Abstract
Background and Aims
Merwilla plumbea is an important African medicinal plant. As the plants grow in soils contaminated with metals from mining activities, the danger of human intoxication exists. An experiment with plants exposed to cadmium (Cd) was performed to investigate the response of M. plumbea to this heavy metal, its uptake and translocation to plant organs and reaction of root tissues.
Methods
Plants grown from seeds were cultivated in controlled conditions. Hydroponic cultivation is not suitable for this species as roots do not tolerate aquatic conditions, and additional stress by Cd treatment results in total root growth inhibition and death. After cultivation in perlite the plants exposed to 1 and 5 mg Cd L−1 in half-strength Hoagland's solution were compared with control plants. Growth parameters were evaluated, Cd content was determined by inductively coupled plasma mass spectroscopy (ICP-MS) and root structure was investigated using various staining procedures, including the fluorescent stain Fluorol yellow 088 to detect suberin deposition in cell walls.
Key Results
The plants exposed to Cd were significantly reduced in growth. Most of the Cd taken up by plants after 4 weeks cultivation was retained in roots, and only a small amount was translocated to bulbs and leaves. In reaction to higher Cd concentrations, roots developed a hypodermal periderm close to the root tip. Cells produced by cork cambium impregnate their cell walls by suberin.
Conclusions
It is suggested that the hypodermal periderm is developed in young root parts in reaction to Cd toxicity to protect the root from radial uptake of Cd ions. Secondary meristems are usually not present in monocotyledonous species. Another interpretation explaining formation of protective suberized layers as a result of periclinal divisions of the hypodermis is discussed. This process may represent an as yet unknown defence reaction of roots when exposed to elemental stress.
Keywords: Cadmium, environmental stress, medicinal plants, Merwilla plumbea, monocotyledonous plants, periderm, plant anatomy, root structure
INTRODUCTION
South Africa has a large concentration of metal, mineral and coal-mining industries with high rates of waste disposal (Cooke and Johnson, 2002). The gold and coal-mining industries are responsible for lowering pH levels of South African rivers by the phenomenon known as ‘acid mine drainage’ (Coetzee, 1995). The waste from mining industries contains heavy metals such as lead, cadmium (Cd), mercury, aluminium, zinc, copper, magnesium and manganese (Department of Water Affairs, 1986; Wren and Stephenson, 1991). Compared with other metals or metalloids, Cd has a higher tendency to accumulate in plant tissues (Kabata-Pendias and Pendias, 1984) and is considered as highly mutagenic and carcinogenic by the International Agency for Research on Cancer (IARC, 1993; Filipic and Hei, 2004). Consumption of edible plant material with high Cd content may cause toxicity in humans (Jackson and Alloway, 1992; FAO/WHO, 1995). Due to its high toxicity, the maximum permissible limit of Cd in medicinal plants set by the World Health Organization (WHO) is 0·3 p.p.m.
In a recent investigation by Street et al. (2008), Cd exceeded the limits recommended by the WHO (1998) in bulb and tuber samples of South African medicinal plants obtained from street markets. In another study, Cd accumulation in bulbs of small and medium-sized plants of Tulbaghia species used medicinally increased with increasing Cd concentration (Street et al., 2010). Bulbs of mature Merwilla plumbea plants accumulated 24-fold more Cd than the WHO guidelines when irrigated with 2 mg Cd L−1. At the same time, the bulb extracts showed increased antibacterial activity (Street et al., 2009). Cadmium had a detrimental effect on the root growth of Bowiea volubilis, which is another important bulbous medicinal plant in South Africa (Street et al., 2007). In most of these studies, it was indicated that bulbs of medicinal plants have a tendency to accumulate Cd. However, the uptake mechanism of Cd is still unclear.
As roots of seedlings represent the primary contact zone of plants in soils, anatomical alterations occurring during Cd uptake may have an effect on accumulation processes and vegetative growth of the plants when they are exposed to this metal. The root reaction of various plant species was recently reviewed by Lux et al. (2011). Inhibition of root growth and branching as a result of the inhibitory effect of Cd on cell division was observed by Fusconi et al. (2006) and Ma et al. (2010). Species-specific reactions and changes in root tissue organization and development occur after Cd treatment (Lunáčková et al., 2003). Changes in the development of root apoplasmic barriers were shown to be related to Cd uptake and translocation (Lux et al., 2004; Martinka and Lux, 2004; Vaculík et al., 2009). Considering these facts, elevated concentrations of Cd in the rhizosphere may influence root growth and development of M. plumbea plants. In the present contribution, two types of cultivation of an extensively used medicinal plant in South Africa, M. plumbea, were tested for the evaluation of uptake and translocation of Cd. An anatomical study was performed to investigate the possible effect of Cd treatment on root structure.
