Abstract
Objective
We have shown that the chloride-proton antiporter ClC-3 is required for endosome-dependent signaling by the Nox1 NADPH oxidase in SMCs. In this study, we tested the hypothesis that ClC-3 is necessary for proliferation of smooth muscle cells (SMCs) and contributes to neointimal hyperplasia following vascular injury.
Methods and Results
Studies were performed in SMCs isolated from the aorta of ClC-3 null and littermate control (WT) mice. Thrombin and TNF-α each caused activation of both mitogen activated protein kinase ERK1/2 and the matrix-degrading enzyme MMP-9, and cell proliferation of WT SMCs. Whereas responses to thrombin were preserved in ClC-3 null SMCs, the responses to TNF-α were markedly impaired. These defects normalized following gene transfer of ClC-3. Carotid injury increased vascular ClC-3 expression and compared to WT mice, ClC-3 null mice exhibited a reduction in neointimal area of the carotid artery 28 days after injury.
Conclusion
ClC-3 is necessary for the activation of SMCs by TNF-α but not thrombin. Deficiency of ClC-3 markedly reduces neointimal hyperplasia following vascular injury. In view of our previous findings, this observation is consistent with a role for ClC-3 in endosomal Nox1-dependent signaling. These findings identify ClC-3 as a novel target for the prevention of inflammatory and proliferative vascular diseases.
Keywords: proliferation, smooth muscle cells, restenosis, ion channels
There is widespread appreciation that atherosclerosis is a chronic inflammatory disease 1. Proliferation of smooth muscle cells (SMCs) is fundamental to the development of vascular diseases, including arteriosclerosis, restenosis, and bypass graft failure 2, 3. In addition to cell division, these processes require SMCs to secrete proteases to degrade extracellular matrix and facilitate the subsequent migration of cells from the media into the forming neointima. A variety of signaling molecules have been implicated in stimulating SMCs to undergo this process.
Thrombin is a serine protease in the clotting cascade that converts soluble fibrinogen to insoluble fibrin. Thrombin also cleaves and activates the G-protein coupled protease activated receptor 1 (PAR1), which causes proliferation and increased motility via activation of the epidermal growth factor receptor (EGFR) 4, 5. PAR1 activation contributes to the pathogenesis of atherosclerosis and restenosis via a variety of mechanisms 6 many of which are mediated via activation of the EGFR 4. Mice deficient in the PAR1 receptor display an altered response to vascular injury 7.
The proinflammatory cytokine tumor necrosis factor-alpha (TNF-α) localizes to areas of arterial injury 8, 9 and contributes to development of neointimal hyperplasia 10, 11. Although macrophages are a primary source of cytokines, vascular endothelium and SMCs also secrete TNF-α 12, 13. Upon exposure to TNF-α, quiescent SMCs express matrix metalloproteinases and increase migration and proliferation by intracellular signaling pathways which involve activation of the transcription factor NF-κB 14, 15. Animals lacking TNF-α display remarkably reduced activation of NF-κB and neointima formation after carotid injury 10. ClC-3 is a member of the CLC family of chloride-transporting proteins, which includes both anion channels (ClC-1 and ClC-2) and Cl−/H+ antiporters (ClC-3 through 7) 16. ClC-3 is present in all cell types and localized in plasma membranes and in intracellular vesicles 17–19. In early endosomes of SMCs and in secretory vesicles of neutrophils, ClC-3 is necessary for activation of NADPH oxidase by cytokines 20–22.
We have recently demonstrated that both thrombin and TNF-α signaling are dependent on production of reactive oxygen species (ROS) via activation of the Nox1 NADPH oxidase 23. Nox1 plays an essential role in SMC proliferation, motility, and the inflammatory cascade associated with NF-κB activation. It is therefore not surprising that Nox1 signaling contributes to neointima development after acute vascular injury 24. However, we have demonstrated that the mechanism and subcellular location of Nox1 activation by thrombin and TNF-α is very different. Whereas TNF-α induces a Nox1-dependent, ClC-3-dependent, endosomal generation of intracellular ROS, thrombin activates Nox1 such that ROS does not occur within endosomes and does not require ClC-3 23. We have proposed that ClC-3 provides charge neutralization for the NADPH oxidase electron current that is required for generation of ROS in signaling endosomes following cytokine activation 20, 25, 26. Based on these findings, we hypothesized that ClC-3 is critical to the activation of SMCs by TNF-α but not to thrombin. We therefore determined whether ClC-3 was required for activation of ERK1/2 and MMP-9 or for cell proliferation in response to thrombin or TNF-α. In addition, since the contribution of ClC-3 to the development of vascular disease has not been previously tested, we assessed the requirement of this protein for neointimal hyperplasia following carotid injury.
