Abstract
More than two decades of pre-clinical research and two recent clinical trials have shown that progesterone (PROG) and its metabolites exert beneficial effects after traumatic brain injury (TBI) through a number of metabolic and physiological pathways that can reduce damage in many different tissues and organ systems. Emerging data on 1,25-dihydroxyvitamin D3 (VDH), itself a steroid hormone, have begun to provide evidence that, like PROG, it too is neuroprotective, although some of its actions may involve different pathways. Both agents have high safety profiles, act on many different injury and pathological mechanisms, and are clinically relevant, easy to administer, and inexpensive. Furthermore, vitamin D deficiency is prevalent in a large segment of the population, especially the elderly and institutionalized, and can significantly affect recovery after CNS injury. The combination of PROG and VDH in pre-clinical and clinical studies is a novel and compelling approach to TBI treatment.
Keywords: 1, 25-dihydroxyvitamin D3; combination therapy; neurosteroids; progesterone; traumatic brain injury; vitamin D
1. Introduction
In the past twenty years, dozens of phase II and III clinical trials for moderate and severe traumatic brain injury (TBI) have failed. This is in spite of the fact that over 130 drugs have shown some efficacy in animal models of injury [174]. One major reason cited for these disappointing outcomes is that the complex and varied mechanisms associated with different types of TBI are not being addressed by a single drug targeted towards only one or a few receptor sites. While pre-clinical experiments use mostly tightly controlled studies with well-circumscribed injuries and clearly defined outcomes, the pathophysiology of TBI in humans is often much more heterogeneous and systemic, affecting many different tissue systems and not just the brain itself. Treating patients suffering from a constellation of these injury-induced events may require a pleiotropic agent or a combination of drugs that can act simultaneously or even sequentially on the injury cascade without producing serious adverse events and complications.
Pre-clinical and clinical data accumulating over the last several years indicate that progesterone (PROG) may be highly effective in the treatment of TBI [91, 246, 255, 259, 300, 302]. A neuroactive steroid, PROG has been shown to improve behavioral and functional recovery and to reduce inflammation, oxidative damage, cerebral edema, and neuronal cell death [64, 102, 114, 299]. Although specific modes of action have yet to be completely defined, PROG has been shown to lead to improvements via a variety of molecular mechanisms [221, 244, 280], making it likely that interacting pleiotropic actions are responsible for its observed benefits. PROG is therefore a hormone with multiple mechanisms of action and can even be considered a “combination therapy” in itself [174]. Given its demonstrated effectiveness and safety in human patients, it is reasonable to consider PROG as a basis for combinations with other potential therapies.
In this respect it is logical to ask first what contextual conditions might limit the beneficial effects of the hormone in a clinical setting. In other words, what co-morbid conditions might affect TBI patients that could reduce the ability of PROG, or any other drug, to promote recovery? Recent research suggests, for example, that vitamin D deficiency (D-deficiency) can exacerbate injury and potentially reduce the beneficial effects of other treatments for TBI [39, 180]. This is especially the case in older subjects and is no small problem, because it has been reported that well over half of older adults suffer from D-deficiency [206]. There is also increasing evidence that about 30-35% of the general American public also suffers from D-deficiency, so patients of any age, including children, presenting with a TBI might be placed more at risk and have a less favorable outcome if they are D-deficient. In this context something as simple as providing vitamin D supplementation could improve recovery and potentially enhance the neuroprotective benefits of PROG (or any other) treatment. This could be important from a clinical perspective, given that most elderly patients who come to the hospital, with or without TBI, will be D-deficient.
Furthermore, it is becoming apparent in the wake of the failure of most TBI treatment clinical trials that multi-targeted pharmacotherapies hold more promise than drugs targeting specific pathobiological pathways [196] and that treatment may be optimized by combinations of agents acting on different mechanisms or the same mechanisms differently [76, 92]. The concept of multi-therapy has already become a standard approach for HIV/AIDS treatment, and patients are known to respond much more effectively to combinations of drugs, each targeted to different parts of the disease cycle, acting at different sites, and synergistically enhancing potencies and durations of action. The same approach has been suggested for TBI, especially due to its complex manifestation in human patients [174], where the functions of multiple organ systems may be affected by a direct injury to the brain.
Based on the literature, we suggest that 1,25-hydroxyvitamin D3 (or vitamin D hormone, VDH) is potentially a good candidate for a combination agent to be used in conjunction with PROG, since both hormones have high safety profiles, act on many different injury and pathological mechanisms, are readily available, easy to administer, and relatively inexpensive. In this article, we review the evidence for PROG neuroprotection after TBI and the emerging evidence for VDH as a neuroprotective agent, and discuss whether combining the two would be a good step to take in the development of a novel therapy for TBI.
2. Progesterone and Traumatic Brain Injury
2.1. Progesterone and Traumatic Brain Injury in Human Patients
A number of recent publications have demonstrated effectiveness of PROG treatment in experimental models of TBI and stroke [244, 254, 255, 258]. Based on the mounting positive pre-clinical data, two single-center Phase II clinical trials using PROG to treat TBI were recently completed, with promising results. The ProTECT (“Progesterone for Traumatic Brain Injury, Experimental Clinical Treatment”) trial was a randomized, double-blind, placebo-controlled trial of 100 patients with moderate to severe brain injury (Glasgow Coma Scale (GSC) scores of 4-12) performed at Grady Hospital in Atlanta, Georgia, a Level I trauma center [300]. No adverse effects were observed, and the severely injured patients receiving three days of intravenous PROG beginning 6-8 hours after injury showed a greater than 50% reduction in mortality at 30 days over those receiving placebo. The moderately injured group also showed statistically significant “encouraging signs of improvement” on Disability Rating Scale outcome compared to controls at 30 days. The conclusion was that PROG helped patients with both severe and moderate injuries, but that the effect was confounded in the severely injured by the fact that many in the group given PROG survived who otherwise would not have, so the overall recovery process took longer than for the moderately injured group.
These results were supported by another single-center trial of 159 severely brain-injured subjects (GCS ≤ 8) [302] in which patient outcomes were tracked for a longer time. The patients in this study received a five-day treatment with intramuscular injections of PROG within 8 hours of injury and showed substantially better survival and functional outcomes at both 3 and 6 months than controls. It is important to note that in both studies, PROG not only decreased mortality, but significantly enhanced functional outcome measures. The patients did not just survive to be consigned to a vegetative existence. Although these two reports need to be confirmed in larger multi-center studies, taken together they are the first to show a substantial benefit for TBI in human patients [65], making PROG among the most promising of the candidates that have been proposed [279].
2.2. Progesterone Signaling Mechanisms
PROG is produced by the ovaries and the corpus luteum in females during normal reproductive cycling [244] and by the adrenal glands, which are its main source in men [225]. PROG is also locally synthesized in both the peripheral and central nervous systems by neurons and glia, and its synthetic enzymes, cytochrome cholesterol side-chain cleavage enzyme (P450scc), which generates pregnenolone from cholesterol, and 3β-hydroxysteroid dehydrogenase (3β-HSD), which synthesizes PROG from pregnenolone, are both present throughout the brains of animals as diverse as fish and humans [103, 187, 188]. This makes it a neuroactive steroid, or “neurosteroid,” defined as a steroid hormone that is synthesized in and has effects on the nervous system [12].
Like all steroids, PROG exerts its cellular effects by regulating gene transcription in the nucleus. These “classical” actions are mediated by the cytoplasmic progesterone receptor (PR), which occurs in two main splice isoforms, PR-A and PR-B. Ligand binding to these receptors recruits nuclear receptor coregulators such as members of the steroid receptor coactivator (SRC) family, which have been found to be limiting factors in steroid-induced responses in the brain [37]. The entire complex then migrates to the nucleus, where it binds to progesterone response elements (PREs) in the promoters of genes and initiates or inhibits gene expression. The PR is also capable of interacting with the Src tyrosine kinase family in the cell membrane [68].
In addition to the classical cognate PR, PROG also interacts with other signal transduction mechanisms such as the σ1 receptor, for which it is a competitive inhibitor and through which it may reduce N-methyl-D-aspartate (NMDA) glutamate signaling [15, 111]. PROG also signals at the nicotinic acetylcholine receptor (nAChR) [276] and affects gamma-aminobutyric acid (GABA), the main inhibitory transmitter in the brain, through its 5α-reduced metabolite allopregnanolone (or 3α,5α-tertrahydroprogesterone; ALLO) and positive modulation of the GABAA receptor [13]. Both these mechanisms may be responsible for the neuroprotective effects of PROG, as they inhibit the excitotoxic response to injury. PROG metabolites have indeed been shown to be neuroprotective in their own right in models of kainic acid-induced hippocampal injury [44, 45] and after experimental TBI [63, 64, 280]. PROG is also known to activate the pregnane X receptor (PXR), which may also be responsible for some of its protective effects in addition to those achieved through the PR. Finally, recent evidence suggests that PROG may also exert direct signaling effects through activation of a membrane surface receptor, the 25-Dx [104, 185].
All these modes of action—gene transcription, neurotransmission, and signal transduction—are affected by PROG, and are likely to be responsible for its effects in the nervous system. Further complexity is added by the fact that both the synthetic enzymes and receptor/signaling systems are modulated by physiological context such as injury and, potentially, aging [244]. For example, not only may receptors be upregulated (25-Dx) or downregulated (PR) in response to TBI, but certain genes affecting neuronal functioning may develop responsiveness to PROG only after injury [56, 243, 244].
2.3. Progesterone as a Neuroprotective Agent
One reason PROG shows benefits where other drugs have failed is that it is a pleiotropic drug. Attention was first drawn to PROG as a treatment for TBI when it was observed that females exhibited less edema after injury than males, with pseudopregnant females (high in PROG) exhibiting virtually none [6, 234]. A number of subsequent studies have shown that PROG can reduce edema and excitotoxic cell death in the perilesional area of secondary injury [114], and protect against ischemia [52, 240]. One major problem with central nervous system (CNS) damage (in both TBI and stroke) is disruption of blood flow to the local area of injury, leading to loss of oxygen and glucose, energy failure, and eventual cell death. PROG has been shown specifically to protect neurons against cerebral ischemia [33, 97] and to decrease infarct size [130, 146]. There are several observed effects in this resistance to ischemia: 1) maintenance of mitochondrial function, 2) increased pro-survival signaling, and 3) reduced internal and exogenous pro-apoptotic signaling [3, 33, 97, 240, 244]. PROG appears to affect mitochondria in multiple ways. It restores them to normal morphology even after severe vacuolation [56], it inhibits pro-apoptotic cytochrome c release [239] and it upregulates the expression of anti-apoptotic mitochondrial proteins such as B-cell lymphoma 2 (Bcl-2) while decreasing the levels of pro-apoptotic signals such as Bcl-2-associated X protein (Bax), Bcl-2-associated death-promoter (BAD), and caspase-3 activation [3, 63, 83, 205, 298, 305]. PROG may affect the expression of these proteins through activation of the extracellular signal regulated kinase (ERK) signaling pathway, which phosphorylates the cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), upregulates bcl-2, and confers improved resistance to ischemia [77, 78]. PROG and its metabolites have also recently been shown to modulate mitogen-activated protein kinase (MAPK) and phosphoinositide-3 kinase (PI3K) signaling in the hypothalamus, hippocampus, and cerebellum of ovariectomized rats in vivo [105]. Finally, PROG has also been shown to reverse the alterations in mitochondrial respiration [229] and normalize the expression of the Na+,K+-ATPase in experimental autoimmune encephalomyelitis (EAE) and models of spinal cord and nerve crush injury [82, 149, 231]. Since both of these are important issues in the energy failure and loss of ionic gradients that lead to cell death, this normalization of cellular metabolism is a key step in the attenuation of secondary injury.
Increased survival of glial and neuronal cells is associated with elevated levels of trophic factors. PROG has been shown to increase levels of both nerve growth factor (NGF) [213, 272] and brain-derived neurotrophic factor (BDNF) [95, 96, 241] after injury. These proteins are especially necessary for glial survival and remyelination [150, 244]. Most importantly, however, PROG is known to reduce microglial activation and the production of pro-inflammatory and pro-apoptotic cytokines such as tumor necrosis factor α (TNFα) and Interleukin-1 (IL-1) [66, 114, 190, 221]. This is very significant, since prolonged inflammation is the main cause of extended secondary injury [19, 193, 247]. PROG also inhibits activation of complement factors [221, 280], and modulates the coagulation cascade [281], both of which are important mechanisms of inflammatory amplification. PROG has also been shown to push helper T cell (TH) cell differentiation towards the TH2 phenotype, which may also play a role in its anti-inflammatory activity [178].
Improvement of mitochondrial function, increased pro-survival factors, and reduced inflammation are not only beneficial in the injury penumbra but have important systemic effects. TBI-associated systemic inflammation is a key mechanism in mortality, and can lead to multi-organ failure and infection [154, 312]. After TBI, catecholamine-induced necrosis of cardiomyocytes leads to cardiopulmonary dysfunction and is also a significant cause of mortality [312]. A compound like PROG that increases survival signaling and attenuates inflammation has a role in recovery that extends well beyond the brain [40, 219].
There is also evidence that PROG treatment after TBI reduces lipid peroxidation [235], perhaps through upregulation of antioxidant enzymes such as superoxide dismutase (SOD) [191], although the mechanisms of action are not completely understood [244]. A reduction of the damage caused by reactive oxygen and nitrogen species (ROS/RNS) can improve cell survival by maintaining membrane integrity, and helps to maintain the blood-brain barrier (BBB) by limiting oxidative damage to the endothelium. PROG has also been shown to help maintain BBB function by upregulating P-glycoprotein (Pgp), an efflux pump transporter and marker of BBB health that serves to eliminate xenobiotic and toxic substances; in the case of traumatic injury, these consist of inflammatory cytokines and ROS-producing compounds [53]. PROG can also protect neurons from direct toxicity of glutamate, FeSO4, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and β-amyloid [26, 98, 205, 210], the last two of which may have implications for the development and prevention of Parkinson's and Alzheimer's diseases, respectively. As mentioned previously, PROG also attenuates glutamate excitotoxicity through conversion to ALLO and subsequent activation of GABAA and σ1 receptors [13, 15].
These mechanisms—reduced inflammation and lipid peroxidation, maintenance of BBB integrity, and ionic stability–all serve to reduce edema after TBI [233, 236] and stroke [8, 52, 130]. Recent findings also indicate that PROG can regulate expression of aquaporin-4 (AQP4), the water channel present in astrocyte endfeet and thought to be important in edema formation [107]. Since brain swelling is one of the main neurological causes of mortality after TBI, this is an important issue in the clinical management of brain-injured patients.
