Abstract
Adenylyl cyclase signaling pathways have been identified in a model hair cell preparation from the trout saccule, for which the hair cell is the only intact cell type. The use of degenerate primers targeting cDNA sequence conserved across adenylyl cyclase (AC) isoforms, and RT-PCR, coupled with cloning of amplification products, indicated expression of AC9, AC7 and AC5/6, with cloning efficiencies of 11:5:2. AC9 and AC5/6 are inhibited by Ca2+, the former in conjunction with calcineurin, and message for calcineurin has also been identified in the trout saccular hair cell layer. AC7 is independent of Ca2+. Given the lack of detection of calcium/calmodulin-activated isoforms previously suggested to mediate adenylyl cyclase activation in the absence of Gαs in mammalian cochlear hair cells, the issue of hair-cell Gαs mRNA expression was re-examined in the teleost vestibular hair cell model. Two full-length coding sequences were obtained for Gαs/olf in the vestibular type II-like hair cells of the trout saccule. Two messages for Gαi have also been detected in the hair cell layer, one with homology to Gαi1 and the second with homology to Gαi3 of higher vertebrates. Both Gαs/olf protein and Gαi1/Gαi3 protein were immunolocalized to stereocilia and to the base of the hair cell, the latter consistent with sites of efferent input. While a signaling event coupling to Gαs/olf and Gαi1/Gαi3 in the stereocilia is currently unknown, signaling with Gαs/olf, Gαi3, and AC5/6 at the base of the hair cell would be consistent with transduction pathways activated by dopaminergic efferent input. mRNA for dopamine receptors D1A4 and five forms of dopamine D2 were found to be expressed in the teleost saccular hair cell layer, representing information on vestibular hair cell expression not directly available for higher vertebrates. Dopamine D1A receptor would couple to Gαolf and activation of AC5/6. Co-expression with dopamine D2 receptor, which itself couples to Gαi3 and AC5/6, will down-modulate levels of cAMP, thus fine-tuning and gradating the hair-cell response to dopamine D1A. As predicted by the trout saccular hair cell model, evidence has been obtained for the first time that hair cells of mammalian otolithic vestibular end organs (rat/mouse saccule/utricle) express dopamine D1A and D2L receptors, and each receptor co-localizes with AC5/6, with a marked presence of all three proteins in subcuticular regions of type I vestibular hair cells. A putative efferent, presynaptic source of dopamine was identified in tyrosine hydroxylase-positive nerve fibers which passed from underlying connective tissue to the sensory epithelia, ending on type I and type II vestibular hair cells and on afferent calyces.
Keywords: saccular hair cells; dopamine D1A and D2L receptors; adenylyl cyclase isoforms 9, 7, 5/6; Gαs/olf; Gαi1; Gαi3
Adenylyl cyclase second messenger pathways are predicted to modulate both hair cell mechanosensory transduction and receptoneural transmission. Cyclic AMP, produced via adenylyl cyclase (AC), increases the rate of adaptation of hair cell mechanosensory transduction (Ricci and Fettiplace, 1997). Cyclic AMP also directly targets HCN channels (DiFrancesco and Tortora, 1991) and indirectly (via PKA) L-type and N-type voltage-gated calcium channels (Lü and Dunlap, 1999; Rotman et al., 1992) found in vestibular hair cells (Cho et al., 2003; Ramakrishnan et al. 2002; 2006), thus putatively regulating receptoneural transmission (Holt and Eatock, 1995; Sugihara and Furukawa, 1996; Bao et al., 2003; Su et al., 1995). Ten AC isoforms are presently known to underlie AC enzymatic activity. However, only the AC isoforms that are specifically expressed in hair cells with their individual pharmacological profiles and specific coupling to G-proteins will modulate hair cell function. The nature of the AC isoform expressed would necessarily be consistent with mechanisms available for its activation (and inhibition). In the cochlea, evidence has been obtained that message and protein for Gαi (Tachibana et al., 1994; Barritt and Beisel, 1995; Adachi et al., 1996) and Gαo (Canlon et al., 1991) are expressed in hair cells. However, there is no evidence supporting Gαs expression in cochlear hair cells (Mizuta et al., 1995). The identification of calmodulin as a hair cell marker localized to the stereocilia (Slepecky et al., 1988; Shepherd et al., 1989; Slepecky and Ulfendahl, 1993; Ogata and Slepecky, 1998) suggested an alternative mechanism to activate AC isoforms in the absence of Gαs. Message and protein for ACI, one of the Ca2+/calmodulin-activated AC isoforms, was subsequently localized to cochlear inner hair cells (Drescher et al., 1997). Little information on adenylyl cyclase signal transduction pathways in vestibular hair cells has been available.
The question of AC isoform expression and Gαs/olf subunit expression in vestibular hair cells has now been directly addressed for a model hair cell preparation from the trout saccule for which the hair cell is the only intact cell type, a primary source of full-length sequence of proteins and splice variants expressed in hair cells (Ramakrishnan et al., 2002; Cho et al., 2003; Drescher DG et al., 2004; Ramakrishnan et al., 2006). With this hair cell model preparation, we have obtained evidence for an adenylyl cyclase pathway comprising the specific AC isoforms and G protein α subunits that are utilized in dopamine receptor signal transduction (Herve et al., 1993; Zhuang et al., 2000; Iwamoto et al., 2003; Lee et al., 2002), pointing to the possibility of dopamine receptor expression by vestibular hair cells. The existence of dopamine receptors on saccular hair cells has been confirmed for both teleost and mammalian models. Dopaminergic innervation of mammalian saccular hair cells would represent an efferent mechanism to modulate hair cell membrane potential via Ih and consequently modulate hair cell spontaneous afferent activity. Preliminary reports have appeared (Oh et al., 2001; Kewson et al., 2003; Drescher MJ et al., 2004; Folbe et al., 2004; Abu-Hamdan et al., 2008).
EXPERIMENTAL PROCEDURES
Isolation of the hair cell layer and cDNA preparation
The method of isolation of the hair cell layer from the trout saccule has been previously described (Drescher et al., 1989; Cho et al., 2003). In brief, the intact sensory epithelium from 10-12 inch rainbow trout (Oncorhynchus mykiss, Imlay City Fish Farm, MI), comprising hair cells alternating with supporting cells, was undermined above the basal lamina. The supporting cells, which extend from the basal lamina to the reticular lamina, were thus sheared, leaving a hair cell sheet of approximately 35,000 hair cells. For RT-PCR, the hair cell sheets from saccules of 15 fish, representing approximately 1,050,000 hair cells, were homogenized in 4 M guanidine thiocyanate containing 1% Sarkosyl at 2-3 °C. Total RNA was isolated with chloroform/phenol (Chomczynski et al., 1987) and genomic DNA removed with DNase I. Reverse transcription was performed with random hexamer/oligo dT12-18 primers and SuperScript reverse transcriptase II (Invitrogen, Carlsbad, CA), or alternatively, with 5’ and 3’ rapid amplification of cDNA ends (5’ and 3’ RACE protocols, SMART RACE cDNA Amplification Kit, Clontech, Palo Alto, CA). All efforts were made to minimize both the suffering and number of animals used. Experimental procedures involving animals reported in this study were performed according to the guidelines issued by the National Institutes of Health, USA, and approved by the Animal Investigation Committee of Wayne State University.
Primer design, PCR, and cloning
AC isoforms
For the determination of AC message in the model hair cell preparation from the trout saccule, we designed degenerate primers targeting AC isoform cDNA sequence highly conserved in the cytoplasmic C2 region across vertebrates and isoforms, corresponding to amino acids 819-828 and 897-905 of mouse AC1 protein (GenBank Accession No. AAC29478, AF053980 for nucleotides, Table 1). The primers were applied in PCR to cDNA from the trout saccular hair sheet. The PCR protocol using Taq DNA polymerase (Invitrogen) included denaturation at 95 °C for 45 s, annealing at 53.6 °C for 60 s, and elongation at 72 °C for 1.5 min, carried out for 35-40 cycles. The combined products falling within the range 200-350 bp on agarose gels and covering the predicted size of AC isoform PCR products were cloned (pGEMT Easy vector systems, Promega, Madison, WI) and sequenced to determine relative abundance of AC isoform message.
Table 1.
PCR primers employed in the present studies.
| Primer Designation | Primer Sequence | Nucleotide Range | Amplicon Size |
|---|---|---|---|
| Adenylyl Cyclase Isoforms1 | 5'-AAGATCAAGACCAT(C,T)GG(C,T)TCCAC(C,T)TACATG-3' | (2456-2485) | |
| 5'-(A,G)TT(A,C)ACGGTGTT(T,G)CCCCAGATGTCGTA-3' | (2690-2716) | Δ 220-300 | |
| Calcineurin2 | 5'-GAYGGWGCCAACATCCTMCG-3'2a | (900-919) | |
| 5'-GCMGSRTAACTGWAGAARTAG-3'2a | (1472-1492) | Δ 593 | |
| 5'-TGYTCCTAYTTCTWCAGTTAYSC-3'2a | (1467-1489) | ||
| 5'-SCYTCCTYTGCTTCTAYGGT-3'2a | (2044-2065) | Δ 599 | |
| Gas/olf3 | 5'-GAYGAGCGYAGGAARTGGAT-3'3a | (720-739) | |
| 5'-AGGTGCATCCTTTGRATAATG-3' | (1178-1198) | Δ 479 | |
| 5'-GGGTCAACAGAGGGATCTAGA-3'3b | (-4-17) | ||
| 5'-TGCATTGAAGCCATTCACATG-3' | (270-290) | Δ 294 | |
| 5'-TTGCGGGAAGATGGGTTGTTT-3'3b | (71-91) | ||
| 5'-TGCATCATCCGGTGTGGTGTA-3' | (986-1007) | Δ 937 | |
| 3’racegsp1up | 5'-GGCCAGTAGCAGCTACAACAT-3'3c-d | ||
| 3’racegsp2up | 5'-GATCCGGGAGGACAATCAGAC-3'3c-d | ||
| 3’upseq.p-1 | 5'-GAGAAGCATCGTGGTGGGCAG-3'3e | ||
| 5’racegsp1down | 5'-TCAGTGTCCACGGCGCAGGTA-3'3f-i | ||
| 5’racegsp2down | 5'-GGTAACAATAGTGCCTGCCAT-3'3f-i | ||
| 5’racegsp3down | 5'-GGTCCTGTTTATTGAGGAAT-3'3f-i | ||
| 5’racegsp4down | 5'-AGGATGACAGAAATGGTCCG-3'3f-i | ||
| 5’dnseq.p-1 | 5'-TGCATTGAAGCCATTCACATG-3'3j | ||
| Gαi4 Gαi-1.us | 5'-TYCTGCTGCTNGGTGCTG-3'4a | (373-391) | |
| Gαi-1.ds | 5'-RATCCACTTYTTCCTCTCTGABC-3'4a | (880-902) | Δ 530 |
| Gαi-2.us | 5'-GACGATGGGGTGTACGCTGAG-3'4b | (18-38) | |
| Gαi-2.ds | 5'-TGATGGACTGGATGGTGTTGC-3'4c | (245-265) | Δ 248 |
| Gαi-3.us | 5'-TCATCGCCATCATCAGAG-3'4c | (263-280) | |
| Gαi-3.ds | 5'-CGTCAAACACAAACTGCACGTT-3'4b | (1012-1033) | Δ 771 |
| Gαi-4.us | 5'-GACGATGGGGTGTACGCTGAG-3'4b | (18-38) | |
| Gαi-4.ds | 5'-CGTCAAACACAAACTGCACGTT-3'4b | (1012-1033) | Δ 1016 |
| Dopamine D1 receptor5 | 5'-GGTVGCBTTTGACATCATGTG-3'5a | (1719-1740) | |
| 5'-GAYRGGGTTGAGYGABGARTT-3'5a | (2387-2408) | Δ 690 | |
| 5'-CTKGTGGCCATYTTGGTRAT-3'5b | (305-324) | ||
| 5'-TGATGAGGGAGGAGGAGATG-3'5b | (676-695) | Δ 391 | |
| 5'-RTCNAAGCGWGTKYTGACWGGYTGT-3'5b | (151-174) | ||
| 5'-AGTTGTCCGGAGGCAGCTCCC-3'5b | (627-647) | Δ 497 | |
| 5'-CCCTCATCAGCTTCTACATCC-3'5c | (830-850) | ||
| 5'-ACRTCYRCATCRCTATCC-3'5c | (1503-1520) | Δ 691 | |
| Dopamine D2 receptor6 | 5'-TGCCCTCTGCTGTTTGGACTCA-3'6a1,2, 3u, 4d | (527-548) | |
| 5'-CTGTGGGTGCAGGCCGTGTCT-3'6a1,2, 3u, 4d | (714-734) | Δ 208 | |
| 5'-TGCCCTCTGCTGTTTGGACTCA-3'6a1,2, 3u, 4d | (527-548) | ||
| 5'-TCTGCATTTCGCTCCCTCCATC-3'6a1,2, 3u, 4d | (1103-1125) | Δ 599 | |
| 5'-TGCCCTCTGCTGTTTGGACTC-3'6a1,2, 3u, 4d | (527-547) | ||
| 5'-YCCGGCYTKSCGGAACTCG-3'a3d | (1282-1302) | Δ 776 | |
| 5'-TCAGYMGGATYCACTGTGATRTTCTG-3'a4u | (330-355) | ||
| 5'-TCTCCCTGTCTGCATTTCGCTC-3'6a1,2, 3u, 4d | (1122-1133) | Δ 804 | |
| 5'-AACACTCGCTACAGCTCCAG-3'6b | (432-452) | ||
| 5'-CTCTTSASRTAMCCCAGCCASGT-3'6b | (1308-1331) | Δ 900 | |
| 5'-CACACAGTATGCCTACAATGAG-3'6b | (23-44) | ||
| 5'- AGAAGGACACAATGGAGGAG-3'6b | (590-609) | Δ 587 | |
| 5'-CACACAGTATGCCTACAATGAG-3'6b | (23-44) | ||
| 5'- AAAGGACAGAACCCACACC-3'6b | (479-497) | Δ 475 | |
| 5'- ATYAAAGAGGTGGCCCACGAAG-3'6b | (834-855) | ||
| 5'- GCACTGTTCACATACCCCAG-3'6b | (1314-1333) | Δ 500 |
Numbering of nucleotides is according to Accession Nos. 1AF053980 for mouse AC1
XM_681081, Danio rerio
X56091 for Gαs, Xenopus laevis
Gαs/olf, trout brain sequence with numbering according to X56091 for Gαs, Xenopus laevis
3’RACE gene specific primers
3’RACE sequencing primer
5’RACE gene specific primers
5’RACE sequencing primer
BC053164, coding for “MGC63957,” a protein similar to Gαi1, Danio rerio
AB001741 coding for Gαi1 subunit, Oryzias latipes
trout hair cell-specific cDNA with numbering according to AB001741 for Gαi1 subunit, Oryzias latipes
X80174 for dopamine D1-like receptor for T. rubripes
Y14626 D1A3 Dopamine receptor, Cyprinus carpio
14627 D1A4 Dopamine receptor, Cyprinus carpio, upstream primer is trout hair cell sequence numbered according to Cyprinus carpio sequence
6aNM_197935 for Danio rerio dopamine receptor D2-like mRNA was used for numbering
AJ347729, Oncorhynchus mykiss dopamine D2 receptor 2 mRNA. Nucleotide sequence for primers
represent sequence obtained from trout brain from the use of degenerate primers based upon carp dopamine D2 receptor sequence, Y14632.
