Abstract
At least five coherent models of carcinogenesis have been proposed in the history of cancer research in the last century. Model 1 is mainly centered around mutations, and its main focus is on the chemical environment, radiation and viruses. Model 2 has to do mainly with genome instability and it focuses on familiality. Model 3 is based on non-genotoxic mechanisms, and clonal expansion and epigenetics are its main features. We propose a fourth model, which can encompass the previous three, based on the concept of a ‘Darwinian’ cell selection (we clarify that the term Darwinian needs to be used cautiously, being a short cut for ‘somatic cellular selection’). Finally, a fifth model has recently become popular, based on the concept of ‘tissue organization’. We describe examples of the five models and how they have been formalized mathematically. The five models largely overlap, both scientifically and historically, but for the sake of clarity, it is useful to treat them separately. We also argue that the five models can be included into a simpler scheme, i.e. two types of models: (i) biological changes in the epithelium alone lead to malignancy and (ii) changes in stroma/extracellular matrix are necessary (along with changes in epithelium) for malignancy. Our description, though simplified, looks realistic, it is able to capture the historical sequence of carcinogenesis theories in the last century and can serve as a frame to make research hypotheses more explicit.
Introduction
Five models of carcinogenesis
Cancer cells are characterized by multiple structural, molecular and behavioural features. According to a recent description, ‘six essential alterations in cell physiology collectively dictate malignant growth: self-sufficiency in growth signals, insensitivity to growth-inhibitory (anti-growth) signals, evasion of programmed cell death (apoptosis), limitless replicative potential, sustained angiogenesis and tissue invasion and metastasis’ (1). The present paper aims at summarizing the main theories of carcinogenesis that have been proposed in the last 60 years. We will use the word ‘model’ instead of ‘theory’ because a model is a theory rooted in observations and encompasses mechanisms.
Models of carcinogenesis have evolved through the interaction of several disciplines. We propose that from 1950, five main models have been the dominant paradigms in cancer research, although we are aware that this is an oversimplification (in particular, the models have overlapped in time and have, themselves, in various ways been integrated and conflated). Table I shows models based on observations and on mathematical descriptions and includes the relevant hypothesized mechanisms. For example, the mutational model proposes mutations as the main feature of carcinogenesis on the basis of mathematical models (2) and of a mechanism (mutation as the main change that leads to the malignant phenotype).
Table I.
Five recent non-exclusive models of carcinogenesis
| Model 1 ‘mutational’ | Model 2 ‘genome instability’ | Model 3 ‘non-genotoxic’ | Model 4 ‘Darwinian’ | Model 5 ‘tissue organization’ | |
| Main focus | Chemical carcinogens | Familiality | Clonal expansion/epigenetics | Clonal expansion/cell selection | Microenvironment |
| Genome instability | Morphostats | ||||
| Viruses | |||||
| Examples | Tobacco and lung | Colon cancer | Diet, hormones | Beta-carotene, folate, chemotherapy | |
| HPV | Rb | ||||
| Mechanisms | DNA adducts | CIN, MIN, MMR, Rb, BRCA1, TSG | Methylation histone acetylation | Selective advantage | |
| Mutations | |||||
| Oncogenes | |||||
| Mathematical models | Armitage–Doll | Knudson | Moolgavkar | Nowak | Baker |
BRCA1, breast cancer 1 gene; HPV, human papillomavirus; MMR, mismatch repair; Rb, retinoblastoma.
As Table I shows, the first model (‘mutational’) was greatly influenced by the discovery of the carcinogenicity of tobacco smoke and some occupational exposures, and (from the beginning of the 20th century) by experimental work in animals with tar, involving exposure to polycyclic aromatic hydrocarbons (PAH), the same compounds found in tobacco smoke and in many occupational settings. This led both to the development of methods for the identification of reaction products between PAHs (and other compounds) and macromolecules (DNA adducts) and to the study of mutations. Viral research played a role in this first period and, in fact, the first oncogene (later shown to be mutated) was found in bladder cancer as a homologue of a viral DNA sequence. Yet, a third stream of research, relevant to the first model, covered induced mutations in bacteria and the development of screening tests for mutagens, the most notable being the Ames test (3–8).
The second model (‘genome instability’) is characterized by two, almost parallel, lines of research, both on cancers occurring in families. One was the theory developed by Knudson for retinoblastoma, which became the basis of the ‘two-hit’ hypothesis and led to the formulation of the theory of ‘tumour suppressor genes’(TSGs) and then to the discovery of Rb1, the TSG that causes retinoblastoma when both copies are mutated (9,10). The two-hit hypothesis also has a somewhat earlier history as John Cairns (11) has noted. The second line of research is similar, in that the investigation of familial colon cancer led to the discovery of microsatellite instability (MIN) and mismatch-repair genes. Thus, the second paradigm puts emphasis on TSG (e.g. p53), genome integrity and repair-related genes (such as BRCA1 and MLH1), in contrast to the first paradigm with its emphasis on point mutations and chemical carcinogens (12–15). The basic idea is that changes in some genes that regulate chromosome stability or repair of DNA damage cause a cascade of events and grossly increase the frequency of mutations downstream. Again, this was not completely new, as John Cairns (16) had earlier proposed that DNA repair might be central in carcinogenesis and Larry Loeb (17) had hypothesized genomic instability as a key feature of cancer progression. Vogelstein et al. (18), and many others, have linked mutations in oncogenes and loss of TS alleles in a relatively coherent, staged model (18).
In the context of this model, an important role is played by haematologic malignancies (leukaemias and lymphomas) because chromosome changes such as translocations have been demonstrated primarily in these cancers (e.g. the 14:18 translocation typical of follicular lymphoma) (19). Chromosomal translocations can result either in the fusion of genes or in bringing genes close to enhancer or promoter elements, hence leading to their altered expression or genomic instability. Interestingly, studies in identical twins, in archived neonatal blood spots of patients and in normal newborn cord bloods support the concept that chromosomal translocations often initiate leukaemia in utero.
It should be noted that the original proposal by Knudson of a two-hit model for retinoblastoma, subsequently confirmed by the discovery of the Rb gene, involves the impact of mutations in recessive TSGs (both alleles need to be mutated), in contrast to the role of mutations in dominant oncogenes (one mutation is sufficient). In the first case, the gene controls genome stability and in the second case, the gene encodes for a gene product directly involved in the control of cell proliferation or other neoplastic phenotypes.