Materials and Methods
A full description of all methods can be found in the supplemental materials. SMCs were isolated from aorta of mice lacking the Clcn3 gene encoding ClC-3 chloride channels 27 (ClC-3 null) and from wild-type (WT) littermate controls as previously described 20. For experiments, SMCs were serum-deprived (0.5% serum) for 24 hours prior to stimulation with TNF-α (10ng/ml) or thrombin (2 U/ml). The long N-terminal isoform of human ClC-3 (P51790) was PCR amplified and cloned into the adenovirus shuttle plasmid pacAd5 CMV behind the CMV promoter and used for reconstitution of ClC-3 expression in the null cells. Cell proliferation was determined by measuring [3H]-thymidine incorporation. Matrix metalloproteinase activity within the media was measured by gelatin zymography. The blood flow cessation model was used to induce carotid neointimal hyperplasia as previously described 28 in ClC-3 null and littermate control mice and carotid arteries harvested 28 days later. Results are expressed as mean ± standard error of the mean. Statistical comparisons were performed by Student’s two-tailed t-tests or analysis of variance with Tukey multiple comparison post-test as appropriate.
Results
Role of ClC-3 in proliferation of SMCs
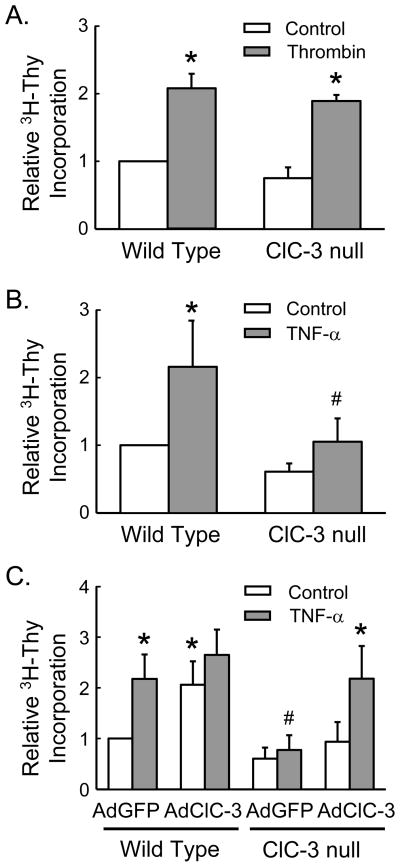
In response to either thrombin or TNF-α, WT SMCs increased 3H-thymidine incorporation more than 2-fold. In contrast, SMCs deficient in ClC-3 responded similarly to thrombin but failed to respond to TNF-α (Fig. 1A and 1B). However, adenoviral-mediated gene transfer of ClC-3 to ClC-3 null SMCs normalized 3H-thymidine incorporation in response to TNF-α (Fig. 1C), suggesting a requirement for ClC-3 in TNF-α-mediated proliferation. The overexpression of ClC-3 in WT SMCs increased 3H-thymidine uptake under basal conditions without affecting the maximal response to TNF-α (Fig. 1C). Further supporting a role for ClC-3 in SMCs growth, the chloride channel inhibitor niflumic acid (NFA, 0.3 mM) inhibited TNF-α-dependent 3H-thymidine incorporation in WT SMCs (2.1 ± 0.3 vs. 0.2 ± 0.1 for DMSO + TNF-α vs. NFA + TNF-α, n = 4, p<0.05).
Figure 1. ClC-3 is necessary for TNF-α-mediated SMC proliferation.
3H-thymidine incorporation was measured in WT and ClC-3 null SMCs in response to (A) thrombin (2 U/ml) or (B) TNF-α (10 ng/ml). * p<0.05 vs. WT control; # p<0.05 vs. WT + TNF-α. (C) WT and ClC-3 null SMCs were treated with adenovirus for the expression of ClC-3 (AdClC-3) or control vector (AdGFP). * p<0.05 vs. WT + AdGFP; # p<0.05 WT + AdGFP + TNF-α. Data are normalized to WT control, n=4 to 6.
Role of ClC-3 in secretion of MMP-9
As detected by gelatin zymography, MMP-9 activity in the culture media increased when SMCs were treated for 24 hours with thrombin (Fig. 2A) or TNF-α respectively (Fig. 2B). SMCs deficient in ClC-3 responded normally to thrombin but failed to produce the normal increase in MMP-9 activity in response to TNF-α. This response was corrected following gene transfer of ClC-3 (Fig. 2B). Consistent with these observations, pre-treatment of WT SMCs with NFA markedly inhibited TNF-α-induced MMP-9 activation (4.2 ± 0.3 vs. 0.7 ± 0.1 for DMSO + TNF-α vs. NFA + TNF-α, n = 4, p<0.05).
Figure 2. ClC-3 is necessary for TNF-α-mediated activation of MMP-9.