Calcineurin
Degenerate primers were designed (Table 1) targeting cDNA sequence from Danio rerio (XM_681081, XM_001338831, and NM_001334533) which corresponded to amino acid sequences 80%, 82% and 82% identical, respectively, to rat calcineurin A subunit alpha, also referred to as protein phosphatase 3, catalytic subunit, alpha isoform (NP_058737). PCR was carried out with Advantage II DNA polymerase (Clontech) with denaturation at 95 °C for 30s, annealing at 62 °C for 30 s, and elongation at 68 °C for 2 min for total of 35-40 cycles.
Gαs/olf
Degenerate primers targeting Gαs cDNA sequence for Xenopus laevis (X56091, Table 1) and Drosophila melanogaster (NM_166640) yielded gene-specific sequence with upstream primer (3a) corresponding to amino acids 214-220 and downstream primer to amino acids 367-373 of Xenopus laevis (X56091). Gαs/olf cDNA sequences for trout saccular hair cells and brain were extended with 3’ and 5’ RACE, utilizing gene-specific primers upstream or downstream coupled with 3’ or 5’ RACE-specific primers, respectively (Table 1). PCR protocols included 35-40 cycles of denaturation for 1 min, annealing at primer-specific temperatures 53-55 °C determined by OLIGO 2 software analysis (National Bio-sciences, Plymouth, MN) with Taq protocols, extension at 72 °C for 1-2 min, and final extension at 72 °C for 5-10 min. RACE protocols entailed the use of “Touchdown” amplification (with Advantage 2 DNA polymerase system, Clontech), with 5 cycles of annealing at 72 °C, 5 cycles at 70 °C, 10 cycles at 68 °C, followed by 30 cycles at 65 °C, with elongation at 72 °C for 2 min per cycle.
Gαi messages
To design primers for use in RT-PCR, we aligned the amino acid sequences for Gαi1 and Gαi3 isoforms from rat with Gαi sequences from Danio rerio (zebrafish) and Oryzias latipes (Japanese medaka) with OLIGO2 software. Regions of sequence conserved across evolution were targeted in design of the first set of degenerate primers for Gαi-1 (Table 1), corresponding to amino acids 36-42 of Danio rerio Gαi1-like protein (MGC63957) for the upstream primer and 205-212 (MGC63957) for the downstream primer (Table 1, BC053164). Additional sets of primers were used to extend trout hair cell sequences based upon Gαi1 for Oryzias latipes (AB001741) (Table 1). PCR products (1,016 bp and 771 bp) were cloned, with ligation into pGEM-T Easy Vector (Promega) and JM109 high efficiency transformation accomplished by heat shock (42 °C).
Dopamine D1 and D2 receptors
Degenerate primers for dopamine D1 receptors were numbered according to cDNA sequence for dopamine D1-like receptor of T. rubripes (X80174) (Table 1). Dopamine D2 receptor message in trout brain was determined initially with specific primers from carp (Y14632), followed by degenerate primers designed with Accelrys software (San Diego, CA) based on Danio rerio dopamine D2-like mRNA (NM_197935) and trout dopamine D2 receptor 2 mRNA (AJ347729) (Table 1). PCR for D1 and D2 was carried out with both Taq and Advantage II protocols.
Western blotting
For the Gαs/olf Western blot, hair-cell layer lysate was prepared in PBST (Sigma, St. Louis, MO) containing 0.1% CHAPS (Sigma) and 1X protease inhibitors (Sigma), and denatured using 1X LDS buffer (NuPAGE LDS Sample Buffer, Invitrogen). For Gαi Western blots, homogenizing solution contained Tris-buffered saline with 1% Tween 20, pH 8.0 (Sigma) and complete mini EDTA-free protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Proteins were resolved by 4-12% SDS gels with MOPS buffer at 120 volts (Invitrogen mini-gel apparatus) and transferred to nitrocellulose with electroblotting (Towbin et al., 1979). Membrane strips were incubated in 5% non-fat milk, 4% goat serum in TBS, and 0.1% Tween for 1 h to block non-specific binding, and incubated overnight at room temperature with primary antibodies targeting Gαs (affinity-purified rabbit polyclonal antibody sc-383, Santa Cruz Biotechnology, Santa Cruz, CA; 1:1,000) or Gαi (affinity-purified rabbit polyclonal, sc-391 Santa Cruz Biotechnology; 1:1,429). The primary antibody for Gαs/olf targeted an 18-aa peptide in the carboxy terminus of rat Gαs/olf, also present in the trout saccular hair cell Gαs/olf-1 and Gαs/olf-2 sequence (see Results). The primary antibody for Gαi was raised against a 20 aa-peptide found in rat Gαi1, conserved across evolution and crossing to Gαi3 (see Results). The primary antibodies for Gαi and Gαs/olf raised in rabbit were coupled to a goat anti-rabbit IgG secondary, tagged with horseradish peroxidase (1:7,000, Santa Cruz) and detected with chemiluminescence (Western Lightning Chemiluminescence Plus, Perkin-Elmer, Waltham MA).
Immunohistochemical analysis
The rapidly isolated intact trout saccular sac underwent fixation in 4% paraformaldehyde, 0.1% glutaraldehyde for 2 hours at 4 °C and was embedded in paraffin (65 °C for 10 min × 3) and sectioned at 4-5 μm according to previously described protocols (Ramakrishnan et al., 2009). The deparaffinized sections were treated with sodium borohydride and hydrogen peroxide to block free aldehydes and peroxidase activity, respectively, blocked in goat serum and incubated for 24 h in primary antibody (sc-383, Santa Cruz Biotechnology, for Gαs/olf; 1:400 or sc-391, Santa Cruz Biotechnology, for Gαi; 1:400 - 1:1000) at 4 °C. Rabbit IgG was detected with a goat anti-rabbit secondary antibody (Vectastain Elite ABC kit, Vector, Burlingame, CA) and 3,3′-diaminobenzidine (DAB) was used as the chromogen (BioGenex, San Ramon, CA).
Immunohistochemical analysis of dopamine receptors in rat and mouse vestibular epithelia was carried out as previously described (Drescher et al., 2006). Dopamine D1 receptor was detected with a rabbit anti-dopamine receptor D1A affinity-purified polyclonal antibody (AB1765P, Chemicon, Temecula, CA; 1:25) raised against a 13 amino-acid sequence (amino acids 403-415) from rat D1A; mouse monoclonal (MAB5290, Chemicon) targeting recombinant rat D1A receptor carboxy terminus; affinity-purified goat polyclonal for dopamine D1DR (sc-31478, Santa Cruz Biotechnology; 1:200) which would detect dopamine D1A as well as dopamine D1B receptor from rat and mouse.
Dopamine receptor D2L immunolocalization was carried out with a rabbit affinity-purified polyclonal antibody (AB1792P, Chemicon; 1:50 - 1:100) which targeted a 27 amino-acid peptide sequence corresponding to the 29 amino-acid peptide found in the third cytoplasmic loop of rat D2 long form. A second rabbit polyclonal antibody was raised against a 28 amino acid sequence in cytoplasmic loop 3, common to dopamine D2S and D2L receptors, which crosses to rat (AB5084P, Chemicon). An additional antibody, a mouse monoclonal antibody (dopamine D2DR, sc-5303, Santa Cruz) targeted amino acids 1-50 of dopamine receptor type 2 of human origin which crosses to mouse and rat.
Adenylyl cyclase type 5/6 was targeted with mouse monoclonal (H00000111-M01, Abnova, Taipei City Taiwan; 1:500), aa 1152-1262 of human AC5/6 sequence with 98% identity to rat AC5/6; an affinity-purified rabbit polyclonal antibody (sc-2550, Santa Cruz) which targeted the amino terminus antigen aa 1-130 of human AC5/6 crossing to rat sequence but not to rat AC1 sequence. Neuronal markers included calretinin for vestibular afferents forming calyxes on vestibular type I hair cells (goat polyclonal, AB1550, Chemicon; 1:1,500) and tyrosine hydroxylase (rabbit polyclonal, CA-101 bTHrab, Protos Biotech, New York, NY; 1:100) for presumptive dopaminergic efferents. Secondary antibodies for immunofluorescence were Alexa Fluor conjugates obtained from Molecular Probes (Eugene, Oregon). Negative controls included pre-absorption with respective antigen or omission of primary antibody and/or replacement of the primary antibody by purified IgG for the species that was used to raise the primary antibody.
Immunoreactivity detected with 3′-diaminobenzidine (DAB) or fluorescence was visualized as previously described (Drescher et al., 2006). Immunostaining with DAB was examined with a Leitz Diaplan microscope (LeItz, Wetzlar, Germany), photographed with an Olympus OM-4T camera and the negatives digitized. Immunofluorescence was examined with an Olympus BX60 microscope, photographed with a PM 20 camera system (Olympus) and the negatives digitized at 300-600 dpi.
RESULTS
Adenylyl cyclase isoform mRNA expression in the trout saccular hair cell sheet
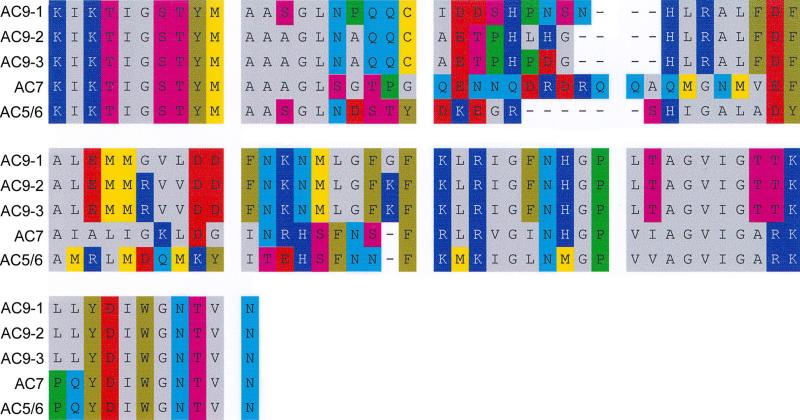
Degenerate 30- and 27-mer primers, targeting a highly-conserved cDNA sequence in the cytoplasmic C2 region across the nine AC isoforms of higher vertebrates, directed amplification in RT-PCR of AC isoform cDNA of trout saccular hair cells. The PCR products ranged in size from 220-300 bp (Fig. 1). Individual PCR products were sequenced and the combined products were cloned and sequenced. Individual amplification products exhibited homology to AC9, AC5/6, and the type 2 AC isoform subfamily. Overall, sequencing of 18 clones indicated a relative distribution of 11:5:2 for AC9:AC7:AC5/6 in the saccular hair cell cDNA (Fig. 2). AC9 clones included three apparent splice variants with 84%, 88% and 88% identity (AC9-1, AC9-2, and AC9-3) to Danio rerio AC9 (XP_001920818), respectively, representing molecular variation in the second cytoplasmic domain predicted to impact catalytic activity. A major presence of AC9 in the teleost hair cell would be consistent with the relatively small, two-fold elevation of intracellular cAMP which we have observed for the hair cell layer in response to forskolin (Drescher et al., 1994), similar to the findings of others for cells stably transfected with AC9 (Premont et al., 1996; Antoni et al., 1998). The trout saccular hair cell AC7 sequence was 90% identical to that of Danio rerio AC7 (NP_00159744), displaying less identity (76%) to Danio rerio AC2a (XP_692173), the next closest AC isoform, also a member of the type 2 AC isoform subfamily. The aa sequence designated AC5/6 was 96% identical to Danio rerio AC5 (ABG34243) and 92% and 91% identical to Takifugu rubripes AC6 (AAB96362) and Danio rerio AC6 (XP_001922749), respectively. The teleost sequence bore similarity to both AC5 and AC6 in rat (89% identity to rat AC5 and 90% identity to rat AC6).
Fig. 1. First Row: First panel shows agarose gel resolution of AC isoform PCR products obtained from trout saccular hair cell cDNA.