MATERIALS AND METHODS
Growth conditions and experimental design
Seeds of Merwilla plumbea (Lindl.) Speta, obtained from the Botanical Garden of the University of KwaZulu-Natal in Pietermaritzburg, South Africa, were germinated on wet filter paper in a Petri dish for 10 d. Thereafter, the young seedlings were transferred to perlite and irrigated with tap water for 4 weeks and then by half-strength Hoagland's solution for 8 weeks in the greenhouse at Comenius University in Bratislava, Slovakia. Two different types of cultivation were applied: (1) cultivation of experimental plants directly in hydroponic solution (hydroponics) and (2) cultivation of plants in perlite, which was irrigated with the same solutions as used for hydroponics. For hydroponics, the first set of prepared young plants with already developed bulbs was transferred to 5 L containers filled with half-strength Hoagland's solution. Another set of plants for perlite was transferred to 1 L pots filled with perlite and placed in a growth chamber. The plants in both experiments were grown for 4 weeks at 25 °C, 60 % humidity, 16/8 h light/dark photoperiod, PAR 150 µmol m−2 s−1.
For both experiments, three different treatments were used: (1) control – irrigated with half-strength Hoagland's solution; (2) Cd 1 – irrigated with half-strength Hoagland's solution containing 1 mg Cd L−1; and (3) Cd 5 – irrigated with half-strength Hoagland's solution containing 5 mg Cd L−1.
Cadmium was applied in the form of Cd(NO3)2·4H2O. The hydroponic solution was changed weekly. The pots containing perlite were irrigated with 200 mL of solution on the first day at the start of the experiment and afterwards with an additional 100 mL of solution every seventh day for 4 weeks.
Growth parameter determination
The plants were harvested and fresh weights of roots, bulbs and leaves were determined. The total root length was recorded and expressed as ‘cumulative root length per plant (CRL)’. The length of individual roots was calculated from the CRL divided by the number of roots on each plant. The same measurements and calculations were done with the leaves. Fresh roots, bulbs and leaves were dried at 70 °C for 3 d and after that the dry weight of these organs was determined.
Determination of Cd concentration
Dried samples of roots, bulbs and leaves were analysed for Cd by inductively coupled plasma mass spectroscopy (ICP-MS). The samples were dissolved in HNO3 and the Cd concentration was determined by an Elan 6000 spectrometer (Pe Sciex, Canada).
Anatomical observations
Series of hand sections of roots were prepared at 1 mm intervals from the root apex to the base. For suberin visualization in fluorescence microscopy, the free hand sections were stained by 0·01 % Fluorol yellow 088 dissolved in lactic acid for 30 min and washed in distilled water according to Lux et al. (2005). The samples were placed into a drop of 0·1 % FeCl3 dissolved in 50 % glycerin prior to observation. The sections for bright-field observations were stained with toluidine blue.
The sections were observed under an Zeiss Axioskop 2 plus epifluorescence microscope (Jena, Germany) and documented by an Olympus DP 72 digital camera.
Statistical analyses
The experimental data shown in the figures are means of 12 replicates ± s.e. from independent experiments repeated three times. The significance was compared at the 0·05 level using Student's t-test (Statgraphics Centurion XV, v. 15·2·05, StatPoint, Inc.).
RESULTS AND DISCUSSION
Growth of the plants
The growth of M. plumbea plants in hydroponics was poor, and only in control conditions did some new adventitious roots form. These exhibited limited growth. The roots of plants treated with Cd were stunted and no new root formation occurred. The majority of roots in both Cd treatments gradually died (Fig. 1). The growth parameters of plants from this experiment are shown in Table 1. It was clear that cultivation in hydroponics is not feasible for this species.
Fig. 1.
Effect of cadmium on 17-week-old Merwilla plumbea plants grown for 4 weeks in (A) hydroponics and (B) perlite. Three different treatments were used: control (C); 1 mg Cd L−1 (Cd1); and 5 mg Cd L−1 (Cd5). Scale bars = 1 cm.
Table 1.
Growth parameters of Merwilla plumbea plants grown for 4 weeks in hydroponics
| Treatment |
|||
|---|---|---|---|
| Growth parameter | Control | Cd1 | Cd5 |
| Root f. wt (g) | 0·0904 ± 0·017a | 0·0534 ± 0·016b | 0·0483 ± 0·019b |
| Root d. wt (g) | 0·0051 ± 0·0009a | 0·0033 ± 0·0012b | 0·0026 ± 0·0008b |
| Leaf f. wt (g) | 0·49 ± 0·083a | 0·131 ± 0·032b | 0·1 ± 0·018b |
| Leaf d. wt (g) | 0·032 ± 0·0045a | 0·0127 ± 0·0009b | 0·0123 ± 0·026b |
| Bulb f. wt (g) | 0·134 ± 0·048a | 0·095 ± 0·029a | 0·011 ± 0·054a |
| Bulb d. wt (g) | 0·013 ± 0·0075a | 0·017 ± 0·0057a | 0·013 ± 0·0024a |
| CRL (cm) | 18·22 ± 3·45a | 10·62 ± 2·21b | 9·38 ± 0·81b |
| ARL (cm) | 4·742 ± 1·46a | 2·948 ± 0·46b | 2·818 ± 0·48b |
Three different treatments were used: control, half-strength Hoagland's solution; Cd1, half-strength Hoagland's solution containing 1 mg kg−1 Cd; and Cd5, half-strength Hoagland's solution containing 5 mg kg−1 Cd.