The third model (Table I) (‘non-genotoxic’) is more recent and is characterized by an emphasis on non-genotoxic effects, in the sense that several important modulators of cancer risk (diet, obesity, hormones and insulin resistance) do not seem to act through a structural change in DNA but rather through functional changes including epigenetic events.
Another characteristic of current thinking is the resurrection of a Darwinian interpretation of carcinogenesis (model 4, Darwinian), which has become popular thanks to the work of Greaves (20) and, from a mathematical point of view, of Nowak (21); this interpretation—like model 3—attributes a greater role to clonal expansion (selection) of cells rather than to mutations but puts emphasis on the role of the environment (both macro and micro) in selecting cells that have some acquired advantage. In fact, the term Darwinian needs to be used cautiously, being a short cut for somatic cellular selection. It has entered into use in the cancer literature, but it should not be used to imply that Darwinian selection at the population (rather than cellular) level is involved in carcinogenesis.
Finally, more recent models have drawn attention to the role of the local or (micro-) environment surrounding the pre-cancerous cells. Model five has two subtypes: models focused on the microenvironment and the model that is based on the theory of morphostats/morphostasis. A common pathway in the natural history of the disease is the appearance of focal proliferative lesions that are known to act as precursors for cancer development. It is becoming increasingly apparent that the emergence of such lesions is not a cell-autonomous phenomenon but is heavily dependent on microenvironmental cues derived from the surrounding tissue (22). The resulting altered tissue architecture translates into the emergence of a unique tumour microenvironment inside these lesions, associated with altered blood vessels and/or blood supply that in turn can trigger biochemical and metabolic changes fuelling tumour progression (23). The theory of morphostats is related to the microenvironment one and is based on observations from embryogenesis. Morphogenetic fields organize tissue morphology in the embryo. Similarly, morphostatic fields maintain normal cell behaviour and normal tissue microarchitecture in the adult. According to this theory, cancer occurs much more frequently when morphostatic influences fail (metaplasia) or at the junction of two different morphostatic fields (24–26).
Clearly, this is a still provisional and rough classification of models, even perhaps a caricature, and there is temporal and conceptual overlap among them. The initiation–promotion model (with mutations as early steps and cell proliferation as a later step) still has important explanatory and predictive power, and there is a Darwinian struggle among competing models (or a competition among ‘paradigms’ in the Kuhnian sense). Also, we do not consider a few other components of the process of carcinogenesis, which, however, are relevant to one or the other of our models: DNA repair, the relationship between ageing and DNA repair and the role of microRNA or copy number variation. The aim is not completeness but to be thought provoking. Finally, the role of inflammation needs to be considered, as this mechanism has proved to be quite important in recent investigations (27). Therefore, we will suggest a way to accommodate inflammation into the models we propose. We were not exhaustive in terms of exposures to carcinogens. For example, we have not gone into details for some specific exposures, such as fibres (e.g. asbestos) because these pose specific challenges concerning mechanisms, which, however, do not invalidate the overall conclusions of the paper.
Mathematical representations
For some of the biologic models, there are also families of mathematical models. In fact, studying time relationships in carcinogenesis has provided important hints on putative mechanisms. Armitage et al. (2) proposed an early (and successful) model, which hypothesized that a power function of age describes the increase of cancer rates with time and that the power integer corresponds to the number of mutations needed to cause a cancer. Thus, it clearly belongs to a mutational class. Knudson's two-hit model (28) was the founder of type-2 models (genome instability).
Subsequent research on mathematical models of carcinogenesis became more sophisticated and introduced the concept of clonal expansion of mutated cells, leaning towards model 3. The latter approaches (29) were based on two assumptions: (i) that carcinogenesis could be described as the effect of ‘initiation’ (mutation) and ‘promotion’ (the latter being interpreted as clonal expansion of initiated cells) and (ii) that carcinogens could be distinguished into those affecting ‘early stages’, with an irreversible effect and those affecting ‘late stages’, with a reversible effect. Such distinctions were largely based on a wealth of experimental research conducted in the previous decades, that showed that initiation—usually with strong carcinogens like PAH—was likely to be due to ‘mutations’, whereas promotion was probably based on ‘non-genotoxic’ mechanisms. Work by Pierce (30), in this regard should be acknowledged.
An early-stage carcinogen was defined as an exposure whose cessation was not followed by a decrease in cancer risk but in fact no real human early-stage carcinogen has ever been clearly identified, except possibly radiation. A controversial example is asbestos, which causes pleural mesothelioma as late as 40 years after first exposure, with little or no change in risk after cessation of exposure. However, this is now believed to be a consequence of the persistent physical presence and lack of clearance of asbestos fibres in the pleura. In contrast, many late-stage carcinogens have been identified: clear examples are arsenic and sex hormones, the carcinogenic effects of which decline rapidly after cessation/reduction of exposure. In the case of tobacco and lung cancer, Doll et al. (3) and others (31,32) have proposed that tobacco—which is a mixture of many different chemicals—may act both early and late because quitting is followed by a reduction in risk, which, however, does not decline to the same level of risk as lifetime non-smokers (33).
The best representation of the third set of models is that of Moolgavkar (29), also known as the two-stage clonal expansion model. In two-stage clonal expansion, normal stem cells can be transformed into cells of an intermediate form at a stochastic event rate μ1 (the first mutation rate). These intermediate cells can divide into two further intermediate cells at a stochastic rate a, then die or differentiate at rate β. In addition, intermediate cells can divide into one intermediate and one malignant cell with a second stochastic event rate μ2. The malignant cells are assumed to develop into a tumour after a deterministic lag time.
In the fourth set of models (these overlap extensively with the third), promotion can be reinterpreted as a somatic cell selection process, i.e. the advantage that mutated cells acquire in comparison with normal cells. Such an interpretation has been proposed in several papers and, recently, in a successful analysis of colon carcinogenesis by Beerenwinkel et al. (34)—based on models developed by Nowak (21). They postulate that cell selection—in addition to mutation—is a driving force in carcinogenesis, according to the following equation:
where s is the selective advantage of cells, Ninit is the initial and Nfin the final, number of cells in the tumour, u is the mutation rate in the relevant genes and d is the number of putative genes involved in the process; finally, tk is the time necessary for a malignant cell to evolve. By fitting the model to data on colon carcinomas, they provided estimates that suggest that the number of genes involved is ∼20, Ninit is 107, Nfin is 109, u is 10−7 and the best estimate for s (selective advantage) is 0.1. Thus, they concluded that selective advantage is much more important than the mutation rate in driving carcinogenesis.
