Abstract
Goal pursuit in humans sometimes involves approaching unpleasant and avoiding pleasant stimuli, such as when a dieter chooses to eat vegetables (although he does not like them) instead of doughnuts (which he greatly prefers). Previous neuroscience investigations have established a left–right prefrontal asymmetry between approaching pleasant and avoiding unpleasant stimuli, but these investigations typically do not untangle the roles of action motivation (approach vs. avoidance) and stimulus valence (pleasant vs. unpleasant) in this asymmetry. Additionally, studies on asymmetry have been conducted almost exclusively using electroencephalography and have been difficult to replicate using functional magnetic resonance imaging (fMRI). The present fMRI study uses a novel goal pursuit task that separates action motivation from stimulus valence and a region-of-interest analysis approach to address these limitations. Results suggest that prefrontal asymmetry is associated with action motivation and not with stimulus valence. Specifically, there was increased left (vs. right) activation in dorsolateral prefrontal cortex during approach (vs. avoidance) actions regardless of the stimulus valence, but no such effect was observed for pleasant compared to unpleasant stimuli. This asymmetry effect during approach–avoidance action motivations occurred in the dorsolateral but not orbito-frontal aspects of prefrontal cortex. Also, individual differences in approach–avoidance motivation moderated the effect such that increasing trait approach motivation was associated with greater left-sided asymmetry during approach actions (regardless of the stimulus valence). Together, these results support the notion that prefrontal asymmetry is associated with action motivation regardless of stimulus valence and, as such, might be linked with goal pursuit processes more broadly.
INTRODUCTION
Goal-relevant stimuli in the environment typically can be categorized into goal-consistent stimuli that should be approached or goal-inconsistent stimuli that should be avoided. Once this categorization takes place, the impetus to move toward (or away from) a stimulus, as well as the action itself, is known as approach (or avoidance) motivation. This triage process is relatively straightforward for most animals: move toward positively valenced stimuli and move away from negatively valenced stimuli. For humans, goal pursuit is not always as easy. Although we share with other animals the same general tendencies to approach positive and avoid negative stimuli, one of the defining qualities of human goal pursuit is that we have the capacity to approach negative and avoid positive stimuli if that behavior serves our ultimate goal. For instance, during dieting, someone with a sweet tooth who generally dislikes vegetables might, nonetheless, have a motive to avoid candy and instead snack on broccoli. Previous work has examined the prefrontal cortical asymmetry associated with approaching positive and avoiding negative stimuli (e.g., Sutton & Davidson, 1997). However, with the exception of studies on anger (discussed below), most of these studies have not shed light on whether this asymmetry is driven by stimulus valence (i.e., positive vs. negative) or action motivation (approach vs. avoidance). The present study separates the roles of stimulus valence and action motivation in hemispheric asymmetry for the first time using fMRI.
Neuroscience investigations that did not separate action from valence have repeatedly observed left–right pre-frontal cortical asymmetry between approaching pleasant and avoiding unpleasant stimuli. Research conducted predominantly using electroencephalography (EEG) has found that approach–pleasant is associated with relatively greater activity in left lateral prefrontal cortex (PFC; relative to the right), and avoid–unpleasant is associated with relatively greater activity in right lateral PFC (compared to left) (Sutton & Davidson, 1997; Davidson, Ekman, Saron, & Senulis, 1990). Lesion studies in rats (Robinson, 1979) and humans (Starkstein et al., 1991) have also supported the link between left PFC and approach–pleasant behaviors. Other EEG studies replicate this finding but also do not distinguish between stimulus valence and action motivation. For example, lateral frontal asymmetry (measured by EEG) has been observed in response to smelling a pleasant vanilla odor compared to a neutral odor (Kline, Blackhart, Woodward, Williams, & Schwartz, 2000), and in two studies comparing monetary rewards compared to losses (Miller & Tomarken, 2001; Sobotka, Davidson, & Senulis, 1992). In both of these examples, the valence of the stimulus (pleasant/unpleasant) covaried completely with the behavioral tendency (approach/avoid).
Several fMRI studies have replicated the basic finding of hemispheric asymmetry being associated with approach–pleasant versus avoid–unpleasant, but as in the EEG literature, these studies did not disentangle action from valence. In the two fMRI studies that explicitly examined asymmetry by directly comparing activation in the right and left hemispheres, participants were either passively shown pleasant and unpleasant words (Herrington et al., 2005) or pictures (Canli, Desmond, Zhao, Glover, & Gabrieli, 1998). These studies suggest that asymmetry during pleasant stimuli is associated with increases in left dorsolateral (Herrington et al., 2005) or throughout the left PFC (Canli et al., 1998), and one of the two found unpleasant stimuli to be associated with activation in right PFC (Canli et al., 1998). Another fMRI study examined the relation between hemispheric asymmetry and promotion/prevention regulatory focus (Eddington, Dolcos, Cabeza, Krishnan, & Strauman, 2007), a construct distinct from approach/avoidance but, nonetheless, related (Cunningham, Raye, & Johnson, 2005; Higgins, 1997). The authors found increases in left orbito-frontal PFC when participants were primed with positively valenced adjectives associated with their “ideal self” (promotion regulatory focus) compared to those associated with their “ought self” (prevention regulatory focus). Together, these findings are broadly consistent with the EEG literature but still cannot identify whether the asymmetry is driven by action tendency or stimulus valence, because in each study, pleasant and unpleasant stimuli were likely to have activated approach and avoidance tendencies, respectively.
