Abstract
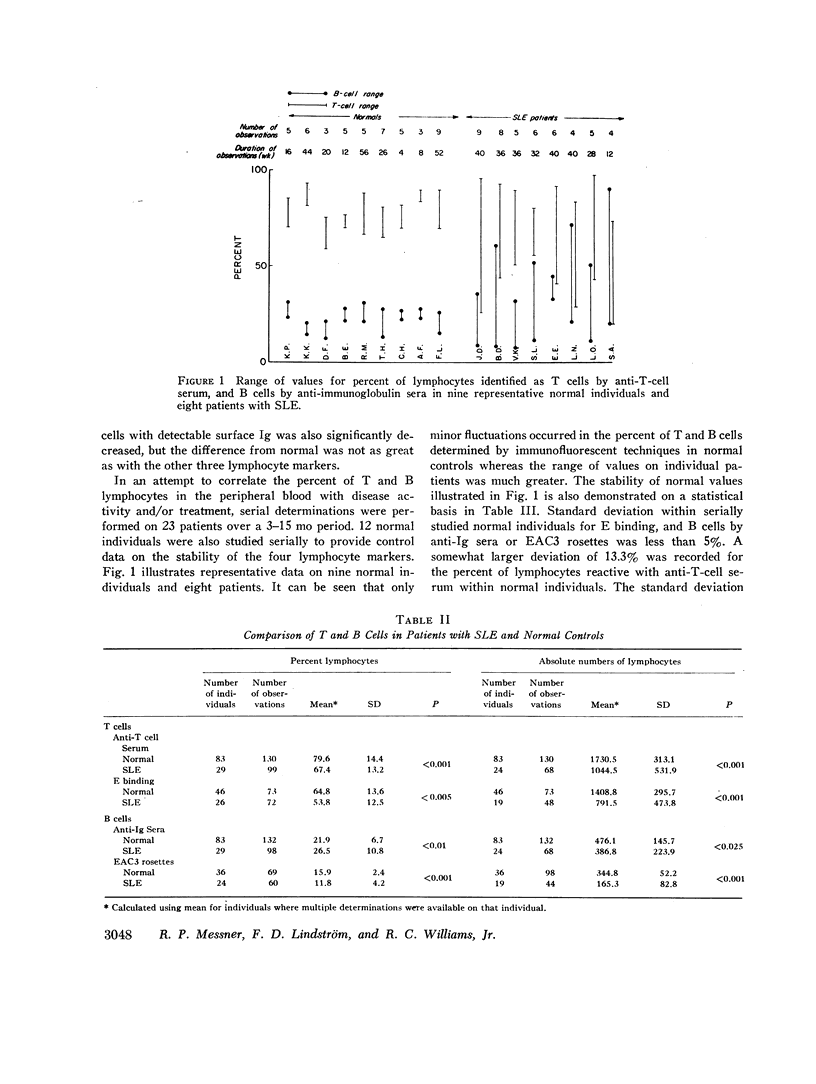
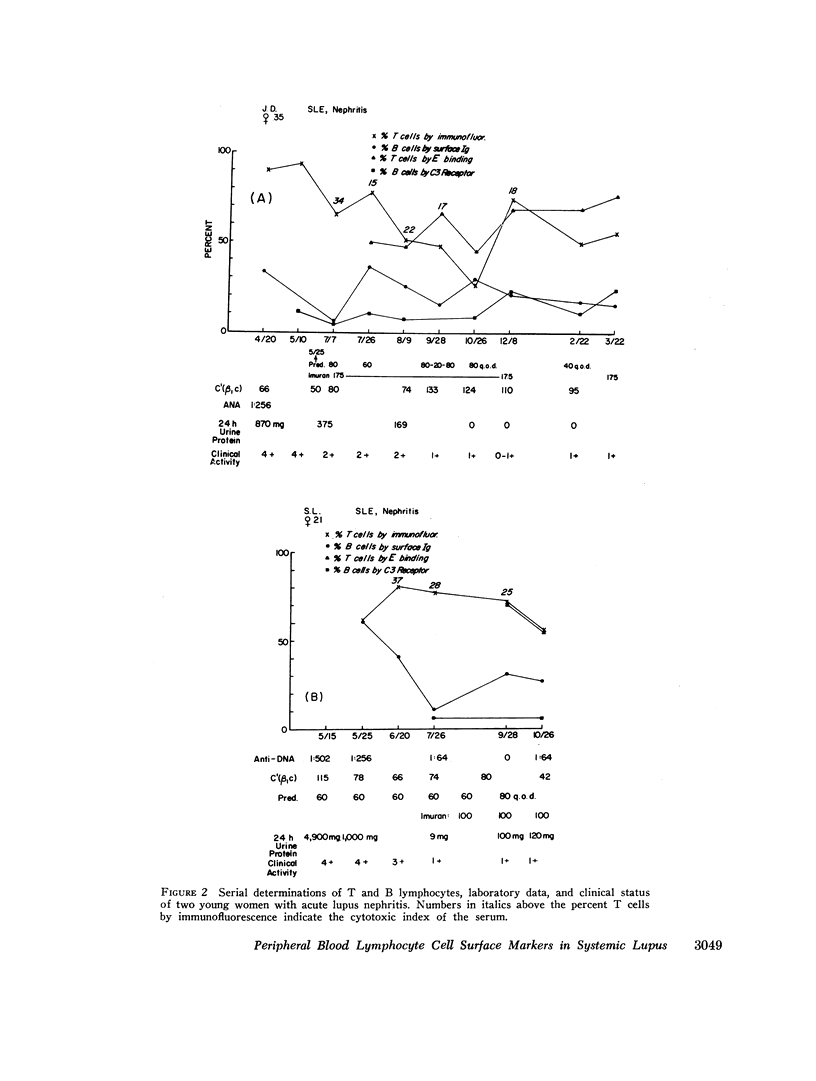
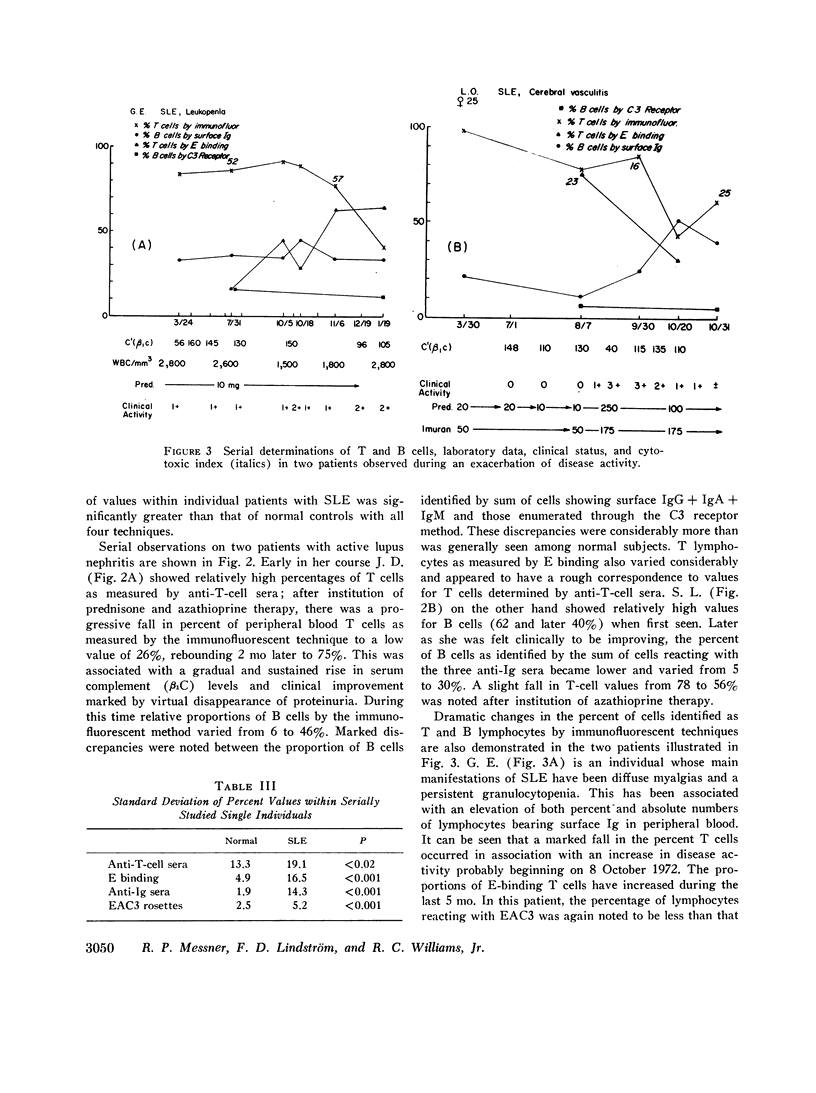
Peripheral blood lymphocytes from 23 patients with active systemic lupus erythematosus (SLE) were serially studied. Changes in bone marrow-derived lymphocytes (B cells), as measured by surface Ig receptors and C3 receptors, and in thymus-derived cells (T cells) measured by rabbit T-cell-specific antiserum and E-binding techniques, were correlated with fluctuations in clinical disease activity and treatment. In normal controls B- and T-cell percentages remained relatively stable, although the situation in SLE was much more labile. A relative and absolute decrease in T lymphocytes and cells bearing a receptor for C3 was found in active lupus. Absolute numbers of cells bearing surface Ig were decreased to a lesser extent, whereas the proportion of these cells was increased. It is postulated that the increase in autoantibody formation and diminished delayed hypersensitivity seen in systemic lupus may be due to a loss of T-lymphocyte function.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Zweiman B., Casella S. R. Effects of azathioprine therapy on bone marrow-dependent and thymus-dependent cells in man. Clin Exp Immunol. 1973 Jan;13(1):55–64. [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Dardenne M. Antigen recognition by T lymphocytes. II. Similar effects of azathioprine, antilymphocyte serum, and anti-theta serum on rosette-forming lymphocytes in normal and neonatally thymectomized mice. Cell Immunol. 1972 Jan;3(1):11–21. doi: 10.1016/0008-8749(72)90221-3. [DOI] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Warner N. L., Pye J. Specific inactivation of thymus-derived (T) and non-thymus-derived (B) lymphocytes by 125I-labelled antigen. Nat New Biol. 1971 May 26;231(21):104–106. doi: 10.1038/newbio231104a0. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. Specific in vitro cytotoxicity of thymus-derived lymphocytes sensitized to alloantigens. Nature. 1970 Dec 26;228(5278):1308–1309. doi: 10.1038/2281308a0. [DOI] [PubMed] [Google Scholar]
