Abstract
The observation that cerebellar Purkinje cells contain type-l benzodiazepine-sensitive GABAA receptors is consistent with findings in the present work that the majority of Purkinje neurons are sensitive to enhancement of GABA by the type-1 benzodiazepine agonist, zolpidem. Previous work has demonstrated a relation between zolpidem and ethanol enhancement of GABA responses in several brain regions, but had not tested Purkinje neurons. Therefore, given that a majority of Purkinje neurons were found to be sensitive to zolpidem, ethanol would have been expected to enhance GABA responses from this cell type. However, in agreement with earlier electrophysiological studies, ethanol enhanced GABA inhibitory responses from only a small proportion of these cerebellar Purkinje neurons. Rather than enhancement of GABA, local application of ethanol either inhibited or did not affect responses to GABA from a majority of cerebellar-Purkinje neurons. Nonetheless, as previously reported, a portion of the Purkinje neurons initially insensitive to ethanol enhancement of GABA became sensitive to this action of ethanol with co-application of the β-adrenergic agonist, isoproterenol. Thus, these results collectively implicate a β-adrenergic input dependency for ethanol enhancement of GABA from some, but not all, cerebellar Purkinje neurons sensitive to zolpidem. Because a β-adrenergic input did not allow ethanol enhancement of GABA from all Purkinje neurons, future studies should explore the possibility that other auxiliary neural inputs to zolpidem-sensitive cerebellar Purkinje neurons may be required for ethanol enhancement of GABA responsiveness when a β-adrenergic input does not have this action. Likewise, knowing that the action of zolpidem can predict ethanol enhancement of GABA in other brain regions, the present findings suggest that a future determination be made concerning whether zolpidem-sensitive neurons in these other regions of brain require a β-adrenergic or an alternative neural input for ethanol enhancement of GABA responses.
Keywords: Cerebellar Purkinje Neurons, Zolpidem Receptors, GABAA Receptors, β-Adrenergic Agonist, Ethanol
Givens and Breese1,2 reported that systemic administration of ethanol enhanced responses to GABA from some, but not all, medial septal neurons, while having no effect on responses from lateral septal neurons. Subsequently, Simson et al.3 reported that local application of ethanol had effects similar to those of systemically administered ethanol on GABA responses from medial septal neurons (i.e., ethanol enhanced some, but not all, responses to GABA). A follow-up report by Criswell et al.4 indicated ethanol enhanced responses to GABA from most, but not all, spontaneously active neurons in the substantia nigra reticulata, inferior colliculus, and ventral pallidum. In contrast to the positive action of ethanol to enhance GABA in the former brain regions, spontaneously active neurons in the lateral septum, ventral tegmental area, and the hippocampus were not sensitive to the action of ethanol to enhance GABA.4,5 During the course of these various investigations, it was noted that a high concentration of zolpidem binding was present in brain regions where neurons were found to be sensitive to ethanol enhancement of GABA, whereas zolpidem binding was low or virtually absent in brain regions where ethanol did not enhance responses to GABA.4–7 Therefore, the action of zolpidem and ethanol on GABA responses was tested on individual neurons in various regions of the brain 4,5 A positive relationship between the action of zolpidem and ethanol to enhance GABA-induced inhibition was found, providing support for the contention that the zolpidem-sensitive GABAA benzodiazepine (BZD) receptor would respond to ethanol enhancement of GABA.8 However, there were a few neurons encountered in the medial septum where this positive relationship between the actions of ethanol and zolpidem did not occur.8
In the survey of zolpidem binding in the brain, the cerebellar Purkinje layer contained a high concentration of zolpidem binding.6,7 Earlier work noted that only a relatively small population of cerebellar Purkinje neurons were sensitive to ethanol enhancement of GABA responses.9–12 In a portion of the Purkinje neurons insensitive to ethanol enhancement of GABA, Lin et al.9,10 reported that iontophoretic application of norepinephrine or isoproterenol allowed ethanol potentiation of GABA-induced inhibition from Purkinje neurons. Furthermore, application of a β-adrenergic antagonist to cerebellar Purkinje neurons initially sensitive to ethanol enhancement of GABA reduced this action of ethanol on GABA responsiveness.11,12 Based upon these previous data that cerebellar Purkinje neurons became sensitive to ethanol enhancement of GABA when a β-adrenergic agonist was added to the cell,9–12 the present investigation determined whether those cerebellar Purkinje neurons sensitive to β-adrenergic influences on ethanol action would be sensitive to zolpidem enhancement of responses to GABA. Thus, this approach was to provide information about the GABAA receptor subtype(s) on cerebellar Purkinje neurons initially sensitive or insensitive to ethanol enhancement of GABA. Given the variable response pattern reported for ethanol enhancement of responses to GABA from cerebellar Purkinje neurons,9,10 the present investigation initially tested the degree to which ethanol sensitivity of this cell type was related to the action of zolpidem to enhance responses to GABA. This investigation allowed an assessment of whether the action of zolpidem would be predictive of the action of ethanol on responses to GABA from cerebellar Purkinje neurons.
METHODS
Animals
Male Sprague-Dawley rats (300 to 500 g) were obtained from Charles River Associates (Raleigh, NC) or bred within the animal colony from Charles River stock. Adult rats were housed two to a cage with food and water ad libitum. The room was temperature-controlled (22 to 25°C) and was maintained on a 12-hr light/dark cycle. Care and procedures were in accordance with the “Guide for the Care and Use of Laboratory Animals” (DHHS Publication No. 85–23; NIH).