One possible way to disentangle valence and action is to study an affective–motivational state in which the two are naturally in opposition—approach–pleasant or avoid–unpleasant. The emotion anger is believed to be one such state because it possesses both an unpleasant valence and an approach motive (Carver & Harmon-Jones, 2009; Harmon-Jones & Allen, 1998). Harmon-Jones et al. (2002) and Harmon-Jones and Sigelman (2001) have capitalized on this fact and examined the effect of both state and trait anger on hemispheric asymmetry. Their studies have revealed increased left lateral prefrontal asymmetry in response to an anger induction such as an insult. Supporting this work, another study found that decreased memory for anger following an experimental reduction of activity in left (but not right) lateral PFC using repetitive transcranial magnetic stimulation (rTMS) (van Honk & Schutter, 2006). Together, these studies suggest that asymmetry is associated with action motivation rather than valence.
Although these studies provide one way to separate action and valence, the extent to which the results might generalize to goal pursuit is unclear. Approaching during anger is different than approaching an unpleasant stimulus during goal pursuit. In the context of a goal, individuals presumably engage in self-regulation in order to approach unpleasant stimuli that they would otherwise avoid (e.g., someone who wants to lose weight but doesn’t like to exercise might nonetheless go to the gym every day). In contrast, the tendency to approach during anger seems to be intrinsic to the experience of anger and does not require overriding a natural tendency to avoid. Further, anger appears to be unique as a mental state that is negatively valenced and also approach-motivated, and there is no parallel affective state that is positively valenced and also avoidance-motivated. Thus, it is important to determine if this is unique to anger or generalizes to action versus valence more broadly.
A second caveat to the broad conclusion that lateral pre-frontal asymmetry is associated with approach–avoidance motivation is that, although several studies have replicated the link between approach motivation and increased left prefrontal cortical activation, many studies fail to replicate the finding that avoidance motivation is associated with increased right prefrontal activation (Amodio, Master, Yee, & Taylor, 2008; Hewig, Hagemann, Seifert, Naumann, & Bartussek, 2006; Pizzagalli, Sherwood, Henriques, & Davidson, 2005; Jackson et al., 2003; Coan, Allen, & Harmon-Jones, 2001; Henriques & Davidson, 2000; Kline et al., 2000). Researchers have speculated that this inconsistency might arise because of the theoretical complexity of the avoidance construct (Coan & Allen, 2004), or because approach is not necessarily the opposite of avoidance (Amodio et al., 2008) and, instead, relies upon distinct psychological processes that might not be elicited by the experimental paradigms used to investigate frontal asymmetry. Another explanation is that EEG is not ideally suited to measuring differences in activation between hemispheres because it relies on comparisons in voltage between two sites that are often nonindependent (Allen, Coan, & Nazarian, 2004). If this were the case, convergent evidence from a different neuroimaging modality such as fMRI would help to clarify the inconsistent findings regarding right lateral prefrontal asymmetry and avoidance.
The key limitation in the current literature on asymmetry is the confounding of action and valence. It is difficult to disentangle action and valence because they are most often confounded in the real world—people typically do approach pleasant things and avoid unpleasant things, and it is typically easy to do so—but they are not always confounded. During goal pursuit, action motivation and stimulus valence are sometimes in opposition. Indeed, one reason why goals are challenging and we often fail at them is because success frequently depends on actions that are in opposition to our natural response to a stimulus, such as when a dieter approaches healthy (but unpleasant) foods or a smoker avoids a cigarette. For this reason, a task that models this type of goal pursuit situation (i.e., approaching unpleasant and avoiding pleasant stimuli) is ideally suited to disentangle action from valence.
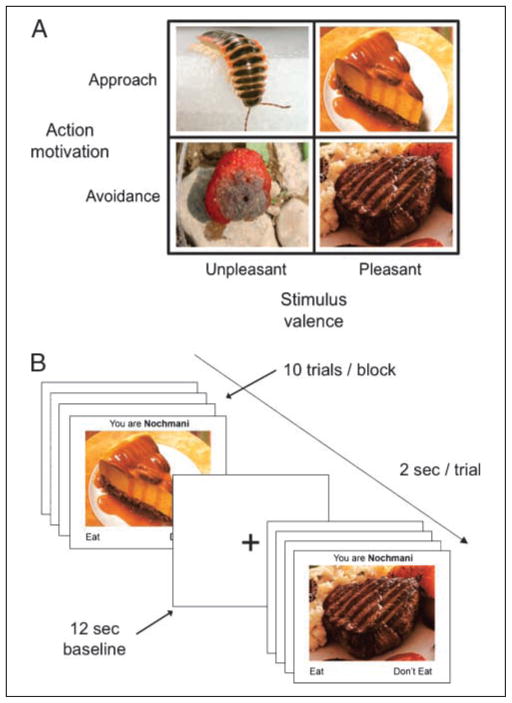
The present study employs a novel task that models goal pursuit in order to cross action motivation (approach/avoidance) with stimulus valence (pleasant/unpleasant) and directly compare the role of these two factors in pre-frontal cortical asymmetry. In the Nochmani paradigm (Berkman, Lieberman, & Gable, 2009), participants first read a fake National Geographic-style article about a newly discovered tribe of people, the Nochmani. From the article, participants learn that the Nochmani are similar to Westerners in their enjoyment of sweets and their distaste for fungi, but are dissimilar in their fondness for eating insects and disgust when eating meats. In the subsequent response time task, Western participants respond whether to “eat” or “not eat” various foods from the perspective of the Nochmani (Figure 1). In this way, the action to “eat” (approach) or “not eat” (avoidance) can be crossed with the valence of the delicious (pleasant) or disgusting (unpleasant) food stimuli into an orthogonal design.
Figure 1.
The Nochmani task. (A) Trials are drawn from one of four experimental conditions defined by the 2 (action motivation: approach/avoidance) × 2 (valence: pleasant/unpleasant) within-subjects factorial design. (B) Two-second trials are presented in blocks of 10 that are composed predominantly (80%) of trials from one cell.