Both the ProTECT trial [300] and that reported by Xiao et al. [302] demonstrated improved functional recovery for patients receiving PROG after TBI. This is an important issue because ultimately the test of a pharmacological intervention is its effect on functional outcome and quality of life. In this context, PROG has shown improved long-term recovery in a number of behavioral paradigms including cognitive, sensory, and spatial learning and memory [232]. These effects have been seen in different models of mild, moderate, and severe experimental injury [21, 244]. The ability to sustain the neuronal circuitry implicated in complex behaviors is an important component of recovery. As expected, PROG has also been shown to attenuate retrograde neuronal degeneration in the nucleus basalis of Meynert (NBM) [114] and to maintain ACh homeostasis by regulating choline acetyltransferase (ChAT) levels in both TBI [63] and spinal cord injury [149]. In addition to affecting connectivity, PROG also helps in signal transmission by promoting myelination and remyelination of injured neurons, and maintaining myelin basic protein (MBP) at control levels [148]. MBP levels are associated with the establishment of a glial scar. These facts suggest that rather than just stopping further damage, PROG in fact initiates repair mechanisms [148, 150].
3. Vitamin D and Neuroprotection
The term “Vitamin D” is something of a misnomer. Although the name is still in use for popular and historical reasons, vitamin D is more properly classed as a secosteroid because it consists of a cholesterol backbone and exerts steroid-like effects throughout the body, directly affecting the expression of over 1,000 genes [70] through the nuclear steroid vitamin D receptor (VDR). It has been shown to affect systems similar to those modulated by other hormones and steroids [89], with which it may interact in a variety of physio-pathological contexts [165, 171, 250, 251, 294]. Vitamin D is also a neuroactive steroid, because both the final activating enzyme and its nuclear receptor are known to be widely distributed throughout the CNS [89].
Vitamin D's physiological role was long believed to be limited to Ca2+ and phosphate homeostasis and the formation and maintenance of bone [58, 59, 89, 117, 122, 306]. Recent evidence, however, suggests a much wider role for this compound, especially in its biologically active form, which includes modulation of the immune system [31, 59, 100, 113, 117, 122, 172], the renin-angiotensin system [227], development of cardiovascular disease [176, 186], neuromuscular function [222], cell cycle control [9, 310], and cancer [220].
3.1. Vitamin D Deficiency and its Consequences
According to the Third National Health and Nutrition Examination Survey, 61% of Caucasian- and 91% of African-Americans are D-deficient [139]. Similar figures have been cited for all segments of the population and in many countries [75, 124, 169]. Although D-deficiency is common in healthy young populations in industrialized nations [58, 89, 122, 306], it is especially frequent in the elderly, especially in resident (nursing) homes and patients with hip fracture [163], with reported prevalence ranging from 65% to 74% in hospital inpatients [38, 50, 269], to 87% in elderly institutionalized patients [153] and 86% in institutionalized postmenopausal women [90]. It is a significant problem that has been called the “silent epidemic” [118], with a number of potential consequences, many of which are still unknown.
Vitamin D levels are commonly determined by serum levels of 25-hydroxyvitamin D3 (25OHD3), with levels below 25nmol/L defined as deficiency, levels between 25nmol/L and 50nmol/L defined as insufficiency, and higher than 50nmol/L as normal [164], although exact cutoff values are still being debated [121, 124]. Levels below 20nmol/L are associated with rickets and adult osteomalacia, the hallmark of deficiency, but recently the value range considered necessary for optimal health has shifted to 100-120nmol/L [223], and a daily intake of at least 2000 IU/day has been suggested [58].
Aside from its classical effects on bone density, D-deficiency has been associated with a number of systemic conditions such as secondary hyperparathyroidism [120, 181], metabolic syndrome [220], hypertension [159, 289], obesity [226], and diabetes mellitus [93, 99], as well as cardiovascular disease events such as stroke and congestive heart failure [189, 285], all of which can significantly affect a patient's ability to recover from severe trauma. Several recent studies also suggest that inadequate vitamin D may predispose towards Parkinson's and other neurodegenerative diseases [72], mood disorders [89, 134], and even tuberculosis infection [308].
The relationship of vitamin D to autoimmune disorders is especially relevant to diseases of the CNS, and deficiency has been associated with increased incidence of multiple sclerosis (MS) [27, 29, 99], Sjögren's syndrome [132], rheumatoid arthritis [2], and Crohn's disease [127, 215, 216]. Systemic vitamin D levels have been suggested as a possible explanation for the latitudinal gradient in MS incidence (nearly zero at the equator and increasing with greater distance from it) [204], and correlations have been observed between circulating vitamin D status and the risk of developing MS [27, 278], as well as a protective effect of vitamin D intake in both human disease [195, 249] and animal models [28, 88, 156]. Vitamin D therapy for MS has been shown to be safe in humans [141] and has recently been recommended for use in double blind controlled clinical trials [204, 248].
A low level of vitamin D is also one of the key markers of frailty, defined as a “global impairment of physiological reserves involving multiple organ systems” [274]. Frailty often results in a reduced capacity to maintain physical and psychosocial homeostasis and greater vulnerability to internal and environmental stressors such as trauma [175, 274]. This could be especially important in the elderly, who are already more vulnerable to TBI, and studies have shown that advanced age is a major predictor of injury severity after TBI [194]. Other potentially exacerbating factors in the aged include systemic issues such as kidney disease, hypertension, atherosclerosis and cardiovascular disease, diabetes, cancer, and hormonal imbalances such as hyperparathyroidism [212]. While all these conditions can independently affect responses to injury, each has also been associated by a growing literature with insufficient serum levels of vitamin D as a key and often ignored underlying problem [99, 124, 220]. Vitamin D status has been specifically associated with functional outcomes in the elderly [18, 55], suggesting that supplementation could be especially helpful for this segment of the population [55].
3.2. Vitamin D Synthesis, Activation, and Metabolism
The first step in the synthesis of VDH is the activation in the epidermis of 7-dehydrocholesterol by sunlight, specifically ultraviolet B (UVB) radiation in the 290-320nm range [108]. Because DNA absorbs UVB in exactly the same spectrum, vitamin D is hypothesized to have evolved as a “sunscreen” for DNA [117]. This is supported by the fact that it is present in animals ranging from phytoplankton to humans [117]. The UVB radiation opens the B ring of the steroid precursor, resulting in the conjugated triene system that characterizes all D vitamins and defines them as secosteroids [67, 207]. Although seven forms of vitamin D exist, D3 is the naturally occurring form in animals and is present in the skin of all higher vertebrates [207].
After its activation by sunlight, vitamin D is activated to VDH in two enzymatic steps. The first of these occurs in the liver by the cytochrome P450 enzyme, vitamin D 25-hydroxylase (CYP2R1) [122]. This step is not tightly regulated and therefore the product, 25OHD3, is a good indicator of overall vitamin D status [207]. The second step requires the 25-hydroxyvitamin D3 1α-hydroxylase enzyme (CYP27 or 1α-OHase) and is a tightly controlled reaction regulated primarily by VDH itself, but also by levels of parathyroid hormone (PTH), calcium, and phosphate [67]. 1α-OHase is most abundant in the kidneys, although recent evidence has shown that it is present throughout the body, including the immune system [113, 268, 277] and the rodent and human brain [7, 73, 74, 94, 116, 152, 183, 261, 262, 275, 286]. The ubiquitous distribution of 1α-OHase suggests that VDH has both local and systemic effects (due to its synthesis by the kidneys and release into the bloodstream), and recent research suggests that a significant percentage of all VDH activity is autocrine or paracrine [163]. VDH is inactivated by 25-dihydroxyvitamin D3 24-hydroxylase (CYP24), which is present in almost all VDH target cells, is induced by VDH, and is regulated in a manner reciprocal to 1α-hydroxylase, allowing for very tight local and global control of VDH levels [67]. The fact that the CNS can locally catalyze both its activation and inactivation makes VDH by definition a neurosteroid [89].
Vitamin D and its metabolites are largely bound in the blood by vitamin D binding protein (DBP), also known as group-specific component of serum or Gc-globulin. DBP serves as the main reservoir and transporter of the vitamin D endocrine system, and binds about 88% of the total 25OHD3 and 85% of the total VDH in serum [207]. This is an important fact in the pharmacokinetics of VDH, since only the free concentration of the hormone is considered to have biological activity [296]. Only about 5% of DBP is bound to vitamin D metabolites, and its serum concentration is about 20-fold that of the various vitamin D species [296]. DBP is an acute phase protein produced by the liver, and is upregulated by estrogen and during pregnancy when PROG is also very elevated [207].
3.3. Vitamin D Signaling Mechanisms
Most action of VDH is mediated by the VDR, a ligand-inducible transcription factor that regulates gene expression by binding to specific vitamin D response elements (VDREs) in DNA [39, 43, 59, 110, 265, 290]. The specificity of the receptor for VDH is some 100 - 1000 times higher than for its precursor 25OHD3 [207]. Like other nuclear steroid receptors, the ligand-receptor complex effects gene transcription after undergoing heterodimerization with the retinoid X receptor (RXR) and recruitment of nuclear receptor coactivation proteins [22, 43, 59, 177, 197, 217, 265, 270]. The VDR belongs generally to the protein superfamily that includes receptors for PROG, estrogen, glucocorticoids, androgens, thyroid hormone, and peroxisome proliferator-activator receptor (PPAR) [207] and more specifically to the NR1I subfamily of orphan nuclear receptors, which also includes the PXR and the constitutive androstane receptor (CAR) [228]. It is interesting to highlight here that the VDR is closely related (60% homology in the DNA-binding domain) to the PXR, a xenobiotic sensor through the activation of which PROG may exert some of its neuroprotective effects [144, 151, 217]. This suggests potential interactions and cross-talk between the two systems.
VDRs are widely distributed throughout the embryonic and adult brain, and appear most prominently in the neuroepithelium and proliferating zones in both rats [152, 263, 265, 282, 286] and humans [74]. Their presence has also been noted in neurons and glia of the human prefrontal and cingulate cortices, thalamus, hypothalamus, cerebellum, substantia nigra, caudate, putamen, amygdala, and hippocampus [24, 74], although notably not in the macrocellular cells within the NBM and the septum [74]. This distribution is mostly coextensive with the presence of 1α-OHase, except in the NBM, where 1α-OHase was present but VDR was not [74]. This expression largely coincides with VDR and 1α-OHase distribution in the rodent brain [24, 74], and also strongly overlaps with the known distributions of receptors for androgens, glucocorticoids, estradiol, and PROG [46, 81, 137, 224]. There is also significant overlap between VDR and 1α-OHase expression in the brain, and VDH synthetic and degradative pathways have been described in neurons and glia [47, 201-203, 309]. This implies that VDRs in the brain are very likely activated by locally synthesized VDH and suggests a functional role for the hormone in the CNS [24].
Like most steroid hormones, VDH is also capable of rapid, non-genomic signaling [67]. These responses are likely mediated by receptors located on the cell surface, and although it has been suggested that these rapid events modulate genomic activity of VDH, the exact function of this signaling pathway has not yet been determined. Although previously thought to be a different receptor protein, the VDH receptor involved in non-genomic signaling now appears to be the VDR, but in this case it is located not in the nucleus or cytosol but rather in membrane caveolae [207]. These caveolae, or lipid rafts, are invaginations in the plasma membrane and believed to be involved in the signal transduction of a number of signaling systems [5]. These rapid effects include activation of phosphoinositide metabolism [17, 192], cyclic guanosine monophosphate (GMP) [106, 284], protein kinase C (PKC) [266], MAPKs [14, 252], opening of Cl- channels [307], and stimulation of cellular Ca2+ levels [160, 167, 192, 264].
3.4. Vitamin D as a Neuroprotective Agent
3.4.1. In vivo models
VDH treatment has shown promising results in a variety of in vivo and in vitro CNS injury paradigms. In a model of stroke, Wang and colleagues showed that VDH pre-treatment for 8 days can significantly increase levels of glial-derived neurotrophic factor (GDNF) and attenuate cortical infarction induced by middle cerebral artery (MCA) ligation in rats [291]. In various modes of Parkinson's disease, a number of researchers have shown that 7 – 8 day pretreatment with VDH can restore levels of dopamine in the substantia nigra of 6-hydroxydopamine lesioned rats [287] and prevent lipid peroxidation and cytosolic cytochrome c in zinc chloride-infused rat substantia nigra [162]. VDH pretreatment also prevented iron-induced oxidative injuries in the locus coeruleus (LC) of the rat [41]. Although these studies used a preventive paradigm by administering VDH for up to 8 days prior to injury, other studies have shown post-injury treatment benefits of VDH as well. Oermann and colleagues found that treatment with VDH after a photothrombotic lesion to the cerebral cortex of rats reduces the expression of glial fibrillary acid protein (GFAP), a key marker of reactive gliosis, in remote areas of secondary damage [209]. One recent report by Chabas and colleagues [34] examined axon regeneration after peripheral (peroneal) nerve injury in rats followed by chronic treatment with vitamin D2. The authors reported that the treatments enhanced the formation of new axons as well as increasing axon diameter and improving sensory responses to metabolic stimulation.
Further research has shown that concurrent administration of VDH with lipopolysaccharide (LPS) significantly inhibited inducible nitric oxide synthase (iNOS) expression in monocytes in the rat brain, suggesting that VDH can also help attenuate immune-induced oxidative damage in the CNS [86]. Lin et al. found a similar effect on zinc-induced toxicity in the CNS, where concurrent administration of VDH reduced apoptosis and oxidative damage [161]. VDH was also found to perform a direct anti-convulsant role in the brains of mice with chemically induced seizures [135].
The majority of in vivo studies with VDH, however, have focused on its effect on MS and its animal model, chronic relapsing EAE. The VDH effect in this model has been known for a long time [89, 156]. VDH has been reported to be able to block the development of disease after onset in both rats [198] and mice [28], an improvement correlated with inhibition of iNOS [84, 88], CD4 antigen expression [198], and IL-12-dependent TH1 cell development in the CNS [179]. VDH also increased levels of transforming growth factor β (TGFβ) and IL-4, which were increased in a mouse model and are anti-inflammatory TH2 immune response cytokines [30]. In another EAE system, VDH significantly reduced acute inflammation and levels of GFAP by inducing inflammatory cell apoptosis [253]. Since a significant component of secondary damage after many types of brain injury including TBI is related to excessive and prolonged inflammation, these data suggest that VDH might be an effective adjunct to treatments for immune disorders of the CNS.
3.4.2. In vitro models
There is also significant in vitro evidence for VDH neuroprotection. Two studies using mesencephalic dopaminergic neuron culture have shown that VDH protects these neurons from glutamate and dopaminergic toxins by increasing neuronal functions that serve to reduce oxidative stress [125, 245]. A similar anti-oxidant effect was described by Garcion et al., who found that VDH treatment increased γ-glutamyl transpeptidase (γ-GT) expression and activity, enhanced glutathione pools, and reduced nitrite production in LPS-stimulated primary rat astrocyte culture [85]. In addition to anti-oxidant activity, VDH has been observed to reduce the production of inflammatory cytokines TNFα, IL-6, and nitric oxide (NO) in stimulated microglia [155]. In addition to neurons, astrocytes, and microglia, VDH has an effect on oligodendrocytes [7] and Schwann cells [51]. VDH also appears to regulate the expression of N-myc, c-myc, PKC, and TGFβ in neuroblastoma cells [283], suggesting that it may affect neural cell growth in ways other than the well-established induction of NGF and its receptors [23, 51, 202, 237, 238, 297].
