Introduction
Slc11a1 (formerly Nramp1) has many pleiotropic effects on macrophage (m ) activation, including regulation of the CXC chemokine KC, interleukin-1β (IL-1β), inducible nitric oxide synthase (iNOS), major histocompatibility complex (MHC) class II molecules, tumour necrosis factor α (TNFα), nitric oxide (NO) release, l-arginine flux, oxidative burst and tumoricidal as well as antimicrobial activity (reviewed by Blackwell and Searle, 1999; Blackwell et al., 2000). A naturally occurring Gly→Asp mutation at amino acid 169 of Slc11a1 makes mice as susceptible to Leishmania donovani, Salmonella typhimurium and Mycobacterium bovis as gene-disrupted mice (Vidal et al., 1995). Hence, the mutation is a functional null. This mutation also confers susceptibility to a range of other pathogens in mice, including Mycobacterium lepraemurium (Brown et al., 1982; Skamene et al., 1984), Mycobacterium intracellulare (Goto et al., 1989), Toxoplasma gondii (Blackwell et al., 1994), Candida albicans (Puliti et al., 1995) and Leishmania infantum (Leclercq et al., 1996). In man, SLC11A1 is linked or associated with multiple infectious (Shaw et al., 1997; Abel et al., 1998; Bellamy et al., 1998; Marquet et al., 1999; Cervino et al., 2000; Gao et al., 2000; Greenwood et al., 2000; Ryu et al., 2000; Mohamed et al., 2001) and autoimmune (Shaw et al., 1996; Hofmeister et al., 1997; Esposito et al., 1998; Maliarik et al., 2000; Sanjeevi et al., 2000; Singal et al., 2000; Yang et al., 2000) diseases. The infectious diseases include viral (HIV), bacterial (tuberculosis, leprosy, meningococcal meningitis) and protozoan (visceral leishmaniasis) pathogens. The autoimmune diseases include rheumatoid arthritis, juvenile rheumatoid arthritis, diabetes, sarcoidosis and Crohn's disease. Mutation in the closely related Slc11a2 (Nramp2) gene causes microcytic anaemia in mice (Fleming et al., 1997), but disease association in man has not been reported. Slc11a1 and Slc11a2 are polytopic integral membrane proteins with 10–12 putative membrane-spanning domains (Vidal et al., 1993; Gunshin et al., 1997). In both, the natural functional null mutation occurs in transmembrane domain 4 (Vidal et al., 1993; Fleming et al., 1997). Both Slc11a1 and Slc11a2 have protein kinase C (PKC) binding sites (Vidal et al., 1993; Barton et al., 1994; Gruenheid et al., 1995), but only Slc11a1 has a Pro–Ser-rich N-terminus (Barton et al., 1994). Here, we review current knowledge on the evolution, function and roles of Slc11a1/SLC11A1 in disease.
) activation, including regulation of the CXC chemokine KC, interleukin-1β (IL-1β), inducible nitric oxide synthase (iNOS), major histocompatibility complex (MHC) class II molecules, tumour necrosis factor α (TNFα), nitric oxide (NO) release, l-arginine flux, oxidative burst and tumoricidal as well as antimicrobial activity (reviewed by Blackwell and Searle, 1999; Blackwell et al., 2000). A naturally occurring Gly→Asp mutation at amino acid 169 of Slc11a1 makes mice as susceptible to Leishmania donovani, Salmonella typhimurium and Mycobacterium bovis as gene-disrupted mice (Vidal et al., 1995). Hence, the mutation is a functional null. This mutation also confers susceptibility to a range of other pathogens in mice, including Mycobacterium lepraemurium (Brown et al., 1982; Skamene et al., 1984), Mycobacterium intracellulare (Goto et al., 1989), Toxoplasma gondii (Blackwell et al., 1994), Candida albicans (Puliti et al., 1995) and Leishmania infantum (Leclercq et al., 1996). In man, SLC11A1 is linked or associated with multiple infectious (Shaw et al., 1997; Abel et al., 1998; Bellamy et al., 1998; Marquet et al., 1999; Cervino et al., 2000; Gao et al., 2000; Greenwood et al., 2000; Ryu et al., 2000; Mohamed et al., 2001) and autoimmune (Shaw et al., 1996; Hofmeister et al., 1997; Esposito et al., 1998; Maliarik et al., 2000; Sanjeevi et al., 2000; Singal et al., 2000; Yang et al., 2000) diseases. The infectious diseases include viral (HIV), bacterial (tuberculosis, leprosy, meningococcal meningitis) and protozoan (visceral leishmaniasis) pathogens. The autoimmune diseases include rheumatoid arthritis, juvenile rheumatoid arthritis, diabetes, sarcoidosis and Crohn's disease. Mutation in the closely related Slc11a2 (Nramp2) gene causes microcytic anaemia in mice (Fleming et al., 1997), but disease association in man has not been reported. Slc11a1 and Slc11a2 are polytopic integral membrane proteins with 10–12 putative membrane-spanning domains (Vidal et al., 1993; Gunshin et al., 1997). In both, the natural functional null mutation occurs in transmembrane domain 4 (Vidal et al., 1993; Fleming et al., 1997). Both Slc11a1 and Slc11a2 have protein kinase C (PKC) binding sites (Vidal et al., 1993; Barton et al., 1994; Gruenheid et al., 1995), but only Slc11a1 has a Pro–Ser-rich N-terminus (Barton et al., 1994). Here, we review current knowledge on the evolution, function and roles of Slc11a1/SLC11A1 in disease.
Localization and tissue distribution
Slc11a1 localizes to membranes of late endosomes and lysosomes in m (Gruenheid et al., 1997; Searle et al., 1998), but not to early endosomes (Gruenheid et al., 1997). This is consistent with the presence of endocytic targeting signals in the 5′ and 3′ ends of Slc11a1 (Atkinson et al., 1997) and suggests that it targets directly from the trans-Golgi network (TGN) to the endosomal compartment rather than becoming incorporated into the phagosome membrane as part of the phagocytic process. Mutant m
(Gruenheid et al., 1997; Searle et al., 1998), but not to early endosomes (Gruenheid et al., 1997). This is consistent with the presence of endocytic targeting signals in the 5′ and 3′ ends of Slc11a1 (Atkinson et al., 1997) and suggests that it targets directly from the trans-Golgi network (TGN) to the endosomal compartment rather than becoming incorporated into the phagosome membrane as part of the phagocytic process. Mutant m express a protein recognized by both anti-N-terminal and anti-C-terminal anti-Slc11a1 antibodies, but the protein is expressed at lower levels in mature bone marrow-derived m
express a protein recognized by both anti-N-terminal and anti-C-terminal anti-Slc11a1 antibodies, but the protein is expressed at lower levels in mature bone marrow-derived m from mutant C57BL/10ScSn mice compared with their wild-type congenic B10.L-Lshr counterparts (Searle et al., 1998). Expression at the mRNA and protein (Searle et al., 1998) levels is upregulated to an equivalent degree in mutant and wild-type m
from mutant C57BL/10ScSn mice compared with their wild-type congenic B10.L-Lshr counterparts (Searle et al., 1998). Expression at the mRNA and protein (Searle et al., 1998) levels is upregulated to an equivalent degree in mutant and wild-type m after treatment with lipopolysac-charide and interferon (IFN)-γ. This is accompanied by an increased proportion of Slc11a1 gold labelling in electron-dense lysosomes compared with the electron-lucent late endosomal compartment (Searle et al., 1998), but the functional significance of this redistribution of the protein has not been addressed. Slc11a1 targets to Leishmania or Mycobacterium avium phagosomes (Searle et al., 1998). Vesicles recognized by anti-Slc11a1 antibodies rapidly migrate to fuse with M. avium-containing phagosomes in resting wild-type but not mutant bone marrow-derived m
after treatment with lipopolysac-charide and interferon (IFN)-γ. This is accompanied by an increased proportion of Slc11a1 gold labelling in electron-dense lysosomes compared with the electron-lucent late endosomal compartment (Searle et al., 1998), but the functional significance of this redistribution of the protein has not been addressed. Slc11a1 targets to Leishmania or Mycobacterium avium phagosomes (Searle et al., 1998). Vesicles recognized by anti-Slc11a1 antibodies rapidly migrate to fuse with M. avium-containing phagosomes in resting wild-type but not mutant bone marrow-derived m (Fig. 1; Searle et al., 1998). This has important implications for any direct role that Slc11a1 might have in determining pathogen survival. For example, previous studies (Sturgill-Koszycki et al., 1994; 1996) examining entry of M. avium and M. tuberculosis into bone marrow-derived m
(Fig. 1; Searle et al., 1998). This has important implications for any direct role that Slc11a1 might have in determining pathogen survival. For example, previous studies (Sturgill-Koszycki et al., 1994; 1996) examining entry of M. avium and M. tuberculosis into bone marrow-derived m from BALB/c (Slc11a1 mutant) mice indicated that maturation of the phagosome is arrested at an early transitional stage. The BALB/c phagosome acquires an immature form of cathepsin D from the TGN but remains accessible to internalized transferrin. Fusion with acidic endosomes or lysosomes and acquisition of proton-ATPases occurs only after activation with IFN-γ. Hence, it might be assumed that Slc11a1 would not have a direct influence on mycobacterial survival in resting macrophage, as the bacterium would not come into direct contact with the Slc11a1 protein. Instead, it seems that normal Slc11a1 function is required for maturation of the mycobacterial phagosome. This is consistent with reduced acidification of Mycobacterium-containing vesicles (Hackam et al., 1998) and reduced phagosome–lysosome fusion (de Chastellier et al., 1993; Hackam et al., 1998) in Mycobacterium-infected mutant m
from BALB/c (Slc11a1 mutant) mice indicated that maturation of the phagosome is arrested at an early transitional stage. The BALB/c phagosome acquires an immature form of cathepsin D from the TGN but remains accessible to internalized transferrin. Fusion with acidic endosomes or lysosomes and acquisition of proton-ATPases occurs only after activation with IFN-γ. Hence, it might be assumed that Slc11a1 would not have a direct influence on mycobacterial survival in resting macrophage, as the bacterium would not come into direct contact with the Slc11a1 protein. Instead, it seems that normal Slc11a1 function is required for maturation of the mycobacterial phagosome. This is consistent with reduced acidification of Mycobacterium-containing vesicles (Hackam et al., 1998) and reduced phagosome–lysosome fusion (de Chastellier et al., 1993; Hackam et al., 1998) in Mycobacterium-infected mutant m . In inactivated wild-type m
. In inactivated wild-type m , normal acidification and phagosome–lysosome fusion occurs (de Chastellier et al., 1993; Hackam et al., 1998; Searle et al., 1998), creating an environment in which Slc11a1 can directly influence antimicrobial activity (see below). These results suggest that the earlier observation (Sturgill-Koszycki et al., 1996) of arrested phagosome development may be a feature peculiar to, or at least unnaturally prolonged in, Slc11a1 mutant m
, normal acidification and phagosome–lysosome fusion occurs (de Chastellier et al., 1993; Hackam et al., 1998; Searle et al., 1998), creating an environment in which Slc11a1 can directly influence antimicrobial activity (see below). These results suggest that the earlier observation (Sturgill-Koszycki et al., 1996) of arrested phagosome development may be a feature peculiar to, or at least unnaturally prolonged in, Slc11a1 mutant m . As Slc11a1 is also reduced in expression on young monocytes, this might also explain why Slc11a1 does not control visceralizing L. major infection that preferentially targets immature monocytes (Davies et al., 1988), and may contribute to the failure of Slc11a1 to regulate M. tuberculosis infection in mice (Medina and North, 1996a,b; North et al., 1999).
