Abstract
Toll-like receptors (TLR) form the major family of pattern recognition receptors (PRR) that are involved in innate immunity. Innate immune responses against microorganisms at the maternal–fetal interface may have a significant impact on the success of pregnancy, as intrauterine infections have been shown to be strongly associated with certain disorders of pregnancy. At the maternal–fetal interface, TLRs are expressed not only in the immune cells but also in non-immune cells such as trophoblasts and decidual cells; moreover, their expression patterns vary according to the stage of pregnancy. Here, we will describe potential functions of TLRs in these cells, their recognition and response to microorganisms, and their involvement in the innate immunity. The impact of TLR-mediated innate immune response will be discussed via animal model studies, as well as clinical observations.
Keywords: Fetus, pre-eclampsia, pregnancy, preterm labor, TLR, trophoblast
Introduction
The maternal–fetal interface is an immunologically unique site that must promote tolerance to the allogeneic fetus, while maintaining host defense against possible pathogens. Clinical studies have shown a strong association between intrauterine bacterial or viral infections and pregnancy disorders such as abortion, preterm labor, intrauterine growth retardation (IUGR) and pre-eclampsia.1–3 Therefore, immediate immune responses against microorganisms at the maternal–fetal interface may have a significant impact on the success of pregnancy.
The innate immune system is the immunological first line of defense that provides an immediate response against invading pathogens through its ability to distinguish between ‘infectious non-self’ and ‘non-infectious self’.4 Furthermore, activation of innate immunity is a critical step to the development of antigen-specific acquired immunity. Therefore, innate immunity at the maternal–fetal interface has fundamental significance for establishing an adequate microenvironment during pregnancy, elimination of ‘infectious non-self’ (bacteria, virus, etc.) and tolerance to ‘non-infectious self’ (mother, placenta and fetus). Indeed, there is growing evidence that the innate immune system is activated in the maternal–fetal interface. For instance, innate immune cells such as natural killer (NK) cells, macrophages and dendritic cells are known to infiltrate the decidua and accumulate around the invading trophoblasts.5–8 In addition to a population increase, these immune cells acquire an activated phenotype during pregnancy.7,9
Cells of the innate immune system express a series of receptors known as pattern recognition receptors (PRRs) which recognize and bind to sequences know as pathogen-associated molecular patterns (PAMPs), which are unique to, and expressed on, the surface of microorganisms. In addition, non-immune cells such as epithelial cells also express PRRs that allow these cells to respond to PAMPs. The ligation of PRRs by PAMPs results in an inflammatory response generated against the invading pathogen.9 There are a number of different PRRs including the mannosebinding receptor and the scavenger receptor;10 however, this review will focus on the major family of PRRs, the Toll-like receptors (TLRs). We will discuss the expression and function of TLRs at the maternal–fetal interface and their roles in the interaction between the trophoblast and the maternal immune system.
Toll-like Receptors
Receptors and Their Ligands
Toll-like receptors (TLR) are transmembrane proteins with extracellular domains of leucine-rich repeat motifs, which are evolutionarily conserved to recognize PAMPs in bacteria, viruses, fungi and parasites. Eleven mammalian TLRs have been identified to date (TLR1 to TLR11);11,12 however, no functional TLR11 proteins have been documented in humans.13,14
Each receptor differs in its specificity (Table I). TLR4 is crucial for effective host cell responses to gram-negative bacterial lipopolysaccharide (LPS).15 TLR2 has the widest specificity, recognizing bacterial lipoproteins, gram-positive bacterial peptidoglycan (PDG), lipoteichoic acid (LTA) and fungal zymosan. 16–18 The range of ligands to which TLR2 responds appears to be broadened by its heterodimerization with other TLRs, so that TLR1/2 heterodimers respond to a panel of lipoproteins different from those recognized by TLR2/6.19,20
Table I.
