Abstract
Objective
To examine whether a lack of prostaglandin E receptor 4 (EP4) on bone marrow-derived cells would increase local inflammation and enhance the formation of abdominal aortic aneurysm (AAA) in vivo.
Methods and Results
Prostaglandin E2 (PGE2), through activation of its receptor EP4, can mute inflammation. Hypercholesterolemic low-density lipoprotein receptor knockout (LDLR−/−) mice transplanted with either EP4+/+ [EP4+/+/LDLR−/−] or EP4−/− [EP4−/−/LDLR−/−] bone marrow received infusions of angiotensin II to induce AAA. Deficiency of EP4 on bone marrow–derived cells increased the incidence (50% of male EP4+/+/LDLR−/− vs. 88.9% male EP4−/−/LDLR−/− developed AAA; and 22% of female EP4+/+/LDLR−/− vs. 83.3% female EP4−/−/LDLR−/− developed AAA) and severity of AAA, increased monocyte chemoattractant protein-1 (2.72-fold in males and 1.64-fold in females), and enhanced infiltration of macrophages (3.8-fold in males and 2.44-fold in females) and T cells (1.88-fold in males and 1.66-fold in females) into AAA lesions. Lack of EP4 on bone marrow–derived cells augmented elastin fragmentation, increased apoptotic markers, and decreased smooth-muscle cell accumulation within AAA lesions.
Conclusions
Deficiency of EP4 on bone marrow–derived cells boosted inflammation and AAA formation induced by angiotensin II in hyperlipidemic mice. This study affirms the pathophysiologic importance of PGE2 signaling through EP4 as an endogenous anti-inflammatory pathway involved in experimental aneurysm formation.
Keywords: Prostaglandin E2, EP4, abdominal aortic aneurysm, inflammation
Introduction
Abdominal aortic aneurysms (AAA) exhibit characteristics of a chronic inflammatory disorder.1–3 While much work has focused on pro-inflammatory pathways participating in the pathogenesis of abdominal aortic aneurysm (AAA), knowledge has lagged regarding the role of endogenous anti-inflammatory pathways in this condition. Microsomal PGE2 synthase-1 (mPGES-1), which catalyzes isomerization of the cyclooxygenase product endoperoxide, produces prostaglandin E2 (PGE2). PGE2 predominately acts on four receptors, denoted as EP1, EP2, EP3, and EP4.4 Human AAA tissues display increased biosynthesis of PGE2.5–7 Mouse and human AAA tissues express abundant EP4.8,9 Studies using mPGES-1–deficient mice have shown suppressed formation of angiotensin II (Ang II)-induced AAA.7 These studies support a role for mPGES-1, PGE2 release, and EPs in the pathogenesis of AAA.
PGE2, through EP4, suppresses the production of inflammatory cytokines (including tumor necrosis factor [TNFα], interleukin-12 [IL-12], and interferon-γ [IFN–γ]) and chemokines (including monocyte chemoattractant protein-1 [MCP-1], macrophage inflammatory proteins [MIP-1α, MIP-1β], IFNγ-inducible protein 10 [IP-10], and interleukin-8 [IL-8]) in macrophages in vitro.10–12 All of these molecules can participate in leukocyte recruitment to AAA lesions. Thus, activation of EP4 may constitute a key endogenous anti-inflammatory pathway. Recent studies have illustrated that an EP4 agonist prolonged cardiac allograft survival13 and improved cardiac function after myocardial ischemia reperfusion injury in rodents by reducing inflammation.14,15 The cardioprotective effects exerted by EP4 agonists render them potentially novel approaches to the treatment or prevention of inflammatory disorders of the cardiovascular system.
AAA lesions commonly exhibit loss of elastin, believed to result from the actions of enzymes such as metalloproteinases (MMP) and certain elastolytic cathepsins. PGE2, through EP4 ligation, can increase the expression of MMP-2 and MMP-9 in cultured monocytes and macrophages.16–18 Another study showed that PGE2 decreased MMP-9 in peritoneal macrophages from patients with endometriosis in vitro.19 Moreover, PGE2 influences cell apoptosis, another important component of AAA development. Studies show that EP4 agonism limits macrophage apoptosis, while deficiency of the receptor augments this process.20 Thus EP4 figures in many of the mechanisms implicated in AAA formation.
Screening studies have shown that 4% to 5% of men >60 years of age have small AAA.21,22 Even in these small aneurysms, the inflammatory process appears well established.23,24 Most of these small AAA continue to expand, and can cause substantial morbidity and mortality. Currently, no therapeutic approach can prevent AAA, leaving patients with invasive treatments as their only option. Accordingly, chemopreventive strategies to prevent the expansion of AAA have considerable appeal.
The importance of EP4 in the pathogenesis of AAA is unknown. We tested the hypothesis that the lack of EP4 on bone marrow–derived cells would increase local inflammation and enhance the formation of experimental aneurysm in vivo.
Methods
Hypercholesterolemic low-density lipoprotein receptor knockout (LDLR−/−) mice transplanted with either EP4+/+ or EP4−/− bone marrow received infusions of angiotensin II to induce AAA. EP4+/+ and EP4−/− bone marrow chimera will be referred as EP4+/+/LDLR−/− and EP4−/−/LDLR−/−, respectively, throughout the manuscript. For detailed methods, see the supplement available online at http://atvb.ahajournals.org.
Results
Congenic C57bl/6 LDLR−/− mice that received transplants from bone marrow of EP4+/+ mice or EP4−/− mice appeared healthy. The body weights for female EP4+/+/LDLR−/− mice and female EP4−/−/LDLR−/− mice at the time of tissue harvest were 21.08 ± 2.21 g and 21.74 ± 1.89 g, respectively (values expressed as means ± SD; n=8–12). The body weights for male EP4+/+/LDLR−/− mice and male EP4−/−/LDLR−/− mice at the time of tissue harvest were 21.50 ± 2.00 g and 22.60 ± 1.80 g, respectively (values expressed as means ± SD; n=8–12). The body weights of wild-type chimeric mice were not significantly different from those of knockout chimeric mice.
Survival rates were comparable between the experimental groups. During the four weeks of Ang II infusion, there were no deaths in the male EP4+/+/LDLR−/− group, while one male EP4−/−/LDLR−/− mouse died. In the female group, there was one death for each mouse genotype. All of these deaths occurred within the first week of Ang II infusion. These deaths were not associated with aneurysmal rupture as qualified by the absence of AAA upon dissection; therefore, the aortas of these deceased mice were not used in subsequent analysis.