Materials
The compounds used were GABA (5 mM; Sigma Chemical Company, St. Louis, MO), isoproterenol (4 mM; Sigma Chemical Company), zolpidem (2.6 μM; Research Biochemicals, Inc., Natick, MA), bicuculline methiodide (5 mM; Sigma), and ethanol (40 mM in saline; Aaper Alcohol and Chemical Co., Shelbyville, KY). All drugs listed herein were dissolved in sterilized water unless otherwise noted. Other chemicals used were protease XXIII (Sigma), Alcian Blue 8GX (Sigma), phosphocreatine (Sigma), and creatine phosphokinase (Sigma).
Surgery
Extracellular single-unit recordings were obtained from cerebellar Purkinje neurons. For recording, rats were anesthetized with urethane (1.5 g/kg ip; Sigma) and placed in a David Kopf (Tujunga, CA) stereotaxic apparatus. The technique of in vivo extracellular single-unit recording has been described in detail elsewhere.3,4,8 Briefly, to record from cerebellar neurons, a piece of skull and dura were removed 2 mm posterior to lambda on the midline, and the brain was covered with 2% agar in saline. A needle hole was also made in the atlanto-occiptal membrane of the cisterna magna to drain the cerebrospinal fluid. Single-unit recordings were made from cerebellar Purkinje cells in the vermis, identified by their characteristic firing patterns.13–15
In Vivo Electrophysiological Recordings
For extracellular single-unit recording, seven-barrel pipettes were pulled and their tips broken back to 5 to 7 μm (the opening of each barrel varies from 1 to 2 μm). Separate barrels were filled with a 0.9% saline solution saturated with Chicago sky blue dye (for recording), 3 M NaCl (for current balance), or drugs (for iontophoretic application). This electrode was then lowered into the brain using a hydraulic microdrive (Trent Well, Inc., Southgate, CA). Current for iontophoretic application of drugs was supplied by a four-channel controlled-current source with a 5 V compliance and automatic current counterbalancing. Timing of ejection and holding currents was computer-controlled. Extracellular potentials were amplified by a locally manufactured amplifier incorporating a remote head-stage mounted field-effect transistor for reduced noise. Filtering was 12 dB/octave with lower and upper ½ amplitude cutoff frequencies of 300 Hz and 10 kHZ, respectively. Output of the amplifier was monitored on a Tektronix (Beaverton, OR) oscilloscope and an audio monitor (Radio Shack). Action potentials isolated from background activity with at least a 3-to-1 ratio and a constant duration and configuration were defined as from a single neuron. Individual spikes were digitized by a window comparitor with the digital pulse output fed into a personal computer that generated rate-meter histograms.
For histological verification of the recording site, a dye spot was deposited in brain by passing a 10 μA d.c. current for 10 min through the recording pipette that contains a 0.9% NaCl solution saturated with Chicago sky blue dye. The brain was removed and freeze-mounted onto a microtome chuck. With the use of a cryostat (Damon/IEG Division), 50 μm slices were sectioned, mounted onto slides, and stained with cresyl violet (Fisher, Springfield, NJ). The locations of recording sites were logged onto a rat brain atlas.
Microiontophoresis
Various drugs in this investigation were applied locally to neurons using microiontophoresis. For this procedure, ethanol was dissolved in saline and retained in the pipette by applying a −60 nA holding current. Because ethanol was ejected locally on neurons by lowering the holding current or applying a positive ejection current, control for these manipulations with saline in the pipette was performed using the same conditions as used for ethanol. In this circumstance, saline did not produce any effect on GABA action from 8 of a total of 9 cerebellar Purkinje cells tested when the holding current was lowered from −60 nA to between −40 and +10 nA (ejection current). However, when the change was to a level above +10 nA, an enhancement of GABA was observed in some neurons. Importantly, in the case of the one Purkinje neuron affected when the current was lowered to −40 nA with saline in the pipette, saline decreased the effect of GABA from this cell. Saline also reduced the baseline firing rate of one cell at 0 nA. Therefore, the effect of ethanol was tested on all neurons at expulsion currents of −40 nA to +10 nA to minimize any effect on neural function due to a change in current. GABA, zolpidem, and isoproterenol were retained by applying a negative holding current (−10 nA) to the pipette. When applied continuously, isoproterenol was ejected using currents of +20 nA or less. A control solution held at −10 nA did not produce any effect on GABA action in 8 of 9 cells investigated at ejection currents of 20 nA or lower. Therefore, zolpidem and isoproterenol were tested at ejection currents of 20 nA or lower in this study to minimize any involvement of “current effects” in drug responses and to provide specificity of zolpidem for the type-1 BZD receptor subtype.
After a spontaneously firing neuron was isolated and a stable baseline was established, GABA was applied to the neuron for 10 sec at 1-min intervals by iontophoresis. After a GABA current that produced a reliable 30% inhibition of activity was identified, the effects of brief local application of ethanol and zolpidem on GABA responses were determined. In these cases, zolpidem or ethanol was applied 30 sec before the onset of GABA iontophoresis, with application of these drugs terminating concurrently with GABA offset (which allows a 10-sec overlap). In a few circumstances, ethanol was also applied continuously to selected neurons at current levels previously used to ensure the same effect on GABA responsiveness as obtained with brief application. Subsequently, isoproterenol, a β-adrenergic agonist, was applied continuously for a period of 10 min and the effect of ethanol on the action of GABA was tested 3 min after the onset of the isoproterenol.
