Abstract
The tumor suppressors phosphatase and tensin homologue deleted on chromosome ten (PTEN) and p53 are closely related to the pathogenesis of breast cancer, yet pathway-specific mechanisms underlying their participation in mediating the protective actions of dietary bioactive components on breast cancer risk are poorly understood. We recently showed that dietary exposure to the soy isoflavone genistein (GEN) induced PTEN expression in mammary epithelial cells in vivo and in vitro, consistent with the breast cancer preventive effects of soy food consumption. Here, we evaluated PTEN and p53 functional interactions in the nuclear compartment of mammary epithelial cells as a mechanism for mammary tumor protection by GEN. Using the non-tumorigenic human mammary epithelial cells MCF10-A, we demonstrate that GEN increased PTEN expression and nuclear localization. We show that increased nuclear PTEN levels initiated an autoregulatory loop involving PTEN-dependent increases in p53 nuclear localization, PTEN–p53 physical association, PTEN–p53 co-recruitment to the PTEN promoter region and p53 transactivation of PTEN promoter activity. The PTEN–p53 cross talk induced by GEN resulted in increased cell cycle arrest; decreased pro-proliferative cyclin D1 and pleiotrophin gene expression and the early formation of mammary acini, indicative of GEN promotion of lobuloalveolar differentiation. Our findings provide support to GEN-induced PTEN as both a target and regulator of p53 action and offer a mechanistic basis for PTEN pathway activation to underlie the antitumor properties of dietary factors, with important implications for reducing breast cancer risk.
Introduction
Breast cancer is the most common malignancy among women in the Western world, affecting one of eight in their lifetime and resulting in ∼50 000 deaths in the USA annually (1). Accumulations of epigenetic and genetic alterations within mammary epithelial cells (MECs) are the triggering events for breast cancer initiation and tumor cell expansion (2). Prevailing evidence suggests that breast cancer development can be influenced by nutrition (3). Epidemiological and case–control studies have shown a 2- to 8-fold lower occurrence of the disease in Asian women whose early intake of soy products is 10–20 times higher than their American counterparts (4,5). Several human, animal and in vitro studies concur that early exposure (pre-pubertal) to soy foods and associated components is correlated with reduced risk of adult breast cancer (6,7). Among the soy products, the isoflavone genistein (GEN) has been identified as an important component that may confer protection against breast tumors (8–10).
Previously, we (11,12) and others (9,13) showed that dietary intake of soy protein isolate (SPI) and control casein (CAS) supplemented with GEN decreased mammary tumor incidence and/or increased tumor latency in rats fed these diets relative to those fed CAS, when exposed to the chemical carcinogens N-methyl-N-nitrosourea or 7,12-dimethyl-benz[a]anthracene. In our studies, breast cancer protective effects were associated with the downregulation of the oncogenic Wnt-signaling pathway and the upregulation of the phosphatase and tensin homologue deleted on chromosome ten (PTEN) expression in the mammary gland by SPI and GEN, relative to CAS, coincident with enhanced MEC differentiation (14,15). Given that these same diets altered several biological and molecular pathways in MECs in vivo (14), additional pathways probably underlie their mammary tumor protective effects.
Next to p53, PTEN is the most common tumor suppressor to be lost or inactivated in human cancers, including breast cancer (16,17). The PTEN gene encodes a dual specificity (lipid and protein) phosphatase that antagonizes phosphatidylinositol 3-kinase (PI3K), preventing activation of the pro-survival protein kinase B/Akt downstream pathway (18). A role for PTEN in mammary gland development and tumorigenesis is supported by reduced cellular proliferation and increased apoptosis in the mammary glands of mice overexpressing PTEN (19). Further, Cowden syndrome patients harboring germ line mutations at the PTEN locus are at high risk of breast cancer and although somatic mutations of PTEN are found in a small fraction of breast cancers (20,21), loss of heterozygosity at the PTEN locus (10q23) occurs frequently (22). Additional studies linking breast carcinoma status with PTEN expression include those demonstrating the predictive value of reduced PTEN in the relapse of tamoxifen-treated estrogen receptor (ER)-α-positive breast cancer patients (23) and the prognostic significance of the gene expression signature of PI3K/Akt activation due to PTEN loss on metastasis and poor survival (24).
One mechanism by which PTEN may protect against mammary tumors is by its interaction with the tumor suppressor p53. PTEN transcription can be enhanced by p53 (25), in turn, PTEN regulates p53 protein stability in two ways: in a phosphatase-dependent manner, by inhibiting PI3K/Akt-induction of Mdm2 nuclear translocation and in a phosphatase-independent manner through its physical interaction with p53 (26). Perhaps, the most convincing evidence supporting complementary functions of PTEN and p53 in tumorigenesis is the unexpected observation that PTEN is oncogenic in the presence of a mutant p53 protein (27).
In this study, we explored a novel mechanism of breast cancer prevention by GEN involving PTEN and p53. Using the non-malignant human mammary epithelial cell line MCF-10A, we show that GEN at physiologically relevant concentrations induced PTEN expression and PTEN and p53 nuclear accumulation. We demonstrate that GEN increased nuclear PTEN expression through an autoregulatory loop whereby PTEN’s increased interaction with nuclear p53 enhanced PTEN promoter activity. Further, we establish that a functional consequence of augmented nuclear PTEN signaling by GEN was the promotion of cell cycle arrest and the stimulation of early lobuloalveolar differentiation. Our results point to PTEN as both a target and regulator of p53 action in normal MECs for mammary tumor prevention by the isoflavone GEN.
Materials and methods
Animals, diets and MEC isolation
Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. Time-mated Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) were individually housed in polycarbonate cages under conditions of 24°C, 40% humidity and a 12 h light–dark cycle. At gestation day 4, dams were randomly assigned to one of two semi-purified isocaloric diets made according to the American Institute of Nutrition-93 G formulation (28), with corn oil substituting for soybean oil. These diets are: (i) CAS diet, containing casein (New Zealand Milk Products, Santa Rosa, CA) as the only protein source and (ii) SPI diet containing soy protein isolate (Solae, St Louis, MO) with isoflavones GEN (216 ± 2 mg/kg) and daidzein (160 ± 6 mg/kg) as aglycone equivalents. Female pups were weaned to the same diets as their dams until isolation of MECs at postnatal day 50. Mammary gland pair #3 was processed for MEC isolation following protocols described by Dr Jeffrey Rosen’s laboratory (http://www.bcm.edu/rosenlab/protocols/primaryMEC.pdf; Baylor College of Medicine, Houston, TX).