Degenerate primers, targeting C2 domain sequence conserved across the nine AC isoforms of higher vertebrates (Table 1), directed amplification of 220-300 bp products. Second and third panels show agarose gel separation of calcineurin amplification products from trout saccular hair cell cDNA. Degenerate primers targeting calcineurin from the trout saccular hair cell cDNA yielded PCR products of predicted size, 599 bp (Calcn Δ599) (Table 1, 2b) and 593 bp (Calcn Δ593) (Table 1, 2a) for hair cell preparations. No amplification was observed for negative control in the absence of reverse transcriptase. The PCR products were cloned with pGEM T-easy vector and sequenced, with the sequence deposited into GenBank (GU073385). Last four panels show Gαs/olf messages in the trout saccular hair cell preparation. Application of degenerate primers targeting Gαs sequence for Xenopus laevis and Drosophila melanogaster (Table 1, 3a) in PCR yielded an amplification product of predicted size, 479 bp (Gαs Δ479), for trout saccular hair cell and trout brain cDNA. A 5’ RACE gene-specific primer 4 (5’racegsp4down, Table 1) directed amplification of hair cell Gαs/olf-2 cDNA (Gαs Δ1300) but not brain Gαs/olf-2 cDNA, presumably due to one nucleotide difference in the respective primer regions for hair cell and brain. The 5’ RACE gene-specific primer 3 (5’racegsp3 down, Table 1), reflecting sequence of Gas/olf-1 and not Gas/olf-2, elicited amplification of brain cDNA at ~ 2 Kb (Gαs Δ2000) but not for hair cell cDNA. Primers specific to trout brain Gas/olf-1 sequence directed amplification of the predicted 294 bp product (Gαs Δ294), with sequence confirmation for hair cell cDNA and brain cDNA.
Second Row: Amplification of saccular hair cell Gαi cDNA with degenerate and specific primers (see Table 1, Gαi-1 through Gαi-4 refer to primer sets and not Gαi isoforms). First panel. Agarose gel shows a PCR product of predicted size 530 bp produced with degenerate primers (Gαi-1, Table 1) from saccular hair cells with reverse transcriptase but not in its absence (Gαi-1, Δ530). Second panel. A PCR product of predicted size, 248 bp, was obtained for hair cell cDNA with medaka-specific upstream and trout-specific downstream primers (Gαi-2, Table 1) (Gαi-2, Δ248). Third panel. A 771 bp predicted product resulted from the use of trout specific primer upstream coupled with medaka sequence downstream on hair cell cDNA (Gαi-3 primers, Table 1) (Gαi-3, Δ771). Fourth panel. A 1,016 bp PCR product was obtained with Gαi-4 primers (Table 1) (Gαi-4, Δ1016) in the presence of reverse transcriptase but not in its absence. The 771 bp and 1,016 bp products were later cloned and sequenced.
Third Row: Agarose gels of dopamine D1 PCR products from the trout saccular hair cell layer cDNA. First and second panels. Amplification results for dopamine D1 receptor transcripts from RT-PCR applied to trout brain and trout saccular hair cells from the use of degenerate primers (Table 1). The predicted 690 bp PCR products (D1 Δ690, panel 1 and panel 2) not present in negative controls. Last three panels. Predicted PCR products amplified from trout saccular hair cell cDNA, 391 bp (D1 Δ391), 497 bp (D1 Δ497), and 691 bp (D1 Δ691).
Fourth Row: Agarose gel separation of dopamine D2 PCR products amplified from the trout saccular hair cell cDNA. Dopamine D2 receptor primers (see Table 1) yielded predicted amplification products (D2 Δ208, D2 Δ599, D2 Δ776, D2 Δ804, D2 Δ900, D2 Δ587, D2 Δ475, D2 Δ500) with reverse transcription for the trout saccular hair cell layer with sequence confirmation. S = standards; H + = hair cell sheet with reverse transcriptase; H - = hair cell sheet without reverse transcriptase; Br = trout brain with reverse transcriptase, a positive control. B = water blank.
Fig. 2. Sequence from cloning of the combined AC isoform PCR products from application of degenerate primers.
Amino acid sequences are presented for AC isoforms expressed in the saccular hair cell layer including three apparent splice variants of AC9 (lines 1-3), AC7 (line 4) and AC5/6 (line 5). The relative number of clones for AC9:AC7:AC5/6 was 11:5:2.
The three AC isoforms found in teleost saccular hair cells differ in their pharmacological properties, impacting the mechanisms available to modulate specific hair cell function. AC9 and AC5/6 are inhibited by Ca2+. Calcium-mediated inhibition of AC9 occurs via calcineurin, the iserine/threonine protein phosphatase 3 (formerly 2B), which is also expressed in the trout saccular hair cell layer (Fig. 1). Sequence from cloned amplification products (not illustrated) covering 68% of full length coding sequence (GU073385) indicated high evolutionary conservation, with 97% and 91% amino acid identity to calcineurin A of Danio rerio (NP_001074063, CAM46977) and human/rat amino acid sequence (NP_000935, NP_058737), respectively. AC7 is a member of the AC2 isoform subfamily, whose enzymatic activity is independent of Ca2+. We have obtained no evidence of message in the trout saccular hair cell sheet for the Ca2+/calmodulin-activated AC isoforms, AC1 and AC8. Activation of AC isoforms 9, 7 and 5/6 occurs through an intervening Gαs/olf protein, which heretofore has not been detected in any hair cells.
G protein Gαs/olf mRNA expression in the trout saccular hair cell layer
Degenerate primers were designed to target cDNA sequence in frog and Drosophila for Gαs which is highly conserved across evolution (Table 1, 3a). These primers directed amplification of a 479 bp product with RT-PCR from both the saccular hair cell sheet and brain (Fig. 1). The sequence of the original products formed the basis of gene-specific primers subsequently utilized in sequence extension for both trout saccular hair cell and brain cDNA. The complete coding sequence has been obtained for two variants of Gαs/olf (thc1, thc2) expressed in the trout saccular hair-cell sheet and characterized by distinctly different amino termini (Fig. 3, EF554329, EF554330). These two variants are 89% identical to each other. The thc2 variant incorporated a 10 aa insert in the amino terminus which appears to be unique, not replicated in Gαs or Gαolf sequences of higher vertebrates. This insert would represent an extension of human Gαolf-2 (compare Fig. 3, lines 3 and 4), but is not the same sequence as the extension seen in human Gαolf-1 (Fig. 3, line 5). The choice otherwise in the hair cell amino acid sequences was relatively equally divided between Gαs and Gαolf, compared to mammalian sequence. A 15-amino-acid deletion between aa 157-171 found in Gαolf across all vertebrates is present in both teleost saccular hair cell forms. The identical carboxy terminus of Gαs and Gαolf sequences of higher vertebrates was conserved in trout saccular hair cell sequences. Overall, the full-length sequence obtained for trout saccular hair cell Gαs/olf-1 exhibited 86% identity to human Gαs, 77% identity to human Gαolf-1 and 80% identity to human Gαolf-2. Trout saccular hair cell Gαs/olf-2 exhibited 81% identity to human Gαs, 79% identity to human Gαolf-1 and 78% identity to human Gαolf-2. With these two full-length sequences, we have therefore obtained evidence, for the first time, that Gαs/olf cDNA is expressed in at least one hair cell type, the saccular type II vestibular hair cell.
Fig. 3. Amino acid sequences for Gαs/olf expressed in trout saccular hair cell preparation compared with sequences for Gαs and Gαolf in human.
Line 1, human Gαs (NP_000517). Line 2, trout hair cell Gαs/olf-1 (ABU45775). Line 3, trout hair cell Gαs/olf-2 (ABU45776). Line 4, human Gαolf-2 (NP_002062). Line 5, human Gαolf-1 (NP_892023).
Gαs/olf immunolocalization in the trout saccular sensory epithelium
An 18-aa peptide found in evolutionarily highly-conserved carboxy terminus of mouse, rat, human and trout saccular hair cell Gαs/olf was targeted by an affinity-purified rabbit polyclonal antibody. Western blots carried out on hair cell extracts indicated a protein band at ~ 45 kDa, the predicted mass of Gαs/olf (Fig. 4a, arrow; Fig. 3, thc-1 and thc-2 sequences corresponded to molecular masses of 44.6 kDa and 46 kDa, respectively, by amino acid calculation). A second, less intense band of higher molecular weight, ~ 60 kDa, was also observed, possibly corresponding to a larger Gαs or Gαolf isoform with a range of sizes reported for zebrafish and higher vertebrates. Gαolf-2 proteins as a group fall in the molecular mass range that would include 60 kDa, as opposed to guanine nucleotide-binding protein G(s) subunit alpha isoforms XLas, which are larger yet.
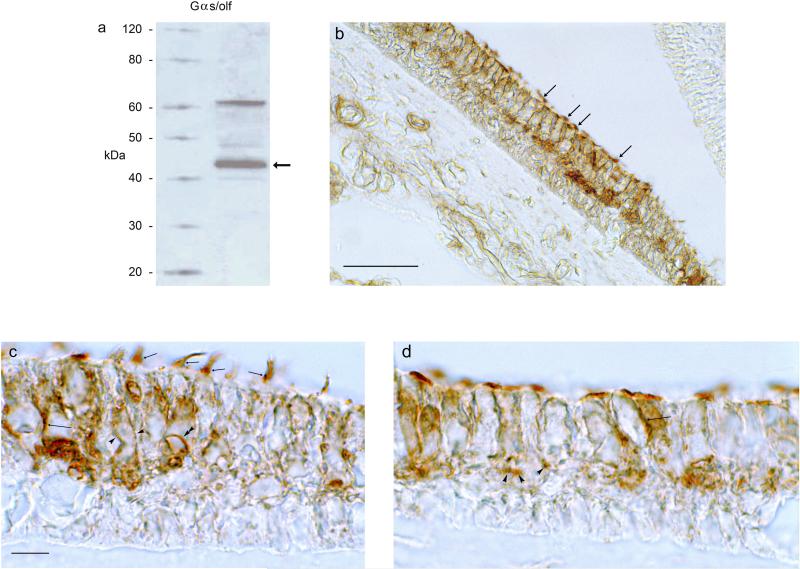
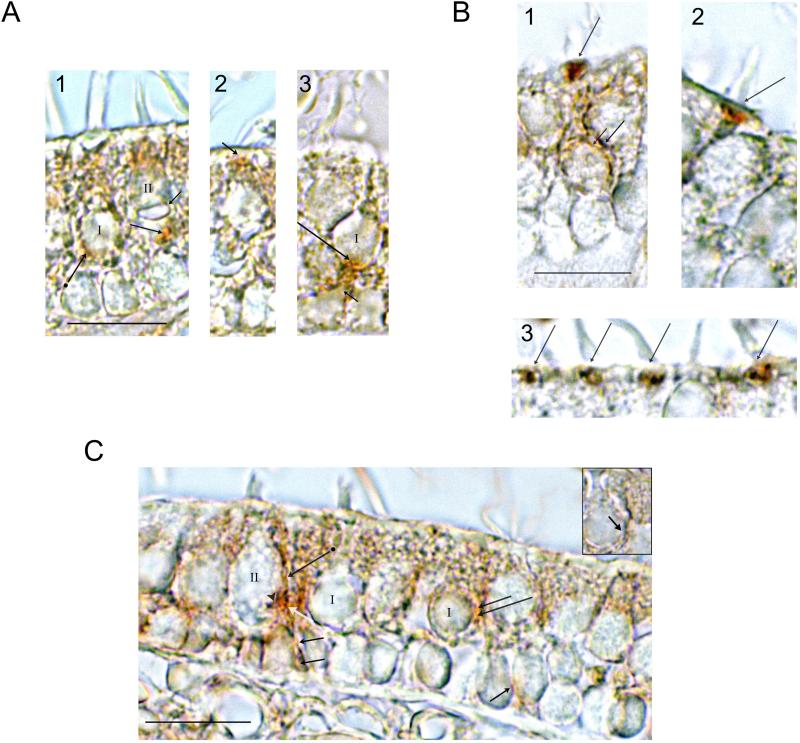
Fig. 4. Western blot and immunohistochemical localization of Gαs/olf proteins in the trout saccule.
a. Western blot for Gαs/olf protein in the trout saccular hair cell layer. Hair-cell layer lysate was probed with Gαs/olf antibody. Lane 1, molecular size standard (Magic Mark, Invitrogen); Lane 2, trout hair cell layer. The band (arrow) corresponds to the estimated molecular mass for Gαs/olf of 45 kDa, based on the deduced trout Gαs/olf-1/Gαs/olf-2 amino acid sequence. b. Immunohistochemical localizations were conducted with transverse sections cut perpendicular to the longitudinal axis of the trout saccule. At low magnification, Gαs/olf immunoreactivity was localized to the upper two-thirds of the sensory epithelium, with immunostaining of hair cells and neural components. Gαs/olf immunoreactivity was found in hair cell stereocilia throughout the sensory epithelium (arrows) and not observed associated with supporting cells whose nuclei lie just above the basal lamina in the lower third of the epithelium. Scale bar = 50 μm. c-d. At higher magnification, Gαs/olf immunoreactivity was localized to the lateral membrane (intermediate-length arrows) and stereocilia (short arrows) of saccular hair cells. Immunoreactivity was also associated with large afferent fibers (c, single arrowheads encompass large afferent) with circles of immunoreactivity at sites of afferent nerve contacts on hair cells (c, double arrowhead). Small-caliber efferent fibers were immunoreactive for Gαs/olf (d, arrowheads). Scale bar in c, applicable also for d = 10 μm.
