-3568(2004)054[1071:TPOPCA]2.0.CO;2) [DOI] [Google Scholar]
- 10.Sheriff M. J., Krebs C. J., Boonstra R. 2010. The ghosts of predators past: population cycles and the role of maternal programming under fluctuating predation risk. Ecology 91, 2983–2994 10.1890/09-1108.1 (doi:10.1890/09-1108.1) [DOI] [PubMed] [Google Scholar]
- 11.Oksanen T., Oksanen L., Dahlgren J., Olofsson J. 2008. Arctic lemmings, Lemmus spp. and Dicrostonyx spp.: integrating ecological and evolutionary perspectives. Evol. Ecol. Res. 10, 415–434 [Google Scholar]
- 12.Klemola T., Tanhuanpaa M., Korpimäki E., Ruohomaki K. 2002. Specialist and generalist natural enemies as an explanation for geographical gradients in population cycles of northern herbivores. Oikos 99, 83–94 10.1034/j.1600-0706.2002.990109.x (doi:10.1034/j.1600-0706.2002.990109.x) [DOI] [Google Scholar]
- 13.Smith M. J., White A., Sherratt J. A., Telfer S., Begon M., Lambin X. 2008. Disease effects on reproduction can cause population cycles in seasonal environments. J. Anim. Ecol. 77, 378–389 10.1111/j.1365-2656.2007.01328.x (doi:10.1111/j.1365-2656.2007.01328.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs C. J., Lambin X., Wolff J. O. 2007. Social behavior and self-regulation in murid rodents. In Rodent societies: an ecological and evolutionary perspective (eds Wolff J. O., Sherman P. W.), pp. 173–181 Chicago, IL: University of Chicago Press [Google Scholar]
- 15.Ginzburg L. R., Taneyhill D. E. 1995. Higher growth rate implies shorter cycle, whatever the cause: a reply to Berryman. J. Anim. Ecol. 64, 294–295 10.2307/5764 (doi:10.2307/5764) [DOI] [Google Scholar]
- 16.Turkington R., John E., Dale M. R. T. 2001. Herbs and grasses. In Ecosystem dynamics of the boreal forest (eds Krebs C. J., Boutin S., Boonstra R.), pp. 69–91 New York, NY: Oxford University Press [Google Scholar]
- 17.Turkington R., John E., Watson S., Seccomb-Hett P. 2002. The effects of fertilization and herbivory on the herbaceous vegetation of the boreal forest in north-western Canada: a 10-year study. J. Ecol. 90, 325–337 10.1046/j.1365-2745.2001.00666.x (doi:10.1046/j.1365-2745.2001.00666.x) [DOI] [Google Scholar]
- 18.Burn C. R., Zhang Y. 2009. Permafrost and climate change at Herschel Island (Qikiqtaruk), Yukon Territory, Canada. J. Geophys. Res. 114, F02001. 10.1029/2008JF001087 (doi:10.1029/2008JF001087) [DOI] [Google Scholar]
- 19.Wilson D., Krebs C. J., Sinclair A. R. E. 1999. Limitation of collared lemming populations during a population cycle. Oikos 87, 382–398 10.2307/3546754 (doi:10.2307/3546754) [DOI] [Google Scholar]
- 20.Hodges K. E., Krebs C. J., Hik D. S., Stefan C. I., Gillis E. A., Doyle C. E. 2001. Snowshoe hare demography. In Ecosystem dynamics of the boreal forest: the Kluane project (eds Krebs C. J., Boutin S., Boonstra R.), pp. 141–178 New York, NY: Oxford University Press [Google Scholar]
- 21.Borchers D. L., Efford M. G. 2008. Spatially explicit maximum likelihood methods for capture–recapture studies. Biometrics 64, 377–385 10.1111/j.1541-0420.2007.00927.x (doi:10.1111/j.1541-0420.2007.00927.x) [DOI] [PubMed] [Google Scholar]