Cell culture and treatments
The human non-tumorigenic mammary epithelial cell line, MCF-10A (American Type Culture Collection, Manassas, VA), was propagated as described (29). Phenol red-free media supplemented with charcoal-stripped fetal bovine serum was used for serum starvation (0.5% charcoal-stripped fetal bovine serum) and for treatments (2.5% charcoal-stripped fetal bovine serum) with GEN (Sigma Chemical Co., St Louis, MO) or vehicle (dimethyl sulfoxide). For small interfacing RNA (siRNA) targeting studies, cells were grown to 30–50% confluency prior to transfecting with PTEN siGENOME SMART pool or siCONTROL Non-targeting siRNA pool (Dharmacon, Lafayette, CO) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), following the manufacturer’s protocol.
Quantitative real-time polymerase chain reaction
Total RNA was isolated from cells using Trizol reagent (Invitrogen), quantified and reverse transcribed to complementary DNA as described (14). SYBR Green detection system (Applied Biosystems, Foster City, CA) was used for quantitative real-time polymerase chain reaction (QPCR). Human primer sequences and corresponding genes were [gene, forward and reverse primer; amplicon size (bp)]: PTEN (5′-GCTATGGGATTTCCTGCAGAA-3′ and 5′-GGCGGTGTCATAATGTCTTTCA-3′; 138), p53 (5′-GGCGCACAGAGGAAGAGAAT-3′ and 5′-GGAGAGGAGCTGGTGTTGTTG-3′; 103), 18S (5′-TCTTAGCTGAGTGTCCCGCG-3′ and 5′-ATCATGGCCTCAGTTCCGA-3′; 151), pleiotrophin (5′-TGCCAGAAGACTGTCACCATCT-3′ and 5′-TCCTGTTTCTTGCCTTCCTTTT-3′; 101) and cyclin D1 (5′-AATGACCCCGCACGATTTC-3′ and 5′-ATGGAGGGCGGATTGGAA-3′; 144). Rat primers used were: PTEN (5′-CAATGTTCAGTGGCGGAACTT-3′ and 5′-GGCAATGGCTGAGGGAACT-3′; 133) and 18S (5′-ATTCGAACGTCTGCCCTATCAA-3′ and 5′-CGGGAGTGGGTAATTTGCG-3′; 151). 18S ribosomal RNA was used as a normalizing control and data are expressed as means ± SEM relative to control (vehicle).
Immunoprecipitation and immunoblotting
Immunoprecipitation was performed in whole cell lysates using the Catch and Release Immunoprecipitation System following the manufacturer’s instructions (Upstate Biotechnology, Lake Placid, NY). Briefly, 500 μg of cell lysate were incubated with 2 μg of PTEN antibody (A2B1; Santa Cruz, Santa Cruz Biotechnology, CA), p53 antibody (Cell Signaling Technology, Danvers, MA) or control IgG (Santa Cruz) and 1 μg of Antibody Capture Affinity Ligand on a rocking platform overnight at 4°C. Eluted proteins were analyzed by western blot using anti-PTEN (A2B1) and anti-p53 (Cell Signaling Technology) antibodies as described (30).
Immunofluorescence
Cells were seeded on sterile 22 mm glass cover slides placed on a six-well plate and allowed to attach overnight. Cells were treated twice with GEN (40 nM or 2 μM, as indicated for each study) at t = 0 and 24 h, fixed and permeabilized in ice-cold methanol for 10 min. Immunofluorescence was done using the Vectastain elite ABC kit (Vector Laboratory, Burlingame, CA) as described (30). Antibodies used were: PTEN (1:200); phospho-PTEN (Ser 380) (1:200; Cell Signaling Technology) and p53 (1:500). Cells were mounted with Vectashield Mounting Medium with 4′,6-diamidino-2-phenylindole (nuclear stain) and analyzed for immunofluorescence under a Carl Zeiss Axiovision microscope (Carl Zeiss AG, Oberkochen, Germany). At least 500 cells were counted from five random areas per slide (×20 objective), with three slides for each treatment group.
Cell proliferation assay
Cell proliferation was evaluated using the 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide assay following the manufacturer’s protocol (American Type Culture Collection). Cells were seeded in 96-well plates and treated with GEN (2 μM) or vehicle (dimethyl sulfoxide) every 2 days for 6 days. Absorbance values (570 nm) reflect the ability of metabolically active cells to reduce the yellow tetrazolium 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide salts into a purple precipitate.
Transient transfection and luciferase assays
The PTEN-luc reporter construct, human PTEN in pGL3b vector, was a gift from Dr Eileen D.Adamson [The Burnham Institute, La Jolla, CA (31)]. Cells were transfected using Lipofectamine 2000 (Invitrogen) with PTEN-luc reporter plasmid or empty (pGL3b) vector (each added at 0.2 μg/well) as described (32). After treatment with vehicle with and without added GEN (2 μM) post-transfection using either scrambled RNA (scRNA) or PTEN siRNAs (50 nM), cells were lysed in lysis buffer (Promega, Madison, WI), and quantitative determination of luciferase activity was carried out using a MLX Microplate Luminometer (Dynex Technologies, Chantilly, VA). Renilla luciferase (8 ng/well) activity was used as an internal control for transfection efficiency among cells and was measured using a Dual-Luciferase Reporter Assay System (Promega). Luciferase activity was normalized to Renilla luciferase for each sample. Data are presented as means ± SEMs from three independent experiments performed in triplicate.
Chromatin immunoprecipitation assays
Cells were treated similarly as for the immunoprecipitation studies (above) and then processed for chromatin immunoprecipitation using the ChIP-IT Express Enzymatic Kit, following the manufacturer’s recommendations (Active Motif, Carlsbad, CA). Polymerase chain reaction (PCR) with primers spanning the p53 binding sites on the PTEN promoter (33) (forward, 5′-CAAAAGCCGCAGCAAGTG-3′ and reverse, 5′-GAGCGCAGAGTCCCCAAG-3′; 115 bp) was carried out under the following conditions: hot start at 94°C for 5 min and then 35 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 30 s with final extension at 72°C for 10 min. PCR products were resolved on a 3% agarose gel containing ethidium bromide.
Fluorescence-activated cell sorting
For each group, at least 10 000 cells were stained with propidium iodide and analyzed with a Becton Dickinson FACSCalibur. The proportion of cells in sub-G1/G0, G1/G0, S and G2/M phases were determined with the Cell Quest software program (BD Biosciences, San Jose, CA).
