Abstract
Reduced levels of β-amyloid1-42 (Aβ1-42) and increased levels of tau proteins in the cerebrospinal fluid (CSF) are found in Alzheimer’s disease (AD), likely reflecting Aβ deposition in plaques and neuronal and axonal damage. It is not known whether these biomarkers are associated with brain atrophy also in healthy aging. We tested the relationship between CSF levels of Aβ1-42 and tau (total tau and tau phosphorylated at threonine 181) proteins and 1-year brain atrophy in 71 cognitively normal elderly individuals. Results showed that under a certain threshold value, levels of Aβ1-42 correlated highly with 1-year change in a wide range of brain areas. The strongest relationships were not found in the regions most vulnerable early in AD. Above the threshold level, Aβ1-42 was not related to brain changes, but significant volume reductions as well as ventricular expansion were still seen. It is concluded that Aβ1-42 correlates with brain atrophy and ventricular expansion in a subgroup of cognitively normal elderly individuals but that reductions independent of CSF levels of Aβ1-42 is common. Further research and follow-up examinations over several years are needed to test whether degenerative pathology will eventually develop in the group of cognitively normal elderly individuals with low levels of Aβ1-42.
Keywords: aging, amyloid, cerebral cortex, CSF biomarkers, MRI
Even healthy elderly adults have, on average, thinner cerebral cortex, smaller volume of most subcortical structures, and expanded cerebral ventricles compared with the younger (Resnick et al. 2003; Raz et al. 2004; Allen et al. 2005; Fjell et al. 2009; Walhovd et al. forthcoming). A crucial question regards whether these atrophic processes in the brains of healthy elderly individuals are related to the cerebrospinal fluid (CSF) biomarkers β-amyloid1-42 (Aβ1-42) and tau proteins, potentially reflecting age-related neurodegenerative mechanisms. Depositions of extracellular plaques (Aβ1-42) and intracellular neurofibrillary tangles (tau) are believed to play causative roles in neurodegeneration in Alzheimer’s disease (AD; Goedert and Spillantini 2006; Spires-Jones et al. 2009a), and CSF levels of these biomarkers correlate with rates of atrophy and ventricular expansion (Hampel et al. 2005; de Leon et al. 2006; Chou et al. 2009; Schuff et al. 2009). However, it is unclear whether and to what extent these CSF biomarkers are related to neurodegenerative effects also in healthy elderly individuals. Improved knowledge of the role played by these CSF biomarkers will greatly enhance our understanding of the neurobiological correlates of brain atrophy in healthy aging and of the specificity of the role of such biomarkers in age-related degenerative diseases. Thus, the aim of the present study was to examine the relationship between longitudinal brain changes in healthy aging and CSF levels of Aβ1-42 and tau proteins.
The CSF level of the different biomarkers likely reflects specific pathogenic processes in the brain. Total tau (T-tau) is probably related to the intensity of the neuronal damage and degeneration because a marked transient increase is found in acute conditions such as stroke, and the magnitude of the increase correlates with infarct size (Hesse et al. 2000). The degree of increase in CSF T-tau in chronic disorders is highest in conditions with the most intense neuronal degeneration, such as Creutzfeldt–Jakob disease (Otto et al. 1997). A moderate increase is found in AD, where degeneration is less intense, and normal levels are found in patients with Parkinson’s disease, where degeneration is limited to a small brain region (Sjogren et al. 2001b). In addition, CSF T-tau levels increase strongly with age even in healthy controls (Sjogren et al. 2001b). Tau phosphorylated at threonine 181 (P-tau), in contrast, does not reflect general neurodegeneration because increased CSF levels have so far only been found in AD, with normal levels both in acute conditions (Hesse et al. 2001) and in intense chronic neurodegenerative disorders (Riemenschneider et al. 2003). Instead, CSF P-tau correlates with tangle load in neocortex (Buerger et al. 2006), suggesting that it is a marker for tau phosphorylation and tangle formation.
CSF Aβ1-42 is reduced in several neurological conditions (Winblad et al. 2008), including AD (Shaw et al. 2009). The reason for the decrease in CSF Aβ1-42 in AD is not clear, but the most probable explanation is that Aβ1-42 is deposited in plaques, with lower amounts of Aβ being free to diffuse into CSF. This explanation is supported by the finding of a strong correlation between low Aβ1-42 in CSF and high numbers of plaques in the neocortex and hippocampus (Strozyk et al. 2003), and between low Aβ1-42 in CSF and high retention of Pittsburgh compound-B (PIB) on positron emission tomography (PET) scanning (Fagan et al. 2006).
In sum, evidence indicates that T-tau and Aβ1-42 are related to axonal and neuronal damage in AD as well as in other conditions (Winblad et al. 2008; Spires-Jones et al. 2009b) and that T-tau increases with higher age even in healthy individuals (Sjogren et al. 2001b). Still, the relationship between the CSF biomarkers and brain atrophy has received little attention in the literature, and the results that have been reported are mixed. One study found whole-brain volume to be positively correlated with CSF levels of Aβ1-42 but not of tau (Fagan et al. 2009), one did not find any relationship between the CSF biomarkers and whole-brain atrophy (Sluimer et al. 2008), and one found that low CSF Aβ correlated with brain atrophy in a cross-sectional sample, whereas high T-tau predicted more marked ventricular widening during follow-up (Wahlund and Blennow 2003). A final study did not find correlations between hippocampal atrophy and CSF biomarkers (de Leon et al. 2006). No studies have examined brain measures other than the whole-brain, ventricular, or hippocampal volume. Of special relevance is the study by Fagan and colleagues, where low levels of Aβ1-42 were found to be associated with smaller whole-brain volume in a large sample (n = 69) of healthy elderly individuals (Clinical Dementia Rating [CDR] = 0). Further, all PIB-positive participants had low CSF levels of Aβ1-42, whereas almost all PIB-negative participants had high CSF levels of Aβ1-42. As mentioned previously, high retention of PIB indicates high brain amyloid plaque load. The authors interpreted their findings to indicate that there is toxicity associated with Aβ aggregation before the onset of clinically detectable disease, whereas increases in tau may occur with clinical onset and progression.
In the present study, we used data from the publicly available Alzheimer’s Disease Neuroimaging Initiative (ADNI) database to examine 71 healthy elderly individuals with CSF and magnetic resonance (MR) data at baseline and MR follow-up after 1 year. We used a newly developed nonlinear registration algorithm that enables precise registration of serial scans, yielding a one-to-one correspondence between each vertex in the baseline and the follow-up scan. Change was measured continuously across the cortical surface, as well as in 15 subcortical and 33 cortical regions of interest (ROIs) in each hemisphere, and related to CSF levels of tau proteins and Aβ1-42. We found that Aβ1-42 modulated volumetric brain reductions and ventricular expansion in healthy elderly individuals when a certain threshold value was reached and that the strongest relationships existed in regions not especially vulnerable to AD.
Materials and Methods
The raw data used in the preparation of this article were obtained from the ADNI database (www.loni.ucla.edu/ADNI). ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies, and nonprofit organizations. The primary goal of ADNI has been to test whether serial MR imaging (MRI), PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD. The principal investigator of this initiative is Michael W. Weiner, VA Medical Center and University of California—San Francisco. There are many coinvestigators, and subjects have been recruited from more than 50 sites across the United States and Canada. The initial goal of ADNI was to recruit 800 adults, including healthy elderly individuals and MCI and AD patients to participate and be followed for 2–3 years. For more information see www.adni-info.org
Sample
Participants are 55–90 years of age, had an informant able to provide an independent evaluation of functioning, and spoke either English or Spanish. General inclusion/exclusion criteria are as follows for normal subjects: Mini-Mental State Examination (MMSE; Folstein et al. 1975) scores between 24 and 30 (inclusive), CDR (Morris 1993) of 0, nondepressed, non-MCI, and nondemented. In addition, to minimize the possibility of including individuals with early preclinical AD, only participants who had the same or better score on CDR sum of boxes (CDR-sb) at the time of follow-up were included. The subject pool was further restricted to those subjects for whom adequate processed and quality checked MR and CSF baseline data were available by February 2009. The total sample consisted of 71 healthy elderly individuals (31 women/40 men), with mean age 75.6 years (62.2–90.2) at baseline, mean MMSE score of 29.9 (25–30) at baseline and 28.9 (25–30) at follow-up, and CDR-sb 0.02 (0–0.5) at baseline and 0.0 (0–0) at follow-up. For assessment of memory, Auditory Verbal Learning Test (AVLT; Lezak 1995) was administered. The 5 learning trials were summed to 1 learning score, and we subtracted the number of intrusions. The same was done for 30-min free recall. In addition, information about number of apolipoprotein E (APOE) ϵ4 alleles was available. Participants with at least 1 ϵ2 allele were excluded (resulting distribution: 37 ϵ3/ϵ3, 20 ϵ3/ϵ4, 1 ϵ4/ϵ4).