- 22.Efford M. G., Borchers D. L., Byrom A. E. 2009. Density estimation by spatially explicit capture–recapture: likelihood-based methods. In Modeling demographic processes in marked populations (eds Thomson E. G. C. D. L., Conroy M. J.), pp. 255–269 New York, NY: Springer [Google Scholar]
- 23.Morris D. W., Davidson D. L., Krebs C. J. 2000. Measuring the ghost of competition: insights from density-dependent habitat selection on the coexistence and dynamics of lemmings. Evol. Ecol. Res. 2, 41–67 [Google Scholar]
- 24.Kausrud K. L., et al. 2008. Linking climate change to lemming cycles. Nature 456, 93–97 10.1038/nature07442 (doi:10.1038/nature07442) [DOI] [PubMed] [Google Scholar]
- 25.Gruyer N., Gauthier G., Berteaux D. 2010. Demography of two lemming species on Bylot Island, Nunavut, Canada. Polar Biol. 33, 725–736 10.1007/s00300-009-0746-7 (doi:10.1007/s00300-009-0746-7) [DOI] [Google Scholar]
- 26.Chitty H. 1950. Canadian Arctic wild life enquiry, 1943–1949: with a summary of results since 1933. J. Anim. Ecol. 19, 180–193 10.2307/1527 (doi:10.2307/1527) [DOI] [Google Scholar]
- 27.Krebs C. J., Kenney A. J., Gilbert S., Danell K., Angerbjörn A., Erlinge S., Bromley R. G., Shank C., Carriere S. 2002. Synchrony in lemming and vole populations in the Canadian Arctic. Can. J. Zool. 80, 1323–1333 10.1139/z02-120 (doi:10.1139/z02-120) [DOI] [Google Scholar]
- 28.Gilg O., Sittler B., Hanski I. 2009. Climate change and cyclic predator–prey population dynamics in the high Arctic. Global Change Biol. 15, 2634–2652 10.1111/j.1365-2486.2009.01927.x (doi:10.1111/j.1365-2486.2009.01927.x) [DOI] [Google Scholar]
- 29.Reid D. G., Krebs C. J., Kenney A. J. 1997. Patterns of predation on noncyclic lemmings. Ecol. Monogr. 67, 89–108 10.1890/0012-9615(1997)067[0089:POPONL]2.0.CO;2 (doi:10.1890/0012-9615(1997)067[0089:POPONL]2.0.CO;2) [DOI] [Google Scholar]
- 30.Millar J. S. 2001. On reproduction in lemmings. Ecoscience 8, 145–150 [Google Scholar]
- 31.Reid D. G., Krebs C. J., Kenney A. J. 1995. Limitation of collared lemming population growth at low densities by predation mortality. Oikos 73, 387–398 10.2307/3545963 (doi:10.2307/3545963) [DOI] [Google Scholar]
- 32.Gilg O., Hanski I., Sittler B. 2003. Cyclic dynamics in a simple vertebrate predator–prey community. Science 302, 866–868 10.1126/science.1087509 (doi:10.1126/science.1087509) [DOI] [PubMed] [Google Scholar]
- 33.Speakman J., Król E. 2005. Limits to sustained energy intake IX: a review of hypotheses. J. Comp. Physiol. B 175, 375–394 10.1007/s00360-005-0013-3 (doi:10.1007/s00360-005-0013-3) [DOI] [PubMed] [Google Scholar]
- 34.Mallory F. F., Brooks R. J. 1978. Infanticide and other reproductive strategies in the collared lemming, Dicrostonyx groenlandicus. Nature 273, 144–146 10.1038/273144a0 (doi:10.1038/273144a0) [DOI] [PubMed] [Google Scholar]
- 35.Millar J. S. 2007. Nest mortality in small mammals. Ecoscience 14, 286–291 10.2980/1195-6860(2007)14[286:nmism]2.0.co;2 (doi:10.2980/1195-6860(2007)14[286:nmism]2.0.co;2). [DOI] [Google Scholar]
