Abstract
Background and Aims
Mechanical perturbation is known to inhibit elongation of the inflorescence stem of Arabidopsis thaliana. The phenomenon has been reported widely for both herbaceous and woody plants, and has implications for how plants adjust their size and form to survive in mechanically perturbed environments. While this response is an important aspect of the plant's architecture, little is known about how mechanical properties of the inflorescence stem are modified or how its primary and secondary tissues respond to mechanical perturbation.
Methods
Plants of the Columbia-0 ecotype were exposed to controlled brushing treatments and then submitted to three-point bending tests to determine stem rigidity and stiffness. Contributions of different tissues to the inflorescence stem geometry were analysed.
Key Results
Perturbed plants showed little difference in stem diameter, were 50 % shorter, 75 % less rigid and 70 % less stiff than controls. Changes in mechanical properties were linked to significant changes in tissue geometry – size and position of the pith, lignified interfascicular tissue and cortex – as well as a reduction in density of lignified cells. Stem mechanical properties were modified by changes in primary tissues and thus differ from changes observed in most woody plants tested with indeterminate growth – even though a vascular cambium is present in the inflorescence axis.
Conclusions
The study suggests that delayed development of key primary developmental features of the stem in this ecotype of Arabidopsis results in a ‘short and flexible’ rather than a ‘short and rigid’ strategy for maintaining upright axes in conditions of severe mechanical perturbation. The mechanism is comparable with more general phenomena in plants where changes in developmental rate can significantly affect the overall growth form of the plant in both ecological and evolutionary contexts.
Keywords: Arabidopsis thaliana, biomechanics, interfascicular extraxylary tissue, mechanical perturbation, stem rigidity and stiffness
INTRODUCTION
Plant stems are constantly affected by mechanical perturbation in the natural environment, with the result that timely and appropriate reactions of the plant stem are necessary for acclimatized growth, reproduction and even survival. Morphological changes resulting from the net combination of (a) repeated mechanical solicitations on the plant body and (b) changes engendered by the plant produce the phenotype that will or will not survive in a mechanically perturbed environment.
Variation of the response and growth form diversity
Since the earliest studies of the phenomenon it is known that different species respond differently to mechanical perturbation (Jaffe et al., 1984; Biddington, 1986). This seems likely, since contrasting growth forms and life histories, such as self-supporting and climbing, herbaceous and woody, perennial and annual, and determinate and indeterminate forms, develop different mechanical architectures (Speck and Rowe, 1999) and thus probably do not share the same mechanical constraints.
Inhibition of stem elongation is often cited as a response to mechanical perturbation in many herbaceous and woody species – also known as thigmomorphogenesis, the term used for describing how plants react to mechanical stimulation (Jaffe, 1973). It has been recorded in detailed studies of strictly herbaceous plants such as Zea mays (Goodman and Ennos, 1998), in species with determinate secondary growth such as Helianthus annuus (Smith and Ennos, 2003) and Solanum lycopersicum (=Lycopersicon esculentum; Coutand et al., 2000), in plants with indeterminate woody development including gymnosperms such as Pinus taeda (Telewski and Jaffe, 1986a), woody angiosperms such as Nicotiana tabacum (Anten et al., 2005) and Ulmus americana (Telewski and Pruyn, 1998) as well as the climbing species Phaseolus vulgaris (Jaffe et al., 1984).
Effects of perturbation on primary and secondary growth
In contrast to the almost universally observed shortening effect of mechanical perturbation, changes in stem diameter are more variable and possibly linked to growth form variation (Biddington, 1986). Some studies indicate an increase in stem diameter among self-supporting woody plants such as Pinus (Telewski and Jaffe, 1986a) and Ulmus (Telewski and Pruyn, 1998), as well as partially woody, climbing stems of Phaseolus (Jaffe et al., 1984). Other reports suggest a decrease in stem diameter, for example in a species of the neotropical pioneer genus Cecropia (Cecropia schreberiana; Cordero, 1999). Detailed studies on herbaceous plants with limited or no secondary growth have reported little overall increase or decrease in stem diameter – for example, Helianthus and Zea (Smith and Ennos, 2003), though, interestingly, both species showed increased ellipticity of the stem section in the direction of flexure. In general, responses in terms of stem diameter and the mechanical roles of the tissues comprising the stem have been less well documented than the effects on extension growth, particularly whether changes are due to primary growth and differentiation, secondary growth of the bifacial vascular cambium or periderm, or a combination of these. Such details are crucial for interpreting which tissues are directly affected by mechanical perturbation and how changes in tissue composition influence mechanical properties and ‘strategies’ for surviving chronic perturbation.
Growth form and organization of mechanical tissues in Arabidopsis
Arabidopsis thaliana normally develops for much of its growth cycle as an herbaceous plant with limited secondary tissues. A bifacial vascular cambium can develop significant volumes of wood in the hypocotyl under short-day growth conditions (Chaffey et al., 2002; Nieminen et al., 2004), and is known to develop significantly in the inflorescence stem after decapitation of inflorescences (Lev-Yadun, 1994), or after applying weights to the inflorescence stem (Ko et al., 2004). Arabidopsis shows a marked response to mechanical stimulation that has been documented in terms of overall morphology with bushier phenotypes and shorter inflorescence stems (Braam and Davis, 1990; Braam, 2005; Chehab et al., 2009). Inflorescence stems of A. thaliana are upright and self-supporting, relying on a band of lignified, extraxylary interfascicular tissue that is linked with xylary tissue (xylem and fibres) for mechanical stiffness and rigidity. Recent studies have highlighted the possible roles of auxin (Little et al., 2002) as well as the genes implicated in the differentiation of interfascicular fibre tissue (Zhong et al., 1997; Zhong and Ye, 1999). Studies investigating cellulose and lignin synthesis in Arabidopsis (Turner and Somerville, 1997; Jones et al., 2001) have pointed to the importance of the interfascicular extraxylary fibre tissue for mechanical stability and strength of the inflorescence axis.
