Abstract
Aims
Calmodulin (CaM) is well known to modulate the channel function of the cardiac ryanodine receptor (RyR2). However, the possible role of CaM on the aberrant Ca2+ release in diseased hearts remains unclear. In this study, we investigated the state of RyR2-bound CaM and channel dysfunctions in pacing-induced failing hearts.
Methods and results
The characteristics of CaM binding to RyR2 and the role of CaM on the aberrant Ca2+ release were assessed in normal and failing canine hearts. The affinity of CaM binding to RyR2 was lower in failing sarcoplasmic reticulum (SR) than in normal SR. Addition of FK506, which dissociates FKBP12.6 from RyR2, to normal SR reduced the CaM-binding affinity. Dantrolene restored a normal level of the CaM-binding affinity in either FK506-treated (normal) SR or failing SR, suggesting that the defective inter-domain interaction between the N-terminal domain and the central domain of RyR2 (the therapeutic target of dantrolene) is involved in the reduction of the CaM-binding affinity in failing hearts. In saponin-permeabilized cardiomyocytes, the frequency of spontaneous Ca2+ sparks was much more increased in failing cardiomyocytes than in normal cardiomyocytes, whereas the addition of a high concentration of CaM attenuated the aberrant increase of Ca2+ sparks.
Conclusion
The defective inter-domain interaction between N-terminal and central domains within RyR2 reduces the binding affinity of CaM to RyR2, thereby causing the spontaneous Ca2+ release events in failing hearts. Correction of the defective CaM binding may be a new strategy to protect against the aberrant Ca2+ release in heart failure.
Keywords: Calmodulin, Ryanodine receptor, Sarcoplasmic reticulum, Calcium, Heart failure
1. Introduction
A growing body of evidence in the literature suggests that abnormal channel gating of the sarcoplasmic reticulum (SR) Ca2+ release channel, the cardiac ryanodine receptor (RyR2), is one of the major causative factors of heart failure.1 The RyR2 plays a key role in the regulation of intracellular Ca2+ homeostasis, and its defect may be responsible for the diastolic Ca2+ leak in diseased hearts.1 Several pathogenic mechanisms for the channel disorder of the RyR2 in diseased hearts have been reported, e.g. PKA-mediated hyperphosphorylation and subsequent FKBP12.6 dissociation2,3 and Ca2+–calmodulin (CaM)-dependent protein kinase-II (CaMKII) activation.4
The RyR2 is also linked with some types of inherited form of lethal arrhythmia. Namely, more than 70 mutations have been found in the four regions (i.e. N-terminal: 1–600; central: 2000–2500; C-terminal: 3700–4200; 4500–5000) in patients with arrhythmogenic right ventricular cardiomyopathy type 2 or catecholaminergic polymorphic ventricular tachycardia (CPVT).5 This suggests that these regions are critically important for the regulation of channel opening.
On the basis of the fact that single-point mutation in either the N-terminal domain or the central domain in skeletal RyR1, produces an abnormal channel opening, generally referred to as hyper-activation and hyper-sensitization effects, Ikemoto and Yamamoto6 proposed that these two domains interact with each other to act as the implicit on/off switch for channel opening and closing (the interacting domain pair was designated as ‘domain switch’); zipping of the interacting domains closes the channel and unzipping opens the channel. A mutation in either domain weakens the inter-domain interaction and causes domain unzipping, resulting in channel activation and diastolic Ca2+ leak.6
We have shown that the defective inter-domain interaction between the N-terminal and the central domains is indeed involved in the pathogenic process of heart failure.7 Thus, RyR2 from failing hearts showed a clear indication of domain unzipping of domain switch, diastolic Ca2+ leak, decreased SR Ca2+ content, and contractile dysfunction. Correction of the defective inter-domain interaction (namely, correction of aberrant unzipped state to normal zipped state), by cardioprotective agent K201 (JTV519) or its peptide analogue reduced the frequency of diastolic Ca2+ sparks and improved the function of failing cardiomyocyte.7,8 It is clear from these studies that RyR2 plays an important role in the pathogenic process of heart failure, but the important question remains to be solved about how the pathogenic conformational signal elicited in the domain switch is transmitted to the transmembrane channel domain.
CaM is a ubiquitous Ca2+-binding protein that regulates Ca2+ channel functions by its direct binding to the ryanodine receptor. CaM inhibits both skeletal isoform (RyR1) and cardiac isoform (RyR2) above the threshold concentration of Ca2+ for muscle contraction;9,10 at sub-threshold concentrations, CaM still inhibits RyR2,10 but activates RyR1.9 In both RyR1 and RyR2 isoforms, one CaM binds to one subunit of the RyR.9,11 CaM binding takes place to the residues 3614–3643 of the RyR111 and to the residues 3583–3603 of the RyR2;10 both regions show highly homologous primary structure. The fact that mutation of several critical residues in this region abolished the CaM binding to both RyR1 and RyR210 supports the notion that both RyR1 and RyR2 have at least one common CaM-binding domain (CaMBD) in the corresponding 3614–3643 and 3583–3603 regions, respectively. The critical role of the RyR2-bound CaM in normal muscle function and pathogenic role of CaM dissociation in the development of cardiac disorder have been shown in recent report by Meissner and his colleagues.12 They generated a mouse with three amino acid substitutions of the RyR2 in the CaMBD to make the RyR2 unable to bind CaM and found that the mutant mouse developed hypertrophic cardiomyopathy with severely impaired contractile function and early death. Since the binding site of CaM locates in the midway between the domain switch region and the transmembrane channel region,13 we hypothesize that the RyR2-bound CaM has some control of the cross-talk between the domain switch and the Ca2+ channel.