Infusion of Ang ll for four weeks significantly increased mean arterial blood pressure in high-fat–fed EP4+/+/LDLR−/− and EP4−/−/LDLR−/− mice (blood pressure at week 0 vs. week 4, p<0.01; Figure 1A, 1B). There was no significant difference between the blood pressures of male EP4+/+/LDLR−/− mice and male EP4−/−/LDLR−/− mice, when the entire blood pressure curve was compared by area under curve. Interestingly, for the male mice, the blood pressure of EP4+/+/LDLR−/− mice appeared to be lower than that of EP4−/−/LDLR−/− mice at week 3. Comparison with the non-parametric Mann-Whitney U test at this time point [EP4+/+/LDLR−/− (t = 3 weeks) vs. EP4+/+/LDLR−/− (t = 3weeks)], however, revealed no statistical significance (p=0.0645). For the female group, there was no significant difference whether the blood pressure data of EP4+/+/LDLR−/− mice and EP4−/−/LDLR−/− mice were compared by area under curve or week-by-week (point-by-point during the four separate time points; Figure 1B).
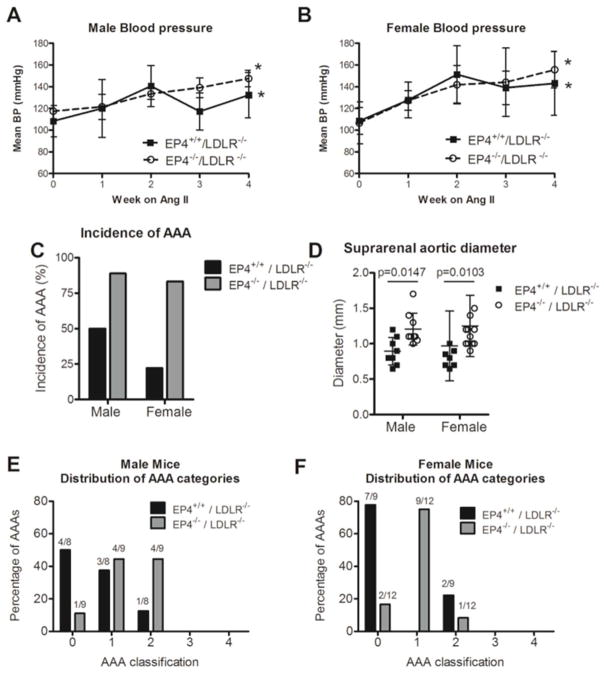
Figure 1.
Blood pressure (BP) response to Ang II infusion in male (A) and female (B) mice. N=5–6 for each experimental group at each time point. *P<0.05 vs. week 0. Absence of EP4 on bone marrow–derived cells enhanced the incidence of AAA formation (C) and increased the severity of disease, as reflected by an increase in the suprarenal aortic diameter (D). Distribution of aneurysm formation into each AAA category for male (E) and female (F) mice. (Type 0 = no aneurysm; Type 1 = suprarenal dilation of 50% increase in aortic diameter without thrombus; Type 2 = suprarenal dilation of 50% increase in aortic diameter with thrombus; Type 3 = multiple aneurysms, including thoracic aneurysms and dissections; Type 4 = death due to aneurysmal rupture.) The number of mice within each category is indicated at the top of the bar. Values are expressed as mean ± SD. Male EP4+/+/LDLR−/− n=8; male EP4−/−/LDLR−/− n=9; female EP4+/+/LDLR−/− n=9; female EP4−/−/LDLR−/− n=12.
Infusion of Ang II yielded aneurysms in the abdominal aorta differently, depending on the donor cell genotype. EP4+/+/LDLR−/− mice had a lower incidence of Ang II-induced AAA compared to EP4−/−/LDLR−/− mice, in both male and female mice (Figure 1C). In male mice, the prevalence of AAA was 50% for EP4+/+/LDLR−/− and 88.9% for EP4−/−/LDLR−/−. In female mice, the prevalence of AAA was 22% for EP4+/+/LDLR−/− and 83.3% for EP4−/−/LDLR−/−. The five-level classification scheme described in the Methods section characterized the complexity of the aneurysms formed. Among males, many EP4+/+/LDLR−/− mice did not develop aneurysms, while the majority of aneurysms in EP4−/−/LDLR−/− fell into class 1 or 2 (Figure 1E). Similarly, for females, many EP4+/+/LDLR−/− mice did not develop observable aneurysms, while most aneurysms in EP4−/−/LDLR−/− mice fell into class 1 (Figure 1F). All aneurysms formed at the suprarenal region of the aorta. The diameters of the suprarenal aortas in both male and female EP4−/−/LDLR−/− mice were wider on average than their EP4+/+/LDLR−/− counterparts (Figure 1D). Over all, deletion of EP4 on bone marrow–derived cells increased the incidence and severity of experimental aneurysm.
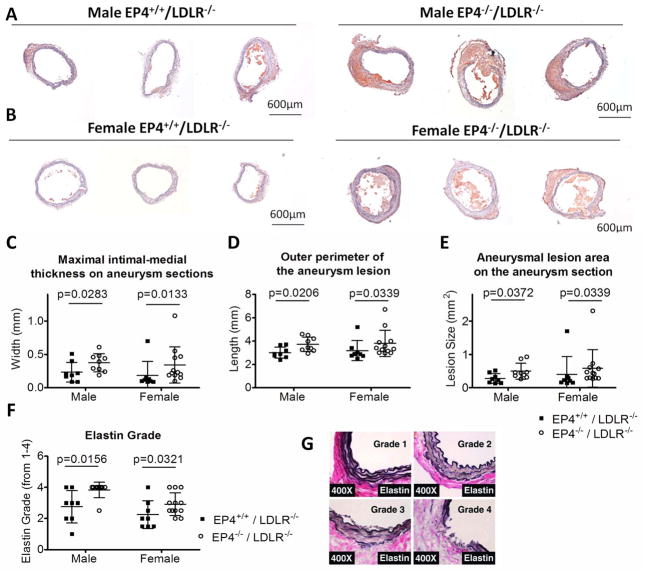
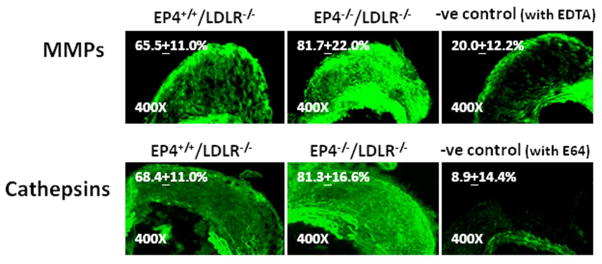
Cross-sectioning of the suprarenal region of the aorta revealed perimedial remodeling in many mice that visual inspection could not identify (Figure 2). EP4−/−/LDLR−/− mice had greater maximal intimal-medial thickness (as measured by the maximal distance from the periphery to the closest part of the lumen on a particular aneurysm section; Figure 2C), greater outer perimeter of the aneurysm section (Figure 2D), and larger aneurysmal lesion area (Figure 2E), compared with EP4+/+/LDLR−/− mice. This pattern applied to both male and female mice. The 0-to-4 scale described in the Methods section graded the degree of elastin fragmentation on AAA lesions among different experimental groups (Figure 2G). In the overall comparison, where sections regardless of AAA grade were included, the aneurysm lesions of EP4−/−/LDLR−/− mice had greater elastin fragmentation than those from EP4+/+/LDLR−/− mice (Figure 2F). Furthermore, when aneurysm lesions of the same grade were compared, EP4−/−/LDLR−/− mice had enhanced MMP and cathepsin elastolytic activity (n=3–4; Figure 3).
