Abstract
A controlled and observable drug delivery system that enables long-term local drug administration is reported. Biodegradable and biocompatible drug-loaded porous Si microparticles were prepared from silicon wafers, resulting in a porous 1-dimensional photonic crystal (rugate filter) approx. 12 micrometers thick and 35 micrometers across. An organic linker, 1-undecylenic acid, was attached to the Si-H terminated inner surface of the particles by hydrosilylation and the anthracycline drug daunorubicin was bound to the carboxy terminus of the linker. Degradation of the porous Si matrix in vitro was found to release the drug in a linear and sustained fashion for 30 d. The bioactivity of the released daunorubicin was verified on retinal pigment epithelial (RPE) cells. The degradation/drug delivery process was monitored in situ by digital imaging or spectroscopic measurement of the photonic resonance reflected from the nanostructured particles, and a simple linear correlation between observed wavelength and drug release was observed. Changes in the optical reflectance spectrum were sufficiently large to be visible as a distinctive red to green color change.
Keywords: Porous silicon, photonic crystal, ophthalmic drug delivery, daunorubicin, sustained drug release
1. Introduction
The need for drug delivery systems capable of providing safe, sustained release to the target tissues is evident in many diseases, including various ocular diseases [1]. The existence of barriers such as the blood-retinal barrier and the tight junctions of the retinal pigment epithelium pose great difficulty in delivering drugs systemically to the vitreous, retina, or choroid [2]. Often, the standard treatment for an ocular disease involves frequent intravitreal injections, risking complications such as infection and vitreous hemorrhaging [3–6]. Proliferative vitreoretinopathy (PVR) is an example of an ocular disease that would benefit from a controlled delivery system. PVR occurs when there is uncontrolled proliferation of non-neoplastic cells that form contractile membranes during the wound healing process after retinal detachment [7, 8]. Efficient drug administration to treat PVR is hindered by the short half-life of the therapeutics in the vitreous coupled with the retinal toxicity caused by a high bolus intravitreal injection [7]. Although an implantable system may provide for more controlled release kinetics, drawbacks include the need for surgery in the implantation and (sometimes) removal of these devices [4]. A biodegradable and injectable system capable of providing long-term sustained release of a therapeutic to the vitreous for the treatment of PVR would therefore be more advantageous.
Porous Si has many advantages as a drug delivery system [9, 10], including the controllability of pore size and volume [11], the ease of chemical modification to load various drugs [12], a high surface area [11], biocompatibility [13, 14] and resorbability [15–17]. Si is required for proper bone and collagen growth, and the degradation product of porous Si in vivo is orthosilicic acid (Si(OH)4), which is the natural form of Si found in the body and is readily excreted by the kidneys [18, 19]. Additionally, previous studies have shown no toxicity from porous Si particles injected into the rabbit or rat eye [3, 20].
The in vitro degradation pathway for porous Si involves two steps: oxidation of the Si matrix to SiO2, followed by hydrolysis to the soluble orthosilicic acid species. The oxidant in the first step can be water or various bioavailable reactive oxygen species (ROS) [21], and this oxidation reaction is significantly slowed if the porous Si surface is modified with a Si-C bonded organic species [12, 22]. Furthermore, the Si-C chemistry provides a convenient means to attach drugs intended for slow release--the low reactivity of Si-C bonds in aqueous media provides an effective means to limit the release of a tethered drug. Si-C chemistry is significantly more stable than the silanol (Si-O) chemistry typically employed to attach molecules to SiO2 surfaces [9, 23]. For example, release of doxorubicin covalently attached to porous Si via Si-C links occurs over several days rather than hours. The rate of release can be increased by addition of ROS [21] and it can be slowed by using more hydrophobic linkers [23] or a more extensive carbonization chemistry [9]. Since water is a competent but kinetically slow oxidant for Si-C modified porous Si matrices, the approach holds potential for long-term in vivo drug release applications [21].
An interesting aspect of the materials science of porous Si is that it can be prepared in the form of multilayers of varying optical density, allowing the fabrication of photonic crystals. In this work we generate a type of photonic crystal known as a rugate filter, in which the porosity varies smoothly and periodically as a function of depth [24]. This gives the porous Si particles an intense reflectivity peak at a predetermined wavelength. Shifts in this wavelength have been used to monitor the loading and release of various biomolecules [25, 26]. Moreover, the reflectivity spectrum can be prepared in the near infrared region, allowing observation through human tissues [27] or other biological media.
In this study, daunorubicin is loaded into particles of porous Si photonic crystals by covalent attachment. Daunorubicin is an anthracycline antibiotic that has been shown to be modestly efficacious as an intraocular anti-proliferative agent for the treatment of experimental PVR [28]. Unfortunately, current use includes an infusion of 7 µg/mL of drug into the eye at the end of surgery, and the eye is exposed to the drug for only several minutes. Even with this application, probably due to the binding of daunorubicin to DNA, there is a weak but real clinical benefit as shown by a reduction in the extent of retinal re-detachment [29]. In order to control PVR, the drug must be able remain in the vitreous long enough to inhibit cell proliferation effectively without exhibiting toxicity to the retina or other tissues [29]. However, daunorubicin has a short half-life in the vitreous and a narrow therapeutic concentration range [7, 30, 31]. Therefore, daunorubicin is not currently used for the treatment of PVR due to the toxicity that can occur with a high initial dose [30]. We are investigating if sustained release of daunorubicin in vitro can be achieved by sequestering the drug in porous Si particles. In addition, we want to develop a method to non-invasively monitor drug release in vivo by the color change of the particles.
2. Materials and Methods
2.1 Materials
Silicon wafers (p++-type) with a resistivity of 0.98 mΩ•cm were purchased from Siltronix, inc. Hydrofluoric acid (48%) was purchased from Fisher. Undecylenic acid (98%) and N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) were purchased from Sigma-Aldrich Chemicals. N-hydroxysulfosuccinimide (Sulfo-NHS) was obtained from Thermo Scientific. Phosphate buffered saline was obtained from Gibco. Dimethylsulfoxide (DMSO, ACS grade) was obtained from Alfa Aesar. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Roche Applied Science. A Sears Kenmore 700 W microwave was used for the hydrosilylation reaction. Fluorescence measurements were obtained on a Gemini XPS spectrofluorometer from Molecular Devices. A Nikon CoolPix 4300 camera fitted to an optical microscope was used to obtain color images of the particles. Reflectivity spectra of particles were acquired using an Ocean Optics CCD spectrometer coupled to an optical microscope by means of fiber optics or using a Nikon LV-150 fluorescence microscope coupled with a SpectraPro 275 scanning monochromator. A plexiglass flow cell with a total volume of 4.5 ml and a Cheminert® M50 liquid handling pump by Valco Instruments were used for drug release experiments.
2.2. Formation of porous Si microparticles
Porous Si microparticles were prepared by electrochemical etch of highly doped, (100)-oriented, p-type Si wafers (Boron-doped, 0.98 mΩ•cm resisitvity) in a 3:1 solution of 48% aqueous hydrofluoric acid (HF):ethanol (CAUTION: HF is highly toxic and contact with skin should be avoided). A Si wafer with an exposed area of 8.04 cm2 was contacted on the backside with a strip of aluminum foil and mounted in a Teflon etching cell with a platinum counter-electrode. The wafer was etched using the current density waveform:
| (1) |
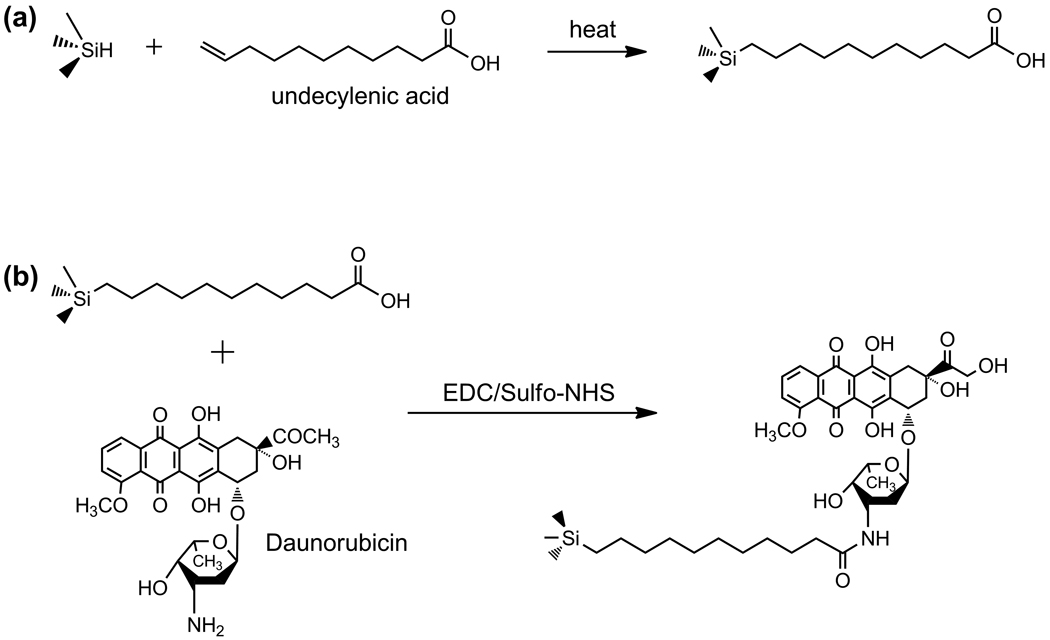
where J is applied current density, A0 is current density offset (in mA/cm2), A is current density amplitude (mA/cm2), k is frequency (s−1), t is time (s), and α is phase shift (s−1). The values used for A0, A, and α were 90.2 mA/cm2, 12.4 mA/cm2, and 0, respectively. Thus the maximum current density value of the waveform was 102.6 mA/cm2 and the minimum was 77.8 mA/cm2. The value of k was adjusted to yield a reflectance peak whose maximum occurred at ~600 nm; a typical value was 2.15. The waveform was etched for a total of 300 s (corresponding to ~80 repeats), producing a layer 12 µm thick. The porous layer was then removed from the substrate by electropolishing in a 3.33% HF in ethanol solution for 2 min at a current density of 6.2 mA/cm2. The etching and electropolishing procedure was repeated 8 times per wafer and the resulting porous layers were placed in absolute ethanol and broken up into microparticles by ultrasound for 30 min. To perform the hydrosilylation chemistry, approx. 40 mg of porous Si microparticles were placed in a 10 mL Pyrex beaker and immersed in 2 mL of undecylenic acid. The mixture was then heated in a commercial consumer microwave oven for 4 min (280 Watts). The particles were rinsed with hexane and ethanol to remove excess undecylenic acid.