ARL, average root length; CRL, cumulative root length (means ± s.e., different letters represent significant differences at P < 0·05).
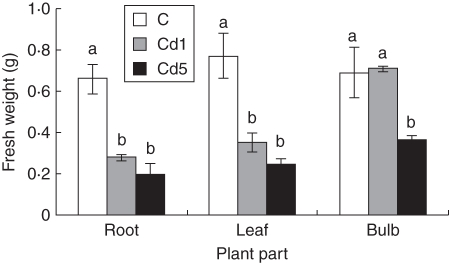
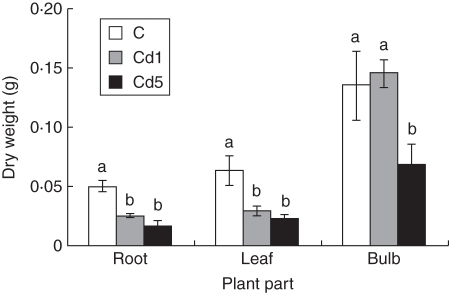
The growth of plants cultivated in perlite under control conditions was adequate and plants exhibited increases in both root and shoot growth (Figs 2 and 3). Use of solid substrate is necessary for this species. Street et al. (2009) used sterilized acid-washed quartz sand wetted with Hoagland's solution for cultivation of this species. In our experiments the growth of M. plumbea plants was retarded after Cd application; the root system was reduced in comparison with the control and leaves were shorter and narrower, and yellowish compared with those of control plants (Fig. 1). Shorter leaves of M. plumbea were observed after CdCl2 treatment, but without statistically significant differences between control and treated plants (Street et al., 2009). However, Cd at 2 mg L−1 resulted in a significant reduction of fresh weight of leaves, bulbs and roots, when compared with the control (Street et al., 2009). Similarly in our experiment in the plants treated with Cd(NO3)2·4H2O, the fresh and dry weight of roots were significantly lower, >50 %, after cultivation in hydroponics with 1 mg Cd L−1 when compared with the control plants. This difference was even higher when the higher Cd concentration (5 mg Cd L−1) was used. However, the difference between 1 and 5 mg Cd L−1 was not significant. The same trend was observed for the leaves. The main differences were observed with bulbs; the fresh and dry weights of bulbs of plants treated with 1 mg Cd L−1 were not different from those of the controls. After higher Cd application (5 mg Cd L−1), the fresh and dry weights of bulbs were lower, about 50 %, when compared with the control and 1 mg Cd L−1-treated plants (Figs 2 and 3).
Fig. 2.
Effect of cadmium on fresh weight of 17-week-old Merwilla plumbea plants grown for 4 weeks in perlite. Three different treatments were used: control (C); 1 mg Cd L−1 (Cd1); and 5 mg Cd L−1 (Cd5). Means ( ± s.e.) with different letters are significantly different (P < 0·05).
Fig. 3.
Effect of cadmium on dry weight of 17-week-old Merwilla plumbea plants grown for 4 weeks in perlite. Three different treatments were used: control (C); 1 mg Cd L−1 (Cd1); and 5 mg Cd L−1 (Cd5). Means ( ± s.e.) with different letters are significantly different (P < 0·05).
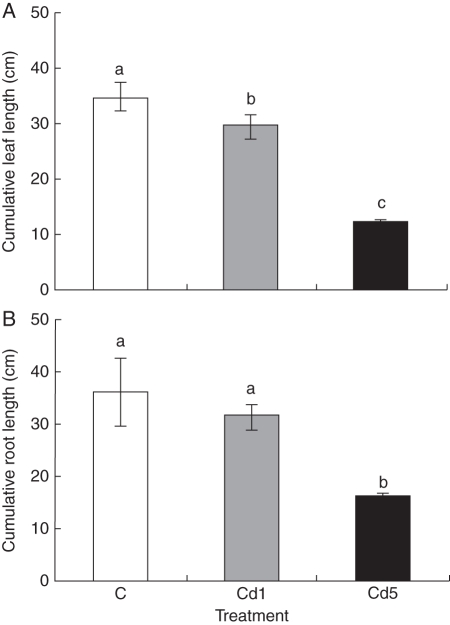
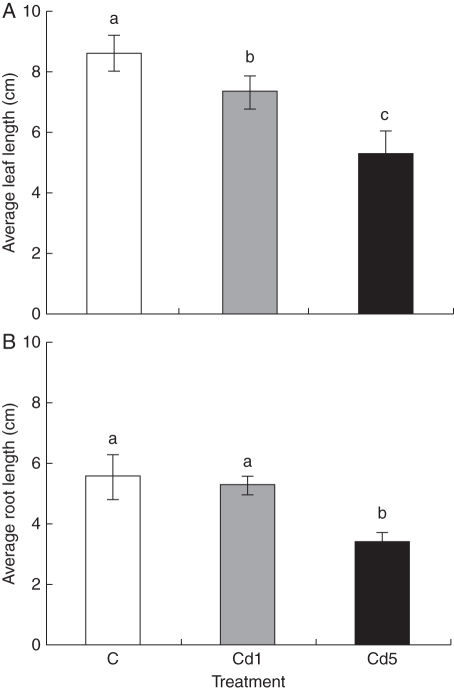
The number of adventitious roots was lower in plants cultivated with Cd; the mean numbers for control, 1 mg Cd L−1 and 5 mg Cd L−1 were 6·5, 6·0 and 4·9, respectively. CRL as well as the average length of roots (ARL) was not different between control and 1 mg Cd L−1-treated plants. However, both CRL and ARL were significantly different between the control and 5 mg Cd L−1 treatments and also between both Cd treatments (Figs 4 and 5). The inhibition of root growth was probably caused by Cd-induced depolymerization of microtubules of the cell cytoskeleton and chromosome aberrations, which resulted in lower mitotic activity of meristematic cells (Fusconi et al., 2006; Seth et al., 2008), and thus inhibition of root elongation. Cumulative leaf length per plant and also the average length of leaves were significantly different between the control and both treatments. The growth of roots of carrot and radish seedlings was inhibited at 20 mg Cd L−1 and this inhibitory effect increased with increasing concentrations of Cd in both liquid culture and pot experiments (Chen et al., 2003). In the present study, the inhibitory effect was more pronounced at 5 mg Cd L−1, suggesting that M. plumbea seedlings are more sensitive to Cd than the seedlings of carrot and radish. Recently, Street et al. (2009) showed that 2 mg Cd L−1 negatively affected the fresh weight of leaves, bulbs and roots of mature plants of M. plumbea exhibiting a high accumulation of Cd in the roots. This suggests that inhibition of root growth at high Cd concentration may affect nutrient and water uptake, resulting in inhibition of shoot elongation. However, there is not enough information on the mechanism of Cd toxicity in the root system, which is the main organ for uptake in plants (Chen et al., 2003).