In addition to NGF, VDH can directly affect the expression of other factors involved in regeneration and recovery after CNS injury, including GDNF [199], neurotrophin 4 (NT-4) [201], and insulin-like growth factor binding proteins (IGFBPs) [177]. The results from these studies suggest that not only does VDH affect oxidative stress, neurotoxicity, oxidative stress, and growth factor expression, but it also works on all cell types involved in the development of and recovery from CNS injury including neurons, astrocytes, oligodendrocytes, and immune cells such as monocytes and microglia.
3.5. Vitamin D Mechanisms of Action
The primary non-calcemic effect of VDH appears to be inhibition of cell proliferation and stimulation of cell differentiation, especially in the immune system, where it acts as a powerful modulator [31, 43, 59, 101, 112, 117, 122, 172, 296]. VDH has been shown to skew all aspects of immune function (T-cell differentiation, macrophage and dendritic cell maturation and antigen-presenting ability, cytokine profiles) towards a type 2 immune response, which is generally anti-inflammatory and regulatory.
This pro-inflammatory versus anti-inflammatory dynamic seems to be an important factor in the development of extended and damaging inflammation and in the genesis of the most likely cause of death for TBI victims, multi-organ system (MOS) dysfunction and failure [154]. Naïve, or TH0, CD4+ cells can differentiate into one of two phenotypes: in the presence of Interleukin-12 (IL-12) they develop pro-inflammatory TH1 characteristics, which consist of production and release of TNF and Interferon-γ (IFNγ), further attraction of macrophages and monocytes, and activation of cell-mediated immunity and inflammation; in the presence of Interleukin-4 (IL-4), TH0 cells develop an anti-inflammatory TH2 phenotype, characterized by further production of IL-4 as well as Interleukin-5 (IL-5) and Interleukin-13 (IL-13), binding to B cells, and activation of antibody-mediated immunity [60, 140, 211, 267]. These two general phenotypes mutually inhibit each other. This TH differentiation appears to be of fundamental importance in the development of pathological inflammation in the hours and days after injury as the damaged system attempts to establish a dynamic equilibrium between the TH1 and TH2 populations and pro- and anti-inflammatory activity. This balancing act has consequences not only on the local injury environment, but on the organism as a whole. [138].
Like PROG, VDH has been shown to decrease levels of pro-inflammatory TH1 cytokines such as TNFα, IL-1β, IL-12, IL-6, IFNγ [48, 126, 168, 172, 268, 311], as well as the downstream reactive oxygen species generated by activated macrophages [133]. Long-term vitamin D deficiency has been shown to lead to generalized inflammatory conditions that compromise the cardiovascular system and glucose metabolism [119, 123, 157, 208, 292], the health of which is essential to survival post-TBI. In acute injury, chronic D-deficiency leads to a more intense pro-inflammatory type 1 reaction, which could exacerbate the processes of damage secondary to the initial traumatic insult. Related to macrophage and microglial activity and TH1 response is the production of reactive species that cause oxidative stress and contribute to secondary injury [19]. By modulating the development of a hyperactive and prolonged inflammatory response through adjusting TH1/TH2 balance and inducing macrophage apoptosis, VDH may limit the secondary injury cascade after TBI. This could be especially important under conditions of D-deficiency, where the underlying physiological state is already skewed towards a type 1 response [126, 172, 178, 179, 273].
Considerable evidence also exists for a direct modulatory effect of VDH on inflammation. VDH is known to down-regulate NFκB [54], the central mediator of inflammation that has also been linked with stress-response in humans [16] and stress-induced neuronal loss in rats [170]. VDH has also been shown to decrease inflammatory cytokine production in a variety of cell types, including endothelial cells [71], keratinocytes [109], monocytes [260], and microglia [155]. Systemic VDH administration has also been noted to lead to lower serum concentrations of TNFα and increased levels of anti-inflammatory IL-10 in heart failure patients [242], as well as lower TNFα and symptom manifestation in a rat model of inflammatory bowel disease (IBD) [311]. Finally, higher pro-inflammatory cytokine levels were found in VDR-KO (knock-out) mice [80], and an inverse correlation was seen between systemic inflammatory markers and 25OHD3 levels [271].
Since increased cellular Ca2+ concentration is the final common step in the initiation of cell death after injury, maintenance of adequate intracellular levels of Ca2+ is important for cell health and survival, not just in neurons but also in astrocytes and oligodendrocytes. VDH helps to regulate these levels and the cellular response through several mechanisms: 1) maintenance of adequate systemic parathyroid hormone (PTH) levels, 2) regulation of L-type voltage-sensitive Ca2+ channel (L-VSCC) expression, and 3) control of intracellular Ca2+ buffering systems. Control of systemic Ca2+ metabolism, along with regulation of parathyroid activity and PTH levels, belongs to the classical set of vitamin D functions; a state of D-deficiency can lead to increased PTH secretion, which in turn leads to increased intracellular Ca2+ concentrations that can increase the likelihood of Ca2+ overload and cell death in case of severe injury. In addition, and very importantly for amelioration of secondary injury after trauma, VDH has been observed to be neuroprotective in primary rat hippocampal cultures through the inhibition of L-VSCCs, which are strongly implicated in the development of glutamate-induced excitotoxic injury [20]. Finally, VDH upregulates proteins of the intracellular Ca2+ buffering system such as calbindin-D28k and parvalbumin [147, 304], thereby improving the ability of cells to cope with increased intracellular Ca2+ levels without entering the irreversible path towards cell death. The general effect of these mechanisms is enhanced resistance to perturbations and improved cellular adaptation. A vitamin D-deficient state, however, can lead to increased susceptibility to Ca2+-induced damage [42, 57, 220].
VDH is also a powerful regulator of the cell cycle: it inhibits cell proliferation and stimulates cell differentiation, and it is most likely this ability to control the cell cycle that makes it effective as an anti-inflammatory and an anti-neoplastic agent [9, 109, 136]. On a molecular level, several different microarray analyses indicate that VDH has effects on cell cycle regulating genes such as p53, p21CIP1/WAF1, p27KIP1, which are involved in apoptosis and control of the G1/S phase transition [128, 131], and growth arrest and DNA-damage-inducible, alpha (GADD45), which is involved in the G2/M phase transition [69, 70, 100, 290, 295]. VDH may control other aspects of the cellular reproductive machinery such as various cyclins and cyclin-dependent kinases [39, 129]. Since terminally differentiated neurons undergoing severe stress are known to re-enter the cell cycle, only to be forced to undergo apoptosis because they have lost their ability to proliferate [32, 61, 79, 145, 184, 310], the ability of VDH to induce cell cycle arrest and DNA repair might also be neuroprotective after TBI. Several studies suggest that this may be the case, and inhibition of cell cycle reentry has been neuroprotective in both experimental TBI [62] and Alzheimer's disease [200] models. Since a significant amount of the damage in TBI is caused by a secondary cascade of injury mechanisms [256, 257], maintaining G0 phase neurons in that state and inducing p53-mediated DNA repair could be a way to reduce post-TBI cell death and improve long-term neuronal survival [145].
4. Why Combine Vitamin D and Progesterone?
4.1. Potential interactions with other neurosteroids, especially PROG
There is growing evidence that vitamin D may interact with other neurosteroids such as PROG and estradiol in a variety of tissues. For example, VDH has been found to stimulate estradiol and PROG secretion in human placenta [10], and it is known to interact with PROG and estrogen in maintaining bone health, especially in post-menopausal women [90, 119]. VDR gene polymorphisms have also been associated with breast and prostate cancer risk [166, 230], suggesting not only that there may be crosstalk among the different steroid signaling pathways, but also that the hormonal context within which a single compound operates may modulate the end effect. Especially intriguing is the finding that xenobiotic activation of the PXR (for which PROG is a ligand and by way of which it may exert some of its neuroprotective effects [11, 151]) can lead to drug-induced osteomalacia by upregulating the expression of CYP24 [218, 303], the chief metabolizing enzyme of VDH. Furthermore, we have also observed that TBI induces lower serum levels of 25OHD3 (unpublished observation), suggesting that injury itself may cause a vitamin D-insufficient state. Given that PROG is a promising treatment for TBI that has been shown to work in a number of model systems and in human patients [258, 259], the possibility that it may interact with vitamin D could have important consequences for treatment outcomes, and opens the possibility of developing a combined TBI treatment that may not only overcome the effects of vitamin D deficiency in the human population but may also enhance the effects of PROG treatment in normal patients with TBI.
From our review of the literature it is growing more apparent that vitamin D and PROG affect many of the same as well as a number of divergent processes involved in the repair of secondary injury following TBI. The similarities may be explained by the fact that VDRs have been found in rodent (and human) microglia, astrocytes, oligodendrocytes and Schwann cells [180], which are known to play a role in inflammation and CNS repair and which are also directly affected by PROG treatment after CNS injuries. It is certainly possible that, if PROG and VDH each work through different pathways to reduce cellular injury and enhance the metabolic processes of repair, then a combination of these agents might lead to more rapid neuronal repair and functional recovery, perhaps even with less dosing and duration of treatment.
A reason to attack the same injury pathways with different compounds lies in the fact that the same repair mechanisms may be modulated through different signals. An example of this would be intracellular Ca2+ levels, which can be independently affected by the reduction of glutamate excitation and by intracellular buffering systems. Here, a combination of PROG's actions through the GABAergic system to inhibit extracellular activity and the action of VDH to increase intracellular Ca2+-binding proteins would both have an effect on calcium metabolism, but via different sub-pathways [239]. Another example would be apoptosis, which may be reduced by a number of different mechanisms: effects on Ca2+ metabolism, induction of trophic factors, inhibition of inflammation and pro-apoptotic signaling, and/or a reduction in lipid peroxidation by reactive species. Two different compounds that affect the same mechanism in this case would be synergistic and presumably enhance the protective effect after a CNS injury.
Another rationale for using a drug combination that affects the same mechanisms is the well-known inverted U-shaped dose response (or hormesis) of steroid action [25, 49], in which the optimal result is obtained with a medium-range dosage while increasing dosages decrease the effectiveness. Why hormesis occurs is not clear, although recent mathematical modeling studies suggest that the artifact may be due to the second-order steroid receptor kinetics that produce a parabolic dose response curve [158]. If the kinetics of the receptor mechanism indeed impose a limit on steroid action, it may be beneficial to attempt to overcome individual system saturation and activate similar protective end mechanisms through different steroid pathways [288, 293]. As an example, PROG and VDH may increase the activity of γ-GT by different mechanisms (PR-PROG activity and VDR-VDH activity), resulting in an increased overall antioxidant capacity. Although this is still not fully confirmed, the suggestion that it may be possible to amplify a neuroprotective effect simply through drug combination is intriguing and worth further exploration.
This concept can be extended to fully divergent mechanisms if one assumes that fewer damaging processes are ultimately better for protection and recovery. A complication may arise here if certain processes, such as inflammation [36], are potentially beneficial in the short term but end up being detrimental in the longer term, in which case an optimal treatment would only be achieved through the use of multiple agents given at different time points in the injury cascade. Another complication may involve non-linear interactions between PROG and VDH such that what may be best doses for treatment with each individually may not work optimally in combination. Some of our initial data (unpublished observations) indeed suggests that this may be the case. This means that dosing parameters may have to be specifically reconfigured for novel combination therapies. Regardless of such considerations, a recent NINDS Workshop on Combination Therapies specifically recommended that, “With its [PROG's] pleiotropic characteristics, it would be advantageous to consider combination therapies for TBI that combine PROG with other agents that 1) protect the intracerebral vasculature, 2) diminish the effects of glutamate release and calcium influx, 3) more directly protect the mitochondria, 4) protect against the toxic effects of heme breakdown products, 5) enhance free radical scavenging, 6) enhance cerebral blood flow, 7) modulate the kalkrein-kinin system, 8) protect the axonal and cytoskeleton infrastructure, and 9) protect against diffuse axonal injury” [174]. VDH meets several of these recommendations for a combinatorial agent:
-
Diminish the effects of glutamate release and calcium influx:
VDH maintains intracellular Ca2+ through downregulating L-VSCCs and upregulating intracellular Ca2+ buffering capacity [57, 147, 304].
-
Protect against the toxic effects of heme breakdown products:
VDH has been reported to upregulate glial heme oxygenase-1 (HO-1) concomitantly with a reduction in GFAP following focal cortical ischemia [209]. HO-1 is one of the rapidly induced heat shock proteins which metabolizes and thus detoxifies free heme to the powerful endogenous antixodants biliverdin, CO and Fe2+ [173, 182]. These studies suggest that HO-1 induction by VDH protects cells from the oxidative toxicity of free heme.
-
Enhance free radical scavenging:
VDH induces the expression of γ-GT and significantly increases intracellular glutathione in response to LPS-induced oxidative stress in astrocytes [85] and protects neurons from chemical toxicity [245].
-
Modulate the renin-angiotensin system:
VDH plays an important role in the regulation of renin biosynthesis and blood pressure homeostasis [143]. It also functions as an endocrine suppressor of renin biosynthesis and genetic disruption of the VDR results in overstimulation of the renin-angiotensin system (RAS), leading to high blood pressure and cardiac hypertrophy [301].
-
Protect the axonal and cytoskeleton infrastructure:
VDH potentiates axon regeneration in a rat model of peripheral nerve injury [35]. Following nerve injury, treatment with vitamin D2 (100 IU/kg/day) significantly increased axogenesis and axon diameter, improved the response of sensory neurons to metabolites such as KCl and lactic acid, and induced a fast-to-slow fiber type transmission of the Tibialis anterior muscle.
It therefore seems clear that VDH not only shares many CNS repair mechanisms with PROG, but also adds to the mechanisms of action that compensate for missing mechanisms in PROG's arsenal.
Finally, in the context of aging and vitamin D deficiency, it makes sense to assume that whatever damage or exacerbation caused by D-deficiency can be at least partially overcome with supplementation to correct the deficiency. To this end, and since we are primarily interested in developing and improving treatment modalities, we recommend that treatment be combined with VDH to correct the potential loss of efficacy of PROG treatment in the D-deficient aged population. If this is effective, it could have significant implications for the treatment of elderly people with TBI.
