Abstract
Background
Lymphatic pump techniques (LPT) are used by osteopathic practitioners for the treatment of edema and infection; however, the mechanisms by which LPT enhances the lymphatic and immune systems are poorly understood.
Methods and Results
To measure the effect of LPT on the rat, the cisterna chyli (CC) of 10 rats were cannulated and lymph was collected during 4 min of 1) pre-LPT baseline, 2) 4 min LPT, and 3) 10 min post-LPT recovery. LPT increased significantly (p < 0.05) lymph flow from a baseline of 24 ± 5 μl/min to 89 ± 30 μl/min. The baseline CC lymphocyte flux was 0.65 ± 0.21 × 106 lymphocytes/min, and LPT increased CC lymphocyte flux to 6.10 ± 0.99 × 106 lymphocytes/min (p < 0.01). LPT had no preferential effect on any lymphocyte population, since total lymphocytes, CD4+ T cells, CD8+ T cells, and B cell numbers were similarly increased. To determine if LPT mobilized gut-associated lymphocytes into the CC lymph, gut-associated lymphocytes in the CC lymph were identified by staining CC lymphocytes for the gut homing receptor integrin α4β7. LPT significantly increased (p < 0.01) the flux of α4β7 positive CC lymphocytes from a baseline of 0.70 ± 0.03 × 105 lymphocytes/min to 6.50 ± 0.10 × 105 lymphocytes/min during LPT. Finally, lymphocyte flux during recovery was similar to baseline, indicating the effects of LPT are transient.
Conclusions
Collectively, these results suggest that LPT may enhance immune surveillance by increasing the numbers of lymphocytes released in to lymphatic circulation, especially from the gut associated lymphoid tissue. The rat provides a useful model to further investigate the effect of LPT on the lymphatic and immune systems.
Introduction
It is well established that compression of lymph vessels by skeletal muscle contraction, respiration, or intestinal peristalsis increases lymph flow.1–4 In addition, cyclic contractions of smooth muscle of the lymph vessels acts at an intrinsic lymph pump.5 Failure of intrinsic lymph pumping can result in certain forms of edema. 2–4 Osteopathic manipulative treatments (OMT), identified specifically as lymphatic pump techniques (LPT), were developed by osteopathic physicians to improve the lymphatic system.1,2
Lymphatic pump techniques (LPT) are thought to enhance lymphatic return by increasing gradients for lymph flow and, thus, assisting the return of lymph from the lung, abdomen, and other tissues.1,3 Theoretically, by improving lymph flow, the interstitial fluid may be drained more efficiently, removing particulate matter, exudates, toxins, and bacteria from tissues.3,4 Improved lymph flow can also reduce excessive accumulation of fluid in the interstitial spaces, and thus lessen edema.3–6
Clinically, LPT has been shown to increase blood leukocyte numbers,7 enhance vaccine specific antibodies,8,9 enhance bronchial clearance during pulmonary infection,10,11 reduce the need for antibiotics during infection,12 and decrease the length of hospital stay in elderly patients with pneumonia.12 In dogs, LPT has been shown to enhance lymph flow and leukocyte numbers in the thoracic13,14 and mesenteric lymph ducts.15 Furthermore, LPT was shown to mobilize lymphocytes from the mesenteric lymph nodes into thoracic duct lymph.15 Collectively, these reports suggest that LPT can enhance function of the lymphatic and immune systems, which may facilitate the clearance of infection; however, the mechanism(s) by which LPT facilitate(s) the clearance of bacteria during infection has not been identified.
While the dog has been used to study the effect of LPT on the lymphatic system,13–15 there are several limitations to the use of dogs for the study LPT on infectious and inflammatory diseases. These limitations include animal cost and the availability of reagents for immunological studies. Importantly, rodents are more commonly used for studies of cancer and infectious disease. Therefore, the objective of this study was to develop a rat model to determine if experimental LPT would increase lymph flow and leukocyte concentration as observed in dogs.14,15 Our results show that LPT increases the flux of lymphocytes in the cisterna chyli (CC) of rats, thus demonstrating that this treatment produces similar effects in two distinct mammalian models. Furthermore, we have developed a small animal model to investigate the effects of LPT on the lymphatic and immune systems.