Fig. 4.
Effect of cadmium on cumulative leaf and root length of 17-week-old Merwilla plumbea plant grown for 4 weeks in perlite. Three different treatments were used: control (C); 1 mg Cd L−1 (Cd1); and 5 mg Cd L−1 (Cd5). Means ( ± s.e.) with different letters are significantly different (P < 0·05).
Fig. 5.
Effect of cadmium on average leaf and root length of 17-week-old Merwilla plumbea plants grown for 4 weeks in perlite. Three different treatments were used: control (C); 1 mg Cd L−1 (Cd1); and 5 mg Cd L−1 (Cd5). Means ( ± s.e.) with different letters are significantly different (P < 0·05).
Cadmium concentration
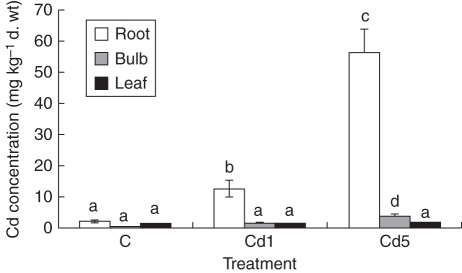
Large differences were found in accumulation of Cd by different plant organs of M. plumbea (Fig. 6). Roots accumulated considerably higher amounts of Cd when compared with the bulbs and leaves. This phenomenon is well known for many plant species. It is assumed that this is one of the main mechanisms to protect the above-ground parts of plants against toxic Cd effects (Lux et al., 2011). The concentration of Cd significantly increased in roots treated with 1 mg Cd L−1 and even more Cd was found in roots when grown at higher Cd stress (5 mg Cd L−1). The same tendency was observed in bulbous species cultivated at 50 and 250 µM Cd (Soudek et al., 2009). Similarly to the roots, the Cd concentrations in the other plant organs were elevated correspondingly with increasing concentration of Cd in the media. However, the concentration of Cd was more than eight times higher in the roots than in the leaves and bulbs when plants were grown in 1 mg Cd L−1. The differences between plant parts were considerably bigger when the higher Cd concentration (5 mg Cd L−1) was used. Comparable results were obtained with CdCl2 treatment of the same species by Street et al. (2009). The concentration of Cd in the roots was approx. 15 times higher compared with the bulbs and about 30 times higher when compared with the leaves. The concentration of Cd was the same in the leaves and the bulbs when a lower Cd concentration (1 mg Cd L−1) was used, and about twice as high in the bulbs than in the leaves at higher Cd treatment (5 mg Cd L−1) (Fig. 6). Soudek et al. (2009) have observed that experimentally tested bulbous plants retained on average 75 % of accumulated Cd in the roots, and only 18 % and 7 % in the bulbs and leaves, respectively. Despite the translocation factor being quite low in M. plumbea, the Cd accumulated in the bulbs exceeded, at all experimental variants, the limit for maximum Cd content (0·3 p.p.m.) set by the WHO for medicinal plants. Another medicinal plant species, Tulbaghia violacea, accumulated Cd in its bulbs in the range of 3·4–8·7 µg g−1 d. wt depending on the size of test plants after 6 weeks treatment with 2 mg L−1 Cd. Tulbaghia violacea accumulated Cd in the leaves at the level of 2·1–1·2 and 5·5–2·9 µg g−1 at 2 and 5 mg L−1, respectively, depending on plant age – the older the plant, the less accumulation (Street et al., 2010). Several plant species, such as Thlaspi caerulescens, Arabidopsis halleri and Sedum alfredii, can accumulate Cd in concentrations exceeding even the level set for hyperaccumulators (which is 100 µg Cd g−1 d. wt) (Salt et al., 1995; Zhao et al., 2006; Deng et al., 2007; Banasova et al., 2008). Usually important crop plants, such as maize, rice, wheat and sunflower, do not reach this hyperaccumulation limit when treated with Cd concentrations < 10 mg kg−1 of soil (Greger and Landberg, 2008; Faessler et al., 2010; Liu et al., 2010). Monocotyledonous bulbous plant species, such as Allium cepa, Allium sativum, Allium schoenoprasum and Allium porrum, behave similarly, but in some cases they can exceed the hyperaccumulation limits when grown under specific conditions, mostly in hydroponics and at high Cd concentrations in the media (Soudek et al., 2009). Allium schoenoprasum was able to accumulate this metal to >1700 and 200 µg g−1 in roots and leaves, respectively, during 28 d of 50 µm Cd treatment (Barazani et al., 2004).