2.3. Characterization of porous Si microparticles
Scanning electron microscope (SEM) images were obtained using a Phillips XL30 ESEM Field Emission Gun (FEG) electron microscope operating at an accelerating voltage of 3 kV. Nitrogen adsorption-desorption isotherms of porous Si microparticles were recorded at 77K using a Micrometrics TriStar volumetric apparatus. Prior to the adsorption experiment, approximately 30 mg of porous Si particles were purged overnight with nitrogen at 40 °C. The surface area of the sample was measured by the Brunnauer-Emmett-Teller (BET) method, which yields the amount of adsorbate corresponding to a molecular monolayer. Pore size distribution was determined using the Barrett-Joyner-Halenda (BJH) method.
2.4. Drug loading
Approximately 5 mg of porous Si microparticles were suspended in 650 µL of 10% dimethyl sulfoxide (DMSO) in Dulbecco’s phosphate buffered saline (PBS) solution containing 50mM N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and 5mM Sulfo-NHS. Afterwards, 200 µL of a 1 mg/mL solution of daunorubicin hydrochloride in water were added. The particles were then vortexed for 2 h at room temperature and rinsed with ethanol 5 times. To determine the amount of daunorubicin loaded, porous Si microparticles were completely dissolved in a solution of 1M NaOH for 10 min. The solution was neutralized with 1M HCl to recover the fluorescence spectrum of the free drug. To quantify the amount of drug released, the solution was excited at 480 nm and the photoluminescence emission spectrum was measured in the range 500–650 nm. The emission maximum at 580 nm was used to calculate the amount of daunorubicin released, based on a calibration curve.
2.5. In vitro porous Si particle degradation and drug release
Porous Si particle degradation and drug release were studied using a custom designed flow cell chamber with a volume of 4.5 mL and a flow rate set to mimic the half-life of daunorubicin in the human eye. A solution of phosphate buffered saline (PBS) was flowed through the chamber containing the drug-loaded particles at a rate of 450 µL/h. The eluted PBS solutions were collected and analyzed for silicon and daunorubicin. Silicon content was determined by inductively coupled plasma optical emission spectrometry (ICP-OES). Samples were filtered through a 0.1 µm filter and 1 mL of the sample was diluted with Millipore water (17.3 mΩ resisitivity) to a total volume of 5 mL. The Si atomic emission peaks at 212.412, 251.611, and 288.158 nm were monitored. Daunorubicin concentration was determined using fluorescence spectrometry as described above. Aliquot volume was typically 150 µL.
2.6. Determining average reflectivity spectra of porous Si particles
Reflectance spectra of porous silicon particles were recorded using an Ocean Optics USB 2000 spectrometer. A tungsten light source with a spot size of approximately 1 mm was focused onto the particles in solution through a bifurcated fiber optic cable. The optical axis was normal to the plane containing the porous Si particles. Results of reflectivity spectra acquired from at least 5 regions per sample were averaged.
2.7. Acquisition and analysis of the reflectivity of individual porous Si particles
A Nikon LV-150 fluorescence microscope was modified to allow illumination of the sample stage with a narrow-band light generated by a 100 W tungsten filament lamp in combination with a SpectraPro 275 scanning monochromator. A CoolSnap HQ2 (Photometrics) 14 bit, monochromatic camera was used to acquire reflectivity images. Metamorph software (Molecular Devices) was used to control the camera and the stepper motor of the monochromator to allow the generation of spectral image stacks. Reflectivity images were acquired as the monochromator was scanned from 500–725 nm in 1 nm steps. All images were acquired using a 10× objective with a live binning of 1 and an exposure time of 20 s to produce 520 × 696 pixel images. The result was a spectral image stack with 226 images. The images were then cropped to 480 × 480 pixels for analysis. Image stacks were analyzed using a code developed in MATLAB, which yielded the wavelength maximum of the reflectivity spectrum for each particle. The value of wavelength maximum for each particle was determined from the average of 5 pixels.
2.8. In vitro cellular proliferation and toxicity assays
Cell proliferation and toxicity experiments were performed on retinal pigment epithelial cells (ARPE-19), grown in DMEM F-12 with 10% FBS and 1% antibiotic-antimyotic (100×). The data were analyzed for 3 samples at various concentrations: (1) daunorubicin as-received, diluted to 0.45 or 0.5 µg/mL in PBS as the highest concentration, (2) daunorubicin released from drug-loaded porous Si particles, with 0.45 or 0.5 µg/mL as the highest concentration, and (3) silicic acid released from non-drug loaded porous Si particles, equivalent in weight to the drug-loaded particles used in (2). Various concentrations of the 3 samples (50 µL) were added to 60–70% confluent cells in a 96-well plate containing 100 µL media in each well. Cells were incubated with the particular sample for 48 h and analyzed. The MTT assay, a quantitative colorimetric assay that measures living, metabolically active cells [32], was used to measure cell proliferation. Cell viability was assessed using the live/dead cell assay with calcein AM and ethidium homodimer-1. Calcein AM is hydrolyzed into the green-flourescent calcein in live cells and ethidium homodimer-1 stains dead cells with compromised membranes [33]. A final concentration of 0.5 µM Calcein AM and 1.5 µM ethidium homodimer-1 was used.
3. Results
3.1 Fabrication and characterization of daunorubicin-loaded porous Si microparticles
Porous Si films possessing a periodic, smoothly varying porosity profile in the z-direction (perpendicular to the wafer surface) were prepared. The periodic gradient was imposed using a cosine etch current waveform. The resulting porous film approximates a rugate optical filter, displaying a single narrow band in the reflectivity spectrum at 600 ± 5 nm [34, 35]. In order to improve stability and provide an attachment point for the drug, the freshly etched particles were functionalized with undecylenic acid using microwave-assisted thermal hydrosilylation [36]. Daunorubicin was subsequently grafted onto the carboxy terminus of the undecanoic acid-modified surface via EDC chemistry, as shown in Scheme 1.
Scheme 1.
Loading of daunorubicin into porous Si microparticles. The process involves functionalization of the hydrogen-terminated porous Si surface (Si-H) by microwave-assisted hydrosilylation of undecylenic acid (a), followed by EDC-mediated coupling of daunorubicin via the pendant amino group (b).
The resulting microparticles are approximately 35 × 45 × 12 µm in size. The specific surface area, porous volume and average pore size were determined using nitrogen adsorption measurements with the application of the BET (Brunauer-Emmett-Teller) and the BJH (Barrett-Joyner-Halenda) methods [37–39]. The BET surface area was determined to be ~260 m2 (STP)/g and the BJH adsorption cumulative pore volume was determined to be ~550 cm3 (STP)/g. The BJH adsorption average pore diameter was found to be 9.6 ± 0.04 nm.