Materials and Methods
Animals
This study was approved by the Institutional Animal Care and Use Committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication no. 85-23, revised 1996). Ten male Sprague-Dawley rats, weighing 250–300 g, and free of clinically evident signs of disease were used for this study.
Cannulation of the thoracic duct
Thirty to forty-five min preoperatively, 1.5 ml of olive oil was administered via oral gavage to increase lipid content, thus making the lymphatic vessels more visible. At the same time, 3 ml of a 32°–36°C, 0.09% sterile saline solution was injected intraperitoneally to compensate for fluid loss during surgery. Anesthesia was induced in a chamber with a combination of 1-1 oxygen and 5% isoflurane. The rat was then transferred onto a heating pad to maintain a body temperature between 36°–37°C. A nose cone with a 1-1 oxygen and 2% isoflurane mixture was used to maintain anesthesia throughout the procedure. The rat was placed in a right lateral recumbent position with its feet secured to the heating pad. The hair on the left side was clipped, and a 70% isopropyl alcohol solution was generously applied to the clipped area.
The abdomen was opened through a left subcostal, mediolateral incision spanning two-thirds of the length of the abdomen. The intestines, left kidney, adrenal, stomach, and spleen were separated from connective tissue and fat with cotton swabs wrapped in warm saline-soaked gauze, which was then retracted to the side. Excessive tension on the renal circulation was avoided. Multiple retractors were used to further visualize the aorta, and the parietal peritoneum was incised just below the diaphragm. Cotton swabs were used to clear fat and connective tissue, thus freeing the aorta from surrounding tissues and exposing the CC containing milky lymph. A silk suture was placed under the aorta, so that the aorta could be retracted during the cannulation procedure. Caution was taken to not damage the thoracic duct or other ducts converging on the CC during this procedure. A 23G hypodermic needle, bent at ∼100° angle, was used to puncture the CC for introduction of MicroRenathane® implantation tubing (MRE033; 0.84 mm O.D. × 0.36 mm I.D.; Braintree Scientific Inc., Braintree, MA) filled with heparinized saline. The insertion site was sealed with 1 or 2 drops of surgical glue, and lymph was allowed to drain by gravity into collection tubes. CC lymph was collected during 4 min pre-LPT, 4 min LPT, and 10 min post-LPT. Lymph flow rates were measured as the volume of lymph collected during each minute, and these values were averaged for each condition.
Lymphatic pump treatment
LPT was performed by a medical student trained in osteopathic lymphatic manipulation. During manipulation, the rat was in a right lateral recumbent position. The retractors were removed from the incision, allowing only the implantation tubing to exit the incision side. To perform LPT, the operator contacted the abdomen of rat with the thumb on one side and index finger and middle finger on the other side of the medial sagittal plane. The fingers were placed bilaterally caudal to the ribs, and attention was paid not to contact the left subcostal, mediolateral incision. Sufficient pressure was exerted medially and cranially to compress the abdomen until significant resistance was met against the diaphragm, then the pressure was released. Compressions were administered at approximately one/sec for the duration of the 4-min treatment.
Leukocyte enumeration
Leukocytes in samples of CC lymph were enumerated using the Hemavet 950 (Drew Scientific, Waterbury, CT). To compute leukocyte flux, the lymphocyte concentration was multiplied by the volume of lymph during each minute for each condition, and these values were averaged.
Flow cytometry
Two-color immunofluorescent staining was performed to identify specific lymphocyte populations using FITC or PE-labeled goat anti-rat isotype control IgG2b, FITC-anti-rat CD3, PE-anti-rat B cell, PE-anti-rat CD4, PE-anti-rat CD8, FitC-anti-rat CD61 (β7), or PE-anti-rat CD 49 (α4) monoclonal antibodies (mAb) (Serotech, Raleigh, NC). Between 2 × 105 and 1 × 106 cells were incubated with the mAb as recommended by the manufacturer. The cells were washed in staining buffer consisting of Mg2+-free, Ca2+-free phosphate buffered saline supplemented with 2% fetal bovine serum (HyClone Laboratories, Logan, UT) and fixed with 0.05% paraformaldehyde until analyzed.