5. Conclusion
Insults to the CNS, including TBI, induce neuroinflammatory and oxidative stress reactions, which then induce the secondary cascade of brain damage. As noted in this review, both PROG and VDH are pleiotropic hormones acting on several common, as well as on independent, CNS pathway mechanisms to reduce CNS damage and enhance CNS repair after TBI. Many studies now show that treatment with PROG significantly improves functional outcome after TBI in rats and humans [91, 246, 254]. PROG has been shown to reduce inflammatory responses [53, 115, 214] and oxidative stress. In addition PROG can activate protective pathways and increase the expression of genes and proteins associated with neuroprotection after brain damage. VDH has also been reported to be neuroprotective in a variety of in vitro and in vivo models including cortical infarction [291], zinc-induced neurotoxicity [162], EAE [87], LPS-induced oxidative stress [85] and Parkinson's disease [245, 287]. VDH has an immunomodulatory effect and regulates the differentiation, growth and function of a broad range of immune system cells [1]. A growing literature demonstrates that VDH restriction impairs a number of physiologic processes associated with healthy CNS functions such as mitosis, mitogenesis, neurite outgrowth, possibly adult neurogenesis in hippocampal cells, and mitochondrial dysfunction [4]. Treatment with VDH induces the expression of NGF, GDNF, pro-apoptotic proteins [142] and upregulation of OH-1 and reduction in GFAP immunoreactivity in injured brain [209]. Given the wide spectrum of action by the two hormones it is likely that a combination of the two, operating through unique and slightly different but compatible molecular mechanisms, might be synergistic in reducing the cytotoxic events associated with the injury cascade and increasing the neuroprotective events related to anti-apoptotic signaling and brain repair.
Supplementary Material
Figure 1. Progesterone.
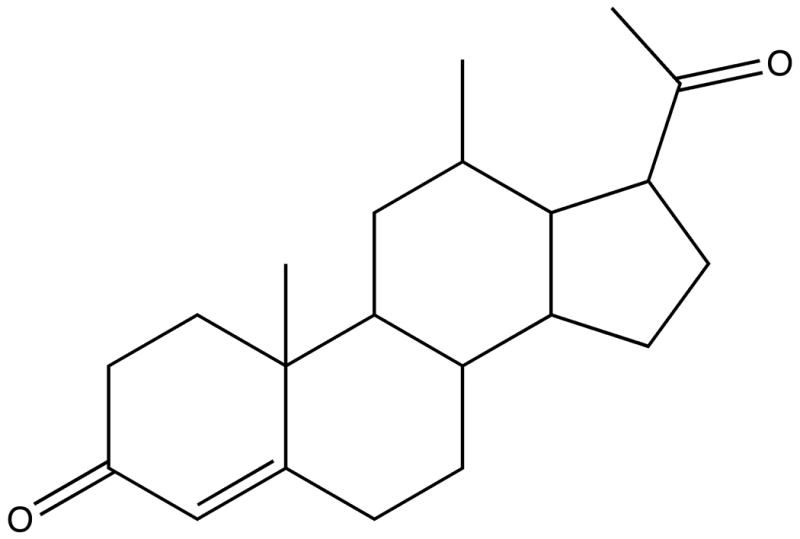
The chemical structure of progesterone.
Figure 2. Brain injury processes affected by PROG and VDH.

Both PROG and VDH are pleiotropic and affect multiple pathways, which may account for their therapeutic effectiveness. Here we show a few of the major pathways involved in injury and discussed in this paper, with the general scheme of blue as beneficial or protective and red as detrimental. 1. Inflammatory pathways consisting of immune cell recruitment and infiltration (macrophages; MΦ), microglial activation and inflammatory cytokine release (TNFα and IL-1), and naive T cell (TH0) differentiation into pro-inflammatory type 1 (TH1) and anti-inflammatory type 2 (TH2). These processes can lead to cell death, edema, and secondary damage; 2. Maintenance of blood-brain barrier (BBB) integrity, including modulation of the expression of channels and transporters such as P-glycoprotein (Pgp) and aquaporin 4 (AQP4) and antioxidant protection for both capillary endothelium and astrocytes. Failure of BBB function is a key component in the development of edema; 3. Glutamate excitotoxicity, mediated primarily by NMDA channels, can be toxic to the cell due to Na+ influx and severe depolarization. These effects can be counteracted by Cl- influx through GABAA channels, leading to repolarization; 4. The balance of cellular pro- and anti-death mechanisms, including release of pro-apoptotic mitochondrial (Bax, BAD, cytochrome c) and anti-apoptotic (Bcl-2) proteins, caspase-3 activation, maintenance of ionic and energy balance, as well as reduction of Ca2+ influx, which is the final common pathway of most mechanisms of cell death including glutamate toxicity. Since the activation of cellular reproductive machinery in terminally differentiated neurons can also lead to apoptosis, arrest of the cell cycle can also be protective; 5. Upregulation of trophic factors, especially NGF and BDNF, which contribute not only to the maintenance of neurons and astrocytes, but also oligodendrocytes and myelination; 6. Antioxidant defenses, which reduce the damage of immune and endogenously released reactive oxygen species (ROS) to cellular components and membranes. L-VSCC: L-type voltage-sensitive Ca2+ channel; Na+,K+-ATPase: Na+/K+ active transport pump.
Figure 3. Metabolism of VDH.

The metabolism of vitamin D and its conversion from 7-dehydrocholesterol to vitamin D hormone (VDH). 25OHD3: 25-hydroxyvitamin D3; UVB: ultraviolet B solar radiation; CYP2R1: vitamin D 25-hydroxylase; 1α-OHase: 25-hydroxyvitamin D3 1α-hydroxylase.
Table 1.
Neuroprotective mechanisms of PROG and VDH.
| MECHANISM | PROGESTERONE | VITAMIN D |
|---|---|---|
| NEURONAL APOPTOSIS | ↓ cytochrome c [239] | ↓ cytochrome c [162] |
| ↓ bad, bax [3, 63, 83, 205, 298, 305] | ↓ cell cycle (neurons) [9, 109, 136, 310] | |
| ↓ caspase-3 [3, 63, 83, 205, 298, 305] | ||
| ↑ bcl-2 [3, 63, 83, 205, 298, 305] | □ n-myc, c-myc [283] | |
| □ mitochondrial function [56] | ||
| TROPHIC FACTORS | ↑ NGF [213, 272] | ↑↑ NGF [23, 51, 202, 237, 238, 297] |
| ↑ BDNF [95, 96, 241] | ↑ GDNF [199, 291], NT-4 [201], TGFβ [283] | |
| □ IGFBPs [177] | ||
| INFLAMMATION | ↓ GFAP [63, 107] | ↓ GFAP [209, 253] |
| ↓ TNFα, IL-1 [66, 114, 190, 221] | ↓ TNFα, IL-1 [48, 126, 155, 168, 172, 268, 311] | |
| ↓ NFκB [221] | ↓ NFκB [54] | |
| TH2 > TH1 [178] | TH2 ≫ TH1 [30, 126, 178, 179, 273] (↑ IL-4, ↓ IL-12, IFNγ) |
|
| ↓ complement (C3, C5) [221, 280] | □ antigen-presenting cells [277] | |
| □ coagulation [281] | □ immune proliferation [126] | |
| OXIDATIVE STRESS | ↓ lipid peroxidation [191, 235, 244] | ↓ lipid peroxidation [161, 162] |
| ↓ iNOS, NO, nitrites [66] | ↓ iNOS, NO, nitrites [84, 86, 88, 155] | |
| ↓ immune ROS [53] | ↓ immune ROS [133] | |
| ↓ toxicity [26, 98, 205, 210] (Fe, MPTP, β-amyloid) |
↓ toxicity [41, 162, 245, 287] (Fe, Zn, 6-OH dopamine) |
|
| ↑ glutathione [21] | ↑ glutathione [85] | |
| ↓ MnSOD [21] | ↑ HO-1 [207] | |
| ↑ SOD [191] | ↑ γ-GT [85] | |
| EXCITOTOXICITY/Ca2+ | ↑ GABAA [13] | ↓ L-VSCCs [20] |
| □ σ1 receptor [15] | ↑ Ca2+ buffering [147, 304] (calbindin, parvalbumin) |
|
| MYELIN/AXONS | □ MBP [148, 150] | ↑ axogenesis [34, 35] |
| ↑ myelination [12, 150] (oligodendrocytes/CNS) |
↑ axon diameter [34, 35] | |
| ↑ remyelination [150, 243] (Schwann cells/PNS) | ||
| OTHER | □ AQP4 [107] | □ Renin-angiotensin [143, 227] |
| □ Pgp (BBB function) [53] | ||
| □ ChAT (NBM) [63, 114, 149] | ||
| □ Na+,K+-ATPase [82, 149, 231] | ||
| ↓ Edema [114] | ||
Notes: Identical mechanisms are identified by gray shading, while divergent mechanisms are white. In cases of a stronger response with reference to one mechanism, a double indicator is used (↑↑ versus ↑). ↑ = increases, ↓ = decreases, > = greater than skew or bias, □ = modulates. Superscript numbers in brackets indicate the references for each effect.
Acknowledgments
The authors would like to thank Leslie McCann for invaluable editorial assistance. This research was supported by funding from NIH grants #1RO1N540825 and #1RO1N538664 and the Emory University Graduate School of Arts and Sciences.
Footnotes
Disclosure statement: The last author (D.G.S.) is entitled to royalty payment from BHR Pharmaceuticals related to research on progesterone and brain injury. His future financial interests may be affected by the outcome of this research. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adorini L. Immunomodulatory effects of vitamin D receptor ligands in autoimmune diseases. Int Immunopharmacol. 2002;2:1017–1028. doi: 10.1016/s1567-5769(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 2.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404–412. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- 3.Alkayed NJ, Goto S, Sugo N, Joh HD, Klaus J, Crain BJ, Bernard O, Traystman RJ, Hurn PD. Estrogen and Bcl-2: gene induction and effect of transgene in experimental stroke. J Neurosci. 2001;21:7543–7550. doi: 10.1523/JNEUROSCI.21-19-07543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeras L, Eyles D, Benech P, Laffite D, Villard C, Patatian A, Boucraut J, Mackay-Sim A, McGrath J, Feron F. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics. 2007;7:769–780. doi: 10.1002/pmic.200600392. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 6.Attella MJ, Nattinville A, Stein DG. Hormonal state affects recovery from frontal cortex lesions in adult female rats. Behav Neural Biol. 1987;48:352–367. doi: 10.1016/s0163-1047(87)90918-6. [DOI] [PubMed] [Google Scholar]
- 7.Baas D, Prufer K, Ittel ME, Kuchler-Bopp S, Labourdette G, Sarlieve LL, Brachet P. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3) Glia. 2000;31:59–68. doi: 10.1002/(sici)1098-1136(200007)31:1<59::aid-glia60>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Bach-y-Rita P. Theoretical and practical considerations in the restoration of function after stroke. Top Stroke Rehabil. 2001;8:1–15. doi: 10.1310/8T1T-ETXU-8PDF-9X7F. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee P, Chatterjee M. Antiproliferative role of vitamin D and its analogs--a brief overview. Mol Cell Biochem. 2003;253:247–254. doi: 10.1023/a:1026072118217. [DOI] [PubMed] [Google Scholar]
- 10.Barrera D, Avila E, Hernandez G, Halhali A, Biruete B, Larrea F, Diaz L. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J Steroid Biochem Mol Biol. 2007;103:529–532. doi: 10.1016/j.jsbmb.2006.12.097. [DOI] [PubMed] [Google Scholar]
- 11.Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66:413–419. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 12.Baulieu EE, Robel P, Schumacher M. Neurosteroids: beginning of the story. Int Rev Neurobiol. 2001;46:1–32. doi: 10.1016/s0074-7742(01)46057-0. [DOI] [PubMed] [Google Scholar]
- 13.Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- 14.Beno DW, Brady LM, Bissonnette M, Davis BH. Protein kinase C and mitogen-activated protein kinase are required for 1,25-dihydroxyvitamin D3-stimulated Egr induction. J Biol Chem. 1995;270:3642–3647. doi: 10.1074/jbc.270.8.3642. [DOI] [PubMed] [Google Scholar]
- 15.Bergeron R, de Montigny C, Debonnel G. Pregnancy reduces brain sigma receptor function. Br J Pharmacol. 1999;127:1769–1776. doi: 10.1038/sj.bjp.0702724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourdeau A, Atmani F, Grosse B, Lieberherr M. Rapid effects of 1,25-dihydroxyvitamin D3 and extracellular Ca2+ on phospholipid metabolism in dispersed porcine parathyroid cells. Endocrinology. 1990;127:2738–2743. doi: 10.1210/endo-127-6-2738. [DOI] [PubMed] [Google Scholar]
- 18.Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc. 2008;56:454–461. doi: 10.1111/j.1532-5415.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 19.Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- 20.Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21:98–108. doi: 10.1523/JNEUROSCI.21-01-00098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown AJ, Dusso A, Slatopolsky E. Vitamin D. Am J Physiol. 1999;277:F157–175. doi: 10.1152/ajprenal.1999.277.2.F157. [DOI] [PubMed] [Google Scholar]
- 23.Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343:139–143. doi: 10.1016/s0304-3940(03)00303-3. [DOI] [PubMed] [Google Scholar]
- 24.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med. 2008;29:415–422. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calabrese EJ, Baldwin LA. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001;22:285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- 26.Callier S, Morissette M, Grandbois M, Pelaprat D, Di Paolo T. Neuroprotective properties of 17beta-estradiol, progesterone, and raloxifene in MPTP C57Bl/6 mice. Synapse. 2001;41:131–138. doi: 10.1002/syn.1067. [DOI] [PubMed] [Google Scholar]
- 27.Cantorna MT. Vitamin D and multiple sclerosis: an update. Nutr Rev. 2008;66:S135–138. doi: 10.1111/j.1753-4887.2008.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229:1136–1142. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 30.Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol. 1998;160:5314–5319. [PubMed] [Google Scholar]
- 31.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–1720S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 32.Cernak I, Stoica B, Byrnes KR, Di Giovanni S, Faden AI. Role of the cell cycle in the pathobiology of central nervous system trauma. Cell Cycle. 2005;4:1286–1293. doi: 10.4161/cc.4.9.1996. [DOI] [PubMed] [Google Scholar]
- 33.Cervantes M, Gonzalez-Vidal MD, Ruelas R, Escobar A, Morali G. Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch Med Res. 2002;33:6–14. doi: 10.1016/s0188-4409(01)00347-2. [DOI] [PubMed] [Google Scholar]
- 34.Chabas JF, Alluin O, Rao G, Garcia S, Lavaut MN, Risso JJ, Legre R, Magalon G, Khrestchatisky M, Marqueste T, Decherchi P, Feron F. Vitamin D2 potentiates axon regeneration. J Neurotrauma. 2008;25:1247–1256. doi: 10.1089/neu.2008.0593. [DOI] [PubMed] [Google Scholar]
- 35.Chabas JF, Alluin O, Rao G, Garcia S, Lavaut MN, Risso JJ, Legre R, Magalon G, Khrestchatisky M, Marqueste T, Decherchi P, Feron F. Vitamin D(2) Potentiates Axon Regeneration. J Neurotrauma. 2008;25:1247–1256. doi: 10.1089/neu.2008.0593. [DOI] [PubMed] [Google Scholar]
- 36.Chan CC. Inflammation: beneficial or detrimental after spinal cord injury? Recent Patents CNS Drug Discov. 2008;3:189–199. doi: 10.2174/157488908786242434. [DOI] [PubMed] [Google Scholar]
- 37.Charlier TD, Ball GF, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. J Neurosci. 2005;25:906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatfield SM, Brand C, Ebeling PR, Russell DM. Vitamin D deficiency in general medical inpatients in summer and winter. Intern Med J. 2007;37:377–382. doi: 10.1111/j.1445-5994.2007.01339.x. [DOI] [PubMed] [Google Scholar]
- 39.Chatterjee M. Vitamin D and genomic stability. Mutat Res. 2001;475:69–87. doi: 10.1016/s0027-5107(01)00080-x. [DOI] [PubMed] [Google Scholar]