Fig. 6.
Effect of cadmium application on cadmium concentration in roots, bulbs and leaves of 17-week-old Merwilla plumbea plants grown for 4 weeks in perlite. Three different treatments were used: control (C); 1 mg Cd L−1 (Cd1); and 5 mg Cd L−1 (Cd5). Means ( ± s.e.) with different letters are significantly different (P < 0·05).
Root anatomy
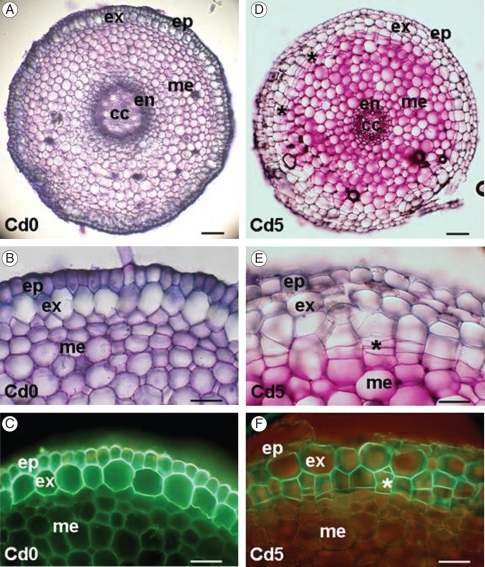
The adventitious roots of M. plumbea seedlings show the typical structure of a monocotyledonous root (Fig. 7A). One layer of a rhizodermis is formed by small cells as the outermost tissue. The cortex is relatively broad, with a one-layered hypodermis. Hypodermal cells are radially extended and develop Casparian bands and suberin lamellae 10–15 mm from the root apex (Fig. 7B, C); this layer can be classified as exodermis (Peterson and Perumala, 1990). Cortical parenchyma is multilayered and cells are alternatively arranged with small intercellular spaces. The single-layered endodermis develops Casparian bands close to the root apex, at a distance of approx. 3 mm. A secondary state of endodermal development (suberin lamellae development) occurs approx. 30 mm from the root apex. The central cylinder is narrow; it consists of a one-layered pericycle, 6–12 radially arranged poles of xylem and phloem elements, and a central parenchymatous region.
Fig. 7.
Cross-sections of adventitious roots of Merwilla plumbea. Plants were grown in control conditions in perlite (A–C) and treated with 5 mg kg−1 Cd (D–F). Roots in control conditions exhibit the typical structure of monocotyledonous roots formed by single-layered epidermis and single-layered exodermis (A, B); exodermal cells develop suberin lamellae close to the root apex, as shown in C after Fluorol yellow 088 staining in fluorescence microscopy. After cadmium treatment the hypodermal periderm is formed in the peripheral cortical zone close to the root apex (D–F). Cork cambium produces cells by periclinal division (E); these become impregnated by suberin, as shown in F after Fluorol yellow 088 staining in fluorescence microscopy. The distances of sections from the root apex are 5 mm (A, B, D, E) and 30 mm (C, F). Abbreviations: ep, epidermis; ex, exodermis; me, mesodermis (mid-cortical layers); en, endodermis; cc, central cylinder; asterisks indicate periclinal divisions in cork cambium. Scale bars: (A, D) = 100 µm, (B, C, E, F) = 50 µm.
The oldest part of M. plumbea root, close to the bulb, is characterized by the presence of a contractile zone. Contractile roots pull bulbs down into the soil. The association between contractile roots, water uptake and habitat aridity was investigated for agaves, yuccas and aloes by North et al. (2008).