MR Acquisition and Analysis
All scans used for the present study were from 1.5 T scanners. Data were collected across a variety of scanners with protocols individualized for each scanner, as defined at http://www.loni.ucla.edu/ADNI/Research/Cores/index.shtml. Raw DICOM MRI scans (including 2 T1-weighted volumes per case) were downloaded from the public ADNI site (http://www.loni.ucla.edu/ADNI/Data/index.shtml) and processed as described elsewhere (Fennema-Notestine et al. 2009). Briefly, these data were reviewed for quality, automatically corrected for spatial distortion due to gradient nonlinearity (Jovicich et al. 2006) and B1 field inhomogeneity (Sled et al. 1998), registered, and averaged to improve signal to noise ratio. Scans were segmented (Fischl et al. 2002), yielding volumetric data for 15 different subcortical structures. The procedure (Fischl et al. 2002, 2004) uses a probabilistic atlas and applies a Bayesian classification rule to assign a neuroanatomical label to each voxel. The cortical surface was reconstructed to measure thickness at each surface point using a semiautomated approach (Dale and Sereno 1993; Dale et al. 1999; Fischl et al. 1999a, 1999b; Fischl and Dale 2000). The measurement technique used here has been validated via histological (Rosas et al. 2002) as well as manual measurements (Kuperberg et al. 2003). The cortical surface was parcellated in 33 cortical sulci and gyri (Fischl et al. 2004; Desikan et al. 2006).
For the analyses of the longitudinal volume changes, each participant’s dual 3D follow-up structural scans were rigid-body aligned, averaged, and affine aligned to the baseline scan. Nonlinear registration of the images was then carried out, where voxel centers are moved about until a good match between the images is made. Several methods exist for effecting this, including fluid deformation (Christensen et al. 1996; Freeborough and Fox 1998; Miller et al. 1993) and tensor-based morphometry (TBM; Ashburner et al. 1999). For the results presented here, however, we applied a method (Holland et al. 2009) based on linear elasticity and closer in spirit to TBM. It proceeds essentially as follows. The images are heavily blurred (smoothed), making them almost identical, and a merit or potential function is calculated. This merit function expresses the intensity difference between the images at each voxel and depends on the displacement field for the voxel centers of the image being transformed; it is also regularized to keep the displacement field spatially smooth. The merit function by design will have a minimum when the displacement field induces a good match between the images. The displacement field in general will turn cubic voxels into displaced irregular hexahedrons whose volumes (Grandy 1997) give the volume change field. The merit function is minimized efficiently using standard numerical methods. Having found a displacement field for the heavily blurred pair of images, the blurring is reduced and the procedure repeated, thus iteratively building up a better displacement field. Two important additions to this are as follows: applying the final displacement field to the image being transformed, then nonlinearly registering the resultant image to the same target, and finally tracing back through the displacement fields thus calculated to find the net displacement field; and restricting to ROIs and zooming when tissue structures are separated by only a voxel or two. These additional features enable very precise registration involving large or subtle deformations, even at small spatial scales with low boundary contrast. Although large deformations are allowed by multiple nonlinear registration (or relaxation) steps, nonphysical deformations are precluded because at each level of blurring the image undergoing deformation is constrained to conform to the target. Note that calculating the deformation field does not depend on initially segmenting tissue. This deformation field was used to align scans at the subvoxel level.
The follow-up aligned image underwent skull stripping and volumetric segmentations (subcortical structures, as well as hippocampal and cerebellar gray matter), with labels applied from the baseline scan. For the cortical reconstructions, surface coordinates for the white matter and pial boundaries were derived from the baseline images and mapped onto the follow-up images using the deformation field. Parcellations from the baseline image were then applied to the follow-up image. This resulted in a one-to-one correspondence between each vertex in the base image and the follow-up image. The procedure produces an estimate of the percent cortical volume loss at each vertex and within each ROI. To the extent that regional cortical areas are relatively stable across time points, the volume change is likely driven almost exclusively by changes in thickness.
As the procedure is fully automated, the test–retest reliability is 1. The method has also been validated in model studies of complex spherical shell geometries with low contrast and noise, where a prescribed volume change is numerically estimated to accuracies of within 0.5% (Holland et al., in preparation).
CSF Acquisition and Analysis
CSF samples were obtained by lumbar puncture using a standardized protocol, as described in the ADNI procedures manual (http://www.adni-info.org/index) at the participating clinical sites. The CSF samples were transferred into polypropylene transfer tubes, freezed on dry ice within 1 h of collection, and shipped overnight to the ADNI Biomarker Core laboratory at the University of Pennsylvania Medical Center. The CSF aliquots were stored in bar code–labeled polypropylene vials at −80 °C. All CSF samples were analyzed over a 14-day period. Test–retest analyses of a subset of the samples showed excellent analytical performance, with r2 values for test–retest results of 0.85–0.98 (Shaw et al. 2009). T-tau, P-tau, and Aβ1-42 levels in CSF were determined by the Luminex xMAP technology using the INNO-BIA AlzBio3 kit (Innogenetics, Ghent, Belgium), as previously described in detail (Olsson et al. 2005). In brief, the method is based on flow cytometric separation of antibody-coated microspheres that are labeled with a specific mixture of 2 fluorescent dyes. After binding of a biotinylated reporter antibody, quantification is made by binding of a third fluorochrome coupled to streptavidin. The method has been shown to have high analytical precision (Olsson et al. 2005).
Statistical Analysis
To reduce the number of comparisons, right and left hemisphere values were averaged for each ROI. Partial correlations controlling for the effect of age were carried out to test the relationship between percentage change in each of the 48 ROIs and CSF levels of Aβ1-42, T-tau, and P-tau, as well as the ratio between each of the tau measures and Aβ1-42. Because it is likely that the CSF biomarker levels will be related to brain changes only when certain concentrations are reached, we tested for nonlinear relationships between the CSF biomarkers and percentage change in brain volume by introducing a quadratic term as an additional predictor in a multiple regression analyses (ROI = β0 + β1age + β2CSF biomarker + β3[CSF biomarker × CSF biomarker] + ϵ). More correlations were found between Aβ1-42 and brain change than between the tau biomarkers and brain change, so this analysis was restricted to Aβ1-42. If β3 was significant, a nonparametric local smoothing technique (the smoothing spline) was used. Given a sequence of data (Xi, Yi); i = 1, …, n, with E (Yi|Xi) = g(xi), the smoothing spline estimate of g minimizes
where the smoothing parameter λ controls the trade-off between fidelity (closeness of g(Xi) to Yi) and smoothness (the size of the average second derivative of g). With no smoothing (λ = 0), g simply interpolates the data, whereas with infinite smoothing (λ → ∞), g corresponds to the line fit by ordinary least squares. Smoothing level was set to 5e5. The smoothing spline was used because the quadratic model is unlikely to represent a reasonable relationship between the CSF biomarkers and brain atrophy.