3.2 In vitro release of daunorubicin covalently attached to porous Si microparticles
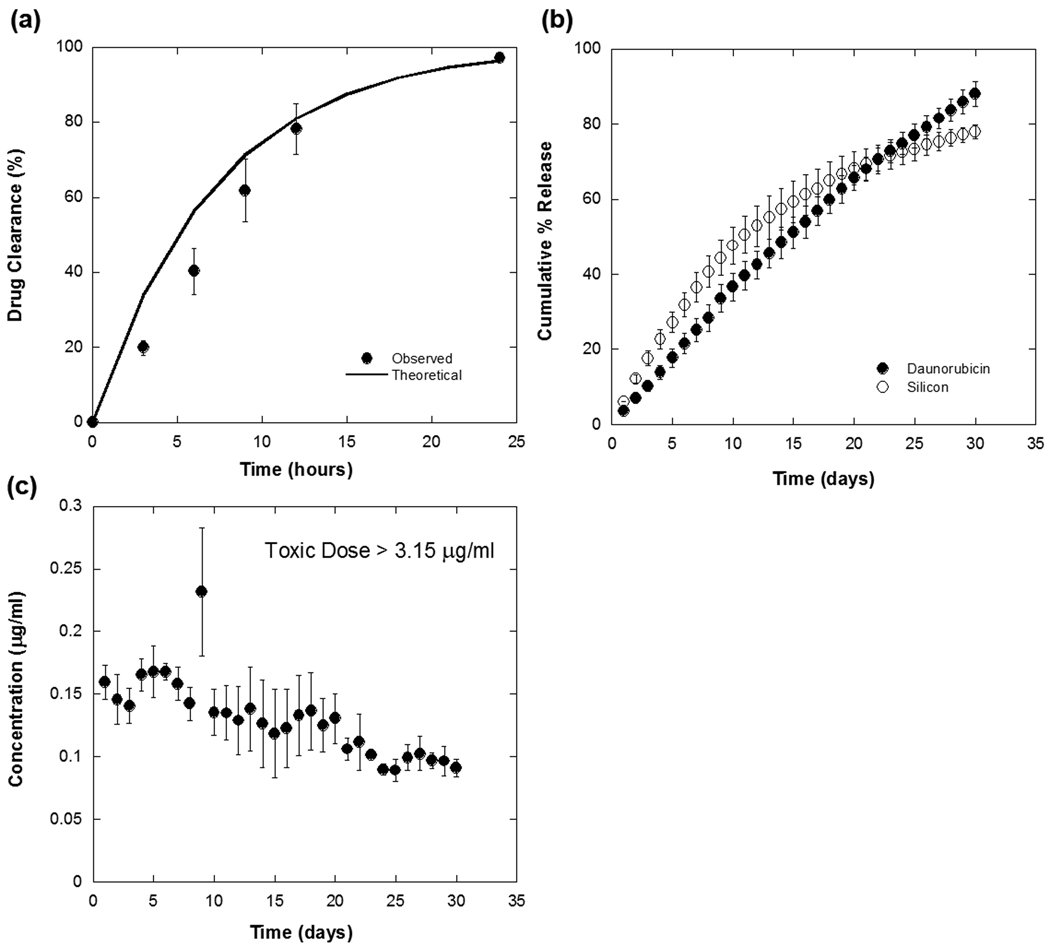
Approximately 20 µg of daunorubicin could be loaded per mg of porous Si particles. The amount of drug loaded was determined by quantifying the amount released upon immersion of the particles in 1M NaOH. In this highly caustic solution, the particles were observed to dissolve completely within 30 min. All 20 µg of daunorubicin are covalently bound, as we found previously that rinsing by ethanol/methanol is able to remove all unbound drug [21]. A reflectivity peak at approximately 660 nm results after immersion of drug loaded particles into buffer (Figure 1). The release rate in pH 7.4 buffer solution (phosphate buffered saline, PBS) is considerably slower, and an experiment to quantify this rate was configured to mimic physiological conditions relevant to an ocular dose: the daunorubicin-loaded particles were confined in a flow cell with a total volume of 4.5 mL (the volume of vitreous contained in the human eye) and maintained at a temperature of 37 °C. Pure PBS solution was flowed through the cell at a rate of 450 µl/hour. At this flow rate, dissolved materials will clear from the cell with a half-life of 5 h. This mimics the half-life for clearance of free of daunorubicin in the vitreous [31]. To test the accuracy of the system, 20 µg of as-received daunorubicin was spiked into the chamber and the concentration of drug in the effluent from the cell was monitored. Experimentally, 97% of the spiked daunorubicin was removed in 24 h (Figure 2a), which agrees well with the amount expected (96.4%) based on a steady-state dilution calculation that assumes the continuous flow rate and reservoir volume of the experiment. To quantify the amount of daunorubicin released from the porous Si particles, the total volume of PBS solution eluted from the cell each day (10.8 mL) was collected and assayed for daunorubicin. A sustained release profile of daunorubicin was observed over a period of 30 d, with approximately 88% of the drug released in that time period (Figure 2b). Thus the porous Si carrier extends the release of drug by a factor of 47×. In addition, the concentration of porous Si throughout the 30 d time period is maintained below the toxic dose (Figure 2c).
Figure 1.
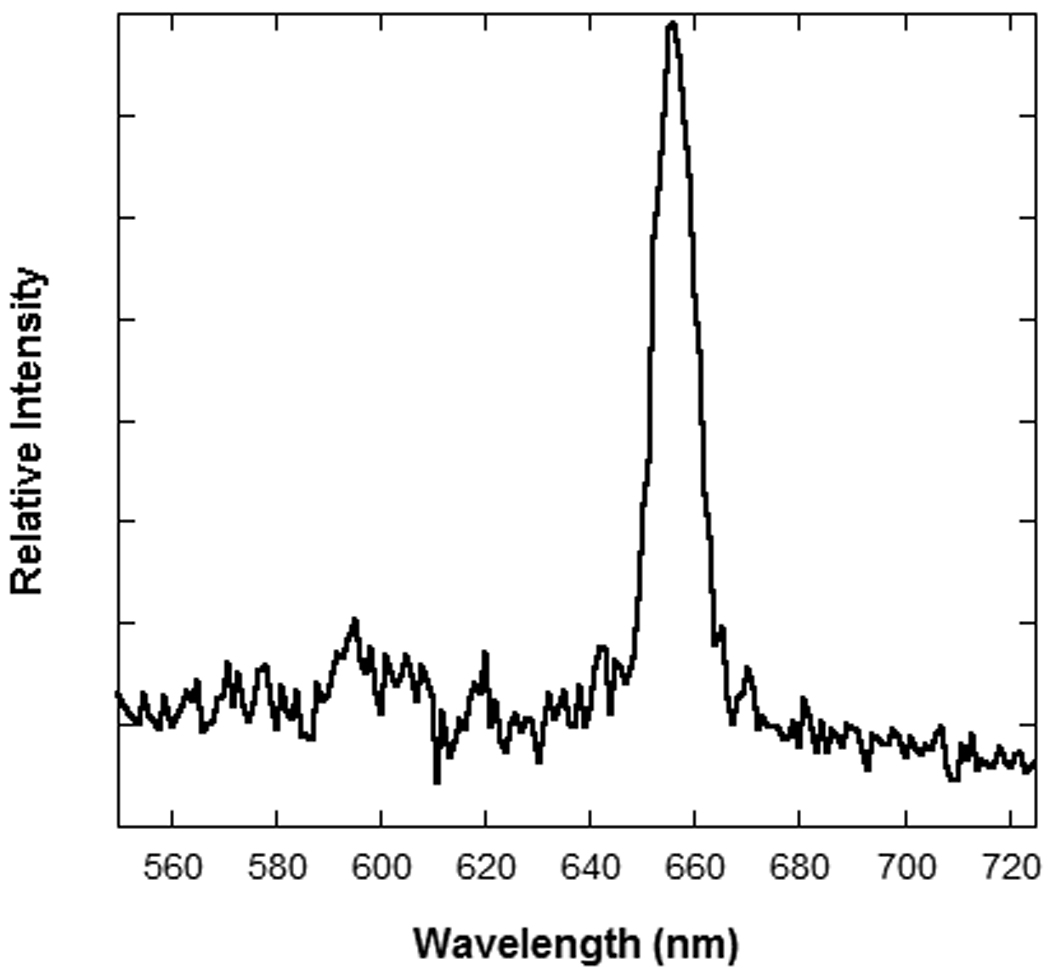
Representative white light reflectance spectrum from a single porous Si particle immersed in buffer solution.
Figure 2.