Toll-like receptors (TLRs) and ligands
| Ligands |
||
|---|---|---|
| TLR | Exogenous | Endogenous |
| TLR1 | Triacetylated lipoproteins (witn TLR2) | |
| TLR2 | Peptidoglycan, Lipopeptides, Lipoteichoic acids, Zymosan | |
| TLR3 | Double-stranded RNA | Host RNA |
| TLR4 | Lipopolysaccharides, paclitaxel | Hsp60, Hsp70, Hsp90, ROS, HMGB1,Surfactant protein A, Fibrinogen, Fibronectin, Hyaluronic acid oligosaccharides, Eosinophil derived neurotoxin |
| TLR5 | Flagellin | |
| TLR6 | Diacylated lipoprotein (with TLR2) | |
| TLR7 | Single-stranded RNA | |
| TLR8 | Single-stranded RNA | |
| TLR9 | Non-methylated CpG DNA, Herpes virus | Autoimmune chromatine-IgG complex |
| TLR10 | – | |
TLRs 3, 7 and 8 appear to play important roles in response to viruses. TLR3 is known to bind viral double-stranded RNA,21 while TLRs 7 and 8 interact with single-stranded RNA.22,23 TLR9 mediates cell responses to bacterial DNA through recognition of cytosine–guanine pairs (‘CpG’ motifs)24 and can also be activated by Herpes virus.23,25
In addition to detecting pathogen-derived ligands, TLRs interact with the hosts’ other endogenous molecules, typically in response to danger. This so-called danger-associated molecular pattern consists of molecules such as reactive oxygen species (ROS),26 proteins released from dead or dying cells such as high-mobility group box protein 1 (HMGB1),27 surfactant protein A,28 fibrinogen,29 breakdown products of extracellular matrix such as fragments of fibronectin,30 hyaluronic acid oligosaccharides31 and eosinophil-derived neurotoxin (EDN).32 Heat-shock proteins (Hsp) such as Hsp60, Hsp70, Hs90 have also been reported to act on TLRs, although much controversy exists in defining the true nature of the interaction.33
TLR Signaling
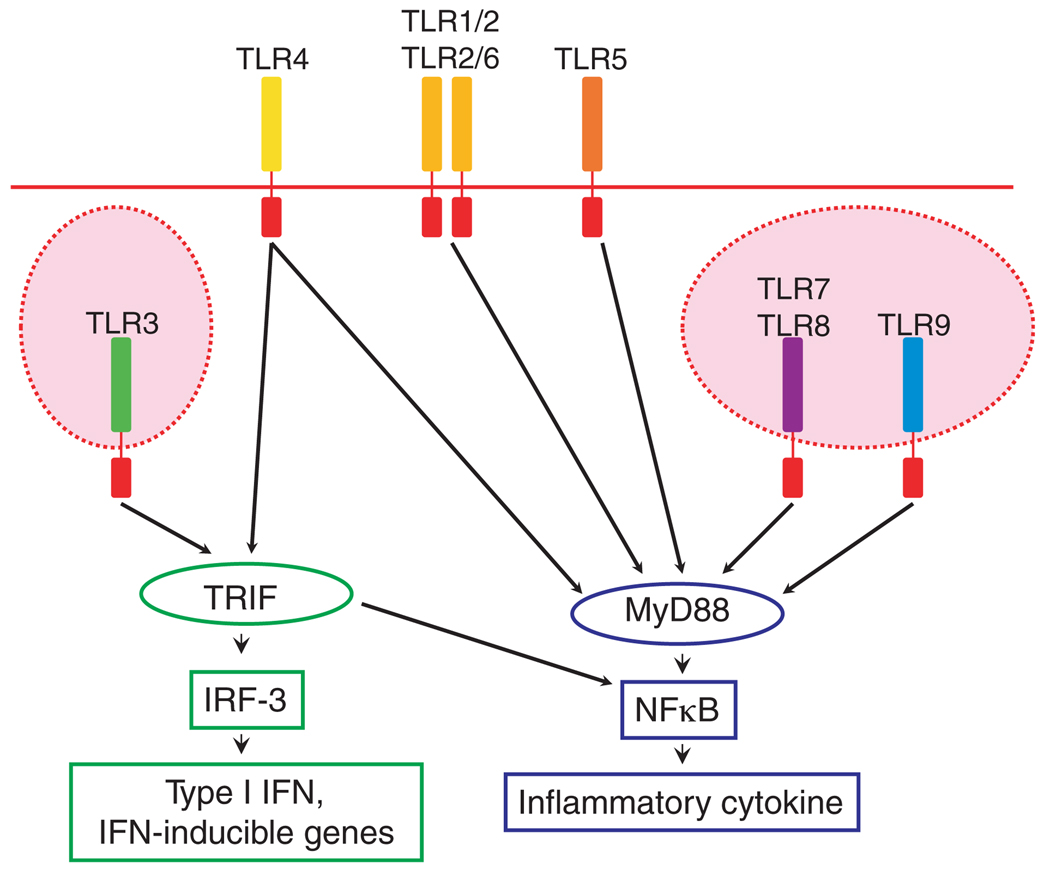
Binding of TLRs often results in the production of cytokines and anti-microbial factors via a common intracellular signaling pathway (Fig. 1). Upon ligand recognition, the TLRs recruit the intracellular signaling adapter protein, myeloid differentiation factor 88 (MyD88), leading to a subsequent kinase cascade, which triggers the activation of NFκB pathway, with resultant generation of an inflammatory response.34
Fig. 1.