Figure 2.
Effect of EP4 deletion on bone marrow–derived cells on aneurysm lesional morphology in LDLR−/− mice. Representative frozen sections show the severity of aneurysm in the male (A) and female (B) experimental mice. All photographs were taken at 4x magnification. The maximal intimal-medial thickness (C), the outer perimeter (D), and the lesion size (E) of the aneurysm lesions, as determined by analysis on frozen cross-sections, are increased in mice deficient in EP4 on their bone marrow–derived cells. The maximal intimal-medial thickness is a measurement of the thickness of the artery walls, and the maximal intimal-medial width on a particular section is used. The aneurysmal lesion size is the total area of the suprarenal aneurysmal section. Severity of medial elastin degradation (determined by Verhoeff-Van Gieson stain) on aneurysm sections of EP4+/+/LDLR−/− mice and EP4−/−/LDLR−/− mice are shown in panel F. Elastin degradation grading (4 grades) keys are indicated in panel G. Male EP4+/+/LDLR−/− n=8; male EP4−/−/LDLR−/− n=9; female EP4+/+/LDLR−/− n=9; female EP4−/−/LDLR−/− n=12. Significant p values are indicated on the graph.
Figure 3.
Elastolytic activity on aneurysms of the same grade was compared between groups. Representative photographs showing in situ elastin activity zymography by MMP (top panel) and cathepsins (bottom panel) on type 1 male EP4+/+/LDLR−/− and type 1 male EP4−/−/LDLR−/− aneurysm lesions. Lesions that lack EP4 emit greater fluorescence, representing greater elastolytic activities. All images were obtained using the same bright field, shutter speed, and magnification. MMP activity was determined at pH 7.4 (with E64 to inhibit cathepsin activities), and cathepsin activity was determined at pH 5.5 (with EDTA to inhibit MMP activities). These conditions allow optimal activity for the respective enzymes. Negative controls (EDTA and E64 were added to MMP and cathepsin measurements, respectively) and no-substrate controls (data not shown; fluorescence emission was negligible) were performed in parallel sections on the same slides for all experiments. Fluorescence intensity (FI), as measured using computer-assisted image quantification, is expressed in percentage of fluorescence area over aneurysm lesion area on each cross-section, excluding the media area due to medial elastin filament autofluoresence. Average FIs (% of aneurysm lesion area) are indicated on the image (FI ± SD; n=3–4).
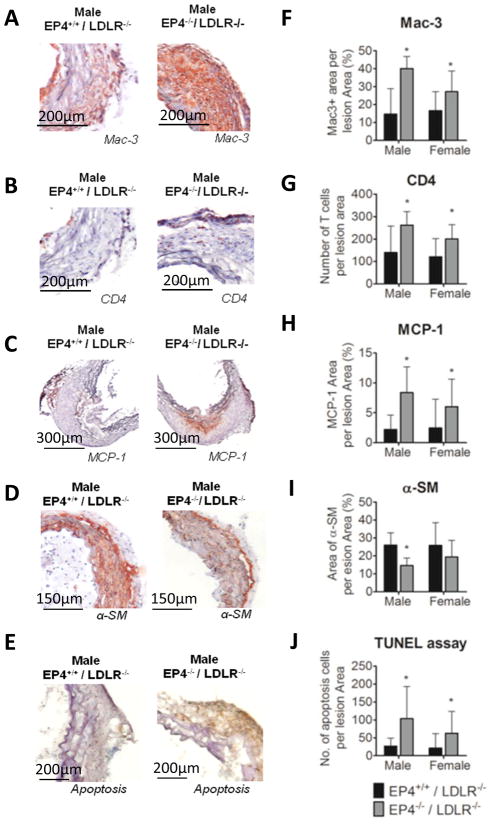
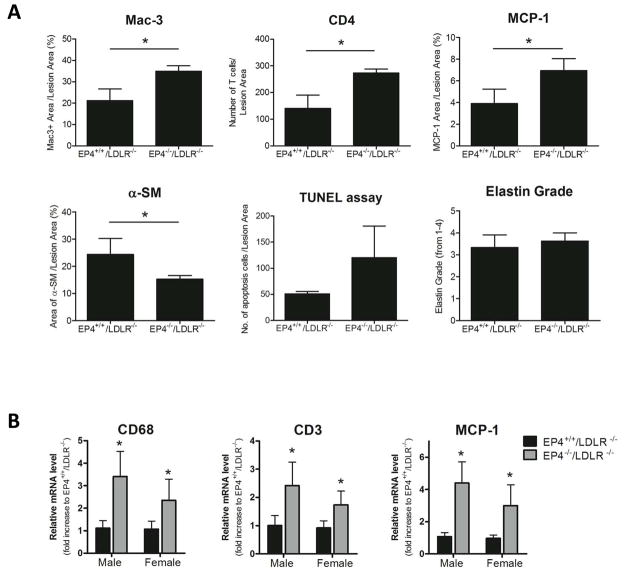
Deletion of EP4 on bone marrow cells increased cells positive for mac-3 (a marker for macrophages; Figure 4A, 4F) and for CD4 (a marker for T cells; Figure 4B, 4G) in AAA lesions. The percentage of mac-3 positive area on aneurysmal lesions of EP4−/−/LDLR−/− mice increased significantly, by 2.72-fold in male mice and by 1.64-fold in female mice, compared to EP4+/+/LDLR−/− mice. The amount of T cells in lesions of EP4−/−/LDLR−/− mice increased significantly — 1.88-fold in male mice and 1.66-fold in female mice — compared to EP4+/+/LDLR−/− mice. The lack of EP4 on bone marrow–derived cells also increased expression of MCP-1 in the aneurysm lesions (Figure 4C, 4H). The percentage of MCP-1 positive area on aneurysm lesions of EP4−/−/LDLR−/− mice was significantly increased — 3.8-fold in male mice and 2.44-fold in female mice — compared to EP4+/+/LDLR−/− mice. These findings indicate that AAA lesions of EP4−/−/LDLR−/− mice have greater inflammation. AAA lesions of EP4−/−/LDLR−/− mice had fewer medial SMC, as reflected by α-actin positive cells (Figure 4D, 4I). Compared to aneurysm lesions of EP4+/+/LDLR−/− mice, those of EP4−/−/LDLR−/− mice had fewer SMC — 43.99% in males, 24.75% in females. More apoptotic cells localized in EP4−/−/LDLR−/− aneurysmal lesions (Figure 4E, 4J), in both male and female mice. Figure 4 illustrates entire group comparisons: the mac-3, CD4, MCP-1, α-smooth muscle, and TUNEL staining on sections; all grades of aneurysm lesion were included.