In vitro release of daunorubicin covalently attached to porous Si microparticles. (a) Control experiment, showing the rapid clearance (t1/2 = 5 h) of an aliquot of free daunorubicin injected into the flow chamber at time t = 0 and then eluted with a continuous flow of 450 µL/h of pure PBS solution. The solid line shown is the calculated % cleared as a function of time in hours, based on the elution rate and chamber volume. (b) Percent of daunorubicin (solid circles, “drug”) and silicon (open circles, “Si”) released from porous Si microparticles into the effluent stream under the same flow conditions as in (a). Note the time axis here is in units of days. The effective t1/2 for clearance of the covalently attached drug from the chamber is increased from 5 h (for free drug) to 15 d. Soluble silicon species (orthosilicate ions) and soluble daunorubicin quantified by ICP-OES and fluorimetry measurements, respectively. (c) Steady-state concentration of daunorubicin in the chamber as a function of time for the experiment described in (b). A therapeutic level of drug is maintained for > 30 d.
The release of covalently attached daunorubicin correlates with degradation of the porous Si microparticle host structure. Particles contained in the flow chamber were continuously flushed with fresh PBS solution over a period of 30 d and the effluent was collected and assayed for soluble Si content by ICP-OES analysis daily (Figure 2b). The amount of Si released per day is approximately constant, although a gradual decrease in the rate of release is noticeable after day 10.
3.3 Correlation of spectral shift with drug release
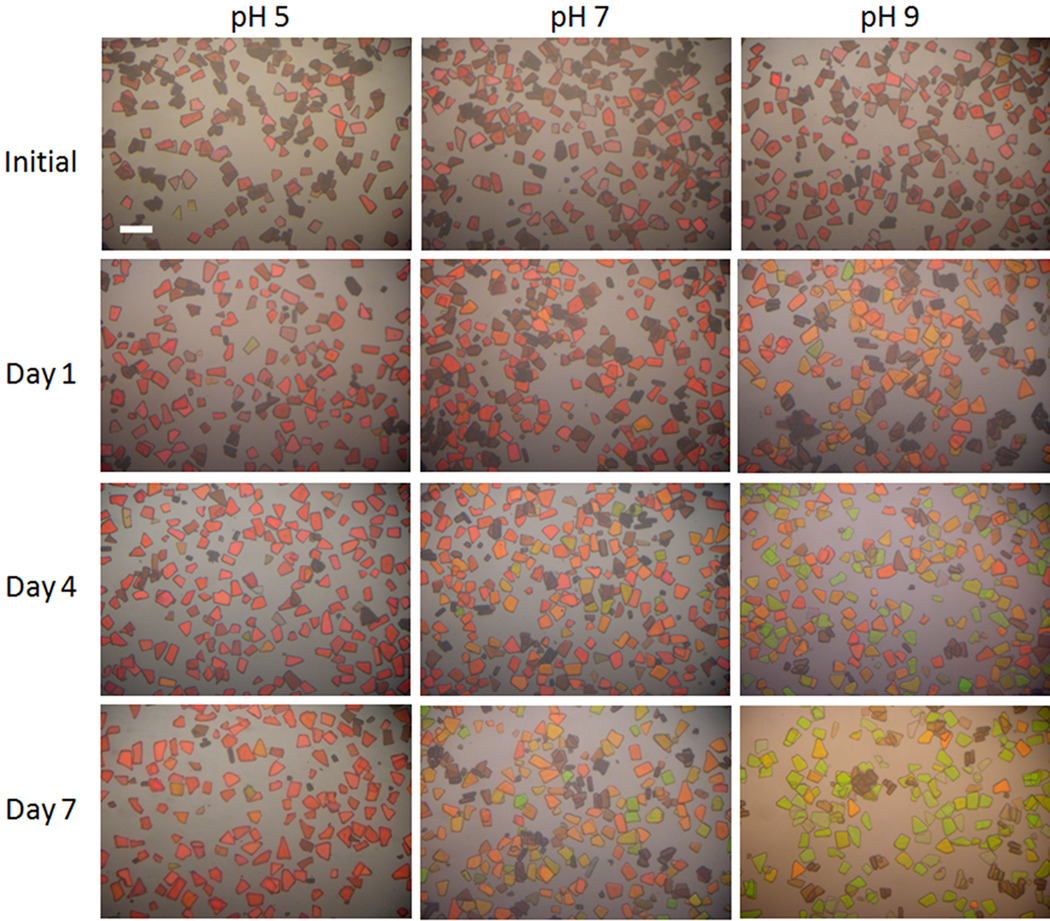
Particles (2 mg) were immersed in 2 mL of buffer solutions in a Petri dish to allow time-resolved microscopic imaging and spectroscopy of the particles as they dissolved in the media. The strong reflectivity peak generated by the rugate optical filter structure gives the particles a distinctive color. The evolution of this color was monitored at three different pH values: pH = 5, 7, and 9, each over a period of 7 d. Although the relevant pH for the eye is 7–7.4, using various pH buffers provided a means to adjust the degradation rate in order to get different drug release rates for different rates of color change. The optical microscope images are presented in Figure 3. For the pH = 9 buffer, the particles turn from red to orange, then yellow, and finally green during the 7 d exposure period. The blue shift is not as extensive in pH = 7 buffer; after 7 d the initially red particles have turned yellow or yellow-green. Particles soaked in pH = 5 buffer display the least apparent color change, turning from red to orange during the 7 d exposure period (Figure 3).
Figure 3.
Optical microscope images showing the color change of particles as they degrade in pH = 5, 7, or 9 buffer solutions. The degree of color change correlates with the amount of drug released (see Figure 4). The scale bar is 100 µm.
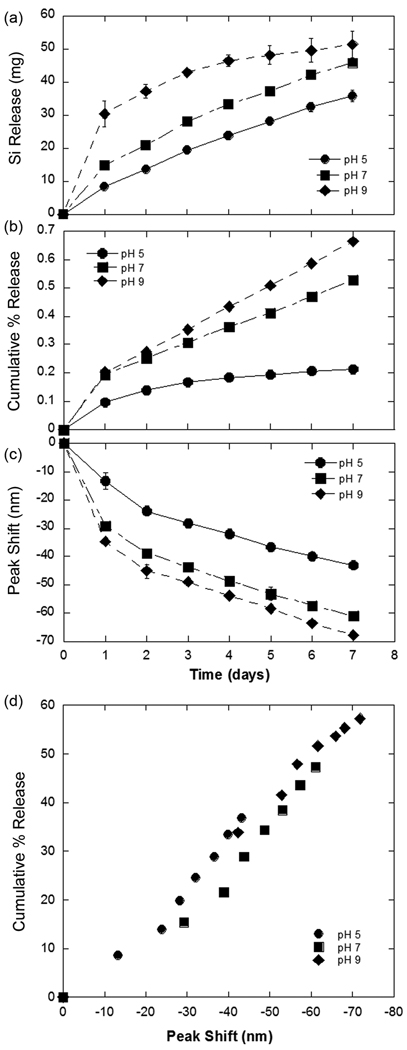
The spectral shifts observed in the images of Figure 3 were quantified using a CCD spectrometer and a tungsten illumination source fitted to an optical microprobe (Figure 4). For each dish, 3 regions were analyzed. The optical probe was focused to a spot of diameter ~1 mm, which collected data from ~150 particles in a scan. Thus spectra from a total of 450 particles were averaged per data point. The largest spectral change was observed for particles immersed in the pH 9 buffer and the smallest was observed for particles in pH 5. After a period of 7 days, a blue shift of ~70 nm was observed for particles in pH 9 buffer, at pH 7 the shift is 60 nm, and at pH 5 the shift is only 40 nm. The corresponding spectra for day 7 are shown in Supplementary Figure 1.
Figure 4.
Quantification of drug release, silicon dissolution, and spectral peak shift as a function of time for drug-loaded porous Si photonic crystal microparticles in aqueous buffer solutions at pH 5, 7, and 9. Degradation of the microparticles releases soluble silicon in the form of orthosilicate (a) and daunorubicin (b) at a rate that increases with increasing pH. The average value of the spectral maximum measured from the particles shifts to shorter wavelengths with time (c), and the rate of this shift also increases with increasing pH. The shift correlates with percent of daunorubicin cumulatively released from the particles in an approximately linear fashion over the range 0–60% drug released (d). All particles were prepared and loaded with drug using the same protocol, and results are presented for the three different solution pH values indicated. The data represent measurements made over a period of 7 days. Peak shifts in (c) and (d) are calculated as λt − λ0, where λt is the average wavelength of the spectral reflectivity peaks determined at time t and λ0 is the average wavelength at time t = 0 (measured on > 100 particles in 5 separate scans). The negative values of peak shift indicate a blue shift in the spectral peak; typical initial peak position was 660 nm.