Top panel shows gelatin zymography for MMP-9 of the culture media from WT and ClC-3 null SMCs after (A) thrombin (2 U/ml) or (B) TNF-α (10 ng/ml). Summary densitometry data is shown in the lower panel and includes ClC-3 null SMCs infected with AdClC-3 or AdGFP. (C) Summary data of SMCs MMP-9 protein expression normalized to GAPDH. All summary data are normalized to WT control, n=4, * p<0.05 vs. WT control, # p<0.05 vs. WT + TNF-α.
We next confirmed that these observations reflected changes in MMP-9 activation and not in expression by ClC-3 null SMCs. Wildtype and ClC-3 null were serum-deprived for 24 hours prior to stimulation with TNF-α or thrombin for 24 hours and cells collected for real time PCR and Western blotting. There were no differences in the mRNA levels of MMP-9 between the genotypes and treatments (n=4, data not shown). Expression of MMP-9 protein was also similar in untreated WT and ClC-3 null cells (Fig. 2C). Following TNF-α and thrombin treatment, protein levels of MMP-9 were reduced in WT cells, consistent with its secretion into the media (as shown in Fig 2A and 2B). In contrast, expression of MMP-9 by ClC-3 deficient cells was not significantly reduced by TNF-α.
Role of ClC-3 in TNF-α-induced activation of ERK1/2
Thrombin and TNF-α have been previously shown to activate multiple signaling pathways in SMCs, including extracellular signal-regulated kinases 1 and 2 (ERK1/2)29, 30. We tested the hypothesis that ClC-3 is necessary for activation of ERK1/2 by these agonists. Five minutes following treatment of SMCs with thrombin (Fig 3A) phosphorylation of ERK1/2 is increased approximately twofold. This effect is unaltered in cells lacking ClC-3. In response to TNF-α (Fig 3B), phosphorylation of ERK1/2 is increased in WT SMCs but ClC-3 null SMCs did not respond. To exclude a delay in signaling, we also found that TNF-α failed to activate ERK1/2 in ClC-3 null SMCs at 10 and 15 minutes after stimulation (data not shown). However, adenoviral-mediated gene transfer of ClC-3 rescued activation of ERK1/2 in ClC-3 deficient SMCs (Fig. 3B). Furthermore, NFA inhibited TNF-α-induced ERK1/2 activation in WT SMCs (1.7 ± 0.1 vs. 0.6 ± 0.1 for DMSO + TNF-α vs. NFA + TNF-α, n = 4, p<0.05).
Figure 3. TNF-α activation of ERK1/2 requires ClC-3.
Representative Western blots showing phospho-ERK1/2 and total ERK1/2 in WT and ClC-3 null SMCs collected five minutes after stimulation with (A) thrombin or (B) TNF-α. Treatments for each lane correspond to the summary data shown in the bottom panel. Densitometry data were normalized to WT control, n=4, * p<0.05 vs. WT control; # p<0.05 vs. WT + TNF-α. TNF-α-mediated (C) 3H-thymidine uptake and (D) MMP-9 activation are inhibited when WT SMCs are pre-treated with the MEK inhibitor U0126 (10 μM). * p<0.05 vs. WT control, # p<0.05 vs. WT + TNF-α, n=3.
We next examined the role of ERK1/2 in cell proliferation and activation of MMP-9 in SMCs following stimulation by TNF-α. The MEK1/2 inhibitor U0126 31 reduced baseline 3H-thymidine incorporation and prevented the proliferative response to TNF-α in WT SMCs (Fig. 3C). Overexpression of ClC-3 failed to restore proliferation in the presence of MEK1/2 inhibition (data not shown). U0126 had a similar effect on MMP-9 activation (Fig. 3D), which is consistent with previous reports 32. Taken together, these observations suggest that in response to TNF-α, ClC-3 is proximal to activation of ERK1/2, which is necessary for the signaling of proliferation and MMP-9 activation.
Role of ClC-3 in neointimal hyperplasia
Expression of Nox1 is increased early after balloon injury of rat arteries and remains elevated in association with the development of neointima 33. We recently demonstrated that activation of NF-κB by Nox1 requires ClC-3 20. Therefore, we next examined expression of ClC-3 in vascular tissues following cytokine activation or vascular injury. Smooth muscle cell expression of ClC-3 increased more than 3-fold following exposure to TNF-α (Fig. 4A). Extending these observations in vivo, the left carotid artery was ligated in mice as a model of neointimal hyperplasia 34. Ten days following surgery, which is prior to the development of neointima 35, ClC-3 expression was increased ~ 2.5-fold in the injured carotid artery, as compared to the contralateral non-injured artery (Fig. 4B).
Figure 4. ClC-3 expression increases in response to TNF-α and carotid injury.