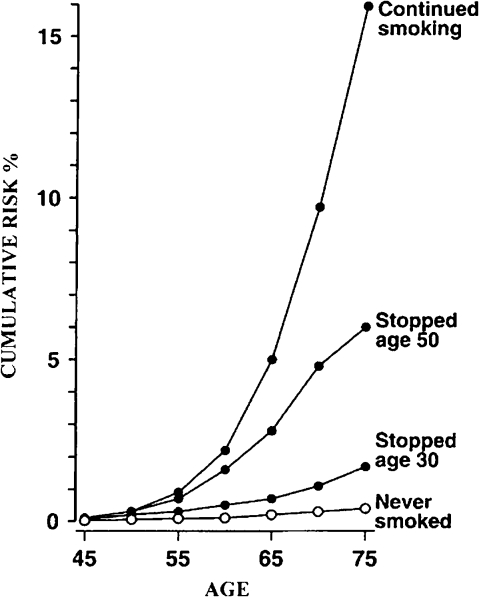
To exemplify the type of data that has been used to fit different families of models, we consider the example of tobacco smoke and lung cancer (Figure 1). Continuing smokers have a cumulative risk of lung cancer of ∼16% in their lifetime; quitting at age 50 years is associated with a cumulative risk by age 75 of 6%; for those quitting at age 30, the cumulative risk is 2%; never smokers have <1% cumulative risk (33). This pattern can be (and has been) interpreted with all classes of mathematical models described above, and fitting empirical data does not allow the identification of one best-fitting model. Clonal expansion (called selection in Nowak's model) is likely to be the driving force because the carcinogenic process seems, at least in part, to be reversible, i.e. quitting smoking ‘freezes’ the risk, a result that would not occur if (irreversible) mutations were to explain the process entirely. However, one should consider that the cumulative risk of lung cancer in smokers and ex-smokers also reflects exposure to other risk factors and the interaction of these with smoking. Moolgavkar's and Nowak's models have similarities in that they postulate a first mutation or set of mutations that leads to intermediate cells, then a predominant process of clonal expansion (‘selection’), followed by a second mutation or set of mutations that leads to the cancer phenotype (invasiveness and metastasis).
Fig. 1.
Cumulative risk of lung cancer according to different smoking habits from ref. (33).
The effects of cigarette smoke are well documented. Tobacco smoke contains DNA-reactive carcinogens, such as nitrosamines, PAHs and pyrolysis products such as carbolines. Enhancing and promotional factors, e.g. catechols, other phenols and terpenes, are also important constituents. Cigarette smoke is a combination of genotoxic and non-genotoxic agents. Experimental results show the active participation of promoters and co-carcinogens as selective agents, in addition to mutagens, in the induction of lung cancer by cigarette smoking.
Model 4: can an evolutionary theory unify models 1 to 3?
Carcinogenesis, at least for some types of cancer, can be interpreted as the consequence of selection of mutated cells similar to the process, in evolutionary theory, that occurs at the population level. Instead of considering a population of organisms, we can refer to a population of cells within multicellular organisms. This interpretation of carcinogenesis (model 4 in our scheme above) is not new, having been proposed by several authors since the 1970s (35–39). In addition, as reading these papers makes clear, cell selection was an integral part of mutational theory because mutation alone was incapable of producing experimental cancers if not followed by cell selection. However, the stress on cell selection characterizes more recent interpretations (as opposed e.g. to the Arrmitage–Doll model) and the Darwinian paradigm may become a unifying theory that explains several biologic phenomena, as Nowak et al. (40) has suggested.
The key concept here is fitness of mutated cells versus normal cells. Ronald Fisher (41) described in a mathematical form the relationship between fitness and time variables (Fisherian fitness):
where Xi is the frequency of the phenotype i in a population and t denotes time. Fitness of the phenotype i is inversely proportional to the frequency at the onset and is directly proportional to the change of the frequency over time. Fitness depends on a set of heritable properties, ai, and environmental factors, E (with vectorial notation). The fitness propensity of an individual (in this case, a stem cell) X, in an environment E, is determined by the expected number of descendants that X will leave in E. Similarities with Nowak–Beerenwinkel's model are obvious.
It has been shown that F-fitness is appropriate in a population with exponential growth (typically, a culture of bacteria with unrestricted space and abundant nutritional support, daughter cells multiplying from a stem cell or cancer cells). This is the case in which the effect of Darwinian selection is observed most evidently (‘survival of the fittest’). When growth is less than exponential (because of limited fuel, space limitations or specific biologic constraints in the cells themselves), Michod and others (42,43) have shown that ‘any’ phenotype can evolve, i.e. new types can increase regardless of their individual capacities (‘fitness of anybody’).
If we accept the Darwinian model of carcinogenesis, in addition to mutagens, we have to introduce the concept of ‘selectogens’, i.e. exposures that select mutated cells, which will have a selective advantage in those circumstances (44).
Examples of selectogens
Beta-carotene supplementation in smokers and lung cancer.
Unexpected results were obtained in randomized controlled trials when heavy smokers were treated with high doses of beta-carotene (which were stopped early for ethical reasons). Cancer occurrence was in fact higher in beta-carotene-treated subjects. No clear explanation has been put forward for this observation, except, perhaps, by one of us (45), who suggested a role for clonal cell selection; the possibility is that certain cells carry mutations, induced by tobacco smoke, that confer a selective advantage in the presence of a single agent, in this case beta-carotene, i.e. the mutated cells have a greater advantage because, for instance, the agent suppresses replication of normal cells but not of cells with specific (but currently unknown) mutations. One parallel is with single-agent chemotherapy, which is known to allow clonal escape and to select malignant cells, whereas polytherapy reduces the likelihood of this occurrence (45). Several vitamins and micronutrients have generally beneficial and protective effects towards several chronic diseases, that have been attributed for example to their antioxidant properties but they also induce mitosis through an action on cell cycle progression and suppress apoptosis (46,47). Such mechanisms can help explain the largely unanticipated results of these trials, as well as the inconsistent results of studies on supplementation with other micronutrients, e.g. flavonoids (48).
Folate and colon cancer.