The present study aimed to investigate how mechanical perturbation influences the development of the mechanical properties of inflorescence stems in A. thaliana. Such information is missing in terms of how chronic mechanical perturbation influences the mechanical properties of the stem or the organization of the tissues that contribute to its mechanical stability. The study therefore aimed to address the following questions. (a) Mechanical perturbation is known to influence extension growth; but does it also influence radial growth and primary and secondary differentiation of the axis? (b) If changes in growth and differentiation occur in the stem, do they involve an increase or decrease in rigidity and stiffness? (c) Do changes in mechanical properties involve changes in tissue composition, geometry or density, or a combination of these? (d) How are responses to mechanical perturbation linked to short-lived, small-bodied, herbaceous life histories and their survival in mechanically perturbed environments? How do these responses compare with strategies of woody species with indeterminate growth?
MATERIALS AND METHODS
Plant growth
Seeds of Arabidopsis thaliana (L.) Heynh [ecotype Columbia-0 (Col-0)] were kept at 4 °C for 1 week and sown in standard horticultural grade compost (Neuhaus Humin Substrat N2) in free-draining pots (9×9 cm wide and 10 cm deep). Plants were grown in a growth chamber and exposed to a cycle of 16 h light at 150 µmol m−2 s−1 and 8 h darkness, with a daytime temperature of 23 °C and 45–60 % humidity. Seedlings were thinned on emergence to leave four plants per pot approx. 2 cm apart and watered every 2–3 d. Plants were cultivated with minimum disturbance and separated into two sets at 12 days after sowing (DAS). One set of plants (16 pots) was cultivated with no perturbation (control plants); the other set (16 pots) was grown in the same cabinet under the same growth conditions but with mechanical perturbation (perturbed plants).
Mechanical perturbation
An electric motor mounted outside the cabinet drove a chariot (40 cm wide) at 0·05 m s−1. The chariot was mounted on rails above the perturbed plants, and a polythene sheet (15 cm length) was suspended along its length. Chariot speed and polythene thickness and length were adjusted so that leaves of emerging rosettes (apparently before the appearance of the inflorescence axis) were lightly brushed without the plants being uprooted. Mid-point inflorescence stems were deflected at 45–65° from vertical on each pass of the chariot in both directions. Plants were brushed for four periods per day at 4 h intervals; in each period the chariot made 20 passes (ten in each direction). At the end of each day, perturbed plants had been brushed 80 times (40 times in each direction).
The aims of the set-up were to ensure the following. (a) Young rosettes received mechanical stimulation prior to the emergence and growth of the inflorescence stem. This would test whether mechanical perturbation played a role in modifying growth and differentiation of the stem from the earliest stage of development. (b) Perturbation would cause flexing and brushing of each stem and plants to brush against each other, thus mimicking the mechanical interactions in an herbaceous community exposed to wind. (c) The relatively high frequency of mechanical stimuli would be consistent with the magnitude of stimulation experienced in a wind-prone environment.
Bending properties of the inflorescence stem
Controls and mechanically perturbed plants (20 of each) were selected randomly after 37 d growth and their stems were submitted to three-point bending tests. This stage of development was selected because (a) plants of both treatments had flowered and (b) inflorescence stems showed no sign of senescence, which was observed to modify measurements of rigidity and stiffness in previous trials. It was necessary to ensure that all plants were tested at precisely the same age, therefore sampling was limited to the number of bending tests possible in 1 d, which was 40.
Inflorescence stems of Arabidopsis are narrow (approx. 0·9 mm diameter), providing little resistance for measuring bending properties on most standard equipment. Also, stems were more conveniently tested using three-point rather than four-point bending procedures (Vincent, 1990). Stems of Arabidopsis contain hydrated parenchyma tissue in the pith and cortex that probably contributes to stem mechanical properties. Initial trials were carried out to evaluate (a) the minimum span-to-depth ratio necessary for avoiding significant levels of shear in the calculation of bending rigidity (Vincent, 1990; Lahaye et al., 2005) and (b) a ‘turgor test’ where excised stem segments were submitted to bending tests at 5 min intervals for up to an hour in order to assess how loss of turgor influenced bending properties. Span tests indicated that stems had to be tested at a span to depth ratio of 35–40; turgor tests indicated a 10 % loss in stiffness after 15 min at room temperature and humidity – extended to 25 min when the cut ends of the stem were sealed with molten paraffin wax. To eliminate significant turgor loss, stems were sealed with wax and tested within 5 min of being cut from the plant.
Each bending test was carried out on 5 cm long segments from the base of each stem on an Instron 5544 testing machine equipped with a 1 N force transducer and at a cross-head speed of 0·5 mm min−1. Software was programmed to record the slope constant (N mm−1) (used for calculating rigidity) from the steepest, linear part of the curve, which occurred early in the test.
Stem bending rigidity (EI; N mm2) was calculated as:
| (1) |
where L is the distance in mm between the two supports in three-point bending and c is the slope of the force deflection curve (N mm−1).
Values of Young's modulus (E; MPa) measured in bending were derived from the above formula by
| (2) |
where I (mm4) represents the axial second moment of area of the stem approximated as an ellipse via:
| (3) |
where a represents the mean vertical radius in the direction of the applied load and b the mean horizontal radius based on the central two-thirds portion of the tested stem using a dissecting microscope. These measurements, obtained as indicated below, were used to calculate the second moment of area used for calculating the bending modulus.
Morphological and anatomical measurements
Inflorescence stem length was measured prior to bending tests. Morphological and anatomical observations were made from the central portion of each basal 5 cm segment of each tested stem. Stem segments were stored in water at 4 °C for up to a week, sectioned by hand to a thickness of approx. 0·2 mm and stained in 0·02 % toluidine blue. Thick sections of fresh Arabidopsis stem material studied here were routinely stained with 0·02 % toluidine blue. This stains lignified cell walls blue as opposed to non-lignified cell walls that were coloured violet. Staining of lignified tissues can vary depending on the species, maturity of tissues and preservation/preparation of the material. The presence of lignified tissues was double checked by staining with HCl (35 %) and phloroglucinol (3 %) in 95 % ethanol.
Stem diameters and whole cross-section second moments of area used for calculating tissue contributions to stem geometry were based on measurements of the major axis and the minor axis of stem segment transverse sections. Second moments of area were calculated as elliptical geometries parallel to the major axis and minor axis of the stem cross-section producing maximum and minimum values of second moment of area.