Acini morphogenesis assay and image acquisition
MCF-10A cells were seeded on a layer of Matrigel (BD Biosciences) in eight-well chamber slides and allowed to form acini as described (29). Culture medium containing 2% charcoal-stripped horse serum and 5 ng/ml epidermal growth factor (EGF) without (vehicle alone) or with added GEN (2 μM) was refreshed every 4 days. Acini number and diameter were assessed at days 6 and 12 of culture using a phase contrast microscope (Carl Zeiss) (×20 objective). Indirect immunofluorescence of acinar structures was performed as described (29). The primary antibodies used were PTEN (1:200) and p53 (1:200). Confocal images were collected on a Zeiss LSM510 confocal microscope (×20 objective).
Data analysis
Statistical analyses was done using StatView version 5.0 for Windows. Data were analyzed using Student’s t-test, one-way analysis of variance or two-way analysis of variance. Differences between means in two-way analysis of variance were further analyzed by Tukey’s test. P values <0.05 were considered statistically significant.
Results
Expression of PTEN in MECs
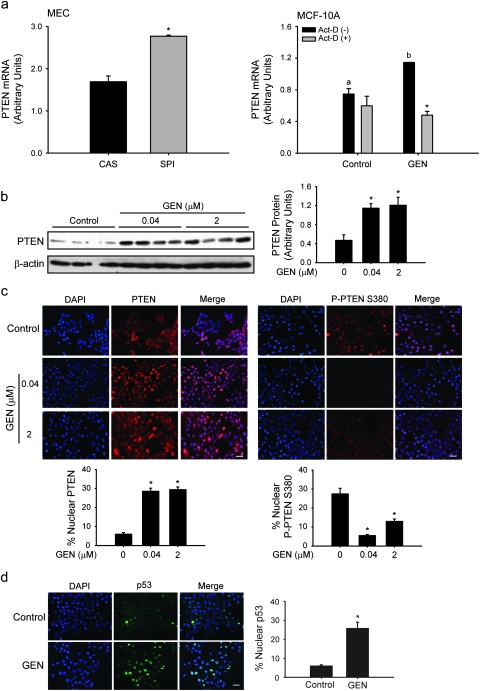
We previously showed that prepubertal dietary exposure to SPI, the main component of infant soy formulas, and to CAS diet supplemented with the major soy isoflavone GEN protected against NMU-induced mammary tumors in rats, relative to the CAS group (11,12). Second to p53, PTEN is the most common tumor suppressor mutated or inactivated in human cancers including breast cancer (17). PTEN expression was assessed in MECs isolated from young adult rats at postnatal day 50 after lifetime exposure to CAS or SPI, by QPCR. Transcript levels of PTEN were increased in MECs of rats fed SPI relative to CAS (Figure 1a, left). To mechanistically dissect the functional implications of increased expression of PTEN in MECs in vivo, the effects of GEN were evaluated in the non-tumorigenic human mammary epithelial cell line, MCF-10A in vitro. The dose of 2 μM GEN is within the concentration range found in plasma of infants fed soy-based formulas (34) and of the Asian population with continuous exposure to GEN from daily soy consumption (35). In addition, this dose is within the range found in the plasma of rats fed a lifetime diet containing SPI or GEN (36). MCF-10A, similar to normal human breast epithelial cells, are ER-negative (37) and unlike the tumorigenic mammary epithelial cell line MCF-7, do not undergo apoptosis with GEN treatment (15). MCF-10A treated with GEN for 6 h had higher transcript levels of PTEN compared with control (vehicle-treated) cells (Figure 1a, right). GEN induction of PTEN transcript levels was blocked by the transcriptional inhibitor actinomycin D (1 μg/ml) added 1 h prior to GEN treatment. Thus, induction of PTEN expression by dietary SPI in vivo was recapitulated by GEN in vitro and results in part, from transcriptional regulation of the PTEN gene.
Fig. 1.
GEN induces nuclear accumulation of PTEN and p53 in MECs. (a) ‘Left’, transcript levels of PTEN in MECs from rats fed CAS or SPI analyzed by QPCR. Values are means ± SEMs; n = 4 rats per diet group (*P < 0.05 relative to CAS). ‘Right’, elevated PTEN gene expression after GEN (2 μM) treatment of non-malignant MCF-10A cells is due to increased transcription. Transcript levels were quantified by QPCR and normalized to 18S rRNA. Means with different letters differed at P < 0.05; *P < 0.05 relative to absence of actinomycin-D (Act-D; 1 μg/ml) within each treatment group. (b) Western blot analysis of whole cell extracts from cells treated with GEN (40 nM and 2 μM). PTEN protein levels were compared with control (vehicle). Each lane represents an individual treatment sample and contains 50 μg of total protein. Immunoreactive bands were quantified by densitometric scanning and values normalized to those of loading control β-actin and are presented as histograms (right panel). (c and d) GEN increases the number of nuclear PTEN (red) and p53 (green)-positive MCF-10A cells, while decreasing nuclear accumulation of inactive Phospho-PTEN (Ser 380). Merge shows nuclear localization (4′,6-diamidino-2-phenylindole; blue); representative images for each group are shown from three independent experiments. Bar, 50 μM; *P < 0.05 relative to control.
GEN increases nuclear levels of PTEN and p53 in MCF-10A cells
PTEN function can be regulated by its subcellular localization, with normal cells preferentially showing nuclear PTEN localization (38). To determine the major site of action of GEN-induced PTEN in MCF-10A cells, PTEN protein levels were assessed by western analysis and immunofluorescence, in cells treated with GEN twice (at t = 0 and 24 h; sample collection at 24 h after last treatment) at 40 nM and 2 μM concentrations. We considered the lower dose of GEN to correspond to the serum concentrations of occasional soy consumers. PTEN protein levels were increased by 3-fold in whole extracts of cells treated with either dose of GEN, when compared with control cells (Figure 1b). Immunofluorescence demonstrated that GEN-treated cells had increased accumulation (by 6-fold) of nuclear PTEN (Figure 1c). The nuclear-localized PTEN is predominantly in the active form since parallel immunofluorescence using a specific antibody against inactive, phosphorylated-PTEN (P-PTEN S380) revealed significantly decreased levels of this protein with GEN treatment at either dose (Figure 1c). Interestingly, nuclear accumulation of p53 protein was significantly enhanced (by 5-fold) similar to PTEN, with GEN treatment (Figure 1d).