In this study, we investigated the state of CaM binding to the RyR2 and its effect on the Ca2+ release activity in failing hearts and demonstrated that the defective inter-domain interaction between the N-terminal (1–600) and the central domain (2000–2500) deteriorates normal activity of CaM binding to the RyR2 and induces diastolic Ca2+ leak.
2. Methods
For a detailed description, see also the expanded materials and methods in Supplementary material online.
2.1. Materials
FK506 was provided by Astellas Pharmaceutical Co. Ltd. (Osaka, Japan).
2.2. Animal model
In beagle dogs weighing 10–13 kg, we induced heart failure by continuous application of rapid ventricular pacing at 250 bpm using an externally programmed miniature pacemaker (Taisho Biomed Instruments Co., Ltd) for 28 days, as described previously.3 This study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The care of the animals and the protocols used were in accord with guidelines laid down by the Animal Ethics Committee of Yamaguchi University School of Medicine.
2.3. Preparation of SR vesicles
We prepared SR vesicles from dog LV muscle as described previously,3 with a slight modification. Briefly, LV homogenate was centrifuged at 5500 g and the resultant supernatant fraction was purified with further extensive centrifugation (12 000 g, 143 000 g × 3), to a final concentration of about 10–20 mg protein/mL.
2.4. Peptides used and peptide synthesis
We used the following domain peptide: DPc10, which harbours CPVT-type mutation site (R2474S), as described previously.7
DPc10: (DPc2460–2495) 2460GFCPDHKAAMVLFLDRVYGIEVQDFLLHLLEVGFLP2495
Peptides were synthesized on an Applied Biosystems model 431A synthesizer employing Fmoc [N-(9-fluorenyl)methoxycarbonyl] as the alpha-amino protecting group, as described previously.14
2.5. Site-directed fluorescent labelling of DPc10 binding site in the RyR2
Specific fluorescence labelling of the RyR2 was performed with the use of a conjugate of the fluorescent conformational probe, sulfosuccinimidyl 2-(7-azido-4-methylcoumarin-3-acetamido) ethyl-1,3′-dithiopropionate (PIERCE) with DPc10 (a site-specific carrier), as described previously.7,14
2.6. Fluorescence quenching assay of the methylcoumarin acetamido probe attached to the binding site of DPc10
The zipping/unzipping mode of regulatory domains within the RyR2 was evaluated as described previously.7,14 The principle of the fluorescence quenching assay of domain unzipping is that a bulky fluorescence quencher (QSY®-BSA conjugate) is not accessible to the attached methylcoumarin acetamido (MCA) in the zipped state, whereas it becomes accessible to the MCA in the unzipped state.
2.7. Preparation of isolated cardiomyocytes and measurement of Ca2+ sparks
Cardiomyocytes were isolated from canine LV, as described previously.7 Briefly, a wedge of LV free wall was perfused with a collagenase-containing buffer solution. Then, rod-shaped adult canine cardiomyocytes were prepared.
Ca2+ sparks were measured using Fluo-3, in saponin-permeabilized cardiomyocytes with a laser scanning confocal microscope (LSM-510, Carl Zeiss).15 Data were analysed with SparkMaster, an automated analysis program which allows rapid and reliable spark analysis.16
2.8. CaM binding to the RyR2
The mammalian CaM cDNA was kindly provided by Dr Z. Grabarek (Boston Biomedical Institute, Boston, MA, USA). Expression and purification of CaM were carried out as described previously.17 We assessed the CaM binding to the RyR2 using a photoreactive crosslinker, sulfosuccinimidyl-6-[4′-azido-2′-nitrophenylamino]hexanoate (Sulfo-SANPAH, PIERCE). First, CaM-SANPAH conjugate was mixed with SR protein in the dark and photolysed with UV light. Then, the mixture was immunoblotted with a monoclonal anti-CaM antibody (Millipore, CA, USA) to detect the RyR2-bound CaM.
2.9. Immunoblot analysis
We performed immunoblot analyses for CaM and RyR2 by using anti-CaM antibody (Millipore), anti-RyR antibody (mouse monoclonal C3-33, Sigma or rabbit polyclonal C-terminal Ab4963, an order-made from Sigma)8 and the antibody against CaMBD (3583–3603) (an order-made from Sigma). The amount of RyR2-bound FKBP12.6 was determined by immunoblot analysis as described previously,3 which involved co-immunoprecipitation of FKBP12.6/RyR2 using the anti-RyR2 antibody and anti-FKBP12 (C-19) antibody (Santa Cruz Biotechnology, CA, USA).