Samples were divided into the high-Aβ1-42 or the low-Aβ1-42 group based on whether their levels were above or below the break point of the smoothing spline function. As brain change is expressed as percentage change relative to baseline, 1-sample t-tests were carried out to determine whether 1-year change in each of the ROIs for each of the 2 groups was significantly different from zero. Intracranial volume was not used as covariate in this analysis because change expressed as percentage inherently is corrected for initial brain size. Independent samples t-tests were used to determine whether percentage change in each ROI differed between the high- and the low-Aβ1-42 group, and whether baseline differences in volume or thickness of each ROI existed between the groups. Percentage change in each ROI for each group was also calculated point by point across the brain surface and shown as an overlay on a template brain. Correlations between Aβ1-42 and percentage change were run separately in each of the groups, and Fisher’s z-transformed correlation coefficients were calculated to test for differences between the groups. Surface maps of the relationships between Aβ1-42 and percentage cortical change within each group were also calculated by general linear models and displayed on the template brain. The statistical results were corrected for multiple comparisons (false discovery rate <0.05). Mean memory performance at baseline, as well as change in memory score over 1 year, for AVLT learning and 30-min delayed recall was compared between the Aβ1-42 groups by independent samples t-tests. Finally, number of APOE ϵ4 alleles was related to CSF biomarker levels by partial correlations controlling for age, and a possible difference in the mean number of ϵ4 alleles in the Aβ1-42 groups was explored by independent samples t-tests. APOE was also correlated with percentage change in each ROI (partial correlations controlling for age), and multiple regressions with APOE, Aβ1-42, and APOE × Aβ1-42 group as simultaneous predictors were carried out to test for possible interaction effects on percentage change in selected ROIs.
Results
Partial correlations between percentage change over 1 year and CSF biomarkers, controlling for the effect of age, are shown in Tables 1 and 2. Aβ1-42 was the CSF biomarker showing the highest correlations with brain change, with significant correlations in 27 ROIs. Correlations greater than 0.30 (P = 0.01) were found for putamen, thalamus, banks of the superior temporal sulcus, isthmus cingulate, caudal middle frontal, pallidum, superior frontal, caudate, accumbens, pars opercularis, and superior temporal cortex, as well as correlations less than −0.30 for the lateral and inferior lateral ventricles. Hippocampal reduction was not significantly related to Aβ1-42 levels (r = 0.19, NS). T-tau was not significantly related to change in any ROI, whereas P-tau was related to change in 9 ROIs (negative correlations with amygdala, paracentral cortex, posterior cingulate, cerebral white matter, and pallidum; positive correlations with the 4 ventricular ROIs). The tau/Aβ1-42 ratio measures were generally sensitive to change in several ROIs. For instance, Aβ1-42–P-tau correlated −0.43 with amygdala, −0.40 with paracentral cortex, and 0.44 or higher with the 4 ventricular ROIs.
Table 1.
Partial correlations between baseline CSF biomarkers and subcortical volumetric reductions and ventricular expansion over 1 year, controlled for the effect of age
| T-tau | P-tau | Aβ1-42 | T-tau–Aβ1-42 | P-tau–Aβ1-42 | |
| Subcortical ROIs | |||||
| Accumbens | −0.15 | −0.16 | 0.33 | −0.37 | −0.30 |
| Amygdala | −0.09 | −0.29 | 0.27 | −0.32 | −0.43 |
| Brainstem | −0.15 | −0.14 | 0.13 | −0.30 | −0.20 |
| Caudate | −0.07 | −0.09 | 0.33 | −0.36 | −0.28 |
| Cerebellar gray matter | 0.01 | −0.06 | 0.11 | −0.16 | −0.13 |
| Cerebellar white matter | 0.02 | −0.19 | 0.17 | −0.13 | −0.24 |
| Cerebral white matter | −0.17 | −0.26 | 0.27 | −0.40 | −0.37 |
| Hippocampus | 0.14 | −0.07 | 0.19 | −0.07 | −0.17 |
| Pallidum | −0.16 | −0.25 | 0.34 | −0.36 | −0.35 |
| Putamen | −0.09 | −0.18 | 0.38 | −0.36 | −0.33 |
| Thalamus | −0.11 | −0.17 | 0.37 | −0.41 | −0.34 |
| Ventricular ROIs | |||||
| Lateral ventricles | 0.13 | 0.33 | −0.33 | 0.38 | 0.46 |
| Inferior lateral ventricles | 0.16 | 0.34 | −0.30 | 0.38 | 0.44 |
| Third ventricle | 0.13 | 0.33 | −0.33 | 0.38 | 0.46 |
| Fourth ventricle | 0.16 | 0.34 | −0.30 | 0.38 | 0.44 |
Note: Values in bold, P < 0.05 (r ≥ 0.24); values in bold and italics, P < 0.001 (r ≥ 0.39). A negative correlation means that higher CSF concentrations are related to more brain atrophy (i.e. negative change in volume) and less ventricular expansion, and a positive correlation means that lower CSF concentrations are related to more atrophy and less ventricular expansion.
Table 2.
Partial correlations between baseline CSF biomarkers and cortical volume reductions over 1 year, controlled for the effect of age
| T-tau | P-tau | Aβ1-42 | T-tau–Aβ1-42 | P-tau–Aβ1-42 | |
| Cingulate, caudal anterior | −0.08 | −0.08 | 0.26 | −0.30 | −0.17 |
| Cingulate, rostral anterior | −0.05 | 0.03 | 0.24 | −0.27 | −0.10 |
| Cingulate, posterior | −0.11 | −0.27 | 0.26 | −0.32 | −0.37 |
| Cingulate, isthmus | −0.09 | −0.09 | 0.35 | −0.40 | −0.25 |
| Frontal, superior | −0.05 | −0.15 | 0.33 | −0.30 | −0.29 |
| Frontal, caudal middle | −0.05 | −0.11 | 0.35 | −0.33 | −0.28 |
| Frontal, rostral middle | −0.04 | −0.10 | 0.26 | −0.29 | −0.22 |
| Frontal, pars opercularis | −0.04 | −0.07 | 0.32 | −0.35 | −0.23 |
| Frontal, pars triangularis | 0.00 | 0.00 | 0.26 | −0.29 | −0.15 |
| Frontal, pars orbitalis | 0.04 | −0.05 | 0.21 | −0.22 | −0.17 |
| Frontal, lateral orbital | 0.05 | −0.01 | 0.25 | −0.20 | −0.17 |
| Frontal, medial orbital | −0.03 | 0.00 | 0.20 | −0.25 | −0.14 |
| Frontal, pole | −0.06 | −0.10 | 0.08 | −0.20 | −0.15 |
| Parietal, precentral gyrus | −0.04 | −0.23 | 0.27 | −0.28 | −0.37 |
| Parietal, postcentral gyrus | 0.09 | −0.14 | 0.12 | −0.07 | −0.23 |
| Parietal, paracentral gyrus | −0.02 | −0.29 | 0.23 | −0.20 | −0.40 |
| Parietal, superior | 0.09 | −0.22 | 0.22 | −0.16 | −0.39 |
| Parietal, inferior | −0.02 | −0.14 | 0.21 | −0.26 | −0.29 |
| Parietal, supramarginal | −0.15 | −0.18 | 0.29 | −0.37 | −0.31 |
| Parietal, precuneus | −0.16 | −0.16 | 0.28 | −0.38 | −0.29 |
| Temporal, parahippocampal | 0.03 | −0.07 | 0.20 | −0.19 | −0.16 |
| Temporal, entorhinal | 0.01 | −0.16 | 0.18 | −0.13 | −0.22 |
| Temporal, pole | −0.07 | −0.18 | 0.16 | −0.20 | −0.24 |
| Temporal, superior | −0.02 | −0.08 | 0.31 | −0.33 | −0.24 |
| Temporal, middle | 0.01 | −0.07 | 0.23 | −0.24 | −0.21 |
| Temporal, inferior | 0.00 | −0.09 | 0.17 | −0.21 | −0.22 |
| Temporal, transverse | −0.05 | 0.15 | 0.14 | −0.26 | 0.05 |
| Temporal, BSTS | −0.11 | −0.10 | 0.36 | −0.40 | −0.27 |
| Temporal, fusiform | −0.09 | −0.12 | 0.25 | −0.34 | −0.26 |
| Occipital, lateral | −0.08 | −0.11 | 0.14 | −0.26 | −0.22 |
| Occipital, pericalcarine | −0.10 | 0.02 | 0.07 | −0.14 | 0.03 |
| Occipital, lingual | −0.17 | 0.00 | 0.22 | −0.36 | −0.12 |
| Occipital, cuneus | −0.13 | −0.11 | 0.13 | −0.23 | −0.15 |
Note: Values in bold, P < 0.05 (r ≥ 0.24); values in bold and italics, P < 0.001 (r ≥ 0.39). BSTS, banks of the superior temporal sulcus. A negative correlation means that higher CSF concentrations are related to more brain atrophy (i.e. negative change in volume) and less ventricular expansion, and a positive correlation means that lower CSF concentrations are related to more atrophy and less ventricular expansion.