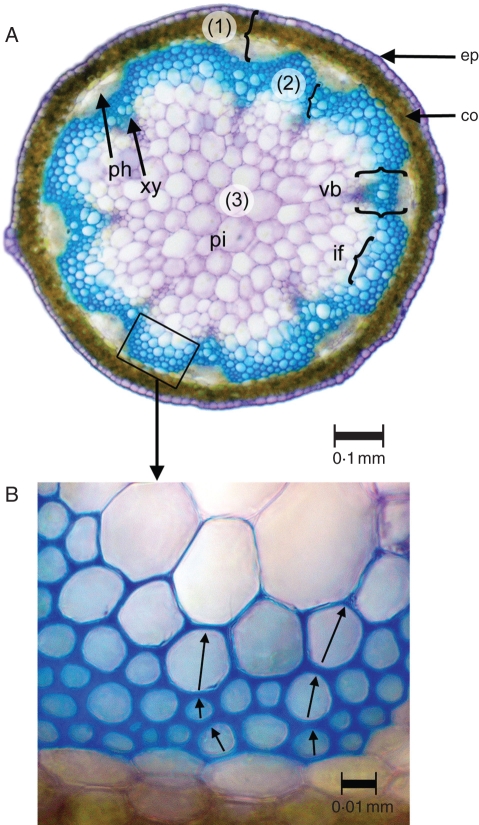
Three tissue areas were considered representative for approximating the mechanical architecture of the stem: (1) the innermost layer of parenchymatous pith (Fig. 1A); (2) the middle, mostly lignified tissues; and (3) the outer layer of thin-walled cortex and epidermis. Areas were measured for each tissue using Image analysis software Optimas.
Fig. 1.
(A) Cross-section of an inflorescence stem of A. thaliana showing three layers of tissues used to define its mechanical architecture and response to mechanical perturbation: (1) outer layer: epidermis (ep), cortex (co), primary phloem (ph); (2) middle layer: primary xylem (xy) and interfascicular fibre tissue (if); (3) inner layer: pith (pi) (staining – toluidine blue). (B) Detail of the main stiffening tissue of the middle layer comprised of interfascicular fibres. Tissue density was measured via wall and lumen dimensions of three-cell transects that crossed the three layers of lignified cells (e.g. arrows).
Vascular cambial activity was recorded by the presence of two or more radially aligned files of cambium-derived cells interpreted as secondary xylem adjacent to the primary xylem in and around the fascicular bundles. Such areas correspond to previously described tissues in basal segments of A. thaliana (Little et al., 2002; Ko et al., 2004; Sehr et al., 2010).
The relative density of the lignified tissues was measured for comparison between control and perturbed plants from high magnification, light microscopy, transverse sections. For each stem segment, four images of the interfascicular tissue were taken at approximately equidistant positions around the stem. For each image, lumen diameters (longest internal diameter and that at 90 ° to it) and double wall thicknesses (the thickness of two adjacent cell walls totalling four thickness measurements per cell) were measured for 18 cells that represented six three-cell transects sampling the outermost, second and third cell row from the outer limit of the tissue (Fig. 1B).
Statistical analyses
Inflorescence stem length, diameter, flexural rigidity, bending modulus and axial second moment of area of controls and mechanically perturbed plants were investigated using non-parametric Mann–Whitney tests because (a) of small sample size (n = 20), (b) some measurements were not normally distributed and (c) some measurement variances were significantly different. Lumen diameter and wall thickness of interfascicular tissue were investigated with linear models using the statistical package R with aov and lm functions (R Development Core Team, 2009). Lumen and cell wall data were log-transformed to meet the assumptions of the analyses.
RESULTS
Inflorescence stem morphology
Mechanical perturbation had a significant effect on stem length. Perturbed plants were 12·38±1·69 cm long, about half the length of the controls which were 24·38±3·26 cm long (P < 0·0001, Table 1, Fig. 2). In contrast, perturbation had little effect on stem diameter. Although measurements suggested that perturbed stems were marginally narrower than controls, the difference was not significant in Mann–Whitney tests for stem major axis diameter (P > 0·05, Table 1) or stem minor axis diameter (P > 0·1, Table 1). Both perturbed plants and controls developed slightly elliptical cross-sectional areas, with values of ellipticity (major axis length/minor axis length) varying around 1·1, which were not significantly different (P > 0·5).
Table 1.
Morphological and biomechanical traits (means±s.d.) with P-values of Mann–Whitney tests comparing medians of control and perturbed plants (n = 20)
| Term | Control plants | Perturbed plants | P-value |
|---|---|---|---|
| Height (cm) | 24·38±3·26 | 12·38±1·69 | <0·0001 |
| Diameter major axis length (mm) | 0·968±0·084 | 0·914±0·104 | 0·052 |
| Diameter breadth (mm) | 0·865±0·069 | 0·825±0·077 | 0·102 |
| Cross-section area (mm2) | 0·651±0·104 | 0·577±0·120 | 0·038 |
| I max (mm4) | 0·040±0·013 | 0·033±0·015 | 0·049 |
| I min (mm4) | 0·032±0·010 | 0·027±0·010 | 0·063 |
| EI (N mm2) | 38·23±15·14 | 9·53±5·158 | <0·0001 |
| E (MPa) | 1103±273 | 336±119 | <0·0001 |
Fig. 2.
Self-supporting growth forms of A. thaliana, 30 d after sowing; (A) controls, (B) perturbed plants after 80 solicitations per day by brushing (1 square = 1 cm).
The stem cross-sectional area of perturbed plants (0·58±0·12 mm2) was slightly less than that of controls (0·65±0·10 mm2; P < 0·05, Table 1), supporting the suggestion that perturbed plants had slightly smaller stem diameters. It was therefore of interest to investigate how much this slight difference in size would influence the rigidity of mechanically perturbed stems. Second moments of area of perturbed plant stems calculated parallel to the major diameter axis differed only slightly (P = 0·049, Table 1) from those of controls; values calculated perpendicular to the main axis were not significantly different (P > 0·05, Table 1).
Mechanical properties
Inflorescence stems of both controls and perturbed plants were self-supporting and vertically stable. Although the brushing treatment systematically flexed and displaced plant stems through angles of up to 45–65° from vertical, perturbed plants returned immediately to an upright self-supporting orientation after each treatment. None of the perturbed plants showed evidence of observable plastic deformation, creep or failure (Fig. 2). Perturbed stems had significantly lower values of flexural rigidity of around 10 N mm2, only a quarter that of controls of around 38 N mm2 (P < 0·0001, Table 1). The Young's modulus of perturbed plants varied around 340 MPa, only a third of that measured for controls of around 1100 MPa (P > 0·0001, Table 1).