2.10. Determination of the binding of exogenous CaM to the RyR2 in saponin-permeabilized cardiomyocytes
The exogenous CaM, fluorescently labelled with Alexa Fluor 488 (Molecular Probes, OR, USA), was added to the saponin-permeabilized normal and failing cardiomyocytes under the same conditions as the above-mentioned Ca2+ spark measurements. We then quantified the distribution of localized CaM through densitometric measurement of CaM-Alexa fluorescence.
2.11. Determination of the binding of endogenous CaM to the RyR2 in intact cardiomyocytes
The isolated cardiomyocytes were fixed with 4% paraformaldehyde and permeabilized in 0.5% Triton X-100 and 1% BSA. Endogenous CaM co-localized with the RyR2 was then detected by immunofluorescent assay.
2.12. Statistics
Unpaired t-test was used for statistical comparison of the data between two different situations. Data are expressed as means ± SD. We accepted a P-value <0.05 as statistically significant.
3. Results
3.1. Cross-linking of CaM-SANPAH to the RyR2 in normal and failing SR vesicles
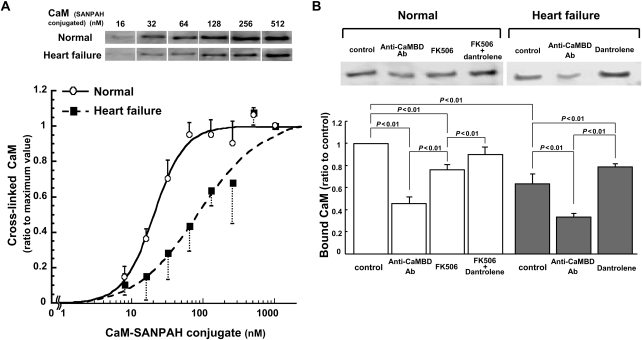
CaM binding to the RyR2 was evaluated by densitometric analysis of the immunoblots of CaM cross-linked to the RyR2. The antibody against CaM detected only RyR2 among many other proteins in the SR, indicating that CaM binding to the RyR2 was very specific (see Supplementary material online, Figure S1). CaM-SANPAH binding to the RyR2 showed Ca2+-dependent increase in a range of 0.03–1 µmol/L free [Ca2+], suggesting that physiological property of CaM was maintained in the CaM-SANPAH (see Supplementary material online, Figure S2). As shown in Figure 1A, the affinity of the CaM binding to the RyR2 is considerably reduced in failing SR compared with normal SR, as indicated by significant right shift of the CaM concentration dependence of CaM binding curve of the failing RyR2. CaM binding to the RyR2 in both normal and failing SRs was markedly inhibited by the addition of the antibody against the CaMBD (3583–3603) (Figure 1B), suggesting that the domain 3583–3603 is indeed the major CaM-binding site in the RyR2. In normal SR, the affinity of CaM binding to the RyR2 was lowered in the presence of FK506 that was previously shown to induce spontaneous Ca2+ leak due to FKBP12.6 dissociation from the RyR2.3 Interestingly, dantrolene, which corrects defective inter-domain interaction between N-terminal and central domains within the RyR2 and inhibits spontaneous Ca2+ leak in failing hearts as shown in our report,18 restored normal CaM binding both in FK506-treated normal SR and failing SR (Figure 1B).
Figure 1.
Cross-linking of CaM to the RyR2 in normal and failing SR vesicles. (A) Representative western blotting of CaM cross-linked to the RyR2 (top) and the summarized data (bottom). The immunoblot density of CaM cross-linked to the RyR2 was determined at various concentrations of CaM-SANPAH as indicated and expressed as the ratio to maximum value obtained at 1 µmol/L CaM. (B) Effects of the antibody against CaMBD (3583–3603; Anti-CaMBD Ab), FK506 (30 µmol/L), and/or dantrolene (1 µmol/L) on the cross-linking of CaM (128 nmol/L) to the RyR2 in normal and failing SR vesicles. Anti-CaMBD Ab: anti-CaMBD antibody. Representative western blots of CaM bound to the RyR2 (top) and the summarized data (bottom). The immunoblot density of CaM cross-linked to the RyR2 was measured and expressed as the ratio to control. Data represent means ± SD of 3–4 SR preparations.