ClC-3 mRNA levels were measured by real time PCR in (A) WT SMCs treated with TNF-α for 6, 24, or 48 hours (* p<0.05 vs. control, n=3) and (B) carotid arteries collected 10 days following ligation (* p<0.05 vs. non-injured, n=3).
The observation that SMCs modulate expression of ClC-3 in response to cytokines and vascular injury, together with our findings that SMCs are dependent on ClC-3 for cellular signaling, matrix degradation, and proliferation, suggests a role for ClC-3 in the vascular response to injury. To test this hypothesis, we performed carotid ligation in WT and ClC-3 null mice. Twenty-eight days following ligation, WT mice developed a robust neointima that was markedly attenuated in the ClC-3 null mice (Fig. 5A). Deficiency of ClC-3 resulted in significant reduction in intimal area (Fig. 5B) and the intima:media ratio as compared to WT mice (Fig. 5C). Flow-induced outward remodeling is characteristic of the right carotid artery after ligation of the left carotid 36. The external elastic lamina (EEL) perimeter was greater in ClC-3 null mice as compared to WT mice (1.08 ± 0.01 vs. 1.01 ± 0.02, n=6, p<0.01).
Figure 5. Deficiency of ClC-3 protects from neointimal formation.

Twenty-eight days following common carotid ligation, right (non-injured) and left (injured) carotid arteries were collected, sectioned 0.5 mm proximal to the ligation, and stained. (A) Representative photomicrographs, original magnification 10x, scale bar represents 50 microns. (B) Intimal area of injured carotid artery. Each symbol represents the mean intimal area of three sections 0.5 mm proximal to the ligation from one animal. Mean ± SEM is shown by bar to right of group data, * p<0.05 vs. WT. (C) Summary data of the ratio of intimal area to medial area of injured carotid in WT and ClC-3 null mice. * p<0.05 vs. WT, n=6.
Discussion
Inflammation, matrix remodeling and SMC proliferation are key features in the development of atherosclerosis, restenosis after angioplasty, vein-graft stenosis and transplant arteriosclerosis. In this study, we show that exposure of cultured SMCs to thrombin or TNF-α enhances secretion of the matrix degrading enzyme MMP-9, activates the MAP kinase signaling cascade ERK1/2, and increases cell proliferation. The primary novel finding of this study is the specific and distinct requirement for the ClC-3 Cl−/H+ antiporter in mediating these effects. Whereas responses to thrombin are maintained, those to TNF-α are markedly attenuated in SMCs deficient in ClC-3. Overexpression of ClC-3 augments the growth of WT SMCs, carotid injury induces vascular expression of ClC-3, and neointima development is impaired after carotid injury in ClC-3 null mice. These observations demonstrate a role for ClC-3 in agonist-specific activation of SMCs and provide the first evidence that ClC-3 contributes to neointimal hyperplasia in response to vascular injury. Our results identify ClC-3 as a potential therapeutic target in inflammatory and proliferative vascular disease.
The majority of ClC-3 protein is localized intracellularly, but cycles through the plasma membrane where it has a half life of less than ten minutes in resting cells 37. In various cell types, ClC-3 has been localized to intracellular vesicles 20, 38–40 and proposed to act as an anion channel and regulate vesicular acidification by providing charge neutralization for the V-ATPase proton pump 38, 41. However, we have recently provided direct evidence that ClC-3 is an antiporter 25, 42 and have proposed that ClC-3 is required for charge neutralization of electron flow through the NADPH oxidase when generation of ROS is within vesicles 20, 26. NADPH oxidases are a primary source of signaling ROS in vascular cells that activate both normal physiologic as well as pathologic processes. We have identified spatially distinct pathways of Nox1 activation and ROS production by TNF-α and thrombin 23. Whereas TNF-α results in dynamin-dependent Nox1 generation of ROS within early endosomes, Nox1 activation by thrombin involves extracellular shedding of EGF-like ligands and activation of the receptor tyrosine kinase EGFR independent of endocytosis. It is expected that these distinct differences in activation of Nox1 provide specificity in cell signaling, possibly distinguishing between inflammatory and non-inflammatory stimuli. Activation of G-protein coupled receptors causes shedding of ligands and subsequent transactivation of EGFR 4. This process may represent a feed-forward mechanism necessary for amplification of intracellular ROS production and cell signaling 43.
Activation of mitogen-activated protein kinases is central to initiating cellular responses in vascular injury. ERK is rapidly phosphorylated in balloon-injured arteries 44 and has been shown to be important in the activation of MMP-9 and in cell proliferation 45. This was confirmed by our observation that inhibition of ERK phosphorylation with U0126, a selective pharmacologic inhibitor of MEK, prevented the TNF-α-dependent increase in MMP-9 and 3H-thymidine incorporation by SMCs. Further highlighting the importance of ERK in vascular injury, gene transfer of wildtype ERK increased, whereas gene transfer of a dominant-negative mutant ERK decreased neointimal hyperplasia in carotid arteries following balloon injury46.