Fluorescently-labeled lymphocytes were analyzed using a Cytomics FC 500 flow cytometer (Beckman Coulter, Fullerton, CA). Lymphocyte gates and detector voltages were set using isotype control stained cells, and stained cell populations were seen as distinct peaks or clusters of cells. The proportion of each cell population was expressed as the percentage of the number of stained cells. To determine the total number of a specific lymphocyte population in a milliliter of lymph, their percentage was multiplied by the total number of cells.
Statistical analyses
Data were subjected to analysis of variance followed by a Tukey-Kramer multiple comparisons post test. Analyses were performed with Graphpad Prism version 5.0 for Windows, (GraphPad Software, San Diego, CA). Differences among mean values with p ≤ 0.05 were considered statistically significant. Data are presented as arithmetic mean ± standard error (SE).
Results
LPT increases CC leukocytes
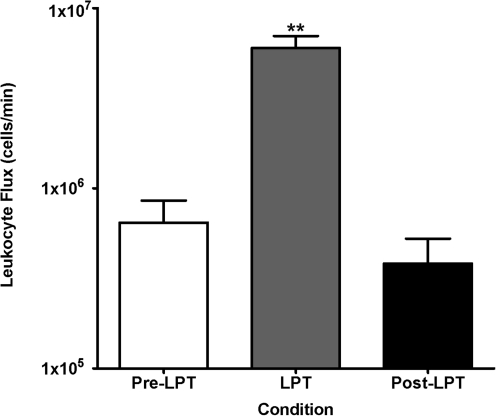
The baseline leukocyte count was 0.30 ± 0.09 × 105 cells/μl of lymph, and LPT significantly (p < 0.05) increased leukocytes to 1.24 ± 0.41 × 105 cells/tl. Furthermore, LPT increased significantly (p < 0.05) lymph flow from a baseline of 24 ± 5 μl/min to 89 ± 30 μl/min. Figure 1 summarizes the effect of LPT on CC leukocyte flux. The baseline CC lymphocyte flux was 0.65 ± 0.21 × 106 lymphocytes/min, and LPT increased CC lymphocyte flux to 6.10 ± 0.99 × 106 lymphocytes/min (p < 0.01 versus Pre- and Post-LPT). Approximately 97% of the white blood cells in the CC lymph were lymphocytes, and LPT had no preferential effect on any lymphocyte populations, since total lymphocytes, CD4+ T cells, CD8+ T cells, and B cell numbers were similarly increased (Table 1). Finally, the leukocyte flux during recovery was similar to pre-LPT, indicating that LPT transiently increases CC lymph flow and leukocyte numbers.
FIG. 1.
Cisterna chyli lymph was collected during 1) 4 min pre-LPT, 2) 4 min LPT, and 3) 10 min post-LPT. Data are means × 106 total leukocytes/min ± SE from 10 animals. **Greater than pre-LPT and post-LPT (p < 0.01).
Table 1.
Lymphatic Pump Treatment Increases Lymphocyte Flux in Cisterna Chyli Lymph
| Lymphocyte Flux (× 106cells/min) | Pre-LPT | LPT | Post-LPT |
|---|---|---|---|
| Total lymphocytesa | 0.63 ± 0.20 | 5.90 ± 0.99** | 0.52 ± 0.13 |
| CD4+ T cellsb | 0.37 ± 0.17 | 3.60 ± 0.54** | 0.26 ± 0.03 |
| CD8+ T cells | 0.11 ± 0.05 | 1.00 ± 0.28** | 0.09 ± 0.06 |
| B cells | 0.14 ± 0.01 | 0.70 ± 0.50* | 0.08 ± 0.02 |
Lymph was collected during 4 min pre-LPT, 4min LPT, and 4 min post-LPT. Values are means ± SE or means × 106 lymphocytes/min from 10 experiments.