3.2. Direct binding of a covalently coupled CaM-Alexa Fluor 488 to the RyR2 in the saponin-permeabilized cardiomyocytes
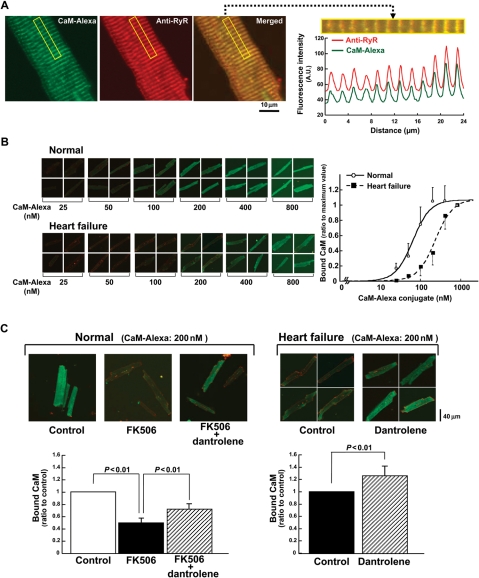
To further examine the relationship between the state of domain switch and CaM binding under a more physiological condition, we evaluated the binding of Alexa labelled CaM to subcellular fractions in the saponin-permeabilized cardiomyocytes (Figure 2A). The Alexa fluorescence signal (green) was detected along sarcomeres in the cardiomyocytes. To confirm the exogenously introduced CaM-Alexa has been bound to the RyR2 in the subcellular fractions, we merged the image of CaM-Alexa fluorescence with the immunofluorescence image of the anti-RyR2 antibody (Figure 2A). As seen, CaM-Alexa fluorescence is co-localized with the immuno-stain with anti-RyR2 antibody, suggesting that exogenously introduced CaM has bound to the RyR2. When we plot the intensity of the Alexa fluorescence along with sarcomeres (see Methods) as a function of the concentration of the exogenous CaM-Alexa, the concentration dependence of CaM binding was shifted towards higher concentrations in failing cardiomyocytes than in normal cardiomyocytes (Figure 2B). These data are consistent with the data of CaM-SANPAH binding to the SR vesicles (Figure 1). In normal cardiomyocytes, addition of FK506 significantly decreased the CaM binding (Figure 2C). In the presence of dantrolene, the decreased CaM binding in FK506-treated normal and failing cardiomyocytes was reversed (Figure 2C). These data again support the idea that defective inter-domain interaction (viz. domain unzipping of domain switch) reduces the affinity of CaM binding to the RyR2.
Figure 2.
Localization and the binding characteristics of exogenously introduced CaM in saponin-permeabilized cardiomyocytes. The CaM, fluorescently labelled with Alexa Fluor 488 (Molecular Probes, OR, USA), was delivered into the cardiomyocytes. The fluorescently labelled cardiomyocytes were laser-scanned with the confocal microscope system (LSM-510, Carl Zeiss). (A) (left) Representative images of exogenously introduced CaM co-localized with the RyR2 in normal cardiomyocytes. Left; CaM-Alexa (green); middle; RyR2 (red); right; merged image. (Right) Periodical increases in the Alexa fluorescence signals of either RyR2 (red) or CaM (green). (B) Delivery of various concentrations of the CaM-Alexa (left) and the summarized data (right). Data represent means ± SD of 22–26 cells from 3–4 hearts. The CaM-Alexa fluorescence was measured and expressed as the ratio to its maximum value. (C) Effect of FK506 (30 µmol/L) or dantrolene (1 µmol/L) on the CaM-Alexa (200 nmol/L) binding on the RyR2 in normal or failing cardiomyocytes. Representative images (top) and the summarized data (bottom). The CaM-Alexa fluorescence was measured and expressed as the ratio to the control value. Data represent means ± SD of 24–40 cells from 3–4 hearts.
Exogenous CaM-Alexa binding to the RyR2 showed [Ca2+]-dependent increase in a range of 0.03–0.3 µmol/L free [Ca2+]; buffered by 1 mmol/L EGTA/calcium buffer. This indicates that the physiological property of CaM is maintained in the CaM-Alexa (see Supplementary material online, Figure S3).
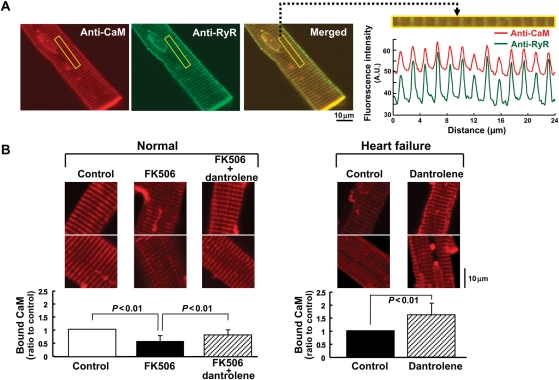
To further assess the amount of endogenous CaM bound with RyR2, we performed the immunofluorescent staining using the anti-CaM antibody (see Methods). As shown in Figure 3A, CaM was detected along with sarcomeres, showing a good co-localization with the RyR2. In the presence of FK506, the intensity of CaM staining was decreased, whereas normal intensity was restored by dantrolene in normal cardiomyocytes (Figure 3B). In failing cardiomyocytes, the intensity of CaM staining was decreased even in the absence of FK506, and it was again restored by dantrolene.
Figure 3.
