Abstract
Purpose
The aim of this study was to evaluate stability and Gd tissue distribution of a biodegradable macromolecular MRI contrast agent, GDCC.
Methods
Kinetic stability of GDCC was evaluated based on transmetallation with endogenous metal ions Zn2+ and Cu2+ in rat plasma in comparison with Omniscan, MultiHance and ProHance. In vivo transmetallation of GDCC was evaluated by determining metal content in the urine samples of Spague-Dawley rats. The biodistribution of the agents was determined in rats at 48 h post-injection.
Results
A new method of using ultrafiltration was developed for study of kinetic stability against transmetallation of Gd(III)-based MRI contrast agents. Both in vitro and in vivo stability of the contrast agents towards transmetallation with Zn2+ was in the order of ProHance > MultiHance ≈ GDCC > Omniscan. No significant transmetallation with Cu2+ was observed for the contrast agents. GDCC had comparable retention to the control agents in most organs and tissues with slightly high retention in the liver and kidneys at 48 hours post-injection.
Conclusions
Ultrafiltration is efficient and accurate for characterizing the kinetic stability of Gd(III)-based MRI contrast agents. The novel biodegradable macromolecular contrast agent GDCC is promising for further development for contrast enhanced MRI.
Keywords: MRI contrast agent, gadolinium, stability, biodistribution, GDCC
INTRODUCTION
Gadolinium-based contrast agents are routinely used to enhance image contrast in magnetic resonance imaging procedures. Currently, the clinical Gd(III) based MRI contrast agents are categorized into two distinct groups, the macrocyclic Gd(III) chelates and linear chelates. In general, macrocyclic chelates, including Gd-DOTA and Gd-(HP-DO3A), are more stable than linear molecules, e.g. Gd-DTPA and Gd-(DTPA-BMA). Macrocyclic Gd(III) chelates have a higher activation energy barrier for both the complexation and dissociation than the linear chelates (1). Transmetallation is considered as the main cause for in vivo dissociation of Gd(III)-based MRI contrast agents, especially for linear chelates, with endogenous metal ions, including Zn2+, Cu2+ and Ca2+ (2–4). As a result, transmetallation can induce release of toxic Gd(III) ions from the chelates in the body and the depletion of the endogenous metal ions with subsequent elimination as a hydrophilic complex through the kidneys. Since Gd3+ ions are highly toxic, the stability of Gd(III) chelates is a critical parameter for design and development of new Gd(III)-based MRI contrast agents. The stability of Gd(III) chelates should include both thermodynamic and kinetic stability. If a Gd(III) chelate has high thermodynamic stability and low kinetic stability, transmetallation with endogenous metal ions such as zinc (Zn2+), copper (Cu2+) and calcium (Ca2+) in the plasma can result in the release of free gadolinium ions (Gd3+), which can deposit in the body (5). Long-term tissue accumulation of toxic Gd3+ can cause toxic side effects, including nephrogenic systemic fibrosis (1,6–11).
Recently, we have designed and developed a new class of biodegradable macromolecular MRI contrast agents based on polydisulfide Gd(III) chelates with improved pharmacokinetic properties (12–14). Our previous studies have shown that the biodegradable macromolecular contrast agents result in prolonged contrast enhancement in the cardiovascular system and tumor tissue, and then gradually degrade in vivo and excrete via renal filtration, resulting minimal long-term tissue accumulation (13–15). The agents are also effective to non-invasively characterize tumor vascularity and assess therapeutic efficacy in dynamic contrast enhanced MRI (16-18). In this study, we developed a new and efficient method for in vitro evaluation of the kinetic stability against transmetallation of Gd(III)-based MRI contrast agents and evaluated the kinetic stability of a lead biodegradable macromolecular contrast agent, Gd-DTPA cystamine copolymers (GDCC), both in vitro and in vivo. Transmetallation of GDCC with endogenous ions Zn2+ and Cu2+ was evaluated in rat plasma and in rats in comparison with clinical contrast agents Omniscan, MultiHance and ProHance. The biodistribution of GDCC at 48 hours after administration was also evaluated in rats in comparison with the clinical agents.
MATERIALS AND METHODS
Contrast Agents
Omniscan (Gd-(DTPA-BMA), 574 Da) was purchased from GE Healthcare. MutiHance (Gd-BOPTA, 1,058 Da) and ProHance (Gd-(HP-DO3A), 558.6 Da) were obtained from Bracco Diagnostics. Gd-DTPA cystamine copolymers with molecular weight of 31, 35, 50 KDa (GDCC-31, GDCC-35, GDCC-50) were prepared as previously described.12 Isotonic saline (0.9 %) solution was used as a negative control.
Animals
Male Sprague-Dawley rats weighing between 200-250 g were purchased from Charles River Laboratories (Wilmington, MA, USA). All animals were housed with a 12-hour day-and-night rhythm and given tap water and standard diet. The animal study was performed according to an animal protocol approved by the Institutional Animal Care and Use Committee of the University of Utah.
Complexation stability of GDCC
GDCC-50 in phosphate buffered saline (PBS) was incubated with 2.5 mM of CaCl2, 0.11 mM of sodium citrate, 50 μM of ZnCl2 and 1 μM of CuCl2 (8 mM of Na2HPO4, 2 mM of KH2PO4 and 140 mM NaCl) at pH 7.4 and 37 °C. This incubation was replicated three times. After 10, 20, 40, 60, 80, 120 and 180 min incubation, incubation mixtures were filtrated through 0.22 μM filters. Then the filtered incubation mixtures run through PD-10 columns and the polymer fractions were collected and freeze-dried. Gd3+, Zn2+, Cu3+ and Ca2+ contents in these freeze-dried samples were analyzed using inductive coupling plasma-optical emission spectrometry (ICP-OES).