Concentration of total lymphocytes.
Concentration of total lymphocytes that were T or B cells.
p < 0.01, compared to pre-LPT and post-LPT. *p < 0.05, compared to pre-LPT and post-LPT.
LPT mobilizes gut-associated lymphocytes into CC lymph
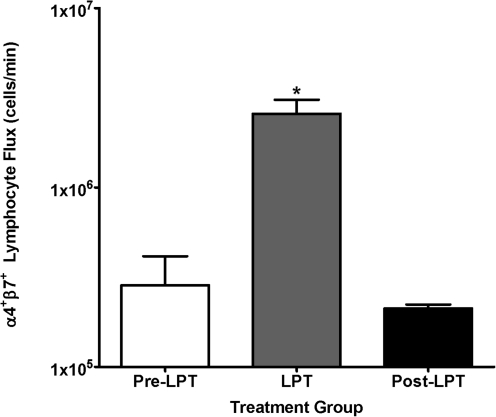
Previously, we demonstrated that LPT mobilizes gut-associated lymphoid tissue (GALT) derived lymphocytes into the thoracic duct lymph of anesthetized dogs.15 Therefore, to determine if LPT mobilizes GALT derived lymphocytes into the CC of rats, we stained lymphocytes collected from the CC for the gut homing leukointegrin α4β7.16 Approximately 40% of the lymphocytes in the CC stained positive for. a4J37 (data not shown). LPT did not increase the percentage of a4J37 positive lymphocytes; however, LPT significantly increased (p < 0.01) the flux of α4β7 positive lymphocytes from a baseline of 0.70 ± 0.03 × 105 leukocytes/min to 6.50 ± 0.10 × 105 leukocytes/min during LPT (Fig. 2). Finally, following LPT, the α4β7 positive lymphocyte flux was similar to that observed pre-LPT, indicating LPT has a transient effect on the mobilization of α4β7 positive lymphocytes. This result demonstrates that the GALT is a tissue source of the lymphocytes mobilized during LPT in rats.
FIG. 2.
Cisterna chyli lymph was collected during 1) pre-LPT, 2) 4 min LPT, and 3) 4 min post-LPT. Data are means × 106 total of α4β7 positive lymphocytes/min ± SE from 10 animals. *Greater than pre-LPT and post-LPT (p < 0.05).
Discussion
This investigation examined for the first time in rats responses to simulated osteopathic manipulation, a therapy advocated to enhance lymph flow and immune function.1,2 The most important findings of this investigation are 1) LPT augmented thoracic duct lymph flow, 2) LPT increased leukocyte concentration in thoracic duct lymph, 3) since both lymph flow and leukocyte count were increased during LPT, leukocyte flux from lymphoid reservoirs was markedly elevated, 4) a significant number of these leukocytes originated from GALT. While these findings are consistent with our previous studies in dogs,13–15 they demonstrate that the rat can be a useful model for future research on mechanisms by which LPT enhances immune function.
Osteopathic philosophy and education suggest that OMT enhances immune function by removing restrictions to blood and lymph flow, by optimizing respiratory mechanics, and by restoring balance between the sympathetic and parasympathetic nervous systems.1,2 Anecdotal reports support the belief that LPT stimulates the immune system and accelerates clearance of infection;7–12 however, the mechanisms responsible for this protection have not been identified. The current finding that LPT greatly increases leukocyte flux in CC lymph provides experimental support for clinical applications of LPT. Furthermore, the increase in lymph flow generated by LPT would enhance distribution of antigens or antibiotics, and thus improve infection control.
It is established that mucosal homing of GALT derived lymphocytes is due to the molecular interaction between integrin α4β7 on lymphocytes and the mucosal vascular addressin, MAdCAM-1.16 The GALT is a major inductive site, and lymphocytes primed in the GALT can migrate into many different effector mucosal tissues, suggesting the existence of a common mucosal immune system.18 Specifically, antigen-specific IgA producing B cells and T cells have been shown to migrate from GALT to other mucosal associated lymphoid tissues, including the bronchus associated lymphoid tissue (BALT).16–19 Of clinical importance, intestinal immunization enhances protective immunity in the lower respiratory tract compared to parenteral immunization.18–21 These studies suggest that GALT primed memory lymphocytes can either recirculate through or reside in the lung; however, the involvement of α4β7 positive lymphocytes in clearance of lower respiratory tract infection is still unclear.
