Abstract
Context
Maternal depressive symptoms during pregnancy have been reported in some, but not all, studies to be associated with an increased risk of preterm birth (PTB), low birth weight (LBW), and intrauterine growth restriction (IUGR).
Objective
To estimate the risk of PTB, LBW, and IUGR associated with antenatal depression.
Data Sources and Study Selection
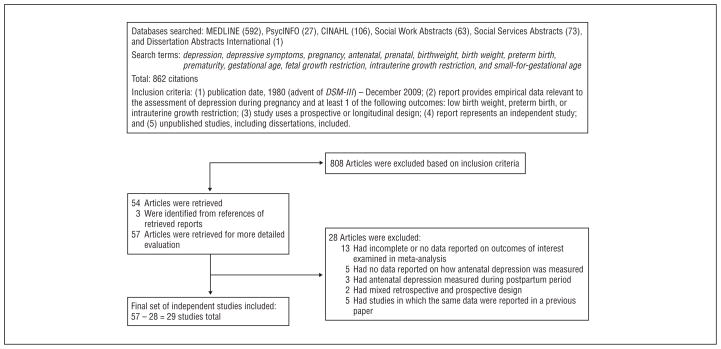
We searched for English-language and non–English-language articles via the MEDLINE, PsycINFO, CINAHL, Social Work Abstracts, Social Services Abstracts, and Dissertation Abstracts International databases (January 1980 through December 2009). We aimed to include prospective studies reporting data on antenatal depression and at least 1 adverse birth outcome: PTB (<37 weeks’ gestation), LBW (<2500 g), or IUGR (<10th percentile for gestational age). Of 862 reviewed studies, 29 US-published and non–US-published studies met the selection criteria.
Data Extraction
Information was extracted on study characteristics, antenatal depression measurement, and other biopsychosocial risk factors and was reviewed twice to minimize error.
Data Synthesis
Pooled relative risks (RRs) for the effect of antenatal depression on each birth outcome were calculated using random-effects methods. In studies of PTB, LBW, and IUGR that used a categorical depression measure, pooled effect sizes were significantly larger (pooled RR [95% confidence interval]=1.39 [1.19–1.61], 1.49 [1.25–1.77], and 1.45 [1.05–2.02], respectively) compared with studies that used a continuous depression measure (1.03 [1.00–1.06], 1.04 [0.99–1.09], and 1.02 [1.00–1.04], respectively). The estimates of risk for categorically defined antenatal depression and PTB and LBW remained significant when the trim-and-fill procedure was used to correct for publication bias. The risk of LBW associated with antenatal depression was significantly larger in developing countries (RR=2.05; 95% confidence interval, 1.43–2.93) compared with the United States (RR=1.10; 95% confidence interval, 1.01–1.21) or European social democracies (RR=1.16; 95% confidence interval, 0.92–1.47). Categorically defined antenatal depression tended to be associated with an increased risk of PTB among women of lower socioeconomic status in the United States.
Conclusions
Women with depression during pregnancy are at increased risk for PTB and LBW, although the magnitude of the effect varies as a function of depression measurement, country location, and US socioeconomic status. An important implication of these findings is that antenatal depression should be identified through universal screening and treated.
Preterm birth (PTB), Low birth weight (LBW), and intrauterine growth restriction (IUGR) are the leading causes of neonatal, infant, and childhood morbidity, mortality, and neurodevelopmental impairments and disabilities worldwide.1–5 Maternal depression during pregnancy has begun to be recognized as a factor that may adversely alter pregnancy outcomes.6–8 Depression also has been linked to known risk factors for adverse pregnancy outcomes such as smoking,9,10 substanceabuse,11 hypertension,12,13 preeclampsia,14,15 and gestational diabetes.16,17 Recent estimates of the prevalence of major depression during pregnancy show that from 8.3%18 to 12.7%19 of US women experience this condition. Moreover, many community-based studies have indicated that poor urban women from minority backgrounds20–22 are at least twice as likely as middle-class women23–25 to meet diagnostic criteria for major and minor depression during pregnancy and the postpartum period (20%–25% vs 9%–13%, respectively). These findings are congruent with epidemiological data showing higher rates of depression in poor young women26 and with data on prevalence rates of perinatal depression for women in developing countries.27–29
Research findings during the last decades on the links between antenatal depression and PTB, LBW, and IUGR have revealed a relatively inconsistent and inconclusive picture.30 Some evidence indicates that depression during pregnancy may be significantly related to PTB,31,32 LBW,33,34 and IUGR,35 whereas other studies have reported no direct association.36–38 Reasons for these contradictory results are most likely related to differences in (1) study design, methods, sample sizes, and the timing, frequency, and type of antenatal depression measurement; (2) misclassification bias with respect to depression or birth outcomes; (3) the populations studied; and (4) the extent to which studies control for confounding factors of PTB, LBW, or IUGR such as socioeconomic status (SES), race/ethnicity, antidepressant use during pregnancy, smoking, substance abuse, previous preterm birth, or obstetric/medical complications.
We therefore conducted a meta-analysis of all available studies to quantify the strength of the relationships between depression during pregnancy and PTB, LBW, and IUGR and examined potential moderators of negative birth outcomes, such as categorical vs continuous measurement of antenatal depression, race or SES of the sample, and country location of the study (ie, whether the study was from the United States, a developing country, or a European social democracy). A social democracy was defined broadly as a democratic welfare state that incorporates both capitalist and socialist practices and provides universal access to health care.39,40 We expected that women with antenatal depression who lived in developing countries, relative to their peers in the United States or social democracies, would show greater disparities in the likelihood of PTB, LBW, and IUGR because of their more limited access to adequate prenatal, health, and mental health care.41 Similarly, we expected that socioeconomically disadvantaged, depressed, pregnant women within the United States would show a higher probability of negative birth outcomes compared with their middle-or upper-class counterparts because they have less access to ongoing adequate health and mental health services.42–44 Finally, given that categorical measures of antenatal depression more closely approximate clinical diagnoses of major depression than do continuous measures, we hypothesized that the former would show a stronger association with adverse birth outcomes.
METHODS
The methods for conducting and reporting the meta-analysis followed state-of-the-art guidelines.45–47
SEARCH STRATEGY AND STUDY SELECTION
Study investigators (N.K.G., A.R.G., J.L.M., and W.J.K.) retrieved potential studies based on a literature search of English-language and non–English-language articles from January 1980 through December 2009 using the MEDLINE, PsycINFO, CINAHL, Social Work Abstracts, Social Services Abstracts, and Dissertation Abstracts International databases. The time frame ensured that the applied standards for the categorical measures of depression were consistent with the Diagnostic and Statistical Manual of Mental Disorders (Third Edition)48 or later criteria. We used the following keywords and their combinations: depression, depressive symptoms, pregnancy, prematurity, antenatal, prenatal, birthweight, birth weight, preterm birth, gestational age, fetal growth restriction, intrauterine growth restriction, and small-for-gestational age. Relevant articles were also identified through references of retrieved articles and contact with prominent investigators in the field.
Published and unpublished English-language and non–English-language observational studies were included in the meta-analysis if they assessed depressive symptoms or unipolar depression diagnoses by means of a depression-screening questionnaire or structured psychiatric interview at 1 or more times during pregnancy. Studies were included if they reported sufficient data to calculate an effect size between depressive symptoms/diagnoses and at least 1 adverse birth outcome: PTB, LBW, or IUGR. Studies were excluded if they used a retrospective design to measure antenatal depression, did not use a prospective or longitudinal design, combined unipolar and bipolar depression diagnoses to measure antenatal depression,49 or reported the same data on antenatal depression and PTB, LBW, or IUGR from a previous article. Of 862 reviewed studies, 29 published studies met the inclusion criteria.
DATA EXTRACTION
Adverse Birth Outcomes
Two investigators (one of whom was N.K.G.) reviewed all the studies. Standardized data collection forms were developed a priori for data extraction.47 In cases of disagreement in coding, we reached agreement through consensus. The mean percentage of disagreement in coding across all 29 studies included in the meta-analysis was 2.1% (95% confidence interval [CI], 1.6%–2.6%). When a report did not contain sufficient data to calculate an effect size, we contacted the primary author up to 3 times to obtain this information. Six of 9 authors contacted provided the requested data. The following types of studies31–38,50–70(Table 1) were included in the meta-analysis: PTB (n=20), LBW (n=11), and IUGR (n=12). The latter included studies examining the keyword topics fetal growth restriction or small-for-gestational age. We extracted information from each study pertinent to each adverse outcome using the authors’ definition of clinical significance. Typically, PTB was defined as less than 37 weeks’ gestation, LBW was defined as less than 2500 g, and IUGR was defined as a fetal weight lower than the 10th percentile for gestational age as determined through an ultrasound.80,81 Alternatively, IUGR was defined in some studies as LBW controlling for gestational age,56,60,64 a fetal weight lower than the 15th percentile for gestational age,70 or a fetal weight lower than the 10th customized percentile.63
Table 1.