- 40.Chen G, Shi J, Ding Y, Yin H, Hang C. Progesterone prevents traumatic brain injury-induced intestinal nuclear factor kappa B activation and proinflammatory cytokines expression in male rats. Mediators Inflamm. 2007;2007:93431. doi: 10.1155/2007/93431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen KB, Lin AM, Chiu TH. Systemic vitamin D3 attenuated oxidative injuries in the locus coeruleus of rat brain. Ann N Y Acad Sci. 2003;993:313–324. doi: 10.1111/j.1749-6632.2003.tb07539.x. discussion 345-319. [DOI] [PubMed] [Google Scholar]
- 42.Choi KC, Jeung EB. Molecular mechanism of regulation of the calcium-binding protein calbindin-D(9k), and its physiological role(s) in mammals: a review of current research. J Cell Mol Med. 2008;12:409–420. doi: 10.1111/j.1582-4934.2007.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- 44.Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J Neuroendocrinol. 2004;16:58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- 45.Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol. 2006;66:916–928. doi: 10.1002/neu.20293. [DOI] [PubMed] [Google Scholar]
- 46.Clancy AN, Bonsall RW, Michael RP. Immunohistochemical labeling of androgen receptors in the brain of rat and monkey. Life Sci. 1992;50:409–417. doi: 10.1016/0024-3205(92)90375-y. [DOI] [PubMed] [Google Scholar]
- 47.Clemens TL, Garrett KP, Zhou XY, Pike JW, Haussler MR, Dempster DW. Immunocytochemical localization of the 1,25-dihydroxyvitamin D3 receptor in target cells. Endocrinology. 1988;122:1224–1230. doi: 10.1210/endo-122-4-1224. [DOI] [PubMed] [Google Scholar]
- 48.Cohen-Lahav M, Douvdevani A, Chaimovitz C, Shany S. The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. J Steroid Biochem Mol Biol. 2007;103:558–562. doi: 10.1016/j.jsbmb.2006.12.093. [DOI] [PubMed] [Google Scholar]
- 49.Conolly RB, Lutz WK. Nonmonotonic dose-response relationships: mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicol Sci. 2004;77:151–157. doi: 10.1093/toxsci/kfh007. [DOI] [PubMed] [Google Scholar]
- 50.Corino A, D'Amelio P, Gancia R, Del Rizzo P, Gabasio S, Limone P, Isaia G. Hypovitaminosis D in internal medicine inpatients. Calcif Tissue Int. 2007;80:76–80. doi: 10.1007/s00223-006-0189-x. [DOI] [PubMed] [Google Scholar]
- 51.Cornet A, Baudet C, Neveu I, Baron-Van Evercooren A, Brachet P, Naveilhan P. 1,25-Dihydroxyvitamin D3 regulates the expression of VDR and NGF gene in Schwann cells in vitro. J Neurosci Res. 1998;53:742–746. doi: 10.1002/(SICI)1097-4547(19980915)53:6<742::AID-JNR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 52.Coughlan T, Gibson C, Murphy S. Modulatory effects of progesterone on inducible nitric oxide synthase expression in vivo and in vitro. J Neurochem. 2005;93:932–942. doi: 10.1111/j.1471-4159.2005.03068.x. [DOI] [PubMed] [Google Scholar]
- 53.Cutler SM, Cekic M, Miller DM, Wali B, VanLandingham JW, Stein DG. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J Neurotrauma. 2007;24:1475–1486. doi: 10.1089/neu.2007.0294. [DOI] [PubMed] [Google Scholar]
- 54.D'Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, Panina-Bordignon P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dawson-Hughes B. Serum 25-hydroxyvitamin D and functional outcomes in the elderly. Am J Clin Nutr. 2008;88:537S–540S. doi: 10.1093/ajcn/88.2.537S. [DOI] [PubMed] [Google Scholar]
- 56.De Nicola AF, Labombarda F, Gonzalez SL, Gonzalez Deniselle MC, Guennoun R, Schumacher M. Steroid effects on glial cells: detrimental or protective for spinal cord function? Ann N Y Acad Sci. 2003;1007:317–328. doi: 10.1196/annals.1286.030. [DOI] [PubMed] [Google Scholar]
- 57.de Viragh PA, Haglid KG, Celio MR. Parvalbumin increases in the caudate putamen of rats with vitamin D hypervitaminosis. Proc Natl Acad Sci U S A. 1989;86:3887–3890. doi: 10.1073/pnas.86.10.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 59.DeLuca HF, Zierold C. Mechanisms and functions of vitamin D. Nutr Rev. 1998;56:S4–10. doi: 10.1111/j.1753-4887.1998.tb01686.x. discussion S 54-75. [DOI] [PubMed] [Google Scholar]
- 60.Desmedt M, Rottiers P, Dooms H, Fiers W, Grooten J. Macrophages induce cellular immunity by activating Th1 cell responses and suppressing Th2 cell responses. J Immunol. 1998;160:5300–5308. [PubMed] [Google Scholar]
- 61.Di Giovanni S, Knoblach SM, Brandoli C, Aden SA, Hoffman EP, Faden AI. Gene profiling in spinal cord injury shows role of cell cycle in neuronal death. Ann Neurol. 2003;53:454–468. doi: 10.1002/ana.10472. [DOI] [PubMed] [Google Scholar]
- 62.Di Giovanni S, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B, Faden AI. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci U S A. 2005;102:8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- 64.Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 65.Doppenberg EM, Choi SC, Bullock R. Clinical trials in traumatic brain injury: lessons for the future. J Neurosurg Anesthesiol. 2004;16:87–94. doi: 10.1097/00008506-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 66.Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111:77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- 67.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 68.Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- 69.Eelen G, Verlinden L, Van Camp M, Mathieu C, Carmeliet G, Bouillon R, Verstuyf A. Microarray analysis of 1alpha,25-dihydroxyvitamin D3-treated MC3T3-E1 cells. J Steroid Biochem Mol Biol. 2004;89-90:405–407. doi: 10.1016/j.jsbmb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Eelen G, Verlinden L, van Camp M, van Hummelen P, Marchal K, de Moor B, Mathieu C, Carmeliet G, Bouillon R, Verstuyf A. The effects of 1alpha,25-dihydroxyvitamin D3 on the expression of DNA replication genes. J Bone Miner Res. 2004;19:133–146. doi: 10.1359/JBMR.0301204. [DOI] [PubMed] [Google Scholar]
- 71.Equils O, Naiki Y, Shapiro AM, Michelsen K, Lu D, Adams J, Jordan S. 1,25-Dihydroxyvitamin D inhibits lipopolysaccharide-induced immune activation in human endothelial cells. Clin Exp Immunol. 2006;143:58–64. doi: 10.1111/j.1365-2249.2005.02961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008;65:1348–1352. doi: 10.1001/archneur.65.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience. 2003;118:641–653. doi: 10.1016/s0306-4522(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 74.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Fabian E, Elmadfa I. Nutritional situation of the elderly in the European Union: data of the European Nutrition and Health Report (2004) Ann Nutr Metab. 2008;52 1:57–61. doi: 10.1159/000115352. [DOI] [PubMed] [Google Scholar]
- 76.Faden AI. Neuroprotection and traumatic brain injury: the search continues. Arch Neurol. 2001;58:1553–1555. doi: 10.1001/archneur.58.10.1553. [DOI] [PubMed] [Google Scholar]
- 77.Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- 78.Freeland K, Boxer LM, Latchman DS. The cyclic AMP response element in the Bcl-2 promoter confers inducibility by hypoxia in neuronal cells. Brain Res Mol Brain Res. 2001;92:98–106. doi: 10.1016/s0169-328x(01)00158-9. [DOI] [PubMed] [Google Scholar]
- 79.Freeman RS, Estus S, Johnson EM., Jr Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of Cyclin D1 during programmed cell death. Neuron. 1994;12:343–355. doi: 10.1016/0896-6273(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 80.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuxe K, Cintra A, Agnati LF, Harfstrand A, Wikstrom AC, Okret S, Zoli M, Miller LS, Greene JL, Gustafsson JA. Studies on the cellular localization and distribution of glucocorticoid receptor and estrogen receptor immunoreactivity in the central nervous system of the rat and their relationship to the monoaminergic and peptidergic neurons of the brain. J Steroid Biochem. 1987;27:159–170. doi: 10.1016/0022-4731(87)90306-2. [DOI] [PubMed] [Google Scholar]
- 82.Garay L, Gonzalez Deniselle MC, Gierman L, Meyer M, Lima A, Roig P, De Nicola AF. Steroid protection in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neuroimmunomodulation. 2008;15:76–83. doi: 10.1159/000135627. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Segura LM, Cardona-Gomez P, Naftolin F, Chowen JA. Estradiol upregulates Bcl-2 expression in adult brain neurons. Neuroreport. 1998;9:593–597. doi: 10.1097/00001756-199803090-00006. [DOI] [PubMed] [Google Scholar]
- 84.Garcion E, Nataf S, Berod A, Darcy F, Brachet P. 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997;45:255–267. doi: 10.1016/s0169-328x(96)00260-4. [DOI] [PubMed] [Google Scholar]
- 85.Garcion E, Sindji L, Leblondel G, Brachet P, Darcy F. 1,25-dihydroxyvitamin D3 regulates the synthesis of gamma-glutamyl transpeptidase and glutathione levels in rat primary astrocytes. J Neurochem. 1999;73:859–866. doi: 10.1046/j.1471-4159.1999.0730859.x. [DOI] [PubMed] [Google Scholar]
- 86.Garcion E, Sindji L, Montero-Menei C, Andre C, Brachet P, Darcy F. Expression of inducible nitric oxide synthase during rat brain inflammation: regulation by 1,25-dihydroxyvitamin D3. Glia. 1998;22:282–294. [PubMed] [Google Scholar]
- 87.Garcion E, Sindji L, Nataf S, Brachet P, Darcy F, Montero-Menei CN. Treatment of experimental autoimmune encephalomyelitis in rat by 1,25-dihydroxyvitamin D3 leads to early effects within the central nervous system. Acta Neuropathol. 2003;105:438–448. doi: 10.1007/s00401-002-0663-0. [DOI] [PubMed] [Google Scholar]
- 88.Garcion E, Sindji L, Nataf S, Brachet P, Darcy F, Montero-Menei CN. Treatment of experimental autoimmune encephalomyelitis in rat by 1,25-dihydroxyvitamin D3 leads to early effects within the central nervous system. Acta Neuropathol (Berl) 2003;105:438–448. doi: 10.1007/s00401-002-0663-0. [DOI] [PubMed] [Google Scholar]
- 89.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 90.Gaugris S, Heaney RP, Boonen S, Kurth H, Bentkover JD, Sen SS. Vitamin D inadequacy among post-menopausal women: a systematic review. Qjm. 2005;98:667–676. doi: 10.1093/qjmed/hci096. [DOI] [PubMed] [Google Scholar]
- 91.Gibson CL, Gray LJ, Bath PM, Murphy SP. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131:318–328. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- 92.Gingrich MB, Traynelis SF. Serine proteases and brain damage - is there a link? Trends Neurosci. 2000;23:399–407. doi: 10.1016/s0166-2236(00)01617-9. [DOI] [PubMed] [Google Scholar]
- 93.Giulietti A, Gysemans C, Stoffels K, van Etten E, Decallonne B, Overbergh L, Bouillon R, Mathieu C. Vitamin D deficiency in early life accelerates Type 1 diabetes in non-obese diabetic mice. Diabetologia. 2004;47:451–462. doi: 10.1007/s00125-004-1329-3. [DOI] [PubMed] [Google Scholar]
- 94.Glaser SD, Veenstra TD, Jirikowski GF, Prufer K. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat olfactory system. Cell Mol Neurobiol. 1999;19:613–624. doi: 10.1023/A:1006932418220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gonzalez SL, Labombarda F, Deniselle MC, Mougel A, Guennoun R, Schumacher M, De Nicola AF. Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J Steroid Biochem Mol Biol. 2005;94:143–149. doi: 10.1016/j.jsbmb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 96.Gonzalez SL, Labombarda F, Gonzalez Deniselle MC, Guennoun R, Schumacher M, De Nicola AF. Progesterone up-regulates neuronal brain-derived neurotrophic factor expression in the injured spinal cord. Neuroscience. 2004;125:605–614. doi: 10.1016/j.neuroscience.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 97.Gonzalez-Vidal MD, Cervera-Gaviria M, Ruelas R, Escobar A, Morali G, Cervantes M. Progesterone: protective effects on the cat hippocampal neuronal damage due to acute global cerebral ischemia. Arch Med Res. 1998;29:117–124. [PubMed] [Google Scholar]
- 98.Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 99.Grant WB. Epidemiology of disease risks in relation to vitamin D insufficiency. Prog Biophys Mol Biol. 2006;92:65–79. doi: 10.1016/j.pbiomolbio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 100.Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 101.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–145. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 102.Grossman KJ, Stein DG. Does endogenous progesterone promote recovery of chronic sensorimotor deficits following contusion to the forelimb representation of the sensorimotor cortex? Behav Brain Res. 2000;116:141–148. doi: 10.1016/s0166-4328(00)00275-8. [DOI] [PubMed] [Google Scholar]
- 103.Guennoun R, Fiddes RJ, Gouezou M, Lombes M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Brain Res Mol Brain Res. 1995;30:287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- 104.Guennoun R, Meffre D, Labombarda F, Gonzalez SL, Deniselle MC, Stein DG, De Nicola AF, Schumacher M. The membrane-associated progesterone-binding protein 25-Dx: Expression, cellular localization and up-regulation after brain and spinal cord injuries. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 105.Guerra-Araiza C, Amorim MA, Pinto-Almazan R, Gonzalez-Arenas A, Campos MG, Garcia-Segura LM. Regulation of the phosphoinositide-3 kinase and mitogen-activated protein kinase signaling pathways by progesterone and its reduced metabolites in the rat brain. J Neurosci Res. 2009;87:470–481. doi: 10.1002/jnr.21848. [DOI] [PubMed] [Google Scholar]
- 106.Guillemant J, Guillemant S. Early rise in cyclic GMP after 1,25-dihydroxycholecalciferol administration in the chick intestinal mucosa. Biochem Biophys Res Commun. 1980;93:906–911. doi: 10.1016/0006-291x(80)91161-4. [DOI] [PubMed] [Google Scholar]
- 107.Guo Q, Sayeed I, Baronne LM, Hoffman SW, Guennoun R, Stein DG. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp Neurol. 2006;198:469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 108.Gupta R, Dixon KM, Deo SS, Holliday CJ, Slater M, Halliday GM, Reeve VE, Mason RS. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol. 2007;127:707–715. doi: 10.1038/sj.jid.5700597. [DOI] [PubMed] [Google Scholar]