In immunohistochemical localizations, Gαs/olf protein in the sensory epithelium, while expected to have a hair cell source, could theoretically also be found in neurons, afferent and/or efferent, and in supporting cells. What was observed was immunolabeling of hair cells and neural processes, but not the supporting cells, with little if any immunoreactivity found in the lower portions of the sensory epithelium immediately above the basal lamina (Fig. 4), a region which only contains supporting cells as an epithelial cell type. For the hair cells, immunoreactivity was localized to the stereocilia (Fig. 4b,c) and to lateral plasma membrane (Fig. 4c,d). The neural immunoreactivity within the sensory epithelium appeared attributable to both the large afferents (Fig. 4c) and smaller efferents (Fig. 4d). The identity of nerve fibers as afferent or efferent in the trout saccule is based on previous electron microscopic correlation of their diameter with presence of synaptic vesicles, post synaptic densities, and other cytochemical architecture within the sensory epithelium (Drescher et al., 1987; Khan et al., 1990, 1991; Drescher et al., 1995).
Identification of Gαi isoform messages expressed in the trout saccular hair cell layer
There is evidence for expression of Gαi isoforms in cochlear hair cells (Tachibana et al., 1994; Barritt and Beisel, 1995; Adachi et al., 1996), with the Gαi isoforms hypothesized to participate in a second messenger response to efferent neurotransmitters. Determination of full-length message sequences with possible attending hair cell-specific variants has not been achievable for mammalian hair cells due to limited number and inaccessibility of the hair cells. The model hair cell preparation which comprises sufficient hair cells for complete sequence determination has been utilized in the present investigation with RT-PCR and degenerate primers designed based upon Gαi cDNA sequences for the zebrafish and Japanese medaka.
Sequence from a 530 bp amplification product for the trout saccular hair cell layer obtained with degenerate primers, Gαi-1 (Table 1), was extended with combination of primers for the medaka sequence and trout-specific sequence (Table 1), yielding a 1,016 bp product (Fig. 1). This cloned product, representing 95% of coding sequence for a Gαi isoform protein (Fig. 5, line 1, EU369353), was closest in amino acid identity to Gαi1: 94%, compared to medaka Gαi1 (NP_001116402, Fig. 5, line 2) and 96%, compared to rat Gαi1 (NP_037277, Fig. 5, line 3). A second amplification product of 771 bp, obtained with the use of Gαi-3 primers (Table 1), yielded Gαi3 sequence when cloned (Fig. 5, line 4) with 98% amino acid identity to Salmo salar sequence (NP_001135256, Fig. 5, line 5) and 89% identity to rat sequence (NP_037238, Fig. 5, line 6). One variant of the trout Gαi3 sequence included a deletion of 16 aa between aa 160-175 (not illustrated). We have not amplified Gαi2 from the hair cell layer. The Gαi1 and Gαi3 isoforms expressed in saccular hair cells would couple differentially to metabotropic neurotransmitter receptors, such as the dopamine D2 and serotonin 5-HT1 receptors, with neurotransmitter receptor expression in saccular hair cells, again, of necessity, consistent with specific Gαi isoform expression.
Fig. 5. Gαi amino acid sequences for the trout saccular hair cell preparation were deduced from nucleotide sequencing of cloned 1,016 bp and 771 bp PCR products.
The 1,016 bp cloned product (line 1), representing 95% of the coding region for a Gαi protein, bore highest amino acid identity to medaka Gαi1 (line 2, NP_001116402) and rat Gαi1 (line 3, NP_037277). A second cloned product 771 bp in length (line 4) was highest in amino acid identity to Salmo salar Gαi3 sequence (line 5) (NP_001135256) and rat Gαi3 (line 6, NP_037238).
Gαi immunolocalization in the trout saccular sensory epithelium
Western blots for Gαi were carried out with a commercial affinity-purified rabbit polyclonal antibody (sc-391, Santa Cruz Biotechnology) targeting a 20 aa-peptide found in rat Gαi1 which was 100% conserved in trout saccular hair cell Gαi1 (IDFGDAARADDARQLFVLAG, Fig. 5, line 1, aa 93-112). Gαi3 sequence from the trout saccular hair cells was 90% identical to the targeted peptide and therefore the antibody would be expected to detect both forms of Gαi expressed in trout saccular hair cells. A major band was observed at ~ 40 kDa (Fig. 6a, arrow), corresponding to a calculated molecular mass of 40,297 for the saccular hair cell Gαi1 based upon actual amino acid sequence obtained from cloned hair cell cDNA. (This calculation included 17 amino acids in the carboxy terminus conserved across Gαi isoforms, from lower vertebrate to higher vertebrate, out of a total of 354 amino acids).
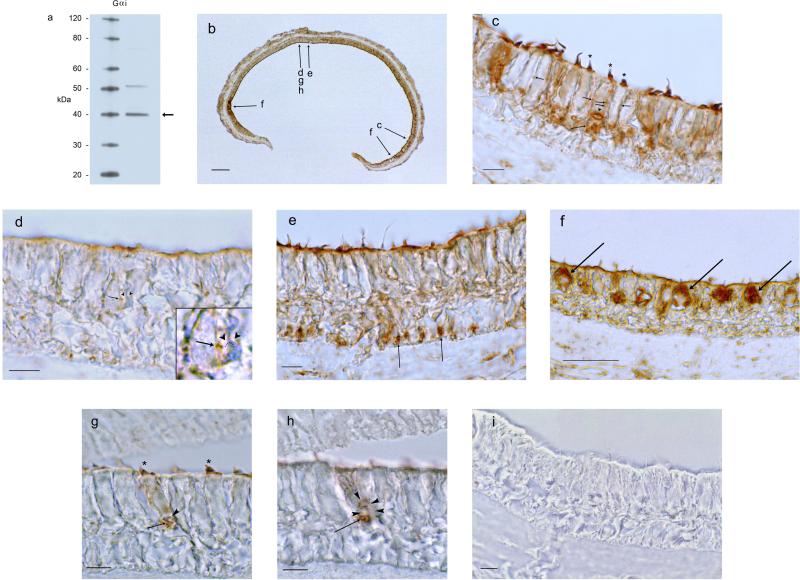
Fig. 6. Western blot and immunohistochemical localization for Gαi protein in the trout saccule.
a. Western blot for Gαi1/ Gαi3 protein in the trout saccular hair cell layer. Lane 1, molecular size standards. Lane 2, trout saccular hair cell layer protein. The band at 40 kDa (arrow) indicates an immunolabeled protein corresponding to calculated molecular mass for cloned saccular hair cell Gαi1. b. Low magnification of immunoreactivity for Gαi (Gαi1/Gαi3) in the trout saccule. Scale bar = 100 μm. Relative positions in the saccule corresponding to more highly-magnified micrographs c-h are indicated by arrows. c. Immunoreactivity for Gαi1/Gαi3 was localized to stereocilia (asterisks) and to the lateral membranes (short arrows) and basal membranes of saccular hair cells (intermediate length arrow). Scale bar = 10 μm. d. Immunoreactivity for Gαi1/Gαi3 was localized to a small diameter presumptive efferent fiber positioned close to the hair cell plasma membrane (short arrow) juxtaposed to the ending of an unreactive nerve fiber (inset: arrowheads and dotted line point to bow-shaped unreactive nerve fiber). Scale bar = 10 μm. e. Gαi immunoreactivity was observed in small nerve fibers entering the sensory epithelium just above the basal lamina passing between supporting cell nuclei (arrows). Scale bar = 10 μm. f. Immunoreactivity for Gαi1/Gαi3 filled the cell bodies of flask-shaped hair cells located close to the border of sensory and non-sensory epithelium. Scale bar = 50 μm. g,h. Immunoreactivity for Gαi at one focal plane was observed in hair cell stereocilia (g, asterisks) and small foci along the basal membrane of hair cell (g, arrow) close to large afferent fiber ending (g, arrowhead). At a lower focal plane, immunoreactivity was associated with efferents (h, arrow). The emergence of the large afferent is indicated (h, arrowheads). Scale bar = 10 μm, for g,h. i. Preabsorption of the antibody with peptide antigen essentially eliminated immunostaining. Scale bar = 10 μm.
Gαi protein was immunolocalized with this antibody to hair cells throughout the saccular macula, however with a differential distribution depending on the position of the hair cell within the sensory epithelium (Fig. 6b). A constant, as for Gαs/olf, was immunolabeling of the stereocilia, often at mid positions of the stereociliary array (Fig. 6c,g). Immunoreactivity for Gαi protein was localized to the hair cell lateral and basal membrane (Fig. 6c,g). Cell bodies of peripheral flask-shaped hair cells, a subpopulation of saccular hair cells bordering non-sensory epithelial cells, were filled with Gαi immunoreactivity (Fig. 6f).
Immunoreactivity was found in efferents (Fig. 6h) in close association with large (unlabeled) afferents (Fig. 6g,h). Smaller-diameter (putative efferent) fibers in close proximity to the hair cell plasma membrane were also immunoreactive (Fig. 6d). These smaller-diameter immunoreactive fibers appeared themselves to be the target of other unlabeled, small-diameter, nerve fibers (Fig. 6d, inset). Small immunoreactive (efferent) fibers were visualized entering the sensory epithelium between nuclei of the supporting cells just above the basal lamina (Fig. 6e). Overall, these results are consistent with expression of Gαi1/Gαi3 in hair cells and efferent nerve, but not in supporting cells.
Expression of message for dopamine D1A4 and D2 receptors in the saccular hair cell layer
The finding that Gαs/olf and adenylyl cyclase type 5/6 were expressed in the saccular hair cell preparation pointed to the possibility of dopaminergic input to vestibular hair cells via a dopamine receptor subtype related to the D1 series, given that all three proteins couple in higher vertebrates (Herve et al., 1993; Zhuang et al., 2000). Further, dopamine and its metabolites had been detected in both the saccular hair cell layer, and relatively more prominently in the paired, accompanying nerve (afferent/efferent) fraction by HPLC analysis coupled with electrochemical detection of biogenic amines (Drescher et al., 2003). A dopaminergic efferent neurotransmitter for the teleost saccule would exert its actions through transmitter receptors located post-synaptically on hair cells/afferent nerve fibers and/or located presynaptically on efferent nerve terminals. The saccular hair cell layer preparation would reflect the post-synaptically expressed hair cell message.
Detection of dopamine D1 receptor subtype cDNA was based on the design of degenerate primers targeting dopamine D1 receptor sequence of the eel (Anguilla anguilla), carp and fugu (Table 1). Amplification products of predicted size were obtained in RT-PCR for the trout hair cell layer, and for trout brain, the latter used as a positive control (Fig. 1). Comparison of amino acid sequences (Fig. 7) indicates that the trout saccular hair-cell amino acid sequence (EU371401) is closest, with 88% amino acid identity, to carp dopamine D1A4 receptor (CAA74971), with 74% amino acid identity to rat dopamine D1A (AAB23803) and 74% identity to human dopamine D1 (NP_000785). Overall, these results indicate mRNA expression of dopamine D1A receptor in saccular hair cells.
Fig. 7. Amino acid sequence for dopamine D1 expressed in the trout saccular hair cell layer.
OMIGA alignment of sequence for dopamine D1A4 expressed in the trout saccular hair cell layer (line 1, EU371401) with sequences for carp dopamine D1A4 (line 2, Y14627), rat dopamine D1A (line 3, S46131), and human dopamine D1A (line 4, CH471062).
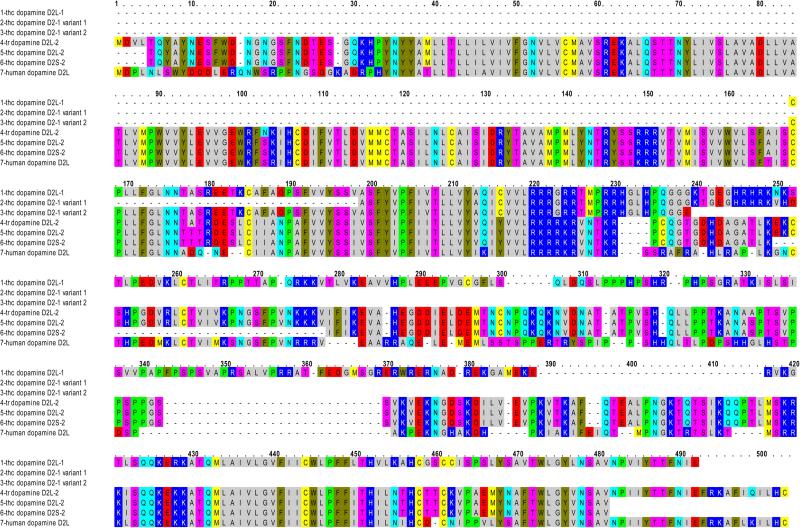
Dopamine D2 receptor primers, based upon dopamine D2 receptor sequence of carp available from GenBank, were applied to cDNA of both trout brain and the trout hair cell layer. The sequence from a 208 bp amplification product from the trout hair cell layer (Fig. 1) was extended with primers based upon trout brain sequence and Danio rerio dopamine D2 sequence (NM_197935, Table 1). Three splice variants of dopamine D2 were obtained: a newly described D2L (Fig. 8, line 1, thc dopamine D2L-1) and two related sequences terminating prematurely within the third intracellular loop region (Fig. 8, lines 2 and 3).
Fig. 8. Amino acid sequence for dopamine D2 receptors expressed in the trout saccular hair cell layer.
Line 1, trout hair cell (thc) dopamine D2L-1 sequence. Line 2, variant 1 of dopamine D2-1 sequence. Line 3, variant 2 of dopamine D2-1 sequence expressed in hair cell layer. Line 4, trout dopamine D2L-2 (CAC79663, Oncorhynchus mykiss dopamine D2 receptor 2). Line 5, trout saccular hair cell dopamine D2L-2. Line 6, trout saccular hair cell D2S-2. Line 7, human dopamine D2L (NP_000786).