The optical and spectral changes in the porous Si microparticles were correlated with the appearance of free daunorubicin and silicon in the buffer solutions, and the results are presented in Figure 4 as a function of time for the three pH values studied. Concurrent with the spectral measurements, 1 mL of the buffer solution was removed and assayed for daunorubicin using the fluorescence method described above. After removal of the assay aliquot, an equal volume of the relevant (fresh) buffer solution was replaced in the dish. The rate of appearance of Si (Figure 4a) and of daunorubicin (Figure 4b) in the solution is dependent on the pH of the buffer. Over a period of 7 d, 60% of the drug was released from particles in pH 9 buffer, 50% of the drug was released from the particles in pH 7 buffer, and 40% of the drug was released from the particles in pH 5 buffer. Degradation of the microparticles releases daunorubicin and soluble silicon in the form of orthosilicate at a rate that increases with increasing pH. A linear relationship between the cumulative amount of daunorubicin released (Figure 4b) and the magnitude of the spectral blue shift (Figure 4c) is observed (Figure 4d) for all pH values tested, for percentages of released drug < 60% of the total amount of loaded drug.
3.4 Distribution of the reflectivity spectra of individual particles
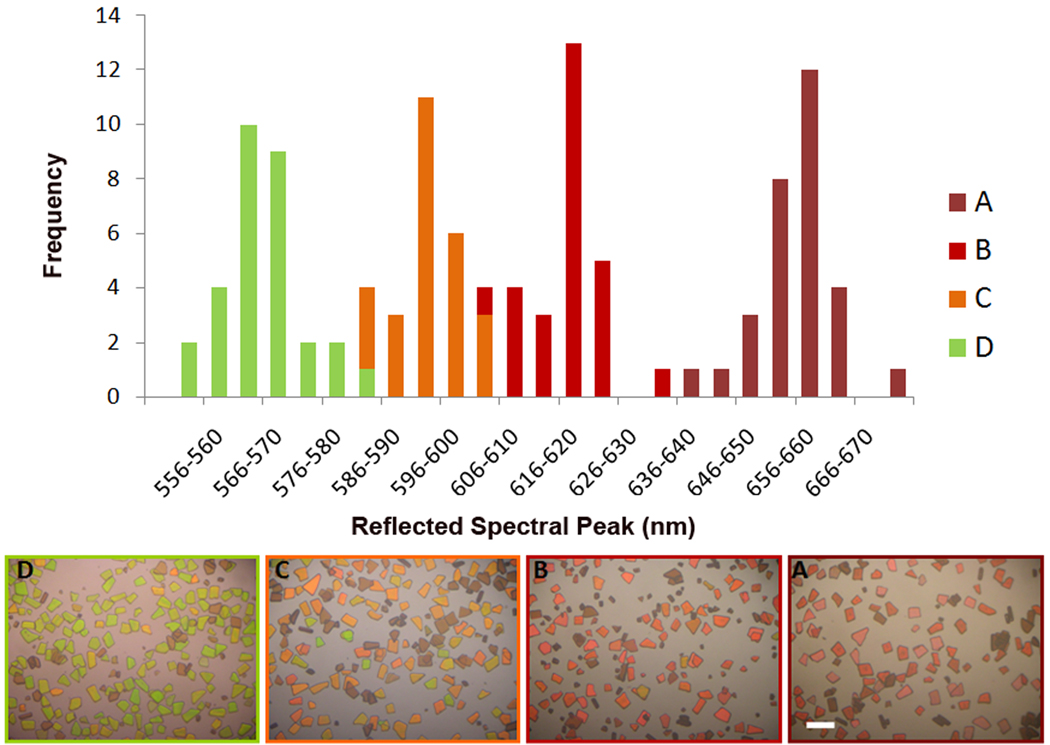
At any given point in time during the dissolution reactions described above, the values of λmax in the reflectivity spectra of the particles displayed a range of values. This distribution was quantified in a separate set of experiments in which several series of microscope images were acquired as a function of illumination wavelength. The same experimental conditions as described in the section above were applied, immersing the porous Si microparticles in pH 5, 7, and 9 buffers. At a given time point, the microscope stage was illuminated by light passed through a 0.275 m monochromator, which was scanned over the wavelength range 500–725 nm in 1 nm steps. Image stacks were then analyzed using a MATLAB code [40] and 5 contiguous pixels were measured and averaged per particle. A total of 30 particles were measured in each set; results are displayed in Figure 5. The distribution of λmax values shifts to the blue end of the visible light spectrum as the particles degrade in the solution. Initially, the distribution approximates a Gaussian, but with increasing time in the buffer solution, the distribution becomes skewed, with the maximum of the distribution appearing closer to the short wavelength. The width of the wavelength distribution does not change significantly with dissolution time.
Figure 5.
Distribution of values of λmax in an ensemble of particles, monitored during degradation in aqueous solution. Data for sample A were obtained on the first day after immersion. Data for samples B, C, and D were obtained on day 7, and they correspond to pH 5, 7, and 9, respectively. Each bar represents a 4 nm wavelength range about the value indicated on the x-axis. Photographic color images of the representative ensembles are shown at the bottom. The scale bar is 100 µm.
3.5 Effect of released daunorubicin on ARPE-19 cells
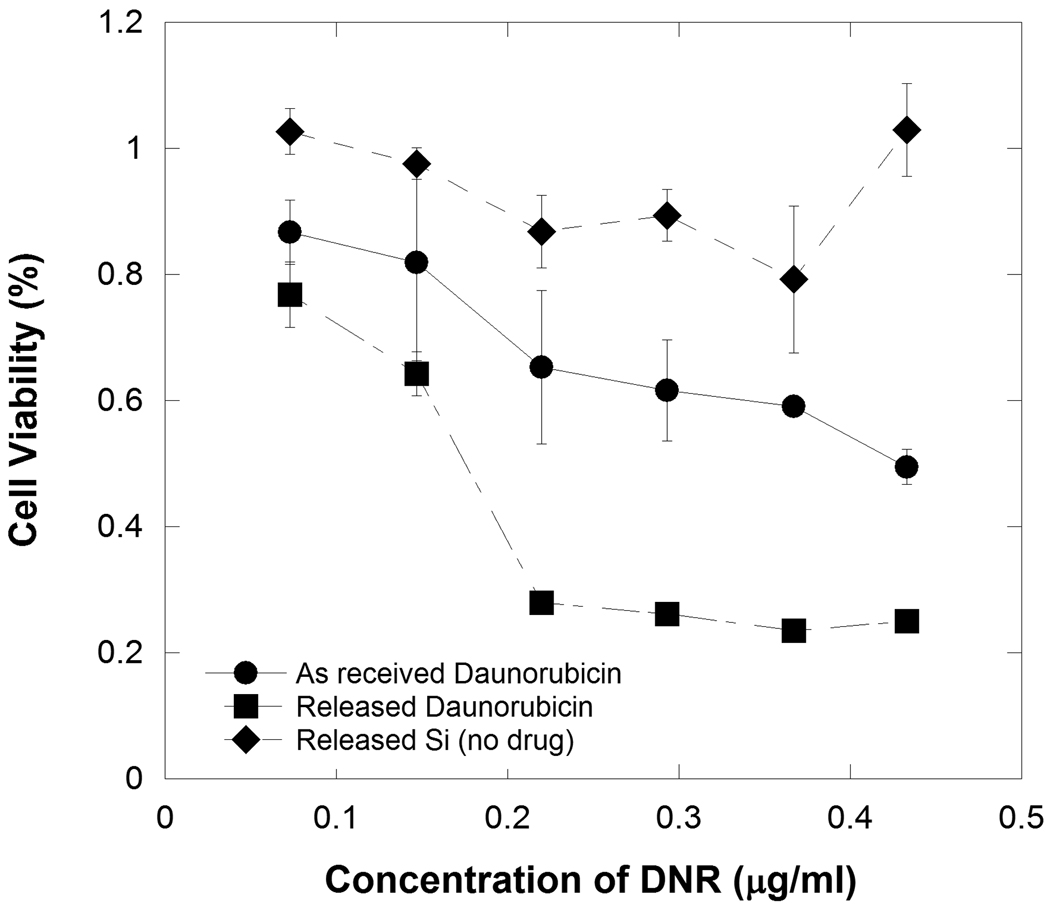
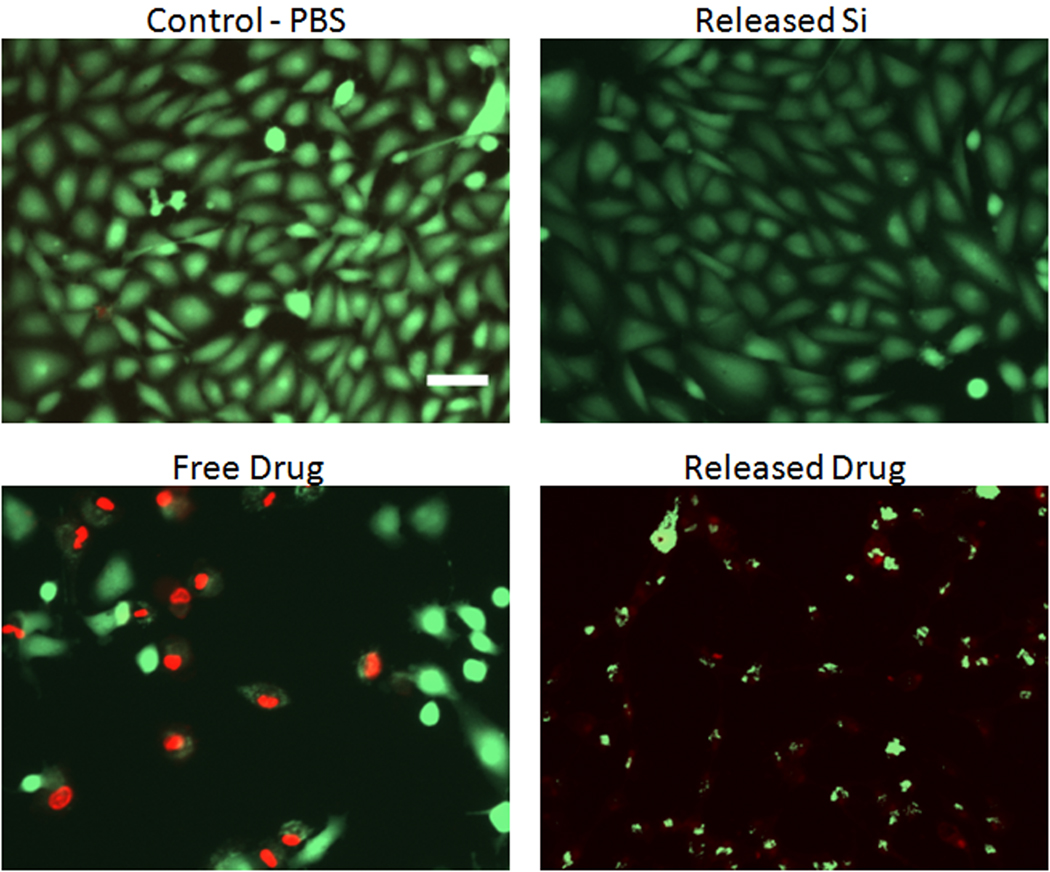
The daunorubicin that is released from porous Si particles into PBS solution was tested for its ability to inhibit cellular proliferation and toxicity on ARPE-19 cells using an MTT assay (Figure 6). The soluble by-products of porous Si dissolution (primarily orthosilicate species) and as-received daunorubicin were also tested. It was found that daunorubicin extracted from porous Si microparticles (previously attached via the coupling chemistry of Scheme 1) inhibits cellular proliferation of RPE cells at concentrations lower than as-received daunorubicin. The soluble by-products of porous Si dissolution (from non-drug loaded particles) do not inhibit cell growth. Fluorescence images resulting from live/dead cell staining experiments also reveal that daunorubicin released from porous Si microparticles exhibits toxicity towards RPE cells (Figure 7). As-received daunorubicin displays the decreased cellular growth and toxic effects expected from this drug, but it shows a lower degree of toxicity relative to the same quantity of daunorubicin released from microparticles.
Figure 6.
Viability of ARPE-19 cells (MTT assay) after exposure to as-received daunorubicin, silicon released (as soluble orthosilicate) from porous Si microparticles (containing the undecylenic acid linker chemistry but not containing any drug), and daunorubicin that had previously been attached to porous Si microparticles and then released by hydrolysis into aqueous solution. The x-axis displays the daunorubicin concentration in terms of the total amount of daunorubicin added per milliliter of media. The “Released Si” trace represents the soluble fraction of material released from a mass of porous Si microparticles that is equal to the mass of porous Si microparticles used in the “Released Daunorubicin” trace. Cell proliferation assessed 48 h after sample introduction.
Figure 7.
Fluorescence microscope images of ARPE-19 cells comparing the toxicity of as-received daunorubicin (“Free Drug”), daunorubicin that had previously been attached to porous Si microparticles (“Released Drug”), and the soluble by-products of porous Si dissolution from non drug loaded porous Si (“Released Si”). A control experiment using PBS buffer only is shown for comparison (“Control-PBS”). The concentration of daunorubicin in the “Free Drug” and “Released Drug” images is 0.53 µg/mL. For the “Released Si” image, the soluble fraction of material released from a mass of porous Si microparticles equal to the mass of porous Si microparticles used in the “Released Drug” image was used. Green and red in images corresponds to calcein AM (live cells) and ethidium homodimer-1 (dead or damaged cells), respectively. The scale bar is 100 µm. Toxicity assessed 48 h after sample introduction.