GEN increases nuclear colocalization and physical interaction of PTEN and p53 in vivo
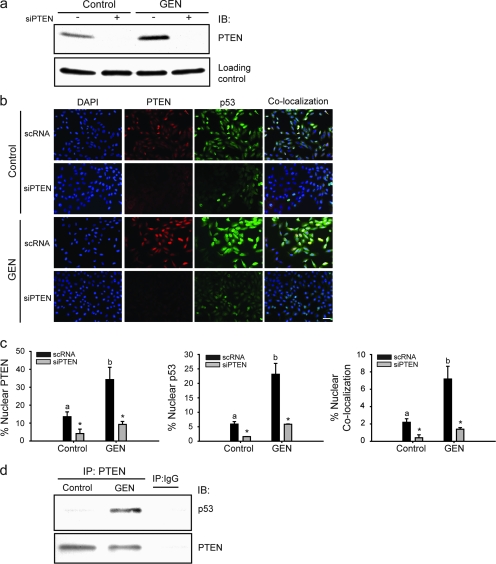
p53 protein levels are reduced in PTEN-null cells and tissues, and reintroduction of wild-type or phosphatase-dead PTEN protein stabilizes p53 protein levels (26). To ascertain whether GEN-induced nuclear p53 levels are dependent on the nuclear levels of PTEN similarly elevated by GEN, cells were transfected with 50 nM of PTEN siRNA or scrambled (non-specific) scRNA, prior to vehicle or GEN (2 μM) treatment and then processed for dual immunofluorescence. Transient knockdown of PTEN by siRNA abolished basal and GEN-induced PTEN protein levels as shown in western blot, without any effect on loading control protein (Figure 2a). A dual immunofluorescence colocalization assay was performed to assess potential interactions between PTEN and p53 based on spatial localization. GEN treatment increased nuclear localization of PTEN and p53 in scRNA-transfected cells (Figure 2b). Treatment with GEN also resulted in increased nuclear colocalization of p53 and PTEN (Figure 2b; scRNA), consistent with PTEN–p53 complex formation predominantly occurring in the nucleus (26). However, not all nuclear-localized p53 or PTEN were found to colocalize (Figure 2b and c). In the presence of PTEN siRNA, nuclear p53 levels were significantly reduced in control and GEN-treated cells. To evaluate whether nuclear colocalized p53 and PTEN are physically associated, whole cell lysates from control and GEN-treated cells were immunoprecipitated with PTEN antibody or control IgG, and p53 and PTEN levels in immunoprecipitates were analyzed by western blots. p53 was co-immunoprecipitated with PTEN in GEN-treated cells but not in control (vehicle-treated) cells (Figure 2d), indicating GEN-mediated promotion of these proteins’ physical association. Non-specific IgG did not immunoprecipitate either p53 nor PTEN protein in the same extracts, indicating specificity of the immune reactions.
Fig. 2.
GEN increases colocalization of nuclear PTEN and p53 and their physical interaction in vivo. (a) siRNAs targeting PTEN abolish basal and GEN-induced PTEN protein levels; loading control is a non-specific protein (140 kDa) present in the same blot. (b) GEN increases nuclear levels of PTEN and p53 in MCF-10A cells and their subsequent colocalization. Cells were immunostained for PTEN (red) and p53 (green) before being counterstained for 4′,6-diamidino-2-phenylindole (blue). Colocalization of anti-PTEN and p53-stained cells coincide with 4′,6-diamidino-2-phenylindole and show an orange color. Representative images for each group are shown from three independent experiments; bar, 50 μM. (c) The percentage of nuclear PTEN, p53 and colocalized PTEN and p53 was calculated by counting five random areas per slide with three slides for each group. Means with different letters (a,b) differed at P < 0.05; *P < 0.05 relative to non-targeting scRNA within each group. (d) Cell lysates from MCF-10A cells were immunoprecipitated with mouse monoclonal PTEN antibody, followed by immunoblotting with anti-p53 or anti-PTEN antibodies. Representative blots from two independent experiments with same results are shown.
GEN induction of PTEN promoter activity requires PTEN and p53
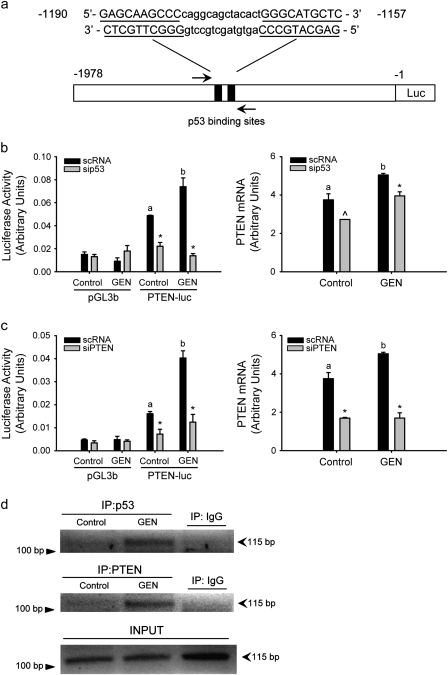
p53 can upregulate PTEN transcription through binding to p53 binding sites in the PTEN promoter (25) (Figure 3a). To determine whether GEN induction of nuclear PTEN leads to enhancement of PTEN transcriptional regulation involving p53, PTEN promoter activity was measured in control and GEN-treated cells by dual luciferase assay in the presence or absence of siRNAs targeting p53 or PTEN (Figure 3b and c). Transfection of PTEN-luc construct significantly increased luciferase activity in both control and GEN-treated cells relative to pGL3b vector-transfected cells. GEN treatment augmented PTEN promoter activity relative to control (vehicle) cells (by 1.5; P = 0.031). siRNA to p53 decreased basal (55% inhibition) and GEN-induced (81% inhibition) PTEN promoter activity (Figure 3b). Interestingly, transient PTEN knockdown by siRNA similarly reduced PTEN promoter activity in control and GEN-treated cells (52 and 70% inhibition, respectively) (Figure 3c). GEN enhancement of PTEN promoter activity and the corresponding reductions in basal and GEN-induced activities with siRNAs to p53 and PTEN were confirmed at the level of the PTEN messenger RNA by QPCR of transfected cells (Figure 3b and c). We next examined the effects of GEN on p53 and PTEN recruitment to the PTEN promoter by chromatin immunoprecipitation assay. Chromatin preparations isolated from control and GEN-treated cells were immunoprecipitated with either anti-p53, anti-PTEN or control IgG antibodies, and immunoprecipitated DNA was analyzed by PCR, using primer sets designed within the region of the PTEN promoter containing the p53 binding sites (Figure 3a). The recruitment of p53 to the PTEN promoter was enhanced by GEN, consistent with GEN regulation of PTEN transcription involving its induction of nuclear p53 levels (Figure 3d, top panel). GEN also increased the recruitment of PTEN to the p53 binding sites of the PTEN promoter (Figure 3d, middle panel). The amounts of PCR product with input DNA were comparable among samples (Figure 3d, bottom panel) and no PCR product was present in samples immunoprecipitated with control IgG, indicating antibody-specific immunoprecipitation procedures.