Consistent with our findings in the dog,14 LPT increased the numbers of α4β7 positive (GALT) derived lymphocytes in the thoracic duct lymph of rats. Mobilization of primed leukocytes from the GALT by LPT should be beneficial during infection. In addition, the increased numbers of circulating leukocytes produced by LPT might improve immune surveillance, which in turn would further boost protection against infectious disease.
It must be recognized that further research is required to determine the migration kinetics of the leukocytes mobilized by LPT, and especially those from GALT. Such research would be most informative if LPT were applied to an animal model with concurrent disease. The present results suggest that the rat would be a useful and practical model for such research.
The result of LPT on lymph flow has been reported in dogs,13–15 but the effect of LPT on lymph flow has not been determined in rodents or humans. In this study, LPT treatment was applied to the rat to simulate, as nearly as possible, how cyclic applications of manual pressure to the abdomen are applied to humans. It is important to note that there are obvious differences between the application of LPT in humans, dogs and rats. This is primarily due to the size, anatomy, and positioning of the animals during treatment as compared to humans. This is an inherent flaw in using animal models to study the mechanisms of human manual medicine treatments, which cannot be overcome at the current state of technology. In spite of the concerns with the use of animal models, they still provide the opportunity to study the simulated effects of LPT on humans.
While LPT was developed to increase lymph flow, it is possible that such maneuvers would mobilize cells directly from lymphoid tissue to the blood independent of the lymphatic circulation. Thus, studies that evaluate effects of LPT only on lymph flow and composition may underestimate the clinical implications of this alternative and complementary therapy for infection.
Footnotes
This research was supported by National Institutes of Health Grant R01 AT004361 (LMH), American Osteopathic Association Grant 06-11-547 (LMH).
Acknowledgments
The expert assistance of Kim Winterrowd during the animal surgery is gratefully acknowledged. The authors thank the Osteopathic Heritage Foundation for their continued support of the Basic Science Research Chair (LMH).
Author Disclosure Statement
No competing financial interests exist for any author.
References
- 1.Seffinger MA. King HH. Ward RC. Jones JM. Rogers FJ. Osteopathic philosophy. In: R. C. Ward., editor. Foundations for Osteopathic Medicine. Baltimore: Lippincott Williams & Wilkins; 2003. pp. 3–12. [Google Scholar]
- 2.Wallace E. McPartland JM. Jones JM., III Kuchera WA. Buser BR. Lymphatic system: Lymphatic manipulative techniques. In: R Ward., editor. Foundations for Osteopathic Medicine. Baltimore: Lippincott Williams & Wilkins; 2003. pp. 1056–1077. [Google Scholar]
- 3.Degenhardt BF. Kuchera ML. Update on osteopathic medical concepts and the lymphatic system. J Am Osteopath Assoc. 1996;96:97–100. doi: 10.7556/jaoa.1996.96.2.97. [DOI] [PubMed] [Google Scholar]
- 4.Olszewski WL. The lymphatic system in body homeostasis: Physiological conditions. Lymphat Res Biol. 2003;1:11–21. doi: 10.1089/15396850360495655. [DOI] [PubMed] [Google Scholar]
- 5.Gashev AA. Wang W. Laine GA. Stewart RH. Zawieja DC. Characteristics of the active lymph pump in bovine prenodal mesenteric lymphatics. Lymphat Res Biol. 2007;5:71–79. doi: 10.1089/lrb.2007.5202. [DOI] [PubMed] [Google Scholar]