. As Slc11a1 is also reduced in expression on young monocytes, this might also explain why Slc11a1 does not control visceralizing L. major infection that preferentially targets immature monocytes (Davies et al., 1988), and may contribute to the failure of Slc11a1 to regulate M. tuberculosis infection in mice (Medina and North, 1996a,b; North et al., 1999).
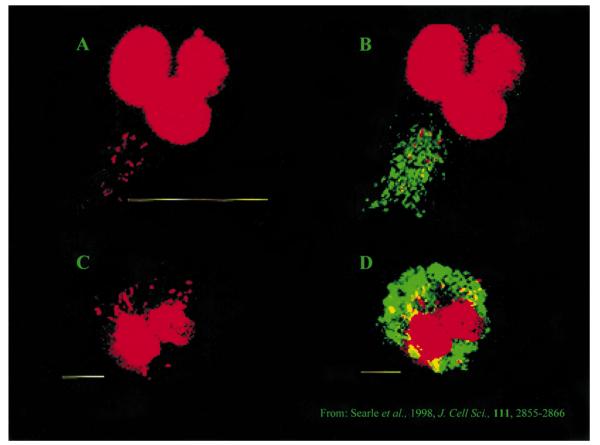
Fig. 1.
Bone marrow-derived m from Slc11a1 wild-type (A and B) versus mutant (C and D) congenic mice infected with M. avium.
from Slc11a1 wild-type (A and B) versus mutant (C and D) congenic mice infected with M. avium.
A and C. Staining of m nuclei and bacterial DNA with propidium iodide.
nuclei and bacterial DNA with propidium iodide.
B and D. The same images merged with the green fluorescence channel showing anti-C-terminal anti-Slc11a1 staining. For wild-type m (B), Slc11a1-positive vesicles have migrated to fuse with mycobacterial phagosomes. For mutant m
(B), Slc11a1-positive vesicles have migrated to fuse with mycobacterial phagosomes. For mutant m (D), no co-localization is observed. Reproduced with permission from Searle et al. (1998).
(D), no co-localization is observed. Reproduced with permission from Searle et al. (1998).
Slc11a2 localizes to early endosomes in m (Gruenheid et al., 1999) and other cell types throughout the body including the intestinal brush border (Fleming et al., 1998; Canonne-Hergaux et al., 1999), i.e. it appears to be expressed ubiquitously. Slc11a1 was thought to be restricted to the myeloid lineage (Vidal et al., 1993), but we have recently localized it to vesicles in neurons (Evans et al., 2001). This may relate directly to differences in behavioural response to stress (Evans et al., 2001), activation of the hypothalamic–pituitary–adrenal axis (HPA) and baseline (Blackwell et al., 1994) and post-stress (Evans et al., 2001) mortality after T. gondii infection in mutant versus wild-type congenic mice. It also relates to the earlier observations of Zwilling and coworkers (Brown et al., 1993) that activation of the HPA axis by restraint stress increased the severity of M. avium infection in Slc11a1 mutant mice but did not affect the ability of congenic Slc11a1 wild-type mice to control the mycobacterial infection. This was attributed to differences in the sensitivity of m
(Gruenheid et al., 1999) and other cell types throughout the body including the intestinal brush border (Fleming et al., 1998; Canonne-Hergaux et al., 1999), i.e. it appears to be expressed ubiquitously. Slc11a1 was thought to be restricted to the myeloid lineage (Vidal et al., 1993), but we have recently localized it to vesicles in neurons (Evans et al., 2001). This may relate directly to differences in behavioural response to stress (Evans et al., 2001), activation of the hypothalamic–pituitary–adrenal axis (HPA) and baseline (Blackwell et al., 1994) and post-stress (Evans et al., 2001) mortality after T. gondii infection in mutant versus wild-type congenic mice. It also relates to the earlier observations of Zwilling and coworkers (Brown et al., 1993) that activation of the HPA axis by restraint stress increased the severity of M. avium infection in Slc11a1 mutant mice but did not affect the ability of congenic Slc11a1 wild-type mice to control the mycobacterial infection. This was attributed to differences in the sensitivity of m from Slc11a1 congenic mice to corticosterone because HPA axis activation also caused increased intracellular growth of M. avium in m
from Slc11a1 congenic mice to corticosterone because HPA axis activation also caused increased intracellular growth of M. avium in m from Slc11a1 mutant but not wild-type mice (Brown and Zwilling, 1995; Brown et al., 1995). These in vivo and in vitro differences were simulated by corticosterone administration and abrogated by surgical or pharmacological adrenalectomy. In our studies (Evans et al., 2001), we observed enhanced levels of mRNA for corticotrophin-releasing hormone in the brain within 30 min of restraint stress. Hence, we hypothesized that, although differences in HPA activation translate into differences in adrenal enlargement and basal circulating corticosterone levels, the primary influence of Slc11a1 is at the level of the neuronal response to stress. These results highlight the importance of HPA activation in neuroimmune regulation of infectious disease and provide new insight into the possible roles of divalent cation transporters of the Slc11a gene family in regulating metal ion homeostasis in the brain and its broader pathological implications. For example, accumulation of metal ions in neurons is a feature of Parkinson's (Jellinger et al., 1993; Gerlach et al., 1994; Hirsch and Faucheux, 1998) and other (Gerlach et al., 1996; Multhaup, 1997) neurodegenerative diseases. Intriguingly, the Drosophila orthologue malvolio also localizes to phagocytes and neurons and plays a role in taste behaviour (Rodrigues et al., 1995), suggesting that this dual cellular localization may be a primitive feature of Slc11a1 expression.
from Slc11a1 mutant but not wild-type mice (Brown and Zwilling, 1995; Brown et al., 1995). These in vivo and in vitro differences were simulated by corticosterone administration and abrogated by surgical or pharmacological adrenalectomy. In our studies (Evans et al., 2001), we observed enhanced levels of mRNA for corticotrophin-releasing hormone in the brain within 30 min of restraint stress. Hence, we hypothesized that, although differences in HPA activation translate into differences in adrenal enlargement and basal circulating corticosterone levels, the primary influence of Slc11a1 is at the level of the neuronal response to stress. These results highlight the importance of HPA activation in neuroimmune regulation of infectious disease and provide new insight into the possible roles of divalent cation transporters of the Slc11a gene family in regulating metal ion homeostasis in the brain and its broader pathological implications. For example, accumulation of metal ions in neurons is a feature of Parkinson's (Jellinger et al., 1993; Gerlach et al., 1994; Hirsch and Faucheux, 1998) and other (Gerlach et al., 1996; Multhaup, 1997) neurodegenerative diseases. Intriguingly, the Drosophila orthologue malvolio also localizes to phagocytes and neurons and plays a role in taste behaviour (Rodrigues et al., 1995), suggesting that this dual cellular localization may be a primitive feature of Slc11a1 expression.
Finding Slc11a1 in non-myeloid cells led us to reassess its expression profile. With both anti-N-and anti-C-terminal Slc11a1 antibodies, we confirmed Slc11a1 expression in cortical pyramidal neurons (principally layers III and V), a subset of striatal neurons, cerebellar Purkinje cell bodies and the anterior pituitary (N. Papo, J. M. Blackwell and J. K. White, unpublished). We also observed Slc11a1 in pancreatic islets and in the adrenal medulla. Expression in pancreatic islets is interesting because Slc11a1 is a candidate diabetes gene (Esposito et al., 1998; Hill et al., 2000).
Slc11a1 is a proton/divalent cation antiporter
Using Xenopus oocytes, we showed that, like Slc11a2 (Gunshin et al., 1997), Slc11a1 is a divalent cation (Fe2+, Zn2+ and Mn2+) transporter (Goswami et al., 2001). Strikingly, however, where Slc11a2 is a symporter of H+ and metal ions, Slc11a1 is an antiporter that can flux divalent cations in either direction against a proton gradient. This provides a new model for metal ion homeostasis in m (Fig. 2). Slc11a2 in early endosomes delivers extracellularly acquired divalent cations into the cytosol. Slc11a1 in late endosomes/lysosomes delivers divalent cations from the cytosol to this acidic compartment. Here, the Fenton reaction can use ferrous iron to generate toxic hydroxyl (OH•) radicals (Zwilling et al., 1999). Mammalian cells, including m
(Fig. 2). Slc11a2 in early endosomes delivers extracellularly acquired divalent cations into the cytosol. Slc11a1 in late endosomes/lysosomes delivers divalent cations from the cytosol to this acidic compartment. Here, the Fenton reaction can use ferrous iron to generate toxic hydroxyl (OH•) radicals (Zwilling et al., 1999). Mammalian cells, including m , contain redoxactive iron in lysosomes (Garner et al., 1998), the exocytosis of which is important for oxidation and uptake of low-density lipoproteins (Yuan et al., 1995). Monocyte-derived m
, contain redoxactive iron in lysosomes (Garner et al., 1998), the exocytosis of which is important for oxidation and uptake of low-density lipoproteins (Yuan et al., 1995). Monocyte-derived m exposed to artificially aged erythrocytes show enhanced oxidation of low-density lipoproteins as a result of exocytosed iron (Yuan et al., 1996).
exposed to artificially aged erythrocytes show enhanced oxidation of low-density lipoproteins as a result of exocytosed iron (Yuan et al., 1996).