In vitro transmetallation
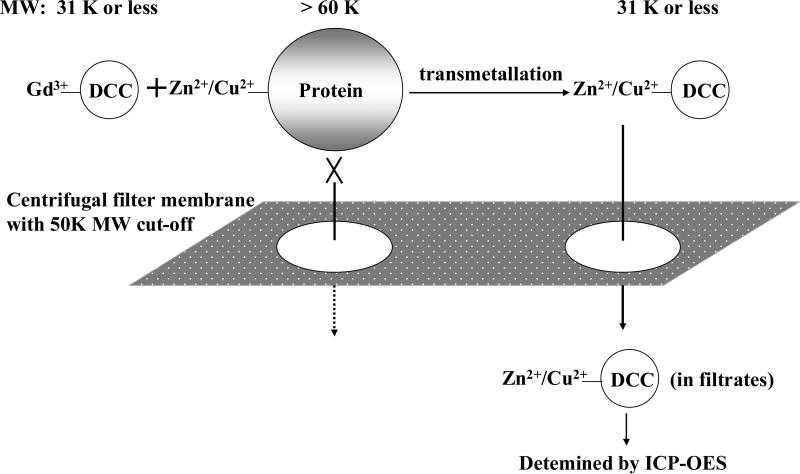
Aqueous solution (0.1 ml, 2 mM-Gd) of GDCC-31, Omniscan, MultiHance or ProHance was mixed with 0.9 ml fresh rat plasma and incubated at room temperature for 2 h. The plasma mixtures were transferred to the sample reservoir of a centrifugal filter CF-50 (molecular weight cut-off 50 KDa) and centrifuged at 4000 rpm and 25 °C for 120 min. Since all Zn2+ or Cu2+ ions bound to proteins, mainly albumin and globulins (>60 KDa), in the plasma, only the chelates smaller than 50 KDa, including Zn2+ or Cu2+ chelates after transmetallation could be filtered through (Figure 1). The protein bound Zn2+ or Cu2+ ions still remained in the upper reservoir of the filter. The efficiency of the ultrafiltration was tested with the aqueous solution of the contrast agents. Ultrafiltration was also performed for rat plasma to test the binding of Zn2+ or Cu2+ ions to plasma proteins. During centrifugation, the solution in the upper reservoir was agitated using a pipette every 20 min for the plasma mixtures. The content of metal ions in both upper reservoir and the filtrates was determined by ICP-OES after appropriate dilution. The degree of transmetallation of the contrast agents with Zn2+ or Cu2+ ions in the plasma was evaluated using the percentage of Zn2+ or Cu2+ ions filtered through the membrane, which was calculated as Zn(Cu)% = (concentration of Zn2+ or Cu2+ in the filtrates)/(total Zn2+ or Cu2+ concentration before centrifugal filtration) × 100%.
Figure 1.
Schematic illustration of the separation mechanism of Zn(II) or Cu(II) exchanged with GDCC-31 in plasma.
In vivo transmetallation
The rats were randomly divided into 5 groups (n=5 each) for four contrast agents, GDCC-31, Omniscan, MutiHance, ProHance and a saline control. The rats were anesthetized with an intraperitoneal injection of a mixture of ketamine (45 mg/kg) and xylazine (6 mg/kg). The contrast agents were injected at a dose of 0.1 mmol-Gd/kg via a tail vein. The rats were immediately placed into metabolic cages after the injection. Urine samples were collected at 12 h pre-injection, 8 h post-injection (0-8 h) and then 24 h post-injection (8-24 h) from the metabolic box. The collected urine samples were centrifuged at 4,000 rpm for 15 min. The contents of Gd(III), Zn(II), Cu(II) and Ca(II) in the supernatant of the urine samples were determined by ICP-OES after appropriate dilutions.
Biodistribution of the contrast agents in rats
The animals in above study were sacrificed with an overdose of isoflurane at 48 h post-injection. The organ and tissue samples, including femur, heart, lung, liver, muscle, spleen and kidney, were collected and weighed. The tissue samples were then cut into small pieces and mixed with ultra-pure nitric acid (1.0 ml, 70%, EMD, Gibbstown, NJ). The tissue samples were liquefied within 2 weeks and the solution was transferred to a centrifuge tube and centrifuged at 14,000 rpm for 15 min. The supernatant (0.2 ml) was diluted 10 times with de-ionized water and further centrifuged at 14,000 rpm for 15 min. The Gd(III) concentration in the final supernatant was measured by ICP-OES. The average Gd(III) content in each organ or tissue was calculated from the measured Gd(III).
Storage stability of GDCC
The storage stability of solid and liquid forms of GDCC-35 was tested at different storage temperature for up to 14 months. For freeze-dried GDCC was stored in nitrogen at room temperature (~ 25 °C), in the refrigerator (~ 5 °C) and the freezer (~ -20 °C). GDCC saline solution at a Gd concentration of 0.33 M was stored in nitrogen at room temperature (~ 25 °C), in the refrigerator (~ 5 °C). All samples were protected from light with aluminum foil. The molecular weight distributions of GDCC-35 under different storage conditions were analyzed with fast protein liquid chromatograph (FPLC, AKTA®) equipped with an analytical Superose 12 column at 1, 2, 5 and 14 months in storage. Gd(III) content in the GDCC was also analyzed by ICP-OES.
Statistical evaluation
Statistical analysis was performed using an unpaired two–tailed Student's t–test (GraphPad Prism; GraphPad Software, San Diego, CA). A confidence interval 95% (P < 0.05) was considered statistically significant.