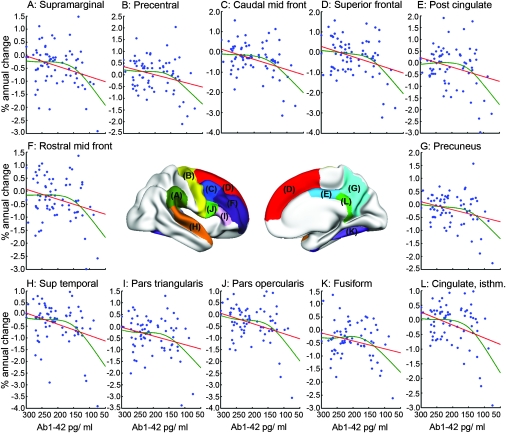
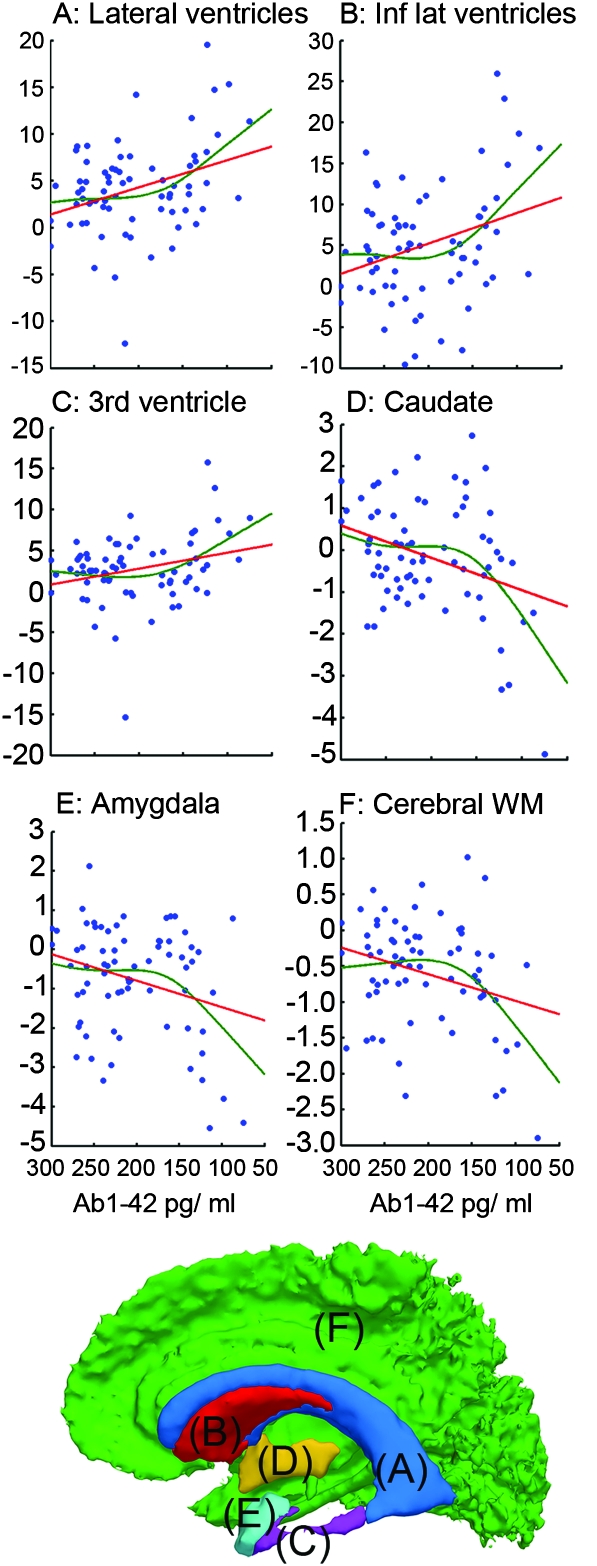
Next, nonlinear relationships between change over 1 year and CSF biomarkers were tested. Regressions were run with the ROIs as dependent variable and age, Aβ1-42, and Aβ1-42 × Aβ1-42 simultaneously as predictors (see Materials and Methods). The results are shown in Supplemental Table 1. Significant nonlinear relationships were found for 20 ROIs (amygdala, accumbens, caudate, cerebral white matter, thalamus, the lateral ventricles, inferior lateral ventricles, third ventricle, caudal middle frontal, fusiform, isthmus cingulate, pars opercularis, posterior cingulate, precentral, precuneus, superior frontal, superior temporal, supramarginal, pars triangularis, and rostral middle frontal). To depict these relationships, a nonparametric local smoothing technique was used (the smoothing spline). The results for 12 selected cortical ROIs are shown in Figure 1 and for 6 subcortical ROIs in Figure 2.
Figure 1.
Nonlinear relationships between Aβ1-42 and volume reductions for cortical ROIs. A nonparametric local smoothing technique (the smoothing spline) was used to depict nonlinear relationships (red lines) for the 71 participants. A relationship between Aβ1-42 and percentage change was found only when the concentration of Aβ1-42 in the CSF was below ≈ 175 pg/mL.
Figure 2.
Nonlinear relationships between Aβ1-42 and volumetric reductions of subcortical ROIs and ventricular expansion. A nonparametric local smoothing technique (the smoothing spline) was used to depict nonlinear relationships (red lines) for the 71 participants.
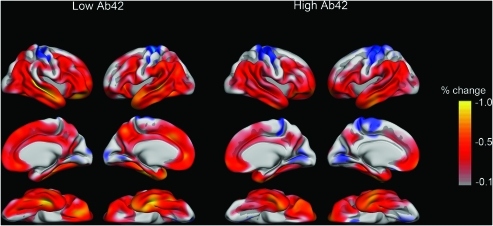
Aβ1-42 was related to percentage change over 1 year when levels were below an apparent break point. By visual inspection of the fit lines, the break point was found to be at Aβ1-42 ≈ 175 pg/mL. Thus, the sample was split into a high (Aβ1-42 >175, n = 45) and a low (Aβ1-42 ≤175, n = 26) group. Mean age was not significantly different between groups (75.0 vs. 76.5 years in the high and the low group, respectively, t(69) = 1.02, P = 0.31). Thinner cortex at baseline in the low group was found in isthmus of the cingulate, precuneus, superior frontal, and superior parietal cortex. Percentage volume change across the cortical mantle for each group is shown in Figure 3. Annual percentage reduction varied across the cortex but was typically around 0.5% in affected areas, including most of the lateral and inferior parts of the temporal lobes, inferior parietal gyrus, precuneus, inferior and middle frontal gyri, medial and lateral orbitofrontal, and the medial parts of the superior frontal gyrus. Brain changes in the low-Aβ1-42 group were generally larger than in the high group. Both groups showed significant 1-year change in numerous ROIs (33 ROIs for the low-Aβ1-42 group and 32 ROIs for the high-Aβ1-42 group; Table 3). The average annual changes seemed larger for almost all ROIs for the low- compared with the high-Aβ1-42 group, but due to the smaller number of participants in the low-Aβ1-42 group, the differences were statistically significant only for 5 ROIs (pallidum, thalamus, lateral and inferior lateral ventricles, banks of the superior temporal sulcus).
Figure 3.
Cortical reductions in the high- and the low-Aβ1-42 groups. Based on the break point of the smoothing spline graphs (see Figs 1 and 2), a high-Aβ1-42 group and a low-Aβ1-42 group were defined. Atrophy was calculated as percentage change in cortex point by point across the brain surface. The reductions are generally larger in the low- than in the high-Aβ1-42 group.
Table 3.