Discussion
It has been suggested that PVR can be prevented by inhibiting cell growth at the cell proliferation stage, which can last for ~6 weeks. Therefore, release of an anti-proliferative should be sustained for >1 month. To mimic the half-life of therapeutics in the vitreous in order to determine if sustained release could be achieved on a timescale relevant to chronic ophthalmic diseases, we designed a flow cell chamber designed to hold approximately the volume of the human vitreous [41]. When loaded into the particles, the residence time of daunorubicin was greatly extended compared to the free drug. Sustained release of daunorubicin was achieved for 30 d, at which time about 88% of the drug had been released. The amount of daunorubicin in solution remained well under the toxic intravitreal dose throughout the duration of the experiment [29]. If it had been administered as free drug, the initial concentration of the drug loaded in the particles would have exceeded the toxic dose (4 µg/mL were injected; the toxic dose is 3.15 µg/mL). Doses of 5 µg/mL per eye (approximately 1 µg/mL) have been applied to the vitreous at the end of surgery and have shown to be efficacious in human PVR[42]. Given that the known half-life of the drug is approximately 5 h and the IC50 on proliferation for daunorubicin is approximately 0.15 ug/mL, it is expected that the drug will fall to subtherapeutic levels in 30 hours. In the present system, therapeutic levels (>0.06 ug/mL) are maintained for 30 days [43–45] (Figure 2c). The data show the drug concentration is maintained at well under the toxic limit for a period of time that is 24 times longer than what could be obtained with a single intravitreal injection of free drug [4, 8], suggesting that this approach may be applicable to PVR treatment.
The rate of dissolution of the porous Si carrier scales with the total exposed surface area of the microparticles. The rate of Si degradation decreased gradually over the 30-day test period, suggesting that the exposed surface area decreased during this time period. Correspondingly, a slight decrease in drug concentration was observed as the total surface area of the particles decreased. There was a strong correlation between the amount of silicon removed and the amount of drug released into solution: over the 30 day period approximately 88% of the drug content and 80% of the silicon content of the microparticles were released.
A non-invasive indicator of the amount of residual drug remaining in the fixture is a desirable feature for drug delivery systems [3], and the present system accomplishes this by harnessing the optical reflectivity properties of the porous Si photonic crystal. A structural color is imparted to the particle during its preparation by application of a sinusoidal current density waveform during the electrochemical etch. This produces a periodic porosity modulation that propagates in the direction of the electrochemical etch, which is the <100> crystallographic direction. In the present case, the stratified refractive index of the resulting particles gives rise to a sharp reflectance peak in the optical spectrum that is responsible for the intense color observed in the photographic images of the particles in Figures 3 and 5. As aqueous solution replaces the drug molecules inside the pores, or as the porous Si matrix degrades, the refractive index in the porous Si layers decreases, resulting in an observable blue shift of the color [46]. As reported previously [21], a primary pathway for release of the attached drug from these hydrosilylated materials is the oxidation and subsequent hydrolysis of the Si-C and Si-Si bonds in these particles. Therefore, in principle, the degradation rate of the particles controls the release rate of daunorubicin. In order to test various rates of porous Si degradation, particles were exposed to 3 different buffer solutions at pH 5, 7, and 9 (Figure 3). Porous Si generally degrades more rapidly in alkaline conditions and more slowly in acidic conditions [9]. Consistent with this relationship, the rate of color change increases with increasing pH (Figure 4).
The reflected spectral peak from the photonic microparticles provides a non-invasive means of monitoring the degree of degradation of the materials, and it provides a surrogate means of monitoring the amount of drug that has been released into solution. We have found the most accurate means of quantifying the photonic resonance is to measure the spectrum of the particles using a CCD spectrometer coupled to a white light source. In the present experiments, the 1 mm diameter spot probed by the optics encompasses hundreds of particles. This allowed the establishment of a statistically significant correlation between spectral peak shift and drug release. The correlation between spectral peak shift and amount of drug released is approximately linear in the range 0–60% of total drug released (Figure 4d), supporting the hypothesis that the photonic signature from the particles could be used to monitor drug release in vivo. However, the clinical utility of the approach would be improved if the spectral change could be monitored by visual inspection through a medical ophthalmoscope or a fundus camera. Thus we imaged the particles under a microscope, using white light illumination and a digital camera. The results clearly indicate that a detectable color change occurs as the particles degrade (Figures 3 and 5), and that this color change correlates with the quantity of drug released (Figure 4). The spectrum and thus the apparent color of individual particles in a given ensemble show some variability. This distribution was quantified by measuring the reflectivity spectra of a large number of individual particles resulting from a single preparation (Figure 5). The center of the distribution of wavelengths from an ensemble of particles shifts to the blue with increasing degradation, as discussed above, whereas the width of the distribution remains relatively constant as the particles degrade.