Figure 4.
Characterization of AAA lesions. Aneurysm lesional sections were stained for mac-3 (A), CD4 (B), MCP-1 (C), α-SM (D), and apoptotic markers (E) to detect macrophages, T cells, chemokines, SMC, and apoptotic cells, respectively. Quantitative analyses of the staining revealed that lesions in EP4−/−/LDLR−/− mice have significantly increased mac-3-positive area (F), number of CD4-positive cells (G), MCP-1-positive area (H), and number of apoptotic cells (J). Aneurysm lesions in EP4−/−/LDLR−/− mice had a smaller area positively stained for α-SM (I). All positive staining is reflected by a reddish-brown color, except apoptotic cells, which appear brown. Male EP4+/+/LDLR−/− n=8; male EP4−/−/LDLR−/− n=9; female EP4+/+/LDLR−/− n=9; female EP4−/−/LDLR−/− n=12; *p<0.05 vs. EP4+/+/LDLR−/− of the same sex.
In addition to overall group-to-group comparison, where sections from all grades of aneurysm lesions were included and analyzed, we compared statistically aneurysms of the same grade between groups. This approach enabled us to segregate whether changes in the parameters studied were mere consequences of an increased incidence of AAA, or direct changes on localized inflammation caused by the absence of EP4 on immune cells. In the male group, 3 of 8 AAA in EP4+/+/LDLR−/− mice were of grade 1, and 4 of 9 EP4−/−/LDLR−/− mice had the same grade. In the female group, 2 of 9 EP4+/+/LDLR−/− mice had type 2 AAA, and only 1 of 12 EP4−/−/LDLR−/− mice had the same grade. Because of the low number of sections of the same grade in female mice, comparison of AAA of the same grade between groups was carried out using sections from male mice only. Mac-3, CD4, MCP-1, α-smooth muscle, TUNEL staining, and elastin grade on type 1 AAA sections of EP4+/+/LDLR−/− mice were compared to type 1 AAA sections of EP4−/−/LDLR−/− mice (Figure 5a). Despite the low number of type 1 aneurysm lesions in male mice (n=3–4), the amount of mac-3 (p=0.0183), CD4 (p=0.0067), and MCP-1 (p=0.0468) staining on type 1 aneurysm lesions of EP4−/−/LDLR−/− mice was significantly greater than those found in type 1 aneurysm lesions from wild-type chimeric mice. Type 1 aneurysm lesions in EP4−/−/LDLR−/− mice had significantly fewer α-actin positive cells than their wild-type counterparts (p=0.0392). The amount of apoptotic cells appeared greater in EP4−/−/LDLR−/− mice compared to wild-type chimeric mice, but this did not reach statistical significance (which may be due to the low number of these lesions (n=3–4), the small effect size, or both). The degree of elastin fragmentation between type 1 lesions of EP4+/+/LDLR−/− mice and EP4−/−/LDLR−/− mice was not significantly different (Figure 5a). Thus, the increase in mac-3, CD4, MCP-1, and apoptotic cell staining, and the decrease in α-smooth muscle in aneurysmal lesions of EP4−/−/LDLR−/− mice, appear to be directly linked to the absence of EP4 on bone marrow–derived cells. However, the enhanced elastin fragmentation in EP4−/−/LDLR−/− mice, observed when sections from all grades of aneurysm lesions were averaged, appears to be a mere consequence of an increase in AAA cadence in the knockout group rather than a direct effect.
Figure 5.
A) Comparison of AAA sections of the same grade between groups. Type I AAA lesional sections from male EP4+/+/LDLR−/− mice (n=3) and EP4−/−/LDLR−/− mice (n=4) stained for mac-3, CD4, MCP-1, α-SM, apoptotic markers and graded for elastin fragmentation were compared; values expressed are mean ± SD; *P<0.05. B) Quantitative RT-PCR analysis of CD68 (a marker for macrophages), CD3 (a marker of T cells), and MCP-1 in suprarenal segments of aortas of EP4+/+/LDLR−/− mice and EP4−/−/LDLR−/− mice. Aortas from all mice regardless of AAA type were used. Expression was quantitated relative to EP4+/+/LDLR−/− mice (of the same sex) and corrected for expression of GAPDH. n=8–12; values shown are means ± SD. *p<0.05 vs. EP4+/+/LDLR−/− (of the same sex).
To consolidate our data further, RT-PCR experiments using suprarenal segments of the aorta where aneurysm occurred were performed (aortas from all mice regardless of AAA type were used for the RT-PCR experiment; Figure 5b). The mRNA expression of CD3 (a marker for T cells), CD68 (a marker for macrophages), and MCP-1 rose significantly in aortas of EP4−/−/LDLR−/− mice, compared to EP4+/+/LDLR−/− mice (both male and female). Comparison of aneurysms of the same grade among groups showed significantly higher mRNA expression of CD3, CD68, and MCP-1 in aortas of EP4−/−/LDLR−/− mice compared to EP4+/+/LDLR−/− mice, despite the small n values (data not shown; n=3–4).