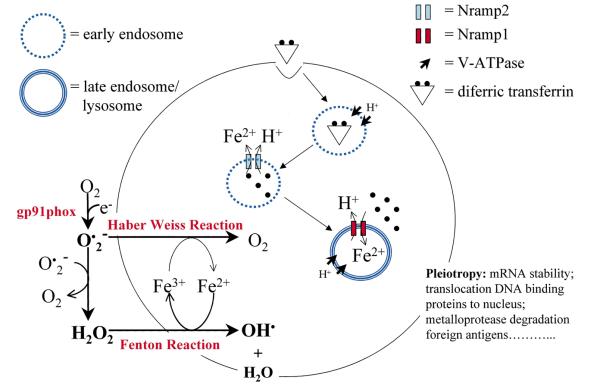
Fig. 2.
Model of divalent cation homeostasis in m and its relationship to oxygen-and nitrogen-dependent antimicrobial pathways. The symport activity of Slc11a2 delivers divalent cations (e.g. Fe2+) to the cytosol across early endosomal membranes after recruitment of V-ATPase and acidification of the vacuole. The antiport activity of Slc11a1 delivers divalent cations from the cytosol to acidic late endosomes and lysosomes, where the Fenton reaction generates toxic antimicrobial radicals. Hydroxyl radicals (OH•) generated from the Fenton reaction may then react with nitric oxide (NO) to produce toxic peroxynitrate.
and its relationship to oxygen-and nitrogen-dependent antimicrobial pathways. The symport activity of Slc11a2 delivers divalent cations (e.g. Fe2+) to the cytosol across early endosomal membranes after recruitment of V-ATPase and acidification of the vacuole. The antiport activity of Slc11a1 delivers divalent cations from the cytosol to acidic late endosomes and lysosomes, where the Fenton reaction generates toxic antimicrobial radicals. Hydroxyl radicals (OH•) generated from the Fenton reaction may then react with nitric oxide (NO) to produce toxic peroxynitrate.
That heavy metals accumulate in, and are exocytosed from, m lysosomes was of interest in relation to the hypothesis (Atkinson and Barton, 1998; Fleming et al., 1998) that Slc11a1 is involved in iron recycling from effete red cells by m
lysosomes was of interest in relation to the hypothesis (Atkinson and Barton, 1998; Fleming et al., 1998) that Slc11a1 is involved in iron recycling from effete red cells by m . With Jeremy Brock (University of Glasgow), we showed that iron phagocytosed via FcR as insoluble 59Fe-labelled transferrin–antitransferrin immune complexes accumulates in mutant-transfected m
. With Jeremy Brock (University of Glasgow), we showed that iron phagocytosed via FcR as insoluble 59Fe-labelled transferrin–antitransferrin immune complexes accumulates in mutant-transfected m but is efficiently recycled to the medium in wild-type m
but is efficiently recycled to the medium in wild-type m (Mulero et al., submitted). This did not occur with 59Fe-transferrin, which is taken up via recycling transferrin receptors, consistent with delivery to late endosomes/lysosomes as necessary for iron recycling by Slc11a1, possibly through lysosomal iron exocytosis. Interestingly, NO generation was essential to trigger iron release. The by-product of the high cytoplasmic iron in mutant m
(Mulero et al., submitted). This did not occur with 59Fe-transferrin, which is taken up via recycling transferrin receptors, consistent with delivery to late endosomes/lysosomes as necessary for iron recycling by Slc11a1, possibly through lysosomal iron exocytosis. Interestingly, NO generation was essential to trigger iron release. The by-product of the high cytoplasmic iron in mutant m is mRNA instability for a range of activation markers (Zwilling et al., 1999; Lafuse et al., 2000), contributing to the pleiotropy associated with Slc11a1 function. A role for Slc11a1 in recycling iron from effete red cells also implies a possible role in regulating iron homeostasis that would be interesting to examine in relation to the anaemias of chronic infection.
is mRNA instability for a range of activation markers (Zwilling et al., 1999; Lafuse et al., 2000), contributing to the pleiotropy associated with Slc11a1 function. A role for Slc11a1 in recycling iron from effete red cells also implies a possible role in regulating iron homeostasis that would be interesting to examine in relation to the anaemias of chronic infection.
Controversy relating to Slc11a1 transport activity in m
Our results using frog oocytes suggest that, depending on membrane topology (see below), antiport activity of Slc11a1 in m will normally deliver divalent cations from the cytosol into acidic late endosomes/lysosomes. However, previous attempts to define Slc11a1 function in m
will normally deliver divalent cations from the cytosol into acidic late endosomes/lysosomes. However, previous attempts to define Slc11a1 function in m using radioisotopes and fluid-phase or particle-bound divalent cation-sensitive fluorescent probes provide contradictory results. Zwilling and colleagues showed that the rate of 55Fe import by latex bead (Kuhn et al., 1999) or M. avium (Zwilling et al., 1999) phagosomes isolated from wild-type transfected m
using radioisotopes and fluid-phase or particle-bound divalent cation-sensitive fluorescent probes provide contradictory results. Zwilling and colleagues showed that the rate of 55Fe import by latex bead (Kuhn et al., 1999) or M. avium (Zwilling et al., 1999) phagosomes isolated from wild-type transfected m was more than twice the rate observed in phagosomes from mutant m
was more than twice the rate observed in phagosomes from mutant m . Phagosomes isolated from wild-type m
. Phagosomes isolated from wild-type m prelabelled with 55Fe-citrate before phagocytosis also contained up to 4× Fe compared with mutant m
prelabelled with 55Fe-citrate before phagocytosis also contained up to 4× Fe compared with mutant m (Kuhn et al., 1999). Using a divalent cation-sensitive fluorescent probe covalently attached to zymosan particles, other researchers (Jabado et al., 2000) found that phagosomes from wild-type m
(Kuhn et al., 1999). Using a divalent cation-sensitive fluorescent probe covalently attached to zymosan particles, other researchers (Jabado et al., 2000) found that phagosomes from wild-type m extrude Mn2+ faster than mutant m
extrude Mn2+ faster than mutant m , a difference eliminated by preventing phagosomal acidification. This was shown by measuring the rate of quenching of particle-bound fluorescence after the addition of exogenous 500 μM Mn2+. Interestingly, the ability (Zwilling et al., 1999) to inhibit mycobacterial growth in wild-type m
, a difference eliminated by preventing phagosomal acidification. This was shown by measuring the rate of quenching of particle-bound fluorescence after the addition of exogenous 500 μM Mn2+. Interestingly, the ability (Zwilling et al., 1999) to inhibit mycobacterial growth in wild-type m by the addition of exogenous iron was dose dependent, reaching a maximum at 0.05 μM iron, decreasing and eventually lost at higher concentrations up to 0.5 μM, suggesting some change in Slc11a1 function at higher exogenous iron concentration. Our data demonstrate that Slc11a1 can flux divalent cations in either direction depending upon the pH on either side of the membrane. Net flux will be determined by the combined electrochemical gradients of the metal and H+, which may explain the discrepancies in results from different laboratories using different techniques and experimental conditions to evaluate the direction of transport across m
by the addition of exogenous iron was dose dependent, reaching a maximum at 0.05 μM iron, decreasing and eventually lost at higher concentrations up to 0.5 μM, suggesting some change in Slc11a1 function at higher exogenous iron concentration. Our data demonstrate that Slc11a1 can flux divalent cations in either direction depending upon the pH on either side of the membrane. Net flux will be determined by the combined electrochemical gradients of the metal and H+, which may explain the discrepancies in results from different laboratories using different techniques and experimental conditions to evaluate the direction of transport across m phagosomal membranes. More refined studies in m
phagosomal membranes. More refined studies in m are required to determine the conditions under which influx versus efflux of divalent cations from Slc11a1-positive vesicles occurs. It is possible that, as in yeast, late endosomes/lysosomes of m
are required to determine the conditions under which influx versus efflux of divalent cations from Slc11a1-positive vesicles occurs. It is possible that, as in yeast, late endosomes/lysosomes of m act as ‘storage vacuoles’ for divalent cations that can be fluxed in or out according to varying conditions of metal ion stress or depletion in the cytosol.
act as ‘storage vacuoles’ for divalent cations that can be fluxed in or out according to varying conditions of metal ion stress or depletion in the cytosol.