Tissue organization
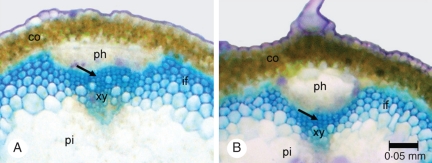
Mechanically perturbed plants differ significantly in terms of tissue organization compared with controls. Perturbed plants developed proportionally less pith and lignified interfascicular tissue but more outer cortex compared with controls, in terms of both cross-sectional area (Table 2) and second moment of area (Table 3). Evidence of cambial activity was identified in both controls and perturbed plant stems, and was largely confined to areas adjacent to fascicular vascular bundles (Fig. 3A, B, arrows). These areas were limited compared with the total area of lignified tissue and were not present in all fascicular bundles in each stem. The presence/absence determinations of cambium-derived tissue indicated that 18/20 controls showed evidence of cambial growth compared with 10/20 perturbed plants (χ2=12·8, P > 0·001).
Table 2.
Contributions of main tissues to stem cross-sectional area (A) (means ± s.d.) with P-values of Mann–Whitney tests comparing medians of control and perturbed plants, n = 20
| Tissue contribution to A (%) |
|||
|---|---|---|---|
| Tissue | Control | Perturbed | P-value |
| Pith | 43·13±2·53 | 39·84±2·28 | 0·0002 |
| Lignified tissues | 24·76±1·85 | 21·38±1·89 | <0·0001 |
| Cortex | 32·12±2·23 | 38·78±1·88 | <0·0001 |
Table 3.
Contributions of main tissues to second moment of area (I) (means±s.d.) with P-values of Mann–Whitney tests comparing medians of control and perturbed plants, n = 20
| Tissue contribution to I (%) |
|||
|---|---|---|---|
| Tissue | Control | Perturbed | P-value |
| Pith | 19·35±2·27 | 16·85±1·98 | 0·0022 |
| Lignified tissues | 27·32±2·19 | 21·32±1·86 | <0·0001 |
| Cortex | 53·32±2·97 | 61·84±2·03 | <0·0001 |
Fig. 3.
Cross-sections of an inflorescence stem with evidence of early cambial growth (arrows). (A) Control plant. (B) Perturbed plant. Cambium-derived tissues occupied only small areas of the total lignified tissue area and were more often expressed in controls than perturbed plants: co, cortex; ph, primary phloem; xy, primary xylem; if, interfascicular fibre tissue; pi, pith (staining – toluidine blue).
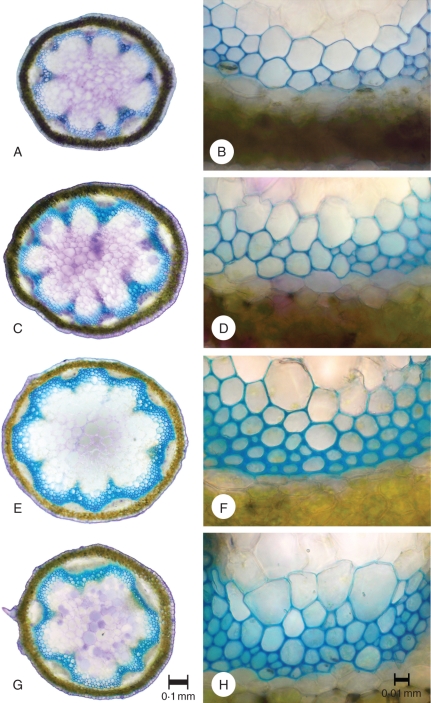
The anatomy of perturbed stems differed from the sub-apical organization of control stems (Fig. 4). The sub-apical parts of the controls (Fig. 4A, B) showed a less mature organization, with narrower, lignified tissue areas with fewer, less variable sized cells than observed in the base of perturbed stems (Fig. 4G, H).
Fig. 4.
Anatomical organization of controls and perturbed plants. (A–F) Control stem anatomy showing variation of the main tissue types from (A, B) 15·5 cm from the apex; (C, D) 20·5 cm below the apex and (E, F) the centre of the mechanically tested segment near the base (25·5 cm from the apex). (G, H) Perturbed stem anatomy at the centre of the mechanically tested segment near the base (11 cm from the apex). Perturbed stem anatomy compares closely with the organization seen at the base of controls, differing largely in terms of tissue density.
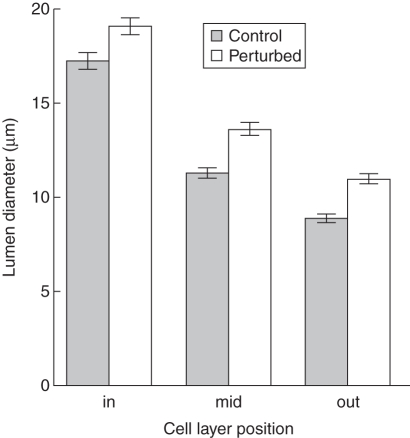
Density of interfascicular tissue
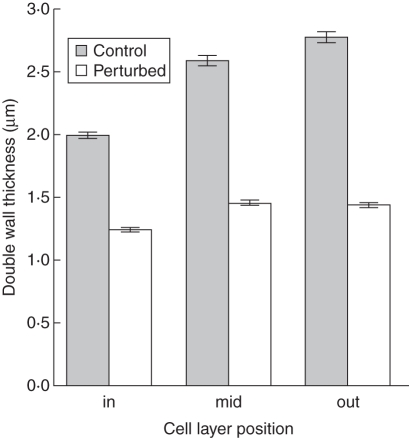
Mechanical perturbation had a significant effect on interfascicular tissue density. In perturbed plants this tissue contained wider lumens (Table 4, Fig. 5) and thinner walls than controls (Table 5, Fig. 6). This was observed consistently in all three outer cell layers of the interfascicular tissue where cell wall thickness approached half that of the controls (Fig. 6).
Table 4.
ANOVA table from the linear model of lumen size with treatment and position fitted as factors
| Term | d.f. | Sum squares | Mean square | F | P-value |
|---|---|---|---|---|---|
| Treatment | 1 | 39·11 | 39·11 | 262·6 | <0·0001 |
| Position | 2 | 363·8 | 181·91 | 1222 | <0·0001 |
| Treatment:position | 2 | 3·01 | 1·5 | 10·094 | <0·0001 |
| Residuals | 5718 | 851·4 | 0·15 | – | – |
Fig. 5.