- 36.Pitelka F. A. 1957. Some characteristics of microtine cycles in the Arctic. In Arctic Biology pp. 73–88 Corvallis, Oregon: Oregon State College Biology Colloquium [Google Scholar]
- 37.Schultz A. M. 1964. The nutrient-recovery hypothesis for Arctic microtine cycles. II. Ecosystem variables in relation to the Arctic microtine cycles. In Grazing in terrestrial and marine environments (ed. Crisp D. J.), pp. 57–68 Oxford, UK: Blackwells [Google Scholar]
- 38.Turchin P., Batzli G. O. 2001. Availability of food and the population dynamics of arvicoline rodents. Ecology 82, 1521–1534 10.1890/0012-9658(2001)082[1521:AOFATP]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[1521:AOFATP]2.0.CO;2) [DOI] [Google Scholar]
- 39.Gauthier G., Berteaux D., Krebs C. J., Reid D. 2009. Arctic lemmings are not simply food limited—a comment. Evol. Ecol. Res. 11, 483–484 [Google Scholar]
- 40.Pitelka F. A., Batzli G. O. 2007. Population cycles of lemmings near Barrow, Alaska: a historical review. Acta Theriol. 52, 323–336 [Google Scholar]
- 41.Ims R. A., Henden J.-A., Killengreen S. T. 2008. Collapsing population cycles. Trends Ecol. Evol. 23, 79–86 10.1016/j.tree.2007.10.010 (doi:10.1016/j.tree.2007.10.010) [DOI] [PubMed] [Google Scholar]
- 42.Elton C., Nicholson M. 1942. The ten-year cycle in numbers of the lynx in Canada. J. Anim. Ecol. 11, 215–244 10.2307/1358 (doi:10.2307/1358) [DOI] [Google Scholar]
- 43.Keith L. B. 1981. Population dynamics of hares. In Proceedings of the World Lagomorph Conference (eds Myers K., MacInnes C. D.), pp. 395–440 Guelph, Ontario: University of Guelph [Google Scholar]
- 44.Krebs C. J., Boutin S., Gilbert B. S. 1986. A natural feeding experiment on a declining snowshoe hare population. Oecologia 70, 194–197 10.1007/BF00379239 (doi:10.1007/BF00379239) [DOI] [PubMed] [Google Scholar]
- 45.Boutin S., Krebs C. J., Boonstra R., Sinclair A. R. E., Hodges K. E. 2002. Understanding the snowshoe hare cycle through large-scale field experiments. In Population cycles: the case for trophic interactions (ed. Berryman A.), pp. 69–91 New York, NY: Oxford University Press [Google Scholar]
- 46.Krebs C. J., Boutin S., Boonstra R., Sinclair A. R. E., Smith J. N. M., Dale M. R. T., Martin K., Turkington R. 1995. Impact of food and predation on the snowshoe hare cycle. Science 269, 1112–1115 10.1126/science.269.5227.1112 (doi:10.1126/science.269.5227.1112) [DOI] [PubMed] [Google Scholar]
- 47.Cary J. R., Keth L. B. 1979. Reproductive changes in the 10-year cycle of snowshoe hares. Can. J. Zool. 57, 375–390 [Google Scholar]
- 48.Boonstra R., Hik D., Singleton G. R., Tinnikov A. 1998. The impact of predator-induced stress on the snowshoe hare cycle. Ecol. Monogr. 68, 371–394 10.1890/0012-9615(1998)068[0371:TIOPIS]2.0.CO;2 (doi:10.1890/0012-9615(1998)068[0371:TIOPIS]2.0.CO;2) [DOI] [Google Scholar]
- 49.Sheriff M. J., Krebs C. J., Boonstra R. 2009. The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. J. Anim. Ecol. 78, 1249–1258 10.1111/j.1365-2656.2009.01552.x (doi:10.1111/j.1365-2656.2009.01552.x) [DOI] [PubMed] [Google Scholar]