Slc11a1 pleiotropy and infectious disease susceptibility
One effect of Slc11a1 is enhanced expression of iNOS (encoded by Nos2A) and generation of toxic NO in wild-type versus mutant m (Roach et al., 1991; Arias et al., 1997). It was assumed that this is crucial to Slc11a1-mediated resistance in vivo, perhaps combining with OH• to produce the more toxic peroxynitrite. Using immunofluorescence, we examined (C. Evans and J. M. Blackwell, unpublished) Slc11a1 and iNOS expression and localization in the liver early in L. donovani infection in Slc11a1 congenic mice. Slc11a1 is expressed in iNOS negative resident Kupffer cells but not in iNOS-positive:- Mac1-positive fresh monocytes entering the liver. In wild-type mice, monocytes (now m
(Roach et al., 1991; Arias et al., 1997). It was assumed that this is crucial to Slc11a1-mediated resistance in vivo, perhaps combining with OH• to produce the more toxic peroxynitrite. Using immunofluorescence, we examined (C. Evans and J. M. Blackwell, unpublished) Slc11a1 and iNOS expression and localization in the liver early in L. donovani infection in Slc11a1 congenic mice. Slc11a1 is expressed in iNOS negative resident Kupffer cells but not in iNOS-positive:- Mac1-positive fresh monocytes entering the liver. In wild-type mice, monocytes (now m ) within developing granulomas become Slc11a1 positive at ≈ 10 days of infection. In mutant mice, they do not. This suggests that iNOS is not important in the early Slc11a1-mediated Kupffer cell regulation (Crocker et al., 1984) of infection, but may play a role later in granuloma-dependent (Stern et al., 1988) control of the parasite. To evaluate further the role of iNOS, we have backcrossed the Nos2A knock-out (KO) (Wei et al., 1995) onto Slc11a1 wild-type and mutant congenic mice on C57BL/10ScSn (= B10), C57BL/6 (= B6) and BALB backgrounds. This will allow us to determine the importance of iNOS in Slc11a1-regulated L. donovani, S. typhimurium and M. avium infections. We are also intercrossing the B6 gp91phox KO (Shiloh et al., 1999) with our B6 Slc11a1/Nos2A KO congenics. Phenotypic analyses will allow us to determine whether NO and O2− contribute separately or syngergistically in Slc11a1-regulated infectious and autoimmune disease phenotypes. The possible role of gp91phox in radical generation becomes more interesting given its contribution of O2− as substrate for superoxide dismutase (SOD)-generated H2O2, which in turn acts as a substrate for Fenton reaction reduction of Fe2+, the latter contributing its electron to OH• (Fig. 2). The reductase gp91phox is crucial for the generation of O2− from O2, but may also act directly as a ferrireductase (C. M. Proctor and N. Robinson, personal communication). Gp91phox is delivered to infected phagosomes by vesicular fusion (Vazquez-Torres et al., 2000), and we are evaluating this using anti-gp91phox antibody and confocal microscopy. Whether its role is solely to generate antimicrobial O2− and/or to act as a ferrireductase to provide ferrous iron for Slc11a1 Fe2+ influx or efflux is not known. Further intercrosses, e.g. crossing in a SOD KO, will tease out the role of gp91phox. Other intercrosses, e.g. knock-outs for TNFα, IL-1β, chemokines or their receptors, will determine the roles of other proinflammatory molecules in Slc11a1-regulated phenotypes.
) within developing granulomas become Slc11a1 positive at ≈ 10 days of infection. In mutant mice, they do not. This suggests that iNOS is not important in the early Slc11a1-mediated Kupffer cell regulation (Crocker et al., 1984) of infection, but may play a role later in granuloma-dependent (Stern et al., 1988) control of the parasite. To evaluate further the role of iNOS, we have backcrossed the Nos2A knock-out (KO) (Wei et al., 1995) onto Slc11a1 wild-type and mutant congenic mice on C57BL/10ScSn (= B10), C57BL/6 (= B6) and BALB backgrounds. This will allow us to determine the importance of iNOS in Slc11a1-regulated L. donovani, S. typhimurium and M. avium infections. We are also intercrossing the B6 gp91phox KO (Shiloh et al., 1999) with our B6 Slc11a1/Nos2A KO congenics. Phenotypic analyses will allow us to determine whether NO and O2− contribute separately or syngergistically in Slc11a1-regulated infectious and autoimmune disease phenotypes. The possible role of gp91phox in radical generation becomes more interesting given its contribution of O2− as substrate for superoxide dismutase (SOD)-generated H2O2, which in turn acts as a substrate for Fenton reaction reduction of Fe2+, the latter contributing its electron to OH• (Fig. 2). The reductase gp91phox is crucial for the generation of O2− from O2, but may also act directly as a ferrireductase (C. M. Proctor and N. Robinson, personal communication). Gp91phox is delivered to infected phagosomes by vesicular fusion (Vazquez-Torres et al., 2000), and we are evaluating this using anti-gp91phox antibody and confocal microscopy. Whether its role is solely to generate antimicrobial O2− and/or to act as a ferrireductase to provide ferrous iron for Slc11a1 Fe2+ influx or efflux is not known. Further intercrosses, e.g. crossing in a SOD KO, will tease out the role of gp91phox. Other intercrosses, e.g. knock-outs for TNFα, IL-1β, chemokines or their receptors, will determine the roles of other proinflammatory molecules in Slc11a1-regulated phenotypes.
In other studies, we showed that Slc11a1 wild-type m have enhanced lipopolysaccharide-dependent antigen processing for presentation to T cells (Lang et al., 1997), which may reflect metal ion requirement for metalloprotease activity and/or endosomal fusion events. This effect on antigen processing is compounded by Slc11a1's influence on molecules regulating (TNFα, IL1β) or directly involved in (MHC class II) antigen presentation. Hence, in mice vaccinated with attenuated S. typhimurium engineered to express tetanus toxoid fragment C, we found polarized T helper 1 (Th1) versus T helper 2 (Th2) responses in congenic wild-type versus mutant mice (Soo et al., 1998). This Th1:Th2 bias was also seen by us in L. donovani infection (Kaye and Blackwell, 1989) and by others in mycobacterial infection (Kramnik et al., 1994).
have enhanced lipopolysaccharide-dependent antigen processing for presentation to T cells (Lang et al., 1997), which may reflect metal ion requirement for metalloprotease activity and/or endosomal fusion events. This effect on antigen processing is compounded by Slc11a1's influence on molecules regulating (TNFα, IL1β) or directly involved in (MHC class II) antigen presentation. Hence, in mice vaccinated with attenuated S. typhimurium engineered to express tetanus toxoid fragment C, we found polarized T helper 1 (Th1) versus T helper 2 (Th2) responses in congenic wild-type versus mutant mice (Soo et al., 1998). This Th1:Th2 bias was also seen by us in L. donovani infection (Kaye and Blackwell, 1989) and by others in mycobacterial infection (Kramnik et al., 1994).
One of the perplexities in SLC11A1 research is the clear association between both 5′ and 3′ haplotypes and global susceptibility to pulmonary tuberculosis in man (Shaw et al., 1997; Bellamy et al., 1998; Cervino et al., 2000; Gao et al., 2000; Greenwood et al., 2000; Ryu et al., 2000), but the lack of any effect of Slc11a1 on primary infection with M. tuberculosis (Medina and North, 1996a,b; North et al., 1999) in mice. One clear difference is that humans, especially in Africa, are repeatedly exposed to environmental mycobacteria before infection with M. tuberculosis. In Malawi, we showed (J. M. Blackwell, S. Floyd, G. Black, H. Dockrell and P. E. M. Fine, unpublished) significant SLC11A1 haplotype associations with IFN-γ responses to M. tuberculosis and Mycobacterium fortuitum antigens, consistent with the murine Th1:Th2 bias. Our results suggest that polymorphism at SLC11A1 influences immune response to ‘priming/vaccinating’ exposures to mycobacteria. This hypothesis is interesting, in that BCG's effectiveness in protecting against leprosy in the same Malawian population is also associated with polymorphism at SLC11A1 (A. V. Hill and P. E. M. Fine, personal communication). No SLC11A1 association with leprosy is observed in non-BCG-vaccinated subjects. Polymorphism at SLC11A1 is also associated with Mitsuda-type skin test reactivity to leprosy antigens (Alcais et al., 2000). These results are also interesting in relation to recent studies suggesting that the adoption of a Western infectious disease-free lifestyle is associated with an increase in the prevalence of allergic and autoimmune diseases, which can also be modulated by immune response to mycobacterial antigens after BCG vaccination (Shirakawa et al., 1997). Stratification by BCG vaccination was recently shown by us to unmask a genetic risk factor for atopy in the region of the SLC11A1 locus (Alm et al., 2001), pointing to the importance of genotype by environment interactions in determining disease susceptibility.
Functional polymorphism at human SLC11A1
Using transient transfection and a luciferase reporter gene, we have shown (Searle and Blackwell, 1999) that a repeat polymorphism, designated (GT)n, identified by us (Blackwell et al., 1995) in the promoter of human SLC11A1 regulates expression. The sequence contains a Z-DNA repeat with four alleles: (i) t(gt)5ac(gt)5ac(gt)11g; (ii) t(gt)5ac(gt)5ac(gt)10g; (iii) t(gt)5ac(gt)5ac(gt)9g; (iv) t(gt)5ac(gt)9g. Alleles (i) and (iv) are rare (gene frequencies ≈0.001); alleles (ii) and (iii) occur at gene frequencies of ≈0.25 and ≈0.75 respectively. In the absence of exogenous stimuli, allele (iii) drives five- to eightfold higher reporter gene expression than alleles (i), (ii) and (iv). All alleles show similar percentage enhancement of expression with IFN-γ, consistent with multiple IFN-γ response elements 5′ and 3′ of the repeat. The addition of lipopolysaccharide has no effect on alleles (i) and (iv), but causes significant reduction in expression driven by allele (ii) and enhances expression driven by allele (iii). Juxtaposition of lipopolysaccharide-related response elements (NFκB, AP-1, NF-IL6) may thus be differentially affected by the two common alleles.
Disease associations with SLC11A1
The multiple pleiotropic effects on m activation prompted us to look for human SLC11A1 association with autoimmune as well as infectious disease (Shaw et al., 1996). This has since been replicated in multiple studies (Hofmeister et al., 1997; Esposito et al., 1998; Maliarik et al., 2000; Sanjeevi et al., 2000; Singal et al., 2000; Yang et al., 2000). We also proposed that the high expressing allele (iii) of the (GT)n polymorphism would be associated with autoimmune disease, and the low expressing allele (ii) with infection. This too has held true across multiple autoimmune (Shaw et al., 1996; Esposito et al., 1998; Sanjeevi et al., 2000) and infectious (Shaw et al., 1997; Bellamy et al., 1998; Gao et al., 2000) disease (specifically tuberculosis) studies, suggesting that there may be some balancing selective forces that keep both alleles in the population. In this respect, it is interesting that allele frequencies for SLC11A1 allele (ii) are in the range 0.14–0.20 throughout western and southern Africa (0.16 South African Cape coloureds; 0.20 Malawi; 0.14 The Gambia) compared with 0.25–0.29 in northern European (0.25 UK; 0.27 Sweden; 0.29 Latvia) populations and 0.36 in Brazil. Recently, we showed (M. Hibbard, M. Levin and J. M. Blackwell, unpublished) that homozygous 3/3 individuals produce higher levels of TNFα and are at significantly higher risk of severe clinical meningococcal disease. This is again consistent with high SLC11A1 expression generating proinflammatory responses during acute bacteraemia. Intriguingly, allele (iii) is also on a 5′ haplotype significantly associated with visceral leishmaniasis and post-kala-azar dermal leishmaniasis in Sudan (Mohamed et al., submitted), consistent with SLC11A1-regulated proinflammatory responses (e.g. high TNFα; Barral-Netto et al., 1991) associated with this disease and with allele (ii) being at a higher frequency (0.27) in this population. Hence, a pathogenic organism's ability or not to elicit an acute proinflammatory response may also contribute to the selective forces influencing the maintenance of SLC11A1 alleles in the population. In many studies, evidence has also been found for polymorphisms on 3′ haplotypes contributing separately to disease susceptibility, suggesting that there are other functional mutations yet to be identified in man. Clearly, more detailed population genetic analyses will be necessary to understand the relationship between more complex haplotypes and disease incidence in different geographic regions. We are currently sequencing across the SLC11A1 to identify new functional coding and/or regulatory polymorphisms contributing to disease susceptibility in different populations.