Barplot of cell lumen diameter according to the cell layer position (in, mid, out) and treatment (control and perturbed plants, as indicated) with 95 % confidence interval bars.
Table 5.
ANOVA table from the linear model of double wall thickness with treatment and position fitted as factors
| Term | d.f. | Sum squares | Mean square | F | P-value |
|---|---|---|---|---|---|
| Treatment | 1 | 925·4 | 925·4 | 8280 | <0·0001 |
| Position | 2 | 132·2 | 66·1 | 591·5 | <0·0001 |
| Treatment:position | 2 | 16·46 | 8·23 | 73·7 | <0·0001 |
| Residuals | 11 442 | 1279 | 0·11 | – | – |
Fig. 6.
Barplot of double wall thickness according to the cell layer position (in, mid, out) and treatment (control and perturbed plants, as indicated) with 95 % confidence interval bars.
Effects of changes in stem diameter, tissue geometry and lignified tissue density on bending properties
A comparison of mean flexural rigidity between treatments indicates that the small difference in mean second moment of area observed (Table 6) could account for up to a 20 % reduction in flexural rigidity but not for the 4-fold difference in rigidity – assuming the same bending modulus in both stems. This suggests that the large reduction in stem rigidity, though at least partly attributable to a difference in size, must also be due to a large change in stiffness of the tissues comprising the stem.
Table 6.
Comparisons of the effect of changes in size, tissue geometry and lignified tissue density on stem rigidity
| I entire stem (mm4) | EI Pith tissue (N mm2) | EI Lignified tissue (N mm2) | EI Outer cortex (N mm2) | EI Entire stem (N mm2) | Reduction in stem rigidity (%) | |
|---|---|---|---|---|---|---|
| Effect of overall size on rigidity* | ||||||
| Control | 0·04 | – | – | – | 44·12 | |
| Perturbed | 0·033 | – | – | – | 36·40 | 17·50 |
| Effect of tissue geometry on rigidity† | ||||||
| Control | 0·04 | 0·15 | 43·71 | 1·28 | 45·15 | |
| Perturbed | 0·04 | 0·13 | 34·11 | 1·48 | 35·73 | 20·86 |
| Effect of infrafascicular tissue density on rigidity‡ | ||||||
| Control | 0·04 | 0·15 | 43·71 | 1·28 | 45·15 | |
| Perturbed | 0·04 | 0·13 | 17·06 | 1·48 | 18·67 | 58·63 |
* Calculation is based on mean the second moment of area (maximum) of control and perturbed stems with the assumption that the Young's modulus of both stems is the same.
† Calculation compares two stems with the same second moment of area (based on maximum value of control stems) but applying the mean relative contributions of second moment of area of the main tissues for each treatment. The comparison based on differences in tissue geometry compares theoretical values of flexural rigidity (EI) based on the same values of Young's modulus for controls and perturbed stems: each tissue type corresponding to Pith = thin-walled parenchyma (20 MN m−2); moderately thick-walled primary fibre tissue (4000 MN m−2) and thin-walled cortical tissue (60 MN m−2). Values are based on experimentally derived values of Young's modulus of parenchyma and moderate–thick-walled fibre tissue (see Rowe et al., 1993).
‡ Calculation uses the same assumptions as the above but investigates the effect of lowering Young's modulus of lignified tissue by 50 % as suggested by density measurements. The first order approximation assumes no substantial changes in cell shape, microfibril angle, wall chemistry and mechanical properties of pith and cortex.
A comparison of recalculated values of EI based on (a) the mean percentage second moment of areas of the main tissues and (b) the same representative values of Young's modulus for each tissue in both control and perturbed tissue configurations (Table 6) indicates that, in terms of tissue geometry alone, the mechanically perturbed organization with a more central lignified tissue could account for a reduction of up to 20 % of flexural stiffness of the stem but not for the 4-fold reduction in EI observed.
Perturbed plants developed thinner walled fibres in all three outer bands of interfascicular tissue where cell density was half that of controls. Stems of Arabidopsis are too small to dissect out effectively and mechanically test each tissue and its influence on stem rigidity (Hepworth and Vincent, 1999; Niklas et al., 2003; Isnard and Rowe, 2008). However, a 50 % drop in density would be expected to modify the elastic modulus of the tissue significantly. First order approximation suggests that the observed reduction in density would reduce tissue stiffness by approx. 50 % assuming that factors such as microfibril angle and cell wall chemistry were approximately the same (Rowe et al., 1993; Speck and Rowe, 2003). A 50 % reduction in stiffness of the main thick-walled tissue of the stem is consistent with the lower Young's moduli of entire stems observed in perturbed plants that is <50 % that of the controls.
In summary, a slightly smaller cross-sectional area recorded for perturbed plants would only reduce rigidity by approx. 20 %, whereas the modified development of the fibre tissue, in terms of both position and density, would be expected to lower the rigidity by >50 %. In other words, rearrangement of tissue geometries and fibre density would account for most of the reduction in rigidity rather than a marginally smaller cross-section.
DISCUSSION
Mechanical perturbation by controlled brushing has a profound effect on the mechanical architecture of the Arabidopsis Col-0 ecotype. Perturbed plants are not only much shorter but develop less rigid stems composed of less stiff material. Brushing plants before and after the appearance of the inflorescence stem influenced primary radial growth and differentiation in terms of tissue geometry and density of lignified fibre tissue. It is these effects on stem differentiation, more than changes in stem diameter, that had a major effect on the mechanical properties of the stem.