A second D2L sequence, detected in saccular hair cells, upon extension yielded 95% of full-length aa coding sequence (Fig. 8, line 5, thc dopamine D2L-2) for a previously described dopamine D2L receptor expressed in trout (GenBank Accession No. CAC79663) (Fig. 8, line 4, tr dopamine D2L-2). A short variant of the second dopamine D2 receptor contained a 29 amino acid deletion in the third intracellular loop orthologous to dopamine D2S in higher vertebrates (Fig. 8, line 6, thc dopamine D2S-2), not previously detected in trout.
The two dopamine D2L receptors expressed in the hair cell preparation were significantly different from each other, with only 42% amino acid identity overall. Diversity occurred primarily in the third intracellular loop. The trout saccular hair cell dopamine D2 sequences display homology to two different zebrafish dopamine D2 sequences corresponding to different evolutionary branches for the dopamine D2 receptor. Trout dopamine D2L-2 (CAC79663) is 73% identical to zebrafish dopamine receptor D2a (NP_898891), which itself is orthologous to human dopamine D2. Trout hair cell D2L-1 is more clearly aligned with zebrafish dopamine D2b (66% identity) as opposed to zebrafish D2a (41% identity). Overall, in our investigation, evidence was obtained for expression of multiple splice variants of dopamine D2 receptors, in addition to dopamine D1A4, in teleost saccular hair cells.
Generality of vestibular hair cell expression of dopamine receptors: Immunolocalization of dopamine D1A and D2L receptors in mammalian vestibular end organs
Molecular results for AC signaling pathways and dopamine receptor expression in the teleost model type II vestibular hair cell preparation clearly pointed to the possibility/generality that vestibular hair cells across evolution may be modulated by dopaminergic input, not previously recognized. To test this hypothesis, we turned to immunolocalization of dopamine receptor protein in mammalian vestibular end organs. Dopamine D1A and D2L receptor immunoreactivity was observed at both hair cell and neural sites in rat saccule and utricle and in mouse utricle (Figs. 9,10). D1A immunoreactivity was concentrated at subcuticular sites of vestibular hair cells, detected with either 3,3′-diaminobenzidine or immunofluorescence in rat saccule (Fig. 9A, panel 2 and Fig. 10c,d1, green). Discrete puncta of immunoreactivity were observed with 3,3′-diaminobenzidine at the inner face of the calyceal afferent endings corresponding to the cell body of the type I vestibular hair cell (Fig. 9A, panel 1), similar to a localization observed with immunofluorescence (Fig. 10c, arrowhead). Dopamine D1 receptors were also immunolocalized to the cell membrane of type II vestibular hair cells (Fig. 10e, red).
Fig. 9. Immunoreactivity for dopamine D1A, D2L and tyrosine hydroxylase in the sensory epithelia of rat saccule and mouse utricle detected with 3,3′-diaminobenzidine.
A. Dopamine D1A immunoreactivity in the rat saccule and mouse utricle. 1. Dopamine D1A immunoreactivity in rat saccule was observed on the inner face of an afferent calyx at its interface with a type I vestibular hair cell (arrow + dot) and in presumptive efferents (intermediate length arrow) at the base of a type II vestibular hair cell identified on basis of the large afferent ending (short arrow; compare afferent in Fig. 9A panel 1 for a type II hair cell with afferent endings in Fig. 4c, Fig. 6c,g,h designated by arrowheads). 2. Dopamine D1A immunoreactivity was found in hair cell subcuticular sites in rat saccule (short arrow). 3. D1A-positive efferents (long arrow) were associated with large afferents (short arrow) at base of putative type I vestibular hair cell in the mouse utricle. Scale bar = 10 μm for 1-3.
B. Dopamine D2L immunolocalization in mouse utricle. 1. Immunoreactivity for dopamine D2L was observed at apical, subcuticular sites on type I vestibular hair cells (long arrow) and at both the outer face (intermediate-length arrow) and inner face (short arrow) of calyceal afferent endings, with the inner face corresponding to the interface of calyx and hair cell membrane. 2,3. Dopamine D2L immunoreactivity at subcuticular sites on vestibular hair cells (arrows). Scale bar = 10 μm for 1-3.
C. Tyrosine hydroxylase immunoreactivity in rat saccule. Immunoreactivity for tyrosine hydroxylase was found within the saccular sensory epithelia in small nerve fibers traveling between supporting cell nuclei just above the basal lamina (short black arrows). Small-diameter immunoreactive fibers contacted the outer membrane of a putative type II vestibular hair cell (long black arrow with dot) and were observed (in cross section, white arrow) to be in close proximity to a larger unlabeled fiber. Small-diameter tyrosine hydroxylase-positive nerve fibers made contact with the outer face of a calyx associated with a type I hair cell (long black arrow) and hair cell body (intermediate-length black arrow). Inset. A grouping of three small-caliber tyrosine hydroxylase-positive nerve fibers (arrow) are identified making contact with the outer face of the afferent calyx, the cell membrane of a type I hair cell, and the cell membrane of an adjacent type II vestibular hair cell. Scale bar = 10 μm.
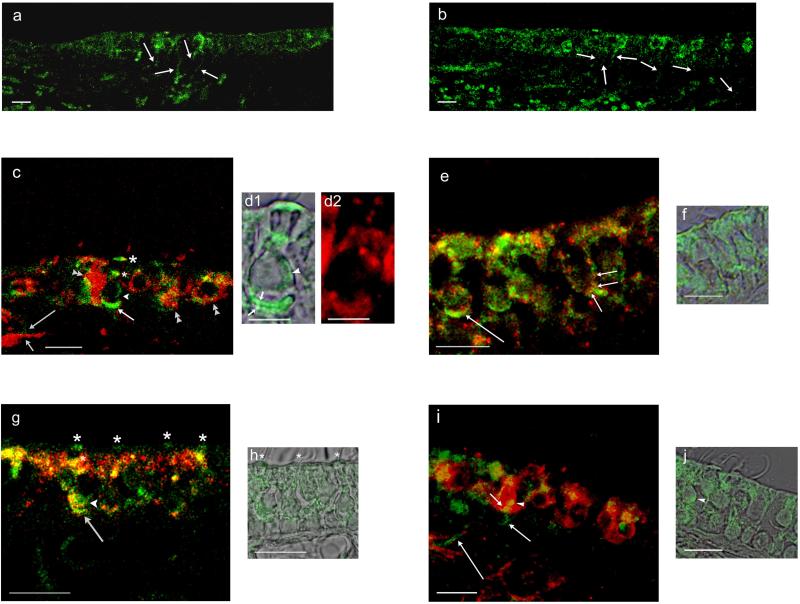
Fig. 10. Dopamine D1 and D2L and tyrosine hydroxylase immunofluorescence in the rat saccule and utricle in comparison with calyceal afferent marker calretinin and AC5/6.
a. Dopamine D1A-positive nerve fibers passed from the connective tissue through the basal lamina to the saccular sensory epithelium of the rat saccule (arrows). b. Dopamine D2L immunofluorescence was localized to nerve fibers which traveled from the connective tissue through the basal lamina to the sensory epithelium of rat saccule (arrows). c. Dopamine D1A immunofluorescence (green) was compared with immunofluorescence for calretinin (red). D1A-positive nerve fibers (long arrow) shadow the calretinin-positive fibers (short arrow) within the connective tissue before passing into the sensory epithelium. Within the sensory epithelium, overlap of dopamine D1A and calretinin immunoreactivity (merge, yellow) occurred on calretinin-containing calyces (double arrowheads) and on calyces at the base of type I vestibular hair cells (intermediate-length arrow). D1A immunoreactivity was found on the cell membrane of the type 1 vestibular hair cell (arrowhead) and at two supranuclear sites (asterisks), one close to the reticular lamina (larger asterisk). d1. D1A immunoreactivity and overlapping differential interference contrast (DIC) image of a type I vestibular hair cell illustrated in c, with immunoreactivity on both faces of the calcyceal afferent (short arrows), on basolateral aspects of the hair cell membrane (arrowhead), and at both intermediate and apical (subcuticular) supranuclear sites. d2. Calretinin immunoreactivity for calyx surrounding the type I vestibular hair cell depicted in d1. e. Dopamine D1 and AC5/6 in rat utricle. AC5 immunofluorescence (green) overlapped/co-localized with dopamine D1 immunofluorescence (red) at a calyceal ending on type I vestibular hair cell (long arrow) (merge, yellow). Co-localization of AC5/6 with dopamine D1 was observed on the basal cell membrane of a type II vestibular hair cell (short arrows) close to a putative efferent nerve ending. f. AC5/6 immunofluorescence image of vestibular type II hair cell illustrated in e overlapping DIC images in adjacent section. g. Dopamine D2L and AC5/6 in rat saccule. Dopamine D2L immunofluorescence (green) co-localized with AC5/6 immunofluorescence (red) at calyceal afferent endings on type I vestibular hair cells (arrow), at the base of type I vestibular hair cell (arrowhead) and at supranuclear sites (asterisks) (merge, yellow). h. DIC and D2L immunoreactivity for the same section. Positions of D2L immunofluorescence at subcuticular sites are marked by asterisks corresponding to first three asterisks in g (left to right). i. Immunofluorescence (green) for tyrosine hydroxylase, a dopaminergic/catecholaminergic (efferent) neuronal marker, was compared to immunofluorescence (red) for calretinin, a calyceal afferent marker. The merged image indicates a small-diameter, tyrosine-positive fiber (green, intermediate-length arrow) in the connective tissue passing through the basal lamina into the sensory epithelium to end (short arrow) on a calyceal afferent (red) of a type I vestibular hair cell (overlap/co-localization in yellow). Tyrosine hydroxylase-positive nerve fibers (presumptive efferents) passed through the connective tissue en route to the sensory epithelium (long arrows). j. DIC and tyrosine hydroxylase immunoreactivity in same section with arrowhead pointing to same site of accumulation of tyrosine hydroxylase immunoreactivity on cell body of a type I vestibular hair cell in i and j. Scale bars = 10 μm, except for d (5 μm).
D1A immunofluorescence was observed in a subpopulation of nerve fibers that passed from underlying connective tissue to the sensory epithelium (Fig. 10a, rat saccule). Within the connective tissue, these fibers (green) appeared to shadow but not overlap calretinin-positive calcyceal afferents (red) (Fig. 10c). Within the sensory epithelium, dopamine D1A immunoreactivity was found in small nerve fibers both at the base of type II vestibular hair cells of rat saccule (Fig. 9A, panel 1) and overlapping large afferents at the base of type I vestibular hair cells in mouse utricle (Fig. 9A, panel 3). D1A immunofluorescence (green) (Fig. 10c) overlapped immunofluorescence of calretinin-containing calyces (red) (Fig. 10c, double arrowheads) with small foci (yellow) consistent with interaction between small D1A-containing fibers and calyces. Overlap also occurred at calyceal sites at the base of type I vestibular hair cells (compare Fig. 10c with Fig. 10d1 and Fig. 10d2), again supporting the possibility of contact between the small-diameter D1A-positive fibers and afferent calyces. The finding of D1A-specific immunoreactivity in small-diameter nerve fibers within the sensory epithelium was not limited to a given rodent species or to one specific type of vestibular end organ, and was found additionally in mouse cristae ampullaris (not illustrated).
Dopamine D2L immunoreactivity was also concentrated at subcuticular sites of vestibular hair cells detected either with 3,3′-diaminobenzidine (Fig. 9B, panels 1-3) or immunofluroescence (Fig. 10g,h, green). Immunolabeling for dopamine D2L was found on both faces of afferent calyces surrounding type I vestibular hair cells (Fig. 9B, panel 1, and Fig. 10g,h), representing sites on the calyceal afferents as well as sites on the cell body of type I vestibular hair cells. Dopamine D2L-positive nerve fibers were observed to pass from underlying connective tissue layer into the sensory epithelium (Fig. 10b). Teleost hair cell expression of dopamine receptor protein was not examined given that sequence targeted by commercially available antibodies does not exist in teleost hair cells.
Comparison of immunoreactivity for dopamine receptors and AC5/6 within the vestibular sensory epithelium of rat saccule and mouse utricle
Molecular analysis of the trout saccular hair cell layer indicated expression in vestibular hair cells of AC 5/6, the AC isoform mediating dopamine receptor signal transduction in higher vertebrates. A corollary is that dopamine receptors in mammalian vestibular end organs and hair cells would colocalize with AC5/6. Immunofluorescence for AC5/6 (Fig. 10e, green) did appear to co-localize with dopamine D1DR (Fig. 10, red) at calyceal afferent endings on vestibular type I hair cells in the rat utricle (Fig. 10e; merge, yellow). AC5/6 also co-localized with D1DR on the basal cell membrane of vestibular type II hair cells (Fig. 10e).
Dopamine D2L immunoreactivity (Fig. 10g,h, green) within the sensory epithelium of the rat saccule co-localized with AC5/6 (Fig. 10g, red), with an overall distribution (Fig. 10g, merge, yellow) at type I vestibular hair cells not dissimilar to that observed for dopamine D1A and calretinin-labeled calyceal afferents (Fig. 10c). Co-localization of immunofluorescence for dopamine D2L and AC5/6 was also observed in nerve fibers entering the sensory epithelium from the connective tissue (not illustrated).
Immunolocalization of tyrosine hydroxylase in mammalian vestibular sensory epithelia
The existence of dopamine D1 and D2 receptors in the vestibular sensory epithelium predicts a source of the agonist, dopamine, putatively in dopaminergic efferent nerve fibers containing tyrosine hydroxylase, the rate-limiting enzyme for biosynthesis of dopamine/catecholamines. In the rat saccule, small-diameter tyrosine hydroxylase-containing fibers were observed to enter the sensory epithelium from underlying connective tissue (Fig. 10i, green), passing between the supporting cell nuclei immediately above the basal lamina (Fig. 9C). These fibers contacted both the outer face of the calyceal afferents (Fig. 9C; Fig. 10i, green) and the hair cell membrane of type I hair cells and type II hair cells (Fig. 9C), consistent with dopaminergic/catecholaminergic input to calyceal afferents and to the cell membrane of type I and type II vestibular hair cells. Tyrosine hydroxylase immunoreactivity was observed close to the reticular lamina within the sensory epithelium (Fig. 10i,j), consistent with the possibility that tyrosine hydroxylase-positive nerve fibers pass through the sensory epithelium to these apical sites.