activation prompted us to look for human SLC11A1 association with autoimmune as well as infectious disease (Shaw et al., 1996). This has since been replicated in multiple studies (Hofmeister et al., 1997; Esposito et al., 1998; Maliarik et al., 2000; Sanjeevi et al., 2000; Singal et al., 2000; Yang et al., 2000). We also proposed that the high expressing allele (iii) of the (GT)n polymorphism would be associated with autoimmune disease, and the low expressing allele (ii) with infection. This too has held true across multiple autoimmune (Shaw et al., 1996; Esposito et al., 1998; Sanjeevi et al., 2000) and infectious (Shaw et al., 1997; Bellamy et al., 1998; Gao et al., 2000) disease (specifically tuberculosis) studies, suggesting that there may be some balancing selective forces that keep both alleles in the population. In this respect, it is interesting that allele frequencies for SLC11A1 allele (ii) are in the range 0.14–0.20 throughout western and southern Africa (0.16 South African Cape coloureds; 0.20 Malawi; 0.14 The Gambia) compared with 0.25–0.29 in northern European (0.25 UK; 0.27 Sweden; 0.29 Latvia) populations and 0.36 in Brazil. Recently, we showed (M. Hibbard, M. Levin and J. M. Blackwell, unpublished) that homozygous 3/3 individuals produce higher levels of TNFα and are at significantly higher risk of severe clinical meningococcal disease. This is again consistent with high SLC11A1 expression generating proinflammatory responses during acute bacteraemia. Intriguingly, allele (iii) is also on a 5′ haplotype significantly associated with visceral leishmaniasis and post-kala-azar dermal leishmaniasis in Sudan (Mohamed et al., submitted), consistent with SLC11A1-regulated proinflammatory responses (e.g. high TNFα; Barral-Netto et al., 1991) associated with this disease and with allele (ii) being at a higher frequency (0.27) in this population. Hence, a pathogenic organism's ability or not to elicit an acute proinflammatory response may also contribute to the selective forces influencing the maintenance of SLC11A1 alleles in the population. In many studies, evidence has also been found for polymorphisms on 3′ haplotypes contributing separately to disease susceptibility, suggesting that there are other functional mutations yet to be identified in man. Clearly, more detailed population genetic analyses will be necessary to understand the relationship between more complex haplotypes and disease incidence in different geographic regions. We are currently sequencing across the SLC11A1 to identify new functional coding and/or regulatory polymorphisms contributing to disease susceptibility in different populations.
Evolution of the Nramp gene family
Following our demonstration that Slc11a1 and Slc11a2 have different modes of action, we were interested in the evolution of Nramp genes. In yeast, Nramp homologues include SMF1/2/3. Smf1p/2p localize to the outer membrane and are involved in Cu2+, high-affinity Mn2+ and, possibly, Fe2+ transport into the cell (Supeck et al., 1996; Cohen et al., 2000). Smf1p and Smf2p localize to distinct cellular compartments under metal starvation (Portnoy et al., 2000): Smf1p accumulates at the cell surface; Smf2p is restricted to intracellular vesicles. Smf3p is quite distinctive. It is downregulated by iron and localizes to vacuolar membranes independently of metal treatment. Yeast lacking Smf3p show symptoms of iron starvation, suggesting that Smf3p helps to mobilize (i.e. efflux) iron from vacuolar stores. Murine Slc11a2 but not Slc11a1 complements the Mn2+ transport and pH-sensitive phenotype in smf1/2 double KO Saccharomyces cerevisiae (Pinner et al., 1997). This could be explained by differential localization (e.g. membrane versus vacuole) in yeast, or symport versus antiport functions. Similarly, human SLC11A2 but not SLC11A1 complements the EGTA-and pH-sensitive phenotype of the divalent metal transporter-deficient pdt1Delta strain of Schizosaccharomyces pombe (Tabuchi et al., 1999). Here, replacement of the N-terminus of SLC11A2 with that of SLC11A1 results in an inactive chimera, indicating that the N-termini of mammalian Nramps differentially regulate function.
To try to determine when mammalian Nramps diverged in localization and function, two orthologues (fSlc11α/fSlc11β) were sequenced by us (Sibthorpe et al., submitted) from the puffer fish Fugu rubripes. Phylogenetic analysis shows that both are similar to mammalian Slc11a2, suggesting that Slc11a2 is ancestral. The gene environment for both is syntenic with mouse chromosome 15, consistent with gene duplication in the ancestral Slc11a2 location. Only fSlc11α has an N-terminal tyrosine-based putative endosomal targeting signal and rudiments of a Pro–Ser-rich N-terminal sequence similar to Slc11a1. Transient transfection of green fluorescent protein-tagged constructs into human epithelial kidney cells confirms a late endosomal/lysosomal localization for fSlc11α, consistent with divergence towards an Slc11a1-like function. Further work is required to see whether fSlc11α is an antiporter. Interestingly, human SLC11A1 complements Drosophila malvolio, suggesting that Slc11a1-like antiport activity emerged earlier in Nramp evolution. Two functional classes of NRAMP proteins also occur in plants: Arabidopsis thaliana (At) SLC11A1 and Oriza sativa (Os) SLC11A1 and 3 represent one class; AtSLC11A2–5 and OsSLC11A2 the other (Curie et al., 2000). AtSlc11a1 and OsSlc11a1 complement the fet3fet4 yeast mutant defective in both low- and high-affinity iron transports, whereas AtSlc11a2 and OsSlc11a2 fail to do so.
Nramps at the host–pathogen interface
The Nramp gene family is highly conserved across prokaryotes and eukaryotes, with orthologues now identified in pathogenic bacteria that come under Slc11a1 control in m (Agranoff et al., 1999; Kehres et al., 2000). This means that the pathogen's own requirement for transition metal ions and genetic variation at its own Nramp-related proteins may be influenced by mammalian Nramp polymorphism and function, providing a dynamic interface upon which the forces of evolution have been acting.
(Agranoff et al., 1999; Kehres et al., 2000). This means that the pathogen's own requirement for transition metal ions and genetic variation at its own Nramp-related proteins may be influenced by mammalian Nramp polymorphism and function, providing a dynamic interface upon which the forces of evolution have been acting.
Role of other divalent cation transporters in disease
Many molecules mediate metal ion homeostasis, including metallochaperones, ferritins, metallothionines and regulatory proteins that adjust the expression or function of metal trafficking transporters. A dysfunction anywhere in the metal-sensing pathway may cause disease. However, the two copper transporters associated with Menkes and Wilson's diseases (reviewed by Harris, 2000) provide particularly intriguing molecular and cell biological parallels with Slc11a functions. For both, yeast complementation has been used successfully to detect defects in copper transport, and mammalian cell assays to identify defects in intracellular trafficking. The Menkes protein (ATP7A) is a P-type ATPase defective in X-linked recessive copper deficiency, leading to decreased copper in the brain, neurodegeneration and early death. More than 150 mutations occur in man. ATP7A normally localizes to the trans-Golgi network (TGN) and constitutively cycles via the plasma membrane in basal copper conditions (Petris and Mercer, 1999). Under copper stress, ATP7A is recruited preferentially to the plasma membrane where it effluxes copper. Endocytosis of ATP7A under both basal and elevated copper is mediated by a C-terminal dileucine motif (Francis et al., 1999; Petris and Mercer, 1999), but other, including transmembrane domain-located, signals are involved in TGN retention and retrieval from endosomes to the TGN (Ambrosini and Mercer, 1999). ATP7A has eight transmembrane domains and a long N-terminus with six copper binding sites (GMxCxxC). These are involved in copper sensing, provide signals for relocalization from the TGN to the plasma membrane (Strausak et al., 1999) and chaperone copper to the centre of the channel, although some copper can be delivered to the channel directly (Voskoboinik et al., 1999). Copper also binds to Cys, Met and His residues on transmembrane domains, forming a transient complex during transport. Mutations in patients influence copper binding, localization, trafficking or transport functions. In autosomal Wilson's disease, more than 200 mutations in ATP7B influence localization and transport function and are associated with high levels of tissue copper, particularly in the liver, brain and kidney. ATP7B localizes to the TGN and late endosomes (Harada et al., 2000) under basal conditions, with high copper influencing redistribution to vesicles and to apical vacuoles/membranes in polarized cells. In this case, copper binding to the N-terminal domain is associated with conformational change that leads to weakening of the interaction between the ATP-binding domain/loop and the N-terminal domain (Vanderwerf et al., 2001). Alteration in domain–domain interactions is coupled to changes in the nucleotide-binding properties of the ATP-binding domain, suggesting that copper binding to the N-terminus modulates transport activity. Increase in copper leads to hyperphosphorylation of ATP7B, and the level of phosphorylation correlates with intracellular localization. Regulation via phosphorylation requires the presence in the protein of the properly folded N-terminal domain.
Using knowledge of ATP7A and ATP7B to relate Slc11a1 structure to function
At present, we know very little about how Slc11a1/SLC11A1 protein sequence and structure relates to function. Figure 3 provides a diagrammatic representation of how Slc11a1 might lie in the late endosomal/lysosomal membrane, highlighting key features of the molecule that may be important to function. Several key questions remain to be addressed.