The Aspirin–Folate Polyp Prevention Study has recently reported that folic acid supplementation (1 mg/day) for up to 6 years in subjects with previous colorectal adenomas (n = 1021) did not prevent the recurrence of colorectal adenomas (relative risk = 1.04). However, in this study, folic acid supplementation significantly increased the number of adenomas by 44% (relative risk = 1.44; 95% CI = 1.03–2.02) and non-significantly increased the incidence of advanced adenomas with a high malignant potential compared with placebo (49). It also resulted in a considerably elevated incidence of prostate cancer. One explanation for this unexpected observation is that folic acid supplementation promotes the progression of already existing, undiagnosed pre-neoplastic lesions or adenomas missed on initial colonoscopy in these predisposed patients at high risk of developing adenomas and colo-rectal carcinoma; as one of us argued in response to these findings, the timing of exposure (before or after the emergence of abnormal cells) can determine the likelihood of benefit or harm (50). This hypothesis is supported by prior observations that addition of folate to established tumours causes an ‘acceleration phenomenon’ in humans. In the 1940s, children treated with folate supplementation experienced an accelerated progression of acute leukaemia and there are data to show similar phenomena in rats (50, 51).
From the same trial of folic acid in adenoma patients, an unexpected excess of prostate cancer was reported (49), with 24 cases (7.3%) in the folic acid group and 9 cases (2.8%) in the placebo group (P = 0.01).
Hyperinsulinemia, peripheral resistance to insulin and cancer.
There is increasing evidence that cancers at different sites (particularly breast, colon, pancreas and prostate) are associated with mechanisms that include hyperinsulinemia, peripheral resistance to insulin and increased production of insulin-like growth factor-1 (52,53). These mechanisms are not directly mutagenic. A possible explanation for the increased risk of cancer is that such mechanisms increase the proportion of cells that undergo mitosis (mitogenic effect). In fact, insulin has several actions including regulation of cell growth, differentiation and metabolism (54). These varied biologic effects of insulin result from the activation of a wide array of intracellular signalling proteins involved in multiple post-receptor pathways (55). Binding of insulin to its receptor activates a tyrosine kinase; such signalling, in turn, upregulates two pathways that lead to the activation of either extracellular-signal-regulated kinase 1/2 or phosphatidylinositol 3-kinase (56). Activation of both pathways has been implicated in the mitogenic effect of insulin in different cell types, whereas metabolic responses elicited by insulin are more closely linked only to the phosphatidylinositol 3-kinase pathway (57). Mutated cells in the colon or pancreas (and, in particular, cells with specific mutations) may be more sensitive than others to the pro-mitogenic activity of insulin (58) accounting, at least in part, for the observed association between hyperinsulinemia and cancer.
Prostate cancer and anti-androgenic therapy.
The international variation in prostate cancer incidence is now largely a function of the use of prostate specific antigen as a screening test and of early-detection practices. However, it is relevant that this international variation is due largely to differences in the occurrence of invasive, infiltrating forms of prostate cancer. Where information on prostate specific antigen-testing patterns is available, the prevalence of latent cancer (detectable with prostate specific antigen) shows much less geographic variation than clinical cancer (59). This observation is important in the light of recent evidence for the ability of anti-androgen therapy (finasteride) to increase more aggressive, infiltrating cancers (60). In a randomized trial, although finasteride reduced prostate cancer incidence overall (a 25% reduction on the active arm), the incidence of Gleason grades ≥7 was higher in the finasteride-treated group (37%) than in the control group (22%, P < 0.001). However, it has been more recently suggested that the observation could be an artefact due to improved diagnosis in men taking finasteride (61).
One possible explanation is that finasteride suppresses androgen-responsive cells and thereby gives androgen-independent cells an advantage. Geographic variation of prostate cancer—which, as noted above, is almost entirely due to infiltrating, high-grade tumours—might be due to environmental agents/behaviours that interfere with androgen metabolism and select more aggressive cells. Unfortunately, the epidemiology of prostate cancer is very little understood. Although the available evidence suggests an involvement of androgens as a risk factor, the distinction between rapidly progressive and clinically more benign tumours has not been made in either epidemiologic or molecular studies (62).
Resistance to chemotherapy.
A recent study has shown that lung cancer cells acquire resistance to the chemotherapeutic agents, Gefitinib or Erlotinib, by developing a double mutation in the epidermal growth factor receptor gene (63). A first, gain-of-function, mutation was already known to confer increased sensitivity to these drugs. The second mutation in the same gene, on the other hand, confers resistance and, in fact, a selective advantage on the cells that carry it. Treatment with Gefitinib or Erlotinib allows doubly mutated (resistant) clones, which otherwise would be extremely rare, to grow out. Although the probability of a double mutation in the same gene occurring spontaneously is extremely low, selection of such mutated clones ultimately makes cells with the double mutation common in the treated cancers.
Resistance to chemotherapy and to carcinogens is now interpreted by some more in terms of gross changes in chromosome number (aneuploidy) or chromosome aberrations (instability) rather than of point mutations (64). The genetic variability permitted as a result of variable chromosome numbers and increased genomic instability is much greater and allows more flexible adaptation to sudden environmental stresses (65), perhaps especially in situations where, as noted above, growth is less than exponential (42).
Cell death contributes to selection of resistant cells.
One of us has conducted an experiment that follows similar lines (66), in the sense that cell death can be a first step for the subsequent selection of damaged cells. We took advantage of cells treated with different carcinogens to assess whether the ‘genetic instability phenotype’ was carcinogen specific. The arylamine 4-aminobiphenyl (4-ABP) is a tobacco smoke constituent, an environmental contaminant, and an established human carcinogen. Bladder, lung, colon and breast cancers have been associated with 4-ABP. We investigated the effects of 4-ABP and N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) on colorectal (HCT116) and bladder (RT112) cancer cells. Cells were treated with carcinogens to generate resistant clones that were then analysed to establish whether they displayed either chromosomal instability (CIN) or microsatellite instability (MIN). We found that 50–60% of cells treated with 4-ABP developed CIN as confirmed by their ability to gain and lose chromosomes, but none developed MIN. In contrast, all MNNG-treated clones (12/12) developed MIN as shown by the microsatellite assay, but none developed CIN. Because MIN has previously been linked to mismatch-repair defects, we used western blotting to analyse the level and pattern of expression of MLH1 and MSH2 in clones resistant to the carcinogens. The results showed that the acquired mechanism of MIN resistance in the MNNG-treated colorectal cells is associated with the reduction or the complete loss of MLH1 expression, strongly suggesting an epigenetic selection process, not mutation, as the cause of the different outcomes.
A similar study had been performed earlier by Bardelli et al. (67) with the bulky-adduct-forming agent 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. They found that cells resistant to 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine exhibited CIN, whereas cells resistant to MNNG exhibited MIN, as in our experiment. These data demonstrate that exposure to specific carcinogens can, indeed, select for tumour cells with distinct forms of genetic instability via non-genotoxic mechanisms.