Characteristics of Studies Included in the Meta-analysisa
| Source | Country/Gini Coefficient | Sample Size | Sample SES | Race/Ethnicity | Depression Measure | Assessment Times/Trimesters | RR (95% CI) |
Control Variables | Quality Rating | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PTB | LBW | IUGR | |||||||||
| Andersson et al,36 2004 | Sweden/25.0 | 1465 | Mixed | White | PRIME-MD71 | Once/second | 1.19 (0.59–2.40) | 1.19 (0.40–3.56) | … | Yes | 12 |
| Berle et al,50 2005 | Norway/25.8 | 680 | Mixed | White | HADS-D72 | Once/third | … | 1.78 (0.23–13.89) | … | No | 8 |
| Chung et al,51 2001 | Hong Kong/43.4 | 642 | Mixed | Chinese | BDI73 | Twice/first, second, and third | … | … | 1.60 (0.69–3.72) | Yes | 8 |
| Copper et al,37 1996 | United States/40.8 | 2593 | Lower | Black | CES-D74 | Once/second and third | 1.03 (0.99–1.06) | 1.02 (1.00–1.04) | 100 (0.97–1.03) | Yes | 10 |
| Dayan et al,52 1999 | France/32.7 | 392 | Mixed | White | EPDS75 | Once/second | 2.10 (1.10–4.10)b | … | … | No | 4 |
| Dayan et al,31 2006 | France/32.7 | 641 | Mixed | White | EPDS75 | Once/second | 4.90 (1.60–14.90)c | … | … | Yes | 10 |
| Diego et al,53 2009 | United States/40.8 | 79 | Mixed | Mixed | SCID76 | Once/second | 2.61 (0.73–9.33) | 4.75 (0.94–24.00) | 11.28 (0.64–197.35) | No | 4 |
| Dole et al,54 2003 | United States/40.8 | 1962 | Mixed | Black/white | CES-D74 | Once/second and third | 1.12 (0.91–1.38) | … | … | Yes | 11 |
| Elsenbruch et al,55 2007 | Germany/28.3 | 896 | Mixed | White | CES-D74 | Once/first | … | 1.16 (0.91–1.47) | … | No | 8 |
| Evans et al,56 2007 | England/30.0 | 10 967 | Mixed | White | EPDS75 | Twice/second and third | … | … | 1.29 (0.87–1.91) | Yes | 9 |
| Gavin et al,57 2009 | United States/40.8 | 3019 | Mixed | White | CES-D74 | Once/second | 1.26 (0.61–2.61) | … | … | Yes | 10 |
| Goldenberg et al,70 1991 | United States/40.8 | 1545 | Lower | Black | CES-D74 | Once/second | … | … | 2.00 (0.94–4.26) | Yes | 8 |
| Haas et al,58 2005 | United States/40.8 | 1619 | Mixed | Mixed | CES-D74 | Twice/second and third | 1.05 (0.66–1.67) | … | … | Yes | 11 |
| Hedegaard et al,59 1993 | Denmark/24.7 | 5872 | Mixed | White | GHQ77 | Twice/second and third | 1.33 (1.10–1.60)c | … | … | Yes | 11 |
| Hoffman and Hatch,60 2000 | United States/40.8 | 666 | Mixed | White | CES-D74 | 3/Second and third | 1.07 (0.87–1.31) | … | 0.69 (0.23–2.07) | Yes | 10 |
| Jesse et al,61 2003 | United States/40.8 | 119 | Lower | White | 2-Item validated screening tool | Once/second | 3.19 (1.08–9.44)b | … | … | Yes | 5 |
| Li et al,62 2009 | United States/40.8 | 791 | Mixed | Mixed | CES-D74 | Once/first and second | 1.60 (0.71–3.63) | … | … | Yes | 8 |
| Neggers et al,33 2006 | United States/40.8 | 3149 | Lower | Black | CES-D74 | Once/second | 1.30 (1.03–1.64)b | 1.40 (1.09–1.79)c | 0.99 (0.75–1.31) | Yes | 10 |
| Nordentoft et al,38 1996 | Denmark/24.7 | 2432 | Mixed | White | GHQ77 | Once/second | 1.01 (0.98–1.05) | … | 1.01 (0.97–1.05) | No | 9 |
| Orr et al,32 2002 | United States/40.8 | 1399 | Lower | Black | CES-D74 | Once/first and second | 1.96 (1.04–3.71)b | … | … | Yes | 10 |
| Paarlberg et al,63 1999 | The Netherlands/30.9 | 396 | Mixed | White | HSCL78 | 3/First, second, and third | … | … | 1.03 (0.98–1.08) | Yes | 9 |
| Patel and Prince,64 2006 | India/32.5 | 245 | Lower | Indian | GHQ77 | Once/third | … | … | 3.49 (1.48–8.23)c | Yes | 8 |
| Perkin et al,65 1993 | United States/40.8 | 1515 | Lower | White | GHQ77 | 3/First, second, and third | 1.28 (0.95–1.73) | … | … | Yes | 10 |
| Rahman et al,34 2004 | Pakistan/30.6 | 265 | Lower | Pakistani | SCAN79 | Once/third | … | 2.10 (1.32–3.35)c | … | Yes | 9 |
| Rondó et al,66 2003 | Brazil/54.0 | 865 | Lower | Brazilian | GHQ77 | 3/Second and third | 2.32 (1.18–4.58)b | 1.97 (1.12–3.47)b | 1.58 (0.84–2.96) | Yes | 9 |
| Steer et al,35 1992 | United States/40.8 | 389d | Lower | Black | BDI73 | Once/third | 1.06 (1.01–1.11)b | 1.07 (1.02–1.12)c | 1.05 (1.00–1.11)b | Yes | 5 |
| Suri et al,67 2007 | United States/40.8 | 90 | Mixed | … | SCID76 | Once/first, second, and third | 1.41 (0.26–7.73) | 1.86 (0.18–19.45) | … | No | 6 |
| Wisner et al,68 2009 | United States/40.8 | 238 | Mixed | Mixed | SCID76 | Continuous/first, second, and third | 2.62 (1.09–6.29)b | … | … | Yes | 7 |
| Zimmer-Gembeck and Helfand,69 1996 | United States/40.8 | 3073 | Lower | Mixed | Nonvalidated | Once/first and second | … | 1.65 (1.12–2.42)b | … | Yes | 8 |
Abbreviations: BDI, Beck Depression Inventory; CES-D, Center for Epidemiologic Studies Depression scale; CI, confidence interval; ellipses, not available; EPDS, Edinburgh Depression Scale; GHQ, General Health Questionnaire; HADS-D, Hospital Anxiety and Depression Scale–Depression; HSCL, Hopkins Symptom Checklist; IUGR, intrauterine growth restriction; LBW, low birth weight; PRIME-MD, Primary Care Evaluation of Mental Disorders; PTB, preterm birth; RR, relative risk; SCAN, Schedules for Clinical Assessment in Neuropsychiatry; SCID, Structured Clinical Interview for DSM-IV; SES, socioeconomic status (as defined by income level, educational level, or type of insurance).
Each row in this table describes an independent study of antenatal depression and adverse birth outcomes. Gini coefficient refers to the numerical coefficient indicating the degree of income inequality in each country (0=perfect equality; 100=perfect inequality). Effect sizes are represented by RRs and 95% CIs. Control variables include (1) demographic (age, SES, parity, race/ethnicity, educational level, marital status, work status, and sex of infant); (2) psychiatric (anxiety, stress, alcohol and substance abuse, selective serotonin reuptake inhibitor use, smoking, and previous depression or psychiatric illness); and (3) obstetric/medical (gestational age; prepregnancy body mass index; previous PTB, LBW, or IUGR; previous or current hypertension, diabetes, or preeclampsia; or other obstetric complications). Study quality ratings range from 0 to 12.
P<.05.
P<.01.
Only the adult (≥18 years) sample was included in the meta-analysis.
Study Characteristics and Antenatal Depression Measures
Information extracted from each study included year of publication, mean maternal age, mean gestational age at first depression assessment, timing and frequency of antenatal depression measurement, country location, country rating of inequality of income distribution (ie, Gini coefficient82), sample size, and the predominant (ie, >60% of the participants) race/ethnicity, SES, parity, marital status, educational level, and work status of the sample. We also recorded the type of antenatal depression measure used (ie, depression-screening questionnaire or structured psychiatric interview).
Other Biopsychosocial Risk Factors for Adverse Birth Outcomes
We coded the extent to which each study controlled for a group of variables observed in the literature to be risk factors for each adverse birth outcome, including demographic variables such as maternal age, SES, parity, race/ethnicity, educational level, marital status, work status, and sex of the infant; psychiatric variables such as antenatal anxiety, stress, drug/alcohol use, antidepressant medication use, and smoking; and obstetric/medical variables such as history of PTB or LBW, current gestational diabetes or preeclampsia, prepregnancy maternal weight or body mass index, and gestational age. We designated the following as key control variables because they have shown the strongest and most consistent associations with adverse birth outcomes in the literature: (1) smoking or substance abuse,10,11,83–85 (2) race/ethnicity or SES,42,60,86 (3) previous PTB,87–89 and (4) selective serotonin reuptake inhibitor (SSRI) antidepressant use.90–94
Methodologic Quality Assessment
Two of the investigators (one of whom was N.K.G.) rated each study on 6 components of methodologic quality, which we developed by modifying the instrument by Downs and Black95 for randomized controlled trials and observational studies. We used a consensus approach in which any differences were resolved before assigning a final rating (intraclass correlation coefficient, 0.97; 95% CI, 0.93–0.98). The 6 components evaluated (1) the size of the sample, (2) the representativeness of the sample, (3) whether the sample was clearly described, (4) the reliability and validity of the measure used to assess antenatal depression, (5) whether the statistical tests were appropriate and controlled for the key variables described previously, and (6) whether the study response rate (ie, those who declined to enter the study) and attrition rate (ie, those who entered the study but dropped out) were reported and taken into account statistically. The 3 levels of quality for each component (not adequate, somewhat adequate, and adequate) received equal weights in scoring. A composite quality score was created for each study (Table 1), which was a sum of the number of the 6 components rated (total score range, 0–12).
DATA ANALYSIS
The association of antenatal depressive symptoms or diagnoses with each adverse birth outcome was examined using relative risks (RRs). To do this, we considered odds ratios (ORs) as surrogates for RRs because when outcomes undergoing study are relatively uncommon, the relative odds approximate RRs. One study55 used the correlation coefficient as the measure of effect size between antenatal depression and LBW; in this case, we computed the RR by means of transformation from the Pear-son correlation to the standardized mean difference and then from this difference to the log OR.96 Two studies of PTB60,62 reported hazard ratios as a measure of association between antenatal depression and time to delivery. Because hazard ratios provide a control for calendar time, we consider an effect size derived from Cox regression as a measure of RR.97 For each birth outcome, there were a sufficient number of studies to calculate an effect size with a corresponding 95% CI.98 We weighted the study-specific RR by the inverse of its variance to compute a pooled RR using random-effects models. A 2-tailed P<.05 was used to determine statistical significance. Statistical analyses were performed with Comprehensive Meta-analysis version 2.2 (Biostat, Englewood, New Jersey) and SPSS version 17.0 (SPSS Inc, Chicago, Illinois) statistical software.