- 50.O'Donoghue M., Boutin S., Krebs C. J., Hofer E. J. 1997. Numerical responses of coyotes and lynx to the snowshoe hare cycle. Oikos 80, 150–162 10.2307/3546526 (doi:10.2307/3546526) [DOI] [Google Scholar]
- 51.Smith J. N. M., Krebs C. J., Sinclair A. R. E., Boonstra R. 1988. Population biology of snowshoe hares II. Interactions with winter food plants. J. Anim. Ecol. 57, 269–286 10.2307/4778 (doi:10.2307/4778) [DOI] [Google Scholar]
- 52.Murray D. L., Boutin S. 1991. The influence of snow on lynx and coyote movements in southwestern Yukon: does morphology affect behaviour? Oecologia 88, 463–469 [DOI] [PubMed] [Google Scholar]
- 53.O'Donoghue M., Boutin S., Krebs C. J., Zuleta G., Murray D. L., Hofer E. J. 1998. Functional responses of coyotes and lynx to the snowshoe hare cycle. Ecology 79, 1193–1208 10.2307/176736 (doi:10.2307/176736) [DOI] [Google Scholar]
- 54.Stenseth N. C., Shabbar A., Chan K.-S., Boutin S., Rueness E. K., Ehrich D., Hurrell J. W., Lingjærde O. C., Jakobsen K. S. 2004. Snow conditions may create an invisible barrier for lynx. Proc. Natl Acad. Sci. USA 101, 10 632–10 634 10.1073/pnas.0308674101 (doi:10.1073/pnas.0308674101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stenseth N. C., et al. 2004. The effect of climatic forcing on population synchrony and genetic structuring of the Canadian lynx. Proc. Natl Acad. Sci. USA 101, 6056–6061 10.1073/pnas.0307123101 (doi:10.1073/pnas.0307123101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mowat G., Poole K. G., O'Donoghue M. 2000. Ecology of lynx in northern Canada and Alaska. In Ecology and conservation of lynx in the United States (eds Ruggiero L. F., Aubry K. B., Buskirk S. W., Koehler G. M., Krebs C. J., McKelvey K. S., Squires J. R.), pp. 265–306 Denver, CO: University Press of Colorado [Google Scholar]
- 57.Poole K. G., Graf R. P. 1996. Winter diet of marten during a snowshoe hare decline. Can. J. Zool. 74, 456–466 10.1139/z96-053 (doi:10.1139/z96-053) [DOI] [Google Scholar]
- 58.Sibly R. M., Hone J. 2002. Population growth rate and its determinants: an overview. Phil. Trans. R. Soc. Lond. B 357, 1153–1170 10.1098/rstb.2002.1117 (doi:10.1098/rstb.2002.1117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindenmayer D. B., Likens G. E. 2009. Adaptive monitoring: a new paradigm for long-term research and monitoring. Trends Ecol. Evol. 24, 482–486 10.1016/j.tree.2009.03.005 (doi:10.1016/j.tree.2009.03.005) [DOI] [PubMed] [Google Scholar]
- 60.Graham I. M., Lambin X. 2002. The impact of weasel predation on cyclic field-vole survival: the specialist predator hypothesis contradicted. J. Anim. Ecol. 71, 946–956 10.1046/j.1365-2656.2002.00657.x (doi:10.1046/j.1365-2656.2002.00657.x) [DOI] [Google Scholar]
- 61.Korpimäki E., Klemola T., Norrdahl K., Oksanen L., Oksanen T., Banks P. B., Batzli G. O., Henttonen H. 2003. Vole cycles and predation. Trends Ecol. Evol. 18, 494–495 10.1016/S0169-5347(03)00159-9 (doi:10.1016/S0169-5347(03)00159-9) [DOI] [Google Scholar]