RESULTS
Complexation stability of GDCC
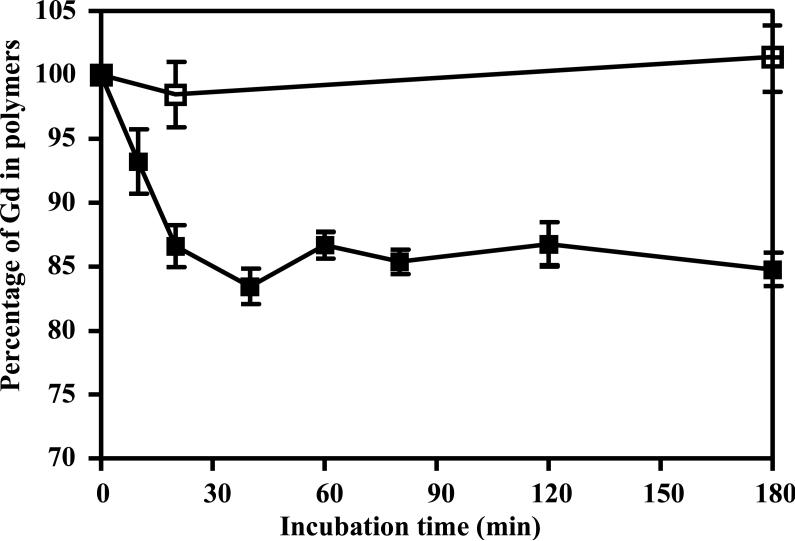
The complexation stability of GDCC and transmetallation effect was first evaluated in PBS buffer and under physiological concentrations with endogenous metal ions, including 2.5 mM of Ca2+, 50 μM of Zn2+, 1 μM of Cu2+ and 0.11 mM of citrate. The dynamic stability of the agent in PBS and in the presence of the metal ions is shown in Figure 2. GDCC gradually lost ~15% of Gd(III) ion within approximately 45 min incubation, and remained steady throughout the duration of study. In comparison, the Gd content in GDCC incubated with PBS did not change during the period of experiment, indicating high complexation stability of GDCC in the absence of the endogenous metal ions.
Figure 2.
Complex stability of GDCC in PBS (□) or in a PBS buffer containing 2.5 mM of CaCl2, 0.11 mM of sodium citrate, 50 μM of ZnCl2 and 1 μM of CuCl2 at pH 7.4 and 37 °C (■). Data presented as mean ± SD.
In vitro transmetallation in rat plasma
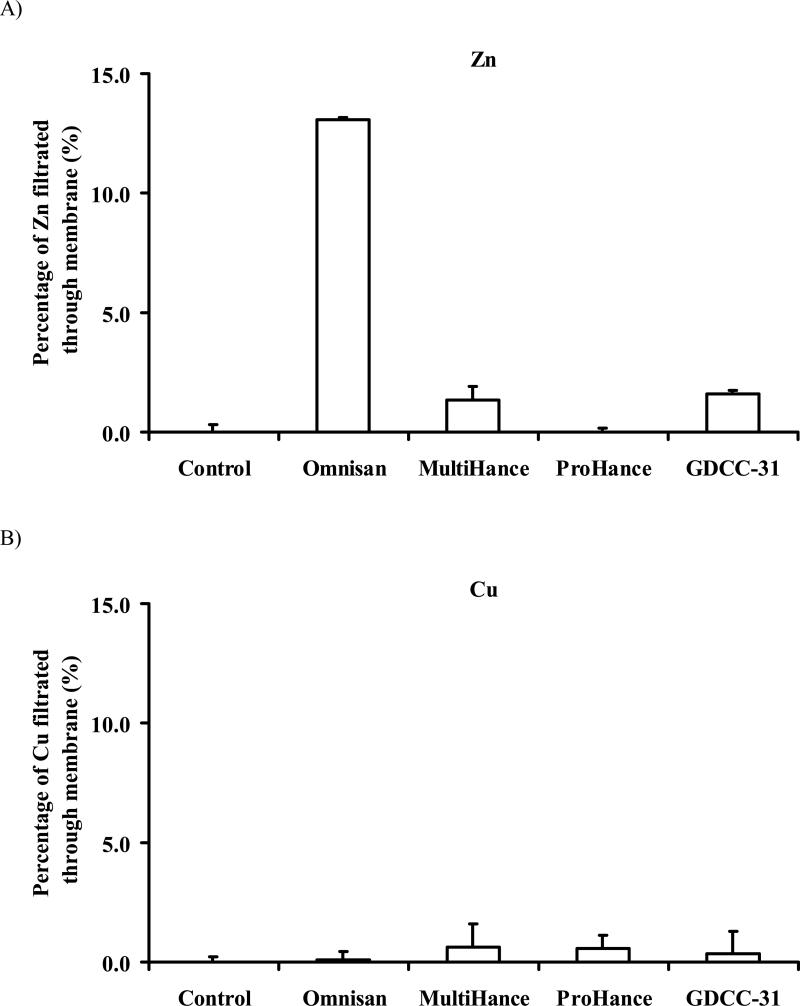
The percentage of GDCC-31, Omniscan, MultiHance and ProHance in aqueous solution filtered through was 96.0%, 98.7%, 96.8% and 98.6%, respectively (Table 1), indicating that the contrast agents could completely filter through CF-50 membrane in ultrafiltration. On the other hand, no Zn2+ and Cu2+ ions were detected in the filtrates of blank plasma, verifying that the Zn2+ and Cu2+ ions bound to the proteins in the plasma and could not filter through CF-50 membrane. The results of the control study validated the effectiveness of the ultrafiltration for evaluating the transmetallation of the contrast agents in the plasma. After transmetallation, the chelates of Zn2+ and Cu2+ formed with ligands of the contrast agents could be readily filtered through the filtration membrane. The degree of transmetallation could be readily determined by measurement of Zn2+ and Cu2+ contents in the filtrates.