In Vitro Electrophysiological Recording
Cerebellar cortex was isolated from 18-to-21-day-old rats, and neurons were dissociated by treatment with protease XXIII (3 mg/ml for 27 min at 24°C). Individual neurons were then isolated by gentle tritruation and allowed to adhere to a glass cover-slip coated with Alcian Blue 8GX. Electrophysiological studies were performed under voltage-clamp in the whole-cell configuration using an Axopatch-ID amplifier. Recording pipettes were fabricated from N 51A capillary glass (Drummond Scientific, Broomall, PA). The internal solution used for measuring ion currents induced by GABA included 150 mM KCI, 3.1 mM MgCl2, 15 mM HEPES, 2 mM K-ATP, 5 mM EGTA, 15 mM phosphocreatine, and 50 units/ml of creatine phosphokinase. Inclusion of the last two items regenerates ATP and GTP and decreases cell rundown.16 This solution was adjusted to pH 7.4 and had an osmolality of 310 (adjusted with sucrose). Seals were formed on the neurons with electrodes having tip resistance of 2 to 4 MΩ. Data were displayed on an oscilloscope, digitized at 50 msec/sample, and stored on a personal computer. Recordings were performed at room temperature in a bath where the neurons were superfused at 0.5 to 1 ml/min with standard external solution [145 mM NaCl, 5 mM KCI, 10 mM HEPES, 2 mM CaCl2, and 10 mM glucose (pH 7.4); 340 mOsm]. GABA (5 μM) and GABA + zolpidem (10, 30, 100, and 300 nM) were applied by a U-tube placed 25 to 50 μM from the neuron for 4-sec intervals, with a minimum of 1 min between applications. This technique allowed a determination of the concentration-response curve for the effect of zolpidem on GABA-gated currents.
Statistics
For each animal, no more than three neurons were sampled in one brain area. If a drug produced at least a 20% change in rate on at least two occasions, the drug was considered to have an effect on the neuron. GABA responses were calculated as the percent change from the baseline firing rate. The formula for the calculation of % change produced by GABA is A = (B − C)/C, where A = % change by GABA, B = firing rate per 10-sec period after GABA, and C = firing rate per 10 sec before GABA. Percent inhibition produced by ethanol, isoproterenol, or zolpidem represents the % change in the difference from baseline firing rate produced by GABA before and after drug application. If a compound reduced or enhanced GABA’s inhibition by at least 20% on at least two occasions, it was considered to be effective. The formula for the calculation of % inhibition by ethanol, isoproterenol, or zolpidem is A = (B − C)/C, where A = % change by drug on GABA responses, B = % change by GABA after drug, and C = % change by GABA before drug. In the cases of continuous application, B and C represent the averages of several tests. To test for differences in the proportion of neurons responsive to test drugs, a χ2 test was used. The means between two groups with and without drug treatment were compared using a two-tailed Student’s t test. Concentration-response curves for the effect of zolpidem on GABA-gated currents from actively dissociated cerebellar Purkinje cells were calculated using GraphPad software. This software used a least-squares algorithm to fit the data to a logistic function with variable slope.
RESULTS
Effect of Zolpidem on Responses to GABA from Cerebellar Purkinje Neurons
A positive relationship has been demonstrated on neurons in several regions of brain between the effectiveness of zolpidem and ethanol to enhance responses to GABA.4,5,8 Although zolpidem binding has been localized to the cerebellar Purkinje cell layer,6 this relationship between the actions of ethanol and zolpidem had not been tested on this cell type. Initially, to establish that GABA was acting on GABAA receptors on the Purkinje neurons, bicuculline was tested against responses to GABA. Local application of bicuculline was found to reduce GABA-induced inhibition by an average of 80.3 ± 4.3% from five cerebellar Purkinje cells, a clear indication that GABAA receptors are present on this cell type.
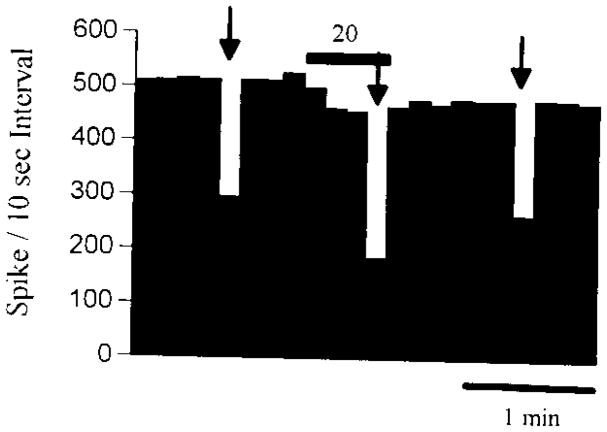
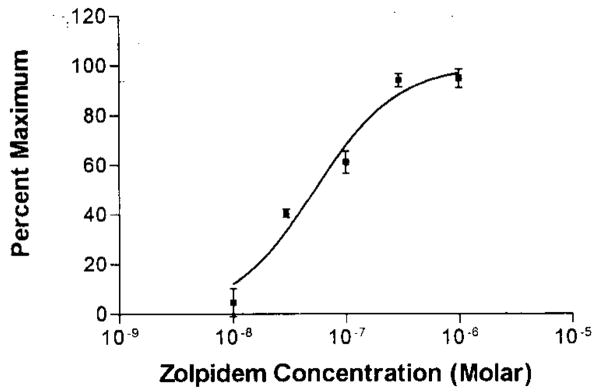
To determine if the GABAA receptors on cerebellar Purkinje neurons were type-1 BZD receptors, the next component of the investigation was to evaluate whether Purkinje neurons would exhibit zolpidem enhancement of responses to GABA after iontophoretic application. Because the concentration of drug reaching a neuron after iontophoretic application cannot be accurately determined, we used very low currents for the release of zolpidem to ensure that the concentration reaching the neuron would have a high probability of being specific for type-1 BZD receptors. Using the currents of zolpidem chosen for this investigation, zolpidem enhanced GABA responses from 12 of 16 (75%) cerebellar Purkinje neurons examined (Table 1). From the neurons, where a positive zolpidem action was observed, the response to GABA was enhanced by 42.3 ± 6.3% (see Fig. 1 for illustration). In addition to the in vivo electrophysiological investigation, we also examined the effect of zolpidem on GABA-gated currents in a limited number (n = 6) of acutely dissociated cerebellar Purkinje cells using whole-cell voltage-clamp recording after direct application of known concentrations of zolpidem and GABA to these neurons. Figure 2 shows the concentration-response curve for the effect of zolpidem on GABA-gated currents from dissociated cerebellar Purkinje neurons. This curve indicates that Purkinje cells are sensitive to low nM concentrations of zolpidem,17 a finding consistent with earlier zolpidem binding data.6,7 Furthermore, a concentration of zolpidem (30 nM) specific for type-1 BZD receptors enhanced responses to GABA from all neurons tested. These latter data provided evidence that the zolpidem enhancement of responses to GABA obtained using in vivo electrophysiological recording was due to an action on type-1 BZD receptors.