We used fMRI to measure neural responses to trials in each of these conditions. We analyzed the data by generating a priori ROIs in left and right dorsolateral and orbito-frontal cortices and directly comparing left to right activation in each of these regions during the approach, avoidance, pleasant, and unpleasant conditions relative to baseline. Activation in these ROIs was also correlated with a trait measure of approach and avoidance motivation. Based on the neuroimaging literature discussed above, we expected asymmetry to be associated with action motivation rather than stimulus valence. There have been few attempts to identify the source of the asymmetry within PFC. The only EEG study to use a formal localization procedure identified three foci in left dorsolateral prefrontal cortex (DLPFC) that were linked to reward preference (Pizzagalli et al., 2005), and several other EEG studies have identified DLPFC more broadly using informal localization procedures (e.g., scalp topography) (Amodio et al., 2008; Harmon-Jones & Allen, 1998; Sutton & Davidson, 1997). The fMRI studies identified either dorsolateral (Herrington et al., 2005) or orbito-frontal (Eddington et al., 2007) PFC, although the Eddington et al. (2007) study used a manipulation of promotion–prevention focus instead of approach–avoidance. Based on these studies, we predicted that the asymmetry would be localized only to DLPFC, and included orbito-frontal cortex as a comparison region. We also predicted that trait approach–avoidance motivation would moderate asymmetry such that higher levels of approach and avoidance would be associated with increased left- and right-sided asymmetry, respectively.
METHODS
Participants
Seventeen right-handed participants (9 men; ages = 19–28 years, M = 23.4 ± 2.7) were recruited from the UCLA community and paid $25 for their participation. The participants were prescreened to be nonvegetarians. All participants provided written informed consent that was approved by the UCLA Office for Protection of Research Subjects.
Procedure
We generated an fMRI-compatible version of a validated paradigm that was designed to separate action motivation and valence (Berkman et al., 2009). Before entering the scanner, participants read a realistic but fake article about the Nochmani (see http://berkman.bol.ucla.edu/Nochmani.pdf for download). Participants were told to read the article carefully because, subsequently, in the scanner they would be completing a memory task about the Nochmani. Although the subjects were led to believe that the task involved memory, success in the forthcoming task depended only on participants remembering two unusual characteristics of the Nochmani—that they enjoyed eating insects, and were disgusted by eating meat. The Nochmani otherwise share Western tastes in food as they enjoy eating desserts and are disgusted by eating fungus-infested foods. Success rates on the present study and a previous study (Berkman et al., 2009) confirm that participants are easily able to retain this information for the duration of the task. After reading the article, participants completed a self-report measure of trait approach and avoidance motivation (described below).
Next, participants completed a computerized response time task during fMRI acquisition. They were shown a series of trials that each displayed a single picture of a dessert, meat, fungus, or insect. Participants were asked to respond as quickly as possible via keypress whether Nochmani would “eat” or “not eat” the food. Trials were arranged into blocks that represented one of the four cells of the 2 (action motivation: approach/avoid) × 2 (valence:pleasant/unpleasant) design. Each block comprised 80% trials from one cell (e.g., approach–pleasant) and 20% “foil” trials from cells with an opposite action (i.e., avoid–pleasant or avoid–unpleasant). These minority foil trials serve to prevent participants from falling into a response set on a given block (e.g., simply responding “eat” to every trial without paying attention to the content of the image). The duration of each trial was set to be sufficiently long (2 sec) so that participants had enough time to consider each trial—including the foil trials—and that these trials would not act as “oddballs” as in a speeded detection task. The four block types were evenly and randomly distributed throughout the experiment.
The key blocks for unconfounding action and valence are those in which the valence of the stimulus conflicts with the direction of the action required for a correct response. Specifically, these trials occur when responding to images of insects (approach–unpleasant) and meat (avoid–pleasant).
Each trial lasted 2 sec, and participants could respond at any point during that time. The images remained on the screen for the duration of the trial following a response. Trials occurred within 20 blocks of 10 trials each that began with 4 sec of instructions per block and 12 sec of resting fixation between blocks. This yielded a total of 200 trials spread across two 6-min functional runs.
Foam padding was placed around participants’ heads to reduce motion. Stimuli were presented on LCD goggles, and responses were recorded on a magnet-safe button box placed in the right hand.
Materials
The pictures of food used in the task belonged to one of four categories: fungi, desserts, meats, or insects. There were 40 pictures in each category for a total of 160 color pictures. The four categories were pre-rated to be equivalent on absolute ratings of valence (i.e., the fungi and insects are equally unpleasant, the desserts and meats were equally pleasant, and all four have equal absolute values on valence; Berkman et al., 2009). The images were standardized on brightness, contrast, and fixed at a resolution of 500 by 375 to maintain a 4:3 width-to-height ratio.
Trait behavioral activation and inhibition was measured using the Behavioral Inhibition/Behavioral Activation Scales (BIS/BAS; Carver & White, 1994). The BAS has three subscales: The BAS-Drive subscale measures persistent pursuit of goals (e.g., “I go out of my way to get things I want”), α = .73; the BAS-Fun Seeking subscale measures desire for new rewards (e.g., “I crave excitement and new sensations”), α = .78; and the BAS-Reward Responsiveness subscale relates to positive responses to reward (e.g., “When I get something I want, I feel excited and energized”), α = .68. The reliability for the overall BAS was .84. The BIS scale is thought to be unidimensional, and taps sensitivity to negative events (e.g., “Criticism or scolding hurts me quite a bit”), α = .82.