Extension growth
Responses observed here on Arabidopsis are not easily compared with previous studies where differing intensities, frequencies and types of perturbation, as well as traits measured, vary widely – a problem pointed out by Coutand et al. (2000). Reduced extension growth at the stem apex is consistent with previous reports of petiole and stem bending in Arabidopsis (Braam and Davis, 1990). Other woody herbaceous plants tested in single bending treatments (Coutand et al., 2000) show a decrease in extension growth as did the monocot Zea (Goodman and Ennos, 1998; Smith and Ennos, 2003). Most reports from experimental bending treatments or open wind-prone conditions demonstrate that self-supporting woody plants also show reduced elongation growth (Telewski and Jaffe, 1986a; Telewski and Pruyn, 1998; Leblanc-Fournier et al., 2008), as has been shown for the climber Phaseolus (Jaffe et al., 1984); though see Telewski (1995), Cordero (1999), Countand et al. (2010) and Jaffe (1973) (for stem rubbing experiments) where this has not been observed or been equivocal. A shortening effect on stem length is consistent with the notion that reduced height will limit the bending moment of the stem and lower the risk of a range of excessive mechanical strains, plastic deformation, uprooting, stem buckling and failure. Reduction of stem length in Arabidopsis is consistent with the response of many herbaceous and woody species with determinate and indeterminate growth or with diverse types of mechanical perturbation at both moderate and chronic frequencies.
Radial growth
The small and equivocal reduction in radial growth was linked to only a marginal decrease in cross-sectional area and second moment of area – even though these properties scale to the second and fourth power, respectively, of the radial dimension. In any case, stem diameter did not increase as a result of mechanical perturbation, which has been widely reported for most woody species (Telewski, 2006; Leblanc-Fournier et al., 2008; Coutand et al., 2010), the relatively large-bodied monocot Zea and the woody herbaceous Helianthus (Goodman and Ennos, 1998; Smith and Ennos, 2003). Furthermore, Arabidopsis does not modify cross-sectional shape in response to bending that has been reported in woody plants (Telewski, 1995) and in Helianthus and Zea (Goodman and Ennos, 1998).
Bending properties
A reduction in stiffness of the stem observed in Arabidopsis is consistent with reports of woody plants including conifers such as Abies fraseri (Pursh) (Telewski and Jaffe, 1986b) and Pinus (Telewski and Jaffe, 1986a), as well as angiosperms such as climbing stems of Phaseolus (Jaffe et al., 1984) and self-supporting species such as Ulmus (Telewski and Pruyn, 1998) and Nicotiana (Hepworth and Vincent, 1999; Anten et al., 2005). Among herbaceous species studied in detail, Zea showed a small increase and the woody herbaceous Helianthus showed a small reduction in modulus (Goodman and Ennos, 1998).
A common feature of tested stems of woody plants with indeterminate growth is an increase in flexural rigidity despite a decrease in elastic modulus (Telewski and Jaffe, 1986b, c; Pruyn et al., 2000; Kern et al., 2005). This results from an increase in diameter of the stem apparently counteracting the reduction in stiffness of the material comprising the stem. The lower elastic modulus of the tissues in such stems might be linked to resistance to failure and fracture propagation (Niklas, 1992).
Determinate and indeterminate growth
In Acer saccharum, leaf petioles of wind-exposed trees are shorter and narrower with less lignified tissues and show a 90 % decrease in rigidity and 50 % decrease in bending modulus (Niklas, 1996). Apart from the notable reduction in petiole diameter, these results are similar to those found in Arabidopsis and consistent with several woody herbs (Biddington and Dearman, 1985). A study on the response of determinate peduncles of Allium sativum to mechanical stress (Niklas, 1990) also showed a significant shortening of the axis, but differed from the results of the Arabidopsis stem by showing no change in values of flexural rigidity or Young's modulus. Determinate axial organs such as fertile shoots and leaf petioles may not respond to mechanical perturbation in the same way as indeterminate woody stems. However, the tendency to produce structures with lower moduli is not always the case: leaves of Festuca submitted to wind-blown perturbation produced higher moduli with higher volumes of fibre tissue (Grace and Russell, 1977).
Effects on primary and secondary development
Some traits observed in mechanically perturbed Arabidopsis that influence stem mechanical properties are consistent with a net inhibition of development. They include elongation growth, wall thickening of lignified tissue cells and a slight reduction in overall stem diameter. The change in tissue geometry of a smaller pith, and a smaller and more central area of lignified tissue, suggest a delay in procambial differentiation and expansion prior to the onset of lignification. Basal segments of perturbed plants (positioned about 10 cm below the apex) do not show the same organization as those 10 cm below the apex of the controls. This suggests that the difference between perturbed plants and controls does not simply reflect the distance from the apex and that an inhibition or a delay in development has affected only certain developmental traits.
From recent studies on woody plants with indeterminate growth, there is evidence that primary meristems and the bifacial vascular cambium might react differently to mechanical perturbation, resulting in a net reduction in stem length and an increase in diameter, which seems to make sense in a mechanically perturbed environment. However, what about herbaceous plants – or to put it more precisely, woody herbaceous plants with highly determinate growth – such as Arabidopsis? The study showed that several primary developmental processes might be interpreted as delays in growth or inhibited growth (Braam and Davis, 1990), but that the effect on establishment growth of the stem diameter was slight.
Interestingly, the presence of cambial growth was observed more frequently in the controls than in perturbed stems. While this might suggest a delay or inhibition in development of secondary tissue, other studies have shown that wood development in inflorescence stems of Arabidopsis is linked to the length and weight of stem supported by the base of the inflorescence axis (Ko et al., 2004). This could correspond to the situation observed here: longer stems bearing heavier loads are more likely to produce wood than the shorter, lighter, perturbed axes. Interestingly it would appear that radial growth in Arabidopsis might be responsive to static load stimulation but unaffected or possibly reduced by frequent bending solicitations, which otherwise stimulate radial growth in most indeterminate woody plants tested. Clearly additional detailed tests on the same ecotype and line are required to explore this further.
Cambial development was not enhanced and certainly did not increase radial dimensions of the stem nor increase stem rigidity in perturbed stems of Arabidopsis. This suggests that the developmental controls that underpin small body size and ‘herbaceousness’ in Arabidopsis and which limit secondary growth were not ‘over-ridden’ by mechanical perturbation. Instead tiny stems of Arabidopsis drastically modified their mechanical properties, producing a lower elastic modulus, which is, nevertheless, one of the mechanical traits achieved by large-bodied woody plants.
From an evolutionary perspective different growth forms and mechanical architectures may be derived within some clades via heterochrony – by adjustments of an existing growth trajectory in terms of developmental rate (Lahaye et al., 2005; Rowe and Speck, 2005). Such adjustments can explain striking changes in size, form and mechanical architecture without implicating complex changes in development and structural novelties. For example, species of shrubs with relatively stiff stems may be phylogenetically derived from flexible lianas simply by expressing and retaining juvenile traits. The responses to mechanical perturbation in Arabidopsis also appear to operate in terms of changes in developmental rate: a tall, rigid mechanical architecture with a shorter more flexible one by relatively simple changes in developmental rate. The results also suggest that primary meristems implicated in extension growth and thickening are affected differently by mechanical perturbation and that the responses of secondary meristems vary according to differences in growth form and life history.