Heterogeneity of effect size was assessed using the Cochran Q χ2 statistic (P≤.10) and the I2 statistic (a transformation of the Cochran Q that indicates the percentage of variation in the effect size estimate attributable to heterogeneity rather than sampling error).99 A nonsignificant χ2 statistic suggests that the obtained pooled RR represents a unitary effect and that any variability in effect sizes is caused by random error rather than the influence of other potential moderator variables. For outcomes in which the test of homogeneity of effect sizes was significant, random-effects meta-regression analyses and moderator analyses were conducted to determine whether 6 study characteristics could explain variability across studies: (1) country location: United States, developing country, or social democracy; (2) the country rating of inequality of income distribution (ie, Gini coefficient); (3) sample SES; (4) sample race (eg, black or white), controlling for SES; (5) study methodologic quality; and (6) categorical vs continuous antenatal depression measurement. Sensitivity analyses100 (known as “leave-one-out”) were conducted by iteratively deleting each study and calculating the resulting effect sizes.
We followed a group of a priori decision rules for pooling data from each study. First, we used the most typical cutoff value for the validated depression scale in each study. Second, when the cutoff score was trichotomized, we used the typical cutoff value to determine the mean of the scores for the medium- and high-risk groups to comprise the group with depression. Third, when a study examined a depression-only group and a depression plus antidepressant medication group, we pooled the effect sizes for the 2 groups in the primary analysis. We then conducted sensitivity analyses100 for the studies that stratified for antidepressant medication use during pregnancy to compare the birth outcomes for depressed women treated and not treated with antidepressants. Fourth, for studies that measured depression more than once at different times or trimesters during pregnancy, we used the mean of the effect sizes in the primary analyses. Fifth, we used both categorical and continuous measures of antenatal depression in the primary analyses and then conducted moderator analyses based on the categorical-continuous distinction. Sixth, in the primary analyses we included 28 studies that used a validated measure of antenatal depression and an additional study that did not report validity or reliability data for its antenatal depression measure.69 We also conducted sensitivity analyses comparing the birth outcomes for the 28 studies using validated measures with the outcomes for the 29 studies used in the primary analyses.
Publication bias was assessed visually using a funnel plot and quantitatively using an adjusted rank correlation test101 and a regression procedure to measure funnel plot asymmetry.102 The trim-and-fill method by Duval and Tweedie103,104 was used to adjust for potential publication bias. The trim-and-fill method assesses asymmetry in the funnel plot, imputes the number of suspected missing studies, and recalculates the adjusted effect size estimate. The adjusted result can be used as a sensitivity analysis to indicate the extent to which publication bias may affect the pooled estimate.105
RESULTS
The study retrieval and selection strategy is illustrated in Figure 1. Of 862 citations meeting initial search criteria, 54 articles were retrieved and 3 were identified from the references of the retrieved articles, making a total of 57. Of these, 28 studies were excluded (23 studies met at least 1 of the exclusion criteria, and 5 studies represented duplicate studies in which the same data were reported in a previous article), leaving a total of 29 articles included in the meta-analysis. Table 1 gives the characteristics of the studies included in the meta-analysis.
Figure 1.
Identification of independent studies for inclusion in meta-analysis (adapted from QUOROM flowchart guidelines46).
ANTENATAL DEPRESSION AND RISK OF ADVERSE BIRTH OUTCOMES
Preterm Birth
Twenty studies evaluated the association between antenatal depression and PTB, with RRs ranging from 1.01 to 4.90 (Table 2). Eleven of the studies found no significant association. Using the random-effects model, depression during pregnancy was significantly associated with PTB (RR=1.13; 95% CI, 1.06–1.21). Significant heterogeneity across studies was noted (Q19 = 49.0; P<.001; I2=61%).
Table 2.
Effect of Antenatal Depression on Outcomes of PTB, LBW, and IUGR
| Outcome | No. of Studies | Relative Risk (95% CI)a | P Value | Heterogeneity |
||
|---|---|---|---|---|---|---|
| Qdf Within | P Value | Variance Explained, % | ||||
| PTB | 20 | 1.13 (1.06–1.21) | <.001 | 49.019 | <.001 | 61 |
| LBW | 11 | 1.18 (1.07–1.30) | .001 | 33.810 | <.001 | 70 |
| IUGR | 12 | 1.03 (0.99–1.08) | .14 | 22.411 | .02 | 51 |
Abbreviations: CI, confidence interval; IUGR, intrauterine growth restriction; LBW, low birth weight; PTB, preterm birth.
Pooled effect size was estimated using the random-effects model.
Low Birth Weight
Eleven studies evaluated the association between antenatal depression and LBW with RRs ranging from 1.02 to 4.75 (Table 2). Six of the studies found no significant association. The random-effects meta-analysis showed that antenatal depression was significantly associated with LBW (RR=1.18; 95% CI, 1.07–1.30). Significant heterogeneity across studies was found (Q10=33.8; P <.001; I2=70%).
Intrauterine Growth Restriction
Twelve studies evaluated the association between antenatal depression and IUGR, with RRs ranging from 0.69 to 11.28 (Table 2). Only 2 studies reported a significant association. The summary RR calculated from the random-effects model showed that antenatal depression was not significantly associated with IUGR (RR=1.03; 95% CI, 0.99–1.08). Significant heterogeneity across studies was noted (Q11=22.4; P =.02; I2=51%).
MODERATORS OF OUTCOME
Moderator analyses were conducted to explore sources of heterogeneity (Table 3). As expected, studies of PTB, LBW, and IUGR that used a categorical depression predictor yielded larger (P<.05 for all) pooled RRs (1.39 [95% CI, 1.19–1.61], 1.49 [1.25–1.77], and 1.45 [1.05–2.02], respectively) than studies that used a continuous depression predictor (1.03 [1.00–1.06], 1.04 [0.99–1.09], and 1.02 [1.00–1.04], respectively). In PTB trials, heterogeneity among studies was reduced by the addition of the depression predictor moderator (categorical: Q15=24.6; P =.06; I2=39%; continuous: Q3=4.7; P =.20; I2=36%).
Table 3.
Moderators of Effect of Antenatal Depression on Outcomes of PTB, LBW, and IUGR
| Moderator | No. of Studies | Within Group |
Effect of Moderator |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Relative Risk (95% CI)a | P Value | Heterogeneity |
|||||||
| Qdf Within | P Value | Variance Explained, % | Qdf Between | P Value | Variance Explained, % | ||||
| PTB | |||||||||
| Depression predictor | 14.21 | .001 | 29 | ||||||
| Categorical | 16 | 1.39 (1.19–1.61) | <.001 | 24.615 | .06 | 39 | |||
| Continuous | 4 | 1.03 (1.00–1.06) | .05 | 4.73 | .20 | 36 | |||
| Study locationb | 1.81 | .18 | 4 | ||||||
| European social democracy | 5 | 1.37 (1.01–1.85) | .04 | 20.04 | <.001 | 80 | |||
| United States | 14 | 1.10 (1.03–1.19) | .005 | 22.213 | .05 | 41 | |||
| Study qualityc | 2.01 | .15 | 4 | ||||||
| ≤6 | 5 | 1.70 (0.99–2.92) | .05 | 10.04 | .04 | 60 | |||
| >6 | 15 | 1.14 (1.06–1.24) | .001 | 37.414 | .001 | 63 | |||
| Effect size | 0.61 | .45 | 1 | ||||||
| Adjusted | 16 | 1.18 (1.08–1.28) | <.001 | 38.415 | .001 | 61 | |||
| Unadjusted | 4 | 1.46 (0.84–2.53) | .18 | 7.03 | .07 | 57 | |||
| LBW | |||||||||
| Depression predictor | 14.61 | <.001 | 43 | ||||||
| Categorical | 9 | 1.49 (1.25–1.77) | <.001 | 9.88 | .28 | 18 | |||
| Continuous | 2 | 1.04 (0.99–1.09) | .10 | 3.31 | .07 | 70 | |||
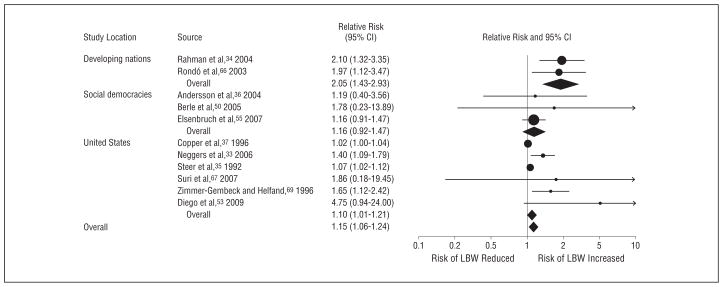
| Study locationd | 10.72 | .005 | 32 | ||||||
| Developing nation | 2 | 2.05 (1.43–2.93) | <.001 | 0.01 | .86 | 0 | |||
| European social democracy | 3 | 1.16 (0.92–1.47) | .20 | 0.22 | .92 | 0 | |||
| United States | 6 | 1.10 (1.01–1.21) | .03 | 18.75 | .002 | 73 | |||
| Study qualityc | 0.11 | .78 | 0 | ||||||
| ≤6 | 3 | 1.59 (0.63–4.00) | .33 | 3.52 | .18 | 42 | |||
| >6 | 8 | 1.39 (1.11–1.73) | .004 | 27.77 | <.001 | 75 | |||
| Effect size | 0.171 | .68 | 0 | ||||||
| Adjusted | 7 | 1.17 (1.06–1.30) | .003 | 29.06 | <.001 | 79 | |||
| Unadjusted | 4 | 1.27 (0.88–1.82) | .20 | 3.13 | .37 | 4 | |||
| IUGR | |||||||||
| Depression predictor | 4.51 | .04 | 20 | ||||||
| Categorical | 8 | 1.45 (1.05–2.02) | .03 | 14.07 | .05 | 50 | |||
| Continuous | 4 | 1.02 (1.00–1.04) | .12 | 3.73 | .29 | 20 | |||
| Study locatione | 4.02 | .14 | 19 | ||||||
| Developing nation | 2 | 2.22 (1.03–4.79) | .04 | 2.11 | .14 | 53 | |||
| European social democracy | 3 | 1.02 (0.99–1.05) | .21 | 1.82 | .41 | 0 | |||
| United States | 6 | 1.03 (0.96–1.10) | .41 | 9.65 | .09 | 48 | |||
| Study qualityc | 0.51 | .49 | 2 | ||||||
| ≤6 | 2 | 2.20 (0.26–18.80) | .47 | 2.61 | .11 | 62 | |||
| >6 | 10 | 1.03 (0.98–1.08) | .30 | 17.39 | .04 | 48 | |||
| Effect size | 0.41 | .52 | 2 | ||||||
| Adjusted | 10 | 1.04 (0.99–1.11) | .14 | 19.49 | .20 | 54 | |||
| Unadjusted | 2 | 2.17 (0.24–19.60) | .49 | 2.71 | .10 | 63 | |||
Abbreviations: CI, confidence interval; IUGR, intrauterine growth restriction; LBW, low birth weight; PTB, preterm birth.