Table 1.
Association of Zolpidem Enhancement of GABA to Ethanol Effects on GABA from all Cerebellar Purkinje Neurons Investigated
| Drug applied | Effect of drug on the GABA response |
||
|---|---|---|---|
| Enhanced | Antagonized | No Effect | |
| Zolpidem* | 12/16 (75%) | 0/16 | 4/16 (25%) |
| Ethanol† | 10/49(20%) | 15/49 (31%) | 24/49(49%) |
Numbers in the table for zolpidem are for all neurons investigated. Zolpidem was tested at a range of currents from 10 to 20 nA. Zolpidem produced 42.3 ± 6.3% (n = 12) enhancement on GABA-induced inhibition.
Numbers in the table are for all neurons where ethanol’s effects on responses to GABA were investigated. Ethanol was tested at the range of −40 to 10 nA. Ethanol produced 65.5 ± 9.0% enhancement on GABA-induced inhibition (n = 10). Ethanol inhibited responses to GABA by 51.6 ± 6.4% (n = 15). The average % change produced by ethanol on 24 neurons that were classified as not affected by ethanol was 0.43 ± 2.6% of control GABA-induced inhibition. Ethanol reduced baseline neural activity in 18 of the 49 neurons, but was without an effect on the remaining 31 neurons. See Fig. 3 for examples of ethanol’s action on neural activity and responses to GABA. Ethanol produced significantly different positive results, compared with saline controls (χ2 test, p < 0.005).
Fig. 1.
Rate histogram demonstrating that zolpidem potentiated the GABA-induced depression from a Purkinje cell. Arrows indicate the GABA application to the neuron, and the horizontal bar represents the period of zolpidem application. See Table 1 and text for the magnitude of the zolpidem enhancement of GABA.
Fig. 2.
Concentration-response curve for the enhancement of GABA-gated currents by zolpidem. A logistic curve was fit to the data by GraphPad software, and the ED50 concentration was 52.9 ± 12.4 nM. Dose-response relationship was determined from six neurons. Each point represents the mean ± SEM.
Effect of Ethanol on GABA Responses from Cerebellar Purkinje Neurons
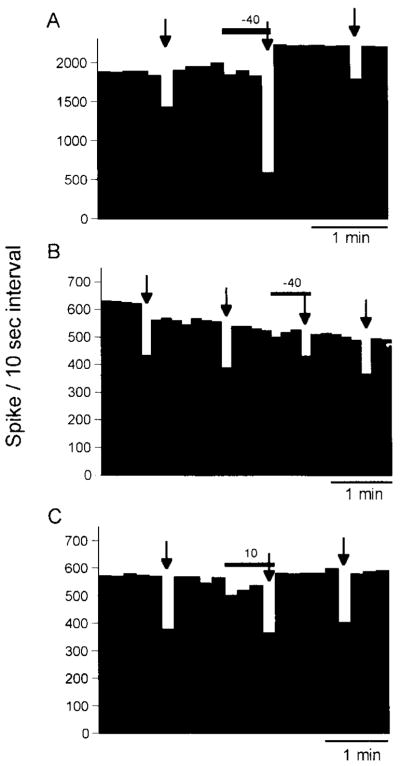
After finding that a majority of cerebellar Purkinje neurons were sensitive to zolpidem enhancement of GABA, the action of ethanol on responses to GABA from cerebellar Purkinje neurons was investigated (Table 1). In addition to reducing baseline neural activity in a portion of neurons (18 of the 49 neurons examined; Table 1), ethanol produced three major patterns of change on GABA inhibition when applied to cerebellar Purkinje neurons (Table 1). Whereas GABA-induced inhibition of cerebellar Purkinje cell activity was enhanced by ethanol, this response pattern occurred on only a small proportion of neurons studied (Table 1). For those neurons where ethanol enhanced GABA responses, the magnitude of the ethanol-induced enhancement was 66.5 ± 9% (n = 10). For the remaining 39 neurons, ethanol either blocked or had no effect on GABA-induced depression of neural activity (Table 1). Examples of these three distinct actions of ethanol on responses to GABA from cerebellar Purkinje neurons are illustrated in Fig. 3. Thus, it was readily apparent from these data that Purkinje neurons sensitive to zolpidem enhancement of GABA were not necessarily sensitive to ethanol enhancement of GABA.