fMRI Data Acquisition and Analysis
Data were acquired on a Siemens Allegra 3-T scanner at the UCLA Ahmanson-Lovelace Brainmapping Center. High-resolution structural T2-weighted echo-planar images (spin-echo; TR = 5000 msec; TE = 33 msec; matrix size 128 × 128; 36 sagittal slices; FOV = 20 cm; 3 mm thick, skip 1 mm) were acquired coplanar with the functional scans. Two functional scans lasting 6 min each were acquired during the task (echo-planar T2*-weighted gradient-echo; TR = 2000 msec; TE = 25 msec; flip angle = 90°; matrix size 64 × 64; 34 axial slices; FOV = 20 cm; 3 mm thick; skip 1 mm). The imaging data were analyzed using SPM5 (Well-come Department of Cognitive Neurology, Institute for Neurology, London, UK). Images from each participant were realigned to correct for head motion, normalized into the Montreal Neurological Institute (MNI) standard stereotactic space, and smoothed with an 8-mm Gaussian kernel, full width at half maximum.
The design was modeled as a blocked 2 (action: approach/avoidance) × 2 (valence: pleasant/unpleasant) factorial design with eight 12-sec fixation-cross periods per run forming a baseline. Linear contrasts were computed to assess the difference in neural activity during each condition compared to baseline. For all analyses, individual-participant contrasts were generated with fixed-effects models and then grouped into a random-effects model for greater generalizability.
A priori ROIs were created for the dorsolateral and orbito-frontal cortices, separately for left and right, using the Wake Forest University Pickatlas Tool (Maldjian, Laurienti, Kraft, & Burdette, 2003) based on the Automated Anatomical Labeling atlas (AAL; Tzourio-Mazoyer et al., 2002). The dorsolateral ROIs were a combination of AAL’s superior and middle frontal gyri, pars opercularis, and pars triangularis, all superior to and including the axial plane at MNI z = 2 (Figure 2A). The orbitofrontal ROIs were a combination of AAL’s orbital subregions of the superior, middle, and inferior frontal gyri, all inferior to and including the axial plane at MNI z = 0 (Figure 2B). All voxels within each of the four resulting ROIs were averaged to create a total of four values per participant per condition.
Figure 2.
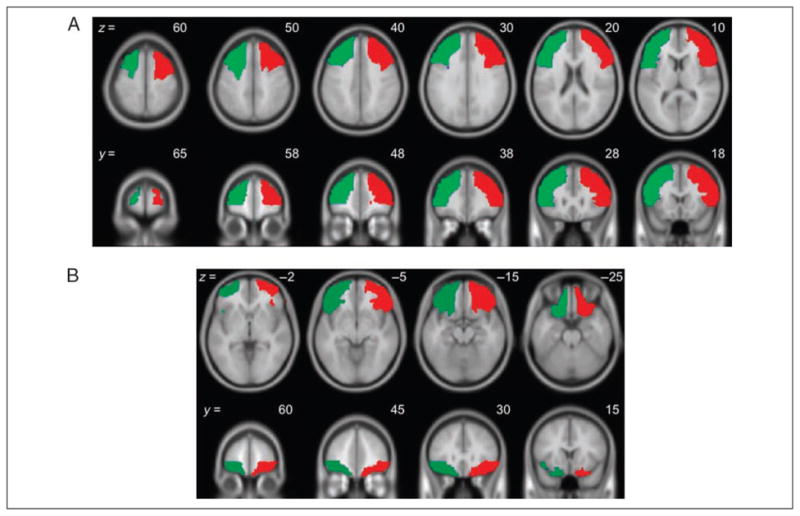
The ROIs for the main analysis. Further details are provided in the text. (A) The dorsolateral region (z ≥ 2) and (B) the orbito-frontal region (z ≤ 0) defined using the AAL atlas.
The primary research questions regarding hemispheric asymmetry were examined in the prefrontal ROIs. Following convention in the EEG literature (e.g., Harmon-Jones et al., 2002), an asymmetry score was calculated for each participant for the dorsolateral and orbito-frontal ROIs. This score was then entered as the dependent measure in a within-subjects factorial ANOVA. Further analyses examined whether trait-level approach and avoidance motivation moderated activation in the ROIs across task conditions. Because of the strong a priori hypotheses about hemispheric asymmetry, and because our dependent measure in the 2 × 2 ANOVA and regression analyses was a single asymmetry score for each of the two regions (instead of thousands of voxels), we used a traditional p value of .05. All neuroimaging results are reported in MNI coordinates.
RESULTS
Behavioral Responses
As in previous work, participants achieved a high rate of accuracy in all conditions (M = 97.4%) and no participant was below 95% accuracy (Berkman et al., 2009). Because of this high rate of accuracy, all trials were included in the blockwise analysis.
Participants were significantly faster to approach [M = 753.6 msec, SD = 25.0 msec] than to avoid [M = 875.8 msec, SD = 59.2 msec; t(16) = 5.31, p < .01]. There were no differences in response time between the pleasant [M = 802.1 msec, SD = 43.7 msec] and unpleasant [M = 826.3 msec, SD = 127.8 msec; t(16) = 0.56, ns] valence conditions. The Action × Valence interaction was not significant [t(16) = 1.60, ns]. Because of the main effect of action, mean response time for each block was entered into the model as a covariate.
Main Effects of Task Condition
The main dependent measures were the left–right asymmetry scores for the dorsolateral and orbito-frontal ROIs. These scores were computed for both of the ROIs for each of the four conditions relative to fixation baseline. For example, the dorsolateral asymmetry score in the approach–pleasant condition was computed as: (approach–pleasant R DLPFC – baseline) − (approach–pleasant L DLPFC -baseline). Once these scores were computed, 2 × 2 within-subjects ANOVAs were run on the asymmetry scores separately for each ROI.