Localization and the binding characteristics of endogenous CaM in normal and failing cardiomyocytes. (A) (left) Representative images of the endogenous CaM, detected by the anti-CaM antibody, co-localized with the RyR2 in normal cardiomyocytes. Left; CaM (red); middle; RyR2 (green); right; merged image. (Right) Periodical increases in the Alexa fluorescence signals of either CaM (red) or RyR2 (green). (B) Effect of FK506 (30 µmol/L) and/or dantrolene (1 µmol/L) on the endogenous CaM binding on the RyR2. Representative images of the endogenous CaM bound to the RyR2 (top) and the summarized data (bottom). The fluorescence signal of the endogenous CaM was measured and expressed as the ratio to control. Data represent means ± SD of 19–22 cells from 3–4 hearts.
To investigate the effect of endogenous CaM on the exogenous CaM-Alexa binding, we assessed the exogenous CaM-Alexa binding with or without pre-incubation by 5 mM EGTA in saponin-permeabilized cardiomyocytes. Endogenous CaM was largely dissociated with pre-incubation by 5 mmol/L EGTA (see Supplementary material online, Figure 4A). Then, after replacing the solution with a 75 nM free [Ca2+] solution (an optimal concentration for CaM binding), we determined the protein concentration dependence of CaM-Alexa binding. As shown in Supplementary material online, Figure S4B, there was no difference in the concentration dependence of the CaM-Alexa binding with or without pre-treatment by 5 mM EGTA. Moreover, upon addition of 200 nmol/L exogenous CaM, there was no change in CaM (detected by anti-CaM antibody) irrespective of pre-incubation by 5 mM EGTA (see Supplementary material online, Figure 4A). This suggests that the endogenous CaM bound to the RyR2 did not significantly affect the exogenous CaM binding, probably because the endogenous CaM is readily replaced with an excess amount of exogenously added CaM-Alexa in a rapid equilibrium.
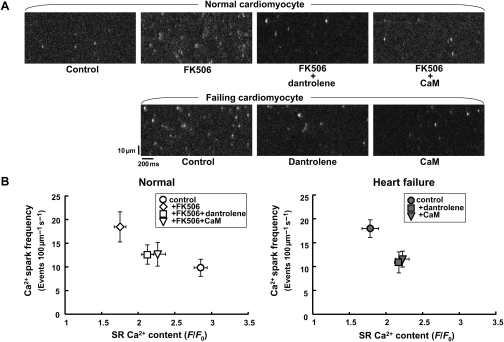
Figure 4.
Effect of FK506 (30 µmol/L), dantrolene (1 µmol/L), and/or CaM (1 µmol/L) on Ca2+ sparks in normal and failing saponin-permeabilized cardiomyocytes. (A) Representative images. (B) Relationship between the Ca2+ spark frequency and SR Ca2+ content. SR Ca2+ content was obtained by the application of 10 mmol/L caffeine. Ca2+ spark images were obtained in the presence of the CaMKII inhibitor KN-93 (1 µmol/L). Data represent means ± SD of 31–54 cells from 4–5 hearts.
3.3. Control of Ca2+ sparks by the RyR2-bound CaM in normal and failing cardiomyocytes
To examine whether CaM binding plays a critical role in the local Ca2+ release events in failing hearts, we measured the Ca2+ sparks in saponin-permeabilized cardiomyocytes. In further support of our recent observation,8 the Ca2+ spark frequency was significantly increased in both FK506-treated normal cardiomyocytes and failing cardiomyocytes (Figure 4A). Addition of a high concentration CaM (1 µmol/L) or dantrolene (1 µmol/L) significantly decreased the Ca2+ spark frequency in both cardiomyocytes. In both FK506-treated normal cardiomyocytes and failing cardiomyocytes, the full width at half maximum (FDHM) was increased, whereas it was decreased by either dantrolene or CaM (see Supplementary material online, Figure S5). These results suggest that as Ca2+ spark frequency increases, Ca2+ spark amplitude decreases, and the duration of each Ca2+ spark prolongs, all of which are restored to normal states by either dantrolene or CaM.
To assess the effect of various compounds on the Ca2+ spark frequency and SR Ca2+ content, we applied caffeine (10 mmol/L) to the saponin-permeabilized cardiomyocytes. As shown in Figure 4B, the dependence of Ca2+ spark frequency on the SR Ca2+ content shifted to the upper and left side in FK506-treated normal cardiomyocytes and in failing cardiomyocytes, whereas it was moved back to lower and right in the presence of either CaM or dantrolene.