Fig. 3.
Rate histograms demonstrating the three major effects of ethanol on responses to GABA. Arrows indicate the GABA application, and horizontal bar represents the period of ethanol application with the current used shown above each bar (see Table 1 for the relative distribution of response patterns). (A) Illustration of ethanol enhancement of GABA responses. (B) Illustration of ethanol antagonism of the response to GABA. (C) Illustration of the absence of an effect of ethanol on responses to GABA.
Modulatory Effect of Isoproterenol on the Action of Ethanol to Affect Responses to GABA from Cerebellar Purkinje Cells
It has been reported that isoproterenol can sensitize the action of ethanol on GABA-induced inhibition from Purkinje cells.9,10 During the course of our investigation, application of isoproterenol to cerebellar Purkinje neurons enhanced GABA-induced depression in 14 of 18 cells (78%), a confirmation of earlier reports that described this action of isoproterenol on responses to GABA.9–12,18 The % enhancement of GABA responses by isoproterenol was 48 ± 14% (n = 14). Isoproterenol had no effect on responses to GABA from 4 of the 18 neurons.
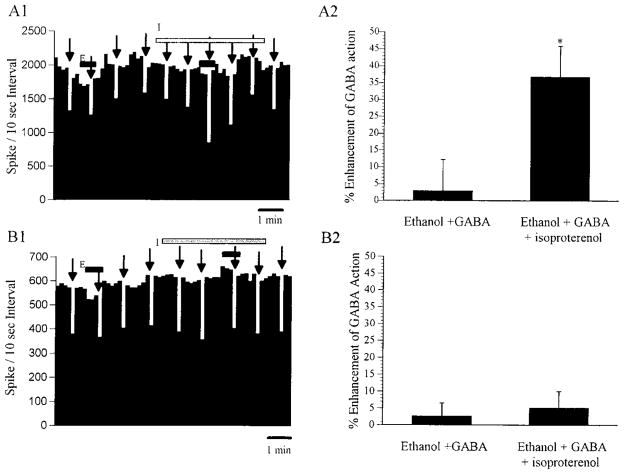
For a set of 15 neurons from which ethanol did not enhance responses to GABA (Table 2), application of ethanol in the presence of continuous application of isoproterenol led to ethanol enhancement of GABA from 8 of these 15 neurons (53%) initially antagonized or not affected by ethanol (Table 2). Compared with a 2.9 ± 9.3% change in the GABA response by ethanol in the absence of isoproterenol, ethanol enhanced the GABA action by 36.7 ± 9.2% in the presence of isoproterenol from these neurons initially insensitive to this action of ethanol. This increased responsiveness in the presence of isoproterenol represented a further enhancement by ethanol over an average of 57 ± 10% enhancement of GABA produced by isoproterenol application alone (n = 8). An illustration of ethanol enhancement of GABA in the presence of isoproterenol is presented in Fig. 4(Al and A2). Thus, these results are consistent with the suggestion that modulation of β-adrenergic receptors can be important for ethanol to potentiate GABA responses from cerebellar Purkinje neurons.9–12 On the other hand, in the presence of isoproterenol, ethanol continued to block the response to GABA from one of those neurons initially antagonized by ethanol (Table 2). Just as important, the presence of isoproterenol did not enable ethanol to enhance responses to GABA from the remaining 6 of the 15 Purkinje neurons insensitive to ethanol. The absence of an effect of isoproterenol on enhancement of GABA by ethanol is illustrated in Fig. 4(B1 and B2).
Table 2.
Effect of Isoproterenol on the Action of Ethanol to Enhance Responses to GABA from Cerebellar Purkinje Neurons
| Ethanol action in the absence of isoproterenol | Ethanol action in the presence of isoproterenol |
|---|---|
| Ethanol enhanced GABA 3/18 (17%) | 2/3 ethanol still enhanced GABA action |
| 1/3 ethanol had no effect on GABA action | |
| Ethanol antagonized GABA 6/18 (33%) | 3/6 ethanol enhanced GABA action |
| 2/6 ethanol had no effect on GABA action | |
| 1/6 ethanol still antagonized GABA action | |
| Ethanol had no effect on GABA 9/18(50%) | 5/9 ethanol enhanced GABA action |
| 4/9 ethanol still had no effect on GABA action |
Isoproterenol was applied at 10 or 20 nA, whereas ethanol was tested at the −40 to 10 nA range.
Fig. 4.
Rate histograms demonstrating the modulatory effects of isoproterenol on ethanol-induced potentiation of GABA inhibition from zolpidem-sensitive cerebellar Purkinje neurons. Closed horizontal bars represent the period of ethanol application, and hatched horizontal bar represents the period of isoproterenol application. (A1) Rate histogram demonstrating that ethanol (10 nA) had no effect on GABA action in the absence of isoproterenol, whereas after the application of isoproterenol (20 nA), ethanol was able to enhance the effects of GABA. (A2) The % enhancement of GABA action before and after the application of isoproterenol. Values represent the mean ± SEM derived from eight neurons in that the GABA actions were enhanced by ethanol in the presence of isoproterenol. Values represent the further enhancement produced by ethanol over the enhancement of GABA action by isoproterenol alone (57 ± 10%). *p < 0.05, compared with control. (B1) Rate histogram demonstrating that ethanol (10 nA) did not enhance the effect of GABA with or without the presence of isoproterenol (20 nA). (B2) The % enhancement of GABA action before and after the application of isoproterenol. Values represent the mean ± SEM derived from seven neurons in that the GABA actions were not further enhanced by ethanol in the presence of isoproterenol.