Based on pharmacological effects of anion channel blockers, anion currents have long been linked to proliferation of multiple cell types 47. Reduction of ClC-3 protein levels using antisense oligonucleotides decreased proliferation of SMCs in response to ET-1, and ClC-3 mRNA expression was proportional to [3H]-thymidine incorporation 48. The current work supports these observations as ClC-3 null cells clearly display impaired TNF-α-induced proliferation. We extend these observations in vivo and show reduced neointima formation in ClC-3 null mice following vascular injury.
In addition, we found that ClC-3 expression increased in cultured SMCs and in carotid arteries following TNF-α and carotid ligation respectively. This is consistent with the previous report that ClC-3 expression is increased in SMCs of pulmonary arteries from rats with experimental pulmonary hypertension, and following incubation with endothelin-1, platelet-derived growth factor, and interleukin-1beta 49. These observations suggest that changes in ClC-3 expression and/or activity may be central in the regulation of cell growth.
Proliferation of SMCs in response to cytokines, including TNF-α, plays an important role in the formation of atherosclerotic and restenotic lesions 1, 3. TNF-α activates the transcription factor NF-κB, which is widely recognized as a key regulatory step in vascular inflammation 50. In vivo transfection of NF-κB decoy oligodeoxynucleotides into rat carotid artery inhibits neointimal formation after injury51. We previously showed that NF-κB activation was markedly inhibited in ClC-3 null cells, and that the defect was a function of impaired ROS production following cytokine stimulation 20. The current data showing defects in ERK1/2 and MMP-9 activation as well as proliferation are consistent with prior results. ERK1/2 activation is linked to ROS production 52, and as we have demonstrated here ERK1/2 is upstream of MMP-9 activation and proliferation.
The responses to vascular injury are complex and dependent on the model studied. The most commonly used murine models to induce neointimal hyperplasia are perivascular cuff, wire injury, and carotid ligation. A potential limitation of our in vivo findings is that ligation results in cessation of blood flow and thrombus formation which may activate cellular processes distinct from models of mechanical injury. Nevertheless, vascular lesions in humans often develop at sites of low shear stress 53 and the carotid ligation model has advantages of reproducibility and simplicity.
In summary, this study presents in vitro data supporting a role for the chloride antiporter ClC-3 in MMP-9 activation and SMC proliferation via impaired TNF-α-mediated activation of ERK1/2 (Fig. 6). In addition, in vivo data demonstrate that deficiency of ClC-3 inhibits neointimal hyperplasia in a model of carotid injury. Based on these observations, we propose that ClC-3 is a new therapeutic target for the prevention of vascular disease.
Figure 6. Proposed role for ClC-3 in the activation of SMCs.
TNF-α causes Nox1 generation of ROS into endosomes whereas thrombin increases intracellular ROS via EGFR activation23. ClC-3 is necessary for ERK1/2 phosphorylation, MMP-9 activation, and cell proliferation in response to TNF-α but not thrombin. This observation is consistent with a Cl−/H+ antiporter function of ClC-3 to provide charge neutralization for Nox1 generation of endosomal ROS 20, 26.
Supplementary Material
Acknowledgments
a) The authors wish to thank associates of the University of Iowa Roy J. and Lucille A. Carver College of Medicine Central Microscopy Research Facility, the Flow Cytometry Facility, and the Gene Transfer Vector Core Facility of the University of Iowa Center for Gene Therapy of Cystic Fibrosis and Other Genetic Diseases (supported by NIH/NIDDK P30 DK 54759).
b) Sources of Funding:
This material is based upon work supported in part by the Office of Research and Development, Department of Veterans Affairs (FJM), and by NIH grants HL062483 (FSL, FJM), HL081750 (FJM), and by the American Heart Association (FSL).