- 109.Gurlek A, Pittelkow MR, Kumar R. Modulation of growth factor/cytokine synthesis and signaling by 1alpha,25-dihydroxyvitamin D(3): implications in cell growth and differentiation. Endocr Rev. 2002;23:763–786. doi: 10.1210/er.2001-0044. [DOI] [PubMed] [Google Scholar]
- 110.Hannah SS, Norman AW. 1 alpha,25(OH)2 vitamin D3-regulated expression of the eukaryotic genome. Nutr Rev. 1994;52:376–382. doi: 10.1111/j.1753-4887.1994.tb01368.x. [DOI] [PubMed] [Google Scholar]
- 111.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hayes CE, Cantorna MT, DeLuca HF. Vitamin D and multiple sclerosis. Proc Soc Exp Biol Med. 1997;216:21–27. doi: 10.3181/00379727-216-44153a. [DOI] [PubMed] [Google Scholar]
- 113.Hayes CE, Nashold FE, Spach KM, Pedersen LB. The immunological functions of the vitamin D endocrine system. Cell Mol Biol (Noisy-le-grand) 2003;49:277–300. [PubMed] [Google Scholar]
- 114.He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004;189:404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 115.He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004;22:19–31. [PubMed] [Google Scholar]
- 116.Hewison M, Zehnder D, Bland R, Stewart PM. 1alpha-Hydroxylase and the action of vitamin D. J Mol Endocrinol. 2000;25:141–148. doi: 10.1677/jme.0.0250141. [DOI] [PubMed] [Google Scholar]
- 117.Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res. 2003;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- 118.Holick MF. McCollum Award Lecture, 1994: vitamin D--new horizons for the 21st century. Am J Clin Nutr. 1994;60:619–630. doi: 10.1093/ajcn/60.4.619. [DOI] [PubMed] [Google Scholar]
- 119.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 120.Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135:2739S–2748S. doi: 10.1093/jn/135.11.2739S. [DOI] [PubMed] [Google Scholar]
- 121.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 122.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 123.Holick MF. Vitamin D: important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med J. 2005;98:1024–1027. doi: 10.1097/01.SMJ.0000140865.32054.DB. [DOI] [PubMed] [Google Scholar]
- 124.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 125.Ibi M, Sawada H, Nakanishi M, Kume T, Katsuki H, Kaneko S, Shimohama S, Akaike A. Protective effects of 1 alpha,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology. 2001;40:761–771. doi: 10.1016/s0028-3908(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 126.Imazeki I, Matsuzaki J, Tsuji K, Nishimura T. Immunomodulating effect of vitamin D3 derivatives on type-1 cellular immunity. Biomed Res. 2006;27:1–9. doi: 10.2220/biomedres.27.1. [DOI] [PubMed] [Google Scholar]
- 127.Jahnsen J, Falch JA, Mowinckel P, Aadland E. Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand J Gastroenterol. 2002;37:192–199. doi: 10.1080/003655202753416876. [DOI] [PubMed] [Google Scholar]
- 128.Jensen SS, Madsen MW, Lukas J, Binderup L, Bartek J. Inhibitory effects of 1alpha,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol Endocrinol. 2001;15:1370–1380. doi: 10.1210/mend.15.8.0673. [DOI] [PubMed] [Google Scholar]
- 129.Jian Y, Yan J, Wang H, Chen C, Sun M, Jiang J, Lu J, Yang Y, Gu J. Cyclin D3 interacts with vitamin D receptor and regulates its transcription activity. Biochem Biophys Res Commun. 2005;335:739–748. doi: 10.1016/j.bbrc.2005.07.141. [DOI] [PubMed] [Google Scholar]
- 130.Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735:101–107. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- 131.Johnson CS, Muindi JR, Hershberger PA, Trump DL. The antitumor efficacy of calcitriol: preclinical studies. Anticancer Res. 2006;26:2543–2549. [PubMed] [Google Scholar]
- 132.Johnson EO, Skopouli FN, Moutsopoulos HM. Neuroendocrine manifestations in Sjogren's syndrome. Rheum Dis Clin North Am. 2000;26:927–949. doi: 10.1016/s0889-857x(05)70177-0. [DOI] [PubMed] [Google Scholar]
- 133.Jung WJ, Sung MK. Effects of major dietary antioxidants on inflammatory markers of RAW 264.7 macrophages. Biofactors. 2004;21:113–117. doi: 10.1002/biof.552210122. [DOI] [PubMed] [Google Scholar]
- 134.Kalueff AV, Lou YR, Laaksi I, Tuohimaa P. Increased anxiety in mice lacking vitamin D receptor gene. Neuroreport. 2004;15:1271–1274. doi: 10.1097/01.wnr.0000129370.04248.92. [DOI] [PubMed] [Google Scholar]
- 135.Kalueff AV, Minasyan A, Tuohimaa P. Anticonvulsant effects of 1,25-dihydroxyvitamin D in chemically induced seizures in mice. Brain Res Bull. 2005;67:156–160. doi: 10.1016/j.brainresbull.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 136.Kalueff AV, Tuohimaa P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr Opin Clin Nutr Metab Care. 2007;10:12–19. doi: 10.1097/MCO.0b013e328010ca18. [DOI] [PubMed] [Google Scholar]
- 137.Kawata M. Roles of steroid hormones and their receptors in structural organization in the nervous system. Neurosci Res. 1995;24:1–46. doi: 10.1016/0168-0102(96)81278-8. [DOI] [PubMed] [Google Scholar]
- 138.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 139.Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110–117. doi: 10.1007/s11926-008-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 141.Kimball SM, Ursell MR, O'Connor P, Vieth R. Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr. 2007;86:645–651. doi: 10.1093/ajcn/86.3.645. [DOI] [PubMed] [Google Scholar]
- 142.Kiraly SJ, Kiraly MA, Hawe RD, Makhani N. Vitamin D as a neuroactive substance: review. Scientific World Journal. 2006;6:125–139. doi: 10.1100/tsw.2006.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kong J, Li YC. Effect of ANG II type I receptor antagonist and ACE inhibitor on vitamin D receptor-null mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R255–261. doi: 10.1152/ajpregu.00517.2002. [DOI] [PubMed] [Google Scholar]
- 144.Krasowski MD, Yasuda K, Hagey LR, Schuetz EG. Evolutionary selection across the nuclear hormone receptor superfamily with a focus on the NR1I subfamily (vitamin D, pregnane X, and constitutive androstane receptors) Nucl Recept. 2005;3:2. doi: 10.1186/1478-1336-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kuan CY, Schloemer AJ, Lu A, Burns KA, Weng WL, Williams MT, Strauss KI, Vorhees CV, Flavell RA, Davis RJ, Sharp FR, Rakic P. Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. J Neurosci. 2004;24:10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kumon Y, Kim SC, Tompkins P, Stevens A, Sakaki S, Loftus CM. Neuroprotective effect of postischemic administration of progesterone in spontaneously hypertensive rats with focal cerebral ischemia. J Neurosurg. 2000;92:848–852. doi: 10.3171/jns.2000.92.5.0848. [DOI] [PubMed] [Google Scholar]
- 147.Kutuzova GD, Deluca HF. Gene expression profiles in rat intestine identify pathways for 1,25-dihydroxyvitamin D(3) stimulated calcium absorption and clarify its immunomodulatory properties. Arch Biochem Biophys. 2004;432:152–166. doi: 10.1016/j.abb.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Labombarda F, Gonzalez S, Gonzalez Deniselle MC, Garay L, Guennoun R, Schumacher M, De Nicola AF. Progesterone increases the expression of myelin basic protein and the number of cells showing NG2 immunostaining in the lesioned spinal cord. J Neurotrauma. 2006;23:181–192. doi: 10.1089/neu.2006.23.181. [DOI] [PubMed] [Google Scholar]
- 149.Labombarda F, Gonzalez SL, Gonzalez DM, Guennoun R, Schumacher M, de Nicola AF. Cellular basis for progesterone neuroprotection in the injured spinal cord. J Neurotrauma. 2002;19:343–355. doi: 10.1089/089771502753594918. [DOI] [PubMed] [Google Scholar]
- 150.Labombarda F, Gonzalez SL, Lima A, Roig P, Guennoun R, Schumacher M, de Nicola AF. Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia. 2008 doi: 10.1002/glia.20814. [DOI] [PubMed] [Google Scholar]
- 151.Langmade SJ, Gale SE, Frolov A, Mohri I, Suzuki K, Mellon SH, Walkley SU, Covey DF, Schaffer JE, Ory DS. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc Natl Acad Sci U S A. 2006;103:13807–13812. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Langub MC, Herman JP, Malluche HH, Koszewski NJ. Evidence of functional vitamin D receptors in rat hippocampus. Neuroscience. 2001;104:49–56. doi: 10.1016/s0306-4522(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 153.Larrosa M, Gratacos J, Vaqueiro M, Prat M, Campos F, Roque M. Prevalence of hypovitaminosis D in elderly institutionalized residents: influence of a substitutive treatment. Med Clin (Barc) 2001;117:611–614. doi: 10.1016/s0025-7753(01)72195-1. [DOI] [PubMed] [Google Scholar]
- 154.Lee CC, Marill KA, Carter WA, Crupi RS. A current concept of trauma-induced multiorgan failure. Ann Emerg Med. 2001;38:170–176. doi: 10.1067/mem.2001.114313. [DOI] [PubMed] [Google Scholar]
- 155.Lefebvre d'Hellencourt C, Montero-Menei CN, Bernard R, Couez D. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J Neurosci Res. 2003;71:575–582. doi: 10.1002/jnr.10491. [DOI] [PubMed] [Google Scholar]
- 156.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87:1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Levin A. Kidneys, hearts, hormones and immunomodulators: integrated understandings. Blood Purif. 2006;24:46–50. doi: 10.1159/000089436. [DOI] [PubMed] [Google Scholar]
- 158.Li L, Andersen ME, Heber S, Zhang Q. Non-monotonic dose-response relationship in steroid hormone receptor-mediated gene expression. J Mol Endocrinol. 2007;38:569–585. doi: 10.1677/JME-07-0003. [DOI] [PubMed] [Google Scholar]
- 159.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lieberherr M. Effects of vitamin D3 metabolites on cytosolic free calcium in confluent mouse osteoblasts. J Biol Chem. 1987;262:13168–13173. doi: 10.1515/9783110846713.769. [DOI] [PubMed] [Google Scholar]
- 161.Lin AM, Chen KB, Chao PL. Antioxidative effect of vitamin D3 on zinc-induced oxidative stress in CNS. Ann N Y Acad Sci. 2005;1053:319–329. doi: 10.1196/annals.1344.028. [DOI] [PubMed] [Google Scholar]
- 162.Lin AM, Fan SF, Yang DM, Hsu LL, Yang CH. Zinc-induced apoptosis in substantia nigra of rat brain: neuroprotection by vitamin D3. Free Radic Biol Med. 2003;34:1416–1425. doi: 10.1016/s0891-5849(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 163.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 164.Lips P. Which circulating level of 25-hydroxyvitamin D is appropriate? J Steroid Biochem Mol Biol. 2004;89-90:611–614. doi: 10.1016/j.jsbmb.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 165.Losem-Heinrichs E, Gorg B, Redecker C, Schleicher A, Witte OW, Zilles K, Bidmon HJ. 1alpha,25-dihydroxy-vitamin D3 in combination with 17beta-estradiol lowers the cortical expression of heat shock protein-27 following experimentally induced focal cortical ischemia in rats. Arch Biochem Biophys. 2005;439:70–79. doi: 10.1016/j.abb.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 166.Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss J, Wilson RG, Colston KW. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005;41:1164–1169. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 167.Lucas PA, Roullet C, Duchambon P, Lacour B, Drueke T. Rapid stimulation of calcium uptake by isolated rat enterocytes by 1,25(OH)2D3. Pflugers Arch. 1989;413:407–413. doi: 10.1007/BF00584491. [DOI] [PubMed] [Google Scholar]
- 168.Lyakh LA, Sanford M, Chekol S, Young HA, Roberts AB. TGF-beta and vitamin D3 utilize distinct pathways to suppress IL-12 production and modulate rapid differentiation of human monocytes into CD83+ dendritic cells. J Immunol. 2005;174:2061–2070. doi: 10.4049/jimmunol.174.4.2061. [DOI] [PubMed] [Google Scholar]
- 169.MacFarlane GD, Sackrison JL, Jr, Body JJ, Ersfeld DL, Fenske JS, Miller AB. Hypovitaminosis D in a normal, apparently healthy urban European population. J Steroid Biochem Mol Biol. 2004;89-90:621–622. doi: 10.1016/j.jsbmb.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 170.Madrigal JL, Hurtado O, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, Leza JC. The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology. 2002;26:155–163. doi: 10.1016/S0893-133X(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 171.Magrassi L, Butti G, Silini E, Bono F, Paoletti P, Milanesi G. The expression of genes of the steroid-thyroid hormone receptor superfamily in central nervous system tumors. Anticancer Res. 1993;13:859–866. [PubMed] [Google Scholar]
- 172.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 173.Maines MD. The Heme Oxygenase System: A regulator of second messenger gases. Ann Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 174.Margulies S, Hicks R. Combination Therapies for Traumatic Brain Injury Workshop. Rockville, MD: 2008. Participants. [Google Scholar]
- 175.Markle-Reid M, Browne G. Conceptualizations of frailty in relation to older adults. J Adv Nurs. 2003;44:58–68. doi: 10.1046/j.1365-2648.2003.02767.x. [DOI] [PubMed] [Google Scholar]
- 176.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 177.Matilainen M, Malinen M, Saavalainen K, Carlberg C. Regulation of multiple insulin-like growth factor binding protein genes by 1alpha,25-dihydroxyvitamin D3. Nucleic Acids Res. 2005;33:5521–5532. doi: 10.1093/nar/gki872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Matsuzaki J, Tsuji T, Imazeki I, Ikeda H, Nishimura T. Immunosteroid as a regulator for Th1/Th2 balance: its possible role in autoimmune diseases. Autoimmunity. 2005;38:369–375. doi: 10.1080/08916930500124122. [DOI] [PubMed] [Google Scholar]