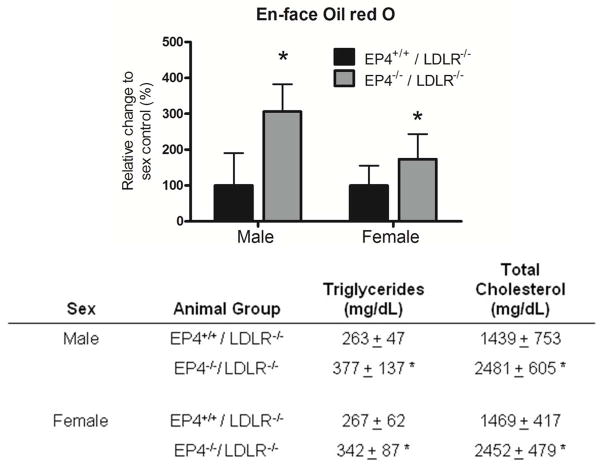
Deletion of EP4 on bone marrow–derived cells increased the amount of lipid in the thoracic aorta, as reflected by the amount of Oil red O staining (Figure 6). The lack of EP4 on bone marrow–derived cells in mice infused with Ang II, produced increased levels of non-fasting plasma triglycerides and total cholesterol (Figure 6). In the absence of Ang II infusion, however, there was no difference in plasma lipids between EP4+/+/LDLR−/− mice and EP4−/−/LDLR−/− mice. The plasma cholesterol levels in the female EP4+/+/LDLR−/− mice and female EP4−/−/LDLR−/− mice (high-fat fed for 5 weeks but without Ang II infusion) were 639 ± 176 mg/dl ± and 758 ± 367 mg/dl, respectively. The plasma triglyceride levels in the female EP4+/+/LDLR−/− mice and female EP4−/−/LDLR−/− mice (high-fat fed for 5 weeks but without Ang II infusion) were 208 ± 50 mg/dl and 244 ± 96 mg/dl, respectively (values are expressed in means ± SD; n=5).
Figure 6.
Deletion of EP4 receptor on bone marrow–derived cells enhanced atherogenesis in LDLR−/− mice infused with Ang II. Quantitative analysis of thoracic aortas stained with oil red O to determine the extent of atherosclerosis (represented as percentage change against same sex controls) for all experimental groups (top); plasma lipid profile of male and female EP4+/+/LDLR−/− and EP4−/−/LDLR−/− mice with Ang II infusion (bottom). Data are shown as mean ± SD. Male EP4+/+/LDLR−/− n=8; male EP4−/−/LDLR−/− n=9; female EP4+/+/LDLR−/− n=9; female EP4−/−/LDLR−/− n=12; *p<0.05 vs. EP4+/+/LDLR−/− of the same sex.
Discussion
Our study demonstrates that mice deficient in EP4 on bone marrow–derived cells have accentuated aneurysm development compared to wild-type mice. Because PGE2 suppresses the production of chemokines such as MCP-1 in human macrophages through EP4 in vitro,12 we tested whether the greater incidence and severity of AAA observed in EP4−/−/LDLR−/− mice was associated with elevated MCP-1. Indeed, expression of MCP-1 in AAA lesions of EP4−/−/LDLR−/− mice increased 3.79-fold in male mice and 2.44-fold in female mice, compared to EP4+/+/LDLR−/− mice. MCP-1 promotes the recruitment of inflammatory cells, and the aneurysm lesions of EP4-deficient bone marrow chimeric mice had markedly increased accumulation of macrophages and T cells, signifying an escalation of local inflammation. The mRNA expression data agree with the histology data. Moreover, comparisons between aneurysms of the same grade showed greater expression of macrophages, T cells, and MCP-1 in aneurysm lesions of EP4−/−/LDLR−/− mice, indicating that these changes result from the lack of EP4 on bone marrow–derived cells.
In overall group-to-group comparison, comparing aneurysms of the same grade, the aneurysm lesions of EP4−/−/LDLR−/− mice had fewer SMC than those of EP4+/+/LDLR−/− mice. SMC constitute a major source of elastin and collagen, and thus contribute to repair and maintenance of the arterial extracellular matrix. Depletion of SMC may weaken the media and favor aneurysm formation. Additionally, aneurysm lesions of EP4−/−/LDLR−/− mice showed more apoptotic cells than EP4+/+/LDLR−/− mice in overall group-to-group comparison, and similar propensity when aneurysms of the same grade were compared. This observation corresponds with a recent study showing that EP4 deficiency increases apoptosis in mouse atheromata.20 SMC loss due to apoptosis may cause accelerated AAA lesion formation in EP4−/−/LDLR−/− mice.
Infiltration of monocytes and macrophages into the vessel wall presents a major source of proteolytic enzymes, including MMPs and cathepsins, which promote matrix degradation, thus impairing the integrity of the artery wall and favoring aneurysm development.25,26 When aneurysm lesions of the same grade were compared, EP4−/−/LDLR−/− mice had greater MMP and cathepsin elastolytic activities than EP4+/+/LDLR−/− mice, as determined by in situ zymography using DQ-elastin as a substrate. This observation could result from the more numerous macrophages residing within the EP4−/−/LDLR−/− aneurysm lesions, or EP4 may directly influence the expression of proteolytic activities. Under some conditions, PGE2 may augment MMP-9 production in macrophages in an EP4-dependent manner.18 Conversely, PGE2 may decrease MMP-9 through EP4.19 The former study performed experiments on resting macrophages obtained from thioglycollate-elicited peritoneal macrophages in mice, while the latter study used peritoneal macrophages from human patients with endometriosis. The experimental settlings of the two studies differed greatly, and the cause of the discrepancy remains unknown. The present in vivo and in situ results support the latter observation, and suggest that EP4-deficient bone marrow–derived cells enhance the ability of MMP and cathepsins to break down elastin within the aneurysm lesions.
Targeted disruption of the gene for cyclooxygenase-2 or mPGES-1 exhibited cardioprotective effects, depressing PGE2 production and retarding AAA formation.7,27 Thus, EP ligand(s) derived from cyclooxygenase-2 and mPGES-1 promote AAA pathogenesis. The specific, responsible EP receptor remains undefined, but activation of EP4 does not likely explain this result. As demonstrated here, EP4 reduces the incidence and severity of AAA. Due to the existence of multiple EP receptors and the opposing effect each one may deliver, selective inhibitors (or agonists, as in the case of EP4) for individual EP receptors may provide a more attractive option than upstream inhibition of cyclooxygenase or mPGES-1 in the treatment or prevention of AAA.
Male and female EP4+/+/LDLR−/− mice exhibited 50% and 22% incidence of AAA formation, respectively — lower rates than those reported in previous studies using the same technique to induce aneurysm.7,28,29 Furthermore, the AAA had lower grades than previously reported. In our study, all recipient mice had irradiation for bone marrow transplantation, which may retard AAA development. In our pilot study, where non-irradiated LDLR−/− mice served as recipient mice to determine the required dose of Ang II for aneurysm generation, 100% (4 of 4) of the mice infused with 1000 ng/kg/min Ang II developed type 3 or type 4 AAA.