Epigenetics: promoter methylation.
Another component of the third and possibly fourth paradigm (Table I) involves epigenetics (functional changes in DNA expression driven by methylation or histone changes, for instance, rather than structural changes like mutations). This hypothesis, supported by both experimental and epidemiologic data, might sooner or later be unified with the Darwinian interpretation suggested above. DNA methylation [the covalent addition of methyl groups (-CH3) to cytosine that precedes a guanosine in the DNA sequence (the CpG dinucleotide)] occurs naturally and has a role in suppressing gene expression, including suppression of incorporated viral and other parasitic sequences. Methylation is an epigenetic modification; it does not change the structure of DNA but the patterns of silencing and expression ‘are heritable’ and pass from one generation of cells to the next in the same organism (there may be rare occasions on which such epigenetic modifications actually pass through the germ line (68) but usually all methylation marks are eliminated very early in embryonic development and reinstated later). Hypermethylation of promoter regions is associated with gene transcriptional silencing and is a common mechanism for the inactivation of TSGs that allows cells a selective growth advantage in cancer. Hypermethylation is known to be associated with the inactivation of several pathways involved in the cancer process, such as DNA repair (hMLH1, BRCA1 and MGMT), cell cyle regulation (p16), apoptosis and carcinogen metabolism (69).
The effects of dietary folate deficiency on methylation patterns may explain an important part of the relationship between diet and cancer. Mechanistic evidence, however, remains sparse. Nutritional changes during pregnancy could also interfere with the subsequent cancer risk through methylation patterns. Relevant experiments have been completed in mice. The dark (agouti) versus yellow colour of agouti mice hair is determined by methylation patterns. If the agouti gene terminal-repeat region is hypermethylated, the mouse is agouti; if it is hypomethylated, the mouse is yellow. When pregnant mice were fed a diet rich in folate and methionine (i.e. high in methyl groups), none of the pups was yellow and the colour was a fixed phenotype. Further, the expression of the yellow coat was linked to an increased risk of obesity, adult diabetes, cancer and mortality. In other words, intrauterine exposure to nutrients associated with epigenetic modifications of the genome in the offspring can lead to increased cancer risk (70).
Example: DNA methylation, lung cancer and smoking
To return to the example of lung cancer, the effect of quitting smoking suggests that epigenetic events are more important than mutations. The involvement of gene methylation is therefore a likely theory to explain the action of tobacco smoke constituents. Several genes are commonly the target of promoter hypermethylation in lung cancer, including p16 (p16INK4a/CDKN2A), DAPK, RARβ, RASSF1 and O6MGMT (a DNA repair gene). Both current and former smoking are associated with aberrant p16, DAPK, RASSF1A and RARβ methylation. In a prospective study, promoter hypermethylation of multiple genes (including those mentioned above) in the sputum was able to predict lung cancer onset with sensitivity and specificity of 64% (71). The role that environmental toxicants might play in TSG hypermethylation is a fertile field for research, analogous to earlier work on the induction of somatic mutations in p53 and oncogenes by chemical carcinogens. Some evidence has been published that environmental agents including metals, cigarette smoking, alcohol and many others may induce hypermethylation of TSGs, suggesting a new molecular mechanism for the carcinogenic effects of environmental agents (72).
It remains to be clarified how an epigenetic mechanism could contribute to a unifying Darwinian theory (model 4) of carcinogenesis.
Model 5: tissue disorganization
There is a feature of evolution that has been neglected, except in developmental studies: self-organization of the living organism. In fact, the contemporary theory of evolution encompasses two major components: selection–adaptation and self-organization (the latter very often overlooked).
One of us has previously observed that adult tissues ‘need to solve the same problems as embryonic tissue: maintaining form even as constituent cells proliferate, move, differentiate and die’. And that the ‘maintenance of epithelial tissues requires, like morphogenesis, a method of relating cell position to function. A morphogenetic field is an evolutionarily well-tried mechanism’ (25). The most obvious feature of cancer, at the tissue level, is the disorganization of microarchitecture, a consequence, we have postulated, of disruption of morphostats, the analogue, in adult-tissue maintenance of morphogens in their role as organizers of tissue morphology and development in the embryo. Evidence for the relevance of morphostats in cancer aetiology comes from, inter alia, the finding that cancer arises more readily in tissues where morphostatic fields have failed, in tissues removed from normal morphostatic influences, and in areas situated at the junction of tissues, where morphostatic fields compete or conflict. Morphostats most plausibly originate in stem cells and in stromal cells that are adjacent to epithelia (25).
Elsewhere, in further exploration of this hypothesis, we have recently built a computer simulation of morphostats, based on simple plausible assumptions about cell renewal: we have shown that disruption of a morphostatic gradient in stroma, with no mutation at all in the epithelium, can generate epithelial cancer precursors (73). This mathematical model is consistent with the possibility that the genetic and epigenetic changes in tumours could arise after the formation of a clone of abnormal cells that has itself arisen as a result of a failure of the morphostatic control of the microarchitecture of mature tissues.
There is a considerable literature consistent, to varying degrees, with these findings including work from Sonnenschein et al. (74), Pierce (30), Bissell et al. (22), Prehn (75) and, most recently, Bizarri et al. (76). Although it is speculative, at this point, to tie together models 4 and 5, both the role of morphostats in maintaining adult-tissue organization and the associated mathematical model are entirely consistent with the concepts in model 4 with, in the case of model 5, the loss of morphostatic control acting as the selectogen.
A model of carcinogenesis based on self-organization has been proposed by Laforge et al. (77) on the basis of Prigogine's theory. The basic idea is that instead of leading to on and off switches, the concentration of transcriptional regulators in cells increases or decreases the probability of gene expression. Based on computer simulations, the authors show that tissue coordination includes at least two basic components: phenotypic autostabilization (differentiated cells stabilize their own phenotype) and interdependence for proliferation (differentiated cells stimulate the proliferation of alien phenotypes). A quantitative equilibrium between the parameters controlling these two processes is proposed, with an imbalance leading to tissue disorganization and invasive cancer-like growth (77).