A main effect of action and no main effect of valence would support the hypothesis that dorsolateral asymmetry is driven by action motivation and not by stimulus valence. In the dorsolateral ROIs, there was a main effect of action [F(1, 16) = 7.07, p < .02], no main effect of valence [F(1, 16) = 0.59, ns], and no interaction [F(1, 16) = 0.24, ns; Figure 3A]. Specifically, there was greater relative left-sided asymmetry in the dorsolateral ROI during approach (M = 0.075, SD = 0.23) than avoidance (M = −0.025, SD = 0.23), but not during pleasant (M = 0.056, SD = 0.34) versus unpleasant (M = 0.005, SD = 0.17) stimuli. The null interaction suggests that the relative left-sided asymmetry during approach versus avoidance for the pleasant stimuli (M = 0.071, SD = 0.35) was not different from the asymmetry between approach and avoidance for unpleasant stimuli (M = 0.128, SD = 0.24).
Figure 3.
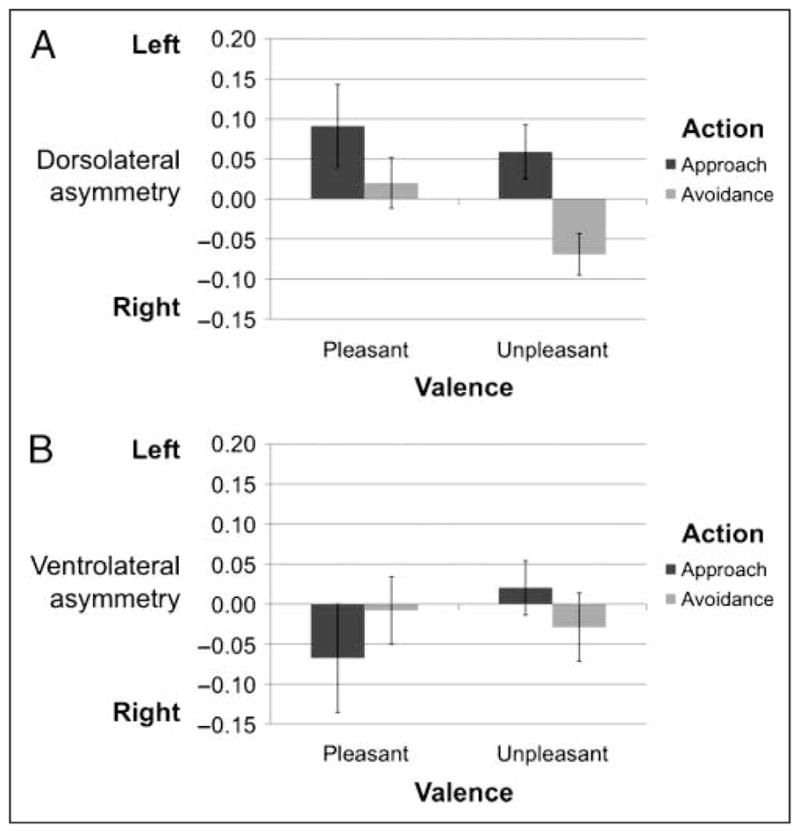
Asymmetry scores in (A) dorsolateral and (B) orbito-frontal PFC. Each score was generated by calculating the average left–right change from baseline during each condition defined by the 2 (action) × 2 (valence) factorial design. The only significant effect is the main effect of action in the dorsolateral region [F(1, 16) = 7.07, p < .02]. This effect was marginally significantly larger than the effect in the orbito-frontal region [F(1, 16) = 3.83, p < .07]. Error bars represent two standard errors.
Next, we tested the same hypotheses in the orbito-frontal region to examine the spatial specificity of the asymmetry. If prefrontal asymmetry was localized only to the dorsolateral region, we would expect to find no effects of task condition on asymmetry. As expected, there were no main effects in the orbito-frontal ROIs of action [F(1, 16) = 0.01, ns] or valence [F(1, 16) = 0.06, ns], and no Action × Valence interaction [F(1, 16) = 0.42, ns; Figure 3B]. The interaction between action and region testing whether the effect of action on asymmetry was greater in the dorsolateral than orbito-frontal region was marginally significant [F(1, 16) = 3.83, p < .07].
We then conducted a series of post hoc paired-samples t tests to investigate whether the increased asymmetry during approach compared to avoidance actions was being driven by increased asymmetry during approach relative to baseline (i.e., left-baseline > right-baseline) or decreased asymmetry during avoidance relative to baseline (i.e., right-baseline > left-baseline). Neither comparison was significant (both ps > .2), suggesting that the main effect of action is driven by a combination of the increase in asymmetry during approach and decrease in asymmetry during avoidance.
Correlations with Trait Measures of Approach–Avoidance
We demonstrated above that, on average, action motivation is associated with dorsolateral prefrontal asymmetry. Next, based on prior findings that individual differences in approach–avoidance motivation are associated with asymmetry as measured by EEG (Amodio, Shah, Sigelman, Brazy, & Harmon-Jones, 2004; Harmon-Jones & Allen, 1998), we tested whether trait-level motivation would moderate the main effect of action motivation on asymmetry described above. To do this, we correlated asymmetry in each ROI during each condition with scores on Carver and White’s (1994) BAS and BIS. For example, a positive correlation between approach motivation and left–right asymmetry during approach actions would suggest that, even though there is a main effect of action on average, individuals with higher approach motivation tend to show greater left-lateralized asymmetry than those with lower approach motivation during that condition.