Table 1.
Gd3+ filtration ratio of different contrast agents from original aqueous solution and Zn2+/Cu2+ filtration ratio from rat plasma.
| Gd3+ | Zn2+ | Cu2+ | |
|---|---|---|---|
| MultiHance | 96.8% ± 2.6% | - | - |
| ProHance | 98.6% ± 0.7% | - | - |
| Omniscan | 98.7% ± 2.1% | - | - |
| GDCC31K | 96.0% ± 3.1% | - | - |
| Rat plasma | - | -1.2% ± 0.3% | -0.5% ± 0.2% |
Figure 3 shows the degree of transmetallation of the contrast agents with Zn2+ and Cu2+ in the rat plasma. The linear neutral contrast agent Omniscan resulted in the highest degree of transmetallation with Zn2+ (13.1% ± 0.1%, Fig. 3A). The transmetallation of Omniscan was almost 10-fold higher than that of anionic linear agent MultiHance (1.4% ± 0.6%). No significant transmetallation with Zn2+ was observed for the macrocyclic agent ProHance. The biodegradable macromolecular contrast agent GDCC-31 resulted in similar degree of transmetallation (1.6% ± 0.1%) with Zn2+ as MultiHance. Transmetallation of Omniscan, MultiHance and GDCC-31 with Cu2+ ions in the plasma was similar to that of ProHance, much less than transmetallation with Zn2+ (Fig. 3B).
Figure 3.
Transmetallation of Gd chelating agents with Zn2+ (A) and Cu2+ (B) in rat plasma.
In vivo transmetallation
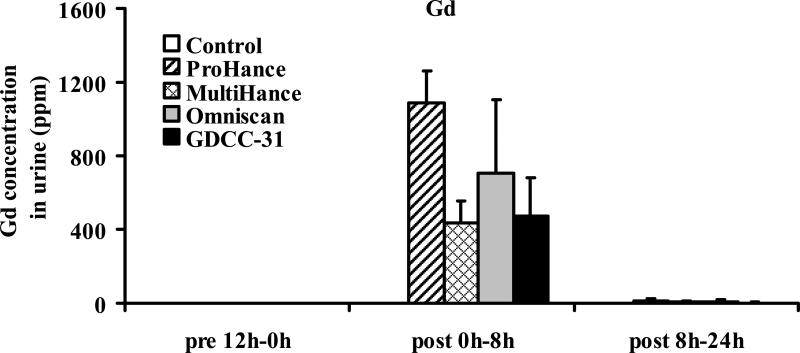
In vivo transmetallation of the contrast agents with Zn2+ or Cu2+ ions in the plasma was evaluated based on the metal ion contents in the urine samples after intravenous administration of the contrast agents. Figure 4 shows the Gd(III) concentration in the urine samples collected before and during 0–8 hours and 8–24 hours after injection of the contrast agents. It appears that the contrast agents mostly excreted in the first 8 hours post-injection. The macrocyclic agent ProHance had the highest concentration in the urine samples.
Figure 4.
Concentrations of gadolinium(III) excreted in urine before and after administration of the contrast agents. Data presented as mean ± SD.
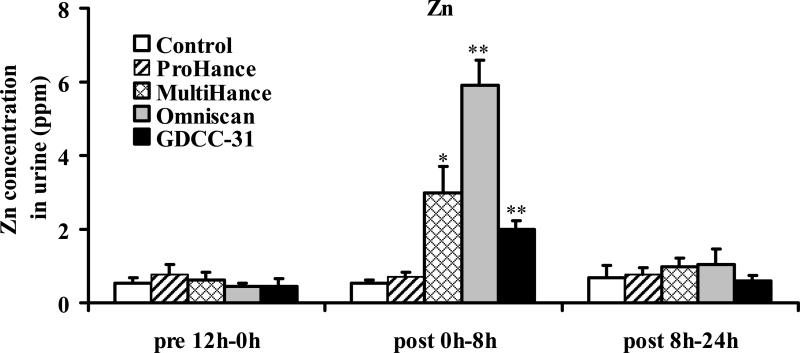
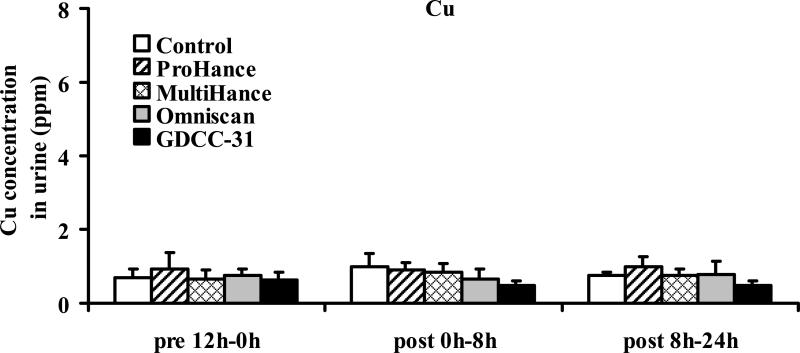
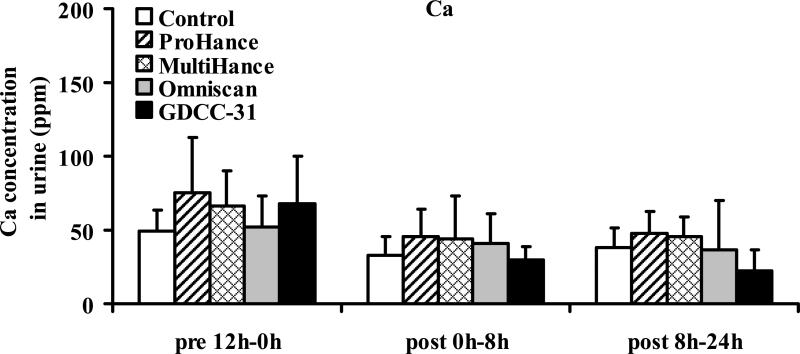
Figure 5 shows the Zn(II) concentration in the urine samples collected before and during 0–8 hours and 8–24 hours after injection of the contrast agents. Significant increase of Zn(II) concentration was detected in the urine samples collected in the first 8 hours post-injection for the linear agents, Omniscan (p < 0.001) and MultiHance (p < 0.005), and GDCC-31 (p < 0.001), as compared to the control group injected with saline and the samples collected before injection. Omniscan resulted in the highest Zn(II) concentration in the urine samples. GDCC-31 resulted in a slightly lower Zn(II) concentration than MultiHance in the urine samples. No significant difference was observed between the ProHance group and the control group (p = 0.92). The Zn(II) concentration in the urine samples collected after 8 hours returned to the baseline level for all agents. No significant change of the Cu(II) or Ca(II) concentration was observed in the urine samples collected after injection of the contrast agents, Figures 6 and 7.