The case of inflammation
Inflammation has gained momentum among the different mechanisms underlying carcinogenesis. We believe it can be used as a paradigmatic example of how different models of carcinogenesis in fact overlap or cooperate. Chronic inflammation is now acknowledged as a major cause of cancer. Mechanisms involved in inflammation-induced cancer include reactive oxygen and nitrogen species, inflammatory cytokines, prostaglandins and specific microRNAs. The activity of these mediators is associated with changes in cell proliferation, cell death, cellular senescence, DNA mutation rates, DNA methylation and angiogenesis (27). Therefore, it seems that inflammation is involved in many of the steps described by Hanahan and Weinberg as constitutive of carcinogenesis, and its action is compatible with our model 4 as encompassing mutation, CIN, cell proliferation and epigenetics. Inflammation, too, is a major cause of disruption of tissue microarchitecture: e.g. ulcerative colitis, Barrett oesophagus and inflammatory atrophic changes that occur in stomach and prostate, all of which are known pre-cancerous lesions.
The case of radiation
Also ionizing radiation is a special case and deserves some comments. It is not included in Table I mainly because ionizing radiation ticks most of the boxes referring to our five models. Irradiated cells show mutations (model 1) as well as genomic instability (model 2). Also adjacent non-irradiated cells show genomic instability, the so-called ‘bystander effect’ (corresponding to model 5, if one interprets the indirect effect on surrounding tissues as an example of tissue disorganization). Referring to the thyroid tumours observed in the Chernobyl population, the author of a recent review suggests that oncogenic rearrangements commonly involve both TSGs (or DNA repair genes) as well as oncogenes (78). The Two- (or Three-) Stage Clonal Expansion model has been successfully applied to lung cancer in uranium miners (79), whereas the importance of the microenvironment (model 5) has been described in experimental studies (80). Finally, a systematic presentation of different biologically based mathematical models has been recently published, suggesting that observations in humans are compatible with several of our models, in the context of a ‘systems biology’ approach (81).
Conclusions and perspectives
At least five coherent models of carcinogenesis have been proposed in the history of cancer research, as summarized in Table I. There is some degree of both conceptual and temporal overlap among them. The question is whether they could be subsumed into a unified view of the cancer process. Science moves forward when a new theory emerges that explains not only previously unexplained findings but also all of the phenomena already explained.
First, the five models can probably be included into a simpler scheme, i.e. two types of models: (i) biological changes in the epithelium alone lead to malignancy and (ii) changes in stroma/extracellular matrix are necessary (along with changes in epithelium) for malignancy. Models 1, 2 and 3 are all variants of A, whereas model 5 is equal to B with two potential versions: (i) tissue disruption as well as DNA changes in both stroma and epithelium are important, which is consistent with the morphostats theory and (ii) DNA mutations are not primary, but may be secondary epiphenomena, whereas tissue disruption is critical. The nature of ‘disruption’, however, is not entirely clear. The key aspect of model 5 is the role of microenvironment/stroma and morphostatic control of tissue architecture.
Within Group A (models 1, 2 and 3), the first two have much in common, and keeping them separate has mainly a historical justification; model 3 involves epigenetics and does differ from 1 and 2. By introducing such distinctions, in fact, we do not reject the classical ‘initiation–promotion’ theory, which has had a central role in the history of carcinogenesis, but we clarify that such theory has been interpreted in different ways. In fact, initiation and promotion would seem to be a combination of models 1/2 with 3.
After these clarifications, we believe that a Darwinian interpretation of carcinogenesis—as described here—might become the unifying view. First, a Darwinian selection theory does unify models 1, 2 and 3. It is in fact compatible with both a mutational theory of carcinogenesis and the role of epigenetics. In addition, it is compatible with the increasingly clear role of cell selection/clonal expansion. And, second, it could help explain many unclear epidemiologic findings, currently not easily assigned to mutations or chromosome aberrations, especially the effects of dietary components or hormones.
A Darwinian model based on mutation/selection is not as easy to reconcile with model 5. This last model links up with modern nonlinear dynamics/chaos/complexity theories, bringing a different broad perspective involving tissue-to-tissue interactions, their potential disruption, spatial-structural organization and disorganization, all elements that are not really part of models 1 to 3. However, there is a way to reconcile the Darwinian interpretation also with model 5, through the concept of self-organization of the living being. Both the selection–adaptation component and the self-organization component (the latter very often overlooked) actually belong to the current theory of evolution. The work on embryonic development and on the genes that control organ formation and that orchestrate the growth of different types of cells is now a central component of the evolutionary theory (82) and is highly relevant to carcinogenesis.
Whether or not a unified view of carcinogenesis, which encompasses the broad views A and B above and the two components of Darwinian theory, mutation/selection and self-organization, is viable will be judged by the next wave of cancer research.
Acknowledgments
Part of this paper was presented at the Seminar on Causality Models in Medicine, 1 April 2008, University of Geneva. We thank an anonymous reviewer for thoughtful suggestions and criticisms.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- 4-ABP
arylamine 4-aminobiphenyl
- CIN
chromosomal instability
- MIN
microsatellite instability
- MNNG
N-methyl-N′-nitro-N-nitrosoguanidine
- PAH
polycyclic aromatic hydrocarbon
- TSG
tumour suppressor gene
References
- 1.Hanahan D, et al. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Armitage P, et al. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br. J. Cancer. 1954;8:1–12. doi: 10.1038/bjc.1954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doll R, et al. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J. Epidemiol. Community Health. 1978;32:303–313. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IARC. IARC monographs on the evaluation of the carcinogenic risk of chemicals to man: some miscellaneous pharmaceutical substances. IARC Monogr. Eval. Carcinog. Risk Chem. Man. 1977;13:1–255. [PubMed] [Google Scholar]