Left-sided asymmetry was correlated with the overall BAS (controlling for BIS) during approach relative to baseline [overall: r(16) = .52, p < .05; approach–pleasant: r(16) = .37, ns; approach–unpleasant: r(16) = .29, ns]. The relationship between the overall BAS and approach actions, controlling for BIS, is shown in Figure 4. Asymmetry was not associated with BAS during avoidance actions relative to baseline [r(16) = .29, ns], or with viewing pleasant [r(16) = .36, ns] or unpleasant [r(16) = .35, ns] stimuli compared to baseline. Because of the relatively small sample size, the nonsignificant correlations were not significantly different from the significant association between BAS and approach action motivation. These correlations indicate that trait approach (but not avoidance) motivation moderates asymmetry specifically during approach actions, but not during viewing of pleasant stimuli or avoidance actions.
Figure 4.
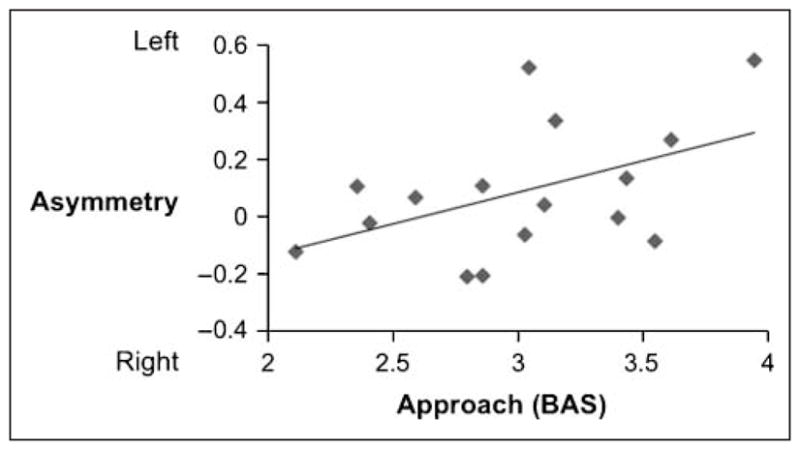
The correlation between left-sided asymmetry and trait approach motivation (as measured by the BAS scale) during approach actions [r(16) = .52, p < .05].
Based on this finding for the overall (i.e., average) BAS, we also examined the correlation of each of the BAS sub-scales during approach baseline. The subscale correlations revealed a similar pattern of results in terms of direction and magnitude, although the only subscale that yielded a significant correlation with asymmetry was the Drive subscale during approach baseline [r(16) = .55, p < .05].
Finally, we examined the relationship of trait avoidance (as measured with the BIS scale) with asymmetry. A negative correlation between avoidance and asymmetry would indicate that higher levels of trait avoidance were associated with greater right-sided asymmetry (because asymmetry is always calculated as left–right). Asymmetry was not significantly correlated with BIS (controlling for overall BAS) in any of the four conditions individually, nor with any of the marginal averages (e.g., approach actions averaging across pleasant and unpleasant valences; all ps > .1).
DISCUSSION
The present study is the first to use fMRI to disentangle the contributions of action motivation and stimulus valence in hemispheric asymmetry. Previous fMRI studies demonstrated that positively and negatively valenced stimuli, that naturally induce a tendency to approach or avoid, respectively, are associated with asymmetry, but these studies could not specify whether the asymmetry was linked to action motivation, valence, or both. Our findings address this limitation by demonstrating that asymmetry is associated with action rather than valence, and specifically, that approach motivation is associated with increased activation in left relative to right PFC. Furthermore, the prior work untangling action from valence has used EEG and has yet to be replicated using an alternative neuroimaging method such as fMRI. Using fMRI in combination with ROIs that were guided by previous work allowed us to replicate findings from the EEG literature to localize the asymmetry to the dorsolateral and not the orbital aspects of PFC. Additionally, consistent with our expectations, trait motivation moderated the asymmetry effect such that individuals with higher levels of trait approach motivation demonstrated increased asymmetry during approach actions.
We used the novel Nochmani experimental paradigm (Berkman et al., 2009) to completely cross two variables that had previously been confounded—action motivation (approach vs. avoidance) and stimulus valence (pleasant vs. unpleasant). This allowed us to independently assess the degree to which hemispheric asymmetry was linked to action and valence. The data suggest that left-sided asymmetry is associated with approach actions and not viewing of pleasant stimuli per se. These results are consistent with findings by Harmon-Jones and Sigelman (2001) and Harmon-Jones and Allen (1997, 1998) that prefrontal asymmetry is associated with state and trait anger, and that anger is an approach-related emotion (Carver & Harmon-Jones, 2009). The fact that we observed increased left-lateral asymmetry in response to nonanger unpleasant emotions (e.g., disgust) when they were coupled with an approach response adds convergent evidence to this view.
Our finding that prefrontal hemispheric asymmetry is tied to action motivation rather than stimulus valence fits with current goal theory and our understanding of the functions of PFC. In a hierarchical model of goal pursuit, behavior can be determined by both proximal and distal motives that vary in abstraction (Elliot, 2006; Elliot & Church, 1997). For example, in the Nochmani paradigm, behavior can be motivated by either the proximal, lower-level, hedonic value of the stimulus (i.e., motivation to approach pleasant and avoid unpleasant stimuli), or by the more distal, higher-level, instrumental goal of success (i.e., motivation to respond correctly even if that sometimes requires approaching unpleasant and avoiding pleasant stimuli). As with many real-world goals, acting in line with the higher-level goal—even when it is in opposition to the lower-level hedonic value—is the only way to reliably attain success on the Nochmani task. Thus, one novel and important feature of the Nochmani task is that it contains conditions that require top–down control to promote higher-level goals over lower-level tendencies. The high rates of success by the participants in the present investigation suggest that they were engaging control by acting according to the higher-level goal and not the lower-level stimulus-driven response. At a neuroanatomical level, there is broad consensus that the main role of PFC is to plan and coordinate precisely this kind of goal-directed action that often requires control (Fuster, 2008; Miller & Cohen, 2001). In contrast, the experience of pleasant and unpleasant affective states is often associated with activation in subcortical structures such as the amygdala, insula, anterior cingulate cortex, and basal ganglia (Phillips et al., 2004; Phan, Wager, Taylor, & Liberzon, 2002; Whalen et al., 2001; Damasio et al., 2000; Mayberg et al., 2000; Morris, Ohman, & Dolan, 1998; Drevets et al., 1997; George, Ketter, Parekh, Herscovitch, & Post, 1996). One meta-analysis explicitly searched for hemispheric asymmetries between pleasant and unpleasant emotions and found no such asymmetries (Wager, Phan, Liberzon, & Taylor, 2003). At least, in the current study, where attaining an abstract goal was linked to action motivation, it makes sense that action motivation, and not stimulus valence, is associated with asymmetrical prefrontal activity. It is an open question whether this interpretation would hold for other findings in this literature.