Fig. 3.
Induction of PTEN promoter by GEN requires PTEN and p53. (a) Schematic representation of the PTEN-luc reporter construct used for luciferase assay containing the two p53 binding sites (black box). MCF-10A cells were cotransfected with 0.2 μg of PTEN promoter–reporter construct (PTEN-luc) or empty vector pGL3-basic vector (pGL3b) and control siRNA (scRNA) or siRNA targeting p53 (b) or PTEN (c). Following overnight serum starvation, cells were treated twice with GEN for 24 h each and then luciferase activity was determined (Materials and Methods). Each siRNA panel represents a separate experiment repeated twice with similar results. PTEN messenger RNA levels were determined under similar treatment conditions and quantified by QPCR. Means with different letters (a,b) differed at P < 0.05; *P < 0.05 and ^P = 0.085 relative to non-targeting scRNA within each group. (d) Cross-linked sheared chromatin was immunoprecipitated with antibody against p53 or PTEN. After reversal of cross-links, DNA was analyzed by PCR using primers that amplify the PTEN promoter region containing the p53 binding sites.
GEN antiproliferative effects are PTEN-dependent
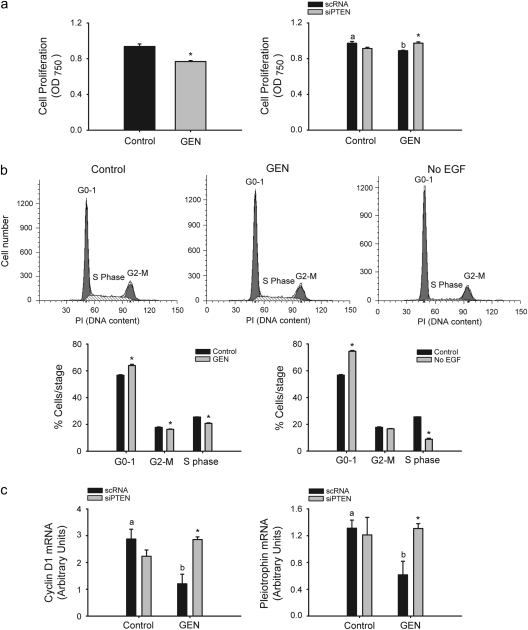
While cytoplasmic PTEN decreases phospho-Akt levels and induces apoptosis, nuclear PTEN has been associated with G0–G1 cell cycle arrest by its downregulation of cyclin D1 (39). To evaluate if GEN induction of nuclear PTEN in MECs (Figures 1c and 2b) results in cell cycle arrest, MCF-10A cells were treated with vehicle or vehicle containing GEN (2 μM), and cell proliferation was measured after 6 days using the 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide assay. GEN modestly but significantly decreased cell proliferation; this effect was reversed in cells pretreated with PTEN siRNAs, showing PTEN dependence (Figure 4a). Since similar treatment with GEN did not change the number of viable cells nor result in induction of apoptosis (data not shown), decreased cell proliferation by GEN may reflect cell cycle arrest, a process attributed to nuclear PTEN (40). To determine whether increased levels of nuclear PTEN with GEN leads to G0–G1 arrest, cell cycle distribution was analyzed in control and GEN-treated cells by fluorescence-activated cell sorting analysis. GEN treatment increased the percentage of cells in G0–G1 phase of the cell cycle (56.72 ± 0.46 versus 62.97 ± 0.51; P = 0.003), whereas lowering that in the S phase (25.54 ± 0.06 versus 20.69 ± 0.05; P = 0.005) (Figure 4b). As a positive control for G0–G1 arrest (41), cells were administered growth media without added EGF for the same period as GEN (Figure 4b, no EGF). Absence of EGF treatment resulted in increased and decreased percentage of cells in the G0–G1 and S phases, respectively. Transcript levels of cyclin D1, a positive regulator of G0/G1–S transition and a nuclear PTEN target (42), and of pleiotrophin, a PTEN (negatively)-regulated growth factor (43), were evaluated in vehicle- and GEN-treated cells transfected with scRNAs or PTEN siRNAs, by QPCR. GEN treatment for 6 h decreased cyclin D1 (58% decrease; P = 0.031) and pleiotrophin (53% decrease; P = 0.042) transcript levels. PTEN siRNA addition abrogated GEN downregulation of these genes’ expression (Figure 4c).
Fig. 4.
The anti-proliferative effect of GEN requires PTEN. (a) Decreased cell proliferation by GEN in a PTEN-dependent manner in MCF-10A cells measured by the 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide assay after 6 days of GEN treatment. (b) Cell cycle distribution determined by fluorescence-activated cell sorting analysis shows G0–G1 arrest and lower percentage of cells in the S phase after GEN treatment. As a positive control for G0–G1 arrest, cells were administered media in the absence of EGF and analyzed at the same time. Values are means ± SEMs from two independent experiments performed with triplicate samples. *P < 0.05 relative to control for each stage of cell cycle. (c) Transcript levels of the positive regulator of G1–S transition, cyclin D1 and the proliferative growth factor pleiotrophin are decreased by GEN in a PTEN-dependent manner. Means with different letters (a,b) differed at P < 0.05; *P<0.05 relative to non-targeting scRNA within each treatment group.
GEN promotes early lobuloalveolar differentiation of MCF-10A cells
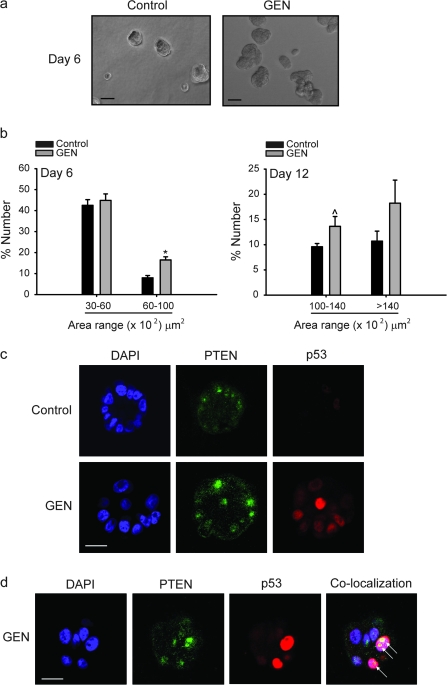
Three-dimensional culture of MCF-10A cells in Matrigel constitutes an excellent model to study mammary differentiation (29). Single-seeded cells plated on Matrigel-coated chamber slides form acini with hollow lumens by day 10 of culture, resembling mammary gland morphology in vivo. To further determine the functional consequence of GEN promotion of PTEN expression and of PTEN–p53 nuclear colocalization in MCF-10A cells, the ability of GEN to induce acini formation was assessed. Cells without or with added GEN (2 μM) were evaluated for acini morphogenesis at days 6 and 12, periods corresponding to pre- and post-differentiated states, respectively (29). At day 6, a significant increase in the numbers of acini in the 60–100 (×102) μm2 range was observed with GEN treatment, indicative of early lobuloalveolar differentiation (Figure 5a and b). The numbers of acini with increasing sizes [(100–140, >140) (×102)] continued to increase at day 12, albeit GEN effects over control only tended to be significant (Figure 5b). PTEN and p53 protein localization/colocalization in acinar structures (day 6) were evaluated by immunofluorescence and confocal imaging. Nuclear PTEN and p53 immunoreactivities were higher in GEN-treated acini relative to corresponding controls (vehicle treated), and a number of these acini showed PTEN–p53 colocalization (Figure 5c and d), similar to the results for GEN-treated cells grown in monolayers (Figure 2b). These results are consistent with the involvement of PTEN and p53 in GEN-induced early lobuloalveolar differentiation of MECs, supporting a recent observation that PTEN regulates acini morphogenesis of MECs (44).