Figure 5.
Concentrations of zinc(II) excreted in urine before and after administration of the contrast agents (** P < 0.001, * P < 0.005). Data presented as mean ± SD.
Figure 6.
Concentrations of copper(II) excreted in urine before and after administration of the contrast agents. Data presented as mean ± SD.
Figure 7.
Concentrations of calcium(II) excreted in urine before and after administration of the contrast agents. Data presented as mean ± SD.
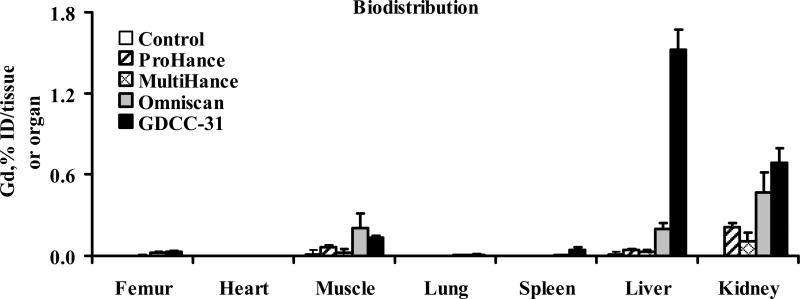
Biodistribution in rats
Figure 8 showed the biodistribution of Gd(III) in the major organs and tissues, including the femur, heart, kidneys, liver, lung, muscle, and spleen of rats 2 days after a single injection of MultiHance, ProHance, Omniscan and GDCC-31 at a dose of 0.1 mmol-Gd/kg. Approximately 1.5% and 0.8% of injected GDCC-31 was measured in the liver and kidneys at 48 hours post-injection, higher than the small molecular contrast agents (p < 0.05). The retention of GDCC-31 in other organs and tissues was at a comparable minimal level as the low molecular weight contrast agents. Omniscan also had a significantly higher retention in the liver and kidneys than other small molecular contrast agents (p < 0.05).
Figure 8.
Biodistribution of gadolinium(III) in rats 2 days after intravenous injection of MultiHance, ProHance, Omniscan and GDCC-31 at a dose of 0.1 mmol Gd/kg. Data presented as mean ± SD.
Storage stability of GDCC
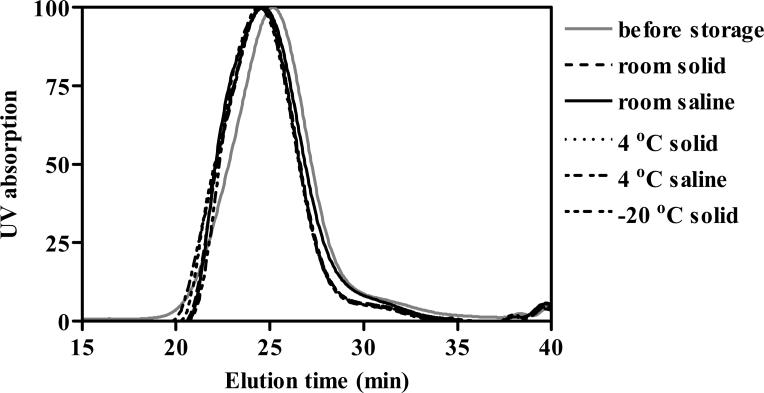
Figure 10 shows the molecular weight distribution of the biodegradable macromolecular contrast agent GDCC after storage for 14 months under different conditions in both solid form and solution form. Since disulfide bonds may be sensitive to oxidation and photo-oxidation, the samples were stored in dark and in the presence of nitrogen. No significant change of the molecular weight distribution was observed for sample after the long-term storage, as shown in size exclusion chromatograms, indicating no breakdown of the polymer chains. The Gd content in GDCC did not change after storage for 14 months. The polydisulfides exhibited good stability for long-term storage in dark and in nitrogen.