Toll-like receptor (TLR) signals. Membranal TLRs; TLR1, 2, 4, 5, 6, can recognize external signals, while cytoplasmic TLRs; TLR3, 7, 8, 9 will recognize intracellular signals. Following ligation, the majority of TLRs induce activation of NFκ B and cytokine production in MyD88-dependent manner. TLR4, like TLR3, can also signal in a MyD88-independent manner, which induces the expression of type I interferons (IFN) and IFN-inducible proteins. IFN, TRIF (Toll/IL-1 receptor domain-containing adaptor inducing IFN-β), IRF3 (IFN regulatory factor).
TLR3 and TLR4 can also signal in a MyD88-independent manner.35 This signaling occurs through an adapter protein Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF), which not only activates the NFκB pathway, but also results in the phosphorylation of IFN regulatory factor-3 (IRF-3). This alternative pathway generates an anti-viral response associated with the production of type I IFNs and IFN-inducible genes.24
Toll-like Receptor Expression at the Maternal–Fetal Interface
Placental Tissue
Expression of all 10 TLRs, as well as various co-receptors and accessory proteins such as CD14, has been described in the human placenta.36,37 Using RT-PCR, Mitsunari et al.37 demonstrated that in cultured cells isolated from term placenta, both cytotrophoblast and syncytiotrophoblast-rich cells express TLR2, 3, 4, 5, 6 and 9. Klaffenbach et al.36 showed that the choriocarcinoma cell lines, JAR and BeWo, express TLR1-10, as well as their co-receptors and accessory proteins: CD14, MyD88, MD-2, TIRP, TRAP and TRIF at the mRNA level. We have previously shown that first-trimester primary trophoblasts as well as trophoblast cell lines, Swan 71, 3A and HTR8, express TLR1, 2, 3 and 4 but not TLR6.38,39 These findings suggest potential roles for TLRs signaling in the placenta during pregnancy.
The expression of TLRs in the placenta is not constant, but seems to be regulated in a temporal and spatial manner. For example, TLR6 is not expressed by first-trimester trophoblasts,39 while it is expressed by third-trimester trophoblasts.37 This suggests TLR6 expression is regulated in a temporal manner. Beijar et al.40 compared term and first-trimester placental TLR4 expression and found that the term placenta expresses higher levels of TLR4 compared to the first trimester. This data suggests that the placenta in early pregnancy may be less responsive to pathogen stimuli compared to term tissue, although the mechanisms that control temporal TLR regulation still need to be elucidated. TLRs also seem to be regulated in a spatial manner. We observed that TLR2 and TLR4 are expressed by villous cytotrophoblast and extravillous trophoblast but not by syncytiotrophoblasts in the first-trimester placenta.39 We speculated that the lack of TLR expression by syncytiotrophoblast, that is, the outer trophoblast layer, allows placental tissue to respond only to a microbe that has broken thorough this outer layer. Thus, a microorganism will only pose a threat to the fetus, if the TLR-negative syncytiotrophoblast layer is breached and the pathogen has entered either the placental villous or the decidual compartments.38,39
TLR expression has also been reported in other types of cells in the placenta. Hofbauer cells, a type of macrophage in the placental villi, were shown to express TLR4 in the term placenta by immunohistochemistry.41 Most recently, Ma et al. evaluated the expression of TLR2 and TLR4 in third-trimester placentas by immunohistochemistry.42 They observed stronger expression of TLR2 in endothelial cells and macrophages and weaker expression in syncytiotrophoblast and fibroblast, while staining for TLR4 was most prominent in syncytiotrophoblast and fibroblast. These findings suggests that not only immune cells but also trophoblasts and other types of cells within the placenta have a capacity to respond to the invading pathogens and may be involved, similar as the innate immune system, in the physiological protection of the placenta.