Fig. 5.
GEN promotes early lobuloalveolar differentiation. (a) MCF-10A cells were cultured in Matrigel to allow acini formation as described in Materials and Methods. The numbers of acini formed were counted at days 6 and 12 of mammary acini morphogenesis from three random areas per chamber with four chambers per treatment group; bar, 50 μM. (b) Results are mean ± SEM from two independent experiments. *P < 0.05 relative to control. (c) Increased nuclear PTEN and p53 in GEN-treated MCF-10A acini at day 6. Subcellular localization of PTEN (green) and p53 (red) was recorded by confocal microscopy. Representative images are from four independent chambers for Control or GEN; bar, 20 μM. (d) Z-section showing colocalization of PTEN and p53 (arrow) in GEN-treated cells at day 6; bar, 20 μM.
Discussion
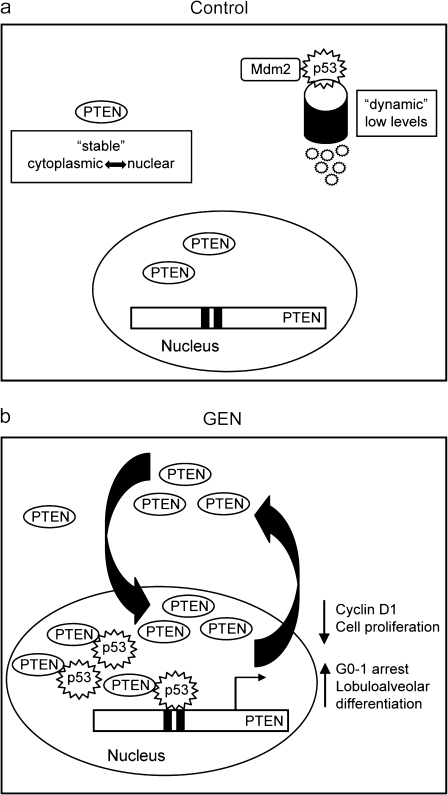
In this study, we establish induction of nuclear PTEN–p53 cross-regulation by GEN in MECs as a novel mechanism of breast cancer prevention by dietary factors. We provide evidence to support a model in which GEN induces an autoregulatory loop between PTEN and p53 to promote mammary epithelial cell cycle arrest and early lobuloalveolar differentiation (Figure 6). GEN induction of PTEN expression and nuclear accumulation elicits a sequence of PTEN-dependent events as follows: (i) increased nuclear p53 accumulation; (ii) enhanced PTEN–p53 physical interaction; (iii) increased recruitment of the PTEN–p53 complex to the p53 binding sites of the PTEN promoter; (iv) higher PTEN promoter activity and (v) promotion of cell cycle arrest and lobuloalveolar differentiation and attenuated expression of proliferative genes cyclin D1 and pleiotrophin. Taken together, our findings identify PTEN pathway activation involving p53 as an early molecular event mediating dietary factor effects in the prevention of mammary tumorigenesis.
Fig. 6.
Proposed model for GEN-induced nuclear PTEN–p53 cross talk in MECs to enhance PTEN signaling and subsequent MEC differentiation. (a) PTEN is constitutively expressed in resting cells and its function is regulated in part by subcellular localization, with nuclear preference in non-malignant cells. On the other hand, p53 is a dynamic protein that is maintained at low levels under normal conditions by Mdm2 degradation. (b) In the presence of GEN, the nuclear pool of PTEN is increased leading to increased levels of nuclear p53, promotion of PTEN–p53 complex formation, increased recruitment of the PTEN–p53 complex to the PTEN promoter, and hence, activation of PTEN transcription. A functional outcome of increased nuclear PTEN is the reduction in the expression of pro-proliferative genes cyclin D1 and pleiotrophin, resulting in G0–G1 arrest and leading to early lobuloalveolar differentiation.
Around 40% of breast cancer cases are preventable by a healthy diet, exercise and weight control alone (45). While it is widely accepted that early (prepubertal) consumption of soy foods is associated with a decreased risk of breast cancer (7), mechanisms underlying dietary protection against mammary tumorigenesis remain poorly understood. Here, we provide strong support for a molecular mechanism involving increased PTEN expression in MECs to underlie mammary tumor protection by diet. The non-tumorigenic, classical ER-negative, human mammary epithelial cell line, MCF-10A, expresses both wild-type PTEN and p53 and represents an ideal in vitro system that mimics prepubertal (estrogen insensitive) mammary gland (46). The current study used GEN at physiologically relevant concentrations mimicking those found in plasma of human subjects regularly consuming soy foods (35). Our results effectively preclude increased DNA damage and apoptosis as underlying the GEN-mediated molecular changes reported here since higher doses of GEN than used in the present study were found to be non-genotoxic to MECs (47).