- 5.McCann J, et al. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals: discussion. Proc. Natl Acad. Sci. USA. 1976;73:950–954. doi: 10.1073/pnas.73.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parada LF, et al. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature. 1982;297:474–478. doi: 10.1038/297474a0. [DOI] [PubMed] [Google Scholar]
- 7.Phillips DH. DNA adducts as markers of exposure and risk. Mutat. Res. 2005;577:284–292. doi: 10.1016/j.mrfmmm.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Slaga TJ. Multistage skin carcinogenesis: a useful model for the study of the chemoprevention of cancer. Acta Pharmacol. Toxicol. (Copenh.) 1984;55(suppl. 2):107–124. doi: 10.1111/j.1600-0773.1984.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 9.Knudson A. Retinoblastoma: teacher of cancer biology and medicine. PLoS Med. 2005;2:e349. doi: 10.1371/journal.pmed.0020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohmann DR. RB1 gene mutations in retinoblastoma. Hum. Mutat. 1999;14:283–288. doi: 10.1002/(SICI)1098-1004(199910)14:4<283::AID-HUMU2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.Cairns J. Familial cancers. Nature. 1984;307:116. doi: 10.1038/307116c0. [DOI] [PubMed] [Google Scholar]
- 12.Duesberg P, et al. Aneuploidy and cancer: from correlation to causation. Contrib. Microbiol. 2006;13:16–44. doi: 10.1159/000092963. [DOI] [PubMed] [Google Scholar]
- 13.Guimaraes DP, et al. TP53: a key gene in human cancer. Biochimie. 2002;84:83–93. doi: 10.1016/s0300-9084(01)01356-6. [DOI] [PubMed] [Google Scholar]
- 14.Norppa H, et al. Chromosomal aberrations and SCEs as biomarkers of cancer risk. Mutat. Res. 2006;600:37–45. doi: 10.1016/j.mrfmmm.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Rowley PT. Inherited susceptibility to colorectal cancer. Annu. Rev. Med. 2005;56:539–554. doi: 10.1146/annurev.med.56.061704.135235. [DOI] [PubMed] [Google Scholar]
- 16.Cairns J. The Leeuwenhoek Lecture, 1978. Bacteria as proper subjects for cancer research. Proc. R. Soc. Lond. B Biol. Sci. 1980;208:121–133. doi: 10.1098/rspb.1980.0046. [DOI] [PubMed] [Google Scholar]
- 17.Loeb LA. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991;51:3075–3079. [PubMed] [Google Scholar]
- 18.Fearon ER, et al. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 19.Greaves MF. Biological models for leukaemia and lymphoma. IARC Sci. Publ. 2004:351–372. [PubMed] [Google Scholar]
- 20.Greaves M. Darwinian medicine: a case for cancer. Nat. Rev. Cancer. 2007;7:213–221. doi: 10.1038/nrc2071. [DOI] [PubMed] [Google Scholar]
- 21.Nowak M. Evolutionary Dynamics: Exploring the Equations of Life. Cambridge: Harvard University Press; 2006. [Google Scholar]
- 22.Bissell MJ, et al. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59:1757s–1763s. discussion 1763s–1764s. [PubMed] [Google Scholar]
- 23.Laconi E, et al. The microenvironments of multistage carcinogenesis. Semin. Cancer Biol. 2008;18:322–329. doi: 10.1016/j.semcancer.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Potter JD. Morphostats: a missing concept in cancer biology. Cancer Epidemiol. Biomarkers Prev. 2001;10:161–170. [PubMed] [Google Scholar]
- 25.Potter JD. Morphogens, morphostats, microarchitecture and malignancy. Nat. Rev. Cancer. 2007;7:464–474. doi: 10.1038/nrc2146. [DOI] [PubMed] [Google Scholar]
- 26.van den Brink GR, et al. The morphogenetic code and colon cancer development. Cancer Cell. 2007;11:109–117. doi: 10.1016/j.ccr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Schetter AJ, et al. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc. Natl Acad. Sci. USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moolgavkar S, et al. Two-event models for carcinogenesis: incidence curves for childhood and adult tumors. Math. Biosci. 1979;47:55–77. [Google Scholar]
- 30.Pierce GB. Relationship between differentiation and carcinogenesis. J. Toxicol. Environ. Health. 1977;2:1335–1342. doi: 10.1080/15287397709529534. [DOI] [PubMed] [Google Scholar]
- 31.Brown CC, et al. Use of multistage models to infer stage affected by carcinogenic exposure: example of lung cancer and cigarette smoking. J. Chronic Dis. 1987;40(suppl. 2):171S–179S. doi: 10.1016/s0021-9681(87)80020-6. [DOI] [PubMed] [Google Scholar]
- 32.Schollnberger H, et al. Analysis of epidemiological cohort data on smoking effects and lung cancer with a multi-stage cancer model. Carcinogenesis. 2006;27:1432–1444. doi: 10.1093/carcin/bgi345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peto R, et al. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321:323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beerenwinkel N, et al. Genetic progression and the waiting time to cancer. PLoS Comput. Biol. 2007;3:e225. doi: 10.1371/journal.pcbi.0030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Gerlache J, et al. Comparison of different models of rat liver carcinogenesis: conclusions from a systemic analysis. Toxicol. Pathol. 1984;12:374–382. doi: 10.1177/019262338401200412. [DOI] [PubMed] [Google Scholar]
- 36.Barbason H, et al. Variations of liver cell control during diethylnitrosamine carcinogenesis. Eur. J. Cancer. 1977;13:13–18. doi: 10.1016/0014-2964(77)90223-7. [DOI] [PubMed] [Google Scholar]
- 37.Farber E. Carcinogenesis—cellular evolution as a unifying thread: Presidential address. Cancer Res. 1973;33:2537–2550. [PubMed] [Google Scholar]
- 38.Lans M, et al. Phenobarbital as a promoter in the initiation/selection process of experimental rat hepatocarcinogenesis. Carcinogenesis. 1983;4:141–144. doi: 10.1093/carcin/4.2.141. [DOI] [PubMed] [Google Scholar]
- 39.Castelain P, et al. Cell population kinetics and ploidy rate of early focal lesions during hepatocarcinogenesis in the rat. Br. J. Cancer. 1989;60:827–833. doi: 10.1038/bjc.1989.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowak MA, et al. The linear process of somatic evolution. Proc. Natl Acad. Sci. USA. 2003;100:14966–14969. doi: 10.1073/pnas.2535419100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher R. The Genetical Theory of Natural Selection. Oxford: Oxford University Press; 1930. [Google Scholar]
- 42.Michod R. Darwinian Dynamics. Princeton, NJ: Princeton University Press; 2000. [Google Scholar]