DISCUSSION
Adenylyl cyclase signaling pathways in saccular hair cells - teleost model
Evidence exists that adenylyl cyclase (AC)-mediated signal transduction impacts mechanosensory transduction (Ricci and Fettiplace, 1997) at apical sites of inner ear hair cells initiating auditory/vestibular signaling. Further, receptoneural transmission at basal sites of hair cells may be modulated via direct actions of cAMP on Ih (Holt and Eatock, 1995; Sugihara and Furukawa, 1996) and indirect actions of cAMP via protein kinase A on VGCC (Bao et al., 2003; Su et al., 1995). The identity of individual, expressed AC isoforms in hair cells will determine the pharmacology of AC enzymatic activity associated with each function. The trout saccular hair cell preparation was utilized to determine hair cell AC transcript expression since it is devoid of contaminating cell types by its method of isolation. This hair cell preparation has singularly provided full-length coding sequence of hair cell proteins, yielding critical sequence information on hair cell splice variants and amino and carboxy termini crucial to the determination of protein-protein interactions which localize hair cell proteins at a molecular level (Ramakrishnan et al., 2009). This preparation, nominally comprising type II vestibular hair cells, does include more than one population of hair cells. Hair cells in striolar and extrastriolar regions can be differentiated in goldfish and frog saccules on the basis of inwardly-rectifying cation channels and cell shape (Holt and Eatock, 1995; Sugihara and Furukawa, 1996). Homology has been noted between striolar and extrastriolar hair cells in fish and type I and type II vestibular hair cells in higher vertebrates, respectively (Popper, 2000).
We have now obtained evidence that message for AC9, AC7 and AC5/6 is expressed in this model hair cell preparation from the trout saccule with message frequency of 11:5:2, respectively. The finding of message for AC9 was consistent with the observed low activation of AC enzymatic activity by forskolin (Drescher et al., 1994). AC9 and AC 5/6 enzymatic activity are inhibited by increases in intracellular Ca2+, the former in conjunction with calcineurin. We now document that calcineurin is also expressed in teleost saccular hair cells. AC7 activity is independent of Ca2+. These results for a type II-like vestibular hair cell differ from cochlear inner hair cell expression of AC isoforms (Drescher et al., 1997), consistent with the hypothesis that hair cells of different types, cochlear inner and outer hair cells, and type I and type II vestibular hair cells, differ in AC-modulated function.
Gαs/olf expression in the saccular hair cell preparation
Biochemical characterizations in other vertebrate systems indicate that the particular AC isoforms that are expressed in the trout saccular hair cells would require Gαs/Gαolf-coupled receptors for activation. AC9 is activated by Gαs, with the degree of activation dependent on its glycosylation state (Cumbay and Watts, 2004). The activation can be down-modulated by Gαi via, for example, dopamine D2L, and also by PKC (Cumbay and Watts, 2004). New information suggests that AC7 is the specific AC isoform coupled to the G12/13 pathway which operates synergistically with Gαs (Jiang et al., 2008). Gα12/13, along with Rho signaling, are activated via protein kinase D (Yuan et al., 2001), which itself is part of the signaling complex formed through protein-protein interactions with AKAP 13 (Wong and Scott, 2004). AKAP 13 is expressed in the teleost saccular hair cell model (MJ Drescher, unpublished results), consistent with the possibility of an AC7-AKAP 13 complex in the type II vestibular hair cells. AC5/6 is activated through Gαs/olf and associates specifically with AKAP79/150, facilitating PKA phosphorylation of AC5/6 isoforms and AC enzymatic inhibition (Bauman et al., 2006). Although the need for Gαs/Gαolf to activate the specific AC isoforms identified in the saccular hair cells is obvious, there has been no previous evidence of expression, either message or protein for Gαs or Gαolf in any vertebrate hair cell. Gαolf message has been found in cDNA from combined cristae and maculae vestibular end organs in rats (Cioffi et al., 2003) which would include multiple cell types. We report here for the first time the complete amino acid sequence of two Gαs/olf proteins expressed in saccular hair cells, sequences which themselves are 89% identical to each other.
Gαs/olf sequences are highly conserved across evolution, with 89% and 84% amino acid identity overall for trout saccular hair cell Gαs/olf-1 and Gαs/olf-2, respectively, compared to human Gαs (NP_001070957). The trout hair cell sequences also are homologous to Gαolf, with 80% and 78% identity, respectively, to human Gαolf-isoform 2. The primary difference between the two trout sequences resided in the first 16 amino acids of the amino terminus (Gαs/olf-2 numbering), with aa 7-16 for Gαs/olf-2 representing a unique 10-amino acid segment that does not exist in other Gαs/olf proteins. Whereas the first 20 aa of trout saccular hair cell Gαs/olf -1 were 95% identical to human Gαs and 65% identical to human Gαolf-2 in sequence, the number dropped for trout saccular hair cell Gαs/olf -2 to 31% for both human Gαs and Gαolf -2. The segment of Gαs/olf-2 from amino acid 2 to 20 has homology (57% identify, 73% positive) to the bird (Taeniopygia guttata) protein kinase NYD-SP5 (XP_002198191), itself an ortholog of IQ motif-containing H isoform 1 (Homo sapiens, NP_001026885) identified as a novel “testis-specific” gene (Yin et al., 2005). The actual amino acid sequence in trout saccular hair cells, exhibiting limited identity to human sequence, does in fact correspond in sequence position to the IQ domain of human NYD-SP5. The possibility of hair cell-specific function for Gαs/olf-2 may relate to this sequence, which in addition contains a WD motif, potentially functioning at the molecular level in protein-protein interactions.
The Gαs/olf proteins were immunolocalized in the trout saccule with a polyclonal antibody targeting the carboxy sequence that is identical for rat Gαs and Gαolf and evolutionarily conserved, appearing in both of the saccular hair cell sequences. Western blot analysis of saccular hair cell proteins indicated immunolabeling of a protein(s) with molecular mass of 45 kDa, the predicted size based on full-length coding sequence of the two expressed forms of Gαs/olf. In immunohistochemical localizations, the hair cells were clearly immunoreactive for Gαs/olf, consistent with molecular results from RT-PCR. Gαs/olf was present in the stereocilia as well as at the base of the teleost hair cells, the latter corresponding to positions of afferent/efferent input. The immunohistochemical signal for Gαs/olf protein in the teleost saccular hair cell stereocilia was substantial, clearly out of the range of what could be present in cochlear hair cell stereocilia based upon immunohistochemical analysis with the same antibodies (Mizuta et al., 1995), suggesting the possibility of different AC signaling in the stereocilia of cochlear hair cells and the stereocilia of type II vestibular hair cells. The extent of immunolabeling of the stereocilia in type II vestibular hair cells would suggest a role for Gαs/olf in a process carried out throughout the stereocilia, as, for example, related to actin filament function.
Gαi isoforms in the saccular hair cell layer
Gαi isoform transcripts are highly conserved across evolution and Gαi transcripts in the trout saccular hair cell model were unequivocally those for cloned Gαi1 and Gαi3, pointing to specificity in the expression of neurotransmitter receptors in saccular hair cells that would selectively couple to Gαi1 and Gαi3. For example, members of the serotonin 5-HT1 receptor subtype family differentially couple to Gαi isoforms, participating in the selectivity of signaling pathways thought to direct distinct cellular functions through, in each instance, inhibition of adenylyl cyclase enzymatic activity (Lin et al., 2002). Serotonin 5-HT1A receptors couple dominantly to Gαi1 and Gαi2 (Kellett et al., 1999; Liu et al., 1999; Lin et al., 2002) and we have previously identified a serotonergic system in the model hair cell preparation including 5-HT1A in a family of expressed 5-HT1 receptors (Drescher et al., 2003) which consequently would represent a candidate receptor coupling to Gαi1. The second form of Gαi expressed in the trout saccular hair cell layer, Gαi3, couples to dopamine D2 activation (Senogles et al., 1994) and AC6/5 (Lee et al., 2002), suggesting the identity of components of a AC pathway in trout saccular hair cells.
Immunoreactivity within the teleost saccular sensory epithelium for Gαi1 and Gαi3 was attributable to hair cell expression, with punctuate immunoreactivity in the stereocilia and along basolateral membranes. As for Gαs/olf, a role for Gαi in stereocilia is unknown. Basolateral positions of Gαi immunoreactivity on the hair cell membrane would correspond to post-synaptic hair cell sites apposed to efferent input. In addition, Gαi immunoreactivity was found in small efferent nerve fibers at the base of the hair cells and in close association with large afferents. Immunoreactivity in these small fibers, designated efferent on the basis of electron microscopic correlation of nerve diameter and presence of synaptic vesicles (Drescher et al., 1995), may relate to presynaptic, receptor-mediated Gαi-associated mechanisms for neurotransmitter release onto hair cells (and possibly onto afferents).
Dopamine D1A4 and D2 receptor transcripts in the trout saccular hair cell preparation
The specific elements of the adenylyl cyclase signal transduction pathway in the trout saccular hair cells are those present in higher vertebrates for dopamine receptor transduction pathways: dopamine D1 coupling to Gαolf (Herve et al., 1993; Zhuang et al., 2000) coupling to adenylyl cyclase type 5/6 (Iwamoto et al., 2003). Dopamine D2 signal transduction also occurs through AC5 (Lee et al., 2002). Further, dopamine D2 receptor activation can down modulate Gαs activation of AC9 (Cumbay and Watts, 2004), and AC9 was one of the detected AC transcripts in trout saccular hair cells. Transcript for dopamine D1 receptor was detected in the model hair cell preparation (EU371401) and determined to resemble most closely dopamine D1A4 and D1A3 of carp (carp D1A4, CAA74971; CarpD1A3, CAA74970).
The dopamine D1 receptor response can be down-modulated and thus gradated by dopamine D2 receptor co-expression (Aizman et al., 2000; Sakolsky and Ashby, 2001). There appear to be five splice variants of dopamine D2 receptors expressed in the teleost hair cell preparation (Abu-Hamdan et al., 2008): two versions of dopamine D2L, one not previously described (D2L-1), and a second (D2L-2) corresponding to a trout sequence expressed during development (NM_001124372 for Oncorhynchus mykiss dopamine D2 receptor 2 mRNA). Three additional splice variants included a short version of dopamine D2 (NM_001124372), whose existence is documented in higher vertebrates but not previously described in teleosts (Boehmler et al., 2004). Two premature terminations within the third cytoplasmic loop for the trout saccular hair cell dopamine D2-1 receptor were also detected.
Curiously, the trout saccular hair cell dopamine D2L receptors, thc D2L-1 and D2L-2, likely represent two separate dopamine D2 genes, corresponding to zebrafish genes dopamine drd2b and drd2a (Boehmler et al., 2004), respectively. Zebrafish dopamine drd2b and drd2a share only 65% amino acid identity, with drd2b clustering with carp D2 and a newly identified fugu sequence on a branch of the phylogenetic tree separate from drd2a. The trout D2L-2 is related to drd2a and human D2L. Drd2b was singularly not syntenic with regions of human chromosomes containing the orthologs of zebrafish dopamine receptor genes, and its function in higher vertebrates is unknown. During development of zebrafish, both drd2b and drd2a have been traced to the pineal gland where they are differentially expressed and proposed to have distinct functions. An association between mRNA expression in the trout saccular hair cells and pineal gland mRNA expression has been previously noted: CNGA3 ion channel splice variants in the trout hair cell preparation are pineal-like (Selvakumar et al., 2010).
CNGA3 and dopamine D2 receptor expression and localization in the trout saccular hair cells may be related. Dopamine D2L and D2S are differentially localized in cultured cells in accord with the expression of heart fatty acid binding protein, H-FABP (Takeuchi and Fukunaga, 2003). Dopamine D2L, with its 29 amino acid insert (exon 5), specifically binds H-FABP with the two proteins co-localized in a perinuclear region around the Golgi apparatus in transfected NG108-15 cells. Dopamine D2S, unable to bind H-FABP, was predominantly localized to the cell membrane. We have determined with yeast two-hybrid analysis that the carboxy terminus of saccular hair cell CNGA3 also specifically binds H-FABP (Selvakumar et al., unpublished results), which thus may act as an intermediary between CNGA3 and dopamine D2L.
Although dopamine D2 receptor coupling to Gαi-isoforms is a subject of debate (Liu et al., 1994, 1999), there is evidence that dopamine D2L couples to Gαi3 (Senogles, 1994), whereas dopamine D2S may operate through Gαi1 (Grunewald et al., 1996). We have obtained evidence of expression of both Gαi1 and Gαi3 isoforms, but not of Gαi2. The third cytoplasmic loop of the D2 dopamine receptor is crucial for determining Gi protein-coupling specificity. Interruption of the alpha-helical character of this region may contribute to changes in selectivity at the receptor-G protein interface (Senogles et al., 2004). Thus, the two novel dopamine D2 receptors that terminate prematurely related to drd2b (D2-1 variants 1 and 2) may have altered ability to interact with Gi protein.