Pooled effect size was estimated using the random-effects model.
Only 1 study was conducted in a developing nation.
Study quality ratings range from 0 to 12.
Pairwise effect of moderator for LBW: developing nation vs European social democracy, Q1=7.0, P =.01; developing nation vs United States, Q1=10.7, P =.001; European social democracy vs United States, Q1=0.2, P =.68.
Pairwise effect of moderator for IUGR: developing nation vs European social democracy, Q1=3.9, P =.048; developing nation vs United States, Q1=3.8, P =.05; European social democracy vs United States, Q1=0.0, P =.84.
As expected, country location (developing nation, social democracy, or United States) was also a significant moderator of the association between antenatal depression and LBW (Table 3 and Figure 2). In developing nations, 2 studies of antenatal depression and LBW yielded a pooled RR of 2.05 (95% CI, 1.43–2.93).34,66 In studies from social democracies36, 50, 55 and the United States,33,35,37,53,67,69 the resulting summary RRs were 1.16 (95% CI, 0.92–1.47) and 1.10 (95% CI, 1.01–1.21), respectively. Significant heterogeneity was still present in US studies (Q5=18.7; P =.002; I2=73%). A similar effect (Q2=7.1; P =.03) was found after excluding 2 US studies35,37 that used a continuous depression predictor, which yielded a pooled RR of 1.50 (95% CI, 1.22–1.84), and that eliminated heterogeneity across US trials (P>.45; I2=0%). Table 3 reveals a similar pattern for studies evaluating the association between antenatal depression and IUGR, with a significant pairwise difference in RR between developing countries and social democracies (P=.048) and a near significant pairwise difference in RR between developing countries and the United States (P=.05). These data indicate that depressed pregnant women in the developing world are twice as likely to experience IUGR as their European or US counterparts.
Figure 2.
Effect of antenatal depression on the risk of low birth weight (LBW) in developing nations, European social democracies, and the United States. CI indicates confidence interval.
Limiting the analysis to the 10 US studies that used a categorically defined depression predictor showed a trend (Q1=3.54; P =.06) for antenatal depression to be associated with an increased risk of PTB among women of lower SES (RR=1.69; 95% CI, 1.14–2.50) but not in women of middle- or upper-income status (RR=1.13; 95% CI, 0.99–1.29).
Inequality of income across countries, study quality, type of depression severity measurement, and use of adjusted vs unadjusted effect size estimates were not significant moderators of PTB, LBW, or IUGR. Race was not a significant moderator of PTB in US studies of predominantly low SES populations. Type of IUGR measurement was not a significant moderator of outcome in IUGR studies. In the 5 studies of PTB31,57,62,67,68 for which stratification by antidepressant medication treatment during pregnancy was possible, the summary RR was comparable for depressed women treated and not treated with antidepressants.
Leave-One-Out Analyses
Sensitivity analyses revealed that no single study unduly influenced the pooled RR estimates of the association between antenatal depression and PTB, LBW, and IUGR (data available on request). In particular, leaving out the 1 study that did not report validity or reliability data for its measure of antenatal depression did not significantly alter the findings observed for LBW in the primary analyses.
Publication Bias
For all 3 outcome measures (PTB, LBW, and IUGR), visual inspection of funnel plots in which each study’s effect size was plotted against the standard error showed marked asymmetry, suggesting that small studies with negative results may not have been published. Formal testing using the regression intercept approach102 confirmed the possibility of publication bias for PTB (P <.001), LBW (P=.001), and IUGR (P=.008). As indicated in Table 4, the trim-and-fill adjusted RRs for each adverse birth outcome are generally lower than the unadjusted RRs. More important, however, the estimates of RR for categorically defined antenatal depression and PTB and LBW were robust to the effects of publication bias.
Table 4.
Comparison of Unadjusted Pooled RRs and Trim-and-Fill Adjusted Pooled RRs
| Depression Predictor | No. of Studies | Unadjusted Pooled RR (95% CI)a | No. of Missing Studies | Trim-and-Fill Adjusted Pooled RR (95% CI)b |
|---|---|---|---|---|
| PTB | ||||
| Overall | 20 | 1.13 (1.06–1.21) | 10 | 1.07 (0.99–1.15) |
| Categorical | 16 | 1.39 (1.19–1.61) | 6 | 1.24 (1.04–1.47) |
| Continuous | 4 | 1.03 (1.00–1.06) | 1 | 1.03 (1.00–1.07) |
| LBWc | ||||
| Overall | 11 | 1.18 (1.07–1.30) | 6 | 1.10 (1.00–1.22) |
| Categorical | 9 | 1.49 (1.25–1.77) | 4 | 1.34 (1.10–1.64) |
| IUGR | ||||
| Overall | 12 | 1.03 (0.99–1.08) | 4 | 1.03 (0.97–1.09) |
| Categorical | 8 | 1.45 (1.05–2.02) | 3 | 1.17 (0.82–1.68) |
| Continuous | 4 | 1.02 (1.00–1.04) | 2 | 1.00 (0.98–1.03) |
Abbreviations: CI, confidence interval; IUGR, intrauterine growth restriction; LBW, low birth weight; PTB, preterm birth; RR, relative risk.
Using random-effects models.
Using random-random effects trim-and-fill models.
Only 2 studies used a continuous depression predictor; the trim-and-fill algorithm requires 3 or more studies.
COMMENT
This meta-analysis showed that depression during pregnancy, regardless of the type of antenatal depression measurement (ie, categorical or continuous), is associated with modest but statistically significant risks of PTB and LBW. Furthermore, the estimates of risk for PTB and LBW from categorically defined antenatal depression appear resilient to the effects of publication bias. Sensitivity analyses showed that the magnitude of RR for these adverse birth outcomes was consistent within the set of 20 studies examining PTB and the set of 11 studies investigating LBW. Although the sizes of the significant RRs for PTB and LBW posed by antenatal depression are modest, the test of relevance in our study is not statistical significance but public health significance. That is, given the prevalence of antenatal depression in a population of pregnant women, what will be the likely burden of PTB or LBW for their infants? Thus, a relatively small effect size magnified by a large population base can have a considerable and noteworthy effect on public health.
We also found evidence that the type of depression measurement (categorical vs continuous) moderated the strength of the associations between antenatal depression and PTB, LBW, and IUGR, thereby eliminating or reducing the heterogeneity associated with these findings. Results for categorical measures of antenatal depression revealed that having major depression or clinically significant depressive symptoms significantly increased the RR of PTB by 39%, the risk of LBW by 49%, and the risk of IUGR by 45%. As expected, continuous measures of antenatal depression showed a similar albeit weaker pattern, indicating that every 1-point increase in depression severity was associated with a 3% significantly increased risk of PTB and nonsignificantly increased risks of LBW (4%) and IUGR (2%). To place the categorical results for antenatal depression in context, we note that smoking was observed to have a dose-dependent relationship with PTB,106,107 with smoking more than 10 cigarettes a day shown to increase the likelihood of PTB between 33 and 36 weeks by 40% and of PTB at 32 weeks or less by 60%. Furthermore, in a cohort analysis of a large, ethnically diverse population,11 substance use disorders were associated with a 2.4-fold higher risk of PTB and a 3.7-fold higher risk of LBW, and black race increased the likelihood of PTB by 60% and of LBW by 2-fold. Thus, the magnitude of risk for PTB and LBW posed by antenatal depression is comparable to the risk of smoking 10 or more cigarettes a day for PTB but is relatively modest contrasted to the greater risks of black race and substance abuse associated with PTB and LBW.