The notion that there are hemispheric asymmetries in approach compared to avoidance action motivation is broadly consistent with prior work investigating processes that can be characterized as predominantly approach or avoidance. For instance, engaging in an avoidant emotion regulation strategy (e.g., by taking a distant or detached perspective on emotional stimuli) has been shown to produce activation in right lateral PFC (Kalisch et al., 2005). Being distracted during a painful stimulus has also been shown to increase activation in this region (Bantick et al., 2002). Finally, Mitchell et al. (2007) found increases in right dorsolateral PFC when participants attempt to avoid thinking about white bears. On the other hand, researchers have noted left lateral prefrontal activation during approach-oriented tasks such as the planning and execution of tool use (Kroliczak & Frey, 2009; Frey, 2007, 2008), action selection (Schluter, Krams, Rushworth, & Passing-ham, 2001), and action planning (Bohlhalter et al., 2008). Although suggestive, these studies often do not explicitly compare left to right activation, and thus, the current study makes an important contribution by doing so. It is also unclear whether action planning is intrinsically approach-oriented, or whether the planning of avoidance actions is different but simply not yet been investigated. Future research can build upon these studies with the aim of clarifying the action motivation of their tasks and directly assessing hemispheric asymmetries.
Our use of a priori ROIs to directly compare activation in left and right PFC during approach and avoidance actions is novel, and allows for several interesting inferences. First, although functionally defined ROIs have been compared in the past (e.g., Herrington et al., 2005), the regions used in the present article are relatively large for fMRI. The fact that we were able to replicate findings from EEG, which has a larger spatial extent than fMRI, suggests that using large ROIs can be a useful tool to link across the two methods. Second, it is interesting to note that asymmetries emerge in an ROI analysis although there have been no obvious asymmetries in previous fMRI studies of action motivation (e.g., Wager et al., 2003). It might be the case that asymmetries between approach- and avoidance-related action motivations may be somewhat distributed across DLPFC, or occur consistently at small number of sites that are each below traditional significance thresholds. Besides pointing to the general utility in comparing the results from ROI and whole-brain analyses, this observation highlights the need for future research to better understand the psychological and neural components of approach-related motivation and actions.
Finally, we found that individuals with higher trait-level approach motivation showed increased left-sided asymmetry during approach actions. This correlation was specific to approach actions and did not generalize to avoidance actions, or to either valence condition averaging across action motivation. Interestingly, trait-level avoidance motivation (as measured by Carver & White’s, 1994 BIS scale) was not associated with asymmetry in any condition. The finding that BIS scores were not associated with asymmetry replicates other findings (e.g., Amodio et al., 2008), and is consistent with the notion that this scale is relevant to conflict monitoring and detection more than avoidance actions per se. Thus, the fact that the present study was designed to maximize correct responses may contribute to this null finding. Indeed, Amodio et al. (2008) found that during a go/no-go task, BIS correlated with one ERP signal (the N2) during successful inhibition (“no-go” trials) but not successful execution (“go” trials), and with another signal (the error-related negativity) during failed inhibition but not during successful inhibition trials. Each of these findings supports the role of BIS in conflict monitoring and error detection rather than avoidance behaviors more broadly.
Future research can build on the current study in a more realistic goal pursuit context. Although one of the strengths of the Nochmani paradigm is the external validity of the stimuli, the task itself is meant to be a laboratory model for real-world goal pursuits such as dieting or smoking cessation. Based on the current results, it might be the case that prefrontal asymmetry plays a role in coordinating goal-directed approach and avoidance actions, and further, that individual differences in approach–avoidance motivation moderate asymmetry and are thus relevant to goal pursuit. We hope that the insights from the present study will inform future work not only on the neuroanatomical underpinnings of action motivation but also on the complex goals to which those behaviors and motivations are applied.
Acknowledgments
This research was supported by NIDA grants T90DA022768 and F31DA024904 (E. T. B.) and NIMH grants R21MH071521 and R01MH084116 (M. D. L.).