- Cohen A. S., Canoso J. J. Criteria for the classification of systemic lupus erythematosus--status 1972. Arthritis Rheum. 1972 Sep-Oct;15(5):540–543. doi: 10.1002/art.1780150512. [DOI] [PubMed] [Google Scholar]
- Crone M., Koch C., Simonsen M. The elusive T cell receptor. Transplant Rev. 1972;10:36–56. doi: 10.1111/j.1600-065x.1972.tb01538.x. [DOI] [PubMed] [Google Scholar]
- Fröland S. S. Binding of sheep erythrocytes to human lymphocytes. A probable marker of T lymphocytes. Scand J Immunol. 1972;1(3):269–280. doi: 10.1111/j.1365-3083.1972.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Fröland S., Natvig J. B., Berdal P. Surface-bound immunoglobulin as a marker of B lymphocytes in man. Nat New Biol. 1971 Dec 22;234(51):251–252. doi: 10.1038/newbio234251a0. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbeck R. J., Bardana E. J., Kohler P. F., Carr R. I. DNA:anti-DNA complexes: their detection in systemic lupus erythematosus sera. J Clin Invest. 1973 Apr;52(4):789–795. doi: 10.1172/JCI107242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. A. Impaired delayed hypersensitivity in systemic lupus erythematosus. Arthritis Rheum. 1972 Jul-Aug;15(4):353–359. doi: 10.1002/art.1780150406. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler D., Agnello V., Thoburn R., Kunkel H. G. Systemic lupus erythematosus: prototype of immune complex nephritis in man. J Exp Med. 1971 Sep 1;134(3 Pt 2):169s–179s. [PubMed] [Google Scholar]
- Lies R. B., Messner R. P., Williams R. C., Jr Relative T-cell specificity of lymphocytotoxins from patients with systemic lupus erythematosus. Arthritis Rheum. 1973 May-Jun;16(3):369–375. doi: 10.1002/art.1780160312. [DOI] [PubMed] [Google Scholar]