Although PTEN is known as a potent inhibitor of the PI3K–Akt pathway, recent emerging data support phosphatase-independent and cellular localization-dependent roles of PTEN. For example, while cytoplasmic PTEN is considered to mediate apoptosis, nuclear PTEN plays a key role in chromosomal integrity, cell cycle arrest and differentiation (42). Loss of nuclear PTEN has been correlated with decreased differentiation of normal cells, favoring neoplastic transformation (48). We demonstrate here that PTEN is both a target and regulator of p53 action, leading to increased MEC differentiation. The GEN-induced PTEN autoregulatory loop is initiated by increased nuclear localization of PTEN, which promotes nuclear retention of p53 and the subsequent transactivation by the PTEN–p53 complex of the PTEN promoter. Several features of this novel mechanism are noteworthy. First, GEN effects on p53 largely occur through PTEN, as demonstrated by the highly significant PTEN-mediated increase in p53 nuclear localization in the relative absence of GEN effects on p53 expression (data not shown). These results are consistent with the observed lack of apoptosis in GEN-treated MCF10A cells (undetectable sub-G0/G1 population), which would be predicted otherwise if GEN leads to increased DNA damage concomitant with elevated p53 expression. Second, while p53 is known to transactivate the PTEN gene (25) and that PTEN has been demonstrated to regulate p53 levels in both phosphatase-dependent and -independent manners (26,49), the present results to the best of our knowledge, constitute the first report of a functional PTEN–p53 transcriptional complex underlying increased PTEN promoter activity in MECs. The latter is consistent with the findings that PTEN and p53 mutations are mutually exclusive in human breast cancers (50), suggesting that loss of either tumor suppressor is sufficient to facilitate tumorigenesis, partly due to the eventual loss of PTEN expression. Finally, while the initiation of the PTEN autoregulatory loop by GEN leads to a significant increase in cell cycle arrest and promotion of early lobuloalveolar differentiation, the biological changes are modest, suggesting multiple pathways underlying dietary effects against tumorigenesis. In agreement with this, we (14,51) and others (52) have identified numerous genes and signaling pathways that are regulated by dietary factors in a variety of tissues and cell types.
Our work links the two most common tumor suppressor pathways, namely PTEN and p53, in the context of breast cancer prevention by soy isoflavone GEN. Albeit the mechanism for induction of PTEN levels by GEN is currently unknown, it is possible that GEN may be acting through classical ER-independent mechanisms via G protein-coupled receptor 30, a membrane receptor for estrogen (53), based on our preliminary results indicating GEN upregulation of G protein-coupled receptor 30 expression in MECs (data not shown). Our studies offer a mechanistic basis to target the PTEN activation pathway by the identification of dietary components with PTEN-promoting activity as a preventative strategy to reduce breast cancer risk.
Funding
United States Department of Agriculture (CRIS 6251-5100002-06S, Arkansas Children’s Nutrition Center) to R.C.M.S.; Children’s University Medical Group award to R.C.M.S. University of Arkansas Graduate Student Research Fund, Arkansas Children’s Hospital Research Institute Student and Clinical Staff Research Intramural Grant to O.R. Pre-doctoral fellowship from the Department of Defense Breast Cancer Research Program (W81XWH-10-1-0047) to O.R.
Acknowledgments
We thank Dr Frank A. Simmen, Dr Shanmugam Nagarajan and laboratory members for helpful discussions and critical reading of this manuscript. We also acknowledge the use of the Confocal Microscopy Laboratory, University of Arkansas for Medical Sciences (Supported by National Institutes of Health Grants P20 RR16460; Dr Larry Cornett, Principal Investigator and NCCR-S10RR19395; Dr Richard Kurten, Principal Investigator).
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CAS
casein
- EGF
epidermal growth factor
- ER
estrogen receptor
- GEN
genistein
- MEC
mammary epithelial cells
- PI3K
phosphatidylinositol 3-kinase
- PTEN
phosphatase and tensin homologue deleted on chromosome ten
- PCR
polymerase chain reaction
- QPCR
quantitative real-time polymerase chain reaction
- scRNA
scrambled RNA
- siRNA
small interfering RNA
- SPI
soy protein isolate
References
- 1.Jemal A, et al. Cancer Statistics 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Vargo-Gogola T, et al. Modeling breast cancer: one size does not fit all. Nat. Rev. Cancer. 2007;7:659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- 3.de Assis S, et al. Timing of dietary estrogenic exposures and breast cancer risk. Ann. N.Y. Acad. Sci. 2006;1089:14–35. doi: 10.1196/annals.1386.039. [DOI] [PubMed] [Google Scholar]
- 4.Shu XO, et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol. Biomarkers Prev. 2001;10:483–488. [PubMed] [Google Scholar]
- 5.Wu AH, et al. Adolescent and adult soy intake and risk of breast cancer in Asian Americans. Carcinogenesis. 2002;23:1491–1496. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 6.Warri A, et al. The role of early life genistein exposures in modifying breast cancer risk. Br. J. Cancer. 2008;98:1485–1493. doi: 10.1038/sj.bjc.6604321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu AH, et al. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamartiniere CA. Protection against breast cancer with genistein: a component of soy. Am. J. Clin. Nutr. 2000;71:1705S–1707S. doi: 10.1093/ajcn/71.6.1705S. [DOI] [PubMed] [Google Scholar]
- 9.Lamartiniere CA, et al. Genistein chemoprevention: timing and mechanisms of action in murine mammary gland and prostate. J. Nutr. 2002;132:552S–558S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 10.Verheus M, et al. Plasma phytoestrogens and subsequent breast cancer risk. J. Clin. Oncol. 2007;25:648–655. doi: 10.1200/JCO.2006.06.0244. [DOI] [PubMed] [Google Scholar]
- 11.Simmen RCM, et al. Inhibition of NMU-induced mammary tumorigenesis by dietary soy. Cancer Lett. 2005;224:45–52. doi: 10.1016/j.canlet.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Su Y, et al. In utero exposure to maternal diets containing soy protein isolate, but not genistein alone, protects young adult rat offspring from NMU-induced mammary tumorigenesis. Carcinogenesis. 2007;28:1046–1051. doi: 10.1093/carcin/bgl240. [DOI] [PubMed] [Google Scholar]
- 13.Hakkak R, et al. Diets containing whey proteins or soy protein isolate protect against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in female rats. Cancer Epidemiol. Biomarkers Prev. 2000;9:113–117. [PubMed] [Google Scholar]
- 14.Su Y, et al. Expression profiling of rat mammary epithelial cell reveals candidate signaling pathways in dietary protection from mammary tumors. Physiol. Genomics. 2007b;30:8–16. doi: 10.1152/physiolgenomics.00023.2007. [DOI] [PubMed] [Google Scholar]