Baseline volume and thickness, and annual percentage volume change for the low-Aβ1-42 (n = 26) and the high-Aβ1-42 (n = 45) groups
| Baseline | 1-year change | |||||
| Low Aβ1-42 | High Aβ1-42 | Difference P | Low Aβ1-42 | High Aβ1-42 | Difference P | |
| Subcortical ROIs | ||||||
| Accumbens | 884 | 902 | NS | −0.86 | −0.36 | * |
| Amygdala | 2919 | 2943 | NS | −1.02 | −0.61 | NS |
| Brainstem | 21 033 | 20 705 | NS | −0.38 | −0.32 | NS |
| Caudate | 6430 | 6562 | NS | −0.40 | 0.00 | NS |
| Cerebellar gray matter | 99 106 | 95 857 | NS | −0.38 | −0.33 | NS |
| Cerebellar white matter | 25 184 | 24 708 | NS | −0.78 | −0.52 | NS |
| Cerebral white matter | 431 469 | 431 316 | NS | −0.80 | −0.47 | * |
| Hippocampus | 7398 | 7346 | NS | −1.01 | −0.73 | NS |
| Pallidum | 3190 | 3296 | NS | −0.60 | −0.21 | ** |
| Putamen | 8868 | 9256 | NS | −0.62 | −0.26 | * |
| Thalamus | 12 300 | 12 272 | NS | −0.99 | −0.51 | ** |
| Ventricular ROIs | ||||||
| Lateral ventricles | 37 531 | 33 504 | NS | 5.92 | 3.26 | ** |
| Inferior lateral ventricles | 2448 | 2279 | NS | 7.33 | 3.83 | ** |
| Third ventricle | 1840 | 1780 | NS | 3.94 | 2.03 | * |
| Fourth ventricle | 2125 | 1969 | NS | 1.06 | 0.29 | NS |
| Cortical ROIs | ||||||
| Cingulate, caudal anterior | 2.59 | 2.55 | NS | −0.50 | −0.20 | ** |
| Cingulate, rostral anterior | 2.70 | 2.76 | NS | −0.51 | −0.27 | NS |
| Cingulate, posterior | 2.33 | 2.37 | NS | −0.34 | −0.06 | NS |
| Cingulate, isthmus | 2.30 | 2.38 | ** | −0.37 | −0.03 | NS |
| Frontal, superior | 2.29 | 2.41 | ** | −0.42 | −0.09 | NS |
| Frontal, caudal middle | 2.14 | 2.20 | NS | −0.63 | −0.18 | * |
| Frontal, rostral middle | 1.96 | 2.02 | NS | −0.45 | −0.20 | NS |
| Frontal, pars opercularis | 2.21 | 2.24 | NS | −0.57 | −0.24 | * |
| Frontal, pars triangularis | 2.04 | 2.07 | NS | −0.59 | −0.29 | NS |
| Frontal, pars orbitalis | 2.43 | 2.48 | NS | −0.58 | −0.39 | NS |
| Frontal, lateral orbital | 2.43 | 2.49 | NS | −0.64 | −0.37 | NS |
| Frontal, medial orbital | 2.16 | 2.23 | * | −0.54 | −0.32 | NS |
| Frontal, pole | 2.54 | 2.59 | NS | −0.58 | −0.68 | NS |
| Parietal, precentral gyrus | 2.02 | 2.05 | NS | −0.15 | 0.12 | NS |
| Parietal, postcentral gyrus | 1.67 | 1.70 | NS | 0.22 | 0.22 | NS |
| Parietal, paracentral gyrus | 1.93 | 1.99 | NS | −0.17 | 0.07 | NS |
| Parietal, superior | 1.78 | 1.84 | ** | −0.23 | 0.01 | NS |
| Parietal, inferior | 2.02 | 2.08 | * | −0.48 | −0.32 | NS |
| Parietal, supramarginal | 2.16 | 2.18 | NS | −0.60 | −0.29 | NS |
| Parietal, precuneus | 1.92 | 1.99 | ** | −0.32 | −0.12 | NS |
| Temporal, parahippocampal | 2.41 | 2.44 | NS | −0.58 | −0.31 | NS |
| Temporal, entorhinal | 3.28 | 3.29 | NS | −0.73 | −0.48 | NS |
| Temporal, pole | 3.56 | 3.62 | NS | −0.61 | −0.46 | NS |
| Temporal, superior | 2.35 | 2.41 | NS | −0.61 | −0.27 | NS |
| Temporal, middle | 2.56 | 2.61 | NS | −0.62 | −0.39 | NS |
| Temporal, inferior | 2.59 | 2.64 | NS | −0.57 | −0.41 | NS |
| Temporal, transverse | 1.84 | 1.94 | * | −0.28 | −0.21 | NS |
| Temporal, BSTS | 2.11 | 2.14 | NS | −0.78 | −0.36 | NS |
| Temporal, fusiform | 2.35 | 2.41 | NS | −0.58 | −0.29 | * |
| Occipital, lateral | 1.83 | 1.86 | NS | −0.21 | −0.13 | NS |
| Occipital, pericalcarine | 1.33 | 1.33 | NS | 0.03 | 0.16 | NS |
| Occipital, lingual | 1.68 | 1.71 | NS | −0.13 | 0.10 | NS |
| Occipital, cuneus | 1.65 | 1.66 | NS | 0.00 | 0.04 | NS |
Note: Values in bold indicate that the change is different from zero at P < 0.05. “*” indicates a trend toward significance (P < 0.10) between low- and high-Aβ1-42 groups; “**” indicates significance (P < 0.05) between low- and high-Aβ1-42 groups. NS, not significant (P > 0.10); BSTS, banks of the superior temporal sulcus. The differences in baseline volume/thickness and volume change between the groups are tested by independent samples t-tests. Baseline volumes are in mm3 and baseline thickness is in mm, whereas atrophy is expressed as annual percentage change.
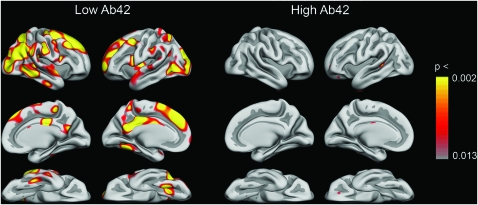
Interestingly, percentage change did not significantly correlate with Aβ1-42 level in the high-Aβ1-42 group (all r’s ≤ 0.28), but changes in 43 ROIs were significantly correlated with Aβ1-42 level in the low-Aβ1-42 group (Tables 4 and 5). In 31 of these ROIs, correlations in the low-Aβ1-42 group were significantly stronger than in the high-Aβ1-42 group. Figure 4 shows the relationship between Aβ1-42 and cortical changes point by point across the brain surface in each group separately. For the high group, no significant effects were seen. For the low group, large and scattered effects were seen, especially strong in superior frontal gyrus, posterior and isthmus cingulate, and occipital and inferior parietal cortex.
Table 4.
Correlations between subcortical volume change and ventricular expansion over 1 year and Aβ1-42 within the low-Aβ1-42 (n = 26) and the high-Aβ1-42 (n = 45) groups
| Low Aβ1-42 | High Aβ1-42 | Difference z-score | |
| Subcortical ROIs | |||
| Accumbens | 0.64 | 0.17 | 2.27 |
| Amygdala | 0.70 | 0.10 | 2.97 |
| Brainstem | 0.41 | 0.14 | 1.14 |
| Caudate | 0.67 | 0.22 | 2.27 |
| Cerebellar gray matter | 0.48 | 0.05 | 1.83 |
| Cerebellar white matter | 0.09 | 0.06 | 0.12 |
| Cerebral white matter | 0.65 | −0.06 | 3.23 |
| Hippocampus | 0.42 | 0.19 | 0.99 |
| Pallidum | 0.36 | 0.15 | 0.87 |
| Putamen | 0.54 | 0.28 | 1.23 |
| Thalamus | 0.64 | 0.11 | 2.51 |
| Ventricular ROIs | |||
| Lateral ventricles | −0.63 | −0.02 | 2.79 |
| Inferior lateral ventricles | −0.63 | 0.07 | 3.14 |
| Third ventricle | −0.57 | 0.08 | 2.82 |
| Fourth ventricle | −0.55 | −0.05 | 2.20 |
Note: Values in bold, P < 0.05; values in bold and italics, P < 0.01. The differences between the correlations were tested with Fisher’s z-transformed correlation coefficients. A positive correlation indicates that lower CSF concentrations are associated with more atrophy and less ventricular expansion.
Table 5.