- 6.Prajapati P. Shah P. King HH. Williams AG., Jr Desai P. Downey HF. Lymphatic pump treatment increases thoracic duct lymph flow in conscious dogs with edema due to constriction of the inferior vena cava. Lymphat Res Biol. 2010;8:149–154. doi: 10.1089/lrb.2009.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castlio Y. Ferris–Swift L. Effects of splenic stimulation in normal individuals on the active and differential blood cell counts and the opsonotic index. Kansas City College of Osteopathy and Surgery. 1932;16:10–16. [Google Scholar]
- 8.Jackson KM. Steele TF. Dugan EP. Kukulka G. Blue W. Roberts A. Effect of lymphatic and splenic pump techniques on the antibody response to Hepatitis B vaccine: A pilot study. J Am Osteopath Assoc. 1998;98:155–160. [PubMed] [Google Scholar]
- 9.Measel JW., Jr The effect of lymphatic pump on the immune response: Preliminary studies on the antibody response to pneumococcal polysaccharide assayed by bacterial agglutination and passive hemagglutination. J Am Osteopath Assoc. 1982;82:28–31. [PubMed] [Google Scholar]
- 10.Allen TW. Pence TK. The use of the thoracic pump in treatment of lower respiratory tract disease. J Am Osteopath Assoc. 1967;67:408–411. [PubMed] [Google Scholar]
- 11.Kline CA. Osteopathic manipulative therapy, antibiotics, and supportive therapy in respiratory infections in children: comparative study. J Am Osteopath Assoc. 1965;65:278–281. [PubMed] [Google Scholar]
- 12.Noll DR. Shores JH. Gamber RG. Herron KM. Swift J., Jr Benefits of osteopathic manipulative treatment for hospitalized elderly patients with pneumonia. J Am Osteopath Assoc. 2000;100:776–782. [PubMed] [Google Scholar]
- 13.Knott EM. Tune JD. Stoll ST. Downey HF. Increased lymphatic flow in the thoracic duct during manipulative intervention. J Am Osteopath Assoc. 2005;105:447–456. [PubMed] [Google Scholar]
- 14.Hodge LM. King HH. Williams AG, Jr, et al. Abdominal lymphatic pump treatment increases leukocyte count and flux in thoracic duct lymph. Lymphat Res Biol. 2007;5:127–133. doi: 10.1089/lrb.2007.1001. [DOI] [PubMed] [Google Scholar]
- 15.Hodge LM. Bearden MK. Schander A. Huff JB. Williams A., Jr King HH. Downey HF. Abdominal lymphatic pump treatment mobilizes leukocytes from the gastrointestinal associated lymphoid tissue into lymph. Lymphat Res Biol. 2010;8:103–110. doi: 10.1089/lrb.2009.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iijima H. Takahashi I. Kiyono H. Mucosal immune network in the gut for the control of infectious diseases. Rev Med Virol. 2001;11:117–133. doi: 10.1002/rmv.307. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi I. Kiyono H. Gut as the largest immunologic tissue. J Parenter Enteral Nutr. 1999;23:S7–12. doi: 10.1177/014860719902300503. [DOI] [PubMed] [Google Scholar]
- 18.Wallace FJ. Cripps AW. Clancy RL. Husband AJ. Witt CS. A role for intestinal T lymphocytes in bronchus mucosal immunity. Immunology. 1991;74:68–73. [PMC free article] [PubMed] [Google Scholar]
- 19.Sato J. Chida K. Suda T. Sato A. Nakamura H. Migratory patterns of thoracic duct lymphocytes into bronchus-associated tissue of immunized rats. Lung. 2000;178:295–308. doi: 10.1007/s004080000033. [DOI] [PubMed] [Google Scholar]
- 20.DiGiandomenico A. Rao J. Goldberg JB. Oral vaccination of BALB/c mice with Salmonella enterica serovar typhimurium expressing Pseudomonas aeruginosa O antigen promotes increased survival in an acute fatal pneumonia model. Infect Immun. 2004;72:7012–7021. doi: 10.1128/IAI.72.12.7012-7021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stokes MG. Titball RW. Neeson BN, et al. Oral administration of a Salmonella enterica-based vaccine expressing Bacillus anthracis protective antigen confers protection against aerosolized B. anthracis. Infect Immun. 2007;75:1827–1834. doi: 10.1128/IAI.01242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]