- 179.Mattner F, Smiroldo S, Galbiati F, Muller M, Di Lucia P, Poliani PL, Martino G, Panina-Bordignon P, Adorini L. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3) Eur J Immunol. 2000;30:498–508. doi: 10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 180.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 181.McCarty MF. Secondary hyperparathyroidism promotes the acute phase response -- a rationale for supplemental vitamin D in prevention of vascular events in the elderly. Med Hypotheses. 2005;64:1022–1026. doi: 10.1016/j.mehy.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 182.McCoubrey WK, Jr, Huang TJ, Maines MD. Heme oxygenase-2 is a hemoprotein and binds heme through heme regulatory motifs that are not involved in heme catalysis. J Biol Chem. 1997;272:12568–12574. doi: 10.1074/jbc.272.19.12568. [DOI] [PubMed] [Google Scholar]
- 183.McGrath JJ, Feron FP, Burne TH, Mackay-Sim A, Eyles DW. Vitamin D3-implications for brain development. J Steroid Biochem Mol Biol. 2004;89-90:557–560. doi: 10.1016/j.jsbmb.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 184.McPherson CA, Kubik J, Wine RN, D'Hellencourt CL, Harry GJ. Alterations in cyclin A, B, and D1 in mouse dentate gyrus following TMT-induced hippocampal damage. Neurotox Res. 2003;5:339–354. doi: 10.1007/BF03033154. [DOI] [PubMed] [Google Scholar]
- 185.Meffre D, Delespierre B, Gouezou M, Leclerc P, Vinson GP, Schumacher M, Stein DG, Guennoun R. The membrane-associated progesterone-binding protein 25-Dx is expressed in brain regions involved in water homeostasis and is up-regulated after traumatic brain injury. J Neurochem. 2005;93:1314–1326. doi: 10.1111/j.1471-4159.2005.03127.x. [DOI] [PubMed] [Google Scholar]
- 186.Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, Raggi P. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:1179–1185. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mellon SH, Griffin LD, Compagnone NA. Biosynthesis and action of neurosteroids. Brain Res Brain Res Rev. 2001;37:3–12. doi: 10.1016/s0165-0173(01)00109-6. [DOI] [PubMed] [Google Scholar]
- 188.Mensah-Nyagan AG, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Anatomical and biochemical evidence for the synthesis of unconjugated and sulfated neurosteroids in amphibians. Brain Res Brain Res Rev. 2001;37:13–24. doi: 10.1016/s0165-0173(01)00110-2. [DOI] [PubMed] [Google Scholar]
- 189.Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008;11:7–12. doi: 10.1097/MCO.0b013e3282f2f4dd. [DOI] [PubMed] [Google Scholar]
- 190.Miller L, Hunt JS. Regulation of TNF-alpha production in activated mouse macrophages by progesterone. J Immunol. 1998;160:5098–5104. [PubMed] [Google Scholar]
- 191.Moorthy K, Sharma D, Basir SF, Baquer NZ. Administration of estradiol and progesterone modulate the activities of antioxidant enzyme and aminotransferases in naturally menopausal rats. Exp Gerontol. 2005;40:295–302. doi: 10.1016/j.exger.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 192.Morelli S, de Boland AR, Boland RL. Generation of inositol phosphates, diacylglycerol and calcium fluxes in myoblasts treated with 1,25-dihydroxyvitamin D3. Biochem J. 1993;289(Pt 3):675–679. doi: 10.1042/bj2890675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 194.Mosenthal AC, Lavery RF, Addis M, Kaul S, Ross S, Marburger R, Deitch EA, Livingston DH. Isolated traumatic brain injury: age is an independent predictor of mortality and early outcome. J Trauma. 2002;52:907–911. doi: 10.1097/00005373-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 195.Munger KL, Zhang SM, O'Reilly E, Hernan MA, Olek MJ, Willett WC, Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 196.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, Coplin WM, Dietrich WD, Ghajar J, Grady SM, Grossman RG, Hall ED, Heetderks W, Hovda DA, Jallo J, Katz RL, Knoller N, Kochanek PM, Maas AI, Majde J, Marion DW, Marmarou A, Marshall LF, McIntosh TK, Miller E, Mohberg N, Muizelaar JP, Pitts LH, Quinn P, Riesenfeld G, Robertson CS, Strauss KI, Teasdale G, Temkin N, Tuma R, Wade C, Walker MD, Weinrich M, Whyte J, Wilberger J, Young AB, Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Narayanan R, Sepulveda VA, Falzon M, Weigel NL. The functional consequences of cross-talk between the vitamin D receptor and ERK signaling pathways are cell-specific. J Biol Chem. 2004;279:47298–47310. doi: 10.1074/jbc.M404101200. [DOI] [PubMed] [Google Scholar]
- 198.Nataf S, Garcion E, Darcy F, Chabannes D, Muller JY, Brachet P. 1,25 Dihydroxyvitamin D3 exerts regional effects in the central nervous system during experimental allergic encephalomyelitis. J Neuropathol Exp Neurol. 1996;55:904–914. doi: 10.1097/00005072-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 199.Naveilhan P, Neveu I, Wion D, Brachet P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport. 1996;7:2171–2175. doi: 10.1097/00001756-199609020-00023. [DOI] [PubMed] [Google Scholar]
- 200.Neve RL, McPhie DL. The cell cycle as a therapeutic target for Alzheimer's disease. Pharmacol Ther. 2006;111:99–113. doi: 10.1016/j.pharmthera.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 201.Neveu I, Naveilhan P, Baudet C, Brachet P, Metsis M. 1,25-dihydroxyvitamin D3 regulates NT-3, NT-4 but not BDNF mRNA in astrocytes. Neuroreport. 1994;6:124–126. doi: 10.1097/00001756-199412300-00032. [DOI] [PubMed] [Google Scholar]
- 202.Neveu I, Naveilhan P, Jehan F, Baudet C, Wion D, De Luca HF, Brachet P. 1,25-dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res Mol Brain Res. 1994;24:70–76. doi: 10.1016/0169-328x(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 203.Neveu I, Naveilhan P, Menaa C, Wion D, Brachet P, Garabedian M. Synthesis of 1,25-dihydroxyvitamin D3 by rat brain macrophages in vitro. J Neurosci Res. 1994;38:214–220. doi: 10.1002/jnr.490380212. [DOI] [PubMed] [Google Scholar]
- 204.Niino M, Fukazawa T, Kikuchi S, Sasaki H. Therapeutic potential of vitamin D for multiple sclerosis. Curr Med Chem. 2008;15:499–505. doi: 10.2174/092986708783503159. [DOI] [PubMed] [Google Scholar]
- 205.Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 206.Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103:204–205. doi: 10.1016/j.jsbmb.2006.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Norman AW, Henry HL. Vitamin D. In: Zempleni J, Rucker RB, McCormick DB, Suttie JW, editors. Handbook of Vitamins. CRC Press; Boca Raton, FL: 2007. pp. 41–110. [Google Scholar]
- 208.Norman PE, Powell JT. Vitamin D, shedding light on the development of disease in peripheral arteries. Arterioscler Thromb Vasc Biol. 2005;25:39–46. doi: 10.1161/01.ATV.0000148450.56697.4a. [DOI] [PubMed] [Google Scholar]
- 209.Oermann E, Bidmon HJ, Witte OW, Zilles K. Effects of 1alpha,25 dihydroxyvitamin D3 on the expression of HO-1 and GFAP in glial cells of the photothrombotically lesioned cerebral cortex. J Chem Neuroanat. 2004;28:225–238. doi: 10.1016/j.jchemneu.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 210.Ogata T, Nakamura Y, Tsuji K, Shibata T, Kataoka K. Steroid hormones protect spinal cord neurons from glutamate toxicity. Neuroscience. 1993;55:445–449. doi: 10.1016/0306-4522(93)90513-f. [DOI] [PubMed] [Google Scholar]
- 211.Onoe K, Yanagawa Y, Minami K, Iijima N, Iwabuchi K. Th1 or Th2 balance regulated by interaction between dendritic cells and NKT cells. Immunol Res. 2007;38:319–332. doi: 10.1007/s12026-007-0011-5. [DOI] [PubMed] [Google Scholar]
- 212.Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- 213.Oyesiku NM, Evans CO, Houston S, Darrell RS, Smith JS, Fulop ZL, Dixon CE, Stein DG. Regional changes in the expression of neurotrophic factors and their receptors following acute traumatic brain injury in the adult rat brain. Brain Res. 1999;833:161–172. doi: 10.1016/s0006-8993(99)01501-2. [DOI] [PubMed] [Google Scholar]
- 214.Pan DS, Liu WG, Yang XF, Cao F. Inhibitory effect of progesterone on inflammatory factors after experimental traumatic brain injury. Biomed Environ Sci. 2007;20:432–438. [PubMed] [Google Scholar]
- 215.Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, Grand RJ. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118:1950–1961. doi: 10.1542/peds.2006-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pappa HM, Grand RJ, Gordon CM. Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis. 2006;12:1162–1174. doi: 10.1097/01.mib.0000236929.74040.b0. [DOI] [PubMed] [Google Scholar]
- 217.Pascussi JM, Gerbal-Chaloin S, Drocourt L, Maurel P, Vilarem MJ. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta. 2003;1619:243–253. doi: 10.1016/s0304-4165(02)00483-x. [DOI] [PubMed] [Google Scholar]
- 218.Pascussi JM, Robert A, Nguyen M, Walrant-Debray O, Garabedian M, Martin P, Pineau T, Saric J, Navarro F, Maurel P, Vilarem MJ. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest. 2005;115:177–186. doi: 10.1172/JCI21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Peltier MR, Tee SC, Smulian JC. Effect of progesterone on proinflammatory cytokine production by monocytes stimulated with pathogens associated with preterm birth. Am J Reprod Immunol. 2008;60:346–353. doi: 10.1111/j.1600-0897.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- 220.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35:290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 221.Pettus EH, Wright DW, Stein DG, Hoffman SW. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049:112–119. doi: 10.1016/j.brainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 222.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13:187–194. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- 223.Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr. 2008;88:500S–506S. doi: 10.1093/ajcn/88.2.500S. [DOI] [PubMed] [Google Scholar]
- 224.Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16:135–145. doi: 10.1016/s0891-0618(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 225.Puder JJ, Freda PU, Goland RS, Ferin M, Wardlaw SL. Stimulatory effects of stress on gonadotropin secretion in estrogen-treated women. J Clin Endocrinol Metab. 2000;85:2184–2188. doi: 10.1210/jcem.85.6.6629. [DOI] [PubMed] [Google Scholar]
- 226.Rajakumar K, Fernstrom JD, Holick MF, Janosky JE, Greenspan SL. Vitamin D status and response to Vitamin D(3) in obese vs. non-obese African American children. Obesity (Silver Spring) 2008;16:90–95. doi: 10.1038/oby.2007.23. [DOI] [PubMed] [Google Scholar]
- 227.Rammos G, Tseke P, Ziakka S. Vitamin D, the renin-angiotensin system, and insulin resistance. Int Urol Nephrol. 2008 doi: 10.1007/s11255-007-9244-4. [DOI] [PubMed] [Google Scholar]
- 228.Reschly EJ, Krasowski MD. Evolution and function of the NR1I nuclear hormone receptor subfamily (VDR, PXR, and CAR) with respect to metabolism of xenobiotics and endogenous compounds. Curr Drug Metab. 2006;7:349–365. doi: 10.2174/138920006776873526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp Neurol. 2006;197:235–243. doi: 10.1016/j.expneurol.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 230.Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway) Cancer Causes Control. 2004;15:149–158. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 231.Roglio I, Bianchi R, Gotti S, Scurati S, Giatti S, Pesaresi M, Caruso D, Panzica GC, Melcangi RC. Neuroprotective effects of dihydroprogesterone and progesterone in an experimental model of nerve crush injury. Neuroscience. 2008;155:673–685. doi: 10.1016/j.neuroscience.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 232.Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129:64–69. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- 233.Roof RL, Duvdevani R, Heyburn JW, Stein DG. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp Neurol. 1996;138:246–251. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- 234.Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1993;607:333–336. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- 235.Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol. 1997;31:1–11. doi: 10.1007/BF02815156. [DOI] [PubMed] [Google Scholar]
- 236.Roof RL, Stein DG. Progesterone treatment attenuates brain edema following contusion injury in male and female rats. Restor Neurol Neurosc. 1992;4:425–427. doi: 10.3233/RNN-1992-4608. [DOI] [PubMed] [Google Scholar]
- 237.Samina Riaz S, Tomlinson DR. Pharmacological modulation of nerve growth factor synthesis: a mechanistic comparison of vitamin D receptor and beta(2)-adrenoceptor agonists. Brain Res Mol Brain Res. 2000;85:179–188. doi: 10.1016/s0169-328x(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 238.Saporito MS, Brown ER, Hartpence KC, Wilcox HM, Vaught JL, Carswell S. Chronic 1,25-dihydroxyvitamin D3-mediated induction of nerve growth factor mRNA and protein in L929 fibroblasts and in adult rat brain. Brain Res. 1994;633:189–196. doi: 10.1016/0006-8993(94)91539-3. [DOI] [PubMed] [Google Scholar]
- 239.Sayeed I, Parvez S, Wali B, Siemen D, Stein DG. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism for better neuroprotective effects of allopregnanolone over progesterone. Brain Research. doi: 10.1016/j.brainres.2009.01.045. In press. [DOI] [PubMed] [Google Scholar]
- 240.Sayeed I, Wali B, Stein DG. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor Neurol Neurosci. 2007;25:151–159. [PubMed] [Google Scholar]
- 241.Scharfman HE, Maclusky NJ. Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue? Trends Neurosci. 2005;28:79–85. doi: 10.1016/j.tins.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 242.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 243.Schumacher M, Guennoun R, Robert F, Carelli C, Gago N, Ghoumari A, Gonzalez Deniselle MC, Gonzalez SL, Ibanez C, Labombarda F, Coirini H, Baulieu EE, De Nicola AF. Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Horm IGF Res. 2004;14 A:S18–33. doi: 10.1016/j.ghir.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 244.Schumacher M, Guennoun R, Stein DG, De Nicola AF. Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacol Ther. 2007;116:77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 245.Shinpo K, Kikuchi S, Sasaki H, Moriwaka F, Tashiro K. Effect of 1,25-dihydroxyvitamin D(3) on cultured mesencephalic dopaminergic neurons to the combined toxicity caused by L-buthionine sulfoximine and 1-methyl-4-phenylpyridine. J Neurosci Res. 2000;62:374–382. doi: 10.1002/1097-4547(20001101)62:3<374::AID-JNR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 246.Singh M, Sumien N, Kyser C, Simpkins JW. Estrogens and progesterone as neuroprotectants: what animal models teach us. Front Biosci. 2008;13:1083–1089. doi: 10.2741/2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Skaper SD. The brain as a target for inflammatory processes and neuroprotective strategies. Ann N Y Acad Sci. 2007;1122:23–34. doi: 10.1196/annals.1403.002. [DOI] [PubMed] [Google Scholar]