Correlations between cortical volume reductions over 1 year and Aβ1-42 within the low-Aβ1-42 (n = 26) and the high-Aβ1-42 (n = 45) groups
| Low Aβ1-42 | High Aβ1-42 | Difference z-score | |
| Cingulate, caudal anterior | 0.46 | 0.24 | 0.97 |
| Cingulate, rostral anterior | 0.44 | 0.22 | 0.96 |
| Cingulate, posterior | 0.69 | 0.07 | 3.00 |
| Cingulate, isthmus | 0.65 | 0.12 | 2.54 |
| Frontal, superior | 0.63 | 0.23 | 1.96 |
| Frontal, caudal middle | 0.57 | 0.06 | 2.26 |
| Frontal, rostral middle | 0.61 | 0.09 | 2.38 |
| Frontal, pars opercularis | 0.59 | 0.15 | 2.03 |
| Frontal, pars triangularis | 0.54 | 0.04 | 2.17 |
| Frontal, pars orbitalis | 0.58 | 0.13 | 2.05 |
| Frontal, lateral orbital | 0.60 | 0.13 | 2.17 |
| Frontal, medial orbital | 0.47 | 0.17 | 1.30 |
| Frontal, pole | 0.60 | 0.04 | 2.52 |
| Parietal, precentral gyrus | 0.59 | 0.06 | 2.38 |
| Parietal, postcentral gyrus | 0.59 | 0.17 | 1.95 |
| Parietal, paracentral gyrus | 0.58 | 0.06 | 2.32 |
| Parietal, superior | 0.58 | −0.02 | 2.63 |
| Parietal, inferior | 0.72 | 0.01 | 3.46 |
| Parietal, supramarginal | 0.64 | 0.07 | 2.65 |
| Parietal, precuneus | 0.58 | 0.19 | 1.81 |
| Temporal, parahippocampal | 0.51 | −0.04 | 2.32 |
| Temporal, entorhinal | 0.40 | 0.18 | 0.93 |
| Temporal, pole | 0.65 | 0.06 | 2.75 |
| Temporal, superior | 0.67 | 0.10 | 2.74 |
| Temporal, middle | 0.67 | 0.02 | 3.04 |
| Temporal, inferior | 0.64 | −0.08 | 3.23 |
| Temporal, transverse | 0.30 | 0.28 | 0.08 |
| Temporal, BSTS | 0.58 | 0.24 | 1.61 |
| Temporal, fusiform | 0.60 | −0.10 | 3.06 |
| Occipital, lateral | 0.62 | −0.04 | 2.95 |
| Occipital, pericalcarine | −0.12 | 0.11 | 0.89 |
| Occipital, lingual | 0.51 | −0.09 | 2.51 |
| Occipital, cuneus | 0.36 | 0.16 | 0.83 |
Note: Values in bold, P < 0.05; values in bold and italics, P < 0.01. The differences between the correlations were tested with Fisher’s z-transformed correlation coefficients. BSTS, banks of the superior temporal sulcus. A positive correlation indicates that lower CSF concentrations are associated with more atrophy and less ventricular expansion.
Figure 4.
Correlations between Aβ1-42 and annual cortical change. Correlations between cortical change point by point across the brain surface and Aβ1-42 were calculated, color coded, and displayed as an overlay on the template brain. This was done for the high- and the low-Aβ1-42 groups separately. The lower P value threshold is equal to a false discovery rate of <0.05 (corrected for multiple comparisons).
Finally, independent samples t-tests were used to compare memory performance in the Aβ1-42 groups. No significant differences were found for total learning (42.0 vs. 40.9 in the low and high groups, respectively, t(69) = 0.53, P = 0.60) and 30-min delayed recall (7.3 vs. 7.2, t(69) = 0.14, P = 0.89). Furthermore, no significant group differences in change in scores over 1 year (score year 3 – score year 1) were found for total learning (−2.12 vs. −0.76 in the low and high groups, respectively, t(69) = −0.68, P = 0.50) or 30-min delayed recall (0.42 vs. −0.20, t(69) = 0.71, P = 0.48).
APOE Analyses
Presence of APOE ϵ4 alleles (0, 1, or 2) correlated significantly with the CSF biomarker levels of Aβ1-42 (r = −0.57, P < 0.10−5) and marginally with P-tau (r = 0.26, P = 0.051) and T-tau (r = 0.24, P = 0.075), all analyses controlled for age. t-Tests showed that the low-Aβ1-42 group had significantly higher mean number of ϵ4 alleles than the high-Aβ1-42 group (0.71 vs. 0.15, respectively, t(36.64) = 4.38, P < 0.0005).
APOE correlated significantly (P < 0.05) with volume reductions in thalamus (r = −0.27), pallidum (−0.34), and amygdala (−0.28). Multiple regressions with APOE, Aβ1-42, and APOE × Aβ1-42 were run for the significant ROIs to test whether APOE interacted with Aβ1-42 in predicting volume reductions. In no case did the interaction term approach significance.
Discussion
CSF levels of Aβ1-42 and tau proteins have been related to atrophy in AD and other degenerative conditions (Braak and Braak 1991; Winblad et al. 2008), but it has not been known how these biomarkers relate to brain changes in healthy elderly individuals. The present study showed that levels of CSF biomarkers, especially Aβ1-42, correlated with ventricular expansion and volumetric reductions of large areas of the brain, not restricted to the structures most vulnerable to the effects of AD. To our knowledge, this is the first study to test the relationship between the CSF biomarkers and MRI-derived brain measures other than the whole-brain, ventricular, or hippocampal volume in healthy elderly individuals. In addition, the sample size allowed for testing of nonlinear relationships, which revealed that only when the CSF level of Aβ1-42 was below a certain threshold did low Aβ1-42 correlate with volumetric reductions and ventricular expansion. This suggests that levels above this break point may reflect naturally occurring individual differences in the amount of CSF Aβ1-42, which are not indicative of neuronal damage. However, levels of Aβ1-42 below this threshold, likely reflect or are causally related to brain atrophy.
We hypothesized that a relationship between the CSF biomarkers and brain atrophy would exist in healthy aging, as T-tau and Aβ1-42 are also related to other conditions involving neuronal or axonal damage than AD (Winblad et al. 2008; Spires-Jones et al. 2009b), and T-tau correlates with age in healthy samples (Sjogren et al. 2001b). The present results, which show that low CSF levels of Aβ1-42 correlated with ventricular expansion and volumetric reductions in widespread areas, indicate that Aβ1-42 may play a role in the brain changes observed in healthy aging. However, the nonlinear analyses revealed that this was true for the participants with low levels of Aβ1-42 only. Thus, it seems that Aβ1-42 is related to accelerated brain changes only in a subgroup of healthy elderly individuals.
The one large study of the relationship between the CSF biomarkers and brain volume found a relationship between low levels of Aβ1-42 and smaller whole-brain volumes (Fagan et al. 2009). The present longitudinal results are in general accordance with this finding, showing relationships between Aβ1-42 and annual percentage change in several brain regions. Furthermore, all PIB-positive participants in that study had Aβ1-42 values of less than 500 pg/mL, whereas most PIB-negative participants had higher values. The cutoff point of 500 pg/mL obtained from the enzyme-linked immunosorbent Aβ1-42 assay used in that study is roughly equivalent to the empirically established break point value of 175 pg/mL obtained with the Luminex Aβ1-42 assay used in the present study. Fagan and colleagues, however, did not observe a correlation between levels of tau and brain size. The present findings indicate that P-tau, but not T-tau, is related to ventricular expansion and volume reductions in healthy elderly individuals, although to a lesser extent than Aβ1-42. The use of longitudinal brain measures in the current study, which may be more sensitive than cross-sectional measures, and the analysis of specific brain structures rather than whole-brain volume, may account for this discrepancy.