References
- Allen JJ, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. Psychophysiology. 2008;45:11–19. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Shah JY, Sigelman J, Brazy PC, Harmon-Jones E. Implicit regulatory focus associated with asymmetrical frontal cortical activity. Journal of Experimental Social Psychology. 2004;40:225–232. [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD, Gable SL. BIS, BAS, and response conflict: Testing predictions of the revised reinforcement sensitivity theory. Personality and Individual Differences. 2009;46:586–591. doi: 10.1016/j.paid.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Hattori N, Wheaton L, Fridman E, Shamim EA, Garraux G, et al. Gesture subtype-dependent left lateralization of praxis planning: An event-related fMRI study. Cerebral Cortex. 2008;19:1256–1262. doi: 10.1093/cercor/bhn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JD. Hemispheric asymmetry for emotional stimuli detected with fMRI. NeuroReport. 1998;9:3233–3239. doi: 10.1097/00001756-199810050-00019. [DOI] [PubMed] [Google Scholar]
- Carver CS, Harmon-Jones E. Anger is an approach-related affect: Evidence and implications. Psychological Bulletin. 2009;135:183–204. doi: 10.1037/a0013965. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality & Social Psychology. 1994;67:319–333. [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, Harmon-Jones E. Voluntary facial expression and hemispheric asymmetry over the frontal cortex. Psychophysiology. 2001;38:912–925. doi: 10.1111/1469-8986.3860912. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Neural correlates of evaluation associated with promotion and prevention regulatory focus. Cognitive, Affective, and Behavioral Neuroscience. 2005;5:202–211. doi: 10.3758/cabn.5.2.202. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA. Approach/withdrawal and cerebral asymmetry: Emotional expression and brain physiology: I. Journal of Personality & Social Psychology. 1990;58:330–341. [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Eddington KM, Dolcos F, Cabeza R, Krishnan KR, Strauman TJ. Neural correlates of promotion and prevention goal activation: An fMRI study using an idiographic approach. Journal of Cognitive Neuroscience. 2007;19:1152–1162. doi: 10.1162/jocn.2007.19.7.1152. [DOI] [PubMed] [Google Scholar]
- Elliot AJ. The hierarchical model of approach–avoidance motivation. Motivation & Emotion. 2006;30:111–116. [Google Scholar]
- Elliot AJ, Church MA. A hierarchical model of approach and avoidance achievement motivation. Journal of Personality & Social Psychology. 1997;72:218–232. doi: 10.1037//0022-3514.76.4.628. [DOI] [PubMed] [Google Scholar]
- Frey SH. What puts the how in where? Tool use and the divided visual streams hypothesis. Cortex. 2007;43:368–375. doi: 10.1016/s0010-9452(08)70462-3. [DOI] [PubMed] [Google Scholar]
- Frey SH. Tool use, communicative gesture and cerebral asymmetries in the modern human brain. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2008;363:1951–1957. doi: 10.1098/rstb.2008.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 4. Boston, MA: Academic Press/Elsevier; 2008. [Google Scholar]
- George MS, Ketter TA, Parekh PI, Herscovitch P, Post RM. Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biological Psychiatry. 1996;40:859–871. doi: 10.1016/0006-3223(95)00572-2. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Sigelman J, Bohlig A, Hogan ME, Harmon-Jones C. Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. Journal of Personality & Social Psychology. 2002;82:610–618. [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106:159–163. doi: 10.1037//0021-843x.106.1.159. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality & Social Psychology. 1998;74:1310–1316. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman J. State anger and prefrontal brain activity: Evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. Journal of Personality & Social Psychology. 2001;80:797–803. [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition & Emotion. 2000;14:711–724. [Google Scholar]
- Herrington JD, Mohanty A, Koven NS, Fisher JE, Stewart JL, Banich MT, et al. Emotion-modulated performance and activity in left dorsolateral prefrontal cortex. Emotion. 2005;5:200–207. doi: 10.1037/1528-3542.5.2.200. [DOI] [PubMed] [Google Scholar]
- Hewig J, Hagemann D, Seifert J, Naumann E, Bartussek D. The relation of cortical activity and BIS/BAS on the trait level. Biological Psychology. 2006;71:42–53. doi: 10.1016/j.biopsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Higgins ET. Beyond pleasure and pain. American Psychologist. 1997;52:1280–1300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Mueller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, et al. Now you feel it, now you don’t: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Seymour B, O’Doherty JP, Oakley DA, et al. Anxiety reduction through detachment: Subjective, physiological, and neural effects. Journal of Cognitive Neuroscience. 2005;17:874–883. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Kline JP, Blackhart GC, Woodward KM, Williams SR, Schwartz GE. Anterior electroencephalographic asymmetry changes in elderly women in response to a pleasant and an unpleasant odor. Biological Psychology. 2000;52:241–250. doi: 10.1016/s0301-0511(99)00046-0. [DOI] [PubMed] [Google Scholar]
- Kroliczak G, Frey SH. A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhn261. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biological Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Miller A, Tomarken AJ. Task-dependent changes in frontal brain asymmetry: Effects of incentive cues, outcome expectancies, and motor responses. Psychophysiology. 2001;38:500–511. [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Kelley WM, Wyland CL, Wegner DM, Neil Macrae C. Separating sustained from transient aspects of cognitive control during thought suppression. Psychological Science. 2007;18:292–297. doi: 10.1111/j.1467-9280.2007.01891.x. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, et al. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage. 2004;21:1484–1496. doi: 10.1016/j.neuroimage.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: A source-localization study. Psychological Science. 2005;16:805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Robinson RG. Differential behavioral and biochemical effects of right and left hemispheric cerebral infarction in the rat. Science. 1979;205:707–710. doi: 10.1126/science.462179. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: The selection of actions. Neuropsychologia. 2001;39:105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Sobotka SS, Davidson RJ, Senulis JA. Anterior brain electrical asymmetries in response to reward and punishment. Electroencephalography and Clinical Neurophysiology. 1992;83:236–247. doi: 10.1016/0013-4694(92)90117-z. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Bryer JB, Berthier ML, Cohen B, Price TR, Robinson RG. Depression after stroke: The importance of cerebral hemisphere asymmetries. Journal of Neuropsychiatry and Clinical Neurosciences. 1991;3:276–285. doi: 10.1176/jnp.3.3.276. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8:204–210. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ. From affective valence to motivational direction. Psychological Science. 2006;17:963–965. doi: 10.1111/j.1467-9280.2006.01813.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]