- 248.Smolders J, Damoiseaux J, Menheere P, Hupperts R. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol. 2008;194:7–17. doi: 10.1016/j.jneuroim.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 249.Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler. 2008;14:1220–1224. doi: 10.1177/1352458508094399. [DOI] [PubMed] [Google Scholar]
- 250.Somjen D. Vitamin D modulation of the activity of estrogenic compounds in bone cells in vitro and in vivo. Crit Rev Eukaryot Gene Expr. 2007;17:115–147. doi: 10.1615/critreveukargeneexpr.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- 251.Somjen D, Katzburg S, Stern N, Kohen F, Sharon O, Limor R, Jaccard N, Hendel D, Weisman Y. 25 hydroxy-vitamin D(3)-1alpha hydroxylase expression and activity in cultured human osteoblasts and their modulation by parathyroid hormone, estrogenic compounds and dihydrotestosterone. J Steroid Biochem Mol Biol. 2007;107:238–244. doi: 10.1016/j.jsbmb.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 252.Song X, Bishop JE, Okamura WH, Norman AW. Stimulation of phosphorylation of mitogen-activated protein kinase by 1alpha,25-dihydroxyvitamin D3 in promyelocytic NB4 leukemia cells: a structure-function study. Endocrinology. 1998;139:457–465. doi: 10.1210/endo.139.2.5747. [DOI] [PubMed] [Google Scholar]
- 253.Spach KM, Pedersen LB, Nashold FE, Kayo T, Yandell BS, Prolla TA, Hayes CE. Gene expression analysis suggests that 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by stimulating inflammatory cell apoptosis. Physiol Genomics. 2004;18:141–151. doi: 10.1152/physiolgenomics.00003.2004. [DOI] [PubMed] [Google Scholar]
- 254.Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Research Reviews. 2008;57:386–397. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. 2008;57:386–397. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Stein DG, Glasier MM, Hoffman SW. Conceptual and practical issues in the pharmacological treatment of brain injury. J Neural Transplant Plast. 1993;4:227–237. doi: 10.1155/NP.1993.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Stein DG, Hoffman SW. Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr Rehabil. 2003;6:13–22. doi: 10.1080/1363849031000095279. [DOI] [PubMed] [Google Scholar]
- 258.Stein DG, Hurn PD. Effects of Sex Steroids on Damaged Neural Systems. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Elsevier; Oxford: In press. [Google Scholar]
- 259.Stein DG, Wright DW, Kellermann AL. Does progesterone have neuroprotective properties? Ann Emerg Med. 2008;51:164–172. doi: 10.1016/j.annemergmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 260.Stio M, Treves C, Martinesi M, Bonanomi AG. Biochemical effects of KH 1060 and anti-TNF monoclonal antibody on human peripheral blood mononuclear cells. Int Immunopharmacol. 2005;5:649–659. doi: 10.1016/j.intimp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 261.Stumpf WE, Clark SA, O'Brien LP, Reid FA. 1,25(OH)2 vitamin D3 sites of action in spinal cord and sensory ganglion. Anat Embryol (Berl) 1988;177(4):307–310. doi: 10.1007/BF00315837. [DOI] [PubMed] [Google Scholar]
- 262.Stumpf WE, O'Brien LP. 1,25 (OH)2 vitamin D3 sites of action in the brain. An autoradiographic study. Histochemistry. 1987;87:393–406. doi: 10.1007/BF00496810. [DOI] [PubMed] [Google Scholar]
- 263.Stumpf WE, Sar M, Clark SA, DeLuca HF. Brain target sites for 1,25-dihydroxyvitamin D3. Science. 1982;215:1403–1405. doi: 10.1126/science.6977846. [DOI] [PubMed] [Google Scholar]
- 264.Sugimoto T, Ritter C, Ried I, Morrissey J, Slatopolsky E. Effect of 1,25-dihydroxyvitamin D3 on cytosolic calcium in dispersed parathyroid cells. Kidney Int. 1988;33:850–854. doi: 10.1038/ki.1988.76. [DOI] [PubMed] [Google Scholar]
- 265.Sutton AL, MacDonald PN. Vitamin D: more than a “bone-a-fide” hormone. Mol Endocrinol. 2003;17:777–791. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 266.Sylvia VL, Schwartz Z, Ellis EB, Helm SH, Gomez R, Dean DD, Boyan BD. Nongenomic regulation of protein kinase C isoforms by the vitamin D metabolites 1 alpha,25-(OH)2D3 and 24R,25-(OH)2D3. J Cell Physiol. 1996;167:380–393. doi: 10.1002/(SICI)1097-4652(199606)167:3<380::AID-JCP2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 267.Tausk F, Elenkov I, Moynihan J. Psychoneuroimmunology. Dermatol Ther. 2008;21:22–31. doi: 10.1111/j.1529-8019.2008.00166.x. [DOI] [PubMed] [Google Scholar]
- 268.Thien R, Baier K, Pietschmann P, Peterlik M, Willheim M. Interactions of 1 alpha,25-dihydroxyvitamin D3 with IL-12 and IL-4 on cytokine expression of human T lymphocytes. J Allergy Clin Immunol. 2005;116:683–689. doi: 10.1016/j.jaci.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 269.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 270.Thummel KE, Brimer C, Yasuda K, Thottassery J, Senn T, Lin Y, Ishizuka H, Kharasch E, Schuetz J, Schuetz E. Transcriptional control of intestinal cytochrome P-4503A by 1alpha,25-dihydroxy vitamin D3. Mol Pharmacol. 2001;60:1399–1406. doi: 10.1124/mol.60.6.1399. [DOI] [PubMed] [Google Scholar]
- 271.Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 272.Tometten M, Blois S, Arck PC. Nerve growth factor in reproductive biology: link between the immune, endocrine and nervous system? Chem Immunol Allergy. 2005;89:135–148. doi: 10.1159/000087962. [DOI] [PubMed] [Google Scholar]
- 273.Topilski I, Flaishon L, Naveh Y, Harmelin A, Levo Y, Shachar I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur J Immunol. 2004;34:1068–1076. doi: 10.1002/eji.200324532. [DOI] [PubMed] [Google Scholar]
- 274.Topinkova E. Aging, disability and frailty. Ann Nutr Metab. 2008;52 1:6–11. doi: 10.1159/000115340. [DOI] [PubMed] [Google Scholar]
- 275.Townsend K, Evans KN, Campbell MJ, Colston KW, Adams JS, Hewison M. Biological actions of extra-renal 25-hydroxyvitamin D-1alpha-hydroxylase and implications for chemoprevention and treatment. J Steroid Biochem Mol Biol. 2005;97:103–109. doi: 10.1016/j.jsbmb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 276.Valera S, Ballivet M, Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1992;89:9949–9953. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 278.VanAmerongen BM, Dijkstra CD, Lips P, Polman CH. Multiple sclerosis and vitamin D: an update. Eur J Clin Nutr. 2004;58:1095–1109. doi: 10.1038/sj.ejcn.1601952. [DOI] [PubMed] [Google Scholar]
- 279.Vandromme M, Melton SM, Kerby JD. Progesterone in traumatic brain injury: time to move on to phase III trials. Crit Care. 2008;12:153. doi: 10.1186/cc6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.VanLandingham JW, Cekic M, Cutler S, Hoffman SW, Stein DG. Neurosteroids reduce inflammation after TBI through CD55 induction. Neurosci Lett. 2007;425:94–98. doi: 10.1016/j.neulet.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.VanLandingham JW, Cekic M, Cutler SM, Hoffman SW, Washington ER, Johnson SJ, Miller D, Stein DG. Progesterone and its metabolite allopregnanolone differentially regulate hemostatic proteins after traumatic brain injury. J Cereb Blood Flow Metab. 2008;28:1786–1794. doi: 10.1038/jcbfm.2008.73. [DOI] [PubMed] [Google Scholar]
- 282.Veenstra TD, Prufer K, Koenigsberger C, Brimijoin SW, Grande JP, Kumar R. 1,25-Dihydroxyvitamin D3 receptors in the central nervous system of the rat embryo. Brain Res. 1998;804:193–205. doi: 10.1016/s0006-8993(98)00565-4. [DOI] [PubMed] [Google Scholar]
- 283.Veenstra TD, Windebank AJ, Kumar R. 1,25-dihydroxyvitamin D3 regulates the expression of N-myc, c-myc, protein kinase C, and transforming growth factor-beta2 in neuroblastoma cells. Biochem Biophys Res Commun. 1997;235:15–18. doi: 10.1006/bbrc.1997.6718. [DOI] [PubMed] [Google Scholar]
- 284.Vesely DL, Juan D. Cation-dependent vitamin D activation of human renal cortical guanylate cyclase. Am J Physiol. 1984;246:E115–120. doi: 10.1152/ajpendo.1984.246.1.E115. [DOI] [PubMed] [Google Scholar]
- 285.Vieth R, Kimball S. Vitamin D in congestive heart failure. Am J Clin Nutr. 2006;83:731–732. doi: 10.1093/ajcn/83.4.731. [DOI] [PubMed] [Google Scholar]
- 286.Walbert T, Jirikowski GF, Prufer K. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the limbic system of the rat. Horm Metab Res. 2001;33:525–531. doi: 10.1055/s-2001-17210. [DOI] [PubMed] [Google Scholar]
- 287.Wang JY, Wu JN, Cherng TL, Hoffer BJ, Chen HH, Borlongan CV, Wang Y. Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 2001;904:67–75. doi: 10.1016/s0006-8993(01)02450-7. [DOI] [PubMed] [Google Scholar]
- 288.Wang Q, Blackford JA, Jr, Song LN, Huang Y, Cho S, Simons SS., Jr Equilibrium interactions of corepressors and coactivators with agonist and antagonist complexes of glucocorticoid receptors. Mol Endocrinol. 2004;18:1376–1395. doi: 10.1210/me.2003-0421. [DOI] [PubMed] [Google Scholar]
- 289.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 291.Wang Y, Chiang YH, Su TP, Hayashi T, Morales M, Hoffer BJ, Lin SZ. Vitamin D(3) attenuates cortical infarction induced by middle cerebral arterial ligation in rats. Neuropharmacology. 2000;39:873–880. doi: 10.1016/s0028-3908(99)00255-5. [DOI] [PubMed] [Google Scholar]
- 292.Wang Y, De Keulenaer GW, Lee RT. Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem. 2002;277:26496–26500. doi: 10.1074/jbc.M202133200. [DOI] [PubMed] [Google Scholar]
- 293.Webb P, Lopez GN, Greene GL, Baxter JD, Kushner PJ. The limits of the cellular capacity to mediate an estrogen response. Mol Endocrinol. 1992;6:157–167. doi: 10.1210/mend.6.2.1569962. [DOI] [PubMed] [Google Scholar]
- 294.Weigel NL. Interactions between vitamin D and androgen receptor signaling in prostate cancer cells. Nutr Rev. 2007;65:S116–117. doi: 10.1111/j.1753-4887.2007.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 295.White JH. Profiling 1,25-dihydroxyvitamin D3-regulated gene expression by microarray analysis. J Steroid Biochem Mol Biol. 2004;89-90:239–244. doi: 10.1016/j.jsbmb.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 296.White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol Metab. 2000;11:320–327. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- 297.Wion D, MacGrogan D, Neveu I, Jehan F, Houlgatte R, Brachet P. 1,25-Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J Neurosci Res. 1991;28:110–114. doi: 10.1002/jnr.490280111. [DOI] [PubMed] [Google Scholar]
- 298.Wise PM. Estrogen therapy: does it help or hurt the adult and aging brain? Insights derived from animal models. Neuroscience. 2006;138:831–835. doi: 10.1016/j.neuroscience.2014.09.056. [DOI] [PubMed] [Google Scholar]
- 299.Wright DW, Bauer ME, Hoffman SW, Stein DG. Serum progesterone levels correlate with decreased cerebral edema after traumatic brain injury in male rats. J Neurotrauma. 2001;18:901–909. doi: 10.1089/089771501750451820. [DOI] [PubMed] [Google Scholar]
- 300.Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. 402 e391-392. [DOI] [PubMed] [Google Scholar]
- 301.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2004;288:E125–132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 302.Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Xu Y, Hashizume T, Shuhart MC, Davis CL, Nelson WL, Sakaki T, Kalhorn TF, Watkins PB, Schuetz EG, Thummel KE. Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1alpha,25-dihydroxyvitamin D(3): implications for drug-induced osteomalacia. Mol Pharmacol. 2006;69:56–65. doi: 10.1124/mol.105.017392. [DOI] [PubMed] [Google Scholar]
- 304.Xu Y, Zhang W, Klaus J, Young J, Koerner I, Sheldahl LC, Hurn PD, Martinez-Murillo F, Alkayed NJ. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc Natl Acad Sci U S A. 2006;103:14489–14494. doi: 10.1073/pnas.0602932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yao XL, Liu J, Lee E, Ling GS, McCabe JT. Progesterone differentially regulates pro- and anti-apoptotic gene expression in cerebral cortex following traumatic brain injury in rats. J Neurotrauma. 2005;22:656–668. doi: 10.1089/neu.2005.22.656. [DOI] [PubMed] [Google Scholar]
- 306.Ylikomi T, Laaksi I, Lou YR, Martikainen P, Miettinen S, Pennanen P, Purmonen S, Syvala H, Vienonen A, Tuohimaa P. Antiproliferative action of vitamin D. Vitam Horm. 2002;64:357–406. doi: 10.1016/s0083-6729(02)64010-5. [DOI] [PubMed] [Google Scholar]
- 307.Zanello LP, Norman AW. 1 alpha,25(OH)2 vitamin D3-mediated stimulation of outward anionic currents in osteoblast-like ROS 17/2.8 cells. Biochem Biophys Res Commun. 1996;225:551–556. doi: 10.1006/bbrc.1996.1210. [DOI] [PubMed] [Google Scholar]
- 308.Zasloff M. Fighting infections with vitamin D. Nat Med. 2006;12:388–390. doi: 10.1038/nm0406-388. [DOI] [PubMed] [Google Scholar]
- 309.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 310.Zhu X, Raina AK, Smith MA. Cell cycle events in neurons. Proliferation or death? Am J Pathol. 1999;155:327–329. doi: 10.1016/S0002-9440(10)65127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35:217–224. doi: 10.1002/eji.200425491. [DOI] [PubMed] [Google Scholar]
- 312.Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. 2005;33:654–660. doi: 10.1097/01.ccm.0000155911.01844.54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