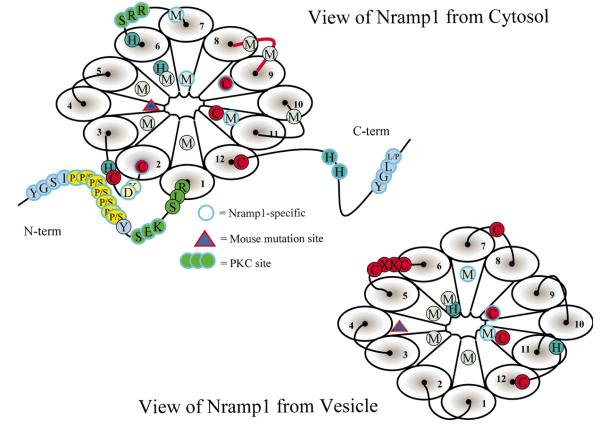
Fig. 3.
Slc11a1 as viewed from the cytosol or the vesicle assuming a topology in which N-and C-termini are cytosolic. This diagrammatic representation shows the locations of: conserved and Slc11a1-specific Cys (C), His (H) and Met (M) residues; N-and C-terminal YXXZ putative endosomal targeting motifs (where X = any amino acid, Z = bulky, hydrophobic residue); the Pro–Ser-rich N-terminus; PKC binding sites; and the position of the murine transmembrane domain 4 functional null mutation.
Question 1 is to determine the topology of Slc11a1 and Slc11a2 in early endosome versus late endosome/lysosome vesicular membranes. Logic dictates that both will have N-and C-termini and conserved transport motif intertransmembrane loop 8 on the cytoplasmic face, with intertransmembrane loops 1, 3, 5, 7, 9 and 11 facing into the vesicle. Symport versus antiport activity then determines that divalent cation flows from vesicle to cytosol for Slc11a2-positive early endosomes and from cytosol to vesicle when Slc11a1-positive late endosomes/lysosomes are acidic. However, Kuhn et al. (2001) have reported that antibody to intertransmembrane loop 7 inhibited divalent cation uptake into isolated m phagosomes, whereas an anti-C-terminal antibody did not. Although the latter may be because the antibody did not provide the correct steric hindrance, the former could only be accounted for by the antibody accessing the intravesicular compartment under the ‘logical’ model. Paradoxically, Kuhn et al. (2001) also report that inhibitors of PKC diminished iron import to phagosomes. This would be consistent with PKC access to binding sites on Slc11a1, all of which lie on the same face as N-and C-termini. Unless PKC could, in this case, gain intravesicular access, there is internal inconsistency in the data. To determine topology, we are currently analysing fluorescence in selectively permeabilized cells after transient transfection of intertransmembrane loop FLAG-tagged Slc11a1 constructs.
phagosomes, whereas an anti-C-terminal antibody did not. Although the latter may be because the antibody did not provide the correct steric hindrance, the former could only be accounted for by the antibody accessing the intravesicular compartment under the ‘logical’ model. Paradoxically, Kuhn et al. (2001) also report that inhibitors of PKC diminished iron import to phagosomes. This would be consistent with PKC access to binding sites on Slc11a1, all of which lie on the same face as N-and C-termini. Unless PKC could, in this case, gain intravesicular access, there is internal inconsistency in the data. To determine topology, we are currently analysing fluorescence in selectively permeabilized cells after transient transfection of intertransmembrane loop FLAG-tagged Slc11a1 constructs.
Question 2 is to determine when symport versus antiport activity diverged in the Nramp gene family. In our laboratory, we are approaching this in two ways: (i) allowing evolution to inform us of divergence in function by testing fSlc11α/β in oocyte functional screens; and (ii) testing chimeric Slc11a1/Slc11a2 constructs to identify regions that determine antiport versus symport function. We can then pinpoint conserved sequences in these regions that might inform us further as to the molecular evolution of divergent functions.
Question 3 is to ascertain the key amino acid and motifs that determine the range and specificity of Slc11a1 divalent cation transport. By analogy with other metal ion-binding and transport proteins, conserved Cys, His and Met residues are likely to be important. Figure 3 demonstrates the potential juxtaposition of conserved residues around a putative pore formed by the 12 transmembrane domains in the vesicular membrane. Conserved residues, and a conserved CxxC motif, also occur on intertransmembrane loops on both faces of the molecule. Five Slc11a1-specific Cys, His and Met residues are conserved across mammalian and chicken Slc11a1. We are currently testing transport function and divalent cation specificity using constructs with targeted mutation in conserved Slc11a1/Slc11a2 and Slc11a1-specific, residues.
Question 4 relates to changes in Slc11a1 function with m activation and the potential importance of PKC binding sites and the N-terminal Pro–Ser-rich domain. PKC activity has been implicated in regulating iron uptake by phagosomes (Kuhn et al., 2001). Using mutated constructs, we are looking at effects on protein phosphorylation, determining which PKC sites affect transport function, and using glutathione-S-transferase pull-down assays to look for interactions between the N- and/or C-terminal domains and putative cytosolic loops of Slc11a1.
activation and the potential importance of PKC binding sites and the N-terminal Pro–Ser-rich domain. PKC activity has been implicated in regulating iron uptake by phagosomes (Kuhn et al., 2001). Using mutated constructs, we are looking at effects on protein phosphorylation, determining which PKC sites affect transport function, and using glutathione-S-transferase pull-down assays to look for interactions between the N- and/or C-terminal domains and putative cytosolic loops of Slc11a1.
Furthering our understanding of the role of SLC11A1 in disease
Transition metal ions are essential for life and participate in many cellular functions. These include regulation of transcription through DNA-binding proteins and metal response elements, the functions of hundreds of enzymes, including metalloproteases, SOD and iNOS, and cellular functions such as endosomal fusion (Aballay et al., 1995). The fact that Slc11a1 transports Fe2+, Zn2+ and Mn2+ and possibly other divalent cations provides the crucial clue to pleiotropy. This reflects not only direct regulation of vesicular divalent cation content, but also secondary effects on concentrations in the cytosol and surrounding cellular milieu. The challenge for the future is to determine precisely which of the direct or indirect pleiotropic effects of SLC11A1 are most important in determining each of the multiple disease associations now observed in man.
Acknowledgements
Our work on Nramps is supported by The Wellcome Trust
References
- Aballay A, Sarrouf MN, Colombo MI, Stahl PD, Mayorga LS. Zn2+ depletion blocks endosome fusion. Biochem J. 1995;312:919–923. doi: 10.1042/bj3120919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel L, Sanchez FO, Oberti J, Thuc NV, Hoa LV, Lap VD, et al. Susceptibility to leprosy is linked to the human NRAMP1 gene. J Infect Dis. 1998;177:133–145. doi: 10.1086/513830. [DOI] [PubMed] [Google Scholar]
- Agranoff D, Monahan IM, Mangan JA, Butcher PD, Krishna S. Mycobacterium tuberculosis expresses a novel pH-dependent divalent cation transporter belonging to the Nramp family. J Exp Med. 1999;190:717–724. doi: 10.1084/jem.190.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcais A, Sanchez FO, Thuc NV, Lap VD, Oberti J, Lagrange PH, et al. Granulomatous reaction to intradermal injection of lepromin (Mitsuda reaction) is linked to the human NRAMP1 gene in Vietnamese leprosy sibships. J Infect Dis. 2000;181:302–308. doi: 10.1086/315174. [DOI] [PubMed] [Google Scholar]
- Alm JS, Sanjeevi CB, Miller EN, Dabadghao P, Lilja G, Pershagen G, et al. Atopy in children in relation to BCG vaccination and genetic polymorphisms at SLC11A1 (formerly NRAMP1) and D2S1471. Genes Immun. 2001 doi: 10.1038/sj.gene.6363834. (in press) [DOI] [PubMed] [Google Scholar]
- Ambrosini L, Mercer JF. Defective copper-induced trafficking and localization of the Menkes protein in patients with mild and copper-treated classical Menkes disease. Hum Mol Genet. 1999;8:1547–1555. doi: 10.1093/hmg/8.8.1547. [DOI] [PubMed] [Google Scholar]
- Arias M, Rojas M, Zabaleta J, Rodriguez JI, Paris SC, Barrera LF, Garcia LF. Inhibition of virulent Mycobacterium tuberculosis by Bcg(r) and Bcg(s) macrophages correlates with nitric oxide production. J Infect Dis. 1997;176:1552–1558. doi: 10.1086/514154. [DOI] [PubMed] [Google Scholar]
- Atkinson PGP, Barton CH. Ectopic expression of Nramp1 in COS-1 cells modulates iron accumulation. FEBS Lett. 1998;425:239–242. doi: 10.1016/s0014-5793(98)00236-1. [DOI] [PubMed] [Google Scholar]
- Atkinson PGP, Blackwell JM, Barton CH. Nramp1 locus encodes a 65 kDa interferon-g inducible protein in murine macrophages. Biochem J. 1997;325:779–786. doi: 10.1042/bj3250779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral-Netto M, Badaro R, Barral A, Almeida RP, Santos SB, Badaro F, et al. Tumor necrosis factor (cachectin) in human visceral leishmaniasis. J Infect Dis. 1991;163:853–857. doi: 10.1093/infdis/163.4.853. [DOI] [PubMed] [Google Scholar]
- Barton CH, White JK, Roach TIA, Blackwell JM. NH2-terminal sequence of macrophage-expressed natural resistance-associated macrophage protein (Nramp) encodes a proline/serine-rich putative Src homology 3-binding domain. J Exp Med. 1994;179:1683–1687. doi: 10.1084/jem.179.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy R, Ruwende C, Corrah T, McAdam KPWJ, Whittle HC, Hill AVS. Variation in the NRAMP1 gene is associated with susceptibility to tuberculosis in West Africans. N Engl J Med. 1998;338:640–644. doi: 10.1056/NEJM199803053381002. [DOI] [PubMed] [Google Scholar]
- Blackwell JM, Searle S. Genetic regulation of macrophage activation: understanding the function of Nramp1 (= Ity/Lsh/Bcg) Immunol Lett. 1999;65:73–80. doi: 10.1016/s0165-2478(98)00127-8. [DOI] [PubMed] [Google Scholar]