The mechanisms and controls underlying plant responses to mechanical perturbation are clearly complex and possibly require a coordinated effort in terms of methods and approaches. Future studies on the morphological, molecular and developmental aspects of the phenomenon should all contribute to uncovering not only how these mechanisms allow plants to respond and survive in perturbed environments but also the processes underlying the evolution of such a wide diversity of mechanical architectures.
ACKNOWLEDGEMENTS
Seeds of Arabidopsis were generously supplied by Cécilia Ramirez (CNRS, Paris). Michaël Gueroult (AMAP, Montpellier) assisted in setting up the growth cabinet, and installation of the automatic electronic brushing device was kindly provided by Jérôme Chopard (DAP, Montpellier). N.R. acknowledges advice on the set-up from Frank Telewski (East Lansing, Michigan). A macro for determining second moment of area from transverse sections was kindly provided by Tancrède Alméras (LMGC, Montpellier). AMAP (Botany and Computational Plant Architecture) is a joint research unit which associates CIRAD (UMR51), CNRS (UMR5120), INRA (UMR931), IRD (R123) and Montpellier 2 University (UM27); http://amap.cirad.fr/; which gratefully acknowledges a post-doctoral fellowship [PBZH-3-125536] from the Schweizerischen Nationalfonds (SNF) of Zurich.
LITERATURE CITED
- Anten NPR, Casado-Garcia R, Nagashima H. Effects of mechanical stress and plant density on mechanical characteristics, growth, and lifetime reproduction of tobacco plants. American Naturalist. 2005;166:650–660. doi: 10.1086/497442. [DOI] [PubMed] [Google Scholar]
- Biddington NL. The effects of mechanically-induced stress in plants – a review. Plant Growth Regulation. 1986;4:103–123. [Google Scholar]
- Biddington NL, Dearman AS. The effect of mechanically induced stress on the growth of cauliflower, lettuce and celery seedlings. Annals of Botany. 1985;55:109–119. [Google Scholar]
- Braam J. In touch: plant responses to mechanical stimuli. New Phytologist. 2005;165:373–389. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- Braam J, Davis RW. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990;60:357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- Chaffey N, Cholewa E, Regan S, Sundberg B. Secondary xylem development in Arabidopsis: a model for wood formation. Physiologia Plantarum. 2002;114:594–600. doi: 10.1034/j.1399-3054.2002.1140413.x. [DOI] [PubMed] [Google Scholar]
- Chehab EW, Eich E, Braam J. Thigmomorphogenesis: a complex plant response to mechano-stimulation. Journal of Experimental Botany. 2009;60:43–56. doi: 10.1093/jxb/ern315. [DOI] [PubMed] [Google Scholar]
- Cordero RA. Ecophysiology of Cecropia schreberiana saplings in two wind regimes in an elfin cloud forest: growth, gas exchange, architecture and stem biomechanics. Tree Physiology. 1999;19:153–163. doi: 10.1093/treephys/19.3.153. [DOI] [PubMed] [Google Scholar]
- Coutand C, Julien JL, Moulia B, Mauget JC, Guitard D. Biomechanical study of the effect of a controlled bending on tomato stem elongation: global mechanical analysis. Journal of Experimental Botany. 2000;51:1813–1824. doi: 10.1093/jexbot/51.352.1813. [DOI] [PubMed] [Google Scholar]
- Coutand C, Chevolot M, Lacointe A, Rowe NP, Scotti I. Mechanosensing of stem bending and its interspecific variability in five neotropical rainforest species. Annals of Botany. 2010;105:341–347. doi: 10.1093/aob/mcp286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AM, Ennos AR. Responses of the root systems of sunflower and maize to unidirectional stem flexure. Annals of Botany. 1998;82:347–357. [Google Scholar]
- Grace J, Russell G. The effect of wind on grasses: III. Influence of continuous drought or wind on anatomy and water relations in Festuca arundinacea schreb. Journal of Experimental Botany. 1977;28:268–278. [Google Scholar]
- Hepworth DG, Vincent JFV. The growth response of the stems of genetically modified tobacco plants (Nicotiana tabacum ‘Samsun’) to flexural stimulation. Annals of Botany. 1999;83:39–43. [Google Scholar]
- Isnard S, Rowe NP. The mechanical role of the leaf sheath in rattans. New Phytologist. 2008;177:643–652. doi: 10.1111/j.1469-8137.2007.02308.x. [DOI] [PubMed] [Google Scholar]
- Jaffe MJ. Thigmomorphogenesis: the response of plant growth and development to mechanical stimulation. Planta. 1973;114:143–157. doi: 10.1007/BF00387472. [DOI] [PubMed] [Google Scholar]
- Jaffe MJ, Telewski FW, Cooke PW. Thigmomorphogenesis: on the mechanical properties of mechanically perturbed bean plants. Physiologia Plantarum. 1984;62:73–78. doi: 10.1111/j.1399-3054.1984.tb05925.x. [DOI] [PubMed] [Google Scholar]
- Jones L, Ennos AR, Turner SR. Cloning and characterization of irregular xylem4 (irx4): a severely lignin-deficient mutant of Arabidopsis. The Plant Journal. 2001;26:205–216. doi: 10.1046/j.1365-313x.2001.01021.x. [DOI] [PubMed] [Google Scholar]
- Kern KA, Ewers FW, Telewski FW, Koehler L. Mechanical perturbation affects conductivity, mechanical properties and aboveground biomass of hybrid poplars. Tree Physiology. 2005;25:1243–1251. doi: 10.1093/treephys/25.10.1243. [DOI] [PubMed] [Google Scholar]
- Ko J-H, Han K-H, Park S, Yang J. Plant body weight-induced secondary growth in Arabidopsis and its transcription phenotype revealed by whole-transcriptome profiling. Plant Physiology. 2004;135:1069–1083. doi: 10.1104/pp.104.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahaye R, Civeyrel L, Speck T, Rowe NP. Evolution of shrub-like growth forms in the lianoid subfamily Secamonoideae (Apocynaceae s.l.) of Madagascar: phylogeny, biomechanics and development. American Journal of Botany. 2005;92:1381–1396. doi: 10.3732/ajb.92.8.1381. [DOI] [PubMed] [Google Scholar]