Footnotes
Disclosures:
None
References
- 1.Ross R. Atherosclerosis is an inflammatory disease. American Heart Journal. 1999;138:S419–S420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz SM, deBlois D, O’Brien ERM. The intima. Soil for atherosclerosis and restenosis. Circulation Research. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 3.Andrew C. Newby ABZ. Molecular mechanisms in intimal hyperplasia. The Journal of Pathology. 2000;190:300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.Kalmes A, Vesti BR, Daum G, Abraham JA, Clowes AW. Heparin Blockade of Thrombin-Induced Smooth Muscle Cell Migration Involves Inhibition of Epidermal Growth Factor (EGF) Receptor Transactivation by Heparin-Binding EGF-Like Growth Factor. Circ Res. 2000;87:92–98. doi: 10.1161/01.res.87.2.92. [DOI] [PubMed] [Google Scholar]
- 5.Darmoul D, Gratio V, Devaud H, Peiretti F, Laburthe M. Activation of proteinase-activated receptor 1 promotes human colon cancer cell proliferation through epidermal growth factor receptor transactivation. Molecular Cancer Research. 2004;2:514–522. [PubMed] [Google Scholar]
- 6.Martorell L, Martinez-Gonzalez J, Rodriguez C, Gentile M, Calvayrac O, Badimon L. Thrombin and protease-activated receptors (PARs) in atherothrombosis. Thromb Haemost. 2008;99:305–315. doi: 10.1160/TH07-08-0481. [DOI] [PubMed] [Google Scholar]
- 7.Cheung WM, D’Andrea MR, Andrade-Gordon P, Damiano BP. Altered vascular injury responses in mice deficient in protease-activated receptor-1. Arterioscler Thromb Vasc Biol. 1999;19:3014–3024. doi: 10.1161/01.atv.19.12.3014. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka H, Sukhova GK, Schwartz D, Libby P. Proliferating arterial smooth muscle cells after balloon injury express TNF-alpha but not interleukin-1 or basic fibroblast growth factor. Arteriosclerosis, Thrombosis & Vascular Biology. 1996;16:12–18. doi: 10.1161/01.atv.16.1.12. [DOI] [PubMed] [Google Scholar]
- 9.Barath P, Fishbein MC, Cao J, Berenson J, Helfant RH, Forrester JS. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol. 1990;65:297–302. doi: 10.1016/0002-9149(90)90291-8. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman MA, Selzman CH, Reznikov LL, Miller SA, Raeburn CD, Emmick J, Meng X, Harken AH. Lack of TNF-alpha attenuates intimal hyperplasia after mouse carotid artery injury. Am J Physiol Regul Integr Comp Physiol. 2002;283:R505–512. doi: 10.1152/ajpregu.00033.2002. [DOI] [PubMed] [Google Scholar]
- 11.Rectenwald JE, Moldawer LL, Huber TS, Seeger JM, Ozaki CK. Direct evidence for cytokine involvement in neointimal hyperplasia. Circulation. 2000;102:1697–1702. doi: 10.1161/01.cir.102.14.1697. [DOI] [PubMed] [Google Scholar]
- 12.Warner SJ, Libby P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. The Journal of Immunology. 1989;142:100–109. [PubMed] [Google Scholar]
- 13.Imaizumi T, Itaya H, Fujita K, Kudoh D, Kudoh S, Mori K, Fujimoto K, Matsumiya T, Yoshida H, Satoh K. Expression of tumor necrosis factor-alpha in cultured human endothelial cells stimulated with lipopolysaccharide or interleukin-1alpha. Arterioscler Thromb Vasc Biol. 2000;20:410–415. doi: 10.1161/01.atv.20.2.410. [DOI] [PubMed] [Google Scholar]
- 14.Selzman CH, Shames BD, Reznikov LL, Miller SA, Meng X, Barton HA, Werman A, Harken AH, Dinarello CA, Banerjee A. Liposomal delivery of purified inhibitory-kappaBalpha inhibits tumor necrosis factor-alpha-induced human vascular smooth muscle proliferation. Circ Res. 1999;84:867–875. doi: 10.1161/01.res.84.8.867. [DOI] [PubMed] [Google Scholar]
- 15.Lee S-O, Jeong Y-J, Yu MH, Lee J-W, Hwangbo MH, Kim C-H, Lee I-S. Wogonin suppresses TNF-a-induced MMP-9 expression by blocking the NF-kB activation via MAPK signaling pathways in human aortic smooth muscle cells. Biochemical and Biophysical Research Communications. 2006;351:118–125. doi: 10.1016/j.bbrc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Jentsch TJ. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol. 2008;43:3–36. doi: 10.1080/10409230701829110. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki M, Uchida S, Monkawa T, Miyawaki A, Mikoshiba K, Marumo F, Sasaki S. Cloning and expression of a protein kinase C-regulated chloride channel abundantly expressed in rat brain neuronal cells. Neuron. 1994;12:597–604. doi: 10.1016/0896-6273(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki M, Fukuma T, Yamauchi K, Sakamoto H, Marumo F, Sasaki S. Identification of an acid-activated Cl(−) channel from human skeletal muscles. Am J Physiol. 1999;277:C948–954. doi: 10.1152/ajpcell.1999.277.5.C948. [DOI] [PubMed] [Google Scholar]