3.4. Addition of saturating concentration of CaM has no effect on defective inter-domain interaction within the domain switch
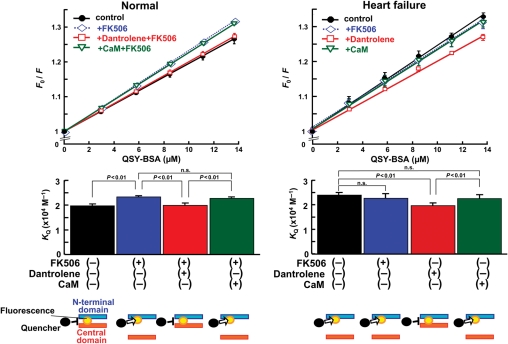
In failing SR or cardiomyocytes, CaM binding to the CaMBD was weakened and diastolic Ca2+ leak was induced, as manifested in the increased spontaneous Ca2+ sparks as shown above (Sections 3.1–3.3). Addition of saturating concentration of CaM restored normal channel function as evidenced by reduced frequency of spontaneous Ca2+ sparks. The next question is then whether the addition of saturating concentration of CaM restores normal mode inter-domain interaction within the domain switch. In order to examine this point, we fluorescently labelled the N-terminal domain of RyR2, by using DPc10 as a carrier, as previously reported.7 DPc10 that was used as a site-directing carrier mediated a specific MCA fluorescence labelling of the RyR2, as indicated by the fact that an excess concentration of DPc10 (10 mmol/L) prevented the DPc10-mediated MCA labelling (‘cold-chase’ effect) (see Supplementary material online, Figure S6, Figure 3A in Oda et al.7). Using the fluorescently labelled SR, we monitored the mode of inter-domain interaction between the N-terminal (0–600) and central (2000–2500) domains. In agreement with our previous report,7 the slope of the Stern–Volmer plot (KQ), which is a measure of the degree of domain unzipping, increased in FK506-treated normal SR and failing SR (Figure 5). Addition of CaM (1 µmol/L) was without effect on the KQ in both FK506-treated normal SR and failing SR. In contrast, dantrolene-inhibited domain unzipping in FK506-treated normal SR (Figure 5) and in failing SR (Figure 5 and Figure 3 in Yamamoto et al.18). These results suggest that unlike dantrolene a saturating concentration of CaM restores normal channel function without modifying inter-domain interaction.
Figure 5.
Fluorescence quenching analysis of the domain unzipping. Evaluation of the domain unzipping between the N-terminal (1–600) and the central domains (2000–2500). The accessibility of the RyR2-bound MCA to a macromolecular fluorescence quencher BSA-QSY conjugate was measured and the Stern–Volmer fluorescence quenching constant (i.e. the slope of the Fo/F vs. [BSA-QSY] plot) was determined as a measure of the degree of domain unzipping. Statistical comparison of the slope of each plot, which is equivalent to the Stern–Volmer quenching constant (KQ) (bottom figures). Data represent means ± SD of four SR preparations. Diagrams for data interpretation are added at the bottom of each figure.
3.5. Dantrolene and a saturating concentration of CaM do not protect FK506-induced dissociation of FKBP12.6 from the RyR2
Since FK506-induced spontaneous Ca2+ sparks seem to be a result from the dissociation of RyR2-bound FKBP12.6, we examined the effect of dantrolene or CaM on FK506-induced dissociation of FKBP12.6 from the RyR2 in normal SR vesicles. The addition of FK506 to the normal SR vesicles completely dissociated FKBP12.6 from the RyR2, regardless of the presence of dantrolene or CaM (see Supplementary material online, Figure S7). Neither dantrolene nor CaM produced any appreciable effect on the RyR2-associated FKBP12.6 in failing SR vesicles. These results exclude the possibility that re-association of FKBP12,6 with RyR2 might be involved in the restoration of normal channel function (less frequent spontaneous Ca2+ sparks) produced by dantrolene or a saturating concentration of CaM.
4. Discussion
We previously demonstrated that a tight interaction between the N-terminal domain and the central domain of the RyR2 (domain zipping) stabilizes the channel gating, and their interaction becomes defectively loose in failing hearts (domain unzipping), resulting in Ca2+ leak and contractile dysfunction of cardiomyocytes.7 We also have shown that both K201 (JTV519)8 and dantrolene18 reverse the mode of inter-domain interaction from a defective unzipped configuration to a normal zipped configuration, and stops Ca2+ leak. As shown in our recent report,8 the specific binding site of K201 is localized in the 2114–2149 residue region of the RyR2, and dantrolene binding site in the 600–620 region. It is worth noting that these two different types of reagents bind to the domain switch and exert the identical effect: correction of defective inter-domain interaction within the RyR2.18 These findings clearly indicate that defective inter-domain interaction plays a crucial role in the pathogenesis of heart failure and correction of the defectiveness is a possible new strategy of the treatment of heart failure. However, the question has remained unsolved about how the pathogenic conformational signal elicited in the domain switch is transmitted to the channel pore region, thereby making the channel leaky.
CaM, one of the accessory proteins of the RyR2, is known to inhibit Ca2+ release in a physiological range of [Ca2+],10 thereby stabilizing the channel in a normal state. The fact that the mutated RyR2, which cannot bind CaM, induced abnormal hypertrophy with dilatation of the left ventricle12 suggests that the CaM binding is a critical factor for the maintenance of normal function and structure of the left ventricle. Moreover, single particle analyses13,19,20 have shown that CaM-binding region (in the so-called ‘handle’ region) locates in the midway between the domain switch (in the so-called ‘clamp’ region) and the channel (in the transmembrane region), suggesting that the RyR2-bound CaM controls the cross-talk between the domain switch and the channel. The present study has provided some new insight into this mechanism.