- Leblanc-Fournier N, Coutand C, Crouzet J, et al. Jr-ZFP2, encoding a Cys2/His2-type transcription factor, is involved in the early stages of the mechano-perception pathway and specifically expressed in mechanically stimulated tissues in woody plants. Plant, Cell and Environment. 2008;31:715–726. doi: 10.1111/j.1365-3040.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- Lev-Yadun S. Induction of sclereid differentiation in the pith of Arabidopsis thaliana (L.) Heynh. Journal of Experimental Botany. 1994;45:1845–1849. [Google Scholar]
- Little CHA, MacDonald JE, Olsson O. Involvement of indole-3-acetic acid in fascicular and interfascicular cambial growth and interfascicular extraxylary fiber differentiation in Arabidopsis thaliana inflorescence stems. International Journal of Plant Sciences. 2002;163:519–529. [Google Scholar]
- Nieminen KM, Kauppinen L, Helariutta Y. A weed for wood? Arabidopsis as a genetic model for xylem development. Plant Physiology. 2004;135:653–659. doi: 10.1104/pp.104.040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ. Determinate growth of Allium sativum peduncles: evidence of determinate growth as a design factor for biomechanical safety. American Journal of Botany. 1990;77:762–771. [Google Scholar]
- Niklas KJ. Plant biomechanics: an engineering approach to plant form and function. Chicago: University of Chicago Press; 1992. [Google Scholar]
- Niklas KJ. Differences between Acer saccharum leaves from open and wind-protected sites. Annals of Botany. 1996;78:61–66. [Google Scholar]
- Niklas KJ, Molina-Freaner F, Tinoco-Ojanguren C, Hogan CJ, Jr, Paolillo DJ., Jr On the mechanical properties of the rare endemic cactus Stenocereus eruca and the related species S. gummosus. American Journal of Botany. 2003;90:663–674. doi: 10.3732/ajb.90.5.663. [DOI] [PubMed] [Google Scholar]
- Pruyn ML, Ewers BJ, Telewski FW. Thigmomorphogenesis: changes in the morphology and mechanical properties of two Populus hybrids in response to mechanical perturbation. Tree Physiology. 2000;20:535–540. doi: 10.1093/treephys/20.8.535. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. 2009 Vienna, Austria, R Foundation for Statistical Computing, http://www.R-project.org . [Google Scholar]
- Rowe NP, Speck T. Plant growth forms: an ecological and evolutionary perspective. New Phytologist. 2005;166:61–72. doi: 10.1111/j.1469-8137.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- Rowe NP, Speck T, Galtier J. Biomechanical analysis of a Palaeozoic gymnosperm stem. Proceedings of the Royal Society B: Biological Sciences. 1993;252:19–28. [Google Scholar]
- Sehr EM, Agusti J, Lehner R, Farmer EE, Schwarz M, Greb T. Analysis of secondary growth in the Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. The Plant Journal. 2010;63:811–822. doi: 10.1111/j.1365-313X.2010.04283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VC, Ennos AR. The effects of air flow and stem flexure on the mechanical and hydraulic properties of the stems of sunflowers Helianthus annuus L. Journal of Experimental Botany. 2003;54:845–849. doi: 10.1093/jxb/erg068. [DOI] [PubMed] [Google Scholar]
- Speck T, Rowe NP. A quantitative approach for analytically defining size, growth form and habit in living and fossil plants. In: Kurmann MH, Hemsley AR, editors. The evolution of plant architecture. Kew: Royal Botanic Gardens; 1999. pp. 447–479. [Google Scholar]
- Speck T, Rowe NP. Modelling primary and secondary growth processes in plants: a summary of the methodology and new data on an early lignophyte. Philosophical Transactions of the Royal Society B: Biological Sciences. 2003;358:1473–1485. doi: 10.1098/rstb.2003.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telewski FW. Wind-induced physiological and developmental responses in trees. In: Coutts MP, Grace J, editors. Wind and trees. Cambridge: Cambridge University Press; 1995. pp. 237–263. [Google Scholar]
- Telewski FW. A unified hypothesis of mechanoperception in plants. American Journal of Botany. 2006;93:1466–1476. doi: 10.3732/ajb.93.10.1466. [DOI] [PubMed] [Google Scholar]
- Telewski FW, Jaffe MJ. Thigmomorphogenesis: anatomical, morphological and mechanical analysis of genetically different sibs of Pinus taeda in response to mechanical perturbation. Physiologia Plantarum. 1986a;66:219–226. doi: 10.1111/j.1399-3054.1986.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Telewski FW, Jaffe MJ. Thigmomorphogenesis: field and laboratory studies of Abies fraseri in response to wind and mechanical perturbation. Physiologia Plantarum. 1986b;66:211–218. doi: 10.1111/j.1399-3054.1986.tb02411.x. [DOI] [PubMed] [Google Scholar]
- Telewski FW, Jaffe MJ. Thigmomorphogenesis: the role of ethylene in the response of Pinus taeda and Abies fraseri to mechanical perturbation. Physiologia Plantarum. 1986c;66:227–233. doi: 10.1111/j.1399-3054.1986.tb02413.x. [DOI] [PubMed] [Google Scholar]
- Telewski FW, Pruyn ML. Thigmomorphogenesis: a dose response to flexing in Ulmus americana seedlings. Tree Physiology. 1998;18:65–68. doi: 10.1093/treephys/18.1.65. [DOI] [PubMed] [Google Scholar]
- Turner SR, Somerville CR. Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. The Plant Cell. 1997;9:689–701. doi: 10.1105/tpc.9.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JFV. Structural biomaterials. Princeton, NJ: Princeton University Press; 1990. [Google Scholar]
- Zhong R, Taylor JJ, Ye Z-H. Disruption of interfascicular fiber differentiation in an Arabidopsis mutant. The Plant Cell. 1997;9:2159–2170. doi: 10.1105/tpc.9.12.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong RQ, Ye ZH. IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. The Plant Cell. 1999;11:2139–2152. doi: 10.1105/tpc.11.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]