Because studies have shown that in healthy elderly individuals, a reduction in CSF Aβ1-42, but not T-tau or P-tau, predicts cognitive decline and development of AD (Gustafson et al. 2003; Skoog et al. 2003; Stomrud et al. 2007), it is possible that the low-Aβ1-42 group reflects preclinical AD. Interestingly, the break point of 175 pg/mL is close to the recently reported mean value of 164 for the MCI group in ADNI (Shaw et al. 2009), and well within 1 standard deviation (±55). We tried to minimize the possibility of including participants with preclinical AD by excluding healthy controls showing any functional decline as indicated by the CDR-sb score. Furthermore, the memory performance did not differ significantly between the groups. However, the only way to exclude with certainty the possibility that the correlations observed in the present study are caused by preclinical AD is by autopsy. Still, one possible interpretation of the present results is that because Aβ1-42 is related to volumetric reductions in healthy elderly individuals in brain areas typically resistant to AD-related atrophy in early stages of the disease, this biomarker may reflect neocortical amyloid deposition and brain injury associated with the process of Aβ aggregation in healthy persons as well as in AD patients. For instance, although accumbens, caudate, pallidum, putamen, and thalamus all may be affected in later stages of AD, these areas are affected to a much lesser extent than the hippocampus in initial phases (Fennema-Notestine et al. 2009). In the present study, hippocampal volume reductions did not correlate more strongly with the CSF biomarker levels than did other subcortical structures. Reductions of the entorhinal cortex, which is also heavily affected in initial phases of AD (Du et al. 2007; Fennema-Notestine et al. 2009; McEvoy et al. 2009), were not significantly related to any of the CSF biomarkers. Furthermore, it has been suggested that P-tau may be a more specific marker for AD than T-tau or Aβ1-42 (Wallin et al. 2006) because elevated levels of P-tau are not found in the CSF of patients with acute stroke (Hesse et al. 2001), Lewy body (Parnetti et al. 2001), or vascular dementia (Sjogren et al. 2001a). Also, autopsy studies have shown more nonspecific distributions of Aβ1-42 than tau (Braak and Braak 1991). In the present study, P-tau showed weaker correlations with volume reductions than Aβ1-42.
The question of whether the correlations between the CSF levels of Aβ1-42 and brain atrophy in seemingly cognitively healthy participants are due to preclinical AD is difficult to settle. One reason for this is that although it is established that low-Aβ1-42 CSF levels are associated with deposition and aggregation of Aβ1-42 in the brain, the main constituent of neuritic plaques, the exact causal mechanisms for the role of Aβ in AD are still not known. For instance, in a recent study it was found that high anti-Aβ titers were related to clearance of amyloid from the brain, but progressive neurodegeneration was not prevented, cognition was not improved, and survival did not increase (Holmes et al. 2008). This indicates that more studies are needed to allow better understanding of the relationship between Aβ oligomers and neurodegeneration in AD (Zetterberg et al. 2009). According to one interesting hypothesis, a major contribution of Aβ to the pathophysiology of AD is its synaptotoxic effects (Shankar et al. 2008), related to a causal chain of events including inhibition of long-term potentiation (LTP), removal of glutamate receptors, and elimination of glutamate synapses (Zetterberg et al. 2009). However, aggregated forms of Aβ in fibrils and plaques seem not to impact synaptic function (Shankar et al. 2008), which may explain why some persons have high amounts of fibrillar Aβ in the brain but are cognitively normal (Reiman et al. 2009). In a recent review paper, Zetterberg and colleagues suggest that extended clinical follow-up is needed before it can be concluded whether such persons are protected from Aβ toxicity by effective sequestration of Aβ in inert aggregates or by other factors, or whether they will eventually show cognitive reductions (Zetterberg et al. 2009). Thus, it is very difficult to determine whether the role played by Aβ1-42 in brain atrophy in nondemented elderly is due to preclinical AD, or whether Aβ1-42 can cause brain changes in healthy elderly individuals who will not develop AD. Senile plaques have been found in elderly individuals without dementia (Bennet et al. 2006; Green et al. 2000), but as preclinical manifestations of AD likely occur years before clinical symptoms are detectable, it is impossible to exclude the possibility that AD-related processes caused the relationship between CSF Aβ1-42 and atrophy observed in the present study. In the largest longitudinal study of healthy aging published to date (Resnick et al. 2003), the authors argued that the observed longitudinal decline was not caused by preclinical dementia, based on the uniformity of the longitudinal changes seen across all individuals in the sample.
In the current study, significant atrophy was also seen in the group of participants with high levels of Aβ1-42. In this group, no relationships between Aβ1-42 and annual brain changes were found. Thus, atrophy in healthy aging seems to be caused by a mixture of processes, where some are related to Aβ1-42, whereas others are not. Importantly, all humans produce Aβ, but only some experience synaptic impairment and AD. The destructive effects of Aβ and amyloid precursor protein (APP) on the brain may be harmful in AD and possibly healthy aging, although they may be important during brain development. Aβ and APP appear to be involved in eliminating synapses (Priller et al. 2006), neuronal cell body death and axonal degeneration (Nikolaev et al. 2009), and restricting mature forms of LTP (Townsend et al. 2006), and it has been suggested that these developmental mechanisms are “hijacked in Alzheimer's disease” (Nikolaev et al. 2009, p. 982). Furthermore, it is possible that only specific forms of Aβ, not expressed in the general population, cause AD, for example, soluble Aβ oligomers (Zetterberg et al. 2009). However, as noted in a recent review, reliable methods for measuring Aβ oligomers in biological fluids are needed to determine the validity of this hypothesis (Zetterberg et al. 2009). The present data indicate that brain atrophy in healthy elderly individuals can be, but is not necessarily, related to CSF levels of Aβ1-42. It is currently unclear whether the atrophy related to Aβ1-42 is caused by preclinical AD.
Presence of APOE ϵ4 alleles is known to increase the risk for AD and also to be related to lower CSF levels of Aβ1-42 (Andersson et al. 2007; Glodzik-Sobanska et al. 2007; Sunderland et al. 2004) and higher PIB distribution volumes (Reiman et al. 2009). A correlation between number of APOE ϵ4 alleles and Aβ1-42 was also found in the present study. Still, APOE was weakly related to percentage brain change over 1 year and did not interact with Aβ1-42 in prediction of atrophy. Thus, the present data do not indicate that levels of Aβ1-42 are related to higher rates of volumetric reductions in ϵ4 carriers than in ϵ3 homozygotes, contrary to what might have been predicted from a vulnerability model of the role of APOE in degenerative brain conditions.
A limitation of the present study is that the number of participants in the low-Aβ1-42 group is relatively low (n = 26). Thus, it is possible that a few individuals with very low Aβ1-42 and accelerated brain changes disproportionally impact the results. Longitudinal CSF data in addition to follow-up MR scans could be used to more accurately map the relationship between atrophy and the changes on CSF Aβ1-42 levels.
In conclusion, ventricular expansion and volumetric brain reductions over 1 year in healthy elderly individuals were related to levels of CSF Aβ1-42 below a certain threshold value, whereas significant atrophy independent of the level of Aβ1-42 was found for participants above this threshold. The strongest relationships between Aβ1-42 and atrophy were found for brain areas not especially vulnerable to AD pathology, but further research and follow-up examinations over several years are needed to test whether degenerative pathology will eventually develop in this group of cognitively normal elderly individuals.
Funding
This work was supported by a grant (#U24 RR021382) to the Morphometry Biomedical Informatics Research Network (http://www.nbirn.net), which is funded by the National Center for Research Resources at the National Institutes of Health (NIH), United States, and by the National Institute on Aging at the NIH (U01 AG024904 and R01 AG031224). The work of K.B.W. and A.M.F. was supported by the Norwegian Research Council. Data collection and sharing for this project was funded by the ADNI (principal investigator: Michael Weiner; NIH grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Pfizer Inc., Wyeth Research, Bristol-Myers Squibb, Eli Lilly & Co., GlaxoSmithKline, Merck & Co. Inc., AstraZeneca AB, Novartis Pharmaceuticals Corp., Alzheimer’s Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Aging, with participation from the US Food and Drug Administration. Industry partnerships are coordinated through the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of California, Los Angeles.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Acknowledgments
We thank Robin Jennings, Michele Perry, Chris Pung, and Elaine Wu for downloading and preprocessing the MRI data. Conflict of Interest: None declared.