- 19.Borsani G, Rugarli EI, Taglialatela M, Wong C, Ballabio A. Characterization of a human and murine gene (CLCN3) sharing similarities to voltage-gated chloride channels and to a yeast integral membrane protein. Genomics. 1995;27:131–141. doi: 10.1006/geno.1995.1015. [DOI] [PubMed] [Google Scholar]
- 20.Miller FJ, Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine Activation of Nuclear Factor-kappa B in Vascular Smooth Muscle Cells Requires Signaling Endosomes Containing Nox1 and ClC-3. Circulation Research. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 21.Moreland JG, Davis AP, Bailey G, Nauseef WM, Lamb FS. Anion channels, including ClC-3, are required for normal neutrophil oxidative function, phagocytosis, and transendothelial migration. J Biol Chem. 2006;281:12277–12288. doi: 10.1074/jbc.M511030200. [DOI] [PubMed] [Google Scholar]
- 22.Moreland JG, Davis AP, Matsuda JJ, Hook JS, Bailey G, Nauseef WM, Lamb FS. Endotoxin priming of neutrophils requires NADPH oxidase-generated oxidants and is regulated by the anion transporter ClC-3. J Biol Chem. 2007;282:33958–33967. doi: 10.1074/jbc.M705289200. [DOI] [PubMed] [Google Scholar]
- 23.Miller FJ, Jr, Chu X, Stanic B, Tian X, Sharma RV, Davisson RL, Lamb FS. A Differential Role for Endocytosis in Receptor-mediated Activation of Nox1. Antioxid Redox Signal. 2010;12:583–593. doi: 10.1089/ars.2009.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MY, Martin AS, Mehta PK, Dikalova AE, Garrido AM, Lyons E, Krause K-H, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of Vascular Smooth Muscle NADPH Oxidase 1 (Nox1) Contribution to Injury-Induced Neointimal Formation. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.108.181925. ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuda JJ, Filali MS, Volk KA, Collins MM, Moreland JG, Lamb FS. Overexpression of CLC-3 in HEK293T cells yields novel currents that are pH dependent. American Journal of Physiology- Cell Physiology. 2008;294:C251. doi: 10.1152/ajpcell.00338.2007. [DOI] [PubMed] [Google Scholar]
- 26.Lamb FS, Moreland JG, Miller FJ., Jr Electrophysiology of reactive oxygen production in signaling endosomes. Antioxidants & Redox Signaling. 2009 June;:1335–1347. doi: 10.1089/ars.2008.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickerson LW, Bonthius DJ, Schutte BC, Yang B, Barna TJ, Bailey MC, Nehrke K, Williamson RA, Lamb FS. Altered GABAergic function accompanies hippocampal degeneration in mice lacking ClC-3 voltage-gated chloride channels. Brain Res. 2002;958:227–250. doi: 10.1016/s0006-8993(02)03519-9. [DOI] [PubMed] [Google Scholar]
- 28.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 29.Schauwienold D, Plum C, Helbing T, Voigt P, Bobbert T, Hoffmann D, Paul M, Reusch HP. ERK1/2-Dependent Contractile Protein Expression in Vascular Smooth Muscle Cells. Hypertension. 2003;41:546–552. doi: 10.1161/01.HYP.0000054213.37471.84. [DOI] [PubMed] [Google Scholar]
- 30.De Keulenaer GW, Ushio-Fukai M, Yin Q, Chung AB, Lyons PR, Ishizaka N, Rengarajan K, Taylor WR, Alexander RW, Griendling KK. Convergence of redox-sensitive and mitogen-activated protein kinase signaling pathways in tumor necrosis factor-alpha-mediated monocyte chemoattractant protein-1 induction in vascular smooth muscle cells. Arteriosclerosis, Thrombosis & Vascular Biology. 2000;20:385–391. doi: 10.1161/01.atv.20.2.385. [DOI] [PubMed] [Google Scholar]
- 31.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. Journal of Biological Chemistry. 1998;273:18623. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 32.Moon SK, Cha BY, Kim CH. ERK1/2 mediates TNF-a-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kB and AP-1: involvement of the Ras dependent pathway. Journal of cellular physiology. 2004;198:417–427. doi: 10.1002/jcp.10435. [DOI] [PubMed] [Google Scholar]
- 33.Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:2238. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 35.Godin D, Ivan E, Johnson C, Magid R, Galis ZS. Remodeling of carotid artery is associated with increased expression of matrix metalloproteinases in mouse blood flow cessation model. Circulation. 2000;102:2861–2866. doi: 10.1161/01.cir.102.23.2861. [DOI] [PubMed] [Google Scholar]