Cadmium application in our experiments at a higher concentration (5 mg Cd L−1) caused changes in the root tissue organization and development. Exposure to Cd induced the formation of cork cambium in cortical tissues internally, adjacent to the exodermis (Fig. 7D). Cells divided periclinally and the derivatives impregnated their cell walls with suberin (Fig. 7E, F). Hypodermal periderm formation occurred in the young, sub-apical part of the root. The first periclinal divisions were present approx. 5 mm from the root apex.
The root of most monocotyledons is covered by the original primary epidermis during their whole life; under some conditions it becomes lignified or even suberized. These cell wall modifications may reduce the growth of plant organs and can affect the transport of nutrients and water. Only a few studies of periderm formation in monocotyledons have been completed, mostly focused on arborescent monocotyledons (Dickinson, 2000). In bulbous plants, wound periderm formation was observed in roots after infection caused by various species of Penicillium (Saaltink, 1971). Exceptional periderm is sometimes present below the exodermis in some species of Asparagales (Kauff et al., 2000). We suggest that formation of hypodermal periderm in young sub-apical parts of Merwilla roots represents a defence reaction of roots exposed to toxic metal treatment. Periderm, with impermeable cell walls, impregnated by suberin may reduce radial uptake of Cd ions by roots.
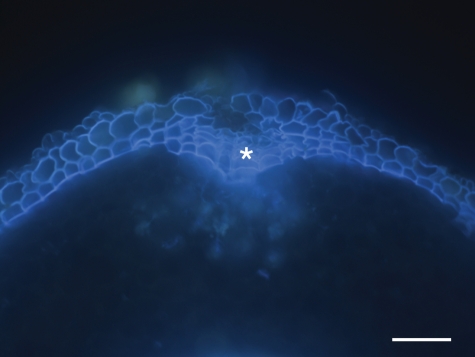
The formation of protective sub-epidermal layers in response to Cd treatment is interpreted here as hypodermal periderm or wound periderm formation. It can probably also be interpreted as multiseriate hypodermis. This interpretation is based on multiseriate hypodermis shown in roots of some monocotyledonous species by Seago and Marsch (1989), Seago et al. (2000) and Heimsch and Seago (2008), and in Iris by Meyer et al. (2009). These studies showed developmental schemes of multiseriate exodermis and hypodermis which can be formed from the root apical meristem (RAM), in some cases many millimetres behind the tip. Exodermis absent in control conditions and induced by salt stress was found in roots of dicotyledonous plant species (cotton) by Reinhardt and Rost (1995). These data suggest the possibility of formation of sub-epidermal layers observed by us as a result of meristematic activity derived from the RAM. We can support our opinion about the formation of secondary meristem in sub-epidermal root layers of Merwilla in response to stress by additional observations. Adventitious roots of adult plants growing in soil (Botanical Garden of University of KwaZulu-Natal, Pietermaritzburg, South Africa) were investigated. In wounded or damaged areas of older root parts, formation of multiseriate cell layers with suberized cell walls resembling typical periderm (wound periderm) of dicotyledonous roots was observed (Fig. 8). Depending on interpretation, we can call these structures in monocotyledonous plant species hypodermal periderm, or regard their formation as a result of additional divisions of cortical cells. Otherwise we can accept the possibility of cork cambium formation resulting in periderm layers in monocotyledons.
Fig. 8.
Cross-section of adventitious roots of an adult Merwilla plant growing in soil (Botanical Garden of University of KwaZulu-Natal, Pietermaritzburg, South Africa) stained with Fluorol yellow 088 in UV light. The formation of multiseriate cell layers with suberized cell walls (*) resembling typical periderm occurs after injury of the root surface. Scale bar = 100 µm.
Conclusions
Accumulation of toxic amounts of heavy metals in plants represents a health threat for humans. Medicinal plants used in traditional medicine are little studied with respect to Cd intake. The capacity of M. plumbea, an intensively traded medicinal plant of South Africa, was studied here. The data show that this plant can accumulate considerable amounts of Cd. Plants limit shoot Cd accumulation by restricting Cd movement to the xylem through both the symplasmic and the apoplasmic pathways. Restriction of apoplasmic movement of Cd was found in various plant species by root cell wall modifications and impregnations, accelerated maturation of apoplasmic barriers – exodermis and endodermis – and lignification of peripheral root tissues. In roots of M. plumbea, early formation of hypodermal periderm close to the root apex occurs after Cd treatment. Periderm is only rarely developed in roots of monocotyledonous plants; therefore, another interpretation may be additional periclinal division of hypodermal layers. We hypothesize that this process and production of secondary tissues impregnated by suberin in M. plumbea treated by Cd have functional significance in reducing the radial transport of toxic Cd ions to the xylem and subsequently to the shoot. This may be a novel defence reaction of plants exposed to Cd.
ACKNOWLEDGEMENTS
We thank the National Research Foundation RSA–Slovak Collaborative Project, South Africa and the Slovak Research and Development Agency (grant APVV SK-ZA-0007-07) for financially supporting the bilateral collaborative project, and the Slovak Grant Agency VEGA (1/0472/10). The authors are grateful to both referees for valuable and stimulating comments and suggestions.