The most important finding of this study in this regard is that domain unzipping between the N-terminal and the central domains causes dissociation of CaM from the RyR2, thereby rendering the channel leaky in failing hearts, and rebinding of CaM to the failing RyR2 corrects its aberrant Ca2+ leak. Thus, in normal SR vesicles or cardiomyocytes domain unzipping between the N-terminal and the central domains induced by FK506 (mimicking defective conformational regulation of the failing RyR2) decreased the affinity of CaM binding, and in turn induced spontaneous Ca2+ sparks. In failing SR or cardiomyocytes, in which domain unzipping between the N-terminal and the central domains has already taken place, CaM-binding affinity was indeed decreased, and correction of defective inter-domain interaction (i.e. domain unzipping to zipping) by dantrolene increased the CaM binding. Interestingly, the addition of an excess concentration of CaM (1 µmol/L) to saturate the CaM-binding site located in the CaMBD could also attenuate diastolic Ca2+ sparks without affecting the state of domain switch (i.e. domain unzipping) in failing cardiomyocytes. These results suggest that the pathological conformational signal elicited in the domain switch is transmitted to the CaMBD, dissociates CaM from the RyR2, and then induces Ca2+ leak. Therefore, restoration of the CaM binding to the RyR2 or correction of the defective inter-domain interaction, or both, may be effective therapeutic treatments of heart failure.
With regard to the mechanism by which the binding of CaM to the RyR inhibits the channel activity, it has been reported that CaMBD (CaMBD: 3614–3643) of the RyR1 interacts with CaM-like domain (CaMLD: 4064–4210) in the RyR1.21–23 On the basis of the observation that both antibodies against the two domains inhibited the RyR1 channel activity, it has been proposed that close contact between CaMBD and CaMLD activates the Ca2+ channel.23 This mechanism accounts for the present finding that CaM dissociation induced increased frequency of spontaneous Ca2+ sparks, because association of CaM with the RyR2 will interfere with the formation of CaMBD–CaMLD link, stabilizing the closed state of the channel, and upon dissociation of CaM the activation link is formed, causing aberrant channel activation.
Because CaM is a ubiquitous protein, the beneficial effect of CaM may be induced by another mechanism unrelated to the RyR2. Therefore, to evaluate the specific effect of CaM bound to the RyR2, we introduced the antibody against the CaMBD (3583–3603) of the RyR2 and examined how the CaM binding to the RyR2 is modified by this antibody. As shown in the present study (Figure 1), in the presence of the antibody most of the CaM binding on the RyR2 was abolished, indicating that CaMBD (3583–3603) seems to be a major target, by which the observed effects have been manifested. CaM is also a cofactor for CaM kinase II-induced phosphorylation of the RyR2, activating the RyR opening and diastolic SR Ca2+ release.24,25 In this study, we inhibited CaMKII activity by using KN93 in order to evaluate the specific effect of the RyR2-bound CaM on the channel gating. Nonetheless, we neither measured the phosphorylation level of the RyR2 nor observed the effect of CaM on channel function under CaMKII stimulation. Because CaMKII has an important effect on normal channel gating, further study is needed to assess the role of CaMKII as well as CaM on channel function.
In conclusion, we demonstrated that CaM plays a critical role as a regulator of transmitting the conformational signal from the domain switch to the transmembrane channel-pore region. Domain unzipping decreases the binding affinity of CaM to the RyR2, rendering the channel leaky. Re-association of CaM to the failing RyR2 restores normal channel gating, even with an unzipped domain switch. Preventing pathological decrease in the CaM-binding affinity to the RyR2 will be a new therapeutic strategy against heart failure.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by grants-in-aid for scientific research from The Ministry of Education in Japan (grant nos. 20390226 to M.Y., 20590868 to T.Y., 20591805 to S.K., 19209030 to M.M.), a grant from Takeda Science Foundation (to M.Y.), and a grant from the National Heart, Lung and Blood Institutes (HL072841 to N.I.).