Relationship between Zolpidem and Ethanol Enhancement of GABA from Individual Neurons in the Presence and Absence of Isoproterenol
The next question explored was whether individual neurons that became sensitive to ethanol enhancement of GABA responsiveness after isoproterenol application would be sensitive to zolpidem. Therefore, the effects of zolpidem and ethanol on the responses to GABA were tested on the same cerebellar Purkinje cells in the absence and presence of isoproterenol. In this regard, zolpidem enhanced GABA responses from a group of 10 Purkinje neurons in which 9 were insensitive to ethanol’s enhancement of GABA inhibition. With the addition of isoproterenol, ethanol enhanced responses to GABA from 5 of the 9 zolpidem-sensitive neurons initially insensitive to ethanol (Fig. 4a). Thus, even though the five neurons where isoproterenol allowed ethanol enhancement of GABA were sensitive to zolpidem, there was a sizeable proportion of zolpidem-sensitive neurons that remained insensitive to the action of ethanol to enhance GABA responses despite the addition of isoproterenol.
DISCUSSION
Ethanol has been demonstrated to enhance responses to GABA in brain regions with [3H]zolpidem binding.3–5 This finding suggested that the zolpidem receptor, classified as a type-1 BZD receptor subtype, was one GABAA receptor sensitive to ethanol.4,5 Further support for this view came from a direct comparison of the action of zolpidem and ethanol on responses to GABA from the same individual neurons, where zolpidem enhancement of GABA was found to be highly predictive of ethanol’s action to enhance GABA responses in several regions of brain.7 In agreement with previous binding data,6,7 the present in vitro data from cerebellar Purkinje neurons confirmed that a relatively high concentration of zolpidem receptors is present on this cell type. Additionally, the mRNAs and proteins for the a,, β2, and γ2 receptor subunits, known to form the type-1 BZD receptor, have been localized to individual cerebellar Purkinje neurons16,19 (unpublished data). In accord with the implication from these earlier neuroanatomic studies that Purkinje neurons would contain functional zolpidem receptors, the majority of Purkinje neurons tested in vitro and in vivo in the present investigation were sensitive to zolpidem enhancement of responses to GABA. For example, voltage-clamp records showed that all Purkinje cells tested responded to 30 nM zolpidem, a dose well below that which acts at type-2 BZD receptors.17 Because some Purkinje neurons did not respond to zolpidem in vivo, it is reasonable to conclude that the concentration of zolpidem reaching those Purkinje cells sensitive to zolpidem enhancement of GABA was low and was likely due to activation of a type-1 BZD receptor. Based on the previous work of Criswell et al.,8 one could have expected the cerebellar Purkinje neurons sensitive to zolpidem to be sensitive to ethanol enhancement of GABA. However, despite the sensitivity of Purkinje neurons to zolpidem, only a small percentage of the cerebellar Purkinje neurons initially exhibited ethanol sensitivity to enhance responses to GABA. Thus, the present results agree with earlier reports that only a portion of cerebellar Purkinje neurons are directly sensitive to ethanol enhancement of GABA.9–12 Consequently, zolpidem does not predict the initial action of ethanol on a large proportion of cerebellar Purkinje neurons, a finding distinct from earlier work where zolpidem was demonstrated to have a high probability of predicting the action of ethanol on a given neuron in other regions of brain.7
The present study confirmed that isoproterenol could enhance responses to GABA from cerebellar Purkinje neurons.8–11 Just as noted for cerebellar Purkinje neurons, Sessler et al.20 found a β-adrenergic input enhanced responses to GABA from neurons in the somatosensory cortex. In agreement with Lin et al.,9–12 application of a β-adrenergic agonist to cerebellar Purkinje neurons, initially insensitive to the action of ethanol to enhance GABA, permitted some Purkinje neurons to become sensitive to ethanol enhancement of GABA responses. On the other hand, application of isoproterenol to a portion of the zolpidem-sensitive neurons did not allow ethanol to enhance responses to GABA. Nonetheless, it should be recognized that, when isoproterenol allowed ethanol to enhance GABA, the neurons were sensitive to zolpidem enhancement of GABA. Obviously, based on the present findings and those of Lin et al.,9–12 the importance of a β-adrenergic input to ethanol enhancement of GABA responses should be explored in other brain regions where zolpidem predicts ethanol’s action to enhance GABA responsiveness.8
Various protein kinases have been implicated in the phosphorylation of GABAA receptors, including protein kinase A,21 protein kinase C,22 and protein tyrosine kinase.23 Freund and Palmer21 found that application of a bromide derivative of cAMP to cerebellar Purkinje neurons allowed ethanol to have an enhancing effect on GABA from neurons previously insensitive to this action of ethanol (i.e., just as seen with isoproterenol). This observation21 was in accord with the view that activation of a cAMP cascade with the subsequent phosphorylation of GABAA receptor protein22 was the basis of the functional changes in ethanol enhancement of GABA in the presence of isoproterenol. In further support of this concept, a protein kinase A (PKA) inhibitor reversed isoproterenol induction of ethanol enhancement of GABA.16 It must be recognized that the zolpidem receptor contains an α1-receptor subunit,17 accompanied by appropriate β- and γ-receptor subunits. Therefore, if isoproterenol allows ethanol enhancement of responses to GABA by phosphorylating a GABAA receptor subunit, a question remaining concerns the exact subunit in the zolpidem receptor that is involved in this process. If, as suggested by the PKA inhibitor investigation,16 a phosphorylation step involving PKA is critical to the action of isoproterenol to allow ethanol enhancement of GABA from cerebellar Purkinje neurons sensitive to zolpidem, this observation would seem to implicate a key role of the β-subunit present in the zolpidem receptor, as this type of subunit can be phosphorylated by PKA.23 Nonetheless, it is emphasized that a portion of neurons sensitive to zolpidem did not become sensitive to ethanol enhancement of GABA with the addition of isoproterenol. This latter observation raises the question whether any other neural inputs could make zolpidem-sensitive cerebellar Purkinje neurons, not initially sensitive to ethanol, sensitive to ethanol enhancement of GABA, just as a portion of the cerebellar Purkinje neurons were affected by isoproterenol. Obviously, future investigations will need to focus on these uncertainties until a clear picture of the basis of the action of ethanol to affect some, but not all, responses from cerebellar Purkinje GABAA receptors sensitive to zolpidem emerges.