Moderator analyses for country location revealed that the RR of delivering an infant with LBW or IUGR was higher among women from developing countries who experienced antenatal depression than their counterparts in the United States or social democracies. Moreover, in US studies, categorically defined antenatal depression tended to be associated with an elevated risk of PTB in women of predominantly lower SES but not in women of middle- or upper-income status. Pregnant women of lower SES in the United States are also twice as likely to experience antenatal major and minor depression as are women from middle- to upper-income strata.20–22 Whereas cross-cultural variability in the prevalence of perinatal depression certainly exists,29 estimates of the prevalence of perinatal depression in several developing countries27,28 are similar to the higher depression rates in pregnant US women of lower SES. Thus, many socioeconomically disadvantaged childbearing women in developing countries and in the United States experience a double-barreled threat: an increased risk of becoming depressed during their pregnancies and an increased likelihood of experiencing adverse birth outcomes once they have antenatal depression. Depressed pregnant women living near or below poverty levels are subject to large amounts of acute and chronic stress, such as living in unsafe neighborhoods, experiencing racial/ethnic or economic discrimination, and confronting food inadequacy in their households.22,108,109 At the same time, despite Medicaid or other public insurance coverage during pregnancy, their mental health problems are seldom accurately diagnosed and they often lack access to specialty mental health services.110–112
Several potential direct and indirect causal pathways through which antenatal depression leads to adverse pregnancy outcomes have been proposed. One possibility is that prenatal stress or depression during pregnancy might promote adverse birth outcomes through the dysregulation of the hypothalamic-pituitary-adrenocortical axis, stimulating the release of stress hormones, such as cortisol and catecholamines. These biological changes may result in placental hypofusion and consequent restriction of oxygen and nutrients to the fetus, leading to fetal growth restriction and/or precipitation of PTB.113–117 Other mechanisms include the possibility that antenatal depression might compromise immune system functioning,118 which in turn may lead to a reproductive tract infection triggering PTB.117 The harmful public health effect of antenatal depression on birth outcomes is further heightened by evidence that depression during pregnancy is associated with risky but modifiable health practices, such as poor nutrition and hygiene, lack of motivation to obtain prenatal care or to follow medical recommendations, and smoking and/or alcohol and substance abuse, all of which adversely affect pregnancy outcomes.11,33,85,119
Clearly, pregnancy is an important time to universally screen women for depression, especially those who are socioeconomically disadvantaged, and to improve their timely access to evidence-based prenatal and mental health services.120 Improved accuracy of diagnosis and treatment of antenatal depression combined with education about harmful but potentially modifiable lifestyle practices could lead to decreased rates of PTB and LBW.
Reducing the rates of these adverse birth outcomes is a critically important public health issue. During childhood, PTB is associated with an increased risk of mortality3; adverse medical outcomes4,121–123 including respiratory distress syndrome, cerebral palsy, chronic lung disease, vision and hearing loss, and neurodevelopmental disabilities; cognitive difficulties124; and psychiatric problems,124 such as internalizing and externalizing behaviors and attention-deficit/hyperactivity disorder. Long-term consequences of PTB in adulthood include diminished rates of reproduction and, for women born preterm, an increased risk of next-generation PTB, fetal stillbirth, and infant mortality.3 Pernicious child and adult outcomes related to LBW125–133 and IUGR134–139 reflect patterns similar to that of PTB. Furthermore, in the United States, the annual economic costs of medically managing the consequences of these adverse birth outcomes are enormous.140,141 For example, approximately 75% of admissions to the neonatal intensive care unit are related to prematurity.142 Daily neonatal intensive care unit costs in the United States exceed $3500 per infant, and it is not unusual for costs to reach $1 million for a prolonged stay.142
A strength of our meta-analysis is that our search included US and non-US English-language studies, as well as a study in French that was translated into English by an expert. Thus, the 29 studies included in our meta-analysis came from 12 non-US countries, indicating significant international representation. Our meta-analysis also included studies that varied in the extent to which they controlled for confounding factors related to PTB, LBW, and IUGR. For example, one-third of the studies (n=10) controlled for SSRI use31,57,62,67,68 or reported that SSRI use was unlikely in their sample.34,36,51,64,66 An increasing number of women with depression are prescribed antidepressant medications during pregnancy.143 These medications, especially SSRIs, have been significantly linked with LBW in some49,92,93,144 but not all studies.145–147 Wisner et al68 recently found that infants who were continuously exposed to either SSRIs or major depression throughout the 3 trimesters of pregnancy were more likely to be born preterm than were infants with partial or no exposure. Differentiating between the effects of depression or depressive symptoms and the effects of antidepressants on birth outcomes is challenging because (1) researchers typically investigate the effects of one without controlling adequately for the other; (2) use of antidepressants during pregnancy occurs at different times, dosages, and durations; (3) recognition and treatment of depression by the physician is often associated with depression severity and persistence; and (4) depression is also associated with the use of additional prescription and nonprescription medications148 and other potential confounders, such as smoking or substance use disorders.
Finally, prior evidence has shown that key variables in addition to depression, such as substance use or abuse, race/ethnicity or SES, and previous PTB, are strongly and consistently predictive of negative birth outcomes. Most of the studies in our meta-analysis (80%) controlled for at least 2 of these key predictors, but few controlled for all of them. In addition, most of the studies did not control for stressful life events and other psychiatric comorbidities of depression, such as antenatal anxiety, which has been linked with adverse birth outcomes in some studies.6 A recent meta-analysis of anxiety symptoms and birth outcomes, however, did not show evidence of this association.149
Only 5 of 29 studies in our meta-analysis assessed major depression during pregnancy by using diagnostic criteria adhering to or compatible with the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).150 Most studies used relatively short screening tools to evaluate levels of depressive symptoms and to set cutoff scores for describing clinically significant depressive symptoms. Although categorizing depressive symptom levels may be related to the diagnosis of clinically significant depression, it is not a substitute nor may it be as accurate as a structured interview. Finally, although we found possible publication bias, the findings of an elevated risk of PTB and LBW associated with categorically defined depression remained robust to trim-and-fill analyses that corrected for this bias.
Limitations of the studies reviewed suggest the need for a large prospective epidemiological study to simultaneously evaluate the RRs during pregnancy of depression, SSRI use, smoking, substance use disorders, key sociodemographic variables, obstetric/medical variables, and important behavioral health practices, including engagement in adequate prenatal and medical care and nutrition. Multiple assessments of major and minor antenatal depression should be performed, using criteria from the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) as well as measures assessing depression severity. The RRs of anxiety disorders and anxiety severity should also be assessed because these psycho-pathologic conditions typically accompany depression and were found to be related in some, but not all, studies to adverse birth outcomes. Ideally, this future study would prospectively gather data during the prepregnancy, pregnancy, and postpartum periods, considering that a broader perspective on a woman’s health status may be necessary to better understand the risk factors associated with harmful birth outcomes.58,151,152
Our overall pattern of findings in this meta-analysis highlights the salient public health risk of PTB and LBW posed by antenatal depression, particularly for socioeconomically disadvantaged women in developing countries and in the United States. Furthermore, mounting evidence from this meta-analysis and other sources68 suggests that untreated major depression during pregnancy is as likely to lead to poor birth outcomes as is treatment with SSRIs. An important implication of these findings is that pregnant women should be universally screened for depression and provided guideline-level treatment before childbirth. Given that untreated antenatal depression is the most robust predictor of postpartum depression and has additional serious adverse consequences for infant and child development beyond harmful birth outcomes, women and their obstetrics professionals will need to weigh the costs and benefits of treating antenatal depression pharmacologically, especially when treatment with evidence-based psychotherapy is not available or desired.
Acknowledgments
Funding/Support: Dr Grote’s role in this study was supported by grant K23-MH 67595 from the National Institute of Mental Health (NIMH), National Institutes of Health (NIH). Dr Bridge’s role was supported by grant K01-MH069948 from the NIMH. Dr Gavin’s role was supported by grant 1KL2RR025015-01 from the National Center for Research Resources, a component of the NIH and NIH Roadmap for Medical Research. Dr Melville’s role was supported by grant K23 MH070704 from the NIMH. Dr Katon’s role was supported by grant K24 MH069471 from the NIMH.
Role of the Sponsor: The NIMH had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Additional Contributions: We gratefully acknowledge the contribution of additional data from some of the investigators of the original studies included in this meta-analysis. We also thank all those who responded to our inquiries, even if data from their studies could not be made available. Finally, we thank Kimberly A. Yonkers, MD, Yale University, for her thoughtful review of the manuscript, for which no compensation was received.
Financial Disclosure: Dr Bridge has received honoraria for participation in a suicidality consensus conference supported by independent educational or other unrestricted grant support from AstraZeneca, Dainippon Sumitomo, Eli Lilly, Forest Research Institute, Ortho-McNeil-Janssen, Pfizer Inc, Roche Pharmaceuticals, Sanofi-Aventis, Schering-Plough, Sepracor, and United BioSource; and he has presented on suicide prevention in children and adolescents at a conference supported in part by Eli Lilly and Lundbeck. Dr Katon has received honoraria for lectures from Lilly, Forest, Wyeth, and Pfizer and has served on advisory boards for Lilly and Wyeth during the last 2 years.