Dopamine D1 and D2L receptor protein and AC5/6 in mammalian vestibular end organs
The expression of dopamine receptors in vestibular hair cells was found to extend from teleost to mammal. Within rat and mouse vestibular end organs for the known dopamine D1A and D2L receptor protein was observed at hair cell sites, providing the first evidence that mammalian vestibular hair cell function may be modulated by dopamine. Receptor protein was localized to the cell membrane of both type I and type 2 vestibular hair cells. Further, both dopamine D1A and D2L receptors were immunolocalized to subcuticular regions of type I vestibular hair cells, with a distribution similar to that of ocsyn, a novel syntaxin-interacting protein. Ocsyn, originally identified in subapical regions of cochlear inner hair cells and thought to be involved in endosome protein recycling (Safieddine et al., 2002), was later detected in type I and type II vestibular hair cells in rat crista, immunolocalized to subcuticular mitochondrialcanalicular complexes (Vautrin et al., 2006). We hypothesize that dopamine D1A and D2 receptors could be recycled from these subcuticular sites.
In addition to hair cell expression, we obtained immunohistochemical evidence supporting dopamine D1 receptor expression by small-caliber efferents contacting calyces and constituting a subpopulation of efferents for type II vestibular hair cells. Further, a presynaptic neural source of dopamine could originate from small-caliber tyrosine hydroxylase-positive fibers passing from the connective tissue layer to end on the cell membrane of type I and type II vestibular hair cells and on the outer face of afferent calyces for type I vestibular hair cells. Direct efferent contact with type I vestibular hair cells has previously been documented although relatively rare (Li et al., 2007). Dopaminergic modulation of afferents for type II-like vestibular hair cells in frog semicircular canal has recently been reported (Andrianov et al., 2009), consistent with the possibility of dopamine D1 and D2 receptors on the “bouton” type afferents and the likely existence of dopaminergic efferent innervation within the cristae ampullaris.
Both dopamine D1A and D2 receptors are known to couple to AC5/6 in higher vertebrates and therefore evidence of co-localization of AC5/6 with dopamine D1 and D2 receptors individually was not surprising and further consistent with a AC-driven mechanism for dopamine receptor signal transduction in mammalian vestibular hair cells. The importance of AC5 in dopaminergic signal transduction is emphasized by the finding that deletion of AC5 in the knockout abolishes both dopamine D2 and D1 modulation of adenylyl cyclase enzymatic activity (Lee et al., 2002; Iwamoto et al., 2003). However, the pharmacology associated with AC5 cannot be considered in isolation from actions of AC6 with which it shares 65% amino acid identity. AC6 expression is enhanced in the knockout for AC5 (Iwamoto et al., 2003) and AC5 protein is reduced in the AC6 knockout (Tang et al., 2008). Further, AC6 participates in increasing the potency of dopamine D2-like receptor agonists (Neve et al., 2004).
Co-localization of dopamine D1 and D2 receptors has been reported in neostriatal neurons (Aizman et al., 2000). There is a reduced response to D1 agonists when D1 and D2 are co-expressed in transfected cells (Sakolsky and Ashby, 2001). Antagonists for dopamine D2 receptors act as functional agonists for dopamine D1 receptor action. In vestibular hair cells, dopamine D2L receptors in conjunction with dopamine D1A receptors could provide a mechanism for a graded post-synaptic adenylyl cyclase response to direct dopaminergic (efferent) input. Adenylyl cyclase-signal transduction pathways in saccular hair cells are believed to up- and down-modulate a hyperpolarization-activated, inwardly rectifying ion channel, Ih which for teleost saccular hair cells is primarily due to expression of HCN1 (Cho et al., 2003; Ramakrishnan et al., 2009). The hyperpolarization-activated, inwardly rectifying cation current Ih has been identified in hair cells of gravity vestibular end organs of both lower and higher vertebrates. For one population of saccular hair cells in frog and fish, Ih is the only inwardly rectifying cation current and was consequently predicted to determine the resting membrane potential and therefore the degree of activation of VGCC and “spontaneous” release of transmitter (Holt and Eatock, 1995; Sugihara and Furukawa, 1996). The second messenger, cAMP, directly gates Ih, shifting the voltage dependence of conductance to more depolarized levels. Adenylyl cyclase enzymatic activity, generating cAMP via AC5/6, would be controlled by dopamine D1A and D2 receptors on hair cells responding to efferent neurotransmitter dopamine. Both dopamine D2 and D1 receptors have been implicated in dopaminergic modulation of neuron excitability via involvement of Ih with several outcomes: excitatory, requiring dopamine D1 + D2 receptors (Wu and Hablitz, 2005) and inhibitory, requiring dopamine D2 receptors (Akopian and Witkovsky, 1996; Vargas and Lucero, 1999; Vandecasteele et al., 2008).
In summary, it has become increasingly evident that the AC signal transduction pathways selectively couple receptor subtypes to Gα isoforms and to AC isoforms, with outcomes dictated by alteration of any one component in the pathway. We have identified in a teleost saccular hair cell preparation the specific elements of the AC signal transduction pathway that in the CNS of higher vertebrates mediates a response to dopamine. Further, we have obtained evidence that mammalian vestibular hair cells also express dopamine D1A and D2L receptors and AC5/6, representing a newly recognized efferent mechanism for regulation of mammalian vestibular hair-cell afferent signaling (Fig. 11).
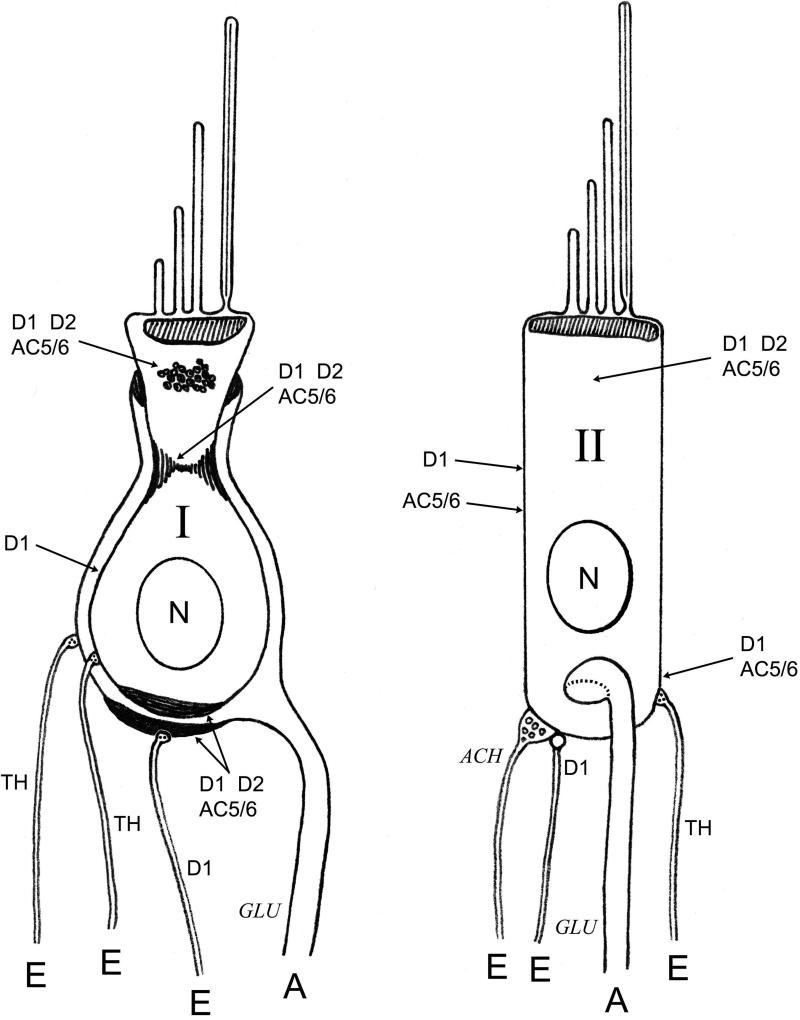
Fig. 11. Proposed dopaminergic innervation of type I and type II vestibular hair cells of the mammalian saccule/utricle.
Localizations of dopamine D1 and D2 receptors and AC5/6 and tyrosine hydroxylase-positive efferent nerve fibers are indicated for type I and type II vestibular hair cells of the saccule/utricle in rat/mouse. Labels reflect localizations for which there is direct evidence from the present study. Putative neurotransmitters in nerve fibers are indicated in italics. E, efferent; A, afferent.
Acknowledgments
This work was supported by NIH DC004076 (MJD) and DC000156 (DGD).
Abbreviations
- AC
adenylyl cyclase
- RT-PCR
reverse transcription-polymerase chain reaction
- Ih
hyperpolarization-activated, inwardly-rectifying cation current
- 5′ and 3′-RACE
5′ and 3′-rapid amplification of cDNA ends
- SMART
switching mechanism at 5′ end of RNA transcript
- taq
thermostable DNA polymerase from Thermus aquaticus
- PBST
phosphate buffered saline containing 0.1% Tween
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate
- SDS
sodium dodecyl sulfate
- DAB
3,3′-diaminobenzidine
- MOPS
3-(N-morpholino)propanesulfonic acid
- VGCC
voltage-gated calcium channels
- DIC
differential interference contrast
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abu-Hamdan MD, Drescher MJ, Hatfield JS, Drescher DG. Expression of novel dopamine D2 receptors in saccular hair cells. Assoc Res Otolaryngol Abstr. 2008;31:73. [Google Scholar]
- Adachi M, Mizuta K, Iwasa KH, Simonds WF, Tachibana M. Ultrastructural localization of Gi-proteins in the organ of Corti of the guinea pig cochlea. Assoc Res Otolaryngol Abstr. 1996;19:185. [Google Scholar]
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- Akopian A, Witkovsky P. D2 dopamine receptor-mediated inhibition of a hyperpolarization activated current in rod photoreceptors. J Neurophysiol. 1996;76:1828–1835. doi: 10.1152/jn.1996.76.3.1828. [DOI] [PubMed] [Google Scholar]
- Andrianov GN, Ryzhova IV, Tobias TV. Dopaminergic modulation of afferent synaptic transmission in the semicircular canals of frogs. Neurosignals. 2009;17:222–228. doi: 10.1159/000224632. [DOI] [PubMed] [Google Scholar]
- Antoni FA, Palkovits M, Simpson J, Smith SM, Leitch AL, Rosie R, Fink G, Paterson JM. Ca2+/calcineurin-inhibited adenylyl cyclase, highly abundant in forebrain regions, is important for learning and memory. J Neurosci. 1998;18:9650–9661. doi: 10.1523/JNEUROSCI.18-23-09650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H, Wong WH, Jay M, Goldberg JM, Eatock RA. Voltage-gated calcium channel currents in type I and type II hair cells isolated from the rat crista. Neurophysiol. 2003;90:155–164. doi: 10.1152/jn.00244.2003. [DOI] [PubMed] [Google Scholar]
- Barritt LC, Beisel KW. G-protein expression in rat inner ear hair cells. Mol Biol Hear Deaf Abstr. 1995;2:134. [Google Scholar]
- Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DM, Dessauer CW, Scott JD. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmler W, Obrecht-Pflumio S, Canfield V, Thisse C, Thisse B, Levenson R. Evolution and expression of D2 and D3 dopamine receptor genes in zebrafish. Dev Dyn. 2004;230:481–493. doi: 10.1002/dvdy.20075. [DOI] [PubMed] [Google Scholar]
- Canlon B, Homburger V, Bockaert J. The identification and localization of the guanine nucleotide binding protein Go in the auditory system. Eur J Neurosci. 1991;3:1338–1342. doi: 10.1111/j.1460-9568.1991.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Cho WJ, Drescher MJ, Hatfield JS, Bessert DA, Skoff RP, Drescher DG. Hyperpolarization-activated, cyclic AMP-gated, HCN1-like cation channel: the primary, full-length HCN isoform expressed in a saccular hair-cell layer. Neuroscience. 2003;118:525–534. doi: 10.1016/s0306-4522(02)00913-2. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cioffi JA, Erbe CB, Raphael R, Kwitek AE, Tiwari UK, Jacob HJ, Popper P, Wackym PA. Expression of G-protein alpha subunit genes in the vestibular periphery of Rattus norvegicus and their chromosomal mapping. Acta Otolaryngol. 2003;123:1027–1034. doi: 10.1080/00016480310000773. [DOI] [PubMed] [Google Scholar]
- Cumbay MG, Watts VJ. Novel regulatory properties of human type 9 adenylate cyclase. J Pharmacol Exp Ther. 2004;310:108–115. doi: 10.1124/jpet.104.065748. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- Drescher DG, Khan KM, Arden RL, Drescher MJ, Kerr TP, Hatfield JS. Protein associated with the sensory cell layer of the rainbow trout saccular macula. Brain Res. 1989;485:225–235. doi: 10.1016/0006-8993(89)90565-9. [DOI] [PubMed] [Google Scholar]
- Drescher DG, Ramakrishnan NA, Drescher MJ, Chun W, Wang X, Myers SF, Green GE, Sadrazodi K, Karadaghy AA, Poopat N, Karpenko AN, Khan KM, Hatfield JS. Cloning and characterization of alpha9 subunits of the nicotinic acetylcholine receptor expressed by saccular hair cells of the rainbow trout (Oncorhynchus mykiss). Neuroscience. 2004;127:737–752. doi: 10.1016/j.neuroscience.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Drescher MJ, Cho WJ, Anne S, Hatfield JS, Drescher DG. Adenylyl cyclase-mediated signal transduction in saccular hair cells: Coupling to Gαs/olf. Assoc Res Otolaryngol Abstr. 2004;27:239. [Google Scholar]
- Drescher MJ, Drescher DG, Hatfield JS. Potassium-evoked release of endogenous primary amine-containing compounds from the trout saccular macula and saccular nerve in vitro. Brain res. 1987;417:39–50. doi: 10.1016/0006-8993(87)90177-6. [DOI] [PubMed] [Google Scholar]
- Drescher MJ, Drescher DG, Khan KM, Hatfield JS, Ramakrishnan NA, Abu-Hamdan MD, Lemonnier LA. Pituitary adenylyl cyclase-activating polypeptide (PACAP) and its receptor (PAC1-R) are positioned to modulate afferent signaling in the cochlea. Neuroscience. 2006;142:139–164. doi: 10.1016/j.neuroscience.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Drescher MJ, Kern RC, Hatfield JS, Drescher DG. Cytochemical localization of adenylyl cyclase activity within the sensory epithelium of the trout saccule. Neurosci Lett. 1995;196:145–148. doi: 10.1016/0304-3940(95)11764-n. [DOI] [PubMed] [Google Scholar]