An observation in this study that has received little attention is the ethanol-induced antagonism of GABA-induced depression of neural activity of some cerebellar Purkinje neurons.18 Importantly, in some instances, application of isoproterenol to cerebellar Purkinje neurons altered the character of this action of ethanol to inhibit responses to GABA. Given that cerebellar Purkinje neurons exhibiting this response to ethanol were sensitive to zolpidem enhancement of GABA, the basis of this uncommon effect of ethanol on GABA inhibition is unknown and should receive attention.
In summary, the present investigation found that the majority of cerebellar Purkinje neurons are sensitive to zolpidem enhancement of GABA. Despite sensitivity of these neurons to zolpidem, it was also confirmed that only a small portion of cerebellar Purkinje neurons are sensitive to ethanol enhancement of GABA.9–12 Of those zolpidem-sensitive neurons where ethanol did not enhance the action of GABA, some, but not all, cerebellar Purkinje neurons became sensitive to ethanol enhancement of GABA upon addition of the β-adrenergic agonist isoproterenol, in agreement with earlier findings that described this event.9–11 However, the GABA response from some neurons insensitive to ethanol enhancement was not affected by exposure to isoproterenol. This finding suggested the possibility that other neural inputs may be required to affect the action of ethanol on cerebellar Purkinje neurons sensitive to zolpidem. Collectively, whereas these data continue to be consistent with the view that the type-1 BZD receptor subtype is a GABAA receptor sensitive to ethanol, the present work indicates that factors in addition to the structural components of the zolpidem receptor subtype, such as receptor phosphorylation, may also contribute to the action of ethanol on cerebellar Purkinje neurons. However, a major question to be answered is whether zolpidem-sensitive GABAA receptors in other regions of the brain that are sensitive to ethanol,8 may also require additional factors (such as a β-adrenergic input) to exhibit optimal sensitivity to ethanol. This possibility should be the focus of future research.
Acknowledgments
This study was supported by U.S. Public Health Service Grants AA-09122, AA-10025, AA-11596, AA11605, AA-00214, and HD-07201-15.
References
- 1.Givens BS, Breese GR. Electrophysiological evidence that ethanol alters function of medial septal area without affecting lateral septal function. J Pharmacol Exp Ther. 1990;253:95–103. [PMC free article] [PubMed] [Google Scholar]
- 2.Givens BS, Breese GR. Site-specific enhancement of GABA-mediated inhibition of neural activity by ethanol in the rat medial septal area. J Pharmacol Exp Ther. 1990;254:528–538. [PMC free article] [PubMed] [Google Scholar]
- 3.Simson PE, Criswell HE, Breese GR. Ethanol potentiates GABA-mediated inhibition in the inferior colliculus: Evidence for local ethanol/GABA interactions. J Pharmacol Exp Ther. 1991;259:1288–1293. [PubMed] [Google Scholar]
- 4.Criswell JE, Simson PE, Duncan GE, McCown TJ, Herbert JS, Morrow AL, Breese GR. Molecular basis for regionally specific action of ethanol on γ-aminobutyric acidA receptors: Generalization to other ligand-gated ion channels. J Pharmacol Exp Ther. 1993;267:522–537. [PubMed] [Google Scholar]
- 5.Breese GR, Morrow AL, Simson PE, Criswell HE, McCown TJ, Duncan GE, Keir WJ. The neuroanatomical specificity of ethanol action on ligand-gated ion channels: A hypothesis. Proc ISBRA, Alcohol & Alcoholism. 1993;Suppl 2:309–313. [PubMed] [Google Scholar]
- 6.Duncan GE, Breese GR, Criswell HE, McCown TJ, Herbert JS, Devaud LL, Morrow AL. Distribution of 3H-zolpidem binding sites in relation to mRNA encoding the α1, β2, and γ2 subunits for the GABAA receptor in rat brain. Neuroscience. 1995;64:1113–1128. doi: 10.1016/0306-4522(94)00433-6. [DOI] [PubMed] [Google Scholar]
- 7.Niddam R, Dubois A, Scatton B, Arbilla S, Langer S. Autoradiographic localization of [3H]zolpidem binding sites in the rat CNS: Comparison with the distribution of [3H]flunitrazepam binding sites. J Neurochem. 1987;49:890–899. doi: 10.1111/j.1471-4159.1987.tb00977.x. [DOI] [PubMed] [Google Scholar]