References
- 1.March of Dimes. Perinatal Profile: Statistics for Monitoring State Maternal and Infant Health, 1999 & 2000 editions. White Plains, NY: Perinatal Data Center; 2000. [Google Scholar]
- 2.World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. Geneva, Switzerland: World Health Organization; 1995. WHO Technical Report Series 854. [Google Scholar]
- 3.Swamy GK, Ostbye T, Skjaerven R. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. JAMA. 2008;299(12):1429–1436. doi: 10.1001/jama.299.12.1429. [DOI] [PubMed] [Google Scholar]
- 4.Allen MC, Jones MD., Jr Medical complications of prematurity. Obstet Gynecol. 1986;67(3):427–437. [PubMed] [Google Scholar]
- 5.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115(4):997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 6.Alder J, Fink N, Bitzer J, Hösli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? a critical review of the literature. J Matern Fetal Neonatal Med. 2007;20(3):189–209. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- 7.Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735. doi: 10.1177/070674370404901103. [DOI] [PubMed] [Google Scholar]
- 8.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- 9.Kyrklund-Blomberg NB, Cnattingius S. Preterm birth and maternal smoking: risks related to gestational age and onset of delivery. Am J Obstet Gynecol. 1998;179(4):1051–1055. doi: 10.1016/s0002-9378(98)70214-5. [DOI] [PubMed] [Google Scholar]
- 10.Ellard GA, Johnstone FD, Prescott RJ, Ji-Xian W, Jian-Hua M. Smoking during pregnancy: the dose dependence of birthweight deficits. Br J Obstet Gynaecol. 1996;103(8):806–813. doi: 10.1111/j.1471-0528.1996.tb09878.x. [DOI] [PubMed] [Google Scholar]
- 11.Kelly RH, Russo J, Holt VL, Danielsen BH, Zatzick DF, Walker E, Katon W. Psychiatric and substance use disorders as risk factors for low birth weight and preterm delivery. Obstet Gynecol. 2002;100(2):297–304. doi: 10.1016/s0029-7844(02)02014-8. [DOI] [PubMed] [Google Scholar]
- 12.Haelterman E, Bréart G, Paris-Llado J, Dramaix M, Tchobroutsky C. Effect of uncomplicated chronic hypertension on the risk of small-for-gestational age birth. Am J Epidemiol. 1997;145(8):689–695. doi: 10.1093/aje/145.8.689. [DOI] [PubMed] [Google Scholar]
- 13.Sibai BM, Caritis SN, Hauth JC, MacPherson C, VanDorsten JP, Klebanoff M, Landon M, Paul RH, Meis PJ, Miodovnik M, Dombrowski MP, Thurnau GR, Moawad AH, Roberts J National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Preterm delivery in women with pregestational diabetes mellitus or chronic hypertension relative to women with uncomplicated pregnancies. Am J Obstet Gynecol. 2000;183(6):1520–1524. doi: 10.1067/mob.2000.107621. [DOI] [PubMed] [Google Scholar]
- 14.Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95 (4):487–490. doi: 10.1016/s0029-7844(99)00602-x. [DOI] [PubMed] [Google Scholar]
- 15.Xiong X, Mayes D, Demianczuk N, Olson DM, Davidge ST, Newburn-Cook C, Saunders LD. Impact of pregnancy-induced hypertension on fetal growth. Am J Obstet Gynecol. 1999;180(1 pt 1):207–213. doi: 10.1016/s0002-9378(99)70176-6. [DOI] [PubMed] [Google Scholar]
- 16.Kozhimannil KB, Pereira MA, Harlow BL. Association between diabetes and perinatal depression among low-income mothers. JAMA. 2009;301(8):842–847. doi: 10.1001/jama.2009.201. [DOI] [PubMed] [Google Scholar]
- 17.Evers IM, de Valk HW, Visser GH. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ. 2004;328(7445):915. doi: 10.1136/bmj.38043.583160.EE.. [published online ahead of print April 5, 2004] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vesga-López O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805–815. doi: 10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 pt 1):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 20.Hobfoll SE, Ritter C, Lavin J, Hulsizer MR, Cameron RP. Depression prevalence and incidence among inner-city pregnant and postpartum women. J Consult Clin Psychol. 1995;63(3):445–453. doi: 10.1037//0022-006x.63.3.445. [DOI] [PubMed] [Google Scholar]
- 21.Scholle SH, Haskett RF, Hanusa BH, Pincus HA, Kupfer DJ. Addressing depression in obstetrics/gynecology practice. Gen Hosp Psychiatry. 2003;25(2):83–90. doi: 10.1016/s0163-8343(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 22.Siefert K, Bowman PJ, Heflin CM, Danziger S, Williams DR. Social and environmental predictors of maternal depression in current and recent welfare recipients. Am J Orthopsychiatry. 2000;70(4):510–522. doi: 10.1037/h0087688. [DOI] [PubMed] [Google Scholar]
- 23.Gotlib IH, Whiffen VE, Wallace PM, Mount JH. Prospective investigation of postpartum depression: factors involved in onset and recovery. J Abnorm Psychol. 1991;100(2):122–132. doi: 10.1037//0021-843x.100.2.122. [DOI] [PubMed] [Google Scholar]
- 24.O’Hara MW, Neunaber DJ, Zekoski EM. Prospective study of postpartum depression: prevalence, course, and predictive factors. J Abnorm Psychol. 1984;93(2):158–171. doi: 10.1037//0021-843x.93.2.158. [DOI] [PubMed] [Google Scholar]
- 25.O’Hara MW, Swain AM. Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996;8(1):18–37. [Google Scholar]
- 26.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 27.Patel V, Rodrigues M, DeSouza N. Gender, poverty, and postnatal depression: a study of mothers in Goa, India. Am J Psychiatry. 2002;159(1):43–47. doi: 10.1176/appi.ajp.159.1.43. [DOI] [PubMed] [Google Scholar]
- 28.Rahman A, Iqbal Z, Harrington R. Life events, social support and depression in childbirth: perspectives from a rural community in the developing world. Psychol Med. 2003;33(7):1161–1167. doi: 10.1017/s0033291703008286. [DOI] [PubMed] [Google Scholar]
- 29.Halbreich U, Karkun S. Cross-cultural and social diversity of prevalence of postpartum depression and depressive symptoms [published online ahead of print February 7, 2006] J Affect Disord. 2006;91(2–3):97–111. doi: 10.1016/j.jad.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 30.Yonkers K, Wisner K, Stewart D, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C. Management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413. doi: 10.1016/j.genhosppsych.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dayan J, Creveuil C, Marks MN, Conroy S, Herlicoviez M, Dreyfus M, Tordjman S. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68(6):938–946. doi: 10.1097/01.psy.0000244025.20549.bd. [DOI] [PubMed] [Google Scholar]
- 32.Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- 33.Neggers Y, Goldenberg R, Cliver S, Hauth J. The relationship between psychosocial profile, health practices, and pregnancy outcomes. Acta Obstet Gynecol Scand. 2006;85(3):277–285. doi: 10.1080/00016340600566121. [DOI] [PubMed] [Google Scholar]
- 34.Rahman A, Iqbal Z, Bunn J, Lovel H, Harrington R. Impact of maternal depression on infant nutritional status and illness: a cohort study. Arch Gen Psychiatry. 2004;61(9):946–952. doi: 10.1001/archpsyc.61.9.946. [DOI] [PubMed] [Google Scholar]
- 35.Steer RA, Scholl TO, Hediger ML, Fischer RL. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093–1099. doi: 10.1016/0895-4356(92)90149-h. [DOI] [PubMed] [Google Scholar]
- 36.Andersson L, Sundström-Poromaa I, Wulff M, Aström M, Bixo M. Neonatal outcome following maternal antenatal depression and anxiety: a population-based study. Am J Epidemiol. 2004;159(9):872–881. doi: 10.1093/aje/kwh122. [DOI] [PubMed] [Google Scholar]
- 37.Copper RL, Goldenberg RL, Das A, Elder N, Swain M, Norman G, Ramsey R, Cotroneo P, Collins BA, Johnson F, Jones P, Meier AM National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The preterm prediction study: maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. Am J Obstet Gynecol. 1996;175(5):1286–1292. doi: 10.1016/s0002-9378(96)70042-x. [DOI] [PubMed] [Google Scholar]
- 38.Nordentoft M, Lou HC, Hansen D, Nim J, Pryds O, Rubin P, Hemmingsen R. Intrauterine growth retardation and premature delivery: the influence of maternal smoking and psychosocial factors. Am J Public Health. 1996;86(3):347–354. doi: 10.2105/ajph.86.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huber E, Stephens JD. Development and Crisis of the Welfare State: Parties and Policies in Global Markets. Chicago, Illinois: University of Chicago Press; 2001. [Google Scholar]
- 40.Esping-Andersen G. Welfare States in Transition: National Adaptations in Global Economies. Thousand Oaks, California: Sage Publications; 1996. [Google Scholar]
- 41.Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, Rahman A. No health without mental health. Lancet. 2007;370(9590):859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- 42.Blackmore-Prince C, Kieke B, Jr, Kugaraj KA, Ferré C, Elam-Evans LD, Krule-witch CJ, Gaudino JA, Overpeck M. Racial differences in the patterns of singleton preterm delivery in the 1988 National Maternal and Infant Health Survey. Matern Child Health J. 1999;3(4):189–197. doi: 10.1023/a:1022373205005. [DOI] [PubMed] [Google Scholar]
- 43.Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):629–640. doi: 10.1001/archpsyc.62.6.629. [DOI] [PubMed] [Google Scholar]
- 44.Murray CJ, Kulkarni S, Ezzati M. Eight Americas: new perspectives on US health disparities. Am J Prev Med. 2005;29(5 suppl 1):4–10. doi: 10.1016/j.amepre.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 45.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 46.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF the QUORUM Group. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet. 1999;354(9193):1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 47.Lipsey MW, Wilson DB. Practical Meta-analysis. Thousand Oaks, California: Sage Publications; 2001. [Google Scholar]
- 48.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 49.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63(8):898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 50.Berle JO, Mykletun A, Daltveit AK, Rasmussen S, Holsten F, Dahl AA. Neonatal outcomes in offspring of women with anxiety and depression during pregnancy: a linkage study from The Nord-Trøndelag Health Study (HUNT) and Medical Birth Registry of Norway. Arch Womens Ment Health. 2005;8(3):181–189. doi: 10.1007/s00737-005-0090-z. [DOI] [PubMed] [Google Scholar]