LITERATURE CITED
- Banasova V, Horak O, Nadubinska M, Ciamporova M, Lichtscheidl I. Heavy metal content in Thlaspi caerulescens J. et C. Presl growing on metalliferous and non-metalliferous soils in Central Slovakia. International Journal of Environment and Pollution. 2008;33:133–145. [Google Scholar]
- Barazani O, Dudai N, Khadka UR, Golan-Goldhirsh A. Cadmium accumulation in Allium schoenoprasum L. grown in an aqueous solution. Chemosphere. 2004;57:1213–1218. doi: 10.1016/j.chemosphere.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Chen YX, He YF, Luo YM, Yu YL, Lin Q, Wong MH. Physiological mechanism of plant roots exposed to cadmium. Chemosphere. 2003;50:789–793. doi: 10.1016/s0045-6535(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Coetzee MAS. Water pollution in South Africa: its impact on wetland biota. In: Cowan G, editor. Wetlands of South Africa. Pretoria, South Africa: Department of Environmental Affairs and Tourism; 1995. [Google Scholar]
- Cooke JA, Johnson MS. Ecological restoration of land with particular reference to the mining of metals and industrial minerals: a review of theory and practice. Environmental Reviews. 2002;10:41–71. [Google Scholar]
- Deng DM, Shu WS, Zhang J, et al. Zinc and cadmium accumulation and tolerance in populations of Sedum alfredii. Environmental Pollution. 2007;147:381–386. doi: 10.1016/j.envpol.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Department of Water Affairs. Management of the water resources of the Republic of South Africa. Pretoria, South Africa: Department of Water Resources; 1986. [Google Scholar]
- Dickinson WC. Integrative plant anatomy. Academic Press: San Diego; 2000. [Google Scholar]
- Faessler E, Robinson BH, Gupta SK, Schulin R. Uptake and allocation of plant nutrients and Cd in maize, sunflower and tobacco growing on contaminated soil and the effect of soil conditioners under field conditions. Nutrient Cycling in Agroecosystems. 2010;87:339–352. [Google Scholar]
- FAO/WHO. Joint committee on food additives and contaminants. Position paper on cadmium (Prepared by France) 1995 27th session. The Hague, The Netherlands, 20–24 March 1995, p. 32. [Google Scholar]
- Filipic M, Hei TK. Mutagenicity of cadmium in mammalian cells: implication of oxidative DNA damage. Mutation Research. 2004;546:81–91. doi: 10.1016/j.mrfmmm.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Fusconi A, Repetto O, Bona E, et al. Effects of cadmium on meristem activity and nucleus ploidy in roots of Pisum sativum L. cv. Frisson seedlings. Environmental and Experimental Botany. 2006;58:253–260. [Google Scholar]
- Greger M, Landberg T. Role of rhizosphere mechanisms in Cd uptake by various wheat cultivars. Plant and Soil. 2008;312:195–205. [Google Scholar]
- Heimsch C, Seago JL. Organization of the root apical meristem in angiosperms. American Journal of Botany. 2008;95:1–21. doi: 10.3732/ajb.95.1.1. [DOI] [PubMed] [Google Scholar]
- IARC. Beryllium, cadmium, mercury and exposure in glass manufacturing industry: monographs on evaluation of carcinogenic risk to humans. Vol. 58. Lyon, France: IARC; 1993. pp. 119–239. [PMC free article] [PubMed] [Google Scholar]
- Jackson AP, Alloway BJ. The transfer of cadmium from agricultural soils to the human food chain. In: Adriano DC, editor. Biogeochemistry of trace metals. Boca Raton, FL: Lewis Publishers; 1992. pp. 109–158. [Google Scholar]
- Kabata-Pendias A, Pendias H. Trace elements in soils and plants. 1984 CRC, USA. [Google Scholar]
- Kauff F, Rudall PJ, Conran JG. Systematic root anatomy of Asparagales and other monocotyledons. Plant Systematics and Evolution. 2000;223:139–154. [Google Scholar]
- Liu J, Cao C, Wong M, Zhang Z, Chai Y. Variations between rice cultivars in iron and manganese plaque on roots and the relation with plant cadmium uptake. Journal of Environmental Sciences. 2010;22:1067–1072. doi: 10.1016/s1001-0742(09)60218-7. [DOI] [PubMed] [Google Scholar]
- Lunáčková L, Šottníková A, Masarovičová E, Lux A, Streško V. Comparison of cadmium effect on willow and poplar in response to different cultivation conditions. Biologia Plantarum. 2003;47:403–411. [Google Scholar]
- Lux A, Šottníková A, Opatrná J, Greger M. Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiologia Plantarum. 2004;120:537–545. doi: 10.1111/j.0031-9317.2004.0275.x. [DOI] [PubMed] [Google Scholar]
- Lux A, Morita S, Abe J, Kaori I. An improved method for clearing and staining free-hand sections and whole-mount samples. Annals of Botany. 2005;96:989–996. doi: 10.1093/aob/mci266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A, Martinka M, Vaculík M, White PJ. Root responses to cadmium in the rhizosphere: a review. Journal of Experimental Botany. 2011 doi: 10.1093/jxb/erq281. doi:10.1093/jxb/erq281 (in press) [DOI] [PubMed] [Google Scholar]