- 15.Dave B, et al. The soy isoflavone genistein promotes apoptosis in mammary epithelial cells by inducing the tumor suppressor PTEN. Carcinogenesis. 2005;26:1793–1803. doi: 10.1093/carcin/bgi131. [DOI] [PubMed] [Google Scholar]
- 16.Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 17.Yin Y, et al. PTEN: a new guardian of the genome. Oncogene. 2008;27:5443–5453. doi: 10.1038/onc.2008.241. [DOI] [PubMed] [Google Scholar]
- 18.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 19.Dupont J, et al. PTEN overexpression suppresses proliferation and differentiation and enhances apoptosis of the mouse mammary epithelium. J. Clin. Invest. 2002;110:815–825. doi: 10.1172/JCI13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eng C, et al. PTEN and inherited hamartoma-cancer syndromes. Nat. Genet. 1998;19:223. doi: 10.1038/897. [DOI] [PubMed] [Google Scholar]
- 21.Rhei E, et al. Mutation analysis of the putative tumor suppressor gene PTEN/MMAC1 in primary breast carcinomas. Cancer Res. 1997;57:3657–3659. [PubMed] [Google Scholar]
- 22.Li G, et al. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development. 2002;129:4159–4170. doi: 10.1242/dev.129.17.4159. [DOI] [PubMed] [Google Scholar]
- 23.Shoman N, et al. Reduced PTEN expression predicts relapse in patients with breast carcinoma treated by tamoxifen. Mod. Pathol. 2005;18:250–259. doi: 10.1038/modpathol.3800296. [DOI] [PubMed] [Google Scholar]
- 24.Saal LH, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc. Natl Acad. Sci. USA. 2007;104:7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stambolic V, et al. Regulation of PTEN transcription by p53. Mol. Cell. 2001;8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 26.Freeman DJ, et al. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell. 2003;3:117–130. doi: 10.1016/s1535-6108(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, et al. PTEN has tumor-promoting properties in the setting of gain-of-function p53 mutations. Cancer Res. 2008;68:1723–1731. doi: 10.1158/0008-5472.CAN-07-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeves PG, et al. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 29.Debnath J, et al. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 30.Su Y, et al. Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates β-catenin signaling in mammary epithelial cells. Carcinogenesis. 2009;130:331–339. doi: 10.1093/carcin/bgn279. [DOI] [PubMed] [Google Scholar]
- 31.Virolle T, et al. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signaling. Nat. Cell Biol. 2001;3:1124–1128. doi: 10.1038/ncb1201-1124. [DOI] [PubMed] [Google Scholar]
- 32.Velarde MC, et al. Progesterone receptor transactivation of the secretory leukocyte protease inhibitor gene in Ishikawa endometrial epithelial cells involves recruitment of Krüppel-like factor 9/basic transcription element bidning protein-1. Endocrinology. 2006;147:1969–1978. doi: 10.1210/en.2005-1419. [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, et al. Id-1 activates Akt-mediated Wnt signaling and p27(Kip1) phosphorylation through PTEN inhibition. Oncogene. 2009;28:824–831. doi: 10.1038/onc.2008.451. [DOI] [PubMed] [Google Scholar]
- 34.Setchell KD, et al. Exposure of infants to phyto-estrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 35.Morton MS, et al. Phytoestrogens concentrations in serum from Japanese men and women over forty years of age. J. Nutr. 2002;132:3168–3171. doi: 10.1093/jn/131.10.3168. [DOI] [PubMed] [Google Scholar]
- 36.Eason RR, et al. Uterine phenotype of young adult rats exposed to dietary soy or genistein during development. J. Nutr. Biochem. 2005;16:625–632. doi: 10.1016/j.jnutbio.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Soule HD, et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10A. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 38.Lian Z, et al. Class reunion: PTEN joins the nuclear crew. Oncogene. 2005;24:7394–7400. doi: 10.1038/sj.onc.1209089. [DOI] [PubMed] [Google Scholar]
- 39.Chung JH, et al. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res. 2005;65:8096–8100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 40.Chung JH, et al. The ERK1/2 pathway modulates nuclear PTEN-mediated cell cycle arrest by cyclin D1 transcriptional regulation. Hum. Mol. Genet. 2006;15:2553–2559. doi: 10.1093/hmg/ddl177. [DOI] [PubMed] [Google Scholar]
- 41.Collins NL, et al. G1/S cell cycle arrest provides anoikis resistance through Erk-mediated Bim suppression. Mol. Cell. Biol. 2005;25:5282–5291. doi: 10.1128/MCB.25.12.5282-5291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Planchon SM, et al. The nuclear affairs of PTEN. J. Cell Sci. 2008;121:249–253. doi: 10.1242/jcs.022459. [DOI] [PubMed] [Google Scholar]
- 43.Li G, et al. PTEN deletion leads to up-regulation of a secreted growth factor pleiotrophin. J. Biol. Chem. 2006;281:10663–10668. doi: 10.1074/jbc.M512509200. [DOI] [PubMed] [Google Scholar]
- 44.Fournier MV, et al. Interaction of E-cadherin and PTEN regulates morphogenesis and growth arrest in human mammary epithelial cells. Cancer Res. 2009;69:4545–4552. doi: 10.1158/0008-5472.CAN-08-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doll R, et al. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 46.Fendrick JL, et al. Mammary gland growth and development from the postnatal period to postmenopause: ovarian steroid receptor ontogeny and regulation in the mouse. J. Mammary Gland Biol. Neoplasia. 1998;3:7–22. doi: 10.1023/a:1018766000275. [DOI] [PubMed] [Google Scholar]
- 47.Steiner C, et al. Genistein protects human mammary epithelial cells from benzo(a)pyrene-7,8-dihydrodiol-9-10-epoxide and 4-hydroxy-2-nonenal genotoxicity by modulating the glutathione/glutathione S-transferase system. Carcinogenesis. 2007;28:738–748. doi: 10.1093/carcin/bgl180. [DOI] [PubMed] [Google Scholar]
- 48.Gimm O, et al. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am. J. Pathol. 2000;156:1693–1700. doi: 10.1016/s0002-9440(10)65040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Y, et al. PTEN autoregulates its expression by stabilization of p53 in a phosphatase-independent manner. Cancer Res. 2006;66:736–742. doi: 10.1158/0008-5472.CAN-05-1557. [DOI] [PubMed] [Google Scholar]
- 50.Kurose K, et al. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat. Genet. 2002;32:355–357. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 51.Su Y, et al. Early soy exposure via maternal diet regulates rat mammary epithelial differentiation by paracrine signaling from stromal adipocytes. J. Nutr. 2009;139:945–951. doi: 10.3945/jn.108.103820. [DOI] [PubMed] [Google Scholar]
- 52.Lavigne JA, et al. Concentration-dependent effects of genistein on global gene expression in MCF-7 breast cancer cells: an oligo microarray study. Breast Cancer Res. Treat. 2008;110:85–98. doi: 10.1007/s10549-007-9705-6. [DOI] [PubMed] [Google Scholar]
- 53.Olde B, et al. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol. Metab. 2009;20:409–416. doi: 10.1016/j.tem.2009.04.006. [DOI] [PubMed] [Google Scholar]