- 51.Chung TK, Lau TK, Yip AS, Chiu HF, Lee DT. Antepartum depressive symptomatology is associated with adverse obstetric and neonatal outcomes. Psychosom Med. 2001;63(5):830–834. doi: 10.1097/00006842-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 52.Dayan J, Creveuil C, Herlicoviez M, Herbel C, Baranger E. Antenatal depression, a risk factor for prenatal delivery [in French] Presse Med. 1999;28 (31):1698. [PubMed] [Google Scholar]
- 53.Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH. Prenatal depression restricts fetal growth. Early Hum Dev. 2009;85(1):65–70. doi: 10.1016/j.earlhumdev.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. Am J Epidemiol. 2003;157(1):14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 55.Elsenbruch S, Benson S, Rücke M, Rose M, Dudenhausen J, Pincus-Knackstedt MK, Klapp BF, Arck PC. Social support during pregnancy: effects on maternal depressive symptoms, smoking and pregnancy outcome [published online ahead of print November 16, 2006] Hum Reprod. 2007;22(3):869–877. doi: 10.1093/humrep/del432. [DOI] [PubMed] [Google Scholar]
- 56.Evans J, Heron J, Patel RR, Wiles N. Depressive symptoms during pregnancy and low birth weight at term: longitudinal study. Br J Psychiatry. 2007;191:84–85. doi: 10.1192/bjp.bp.105.016568. [DOI] [PubMed] [Google Scholar]
- 57.Gavin AR, Holzman C, Siefert K, Tian Y. Maternal depressive symptoms, depression, and psychiatric medication use in relation to risk of preterm delivery. Womens Health Issues. 2009;19(5):325–334. doi: 10.1016/j.whi.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haas JS, Fuentes-Afflick E, Stewart AL, Jackson RA, Dean ML, Brawarsky P, Escobar GJ. Prepregnancy health status and the risk of preterm delivery. Arch Pediatr Adolesc Med. 2005;159(1):58–63. doi: 10.1001/archpedi.159.1.58. [DOI] [PubMed] [Google Scholar]
- 59.Hedegaard M, Henriksen TB, Sabroe S, Secher NJ. Psychological distress in pregnancy and preterm delivery. BMJ. 1993;307(6898):234–239. doi: 10.1136/bmj.307.6898.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoffman S, Hatch MC. Depressive symptomatology during pregnancy: evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol. 2000;19(6):535–543. [PubMed] [Google Scholar]
- 61.Jesse DE, Seaver W, Wallace DC. Maternal psychosocial risks predict preterm birth in a group of women from Appalachia. Midwifery. 2003;19(3):191–202. doi: 10.1016/s0266-6138(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 62.Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod. 2009;24(1):146–153. doi: 10.1093/humrep/den342. [DOI] [PubMed] [Google Scholar]
- 63.Paarlberg KM, Vingerhoets AJ, Passchier J, Dekker GA, Heinen AG, van Geijn HP. Psychosocial predictors of low birthweight: a prospective study. Br J Obstet Gynaecol. 1999;106(8):834–841. doi: 10.1111/j.1471-0528.1999.tb08406.x. [DOI] [PubMed] [Google Scholar]
- 64.Patel V, Prince M. Maternal psychological morbidity and low birth weight in India. Br J Psychiatry. 2006;188:284–285. doi: 10.1192/bjp.bp.105.012096. [DOI] [PubMed] [Google Scholar]
- 65.Perkin MR, Bland JM, Peacock JL, Anderson HR. The effect of anxiety and depression during pregnancy on obstetric complications. Br J Obstet Gynaecol. 1993;100(7):629–634. doi: 10.1111/j.1471-0528.1993.tb14228.x. [DOI] [PubMed] [Google Scholar]
- 66.Rondó PH, Ferreira RF, Nogueira F, Ribeiro MC, Lobert H, Artes R. Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. Eur J Clin Nutr. 2003;57(2):266–272. doi: 10.1038/sj.ejcn.1601526. [DOI] [PubMed] [Google Scholar]
- 67.Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry. 2007;164(8):1206–1213. doi: 10.1176/appi.ajp.2007.06071172. [DOI] [PubMed] [Google Scholar]
- 68.Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, Perel JM, Jones-Ivy S, Bodnar LM, Singer LT. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimmer-Gembeck MJ, Helfand M. Low birthweight in a public prenatal care program: behavioral and psychosocial risk factors and psychosocial intervention. Soc Sci Med. 1996;43(2):187–197. doi: 10.1016/0277-9536(95)00361-4. [DOI] [PubMed] [Google Scholar]
- 70.Goldenberg RL, Cliver SP, Cutter GR, Hoffman HJ, Copper RL, Gotlieb S, Davis RO. Maternal psychosocial characteristics and intrauterine growth retardation. Pre-Peri-Nat Psychol J. 1991;6(2):129–134. [Google Scholar]
- 71.Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, III, Hahn SR, Brody D, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care: the PRIME-MD 1000 study. JAMA. 1994;272(22):1749–1756. [PubMed] [Google Scholar]
- 72.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 73.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 74.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 75.Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EPDS) J Reprod Infant Psychol. 1990;8(2):99–107. [Google Scholar]
- 76.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: SCID-I—Clinician Version. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 77.Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychol Med. 1979;9(1):139–145. doi: 10.1017/s0033291700021644. [DOI] [PubMed] [Google Scholar]
- 78.Luteijn F, Hamel LF, Bouman TK, Kok AR. HSCL Hopkins Symptom Checklist (Manual) Lisse, the Netherlands: Swets and Zeitlinger; 1984. [Google Scholar]
- 79.World Health Organization, Division of Mental Health. SCAN: Schedules for Clinical Assessment in Neuropsychiatry. Geneva, Switzerland: World Health Organization; 1994. [Google Scholar]
- 80.Brenner WE, Edelman DA, Hendricks CH. A standard of fetal growth for the United States of America. Am J Obstet Gynecol. 1976;126(5):555–564. doi: 10.1016/0002-9378(76)90748-1. [DOI] [PubMed] [Google Scholar]
- 81.Roberton NRC. Textbook of Neonatology. New York, NY: Churchill Living-stone; 1986. [Google Scholar]
- 82.United Nations Development Programme. [Accessed September 1, 2007];Human Development Report 2006: Beyond Scarcity: Power, Poverty, and the Global Water Crisis: Table 15: Inequality in Income or Expenditure. http://hdr.undp.org/hdr2006/
- 83.Cliver SP, Goldenberg RL, Cutter GR, Hoffman HJ, Copper RL, Gotlieb SJ, Davis RO. The relationships among psychosocial profile, maternal size, and smoking in predicting fetal growth retardation. Obstet Gynecol. 1992;80(2):262–267. [PubMed] [Google Scholar]
- 84.Lundsberg LS, Bracken MB, Saftlas AF. Low-to-moderate gestational alcohol use and intrauterine growth retardation, low birthweight, and preterm delivery. Ann Epidemiol. 1997;7(7):498–508. doi: 10.1016/s1047-2797(97)00081-1. [DOI] [PubMed] [Google Scholar]
- 85.Wen SW, Goldenberg RL, Cutter GR, Hoffman HJ, Cliver SP. Intrauterine growth retardation and preterm delivery: prenatal risk factors in an indigent population. Am J Obstet Gynecol. 1990;162(1):213–218. doi: 10.1016/0002-9378(90)90853-y. [DOI] [PubMed] [Google Scholar]
- 86.Lieberman E, Ryan KJ, Monson RR, Schoenbaum SC. Risk factors accounting for racial differences in the rate of premature birth. N Engl J Med. 1987;317(12):743–748. doi: 10.1056/NEJM198709173171206. [DOI] [PubMed] [Google Scholar]
- 87.Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283(12):1591–1596. doi: 10.1001/jama.283.12.1591. [DOI] [PubMed] [Google Scholar]
- 88.Mercer BM, Goldenberg RL, Moawad AH, Meis PJ, Iams JD, Das AF, Caritis SN, Miodovnik M, Menard MK, Thurnau GR, Dombrowski MP, Roberts JM, McNellis D National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. Am J Obstet Gynecol. 1999;181(5 pt 1):1216–1221. doi: 10.1016/s0002-9378(99)70111-0. [DOI] [PubMed] [Google Scholar]
- 89.Esplin MS, O’Brien E, Fraser A, Kerber RA, Clark E, Simonsen SE, Holmgren C, Mineau GP, Varner MW. Estimating recurrence of spontaneous preterm delivery. Obstet Gynecol. 2008;112(3):516–523. doi: 10.1097/AOG.0b013e318184181a. [DOI] [PubMed] [Google Scholar]
- 90.Davis RL, Rubanowice D, McPhillips H, Raebel MA, Andrade SE, Smith D, Yood MU, Platt R. HMO Research Network Center for Education, Research in Therapeutics. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug Saf. 2007;16(10):1086–1094. doi: 10.1002/pds.1462. [DOI] [PubMed] [Google Scholar]
- 91.Källén B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med. 2004;158(4):312–316. doi: 10.1001/archpedi.158.4.312. [DOI] [PubMed] [Google Scholar]
- 92.Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry. 2002;159(12):2055–2061. doi: 10.1176/appi.ajp.159.12.2055. [DOI] [PubMed] [Google Scholar]
- 93.Wen SW, Yang Q, Garner P, Fraser W, Olatunbosun O, Nimrod C, Walker M. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol. 2006;194(4):961–966. doi: 10.1016/j.ajog.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 94.Zeskind PS, Stephens LE. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics. 2004;113(2):368–375. doi: 10.1542/peds.113.2.368. [DOI] [PubMed] [Google Scholar]
- 95.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York, New York: Russell Sage Foundation; 1994. pp. 231–244. [Google Scholar]
- 97.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296(13):1633–1644. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 98.Rosenthal R. Meta-Analytic Procedures for Social Research. Newbury Park, California: Sage Publications; 1991. [Google Scholar]
- 99.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 100.Greenhouse JB, Iyengar S. Sensitivity analyses and diagnostics. In: Cooper HM, Hedges LV, editors. The Handbook of Research Synthesis. New York, New York: Russell Sage Foundation; 1994. pp. 383–398. [Google Scholar]
- 101.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 102.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56 (2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 104.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320(7249):1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007;26(25):4544–4562. doi: 10.1002/sim.2889. [DOI] [PubMed] [Google Scholar]