References
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 1279–1282. [DOI] [PubMed] [Google Scholar]
- Andersson C, Blennow K, Johansson SE, Almkvist O, Engfeldt P, LIndau M, Eriksdotter-Jönhagen M. Differential CSF biomarker levels in APOE-epsilon4-positive and –negative patients with memory impairment. Dement Geriatr Cogn Disord. 2007;23:87–95. doi: 10.1159/000097354. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Andersson JL, Friston KJ. High-dimensional image registration using symmetric priors. Neuroimage. 1999;9:619–628. doi: 10.1006/nimg.1999.0437. [DOI] [PubMed] [Google Scholar]
- Bennet DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, DeBernardis J, Kerkman D, McCulloch C, Soininen H, et al CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- Chou YY, Lepore N, Avedissian C, Madsen SK, Parikshak N, Hua X, Shaw LM, Trojanowski JQ, Weiner MW, Toga AW, et al. Forthcoming Mapping correlations between ventricular expansion and CSF amyloid and tau biomarkers in 240 subjects with Alzheimer's disease, mild cognitive impairment & elderly controls. Neuroimage. 2009;46:394–410. doi: 10.1016/j.neuroimage.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen GE, Rabbitt RD, Miller MI. Deformable templates using large deformation kinematics. IEEE Trans Image Process. 1996;5:1435–1447. doi: 10.1109/83.536892. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, Rusinek H, Li J, Tsui W, Saint Louis LA, et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27:394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin K, Miller BL, Weiner MW. Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Brain. 2007;130:1159–1166. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid Abeta (42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, et al Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Hagler DJ Jr, McEvoy LK, Fleisher AS, Wu EH, Karow DS, Dale AM. Structural MRI biomarkers for preclinical and mild Alzheimer's disease. Hum Brain Mapp. 2009;30:3238–3253. doi: 10.1002/hbm.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat D, Greve D, Fischl B, et al. High consistency of regional cortical thinning in aging across multiple samples. Cerebral Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freeborough PA, Fox NC. Modeling brain deformations in Alzheimer disease by fluid registration of serial 3D MR images. J Comput Assist Tomogr. 1998;22:838–843. doi: 10.1097/00004728-199809000-00031. [DOI] [PubMed] [Google Scholar]
- Glodzik-Sobanska L, Pirraglia E, Brys M, de Santi S, Mosconi L, Rich KE, Switalski R, Saint Louis L, Sadowski MJ, Martiniuk F, et al. The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer's disease. Neurobiol Aging. 2007;30:672–681. doi: 10.1016/j.neurobiolaging.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Grandy J. Efficient Computation of Volume of Hexahedral Cells. Lawrence Livermore National Laboratory; 1997. [Google Scholar]
- Green MS, Kaye JA, Ball MJ. The Oregon brain aging study: neuropathology accompanying healthy aging in the oldest old. Neurology. 2000;54:105–113. doi: 10.1212/wnl.54.1.105. [DOI] [PubMed] [Google Scholar]
- Gustafson DR, Wen MJ, Koppanati BM. Androgen receptor gene repeats and indices of obesity in older adults. Int J Obes Relat Metab Disord. 2003;27:75–81. doi: 10.1038/sj.ijo.0802191. [DOI] [PubMed] [Google Scholar]
- Hampel H, Burger K, Pruessner JC, Zinkowski R, DeBernardis J, Kerkman D, Leinsinger G, Evans AC, Davies P, Moller HJ, et al. Correlation of cerebrospinal fluid levels of tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Arch Neurol. 2005;62:770–773. doi: 10.1001/archneur.62.5.770. [DOI] [PubMed] [Google Scholar]
- Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E, Blennow K. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297:187–190. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- Hesse C, Rosengren L, Vanmechelen E, Vanderstichele H, Jensen C, Davidsson P, Blennow K. Cerebrospinal fluid markers for Alzheimer's disease evaluated after acute ischemic stroke. J Alzheimer’s Dis. 2000;2:199–206. doi: 10.3233/jad-2000-23-402. [DOI] [PubMed] [Google Scholar]
- Holland D, Brewer JB, Hagler DJ, Fenema-Notestine C, Dale AM. Subregional neuroanatomical change as a biomarker for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;8:20954–20959. doi: 10.1073/pnas.0906053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd ed. Oxford: Oxford University Press; 1995. [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Roddey JC, Hagler DJ, Jr, Holland D, Karow DS, Pung CJ, Brewer JB, Dale AM. Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology. 2009;251:195–205. doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Christensen GE, Amit Y, Grenander U. Mathematical textbook of deformable neuroanatomies. Proc Natl Acad Sci USA. 1993;90:11944–11948. doi: 10.1073/pnas.90.24.11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, Rosengren L, Vanmechelen E, Blennow K. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- Otto M, Wiltfang J, Tumani H, Zerr I, Lantsch M, Kornhuber J, Weber T, Kretzschmar HA, Poser S. Elevated levels of tau-protein in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurosci Lett. 1997;225:210–212. doi: 10.1016/s0304-3940(97)00215-2. [DOI] [PubMed] [Google Scholar]
- Parnetti L, Lanari A, Amici S, Gallai V, Vanmechelen E, Hulstaert F. CSF phosphorylated tau is a possible marker for discriminating Alzheimer’s disease from dementia with Lewy bodies. Phospho-Tau International Study Group. Neurol Sci. 2001;22:77–78. doi: 10.1007/s100720170055. [DOI] [PubMed] [Google Scholar]
- Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26:7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JBS, et al. Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemenschneider M, Wagenpfeil S, Vanderstichele H, Otto M, Wiltfang J, Kretzschmar H, Vanmechelen E, Forstl H, Kurz A. Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt-Jakob disease from other dementias. Mol Psychiatry. 2003;8:343–347. doi: 10.1038/sj.mp.4001220. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, Thompson PM, Jack CR, Jr, Weiner MW. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain. 2009;132:1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Shaomin L, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren M, Davidsson P, Tullberg M, Minthon L, Wallin A, Wikkelso C, Granerus AK, Vanderstichele H, Vanmechelen E, Blennow K. Both total and phosphorylated tau are increased in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001a;70:624–630. doi: 10.1136/jnnp.70.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren M, Vanderstichele H, Agren H, Zachrisson O, Edsbagge M, Wikkelso C, Skoog I, Wallin A, Wahlund LO, Marcusson J, et al. Tau and Abeta42 in cerebrospinal fluid from healthy adults 21-93 years of age: establishment of reference values. Clin Chem. 2001b;47:1776–1781. [PubMed] [Google Scholar]
- Skoog I, Davidsson P, Aevarsson O, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid beta-amyloid 42 is reduced before the onset of sporadic dementia: a population-based study in 85-year-olds. Dement Geriatr Cogn Disord. 2003;15:169–176. doi: 10.1159/000068478. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sluimer JD, Bouwman FH, Vrenken H, Blankenstein MA, Barkhof F, van der Flier WM, Scheltens P. Whole-brain atrophy rate and CSF biomarker levels in MCI and AD: a longitudinal study. Neurobiol Aging. Forthcoming 2008 doi: 10.1016/j.neurobiolaging.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Spires-Jones TL, Stoothoff WH, de Calignon A, Jones PB, Hyman BT. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009a;32:150–159. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Spires-Jones TL, Stoothoff WH, de Calignon A, Jones PB, Hyman BT. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009b;32:150–159. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement Geriatr Cogn Disord. 2007;24:118–124. doi: 10.1159/000105017. [DOI] [PubMed] [Google Scholar]
- Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Mirza N, Putnam KT, Linker G, Bhupali D, Durham R, Soares H, Kimmel L, Friedman D, Bergeson J, et al. Cerebrospinal fluid beta-amyloid1-42 and tau in control subjects at risk for Alzheimer's disease: the effect of APOE epsilon4 allele. Biol Psychiatry. 2004;56:670–676. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlund LO, Blennow K. Cerebrospinal fluid biomarkers for disease stage and intensity in cognitively impaired patients. Neurosci Lett. 2003;339:99–102. doi: 10.1016/s0304-3940(02)01483-0. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat D, Greve D, Fischl B, et al Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. Forthcoming doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin AK, Blennow K, Andreasen N, Minthon L. CSF biomarkers for Alzheimer’s disease: levels of beta-amyloid, tau, phosphorylated tau relate to clinical symptoms and survival. Dement Geriatr Cogn Disord. 2006;21:131–138. doi: 10.1159/000090631. [DOI] [PubMed] [Google Scholar]
- Winblad S, Mansson JE, Blennow K, Jensen C, Samuelsson L, Lindberg C. Cerebrospinal fluid tau and amyloid beta42 protein in patients with myotonic dystrophy type 1. Eur J Neurol. 2008;15:947–952. doi: 10.1111/j.1468-1331.2008.02217.x. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Blennow K, Hanse E. Amyloid β and APP as biomarkers for Alzheimer’s disease. Exp Geront. Forthcoming doi: 10.1016/j.exger.2009.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.