DISCUSSION
Polydisulfide Gd(III) complexes have been designed as biodegradable macromolecular MRI contrast agents to alleviate the safety concerns related to the slow excretion of Gd(III)-based macromolecular MRI contrast agents. Macromolecular MRI contrast agents have shown superior enhancement for blood pool imaging and cancer imaging over low molecular weight contrast agents. However, the safety concern related to their slow excretion has limited further development of macromolecular contrast agents. The polydisulfide Gd(III) complexes provide strong blood pool enhancement and can readily breakdown into small Gd(III) chelates via reduction of the disulfide bonds in the polymer chains by endogenous free thiols in the plasma. The resulted small Gd(III) chelates can readily excreted via renal filtration with minimal long-term tissue accumulation after the contrast enhanced MRI.
Our previous studies have shown that Gd-DTPA cystamine copolymer (GDCC) is a promising lead biodegradable macromolecular MRI contrast agent. GDCC initially acts as a macromolecular contrast agent, resulting in superior contrast enhancement over low molecular weight contrast agent, e.g. Gd(DTPA-BMA), for blood pool imaging and tumor imaging. It is effective to determine vascular permeability of angiogenic tumor microvessels, while low molecular weight contrast agents often result in overestimated vascular permeability (15-18). GDCC can also accurately assess therapeutic efficacy of anti-angiogenic therapy and other cancer treatment. In vivo complexation stability of GDCC is a critical parameter for further development of biodegradable macromolecular contrast agent. The Gd(III) chelates approved for clinical applications have high a thermodynamic stability. However, transmetallation of these agents with endogenous Zn2+, Cu2+ and Ca2+ may result in dissociation of the chelates and release of toxic Gd(III) ions (3,4).
In this study, we further investigated the transmetallation stability of a lead biodegradable macromolecular MRI contrast agent, Gd-DTPA cystamine copolymers (GDCC), both in vitro and in vivo in comparison with the clinical contrast agents, MultiHance, Ominscan and ProHance. The results have shown that the structure of the contrast agents have a significant effect on transmetallation with endogenous metal ions, consistent to previous reports1–3. The biodegradable macromolecular contrast agent GDCC had similar in vitro and in vivo kinetic stability as the linear agent MultiHance. GDCC also showed good stability for long-term storage in dark and the absence of oxygen.
The in vitro transmetallation of the agents was investigated by ultrafiltration in rat plasma, a newly developed method in this study. The control study showed that the endogenous metal ions Zn2+ and Cu2+ did not pass through the filtration membrane of the selected size because they bound to the proteins in the plasma. These ions were only detected in the filtrate when they were removed from the proteins after transmetallation. The method was validated by measuring the concentration of the contrast agents in the filtrate after filtration. Centrifugal filtration was a convenient and accurate alternate approach for the study of transmetallation in the plasma along with other reported methods, including instant thin-layer chromatography-silica gel (ITLC-SG) (19), high-performance liquid chromatography (HPLC) (20,21), and relaxometric method (22).
It has been reported that Zn(II) ions are the main cause of transmetallation of gadolinium based MRI contrast agents, especially the linear agents, both in vitro and in vivo (3,4,19–22). We showed here that GDCC was stable in the absence of the endogenous metal ions. Zn(II) in rat plasma resulted in significant release of Gd(III) ions from Omniscan, MultiHance and GDCC via transmetallation. Omniscan resulted in the most transmetallation with Zn(II) and GDCC had similar transmetallation as the MultiHance. The macrocyclic agent ProHance was stable against transmetallation. It was noticed that the degree of transmetallation induced by Zn(II) in the plasma was much less than that by free Zn(II) ions in buffer (19–22). The binding of Zn(II) in the proteins might inhibit further transmetallation with the contrast agents. The order of transmetallation of all the tested agents with plasmic Zn(II) was Omniscan > MultiHance ≈ GDCC > ProHance (Figure 3A).
It was speculated that Cu2+ in the plasma might cause significant transmetallation. The transmetallation of GDCC, MultiHance and Omniscan with plasmic Cu(II) was much less significant than that with Zn(II) (Figure 3B), although the concentrations of Zn2+ and Cu2+ in rat plasma was similar as determined by ICP-OES (data not shown). Slight transmetallation with Cu(II) was observed for the macrocyclic agent ProHance in the plasma. The results indicated that the Gd(III) based contrast agents were generally stable against transmetallation with Cu(II) in plasma. The transmetallation of the contrast agents with Ca2+ in the plasma could not be determined with centrifugal filtration because Ca2+ ions in plasma were mostly in free form and could readily filter through. Nevertheless, the thermodynamic stability constants of the chelates of Ca(II) and the ligands of the MRI contrast agent is approximately 10 orders of magnitude lower than those of corresponding Gd(III) chelates (2). It is considered that Ca2+ is inert for transmetallation with Gd(III) based contrast agents.
The results of in vivo transmetallation of GDCC and other tested agents were consistent with the in vitro data. Omniscan resulted in the highest Zn(II) concentration in the urine samples collected in the first 8 hours post-injection. GDCC resulted in slightly lower Zn(II) concentration than MultiHance. No increase of Zn(II) concentration was observed in the urine samples collected from the rats injected with ProHance. No increase of Ca(II) and Cu(II) concentration was observed in all urine samples, suggesting that the endogenous Zn(II) was the main cause for transmetallation of open-chain Gd(III) chelates. The order of in vivo stability of the contrast agents against transmetallation was the same as what was observed in the in vitro study (Figures 3A and 5). The stability of the contrast agents was clearly correlated to their structural characteristics (23–25). Macrocyclic molecules, where Gd3+ is caged in the pre-organized cavity of the ligand, are much more stable than linear molecules because a much higher activation energy barrier has to be overcome for both the complexation and de-complexation of macrocyclic agents. The linear open-chain Gd(III) chelates had relatively high conformational mobility, while the macrocyclic chelates had tight packing and high conformational rigidity.