PGE2 did not suppress chemokine expression in human EC and SMC treated with lipopolysaccride (LPS) and proinflammatory cytokines (but only in macrophages),12 and such findings justify the rationale for examining EP4 specifically in bone marrow–derived cells in our study. EP4−/− mice do not survive in the congenic C57BL/6 strain; the majority die soon after birth due to ineffective closure of the ductus arteriosus.30 Breeding on a mixed-strain background increases survival of EP4-deficient mice. Thus, bone marrow transplantation was performed using bone marrow cells from mice of a mixed background (129/Olac, C57BL/6, DBA/2). Presumably, the assorted genetic background offers alleles that provide alternative mechanisms for the ductal closure.30 All bone marrow transplantation in our study used EP4−/− mice and their wild-type littermates of the same mixed background. Hence, any observed differences between experimental groups do not result from the bone marrow transplantation mismatch.
It is generally understood,31 and apparent in EP4+/+/LDLR−/− mice, that male mice have greater susceptibility to AAA formation than female mice; interestingly, the incidence of AAA appeared to be equal between the male and female EP4−/−/LDLR−/− recipients in our study. Additionally, AAA lesional sections from male and female EP4+/+/LDLR−/− mice have similar levels of mac-3 (a marker of macrophages) and CD4 (a marker for T cells). However, in EP4−/−/LDLR−/− mice, mac-3 and CD4 staining appeared greater in male mice than in female mice. The mRNA expression data are in line with these histology data. These observations suggest that the differences in AAA incidence between the sexes could relate to the presence of EP4 on bone marrow–derived cells and differences in recruitment of leukocytes into aneurysmal lesions. Androgen is a primary cause for the enhanced propensity of Ang II-induced AAA in male mice, as it increases Ang II type 1A receptor (AT1aR).32 Similarly, castration has been shown to decrease AT1aR abundance in mouse abdominal aortas and prevent AAA formation, whereas administration of dihydrotesterone increases AT1aR expression and promotes AAA incidence in castrated male mice and in female mice.33 Although androgen is one of the primary mediators driving sex differences in Ang II-induced AAA, this does not exclude a role for EP4 in sex differences in Ang II-induced AAA. The expression of EP4 seems subject to regulation by androgen and progesterone in different cell types.34–36 Furthermore, PGE2 may regulate the activity of aromatase enzyme (a key enzyme that converts testerosterone into estradiol).37,38 Judging from these observations, EP4 may contribute to steriodogenesis and circuitously influence AAA prevalence in Ang II-induced AAA mouse models through hormonal regulation.
During harvesting of the aorta for analysis, EP4+/+/LDLR−/− mice and EP4−/−/LDLR−/− mice exhibited unanticipated differences in the degree of lipid deposits on the thoracic aorta. Thus, we stained these lipids with Oil red O to quantify the differences. Indeed, aortas of EP4−/−/LDLR−/− mice had significantly more lipids en face. Profiling plasma for lipids revealed higher triglycerides and total cholesterol in EP4−/−/LDLR−/− mice than in EP4+/+/LDLR−/− mice. The differences in plasma cholesterol and triglycerides between wild-type mice and knockout chimeric mice only occur with Ang II administration. Hence, EP4 contributes to Ang II regulation of lipid metabolism. Ang II modulates lipid metabolism at many levels: it can affect cellular lipid peroxidation, increase cellular oxLDL uptake by macrophages (via lectin-like receptor for oxLDL), alter insulin sensitivity, affect adipocyte differentiation, and inhibit lipolysis in adipose tissues. Resolution of the involvement of EP4 in these Ang II-dependent pathways is beyond the scope of the present study, and is a task for future research.
Hyperlipidemia in EP4−/−/LDLR−/− mice complicates the interpretation of this study. There are two possible explanations for the enhanced AAA formation in EP4−/−/LDLR−/− mice: 1) the lack of EP4 on bone marrow cells results in greater local inflammation; 2) the lack of EP4 on bone marrow cells results in the elevation of plasma lipids. The present study cannot discriminate between the impact of altered lipid metabolism and the role of local inflammatory changes on AAA development in EP4−/−/LDLR−/− mice. Changes in AAA incidence or severity and characteristics in EP4−/−/LDLR−/− mice may result from both mechanisms. We cannot conclude that the enhancement of AAA formation resuts solely from the anti-inflammatory role of EP4, but several observations support this hypothesis: 1) Overall comparison, where all grades of aneurysmal lesions were included in the analysis, revealed greater protein expression of MCP-1, macrophages, and T cells in aneurysmal lesions of EP4−/−/LDLR−/− mice, indicative of an increase in localized inflammation; 2) In analysis of aneurysm lesions of the same grade among groups, the amounts of MCP-1 and inflammatory cells (macrophages and T cells) were increased in sections derived from EP4−/−/LDLR−/− mice, despite the small n values; 3) In RT-PCR experiments using suprarenal sections of the aorta where aneurysms occurred (from all mice regardless of AAA type), the mRNA expression of CD3, CD68, and MCP-1 were increased in aortas of both male and female EP4−/−/LDLR−/− mice; 4) When aneurysms of the same grade among groups were compared, the mRNA expression of CD3, CD68, and MCP-1 remained significantly higher in aortas of EP4−/−/LDLR−/− mice compared to EP4+/+/LDLR−/− mice, despite the low n values. These observations collectively support a role of EP4 in suppressing localized inflammation in aneurysm lesions. In addition, our group has previously demonstrated that PGE2 suppresses macrophage-derived chemokine production via EP4 in vitro.12 In LPS-treated human macrophages, PGE2 attenuated LPS-induced mRNA and protein expression of chemokines, including MCP-1, IL-8, MIP1α, MIP1β, and IP-10. A selective EP4 antagonist completely reversed PGE2-mediated suppression of chemokine production. PGE2 also inhibited TNFα-, IFNγ-, and IL-1β-mediated expression of these chemokines.12 These in vitro data strengthen the idea that the greater AAA formation in EP4−/−/LDLR−/− mice results from greater local inflammation. The possibility that EP4 may modulate lipids is intriguing, and opens an exciting field of future research. Few studies have explored the role of EP4 in lipid metabolism. Reports have suggested that activation of EP4 suppresses food intake in mice,39,40 and in vitro studies have suggested that EP4 suppresses adipocyte differentiation.41 Mechanisms that underlie these effects remain unexplored.