References
- 1.Yano M, Yamamoto T, Ikemoto N, Matsuzaki M. Abnormal ryanodine receptor function in heart failure. Pharmacol Ther. 2005;107:377–391. doi: 10.1016/j.pharmthera.2005.04.003. doi:10.1016/j.pharmthera.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. doi:10.1016/S0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 3.Yano M, Ono K, Ohkusa T, Suetsugu M, Kohno M, Hisaoka T, et al. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca2+ leak through ryanodine receptor in heart failure. Circulation. 2000;102:2131–2136. doi: 10.1161/01.cir.102.17.2131. [DOI] [PubMed] [Google Scholar]
- 4.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. doi:10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 5.Yano M, Yamamoto T, Ikeda Y, Matsuzaki M. Mechanisms of disease: ryanodine receptor defects in heart failure and fatal arrhythmia. Nat Clin Pract Cardiovasc Med. 2006;3:43–52. doi: 10.1038/ncpcardio0419. doi:10.1038/ncpcardio0419. [DOI] [PubMed] [Google Scholar]
- 6.Ikemoto N, Yamamoto T. Regulation of calcium release by interdomain interaction within ryanodine receptors. Front Biosci. 2002;7:d671–d683. doi: 10.2741/A803. doi:10.2741/ikemoto. [DOI] [PubMed] [Google Scholar]
- 7.Oda T, Yano M, Yamamoto T, Tokuhisa T, Okuda S, Doi M, et al. Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure. Circulation. 2005;111:3400–3410. doi: 10.1161/CIRCULATIONAHA.104.507921. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T, Yano M, Xu X, Uchinoumi H, Tateishi H, Mochizuki M, et al. Identification of target domains of the cardiac ryanodine receptor to correct channel disorder in failing hearts. Circulation. 2008;117:762–772. doi: 10.1161/CIRCULATIONAHA.107.718957. doi:10.1161/CIRCULATIONAHA.107.718957. [DOI] [PubMed] [Google Scholar]
- 9.Rodney GG, Williams BY, Strasburg GM, Beckingham K, Hamilton SL. Regulation of RyR1 activity by Ca2+ and calmodulin. Biochemistry. 2000;39:7807–7812. doi: 10.1021/bi0005660. doi:10.1021/bi0005660. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi N, Xu L, Pasek DA, Evans KE, Meissner G. Molecular basis of calmodulin binding to cardiac muscle Ca2+ release channel (ryanodine receptor) J Biol Chem. 2003;278:23480–23486. doi: 10.1074/jbc.M301125200. doi:10.1074/jbc.M301125200. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi N, Xin C, Meissner G. Identification of apocalmodulin and Ca2+-calmodulin regulatory domain in skeletal muscle Ca2+ release channel, ryanodine receptor. J Biol Chem. 2001;276:22579–22585. doi: 10.1074/jbc.M102729200. doi:10.1074/jbc.M102729200. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi N, Takahashi N, Xu L, Smithies O, Meissner G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca release channel. J Clin Invest. 2007;117:1344–1353. doi: 10.1172/JCI29515. doi:10.1172/JCI29515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samsó M, Wagenknecht T. Apocalmodulin and Ca2+-calmodulin bind to neighboring locations on the ryanodine receptor. J Biol Chem. 2002;277:1349–1353. doi: 10.1074/jbc.M109196200. doi:10.1074/jbc.M109196200. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Ikemoto N. Peptide probe study of the critical regulatory domain of the cardiac ryanodine receptor. Biochem Biophys Res Commun. 2002;291:1102–1108. doi: 10.1006/bbrc.2002.6569. doi:10.1006/bbrc.2002.6569. [DOI] [PubMed] [Google Scholar]
- 15.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. doi:10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 16.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol. 2007;293:c1073–c1081. doi: 10.1152/ajpcell.00586.2006. doi:10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- 17.Tan R-Y, Mabuchi Y, Grabarek Z. Blocking the Ca2+-induced conformational transitions in calmodulin with disulfide bonds. J Biol Chem. 1996;271:7479–7483. doi: 10.1074/jbc.271.13.7479. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, et al. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009;53:1993–2005. doi: 10.1016/j.jacc.2009.01.065. doi:10.1016/j.jacc.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Wang R, Zhang J, Chen SR, Wagenknecht T. Localization of a disease-associated mutation site in the three-dimensional structure of the cardiac muscle ryanodine receptor. J Biol Chem. 2005;280:37941–37947. doi: 10.1074/jbc.M505714200. doi:10.1074/jbc.M505714200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang R, Chen W, Cai S, Zhang J, Bolstad J, Wagenknecht T, et al. Localization of an NH(2)-terminal disease-causing mutation hot spot to the ‘clamp’ region in the three-dimensional structure of the cardiac ryanodine receptor. J Biol Chem. 2007;282:17785–17793. doi: 10.1074/jbc.M700660200. doi:10.1074/jbc.M700660200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong L, Zhang JZ, He R, Hamilton SL. A Ca2+-binding domain in RyR1 that interacts with the calmodulin binding site and modulates channel activity. Biophys J. 2006;90:173–182. doi: 10.1529/biophysj.105.066092. doi:10.1529/biophysj.105.066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gangopadhyay JP, Ikemoto N. Role of the Met3534–Ala4271 region of the ryanodine receptor in the regulation of Ca2+ release induced by calmodulin binding domain peptide. Biophys J. 2006;90:2015–2026. doi: 10.1529/biophysj.105.074328. doi:10.1529/biophysj.105.074328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gangopadhyay JP, Ikemoto N. Interaction of the Lys(3614)–Asn(3643) calmodulin-binding domain with the Cys(4114)–Asn(4142) region of the type 1 ryanodine receptor is involved in the mechanism of Ca2+/agonist-induced channel activation. Biochem J. 2008;411:415–423. doi: 10.1042/BJ20071375. doi:10.1042/BJ20071375. [DOI] [PubMed] [Google Scholar]
- 24.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, et al. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. doi:10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 25.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. doi:10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.