The contractile membranes formed in PVR are made up of cellular components, including retinal pigment epithelial (RPE) cells, glial cells, fibroblasts, and inflammatory cells. These cells are capable of synthesizing collagen, which imparts a whitish appearance to the membranes [4, 8]. Preventing the proliferation of these cellular components may inhibit the development of PVR. Pure daunorubicin is known to effectively inhibit growth of RPE cells. However, in the present study this active molecule was attached to the porous Si surface using a linker molecule. It is possible that such a chemically modified drug will not display the same therapeutic efficacy as the parent compound. In addition, porous Si or its degradation byproducts may participate in side-reactions with the drug [47]; for example, the chemically related therapeutic doxorubicin has been shown to be reduced by porous Si under certain conditions [21], and porous Si has been shown to be a competent reductant for benzoquinone [48], which is one of the structural units contained in both daunorubicin and doxorubicin. For these reasons it was important to validate the efficacy of the drug delivered from the microparticles.
In vitro cell toxicity and proliferation assays were performed using ARPE-19 cells. The assays were run on the water-soluble components extracted from the porous Si particles rather than on the particles themselves, in order to simplify interpretation of the results. The MTT cell proliferation assay indicates that daunorubicin released from porous Si inhibits the growth of RPE cells at concentrations lower than as-received daunorubicin (Figure 6). The concentration of drug used in each assay was verified by fluorescence spectroscopy. Similarly, a live/dead assay reveals that daunorubicin released from porous Si is more toxic to RPE cells than as-received daunorubicin (Figure 7). Control experiments established that the source of additional toxicity cannot be attributed to silicic acid released from the porous Si particles; experiments run with extracts from porous Si particles that contained only the linker chemistry (no attached drug) show no significant toxicity (Figures 6 and 7). The redox chemistry of the quinone group with porous Si is thought to play a role in the enhanced cellular toxicity observed. As discussed above, porous Si can reduce quinone to a semiquinone radical[48]. Mass spectra obtained on the water-soluble extracts of the daunorubicin-modified porous Si microparticles display a molecular ion consistent with reduction of the quinone group of daunorubicin to a semiquinone radical (Supplementary Figures 2–3). Chemically modified drug (daunorubicin bound to undecylenic acid linker) was not detected (Supplementary Figure 3). We propose that superoxide radicals, formed as the semiquinone free radical is oxidized back into the parent quinone [49, 50], are responsible for the higher toxicity observed with released daunorubicin relative to the as-received drug. These results suggest that a lower overall quantity of drug may be needed to inhibit RPE cell proliferation, if the drug is delivered via porous Si microparticles. However, the fact that this system shows enhanced toxicity to RPE cells opens the possibility that it may show unwanted toxicity to other cells of the eye, or that it may introduce other deleterious effects. Such a possibility suggests that a thorough in vivo study is needed to assess the efficacy of the porous Si drug delivery system, and it underscores a limitation of the approach--covalent attachment of drugs to a potentially reactive porous Si matrix may not be appropriate for many in-vivo applications.
Conclusions
In summary, daunorubicin can be loaded into porous Si particles via covalent attachment and it is slowly released as the porous Si matrix degrades. Using porous Si microparticles, sustained release of daunorubicin at therapeutic concentrations (for PVR treatment) can be maintained for a period of 30 days, significantly longer than could be obtained with an injection of comparable quantities of free drug. The released drug is able to inhibit the growth of RPE cells at even lower concentrations than as-received daunorubicin, suggesting that lower doses of the drug could be effective with this formulation. The extent of degradation of the porous Si particles can be monitored by the observed color change of the particles, and the spectral shift directly correlates with the amount of drug released. The data presented represent an effective means of providing extended drug release from an intravitreally injected material that can be monitored in a non-invasive clinical setting.
Supplementary Material
Acknowledgements
This project was supported by the U.S. National Science Foundation (Grant # DMR-0806859), the CNRS/DREI program (Call for proposal CNRS/United States 2005 #3312), and through the Jacobs Retina Center (EYO 7366 and EYO 18589). MJS is a member of the Moores UCSD Cancer Center and the UCSD NanoTUMOR Center under which this research was conducted and partially supported by NIH grant U54 CA 119335. Dr. Freeman is a member of the UCSD engineering in Medicine Initiative. The authors gratefully acknowledge Dr. Frederique Cunin, Dr. Daniel Brunel, Dr. Daniel Lerner, and Dr. Sylvie Begu of the CNRS labs at the Institut Charles Gerhardt, Montpellier, France, for helpful discussions, Dr. Yongxuan Su for help with mass spectrometry, as well as Michelle Chen for help with MATLAB programming.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy L, Sahoo Y, Kim K-S, Bergey EJ, Prasad NP. Nanochemistry: synthesis and characterization of multi-functional nanoclinics for biological applications. Chem Mater. 2002;14:3715–3721. [Google Scholar]
- 2.Barar J, Javadzadeh AR, Omidi Y. Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin Drug Deliv. 2008;5:567–581. doi: 10.1517/17425247.5.5.567. [DOI] [PubMed] [Google Scholar]
- 3.Cheng L, Anglin E, Cunin F, Kim D, Sailor MJ, Falkenstein I, et al. Intravitreal properties of porous silicon photonic crystals: a potential self-reporting intraocular drug delivery vehicle. Br J Ophthalmol. 2008;92:705–711. doi: 10.1136/bjo.2007.133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidetti B, Azema J, Malet-Martino M, Martino R. Delivery systems for the treatment of proliferative vitreoretinopathy: materials, devices and colloidal carriers. Curr Drug Delivery. 2008;5:7–19. doi: 10.2174/156720108783331050. [DOI] [PubMed] [Google Scholar]
- 5.Nagarwal RC, Kant S, Singh PN, Maiti P, Pandit JK. Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J Controlled Release. 2009;136:2–13. doi: 10.1016/j.jconrel.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Sahoo SK, Diinawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discovery Today. 2008;13:144–151. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Hui Y-N, Liang H-C, Cai Y-S, Kirchhof B, Heimann K. Corticosteroids and daunomycin in the prevention of experimental proliferative vitreoretinopathy induced by macrophages. Graefe's Arch Clin Exp Ophthalmol. 1993;231:109–114. doi: 10.1007/BF00920223. [DOI] [PubMed] [Google Scholar]
- 8.Peyman GA, Schulman J. Proliferative vitreoretinopathy and chemotherapeutic agents. Surv Ophthalmol. 1985;29:434–442. doi: 10.1016/0039-6257(85)90208-5. [DOI] [PubMed] [Google Scholar]
- 9.Salonen J, Kaukonen AM, Hirvonen J, Lehto V-P. Mesoporous silicon in drug delivery applications. J Pharm Sci. 2008;97:632–653. doi: 10.1002/jps.20999. [DOI] [PubMed] [Google Scholar]
- 10.Anglin EJ, Cheng L, Freeman WR, Sailor MJ. Porous silicon in drug delivery devices and materials. Adv Drug Deliv Rev. 2008;60:1266–1277. doi: 10.1016/j.addr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang XG. Morphology and formation mechanisms of porous silicon. J Electrochem Soc. 2004;151:C69–C80. [Google Scholar]
- 12.Buriak JM. Organometallic chemistry on silicon and germanium surfaces. Chem Rev. 2002;102:1272–1308. doi: 10.1021/cr000064s. [DOI] [PubMed] [Google Scholar]