- 43.Michod RE, et al. Life-history evolution and the origin of multicellularity. J. Theor. Biol. 2006;239:257–272. doi: 10.1016/j.jtbi.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 44.Finette BA, et al. Accumulation of somatic mutations in proliferating T cell clones from children treated for leukemia. Leukemia. 2001;15:1898–1905. doi: 10.1038/sj.leu.2402306. [DOI] [PubMed] [Google Scholar]
- 45.Potter J. Chemoprevention: pharmacology or biology? Oncology. 1996;10:1487–1488. [Google Scholar]
- 46.Bohnsack BL, et al. Nutrient regulation of cell cycle progression. Annu. Rev. Nutr. 2004;24:433–453. doi: 10.1146/annurev.nutr.23.011702.073203. [DOI] [PubMed] [Google Scholar]
- 47.Watson WH, et al. Diet and apoptosis. Annu. Rev. Nutr. 2000;20:485–505. doi: 10.1146/annurev.nutr.20.1.485. [DOI] [PubMed] [Google Scholar]
- 48.Adams KF, et al. Soy protein containing isoflavones does not decrease colorectal epithelial cell proliferation in a randomized controlled trial. Am. J. Clin. Nutr. 2005;82:620–626. doi: 10.1093/ajcn.82.3.620. [DOI] [PubMed] [Google Scholar]
- 49.Cole BF, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 50.Ulrich CM, et al. Folate and cancer—timing is everything. JAMA. 2007;297:2408–2409. doi: 10.1001/jama.297.21.2408. [DOI] [PubMed] [Google Scholar]
- 51.Hoffbrand AV, et al. The history of folic acid. Br. J. Haematol. 2001;113:579–589. doi: 10.1046/j.1365-2141.2001.02822.x. [DOI] [PubMed] [Google Scholar]
- 52.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am. J. Clin. Nutr. 2007;86:s836–s842. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 53.McKeown-Eyssen G. Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol. Biomarkers Prev. 1994;3:687–695. [PubMed] [Google Scholar]
- 54.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 55.Jensen M, et al. Molecular mechanisms of differential intracellular signaling from the insulin receptor. Vitam. Horm. 2009;80:51–75. doi: 10.1016/S0083-6729(08)00603-1. [DOI] [PubMed] [Google Scholar]
- 56.Muoio DM, et al. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 57.Musi N, et al. Insulin resistance and improvements in signal transduction. Endocrine. 2006;29:73–80. doi: 10.1385/ENDO:29:1:73. [DOI] [PubMed] [Google Scholar]
- 58.Borisov N, et al. Systems-level interactions between insulin-EGF networks amplify mitogenic signaling. Mol. Syst. Biol. 2009;5:256. doi: 10.1038/msb.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oliver SE, et al. International trends in prostate-cancer mortality in the "PSA ERA". Int. J. Cancer. 2001;92:893–8. doi: 10.1002/ijc.1260. [DOI] [PubMed] [Google Scholar]
- 60.Thompson IM, et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 61.Redman MW, et al. Finasteride does not increase the risk of high-grade prostate cancer: a bias-adjusted modeling approach. Cancer Prev. Res. (Phila Pa) 2008;1:174–181. doi: 10.1158/1940-6207.CAPR-08-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Platz EA. Epidemiologic musing on statin drugs in the prevention of advanced prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2007;16:2175–2180. doi: 10.1158/1055-9965.EPI-07-0777. [DOI] [PubMed] [Google Scholar]
- 63.Pao W, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duesberg P, et al. Explaining the high mutation rates of cancer cells to drug and multidrug resistance by chromosome reassortments that are catalyzed by aneuploidy. Proc. Natl Acad. Sci. USA. 2000;97:14295–14300. doi: 10.1073/pnas.97.26.14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breivik J, et al. Genomic instability, DNA methylation, and natural selection in colorectal carcinogenesis. Semin. Cancer Biol. 1999;9:245–254. doi: 10.1006/scbi.1999.0123. [DOI] [PubMed] [Google Scholar]
- 66.Saletta F, et al. Exposure to the tobacco smoke constituent 4-aminobiphenyl induces chromosomal instability in human cancer cells. Cancer Res. 2007;67:7088–7094. doi: 10.1158/0008-5472.CAN-06-4420. [DOI] [PubMed] [Google Scholar]
- 67.Bardelli A, et al. Carcinogen-specific induction of genetic instability. Proc. Natl Acad. Sci. USA. 2001;98:5770–5775. doi: 10.1073/pnas.081082898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chong S, et al. Modifiers of epigenetic reprogramming show paternal effects in the mouse. Nat. Genet. 2007;39:614–622. doi: 10.1038/ng2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esteller M. Epigenetics in cancer. N. Engl. J. Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 70.Waterland RA, et al. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Belinsky SA, et al. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006;66:3338–3344. doi: 10.1158/0008-5472.CAN-05-3408. [DOI] [PubMed] [Google Scholar]
- 72.Reamon-Buettner SM, et al. The next innovation cycle in toxicogenomics: environmental epigenetics. Mutat. Res. 2008;659:158–165. doi: 10.1016/j.mrrev.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Baker SG, et al. Plausibility of stromal initiation of epithelial cancers without a mutation in the epithelium: a computer simulation of morphostats. BMC Cancer. 2009;9:89. doi: 10.1186/1471-2407-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sonnenschein C, et al. The Society of Cells: Cancer and Control of Cell Proliferation. New York: Springer Verlag; 1999. [Google Scholar]
- 75.Prehn RT. Cancers beget mutations versus mutations beget cancers. Cancer Res. 1994;54:5296–300. [PubMed] [Google Scholar]
- 76.Bizzarri M, et al. Beyond the oncogene paradigm: understanding complexity in cancerogenesis. Acta Biotheor. 2008;56:173–196. doi: 10.1007/s10441-008-9047-8. [DOI] [PubMed] [Google Scholar]
- 77.Laforge B, et al. Modeling embryogenesis and cancer: an approach based on an equilibrium between the autostabilization of stochastic gene expression and the interdependence of cells for proliferation. Prog. Biophys. Mol. Biol. 2005;89:93–120. doi: 10.1016/j.pbiomolbio.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Williams D. Radiation carcinogenesis: lessons from Chernobyl. Oncogene. 2008;27(suppl. 2):S9–S18. doi: 10.1038/onc.2009.349. [DOI] [PubMed] [Google Scholar]
- 79.Little MP, et al. Modelling lung tumour risk in radon-exposed uranium miners using generalizations of the two-mutation model of Moolgavkar, Venzon and Knudson. Int. J. Radiat. Biol. 2002;78:49–68. doi: 10.1080/09553000110085797. [DOI] [PubMed] [Google Scholar]
- 80.Barcellos-Hoff MH, et al. Radiation carcinogenesis in context: how do irradiated tissues become tumors? Health Phys. 2009;97:446–457. doi: 10.1097/HP.0b013e3181b08a10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Little MP, et al. Systems biological and mechanistic modelling of radiation-induced cancer. Radiat. Environ. Biophys. 2008;47:39–47. doi: 10.1007/s00411-007-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fox C, et al. Evolutionary Genetics. Concepts and Case Studies. Oxford: Oxford University Press; 2006. [Google Scholar]