- 8.Criswell HE, Simson PE, Knapp DJ, Devaud LL, McCown TJ, Duncan GE, Morrow AL, Breese GR. Effect of zolpidem on γ-aminobutyric acid (GABA)-induced inhibition predicts the interaction of ethanol with GABA on individual neurons in several rat brain regions. J Pharmacol Exp Ther. 1995;273:526–536. [PubMed] [Google Scholar]
- 9.Lin AM-Y, Freund RK, Palmer MR. Ethanol potentiation of GABA-induced electrophysiological responses in cerebellum: Requirement for catecholamine modulation. Neurosci Lett. 1991;127:154–158. doi: 10.1016/0304-3940(91)90846-l. [DOI] [PubMed] [Google Scholar]
- 10.Lin AM-Y, Freund RK, Palmer MR. Sensitization of γ-aminobutyric acid-induced depressions of cerebellar Purkinje neurons to the potentiative effects of ethanol by beta adrenergic mechanisms in rat brain. J Pharmacol Exp Ther. 1993;265:426–432. [PubMed] [Google Scholar]
- 11.Lin AM-Y, Bickford PC, Palmer MR. The effect of ethanol on γ-aminobutyric acid-induced depressions of cerebellar Purkinje neurons: Influence of beta adrenergic receptor action in young and aged Fischer 344 rats. J Pharmacol Exp Ther. 1993;264:951–957. [PubMed] [Google Scholar]
- 12.Lin AM-Y, Freund RK, Hoffer BJ, Palmer MR. Ethanol-induced depressions of cerebellar Purkinje neurons are potentiated by β-adrenergic mechanisms in rat brain. J Pharmacol Exp Ther. 1994;271:1175–1180. [PubMed] [Google Scholar]
- 13.de la Garza R, McGuire TJ, Freedman R, Hoffer BJ. The electrophysiological effects of nicotine in the rat cerebellum: Evidence for direct postsynaptic actions. Neurosci Lett. 1987;80:303–308. doi: 10.1016/0304-3940(87)90472-1. [DOI] [PubMed] [Google Scholar]
- 14.de la Garza R, Freedman R, Hoffer J. κ-Bungarotoxin blockade of nicotine electrophysiological actions in cerebellar Purkinje neurons. Neurosci Lett. 1989;99:95–100. doi: 10.1016/0304-3940(89)90271-1. [DOI] [PubMed] [Google Scholar]
- 15.de la Garza R, Freedman R, Hoffer J. Nicotine-induced inhibition of cerebellar Purkinje neurons: Specific actions of nicotine and selective blockade by mecamylamine. Neuropharmacology. 1989;28:495–501. doi: 10.1016/0028-3908(89)90085-3. [DOI] [PubMed] [Google Scholar]
- 16.Forscher P, Oxford G. Modulation of calcium channels by NE in internally dialyzed avian sensory neurons. J Gen Physiol. 1985;85:743–763. doi: 10.1085/jgp.85.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Criswell HE, McCown TJ, Moy SS, Oxford GS, Mueller RA, Morrow AL, Breese GR. Action of zolpidem on responses to GABA in relation to mRNAs for GABAA isoreceptors subunits within single-cells: Evidence for multiple functional GABAA isoreceptors on individual neurons. Neuropharmacology. 1997;36:1641–1652. doi: 10.1016/s0028-3908(97)00169-x. [DOI] [PubMed] [Google Scholar]
- 18.Freund RK, van Hone CG, Harlan T, Palmer MR. Electrophysiological interactions of ethanol with GABAergic mechanisms in the rat cerebellum in vivo. Alcohol Clin Exp Res. 1993;17:321–328. doi: 10.1111/j.1530-0277.1993.tb00770.x. [DOI] [PubMed] [Google Scholar]
- 19.Khan ZU, Gutierrez A, De Bias AL. The subunit composition of a GABAA/benzodiazepine receptor from rat cerebellum. J Neurochem. 1994;63:371–374. doi: 10.1046/j.1471-4159.1994.63010371.x. [DOI] [PubMed] [Google Scholar]
- 20.Sessler FM, Liu W, Kirifides ML, Mouradian RD, Lin RC-S, Waterhouse BD. Noradrenergic enhancement of GABA-induced input resistance changes in layer V regular spiking pyramidal neurons of rat somatosensory cortex. Brain Res. 1995;675:171–182. doi: 10.1016/0006-8993(95)00060-4. [DOI] [PubMed] [Google Scholar]
- 21.Freund RK, Palmer MK. Beta-adrenergic sensitization of GABAA receptors to ethanol involves a cyclic AMP/protein kinase A second messenger mechanism. J Pharmacol Exp Ther. 1997;280:1192–1200. [PubMed] [Google Scholar]
- 22.Macdonald RL. Ethanol, GABAA receptors, and protein kinase C phosphorylation. Proc Natl Acad Sci USA. 1995;92:3633–3635. doi: 10.1073/pnas.92.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan Q, Man HY, Braunton J, Wang W, Salter MW, Becker L, Wang YT. Modulation of GABAA receptor function by tyrosine phosphorylation of β subunits. J Neurosci. 1997;17:5062–5069. doi: 10.1523/JNEUROSCI.17-13-05062.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]