- Blackwell JM, Roberts CW, Roach TIA, Alexander J. Influence of macrophage resistance gene Lsh/Ity/Bcg (candidate Nramp) on Toxoplasma gondii infection in mice. Clin Exp Immunol. 1994;97:107–112. doi: 10.1111/j.1365-2249.1994.tb06587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell JM, Barton CH, White JK, Searle S, Baker A-M, Williams H, Shaw M-A. Genomic organization and sequence of the human NRAMP gene: identification and mapping of a promoter region polymorphism. Mol Med. 1995;1:194–205. [PMC free article] [PubMed] [Google Scholar]
- Blackwell JM, Searle S, Goswami T, Miller EN. Understanding the multiple functions of Nramp1. Microbes Infect. 2000;2:317–321. doi: 10.1016/s1286-4579(00)00295-1. [DOI] [PubMed] [Google Scholar]
- Brown DH, Zwilling BS. Activation of the hypothalamic-pituitary-adrenal axis differentially affects the anti-mycobacterial activity of macrophages from BCG-resistant and susceptible mice. J Neuroimmunology. 1994;53:181–187. doi: 10.1016/0165-5728(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Brown DH, Sheridan J, Pearl D, Zwilling BS. Regulation of mycobacterial growth by the hypothalamus-pituitary-adrenal axis: differential responses of Mycobacterium bovis BCG-resistant and -susceptible mice. Infect Immun. 1993;61:4793–4800. doi: 10.1128/iai.61.11.4793-4800.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DH, LaFuse W, Zwilling BS. Cytokine-mediated activation of macrophages from Mycobacterium bovis BCG-resistant and -susceptible mice: differential effects of corticosterone on antimycobacterial activity and expression of the Bcg gene (candidate Nramp) Infect Immun. 1995;63:2983–2988. doi: 10.1128/iai.63.8.2983-2988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IN, Glynn AA, Plant J. Inbred mouse strain resistance to Mycobacterium lepraemurium follows the Ity/Lsh pattern. Immunology. 1982;47:149–156. [PMC free article] [PubMed] [Google Scholar]
- Canonne-Hergaux F, Gruenheid S, Ponka P, Gros P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood. 1999;93:4406–4417. [PubMed] [Google Scholar]
- Cervino AC, Lakiss S, Sow O, Hill AV. Allelic association between the NRAMP1 gene and susceptibility to tuberculosis in Guinea-Conakry. Ann Hum Genet. 2000;64:507–512. doi: 10.1046/j.1469-1809.2000.6460507.x. [DOI] [PubMed] [Google Scholar]
- de Chastellier C, Frehel C, Offredo C, Skamene E. Implication of phagosome-lysosome fusion in restriction of Mycobacterium avium growth in bone marrow macrophages from genetically resistant mice. Infect Immun. 1993;61:3775–3784. doi: 10.1128/iai.61.9.3775-3784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Nelson H, Nelson N. The family of SMF metal ion transporters in yeast cells. J Biol Chem. 2000;275:33388–33394. doi: 10.1074/jbc.M004611200. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Blackwell JM, Bradley DJ. Expression of the natural resistance gene Lsh in resident liver macrophages. Infect Immun. 1984;43:1033–1040. doi: 10.1128/iai.43.3.1033-1040.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C, Alonso JM, Le Jean M, Ecker JR, Briat JF. Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J. 2000;347:749–755. [PMC free article] [PubMed] [Google Scholar]
- Davies EV, Singleton AM, Blackwell JM. Differences in Lsh gene control over systemic Leishmania major and Leishmania donovani or Leishmania mexicana mexicana infections are caused by differential targeting to infiltrating and resident liver macrophage populations. Infect Immun. 1988;56:1128–1134. doi: 10.1128/iai.56.5.1128-1134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito L, Hill NJ, Pritchard LE, Cucca F, Muxworthy C, Merriman ME, et al. Genetic analysis of chromosome 2 in type 1 diabetes: analysis of putative loci IDDM7, IDDM12, and IDDM13 and candidate genes NRAMP1 and IA-2 and the interleukin-1 gene cluster. IMDIAB Group. Diabetes. 1998;47:1797–1799. doi: 10.2337/diabetes.47.11.1797. [DOI] [PubMed] [Google Scholar]
- Evans CAW, Harbuz MS, Ostenfeld T, Norrish A, Blackwell JM. Nramp1 is expressed in neurons and is associated with behavioural and immune responses to stress. Neurogenetics. 2001;3:69–78. doi: 10.1007/s100480100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MD, Trenor CC, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nature Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: Evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MJ, Jones EE, Levy ER, Martin RL, Ponnambalam S, Monaco AP. Identification of a di-leucine motif within the C terminus domain of the Menkes disease protein that mediates endocytosis from the plasma membrane. J Cell Sci. 1999;112:1721–1732. doi: 10.1242/jcs.112.11.1721. [DOI] [PubMed] [Google Scholar]
- Gao PS, Fujishima S, Mao XQ, Remus N, Kanda M, Enomoto T, et al. Genetic variants of NRAMP1 and active tuberculosis in Japanese populations. International Tuberculosis Genetics Team. Clin Genet. 2000;58:74–76. doi: 10.1034/j.1399-0004.2000.580113.x. [DOI] [PubMed] [Google Scholar]
- Garner B, Roberg K, Brunk UT. Endogenous ferritin protects cells with iron-laden lysosomes against oxidative stress. Free Radic Res. 1998;29:103–114. doi: 10.1080/10715769800300121. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Ben-Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Riederer P, Youdim MB. Molecular mechanisms for neurodegeneration. Synergism between reactive oxygen species, calcium, and excitotoxic amino acids. Adv Neurol. 1996;69:177–194. [PubMed] [Google Scholar]
- Goswami T, Bhattacharjee A, Babal P, Searle S, Moore E, Li M, Blackwell JM. Natural-resistance-associated macrophage protein 1 is an H+/bivalent cation antiporter. Biochem J. 2001;354:511–519. doi: 10.1042/0264-6021:3540511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Buschman E, Skamene E. Regulation of host resistance to Mycobacterium intracellulare in vivo and in vitro by the Bcg gene. Immunogenetics. 1989;30:218–221. doi: 10.1007/BF02421210. [DOI] [PubMed] [Google Scholar]
- Greenwood CM, Fujiwara TM, Boothroyd LJ, Miller MA, Frappier D, Fanning EA, et al. Linkage of tuberculosis to chromosome 2q35 loci, including NRAMP1, in a large aboriginal Canadian family. Am J Hum Genet. 2000;67:405–416. doi: 10.1086/303012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S, Cellier M, Vidal S, Gros P. Identification and characterization of a second mouse Nramp gene. Genomics. 1995;25:514–525. doi: 10.1016/0888-7543(95)80053-o. [DOI] [PubMed] [Google Scholar]
- Gruenheid S, Pinner E, Desjardins M, Gros P. Natural resistance to infections with intracellular pathogens: The Nramp1 protein is recruited to the membrane of the phagosome. J Exp Med. 1997;185:717–730. doi: 10.1084/jem.185.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S, Canonne-Hergaux F, Gauthier S, Hackam DJ, Grinstein S, Gros P. The iron transport protein NRAMP2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. J Exp Med. 1999;189:831–841. doi: 10.1084/jem.189.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Hackam DJ, Rotstein OD, Zhang W, Gruenheid S, Gros P, Grinstein S. Host resistance to intracellular infection: mutation of natural resistance-associated macrophage protein 1 (Nramp1) impairs phagosomal acidification. J Exp Med. 1998;188:351–364. doi: 10.1084/jem.188.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Sakisaka S, Kawaguchi T, Kimura R, Taniguchi E, Koga H, et al. Copper does not alter the intracellular distribution of ATP7B, a copper-transporting ATPase. Biochem Biophys Res Commun. 2000;275:871–876. doi: 10.1006/bbrc.2000.3403. [DOI] [PubMed] [Google Scholar]
- Harris ED. Cellular copper transport and metabolism. Annu Rev Nutr. 2000;20:291–310. doi: 10.1146/annurev.nutr.20.1.291. [DOI] [PubMed] [Google Scholar]
- Hill NJ, Lyons PA, Armitage N, Todd JA, Wicker LS, Peterson LB. NOD Idd5 locus controls insulitis and diabetes and overlaps the orthologous CTLA4/IDDM12 and NRAMP1 loci in humans. Diabetes. 2000;49:1744–1777. doi: 10.2337/diabetes.49.10.1744. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Faucheux BA. Iron metabolism and Parkinson's disease. Mov Disord. 1998;13:39–45. [PubMed] [Google Scholar]
- Hofmeister A, Neibergs HL, Pokorny RM, Galanduik S. The natural resistance-associated macrophage protein gene is associated with Crohn's disease. Surgery. 1997;122:173–179. doi: 10.1016/s0039-6060(97)90006-4. [DOI] [PubMed] [Google Scholar]
- Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med. 2000;192:1237–1248. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Kienzl E, Rumpelmaier G, Paulus W, Riederer P, Stachelberger H, et al. Iron and ferritin in substantia nigra in Parkinson's disease. Adv Neurol. 1993;60:267–272. [PubMed] [Google Scholar]
- Kaye PM, Blackwell JM. Lsh, antigen presentation and the development of CMI. Res Immunol. 1989;140:810–815. doi: 10.1016/0923-2494(89)90038-2. [DOI] [PubMed] [Google Scholar]
- Kehres DG, Zaharik ML, Finlay BB, Maguire ME. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol. 2000;36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- Kramnik I, Radzioch D, Skamene E. T-helper 1-like subset selection in Mycobacterium bovis bacillus Calmette-Guérin-infected resistant and susceptible mice. Immunology. 1994;81:618–625. [PMC free article] [PubMed] [Google Scholar]
- Kuhn DE, Baker BD, Lafuse WP, Zwilling BS. Differential iron transport into phagosomes isolated from the RAW264.7 macrophage cell lines transfected with Nramp1Gly169 or Nramp1Asp169. J Leukoc Biol. 1999;66:113–119. doi: 10.1002/jlb.66.1.113. [DOI] [PubMed] [Google Scholar]
- Kuhn DE, Lafuse WP, Zwilling BS. Iron transport into mycobacterium avium-containing phagosomes from an Nramp1 (Gly169)-transfected RAW264.7 macrophage cell line. J Leukoc Biol. 2001;69:43–49. [PubMed] [Google Scholar]