The biodegradable macromolecular contrast agent showed similar stability as MultiHance against transmetallation with Zn(II) both in vitro and in vivo. Although GDCC had similar non-ionic chelate structure as Omniscan, it resulted in significant less transmetallation with Zn(II) than Omniscan. One possible reason was that Omniscan had an excess of calcium chelated ligand (25 mM) in its formulation. Since the thermodynamic stability constant of Zn(DTPA-BMA) is much higher than that of Ca(DTPA-BMA) (2), the excess Ca(DTPA-BMA) could readily exchange with Zn(II) ions and remove Zn(II) ions from the plasma proteins. Another possible explanation was that GDCC was a polymeric complex with multiple chelating sites in its backbone, which might increase the stability of the agent via polydentate effect.
The short-term tissue retention of GDCC was investigated in rats at 48 hours after the injection. The long-term tissue accumulation of GDCC was reported previously in rats at 10 days post-injection (13). Due to its biodegradability, GDCC resulted in minimal long-term tissue retention similar to low molecular contrast agents independent of its molecular weight (13,14). Its biodistribution at 48 hours post-injection would be helpful to understand how rapid the agent could be eliminated from the body. The study showed that GDCC-31 had comparable low retention as the tested low molecular weight contrast agents in most organs and tissues, including the femur, heart, lung, muscle and spleen. Its retention in the liver and kidneys was only slightly higher than the small molecular agents at 48 hours post-injection (Figure 8), suggesting that in vivo degradation of GDCC macromolecules might be a gradual process. As demonstrated in our previous study, GDCC with molecular weights of 18 and 60 KDa resulted in minimal tissue accumulation comparable to Omniscan at 10 days after intravenous injection (13). In comparison, a nondegradable macromolecular agent, Gd-DTPA 1,6-hexanediamine copolymers, resulted in much higher tissue accumulation in rats at 10 days after injection (14). The results in study showed that over 95% of the injected GDCC was excreted in the first two days post-injection, which was much higher than the excretion of non-degradable macromolecular contrast agents after 10 days post-injection (14). The retention of GDCC was much lower than that of other macromolecular Gd(III) complexes. For example, a Gd-DTPA polypropyleneimine dendrimer (generation 2) conjugate (7 kDa) resulted in the retention of 45% of injected dose in rats 14 days after injection (26). Carboxymethyl hydroxyethyl starch-(Gd-DO3A) had approximately 47% of injected dose in the body of experimental animals seven days after injection (27). Although the biodegradable macromolecular contrast agent excreted a slightly slower than the small molecular contrast agents, it showed much rapid excretion than non-degradable macromolecular contrast agents and could be completely excreted in an extended period post-injection.
CONCLUSIONS
We have established a new method of using ultrafiltration to evaluate transmetallation of the Gd(III) based MRI contrast agents with endogenous metal ions in the blood plasma. The results showed that Omniscan was most sensitive to transmetallation with Zn(II) ions in the plasma and ProHance was most stable against transmetallation. The biodegradable macromolecular contrast agent GDCC demonstrated similar kinetic stability against transmetallation with Zn(II) as MultiHance. The same in vivo stability order was observed for the agents based on elevated excretion of Zn(II) in the urine samples collected with 8 hours after the administration. GDCC resulted in similar short-term tissue retention as the small molecular contrast agents in most organs and tissues with slightly high retention in the kidneys and liver. The biodegradable macromolecular contrast agent GDCC showed good kinetic stability toward transmetallation and rapid excretion via renal filtration. GDCC was stable for storage up to 14 months in dark and the presence of nitrogen. GDCC is promising for further preclinical and clinical development as a biodegradable macromolecular MRI contrast agent.
Figure 9.
Size exclusion chromatograms of GDCC before and after 14 months storage under various conditions.
ACKNOWLEDGEMENTS
The authors thank Dr. Yong-En Sun for the animal handling. This work was supported in part by the NIH grant R01 EB00489.