- Ma LJ, Zhang Y, Bu N, Wang SH. Alleviation effect of alginate-derived oligosaccharides on Vicia faba root tip cells damaged by cadmium. Bulletin of Environmental Contamination and Toxicology. 2010;84:161–164. doi: 10.1007/s00128-009-9914-2. [DOI] [PubMed] [Google Scholar]
- Martinka M, Lux A. Response of roots of three populations of Silene dioica to cadmium treatment. Biologia. 2004;59:185–189. [Google Scholar]
- Meyer CJ, Seago JL, Peterson CA. Environmental effects on the maturation of the endodermis and multiseriate exodermis of Iris germanica roots. Annals of Botany. 2009;103:687–702. doi: 10.1093/aob/mcn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North GB, Brinton EK, Garrett TY. Contractile roots in succulent monocots: convergence, divergence and adaptation to limited rainfall. Plant, Cell and Environment. 2008;31:1179–1189. doi: 10.1111/j.1365-3040.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- Peterson CA, Perumala CJ. A survey of angiosperm species to detect hypodermal Casparian bands. II. Roots with multiseriate hypodermis or epidermis. Botanical Journal of the Linnean Society. 1990;103:113–125. [Google Scholar]
- Reinhardt DH, Rost TL. Salinity accelerates endodermal development and induces an exodermis in cotton seedling roots. Environmental and Experimental Botany. 1995;35:563–574. [Google Scholar]
- Saaltink GJ. The infection of bulbs by Penicillium sp. Acta Horticulturae. 1971;23:235–241. [Google Scholar]
- Salt DE, Blaylock M, Kumar NPBA, et al. Phytoremediation – a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology. 1995;13:468–474. doi: 10.1038/nbt0595-468. [DOI] [PubMed] [Google Scholar]
- Seago JL, Marsh LC. Adventitious root development in Typha glauca, with emphasis on the cortex. American Journal of Botany. 1989;76:909–923. [Google Scholar]
- Seago JL, Peterson CA, Enstone DE. Cortical development in roots of the aquatic plant Pontederia cordata (Pontederiaceae) American Journal of Botany. 2000;87:1116–1127. [PubMed] [Google Scholar]
- Seth CS, Misra V, Chauhan LKS, Singh RR. Genotoxicity of cadmium on root meristem cells of Allium cepa: cytogenetic and Comet assay approach. Ecotoxicology and Environmental Safety. 2008;71:711–716. doi: 10.1016/j.ecoenv.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Soudek P, Kotyza J, Lenikusová I, Petrová Š, Benešová D, Vaněk T. Accumulation of heavy metals in hydroponically cultivated garlic (Allium sativum L.), onion (Allium cepa L.), leek (Allium porrum L.) and chive (Allium schoenoprasum L.) Journal of Food, Agriculture and Environment. 2009;7:761–769. [Google Scholar]
- Street RA, Kulkarni MG, Stirk WA, Southway C, Van Staden J. Toxicity of metal elements on germination and seedling growth of widely used medicinal plants belonging to Hyacinthaceae. Bulletin of Environmental Contamination and Toxicology. 2007;79:371–376. doi: 10.1007/s00128-007-9237-0. [DOI] [PubMed] [Google Scholar]
- Street RA, Kulkarni MG, Stirk WA, Southway C, Van Staden J. Variation in heavy metals and microelements in South African medicinal plants obtained from street markets. Food Additives and Contaminants. 2008;25:953–960. doi: 10.1080/02652030801993605. [DOI] [PubMed] [Google Scholar]
- Street RA, Kulkarni MG, Stirk WA, Southway C, Abdillahi HS, Chinsamy M, Van Staden J. Effect of cadmium uptake and accumulation on growth and antibacterial activity of Merwilla plumbea – an extensively used medicinal plant in South Africa. South African Journal of Botany. 2009;75:611–616. [Google Scholar]
- Street RA, Kulkarni MG, Stirk WA, Southway C, Van Staden J. Effect of cadmium on growth and micronutrient distribution in wild garlic (Tulbaghia violacea) South African Journal of Botany. 2010;76:332–336. [Google Scholar]
- Vaculík M, Lux A, Luxová M, Tanimoto E, Lichtscheidl I. Silicon mitigates cadmium inhibitory effects in young maize plants. Environmental and Experimental Botany. 2009;67:52–58. [Google Scholar]
- World Health Organization. Quality control methods for medicinal plant materials. 1998 Geneva, Switzerland. [Google Scholar]
- Wren CD, Stephenson GL. The effect of acidification on the accumulation and toxicity of metals to freshwater invertebrates. Environmental Pollution. 1991;71:205–241. doi: 10.1016/0269-7491(91)90033-s. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Jiang RF, Dunham SJ, McGrath SP. Cadmium uptake, translocation and tolerance in the hyperaccumulator Arabidopsis halleri. New Phytologist. 2006;172:646–654. doi: 10.1111/j.1469-8137.2006.01867.x. [DOI] [PubMed] [Google Scholar]