- Mellbye O. J., Messner R. P., DeBord J. R., Williams R. C., Jr Immunoglobulin and receptors for C3 on lymphocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1972 Jul-Aug;15(4):371–380. doi: 10.1002/art.1780150408. [DOI] [PubMed] [Google Scholar]
- Mittal K. K., Rossen R. D., Sharp J. T., Lidsky M. D., Butler W. T. Lymphocyte cytotoxic antibodies in systemic lupus erythematosus. Nature. 1970 Mar 28;225(5239):1255–1256. doi: 10.1038/2251255a0. [DOI] [PubMed] [Google Scholar]
- Papamichail M., Brown J. C., Holborow E. J. Immunoglobulins on the surface of human lymphocytes. Lancet. 1971 Oct 16;2(7729):850–852. doi: 10.1016/s0140-6736(71)90224-8. [DOI] [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Rabellino E. M., Polley M. J., Grey H. M. Combined studies of complement receptor and surface immunoglobulin-bearing cells and sheep erythrocyte rosette-forming cells in normal and leukemic human lymphocytes. J Clin Invest. 1973 Feb;52(2):377–385. doi: 10.1172/JCI107194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Mellors R. C. Natural thymocytotoxic autoantibody and reactive antigen in New Zealand black and other mice. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1412–1415. doi: 10.1073/pnas.68.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Yoshiki T., Mellors R. C. Effects of natural thymocytotoxic autoantibody of NZB mice and of specifically prepared antilymphocyte serum on the tissue distribution of 51 Cr-labeled lymphocytes. J Immunol. 1973 Feb;110(2):517–523. [PubMed] [Google Scholar]
- Shirai T., Yoshiki T., Mellors R. C. Thymus dependence of cells in peripheral lymphoid tissues and in the circulation sensitive to natural thymocytotoxic autoantibody in NZB mice. J Immunol. 1972 Jul;109(1):32–37. [PubMed] [Google Scholar]