Reference
- 1.Sieber MA, Lengsfeld P, Frenzel T, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol. 2008;18:2164–2173. doi: 10.1007/s00330-008-0977-y. [DOI] [PubMed] [Google Scholar]
- 2.Idée JM, Port M, Raynal I, et al. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam Clin Pharmacol. 2006;20:563–576. doi: 10.1111/j.1472-8206.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 3.Kimura J, Ishiguchi T, Matsuda J, et al. Human comparative study of zinc and copper excretion via urine after administration of magnetic resonance imaging contrast agents. Radiation Medicine. 2005;23:322–326. [PubMed] [Google Scholar]
- 4.Puttagunta NR, Gibby WA, Smith GT. Human in vivo comparative study of zinc and copper transmetallation after administration of magnetic resonance imaging contrast agents. Invest Radiol. 1996;31:739–742. doi: 10.1097/00004424-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Spencer AJ, Wilson SA, Batchelor J, et al. Gadolinium chloride toxicity in the rat. Toxicol Pathol. 1997;25:245–55. doi: 10.1177/019262339702500301. [DOI] [PubMed] [Google Scholar]
- 6.Sieber MA, Pietsch H, Walter J, et al. A preclinical study to investigate the development of nephrogenic systemic fibrosis: a possible role for gadolinium-based contrast media. Invest Radiol. 2008;43:65–75. doi: 10.1097/RLI.0b013e31815e6277. [DOI] [PubMed] [Google Scholar]
- 7.Sieber MA, Lengsfeld P, Walter J, et al. Gadolinium-based contrast agents and their potential role in the pathogenesis of nephrogenic systemic fibrosis: the role of excess ligand. J Magn Reson Imaging. 2008;27:955–962. doi: 10.1002/jmri.21368. [DOI] [PubMed] [Google Scholar]
- 8.Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol. 2006;18:614–617. doi: 10.1097/01.bor.0000245725.94887.8d. [DOI] [PubMed] [Google Scholar]
- 9.Perazella MA. Nephrogenic systemic fibrosis, kidney disease and gadolinium: is there a link? Clin J Am Soc Nephrol. 2007;2:200–202. doi: 10.2215/CJN.00030107. [DOI] [PubMed] [Google Scholar]
- 10.Morcos SK. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium-based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br J Radiol. 2007;80:73–76. doi: 10.1259/bjr/17111243. [DOI] [PubMed] [Google Scholar]
- 11.Yerram P, Saab G, Karuparthi PR, et al. Nephrogenic systemic fibrosis: a mysterious disease in patients with renal failure--role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin J Am Soc Nephrol. 2007;2:258–263. doi: 10.2215/CJN.03250906. [DOI] [PubMed] [Google Scholar]
- 12.Lu Z-R, Parker DL, Goodrich KC, et al. Extracellular biodegradable macromolecular gadolinium(III) complexes for MRI. Magn Reson Med. 2004;51:27–34. doi: 10.1002/mrm.10656. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Feng Y, Ke T, et al. Pharmacokinetics and tissue retention of (Gd-DTPA)-cystamine copolymers, a biodegradable macromolecular magnetic resonance imaging contrast agent. Pharm Res. 2005;22:596–602. doi: 10.1007/s11095-005-2489-7. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Zong Y, Ke T, et al. Pharmacokinetics, biodistribution and contrast enhanced MR blood pool imaging of Gd-DTPA cystine copolymers and Gd-DTPA cystine diethyl ester copolymers in a rat model. Pharm Res. 2006;23:1736–1742. doi: 10.1007/s11095-006-9028-z. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Jeong E-K, Mohs A, et al. Characterization of tumor angiogenesis with dynamic contrast enhanced magnetic resonance imaging and biodegradable macromolecular contrast agents in mice. Magn Reson Med. 2008;60:1347–1352. doi: 10.1002/mrm.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, Feng Y, Jeong EK, et al. Tumor characterization with dynamic contrast enhanced magnetic resonance imaging and biodegradable macromolecular contrast agents in mice. Pharm Res. 2009;26:2202–8. doi: 10.1007/s11095-009-9935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaidya A, Sun Y, Feng Y, et al. Contrast-enhanced MRI-guided photodynamic cancer therapy with a pegylated bifunctional polymer conjugate. Pharm Res. 2008;25:2002–11. doi: 10.1007/s11095-008-9608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, Jeong EK, Emerson L, et al. Noninvasive Evaluation of Antiangiogenic Effect in a Mouse Tumor Model by DCE-MRI with Gd-DTPA Cystamine Copolymers. Mol Pharm. 2010;7:41–8. doi: 10.1021/mp900153f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tweedle MF, Hagan JJ, Kumar K, et al. Reaction of gadolinium chelates with endogenously available ions. Magn Reson Imaging. 1991;9:409–415. doi: 10.1016/0730-725x(91)90429-p. [DOI] [PubMed] [Google Scholar]
- 20.Puttagunta NR, Gibby WA, Puttagunta VL. Comparative transmetallation kinetics and thermodynamic stability of gadolinium-DTPA bis-glucosamide and other magnetic resonance imaging contrast media. Invest Radiol. 1996;31:619–624. doi: 10.1097/00004424-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Sarka L, Burai L, Brücher E. The rates of the exchange reactions between [Gd(DTPA)]2- and the endogenous ions Cu2+ and Zn2+: a kinetic model for the prediction of the in vivo stability of [Gd(DTPA)]2-, used as a contrast agent in magnetic resonance imaging. Chemistry. 2000;6:719–724. doi: 10.1002/(sici)1521-3765(20000218)6:4<719::aid-chem719>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Laurent S, Elst LV, Copoix F, et al. Stability of MRI paramagnetic contrast media: a proton relaxometric protocol of transmetallation assessment. Invest Radiol. 2001;36:115–112. doi: 10.1097/00004424-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Port M, Idée JM, Medina C, et al. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals. 2008;21:469–490. doi: 10.1007/s10534-008-9135-x. [DOI] [PubMed] [Google Scholar]
- 24.Tweedle MF, Wedeking P, Kumar K. Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate, and gadodiamide in mice and rats. Invest Radiol. 1995;30:372–380. doi: 10.1097/00004424-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Jin T, Comblin V, et al. A kinetic investigation of the lanthanide DOTA chelates. Stability and rates of formation and of dissociation of a macrocyclic gadolinium (III) polyaza polycarboxylic MRI contrast agent. Inorg Chem. 1992;31:1095–1099. [Google Scholar]
- 26.Wang SJ, Brechbiel M, Wiener EC. Characteristics of a new MRI contrast agent prepared from polypropyleneimine dendrimers, generation 2. Invest Radiol. 2003;38:662–668. doi: 10.1097/01.rli.0000084887.47427.75. [DOI] [PubMed] [Google Scholar]
- 27.Helbich TH, Gossman A, Mareski PA, et al. A new polysaccharide macromolecular contrast agent for MR imaging: biodistribution and imaging characteristics. J Magn Reson Imaging. 2000;11:694–701. doi: 10.1002/1522-2586(200006)11:6<694::aid-jmri17>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]