- 36.Korshunov VA, Berk BC. Strain-Dependent Vascular Remodeling: The “Glagov Phenomenon” Is Genetically Determined. Circulation. 2004;110:220–226. doi: 10.1161/01.CIR.0000134958.88379.2E. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Z, Li X, Hao J, Winston JH, Weinman SA. The ClC-3 chloride transport protein traffics through the plasma membrane via interaction of an N-terminal dileucine cluster with clathrin. J Biol Chem. 2007;282:29022–29031. doi: 10.1074/jbc.M703506200. [DOI] [PubMed] [Google Scholar]
- 38.Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 39.Barg S, Huang P, Eliasson L, Nelson DJ, Obermuller S, Rorsman P, Thevenod F, Renstrom E. Priming of insulin granules for exocytosis by granular Cl(−) uptake and acidification. J Cell Sci. 2001;114:2145–2154. doi: 10.1242/jcs.114.11.2145. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Wang T, Zhao Z, Weinman SA. The ClC-3 chloride channel promotes acidification of lysosomes in CHO- K1 and Huh-7 cells. Am J Physiol Cell Physiol. 2002;282:C1483–1491. doi: 10.1152/ajpcell.00504.2001. [DOI] [PubMed] [Google Scholar]
- 41.Hara-Chikuma M, Yang B, Sonawane ND, Sasaki S, Uchida S, Verkman AS. ClC-3 chloride channels facilitate endosomal acidification and chloride accumulation. J Biol Chem. 2005;280:1241–1247. doi: 10.1074/jbc.M407030200. [DOI] [PubMed] [Google Scholar]
- 42.Matsuda JJ, Filali MS, Collins MM, Volk KA, Lamb FS. The ClC-3 Cl−/H+ antiporter becomes uncoupled at low extracellular pH. J Biol Chem. 2010;285:2569–2579. doi: 10.1074/jbc.M109.018002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, Cheng L, Hochleitner BW, Xu Q. Activation of mitogen-activated protein kinases (ERK/JNK) and AP-1 transcription factor in rat carotid arteries after balloon injury. Arteriosclerosis, Thrombosis & Vascular Biology. 1997;17:2808–2816. doi: 10.1161/01.atv.17.11.2808. [DOI] [PubMed] [Google Scholar]
- 45.Sung-Kwon Moon B-YCC-HK. ERK1/2 mediates TNF-α-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-κB and AP-1: Involvement of the ras dependent pathway. Journal of Cellular Physiology. 2004;198:417–427. doi: 10.1002/jcp.10435. [DOI] [PubMed] [Google Scholar]
- 46.Izumi Y, Kim S, Namba M, Yasumoto H, Miyazaki H, Hoshiga M, Kaneda Y, Morishita R, Zhan Y, Iwao H. Gene Transfer of Dominant-Negative Mutants of Extracellular Signal-Regulated Kinase and c-Jun NH2-Terminal Kinase Prevents Neointimal Formation in Balloon-Injured Rat Artery. Circ Res. 2001;88:1120–1126. doi: 10.1161/hh1101.091267. [DOI] [PubMed] [Google Scholar]
- 47.Voets T, Szucs G, Droogmans G, Nilius B. Blockers of volume-activated Cl− currents inhibit endothelial cell proliferation. Pflugers Arch. 1995;431:132–134. doi: 10.1007/BF00374387. [DOI] [PubMed] [Google Scholar]
- 48.Wang GL, Wang XR, Lin MJ, He H, Lan XJ, Guan YY. Deficiency in ClC-3 chloride channels prevents rat aortic smooth muscle cell proliferation. Circ Res. 2002;91:E28–32. doi: 10.1161/01.res.0000042062.69653.e4. [DOI] [PubMed] [Google Scholar]
- 49.Dai YP, Bongalon S, Hatton WJ, Hume JR, Yamboliev IA. ClC-3 chloride channel is upregulated by hypertrophy and inflammation in rat and canine pulmonary artery. Br J Pharmacol. 2005;145:5–14. doi: 10.1038/sj.bjp.0706135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raines EW, Garton KJ, Ferri N. Beyond the endothelium: NF-kappaB regulation of smooth muscle function. Circ Res. 2004;94:706–708. doi: 10.1161/01.RES.0000125646.08156.4D. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimura S, Morishita R, Hayashi K, Yamamoto K, Nakagami H, Kaneda Y, Sakai N, Ogihara T. Inhibition of intimal hyperplasia after balloon injury in rat carotid artery model using cis-element ‘decoy’ of nuclear factor-κB binding site as a novel molecular strategy. Gene therapy(Basingstoke) 2001;8:1635–1642. doi: 10.1038/sj.gt.3301566. [DOI] [PubMed] [Google Scholar]
- 52.Mehdi M, Azar Z, Srivastava A. Role of receptor and nonreceptor protein tyrosine kinases in H2O2-induced PKB and ERK1/2 signaling. Cell Biochem Biophys. 2007;47:1–10. doi: 10.1385/cbb:47:1:1. [DOI] [PubMed] [Google Scholar]
- 53.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. Journal of the American Medical Association. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.