- Lafuse WP, Alvarez GR, Zwilling BS. Regulation of Nramp1 mRNA stability by oxidants and protein kinase C in RAW264.7 macrophages expressing Nramp1 (Gly169) Biochem J. 2000;351:687–696. [PMC free article] [PubMed] [Google Scholar]
- Lang T, Prina E, Sibthorpe D, Blackwell JM. Nramp1 transfection transfers Ity/Lsh/Bcg-related pleiotropic effects on macrophage activation: Influence on antigen processing and presentation. Infect Immun. 1997;65:380–386. doi: 10.1128/iai.65.2.380-386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq V, Lebastard M, Belkaid Y, Louis J, Milon G. The outcome of the parasitic process initiated by Leishmania infantum in laboratory mice. a tissue-dependent pattern controlled by the Lsh and MHC loci. J Immunol. 1996;157:4537–4545. [PubMed] [Google Scholar]
- Maliarik MJ, Chen KM, Sheffer RG, Rybicki BA, Major ML, Popovich J, Jr, Iannuzzi MC. The natural resistance-associated macrophage protein gene in African Americans with sarcoidosis. Am J Respir Cell Mol Biol. 2000;22:672–675. doi: 10.1165/ajrcmb.22.6.3745. [DOI] [PubMed] [Google Scholar]
- Marquet S, Sanchez FO, Arias M, Rodriguez J, Paris SC, Skamene E, et al. Variants of the human NRAMP1 gene and altered human immunodeficiency virus infection susceptibility. J Infect Dis. 1999;180:1521–1525. doi: 10.1086/315091. [DOI] [PubMed] [Google Scholar]
- Medina E, North RA. The Bcg gene (Nramp1) does not determine resistance of mice to virulent Mycobacterium tuberculosis. Ann NY Acad Sci. 1996a;797:257–259. doi: 10.1111/j.1749-6632.1996.tb52970.x. [DOI] [PubMed] [Google Scholar]
- Medina E, North RJ. Evidence inconsistent with a role for the Bcg gene (Nramp1) in resistance of mice to infection with virulent Mycobacterium tuberculosis. J Exp Med. 1996b;183:1045–1051. doi: 10.1084/jem.183.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhaup G. Amyloid precursor protein, copper and Alzheimer's disease. Biomed Pharmacother. 1997;51:105–111. doi: 10.1016/s0753-3322(97)86907-7. [DOI] [PubMed] [Google Scholar]
- North RJ, LaCourse R, Ryan L, Gros P. Consequence of Nramp1 deletion to Mycobacterium tuberculosis infection in mice. Infect Immun. 1999;67:5811–5814. doi: 10.1128/iai.67.11.5811-5814.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petris MJ, Mercer JF. The Menkes protein (ATP7A; MNK) cycles via the plasma membrane both in basal and elevated extracellular copper using a C-terminal dileucine endocytic signal. Hum Mol Genet. 1999;8:2107–2115. doi: 10.1093/hmg/8.11.2107. [DOI] [PubMed] [Google Scholar]
- Pinner E, Gruenheid S, Raymond M, Gros P. Functional complementation of the yeast divalent cation transporter family SMF by NRAMP2, a member of the mammalian natural resistance-associated macrophage protein family. J Biol Chem. 1997;272:28933–28938. doi: 10.1074/jbc.272.46.28933. [DOI] [PubMed] [Google Scholar]
- Portnoy ME, Liu XF, Culotta VC. Saccharomyces cerevisiae expresses three functionally distinct homologues of the nramp family of metal transporters. Mol Cell Biol. 2000;20:7893–7902. doi: 10.1128/mcb.20.21.7893-7902.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puliti M, Radzioch D, Mazzolla R, Barluzzi R, Bistoni F, Blasi E. Influence of the Bcg locus on macrophage response to the dimorphic fungus Candida albicans. Infect Immun. 1995;63:4170–4173. doi: 10.1128/iai.63.10.4170-4173.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach TI, Kiderlen AF, Blackwell JM. Role of inorganic nitrogen oxides and tumor necrosis factor-alpha in killing Leishmania donovani amastigotes in gamma interferon-lipopolysaccharide-activated macrophages from Lshs and Lshr congenic mouse strains. Infect Immun. 1991;59:3935–3944. doi: 10.1128/iai.59.11.3935-3944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues V, Cheah VPY, Ray K, Chia W. malvolio, the Drosophila homologue of mouse Nramp1 (Bcg), is expressed in macrophages and in the nervous system and is required for normal taste behaviour. EMBO J. 1995;14:3007–3020. doi: 10.1002/j.1460-2075.1995.tb07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Park YK, Bai GH, Kim SJ, Park SN, Kang S. 3′UTR. polymorphisms in the NRAMP1 gene are associated with susceptibility to tuberculosis in Koreans. Int J Tuberc Lung Dis. 2000;4:577–580. [PubMed] [Google Scholar]
- Sanjeevi CB, Miller EN, Dabadghao P, Rumba I, Shtauvere A, Denisova A, et al. Polymorphism at NRAMP1 and D2S1471 loci associated with juvenile rheumatoid arthritis. Arthritis Rheum. 2000;43:1397–1404. doi: 10.1002/1529-0131(200006)43:6<1397::AID-ANR25>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Searle S, Blackwell JM. Evidence for a functional repeat polymorphism in the promoter of the human NRAMP1 gene that correlates with autoimmune versus infectious disease susceptibility. J Med Genet. 1999;36:295–299. [PMC free article] [PubMed] [Google Scholar]
- Searle S, Bright NA, Roach TIA, Atkinson PGP, Barton CH, Meloen RH, Blackwell JM. Localisation of Nramp1 in macrophages: modulation with activation and infection. J Cell Sci. 1998;111:2855–2866. doi: 10.1242/jcs.111.19.2855. [DOI] [PubMed] [Google Scholar]
- Shaw M-A, Clayton D, Atkinson SE, Williams H, Miller N, Sibthorpe D, Blackwell JM. Linkage of rheumatoid arthritis to the candidate gene NRAMP1 on 2q35. J Med Genet. 1996;33:672–677. doi: 10.1136/jmg.33.8.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M-A, Collins A, Peacock CS, Miller EN, Black GF, Sibthorpe D, et al. Evidence that genetic susceptibility to Mycobacterium tuberculosis in a Brazilian population is under oligogenic control: linkage study of the candidate genes NRAMP1 and TNFA. Tuberc Lung Dis. 1997;78:35–45. doi: 10.1016/s0962-8479(97)90014-9. [DOI] [PubMed] [Google Scholar]
- Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- Shirakawa T, Enomoto T, Shimazu S-I, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- Singal DP, Li J, Zhu Y, Zhang G. NRAMP. 1 gene polymorphisms in patients with rheumatoid arthritis. Tissue Antigens. 2000;55:44–47. doi: 10.1034/j.1399-0039.2000.550107.x. [DOI] [PubMed] [Google Scholar]
- Skamene E, Gros P, Forget A, Patel PJ, Nesbitt MN. Regulation of resistance to leprosy by chromosome 1 locus in the mouse. Immunogenetics. 1984;19:117–124. doi: 10.1007/BF00387854. [DOI] [PubMed] [Google Scholar]
- Soo S-S, Villarreal Ramos B, Khan CM, Hormaeche CE, Blackwell JM. Genetic control of immune response to recombinant antigens carried by an attenuated Salmonella typhimurium vaccine strain: Nramp1 influences T-helper subset responses and protection against leishmanial challenge. Infect Immun. 1998;66:1910–1917. doi: 10.1128/iai.66.5.1910-1917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JJ, Oca MJ, Rubin BY, Anderson SL, Murray HW. Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol. 1988;140:3971–3977. [PubMed] [Google Scholar]
- Strausak D, La Fontaine S, Hill J, Firth SD, Lockhart PJ, Mercer JF. The role of GMXCXXC metal binding sites in the copper-induced redistribution of the Menkes protein. J Biol Chem. 1999;274:11170–11177. doi: 10.1074/jbc.274.16.11170. [DOI] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schaible UE, Russell DG. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- Supeck F, Supekova L, Nelson H, Nelson N. A. yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc Natl Acad Sci USA. 1996;93:5105–5110. doi: 10.1073/pnas.93.10.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi M, Yoshida T, Takegawa K, Kishi F. Functional analysis of the human NRAMP family expressed in fission yeast. Biochem J. 1999;344:211–219. [PMC free article] [PubMed] [Google Scholar]
- Vanderwerf S, Cooper MJ, Stetsenko IV, Lutsenko S. Copper specifically regulates intracellular phosphorylation of the Wilson's disease protein, a human copper-transporting ATPase. J Biol Chem. 2001;26 doi: 10.1074/jbc.M102055200. July 26 (epub) [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, et al. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- Vidal SM, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Vidal S, Tremblay ML, Govoni G, Gauthier S, Sebastiani G, Malo D, et al. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med. 1995;182:655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskoboinik I, Strausak D, Greenough M, Brooks H, Petris M, Smith S, et al. Functional analysis of the N-terminal CXXC metal-binding motifs in the human Menkes copper-transporting P-type ATPase expressed in cultured mammalian cells. J Biol Chem. 1999;274:22008–22012. doi: 10.1074/jbc.274.31.22008. [DOI] [PubMed] [Google Scholar]
- Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Yang YS, Kim SJ, Kim JW, Koh EM. NRAMP. 1 gene polymorphisms in patients with rheumatoid arthritis in Koreans. J Korean Med Sci. 2000;15:83–87. doi: 10.3346/jkms.2000.15.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan XM, Brunk UT, Olsson AG. Effects of iron- and hemoglobin-loaded human monocyte-derived macrophages on oxidation and uptake of LDL. Arterioscl Throm Vas. 1995;15:1345–1351. doi: 10.1161/01.atv.15.9.1345. [DOI] [PubMed] [Google Scholar]
- Yuan XM, Anders WL, Olsson AG, Brunk UT. Iron in human atheroma and LDL oxidation by macrophages following erythrophagocytosis. Atherosclerosis. 1996;124:61–73. doi: 10.1016/0021-9150(96)05817-0. [DOI] [PubMed] [Google Scholar]
- Zwilling BS, Kuhn DE, Wikoff L, Brown D, LaFuse W. The role of iron in Nramp1 mediated inhibition of mycobacterial growth. Infect Immun. 1999;67:1386–1392. doi: 10.1128/iai.67.3.1386-1392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]