In conclusion, this study shows that absence of EP4 on bone marrow–derived cells dramatically increases AAA formation induced by infusion of Ang II in hyperlipidemic mice. The lack of EP4 on bone marrow cells increased MCP-1 expression, infiltration of macrophages and T cells, elastin fragmentation, apoptosis, and MMP and cathepsin elastolytic activity, and decreased SMC density within the aneurysmal lesions. Our data support the importance of EP4 on bone marrow cells in suppressing local inflammation during the pathogenesis of experimental aneurysm.
Supplementary Material
Acknowledgments
This was supported in part by grants from the National Heart, Lung and Blood Institute (HL-34636 to Dr. Libby, HL-67249 to Dr. Sukhova). Dr. Tang received a fellowship from the Croucher Foundation. We thank Dr. Beverly Koller of the University of North Carolina for providing the EP4 wild-type and knockout mice.
Non-standard Abbreviations and Acronyms
- AAA
abdominal aortic aneurysm
- Ang II
angiotensin II
- BP
blood pressure
- EP
prostaglandin E2 receptor
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- LDLR−/−
low-density lipoprotein receptor knockout mice
- MCP-1
monocyte chemoattractant protein-1
- MMP
metalloproteinases
- mPGES-1
microsomal PGE2 synthase-1
- PGE2
prostaglandin E2
- SMC
smooth-muscle cells
References
- 1.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 2.Middleton RK, Lloyd GM, Bown MJ, Cooper NJ, London NJ, Sayers RD. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: a protein array study. J Vasc Surg. 2007;45:574–580. doi: 10.1016/j.jvs.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Lindeman JH, Abdul-Hussien H, Schaapherder AF, Van Bockel JH, Von der Thüsen JH, Roelen DL, Kleemann R. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurismal from atherosclerotic aorta: IL-6- and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin Sci (Lond) 2008;114:687–697. doi: 10.1042/CS20070352. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 5.Holmes DR, Wester W, Thompson RW, Reilly JM. Prostaglandin E2 synthesis and cyclooxygenase expression in abdominal aortic aneurysms. J Vasc Surg. 1999;25:810–815. doi: 10.1016/s0741-5214(97)70210-6. [DOI] [PubMed] [Google Scholar]
- 6.Walton LJ, Franklin IJ, Bayston T, Brown LC, Greenhalgh RM, Taylor GW, Powell JT. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation. 1999;100:48–54. doi: 10.1161/01.cir.100.1.48. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Lee E, Song W, Ricciotti E, Rader DJ, Lawson JA, Pure E, FitzGerald GA. Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II-induced abdominal aortic aneurysm formation. Circulation. 2008;117:1302–1309. doi: 10.1161/CIRCULATIONAHA.107.731398. [DOI] [PubMed] [Google Scholar]
- 8.Bayston T, Ramessur S, Reise J, Jones KG, Powell JT. Prostaglandin E2 receptors in abdominal aortic aneurysm and human aortic smooth muscle cells. J Vasc Surg. 2003;38:354–359. doi: 10.1016/s0741-5214(03)00339-2. [DOI] [PubMed] [Google Scholar]
- 9.Hristovska AM, Rasmussen LE, Hansen PB, Nielsen SS, Nusing RM, Narumiya S, Vanhoutte P, Skott O, Jensen BL. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension. 2007;50:525–530. doi: 10.1161/HYPERTENSIONAHA.107.088948. [DOI] [PubMed] [Google Scholar]
- 10.Van der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilkens C, Snijders A, Vermeulen H, van der Meide P, Wierenga E, Kapsenberg M. Accessory cell-derived interleukin-12 and prostaglandin E2 determine the level of interferon-gamma produced by activated human CD4+ T cells. Ann N Y Acad Sci. 1996;795:349–350. doi: 10.1111/j.1749-6632.1996.tb52689.x. [DOI] [PubMed] [Google Scholar]
- 12.Takayama K, Garcia-Cardena G, Sukhova G, Comander J, Gimbrone MA, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002;277:44147–44154. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa M, Suzuki J, Kosuge H, Takayama K, Nagai R, Isobe M. The mechanism of anti-inflammatory effects of prostaglandin E2 receptor 4 activation in murine cardiac transplantation. Transplantation. 2009;87:1645–1653. doi: 10.1097/TP.0b013e3181a5c84c. [DOI] [PubMed] [Google Scholar]
- 14.Xiao CY, Yuhki K, Hara A, Fujino T, Kuriyama S, Yamada T, Takayama K, Takahata O, Karibe H, Taniguchi T, Narumiya S, Ushikubi F. Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation. 2004;109:2462–2468. doi: 10.1161/01.CIR.0000128046.54681.97. [DOI] [PubMed] [Google Scholar]
- 15.Hishikari K, Suzuki J, Ogawa M, Isobe K, Takahashi T, Onishi M, Takayama K, Isobe M. Pharmacological activation of the prostaglandin E2 receptor EP4 improves cardiac function after myocardial ischaemia/reperfusion injury. Cardiovasc Res. 2009;81:123–132. doi: 10.1093/cvr/cvn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cipollone F, Prontera C, Pini B, Marini M, fazia M, Cesare DD, Iezzi A, Ucchino S, Boccoli G, Saba V, Chiarelli F, Cuccurullo F, Mezzetti A. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E2-dependent plaque instability. Circulation. 2001;104:921–927. doi: 10.1161/hc3401.093152. [DOI] [PubMed] [Google Scholar]
- 17.Cipollone F, Fazia ML, Iezzi A, Cuccurullo C, De Cesare D, Ucchino S, Spigonardo F, Marchetti A, Buttitta F, Paloscia L, Mascellanti M, Cuccurullo F, Mezzetti A. Association between prostaglandin E receptor subtype EP4 overexpression and unstable phenotype in atherosclerotic plaques in human. Arterioscler Thromb Vasc Biol. 2005;9:1925–1931. doi: 10.1161/01.ATV.0000177814.41505.41. [DOI] [PubMed] [Google Scholar]
- 18.Pavlovic S, Du B, Sakamoto K, Khan KMF, Natarajan C, Breyer RM, Dannenberg AJ, Falcone DJ. Targeting prostaglandin E2 receptors as an alternative strategy to block cyclooxygenase-2-dependent extracellular matrix-induced matrix metalloproteinase-9 expression by macrophages. J Biol Chem. 2006;281:3321–3328. doi: 10.1074/jbc.M506846200. [DOI] [PubMed] [Google Scholar]