- 106.Berkowitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiol Rev. 1993;15(2):414–443. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- 107.Harlow BL, Frigoletto FD, Cramer DW, Evans JK, LeFevre ML, Bain RP, McNellis D the RADIUS Study Group. Determinants of preterm delivery in low-risk pregnancies. J Clin Epidemiol. 1996;49(4):441–448. doi: 10.1016/0895-4356(95)00566-8. [DOI] [PubMed] [Google Scholar]
- 108.Belle D, Doucet J. Poverty, inequality, and discrimination as sources of depression among US women. Psychol Women Q. 2003;27:101–113. [Google Scholar]
- 109.Grote NK, Bledsoe SE, Wellman J, Brown C. Depression in African American and white women with low incomes: the role of chronic stress. Soc Work Public Health. 2007;23(2–3):59–88. doi: 10.1080/19371910802148511. [DOI] [PubMed] [Google Scholar]
- 110.Miranda J, Chung JY, Green BL, Krupnick J, Siddique J, Revicki DA, Belin T. Treating depression in predominantly low-income young minority women: a randomized controlled trial. JAMA. 2003;290(1):57–65. doi: 10.1001/jama.290.1.57. [DOI] [PubMed] [Google Scholar]
- 111.Grote NK, Zuckoff A, Swartz H, Bledsoe SE, Geibel S. Engaging women who are depressed and economically disadvantaged in mental health treatment. Soc Work. 2007;52(4):295–308. doi: 10.1093/sw/52.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kelly R, Zatzick D, Anders T. The detection and treatment of psychiatric disorders and substance use among pregnant women cared for in obstetrics. Am J Psychiatry. 2001;158(2):213–219. doi: 10.1176/appi.ajp.158.2.213. [DOI] [PubMed] [Google Scholar]
- 113.Borders AE, Grobman WA, Amsden LB, Holl JL. Chronic stress and low birth weight neonates in a low-income population of women. Obstet Gynecol. 2007;109(2 pt 1):331–338. doi: 10.1097/01.AOG.0000250535.97920.b5. [DOI] [PubMed] [Google Scholar]
- 114.Lundy BL, Jones NA, Field T, Nearing G, Davalos M, Pietro PA, et al. Prenatal depression effects on neonates. Infant Behav Dev. 1999;22(1):119–129. [Google Scholar]
- 115.Talge NM, Neal C, Glover V Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48(3–4):245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van Goozen SHM, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychol Bull. 2007;133 (1):149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- 117.Federenko IS, Wadhwa PD. Women’s mental health during pregnancy influences fetal and infant developmental and health outcomes. CNS Spectr. 2004;9(3):198–206. doi: 10.1017/s1092852900008993. [DOI] [PubMed] [Google Scholar]
- 118.Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosom Med. 1993;55(4):364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 119.Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160(5 pt 1):1107–1111. doi: 10.1016/0002-9378(89)90170-1. [DOI] [PubMed] [Google Scholar]
- 120.Stratton KR, Howe CJ, Battaglia FC. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 121.Knoches AM, Doyle LW. Long-term outcome of infants born preterm. Baillieres Clin Obstet Gynaecol. 1993;7(3):633–651. doi: 10.1016/s0950-3552(05)80452-3. [DOI] [PubMed] [Google Scholar]
- 122.Marlow ES, Hunt LP, Marlow N. Sensorineural hearing loss and prematurity. Arch Dis Child Fetal Neonatal Ed. 2000;82(2):F141–F144. doi: 10.1136/fn.82.2.F141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Majnemer A, Riley P, Shevell M, Birnbaum R, Greenstone H, Coates AL. Severe bronchopulmonary dysplasia increases risk for later neurological and motor sequelae in preterm survivors. Dev Med Child Neurol. 2000;42(1):53–60. doi: 10.1017/s001216220000013x. [DOI] [PubMed] [Google Scholar]
- 124.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 125.Roussounis SH, Hubley PA, Dear PR. Five-year-follow-up of very low birth-weight infants: neurological and psychological outcome. Child Care Health Dev. 1993;19(1):45–59. doi: 10.1111/j.1365-2214.1993.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 126.Antoniades L, MacGregor AJ, Andrew T, Spector TD. Association of birth weight with osteoporosis and osteoarthritis in adult twins. Rheumatology (Oxford) 2003;42(6):791–796. doi: 10.1093/rheumatology/keg227. [DOI] [PubMed] [Google Scholar]
- 127.Barker DJ, Lackland DT. Prenatal influences on stroke mortality in England and Wales. Stroke. 2003;34(7):1598–1602. doi: 10.1161/01.STR.0000077257.27430.7E. [DOI] [PubMed] [Google Scholar]
- 128.Bhargava SK, Sachdev HS, Fall CHD, Osmond C, Lakshmy R, Barker DJ, Bis-was SK, Ramji S, Prabhakaran D, Reddy KS. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350(9):865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kajantie E, Osmond C, Barker DJ, Forsén T, Phillips DI, Eriksson JG. Size at birth as a predictor of mortality in adulthood: a follow-up of 350 000 person-years [published online ahead of print March 11, 2005] Int J Epidemiol. 2005;34(3):655–663. doi: 10.1093/ije/dyi048. [DOI] [PubMed] [Google Scholar]
- 130.Paneth N. The impressionable fetus? fetal life and adult health. Am J Public Health. 1994;84(9):1372–1374. doi: 10.2105/ajph.84.9.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94(12):3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 132.Ekholm K, Carstensen J, Finnström O, Sydsjö G. The probability of giving birth among women who were born preterm or with impaired fetal growth: a Swedish population-based registry study. Am J Epidemiol. 2005;161(8):725–733. doi: 10.1093/aje/kwi096. [DOI] [PubMed] [Google Scholar]
- 133.Wang X, Zuckerman B, Coffman GA, Corwin MJ. Familial aggregation of low birth weight among whites and blacks in the United States. N Engl J Med. 1995;333(26):1744–1749. doi: 10.1056/NEJM199512283332606. [DOI] [PubMed] [Google Scholar]
- 134.Barker DJ. Early growth and cardiovascular disease. Arch Dis Child. 1999;80(4):305–307. doi: 10.1136/adc.80.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A the Vermont Oxford Network. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. Am J Obstet Gynecol. 2000;182(1 pt 1):198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- 136.Fattal-Valevski A, Leitner Y, Kutai M, Tal-Posener E, Tomer A, Lieberman D, Jaffa A, Many A, Harel S. Neurodevelopmental outcome in children with intrauterine growth retardation: a 3-year follow-up. J Child Neurol. 1999;14(11):724–727. doi: 10.1177/088307389901401107. [DOI] [PubMed] [Google Scholar]
- 137.Ferguson AC. Prolonged impairment of cellular immunity in children with intrauterine growth retardation. J Pediatr. 1978;93(1):52–56. doi: 10.1016/s0022-3476(78)80599-x. [DOI] [PubMed] [Google Scholar]
- 138.Kok JH, den Ouden AL, Verloove-Vanhorick SP, Brand R. Outcome of very pre-term small for gestational age infants: the first nine years of life. Br J Obstet Gynaecol. 1998;105(2):162–168. doi: 10.1111/j.1471-0528.1998.tb10046.x. [DOI] [PubMed] [Google Scholar]
- 139.Malloy MH. Size for gestational age at birth: impact on risk for sudden infant death and other causes of death, USA 2002 [published online ahead of print February 21, 2007] Arch Dis Child Fetal Neonatal Ed. 2007;92(6):F473–F478. doi: 10.1136/adc.2006.107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Committee on Understanding Premature Birth and Assuring Healthy Outcomes, Board on Health Sciences Policy. Societal costs of preterm birth. In: Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. chap 12. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 141.Cuevas KD, Silver DR, Brooten D, Youngblut JM, Bobo CM. The cost of prematurity: hospital charges at birth and frequency of rehospitalizations and acute care visits over the first year of life: a comparison by gestational age and birth weight. Am J Nurs. 2005;105(7):56–64. doi: 10.1097/00000446-200507000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Muraskas J, Parsi K. The cost of saving the tiniest lives: NICUs versus prevention. Virtual Mentor. 2008;10(10):655–658. doi: 10.1001/virtualmentor.2008.10.10.pfor1-0810. [DOI] [PubMed] [Google Scholar]
- 143.Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544.e1–544.e5. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 144.Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335(14):1010–1015. doi: 10.1056/NEJM199610033351402. [DOI] [PubMed] [Google Scholar]
- 145.Chun-Fai-Chan B, Koren G, Fayez I, Kalra S, Voyer-Lavigne S, Boshier A, Shakir S, Einarson A. Pregnancy outcome of women exposed to bupropion during pregnancy: a prospective comparative study. Am J Obstet Gynecol. 2005;192(3):932–936. doi: 10.1016/j.ajog.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 146.Einarson A, Fatoye B, Sarkar M, Lavigne SV, Brochu J, Chambers C, Mastroiacovo P, Addis A, Matsui D, Schuler L, Einarson TR, Koren G. Pregnancy outcome following gestational exposure to venlafaxine: a multicenter prospective controlled study. Am J Psychiatry. 2001;158(10):1728–1730. doi: 10.1176/appi.ajp.158.10.1728. [DOI] [PubMed] [Google Scholar]
- 147.Sivojelezova A, Shuhaiber S, Sarkissian L, Einarson A, Koren G. Citalopram use in pregnancy: prospective comparative evaluation of pregnancy and fetal outcome. Am J Obstet Gynecol. 2005;193(6):2004–2009. doi: 10.1016/j.ajog.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 148.Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79(4):301–308. doi: 10.1002/bdra.20327. [DOI] [PubMed] [Google Scholar]
- 149.Littleton HL, Breitkopf CR, Berenson AB. Correlates of anxiety symptoms during pregnancy and association with perinatal outcomes: a meta-analysis. Am J Obstet Gynecol. 2007;196(5):424–432. doi: 10.1016/j.ajog.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 150.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 151.Misra DP, Guyer B, Allston A. Integrated perinatal health framework: a multiple determinants model with a life span approach. Am J Prev Med. 2003;25 (1):65–75. doi: 10.1016/s0749-3797(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 152.Lu MC, Tache V, Alexander GR, Kotelchuck M, Halfon N. Preventing low birth weight: is prenatal care the answer? J Matern Fetal Neonatal Med. 2003;13(6):362–380. doi: 10.1080/jmf.13.6.362.380. [DOI] [PubMed] [Google Scholar]