- Stobo J. D., Talal N., Paul W. E. Lymphocyte classes in New Zealand mice. II. Decreased frequency of immunoglobulin-bearing lymphocytes and increased frequency of lymphocytes lacking detectable theta or immunoglobulin determinants. J Immunol. 1972 Oct;109(4):701–710. [PubMed] [Google Scholar]
- Stutman O. Lymphocyte subpopulations in NZB mice: deficit of thymus-dependent lymphocytes. J Immunol. 1972 Sep;109(3):602–611. [PubMed] [Google Scholar]
- TERASAKI P. I., MCCLELLAND J. D. MICRODROPLET ASSAY OF HUMAN SERUM CYTOTOXINS. Nature. 1964 Dec 5;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- Terasaki P. I., Mottironi V. D., Barnett E. V. Cytotoxins in disease. Autocytotoxins in lupus. N Engl J Med. 1970 Oct 1;283(14):724–728. doi: 10.1056/NEJM197010012831403. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, DeBoard J. R., Mellbye O. J., Messner R. P., Lindström F. D. Studies of T- and B-lymphocytes in patients with connective tissue diseases. J Clin Invest. 1973 Feb;52(2):283–295. doi: 10.1172/JCI107184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Emmons J. D., Yunis E. J. Studies of human sera with cytotoxic activity. J Clin Invest. 1971 Jul;50(7):1514–1524. doi: 10.1172/JCI106637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Nossal G. J. Identification of human T and B lymphocytes in normal peripheral blood and in chronic lymphocytic leukaemia. Lancet. 1971 Oct 9;2(7728):788–791. doi: 10.1016/s0140-6736(71)92741-3. [DOI] [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. The human rosette-forming cell as a marker of a population of thymus-derived cells. J Clin Invest. 1972 Oct;51(10):2537–2543. doi: 10.1172/JCI107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz M. M., Mellors R. C., Lance E. M. Changes in lymphoid populations of ageing CBA and NZB mice. Clin Exp Immunol. 1971 Mar;8(3):491–500. [PMC free article] [PubMed] [Google Scholar]