- 19.Wu MH, Shoji Y, Wu MG, Chuang PC, Lin CC, Huang MF, Tsai SJ. Suppression of matrix metalloproteinase-9 by prostaglandin E2 in peritoneal macrophage is associated with severity of endometriosis. Am J Pathol. 2005;67:1061–1069. doi: 10.1016/S0002-9440(10)61195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, Oates JA, Fazio S, Linton MF. Macrophages EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metab. 2008;8:492–501. doi: 10.1016/j.cmet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collin J, Walton J, Araujo L, Lindsell D. Oxford screening programme for abdominal aortic aneurysm in men aged 65 to 74 years. Lancet. 1988;2:613–615. doi: 10.1016/s0140-6736(88)90649-6. [DOI] [PubMed] [Google Scholar]
- 22.Scott RAP, Ashton HA, Kay DN. Abdominal aortic aneurysm in 4237 screened patients: prevalence, development and management over 6 years. Br J Surg. 1991;78:1122–1125. doi: 10.1002/bjs.1800780929. [DOI] [PubMed] [Google Scholar]
- 23.Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995;15:1145–1151. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- 24.Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjala H, Airaksinen J, Leinonen M, Saikku PT, Juvonen T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–2847. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 25.Eliason JL, Hannawa KK, Ailawadi G, Sinha I, Ford JW, Deogracias MP, Roelofs KJ, Woodrum DT, Ennis TL, Henke PK, Stanley JC, Thompson RW, Upchurch GR., Jr Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation. 2005;112:232–240. doi: 10.1161/CIRCULATIONAHA.104.517391. [DOI] [PubMed] [Google Scholar]
- 26.Keeling WB, Armstrong PA, Stone PA, Bandyk DF, Shames ML. An overview of matrix metalloproteinases in the pathogensis and treatment of abdominal aortic aneurysms. Vasc Endovascular Surg. 2005;39:457–464. doi: 10.1177/153857440503900601. [DOI] [PubMed] [Google Scholar]
- 27.Gitlin JM, Trivedi DB, Langenbach R, Loftin CD. Genetic deficiency of cyclooxygenase-2 attenuates abdominal aortic aneurysm formation in mice. Cardiovasc Res. 2007;73:227–236. doi: 10.1016/j.cardiores.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogensis in low density lipoprotein receptor −/− mice. Ann N Y Acad Sci. 1999;982:108–118. doi: 10.1111/j.1749-6632.1999.tb07789.x. [DOI] [PubMed] [Google Scholar]
- 29.Daugherty A, Manning MW, Cassis LA. Antagonism of AT2 receptors augments angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001;134:865–870. doi: 10.1038/sj.bjp.0704331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segi E, Sugimoto Y, Yamasaki A, Aze Y, Oida H, Nishimura T, Murata T, Mastuoka T, Ushikubi F, Hirose M, Tanaka T, Yoshida N, Narumiya S, Ichikawa A. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophy Res Comm. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 31.Manning MW, Cassis LA, Huang J, Szilvassy SJ, Daugherty A. Abdominal aortic aneurysms: fresh insights from a novel animal model of the disease. Vasc Med. 2002;7:45–54. doi: 10.1191/1358863x02vm413ra. [DOI] [PubMed] [Google Scholar]
- 32.Henriques TA, Huang J, D’Souza SS, Daugherty A, Cassis LA. Orchiectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E deficient mice. Endocrinology. 2004;145:3866–3872. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 33.Henriques T, Zhang X, Yiannikouris FB, Daugherty A, Cassis LA. Androgen Increases AT1a Receptor Expression in Abdominal Aortas to Promote Angiotensin II–Induced AAAs in Apolipoprotein E–Deficient Mice. Arteriosclerosis, Thrombosis and Vascular Biology. 2008;28:1251–1256. doi: 10.1161/ATVBAHA.107.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Echeverría González FM, Cuasnicú PS, Blaquier JA. Identification of androgen-dependent glycoproteins in the hamster epididymis and their association with spermatozoa. J Reprod Fertil. 1982;64:1–7. doi: 10.1530/jrf.0.0640001. [DOI] [PubMed] [Google Scholar]
- 35.Hinton AC, Grigsby PL, Pitzer BA, Brockman DE, Ittenbach RF, Hinton RB, Myatt L. Hormonal regulation of prostaglandin E2 receptors: localization and expression in rat cervical tissue. Reprod Sci. 2010;17:136–146. doi: 10.1177/1933719109348068. [DOI] [PubMed] [Google Scholar]
- 36.Terada N, Shimizu Y, Kamba T, Inoue T, Maeno A, Kobayashi T, Nakamura E, Kamoto T, Kanaji T, Maruyama T, Mikami Y, Toda Y, Matsuoka T, Okuno Y, Tsujimoto G, Narumiya S, Ogawa O. Identification of EP4 as a potential target for the treatment of castration-resistant prostate cancer using a novel xenograft model. Cancer Res. 2010;70:1606–1615. doi: 10.1158/0008-5472.CAN-09-2984. [DOI] [PubMed] [Google Scholar]
- 37.Sirianni R, Chimento A, De Luca A, Zolea F, Carpino A, Rago V, Maggiolini M, Andò S, Pezzi V. Inhibition of cyclooxygenase-2 down-regulates aromatase activity and decreases proliferation of Leydig tumor cells. J Biol Chem. 2009;284:28905–16. doi: 10.1074/jbc.M109.041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JY, Han EH, Kim HG, Oh KN, Kim SK, Lee KY, Jeong HG. Bisphenol A-induced aromatase activation is mediated by cyclooxygenase-2 up-regulation in rat testicular Leydig cells. Toxicol Lett. 2010;193:200–208. doi: 10.1016/j.toxlet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Ohinata K, Suetsugu K, Fujiwara Y, Yoshikawa M. Activation of prostaglandin E receptor EP4 subtype suppresses food intake in mice. Prostag & Other Lipids Med. 2006;81:31–36. doi: 10.1016/j.prostaglandins.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Ohinata K, Fujiwara Y, Fukumoto S, Iwai M, Horiuchi M, Yoshikawa M. Angiotensin II and III suppress food intake via angiontensin AT2 receptor and prostaglandin EP4 receptor in mice. FEBS Letters. 2008;582:773–777. doi: 10.1016/j.febslet.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 41.Sugimoto Y, Tsuboi H, Okuno Y, Tamba S, Tsuchiya S, Tsujimoto G, Ichikawa A. Microarray evaluation of EP4 receptor-mediated prostaglandin E2 suppression of 3T3-L1 adipocyte differentiation. Biochem & Biophy Res Comm. 2004;322:911–917. doi: 10.1016/j.bbrc.2004.07.194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.