- Drescher MJ, Khan KM, Beisel KW, Karadaghy AA, Hatfield JS, Kim SY, Drescher AJ, Lasak JM, Barretto RL, Shakir AH, Drescher DG. Expression of adenylyl cyclase type I in cochlear inner hair cells. Mol Brain Res. 1997;45:325–330. doi: 10.1016/s0169-328x(97)00007-7. [DOI] [PubMed] [Google Scholar]
- Drescher MJ, Khan KM, Cho WJ, Kewson DT, Hatfield JS, Drescher DG. Serotonin and its receptors may modulate hair cell afferent transmission in the trout saccule. Assoc Res Otolaryngol Abstr. 2003;26:11–12. [Google Scholar]
- Drescher MJ, Margolis DI, Drescher DG. Cyclic AMP in an inner-ear hair cell sheet preparation. Soc Neurosci Abstr. 1994;20:969. [Google Scholar]
- Folbe AJ, Drescher MJ, Ramakrishnan NA, Khan KM, Hatfield JS, Drescher DG. Gαi isoforms in a saccular hair cell preparation: mRNA and protein expression. Assoc Res Otolaryngol Abstr. 2004;27:239. [Google Scholar]
- Grünewald S, Reiländer H, Michel H. In vivo reconstitution of dopamine D2S receptor-mediated G-protein activation in baculovirus-infected insect cells: preferred coupling to Gi1 versus Gi2. Biochemistry. 1996;35:15162–15173. doi: 10.1021/bi960757w. [DOI] [PubMed] [Google Scholar]
- Herve D, Levi-Strauss M, Marey-Semper I, Verney C, Tassin JP, Glowinski J, Girault JA. G(olf) and Gs in rat basal ganglia: possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci. 1993;13:2237–2248. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JR, Eatock RA. Inwardly rectifying currents of saccular hair cells from the leopard frog. J Neurophysiol. 1995;73:1484–1502. doi: 10.1152/jn.1995.73.4.1484. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Okumara S, Iwatsubo K, Kawabe J, Ohtsu K, Sakai I, Hashimoto Y, Izumitani A, Sango K, Ajiki K, Toya Y, Umemura S, Goshima Y, Arai N, Vatner SF, Ishikawa Y. Motor dysfunction in type 5 adenylyl cyclase-null mice. J Biol Chem. 2003;278:16936–16940. doi: 10.1074/jbc.C300075200. [DOI] [PubMed] [Google Scholar]
- Jiang LI, Collins J, Davis R, Fraser ID, Sternweis PC. Regulation of cAMP responses by the G12/13 pathway converges on adenylyl cyclase VII. J Biol Chem. 2008;283:23429–23439. doi: 10.1074/jbc.M803281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett E, Carr IC, Milligan G. Regulation of G protein activation and effector modulation by fusion proteins between the human 5-hydroxytryptamine 1A receptor and the α subunit of Gi1: differences in receptor-constitutive activity imparted by a single amino acid substitution in Gi1α. Mol Pharmacol. 1999;56:684–692. [PubMed] [Google Scholar]
- Kewson DT, Drescher MJ, Hatfield JS, Myers SF, Drescher DG. Dopamine receptor expression in the inner ear. Assoc Res Otolaryngol Abstr. 2003;26:156. [Google Scholar]
- Khan KM, Hatfield JS, Drescher DG. The cell coat of the sensory and supporting cells of the rainbow trout saccular macula as demonstrated by reaction with ruthenium red and tannic acid. J Histochem Cytochem. 1990;11:1615–1623. doi: 10.1177/38.11.1698852. [DOI] [PubMed] [Google Scholar]
- Khan KM, Hatfield JS, Drescher MJ, Drescher DG. The histochemical localization of acetylcholinesterase in the rainbow trout saccular macula by electron microscopy. Neurosci Lett. 1991;131:109–112. doi: 10.1016/0304-3940(91)90348-w. [DOI] [PubMed] [Google Scholar]
- Lee KW, Hong JH, Choi IY, Che Y, Lee JK, Yang SD, Song CW, Kang HS, Lee JH, Noh JS, Shin HS, Han PL. Impaired D2 dopamine receptor function in mice lacking type 5 adenylyl cyclase. J Neurosci. 2002;22:7931–7940. doi: 10.1523/JNEUROSCI.22-18-07931.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GQ, Kevetter GA, Leonard RB, Prusak DJ, Wood TG, Correia MJ. Muscarinic acetylcholine receptor subtype expression in avian vestibular hair cells, nerve terminals and ganglion cells. Neuroscience. 2007;146:384–402. doi: 10.1016/j.neuroscience.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL, Setya S, Johnson-Farley NN, Cowen DS. Differential coupling of 5-HT1 receptors to G proteins of the Gi family. Brit J Pharmacol. 2002;136:1072–1078. doi: 10.1038/sj.bjp.0704809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YF, Ghahremani MH, Rasenick MM, Jakobs KH, Albert PR. Stimulation of cAMP synthesis by Gi-coupled receptors upon ablation of distinct Gαi protein expression. Gi subtype specificity of the 5-HT1A receptor. J Biol Chem. 1999;274:16444–16450. doi: 10.1074/jbc.274.23.16444. [DOI] [PubMed] [Google Scholar]
- Liu YF, Jakobs KH, Rasenick MM, Albert PR. G protein specificity in receptor-effector coupling. Analysis of the roles of Go and Gi2 in GH4C1 pituitary cells. J Biol Chem. 1994;269:13880–13886. [PubMed] [Google Scholar]
- Lü Q, Dunlap K. Cloning and functional expression of novel N-type Ca2+ channels variants. J Biol Chem. 1999;274:34566–34575. doi: 10.1074/jbc.274.49.34566. [DOI] [PubMed] [Google Scholar]
- Mizuta K, Iwasa KH, Simonds WF, Tachibana M. Ultrastructural localization of G-protein Gs in the organ of Corti. Neurosci Lett. 1995;201:147–150. doi: 10.1016/0304-3940(95)12149-8. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Ogata Y, Slepecky NB. Immunocytochemical localization of calmodulin in the vestibular end-organs of the gerbil. J Vestib Res. 1998;8:209–216. [PubMed] [Google Scholar]
- Oh CK, Drescher MJ, Drescher DG, Myers SF. Detection of serotonin 5-HT1A transcript in a teleost hair cell layer. Assoc Res Otolaryngol Abstr. 2001;24:141. [Google Scholar]
- Popper AN. Hair cell heterogeneity and ultrasonic hearing: recent advances in understanding fish hearing. Phil Trans R Soc Lond B Biol Sci. 2000;355:1277–1280. doi: 10.1098/rstb.2000.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Matsuoka I, Mattei MG, Pouille Y, Defer N, Hanoune J. Identification and characterization of a widely expressed form of adenylyl cyclase. J Biol Chem. 1996;271:13900–13907. doi: 10.1074/jbc.271.23.13900. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan NA, Drescher MJ, Sheikhali SA, Khan KM, Hatfield JS, Dickson MJ, Drescher DG. Molecular identification of an N-type Ca2+ channel in saccular hair cells. Neuroscience. 2006;139:1417–1434. doi: 10.1016/j.neuroscience.2006.01.064. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan NA, Drescher MJ, Barretto RL, Beisel KW, Hatfield JS, Drescher DG. Calcium-dependent binding of HCN1 channel protein to hair cell stereociliary tip-link protein protocadherin 15 CD3. J Biol Chem. 2009;284:3227–3238. doi: 10.1074/jbc.M806177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan NA, Green GE, Pasha R, Drescher MJ, Swanson GS, Perin P, Lakhani RS, Ahsan SF, Hatfield JS, Khan KM, Drescher DG. Voltage-gated Ca2+ channel CaV1.3 subunit expressed in the hair cell epithelium of the sacculus of the trout Oncorhynchus mykiss: cloning and comparison across vertebrate classes. Brain Res Mol Brain Res. 2002;109:69–83. doi: 10.1016/s0169-328x(02)00522-3. [DOI] [PubMed] [Google Scholar]
- Ricci AJ, Fettiplace R. The effects of calcium buffering and cyclic AMP on mechano- electrical transduction in turtle auditory hair cells. J Physiol. 1997;501:111–124. doi: 10.1111/j.1469-7793.1997.111bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman EI, De Jongh KS, Florio V, Lai Y, Catterall WA. Specific phosphorylation of a COOH-terminal site on the full-length form of the α1 subunit of the skeletal muscle calcium channel by cAMP-dependent protein kinase. J. Biol. Chem. 1992;267:16100–16105. [PubMed] [Google Scholar]
- Safieddine S, Ly CD, Wang YX, Wang CY, Kachar B, Petralia RS, Wenthold RJ. Ocsyn, a novel syntaxin-interacting protein enriched in the subapical region of inner hair cells. Mol Cell Neurosci. 2002;20:343–353. doi: 10.1006/mcne.2002.1120. [DOI] [PubMed] [Google Scholar]
- Sakolsky DJ, Ashby B. Patterns of cyclic AMP formation by coexpressed D1 and D2L dopamine receptors in HEK 293 cells. Receptors Channels. 2001;7:479–489. [PubMed] [Google Scholar]
- Selvakumar D, Drescher M, Drescher D. Cyclic nucleotide-gated (CNG) ion channels in saccular hair cells. Assoc Res Otolaryngol Abstr. 2010;33:28. [Google Scholar]
- Senogles SE. The D2 dopamine receptor isoforms signal through distinct Gi alpha proteins to inhibit adenylyl cyclase. A study with site-directed mutant Gi alpha proteins. J Biol Chem. 1994;269:23120–23127. [PubMed] [Google Scholar]
- Senogles SE, Heimert TL, Odife ER, Quasney MW. A region of the third intracellular loop of the short form of the D2 dopamine receptor dictates Gi coupling specificity. J Biol Chem. 2004;279:1601–1606. doi: 10.1074/jbc.M309792200. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Barres BA, Corey DP. ‘Bundle blot’ purification and initial protein characterization of hair cell stereocilia. Proc Natl Acad Sci USA. 1989;86:4973–4077. doi: 10.1073/pnas.86.13.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky NB, Ulfendahl M. Evidence for calcium-binding proteins and calcium-dependent regulatory proteins in sensory cells of the organ of Corti. Hear Res. 1993;70:73–84. doi: 10.1016/0378-5955(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Slepecky N, Ulfendahl M, Flock Å . Effects of caffeine and tetracaine on outer hair cell shortening suggest intracellular calcium involvement. Hear Res. 1988;32:11–22. doi: 10.1016/0378-5955(88)90143-8. [DOI] [PubMed] [Google Scholar]
- Su ZL, Jiang SC, Gu R, Yang WP. Two types of calcium channels in bullfrog saccular hair cells. Hear Res. 1995;87:62–68. doi: 10.1016/0378-5955(95)00079-j. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Furukawa T. Inwardly rectifying currents in hair cells and supporting cells in the goldfish sacculus. J Physiol. 1996;495:665–679. doi: 10.1113/jphysiol.1996.sp021624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Asano T, Wilcox E, Yokotani N, Rivolta MN, Fex J. G protein Gi2 α in the cochlea: cloning and selective occurrence in receptor cells. Brain Res Mol Brain Res. 1994;21:355–358. doi: 10.1016/0169-328x(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Fukunaga K. Differential subcellular localization of two dopamine D2 receptor isoforms in transfected NG108-15 cells. J Neurochem. 2003;85:1064–1074. doi: 10.1046/j.1471-4159.2003.01763.x. [DOI] [PubMed] [Google Scholar]
- Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JX, Roth DM, Hammond HK. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation. 2008;117:61–69. doi: 10.1161/CIRCULATIONAHA.107.730069. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandecasteele M, Glowinski J, Deniau JM, Venance L. Chemical transmission between dopaminergic neuron pairs. Proc Natl Acad Sci USA. 2008;105:4904–4909. doi: 10.1073/pnas.0703121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas G, Lucero MT. Dopamine modulates inwardly rectifying hyperpolarization-activated current (Ih) in cultured rat olfactory receptor neurons. J Neurophysiol. 1999;81:149–158. doi: 10.1152/jn.1999.81.1.149. [DOI] [PubMed] [Google Scholar]
- Vautrin J, Travo C, Boyer C, Ventéo S, Favre D, Dechesne CJ. Ocsyn and mitochondrialcanalicular complexes in vestibular hair cells. Hearing Res. 2006;222:28–34. doi: 10.1016/j.heares.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Wu J, Hablitz JJ. Cooperative activation of D1 and D2 dopamine receptors enhances a hyperpolarization-activated inward current in layer I interneurons. J Neurosci. 2005;25:6322–6328. doi: 10.1523/JNEUROSCI.1405-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin LL, Li JM, Zhou ZM, Sha JH. Identification of a novel testis-specific gene and its potential roles in testis development/spermatogenesis. Asian J Androl. 2005;7:127–137. doi: 10.1111/j.1745-7262.2005.00041.x. [DOI] [PubMed] [Google Scholar]
- Yuan J, Slice LW, Rozengurt E. Activation of protein kinase D by signaling through Rho and the α subunit of the heterotrimeric G protein G13. J Biol Chem. 2001;276:38619–38627. doi: 10.1074/jbc.M105530200. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Belluscio L, Hen R. GOLFα mediates dopamine D1 receptor signaling. J Neurosci. 2000;20:RC91. doi: 10.1523/JNEUROSCI.20-16-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]