- 13.Bayliss SC, Buckberry LD, Harris PJ, Tobin M. Nature of the silicon-animal cell interface. J Porous Mater. 2000;7:191–195. [Google Scholar]
- 14.Foraker AB, Walczak RJ, Cohen MH, Boiarski TA, Grove CF, Swaan PW. Microfabricated porous silicon particles enhance paracellular delivery of insulin across intestinal Caco-2 cell monolayers. Pharm Res. 2003;20:110–116. doi: 10.1023/a:1022211127890. [DOI] [PubMed] [Google Scholar]
- 15.Canham LT. Bioactive silicon structure fabrication through nanoetching techniques. Adv Mater. 1995;7:1033–1037. [Google Scholar]
- 16.Anderson SHC, Elliott H, Wallis DJ, Canham LT, Powell JJ. Dissolution of different forms of partially porous silicon wafers under simulated physiological conditions. Phys Status Solidi A-Appl Res. 2003;197:331–335. [Google Scholar]
- 17.Park J-H, Gu L, Maltzahn Gv, Ruoslahti E, Bhatia SN, Sailor MJ. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nature Mater. 2009;8:331–336. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evered D, O'Connor M. Silicon biochemistry. Chichester; New York: Wiley; 1986. [Google Scholar]
- 19.Canham LT. Nanoscale semiconducting silicon as a nutritional food additive. Nanotechnology. 2007;18:1–6. [Google Scholar]
- 20.Low SP, Voelcker NH, Canham LT, Willams KA. The biocompatibility of porous silicon in tissues of the eye. Biomaterials. 2009;30:2873–2880. doi: 10.1016/j.biomaterials.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Wu EC, Park J-H, Park J, Segal E, Cunin F, Sailor MJ. Oxidation-triggered release of fluorescent molecules or drugs from mesoporous Si microparticles. ACS Nano. 2008;2:2401–2409. doi: 10.1021/nn800592q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canham LT, Reeves CL, Newey JP, Houlton MR, Cox TI, Buriak JM, et al. Derivatized mesoporous silicon with dramatically improved stability in simulated human blood plasma. Adv Mater. 1999;11:1505. [Google Scholar]
- 23.Lees IN, Lin H, Canaria CA, Gurtner C, Sailor MJ, Miskelly GM. Chemical stability of porous silicon surfaces electrochemically modified with functional alkyl species. Langmuir. 2003;19:9812–9817. [Google Scholar]
- 24.Cunin F, Schmedake TA, Link JR, Li YY, Koh J, Bhatia SN, et al. Biomolecular screening with encoded porous silicon photonic crystals. Nature Mater. 2002;1:39–41. doi: 10.1038/nmat702. [DOI] [PubMed] [Google Scholar]
- 25.Dancil K-PS, Greiner DP, Sailor MJ. A porous silicon optical biosensor: detection of reversible binding of IgG to a protein A-modified surface. J Am Chem Soc. 1999;121:7925–7930. [Google Scholar]
- 26.Chan S, Horner SR, Miller BL, Fauchet PM. Identification of gram negative bacteria using nanoscale silicon microcavities. J Am Chem Soc. 2001;123:11797–11798. doi: 10.1021/ja016555r. [DOI] [PubMed] [Google Scholar]
- 27.Li YY, Cunin F, Link JR, Gao T, Betts RE, Reiver SH, et al. Polymer replicas of photonic porous silicon For sensing and drug delivery applications. Science. 2003;299:2045–2047. doi: 10.1126/science.1081298. [DOI] [PubMed] [Google Scholar]
- 28.Khawly JA, Saloupis P, Hatchell DL, Machemer R. Daunorubicin treatment in a refined experimental model of proliferative vitreretinopathy. Graefe's Arch Clin Exp Ophthalmol. 1991;229:464–467. doi: 10.1007/BF00166311. [DOI] [PubMed] [Google Scholar]
- 29.Wiedemann P, Sorgente N, Bekhor C, Patterson R, Tran T, Ryan SJ. Daunomycin in the treatment of experimental proliferative vitreoretinopathy: effective doses in vitro and in vivo. Invest Ophthalmol Vis Sci. 1985;26:719–725. [PubMed] [Google Scholar]
- 30.Santana M, Wiedemann P, Kirmani M, Minckler DS, Patterson R, Sorgente N, et al. Daunomycin in the treatment of experimental proliferative vitreoretinopathy - retinal toxicity of intravitreal daunomycin in the rabbit. Graefes Arch Clin Exp Ophthalmol. 1984;221:210–213. doi: 10.1007/BF02134142. [DOI] [PubMed] [Google Scholar]
- 31.Kizhakkethara I, Li X, El-Sayed S, Khoobehi B, Moshfeghi DM, Rahimy M, et al. Noninvasive monitoring of intraocular pharmacokinetics of daunorubicin using fluorophotometry. Int Ophthalmol. 1996;19:363–367. doi: 10.1007/BF00130856. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 33.Moore P, MacCoubrey I, Haugland R. A rapid, pH insensitive, two color fluorescence viability (cytotoxicity) assay. J Cell Biol. 1990;111:304–304. [Google Scholar]
- 34.Link JR, Sailor MJ. Smart Dust: Self-assembling, self-orienting photonic crystals of porous Si. Proc Nat Acad Sci. 2003;100:10607–10610. doi: 10.1073/pnas.1233824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger MG, Arens-Fischer R, Thoenissen M, Krueger M, Billat S, Lueth H, et al. Dielectric filters made of porous silicon: advanced performance by oxidation and new layer structures. Thin Sol Films. 1997;297:237–240. [Google Scholar]
- 36.Boukherroub R, Petit A, Loupy A, Chazalviel JN, Ozanam F. Microwave-assisted chemical functionalization of hydrogen-terminated porous silicon surfaces. J Phys Chem B. 2003;107:13459–13462. [Google Scholar]
- 37.Gregg SJ, Sing KSW. Adsorption, surface area and porosity. 2nd ed. London: Academic Press Inc.; 1982. [Google Scholar]
- 38.Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60:309–319. [Google Scholar]
- 39.Barrett EP, Joyner LG, Halenda PP. The determination of pore volume and area distributions in porous substances. I. computations from nitrogen isotherms. J Am Chem Soc. 1951;73:373–380. [Google Scholar]
- 40.Meade SO, Chen MY, Sailor MJ, Miskelly GM. Multiplexed DNA detection using spectrally encoded porous SiO2 photonic crystal particles. Anal Chem. 2009;81:2618–2625. doi: 10.1021/ac802538x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teichmann KD. Intravitreal injections: Does globe size matter? J Cataract Refract Surg. 2002;28:1886–1889. doi: 10.1016/s0886-3350(02)01671-1. [DOI] [PubMed] [Google Scholar]
- 42.Kumar A, Nainiwal S, Choudhary I, Tewari HK, Verma LK. Role of daunorubicin in inhibiting proliferative vitreoretinopathy after retinal detachment surgery. Clin Exp Ophthalmol. 2002;30:348–351. doi: 10.1046/j.1442-9071.2002.00554.x. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y. Experimental study of the inhibitory effect of daunorubicin and doxorubicin in conjunctival fibroblast proliferation. Zhonghua Yan Ke Za Zhi. 1991;27:112–114. [PubMed] [Google Scholar]
- 44.Rahimy MH, Peyman GA, Fernandes ML, Elsayed SH, Luo Q, Borhani H. Effects of an intravitreal daunomycin implant on experimental proliferative vitreoretinopathy - simultaneous pharmacokinetic and pharmacodynamic evaluations. J Ocul Pharmacol. 1994;10:561–570. doi: 10.1089/jop.1994.10.561. [DOI] [PubMed] [Google Scholar]
- 45.Garweg JG, Wegmann-Burns M, Goldbum D. Effects of daunorubicin, mitomycin C, azathioprine and cyclosporin A on human retinal pigmented epithelial, corneal endothelial and conjunctival cell lines. Graefe’s Arch Clin Exp Ophthalmol. 2006;244:382–389. doi: 10.1007/s00417-005-0017-4. [DOI] [PubMed] [Google Scholar]
- 46.Sailor MJ, Link JR. Smart Dust: nanostructured devices in a grain of sand. Chem Commun. 2005:1375–1383. doi: 10.1039/b417554a. [DOI] [PubMed] [Google Scholar]
- 47.Laaksonen T, Santos H, Vihola H, Salonen J, Riikonen J, Heikkil T, et al. Failure of MTT as a toxicity testing agent for mesoporous silicon microparticles. Chem Res Toxicol. 2007;20:1913–1918. doi: 10.1021/tx700326b. [DOI] [PubMed] [Google Scholar]
- 48.Harper TF, Sailor MJ. Using porous silicon as a hydrogenating agent: derivatization of the surface of luminescent nanocrystalline silicon with benzoquinone. J Am Chem Soc. 1997;119:6943–6944. [Google Scholar]
- 49.Keizer HG, Pinedo HM, Schuurhuis GJ, Joenje H. Doxorubicin (adriamycin): a critical review of free radical-dependent mechanims of cytotoxicity. Pharmac Ther. 1990;47:219–231. doi: 10.1016/0163-7258(90)90088-j. [DOI] [PubMed] [Google Scholar]
- 50.Malisza KL, Hasinoff BB. Inhibition of anthracycline semiquinone formation by ICRF-187 (dexrazoxane) in cells. Free Radic Biol Med. 1996;20:905–914. doi: 10.1016/0891-5849(95)02188-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.