Decidua and Amnion
In contrast to placental tissue, very little is known about the expression of TLRs in the decidua. Recently, two studies described the expression of TLRs in the human decidua. Krikun and coworkers reported the presence of mRNA for all 10 TLRs in first trimester and term decidua. They also demonstrated the protein expression for TLR2 and 4 in first-trimester decidual cells. 1 These results were also confirmed by Canavan and Simhan43, using immunocytochemistry, described the expression of TLR1-6 in primary cultures of decidual cells isolated from third-trimester pregnancies.
As for amnion, one study showed that TLR4 is expressed at the apical side of amniotic epithelium, indicating that TLR4 is poised to monitor amniotic fluid for pathogens.42 Dulay et al.44 demonstrated the presence of soluble TLR2 in amniotic fluid, which may interfere the recognition of TLR2 ligands by TLR2. These results suggest that TLR system plays a role in regulating intra-amniotic inflammatory response to microbial pathogens.
Toll-like Receptor Signaling and Function at the Maternal–Fetal interface
Given that TLRs are widely expressed at the maternal–fetal interface, not only by immune cells but also by non-immune cells such as trophoblasts, decidual cells and amniotic epithelium, the next question is what is the role of TLRs in these cells and their influence in regulating local and systemic immune responses during pregnancy. Here, we will discuss possible functions of TLRs at the maternal–fetal interface.
Differential Responses to TLR4 and TLR2: Ligation in Trophoblast Cells
TLR2 and TLR4 function at the maternal–fetal interface is well described because they are the principal receptors for recognition of bacterial cell wall components. Holmlund et al. firstly reported TLR function in placenta. They showed that stimulation of TLR2 and TLR4 with zymosan and LPS-induced IL-6 and IL-8 production by third-trimester placental cultures, which indicated that trophoblast have a capacity to recognize microorganisms and initiate immune responses by activating immune cells.45
Our studies have shown that TLR-expressing first-trimester trophoblasts elicit very distinct patterns of responses, depending upon the specific TLR that is activated. For example, ligation of TLR4 with LPS in first-trimester trophoblasts produces a slow inflammatory response, characterized by a modest up-regulation of cytokines.39 In contrast, PDG, which signals through TLR2, induces apoptosis in trophoblasts rather than stimulating a cytokine response.39
The pattern of response following TLR ligation also depends on the type of stimuli. While LPS did not induce apoptosis in first-trimester trophobalsts,39 Chlamydia heat shock protein 60 was shown to induce apoptosis in trophoblasts through TLR4.46 This differential effect of different TLR4 ligands may be explained by the diverse downstream signaling events and differential use of adapter molecules by different TLR4 ligands.
This differential response of the same receptor ligation was also observed in TLR2. Induction of apoptosis through TLR2 ligation was demonstrated in first-trimester trophoblasts not only by PDG39 but also by ultraviolet-inactivated human cytomegalovirus (HCMV).47 On the other hand, using third-trimester trophoblasts, Mitsunari et al.37 reported that macrophage-activating lipopeptide-2 (MALP-2) purified from Mycoplasma fementans, signaled TLR2 and induced the expression of cyclooxygenase (COX)-2 and prostaglandin E2. This differential effect between first- and third-trimester trophoblasts may be attributable to the presence of TLR6 in third-trimester trophoblast. As we described, the response following TLR2 stimulation appears to be dependent upon the cooperative receptors, TLR1 and TLR6. Indeed, our in vitro studies suggest that the pro-apoptotic effect observed following PDG treatment is mediated by TLR1 and TLR2 heterodimers, which then activate caspase-8, -9 and -3 through MyD88/FADD pathway, whereas the presence of TLR-6 may shift the type of response; cell death is prevented and a cytokine response ensues through NFκB activation.48
We have also shown that TLR4 ligation by LPS inhibited the migration of trophoblast cells.49 This effect may explain the incomplete invasion of the trophoblast to the spiral arteries in the uterus observed in patients with pre-eclampsia.
TLR Signaling Induces Anti-Viral Response: The Role of TLR3
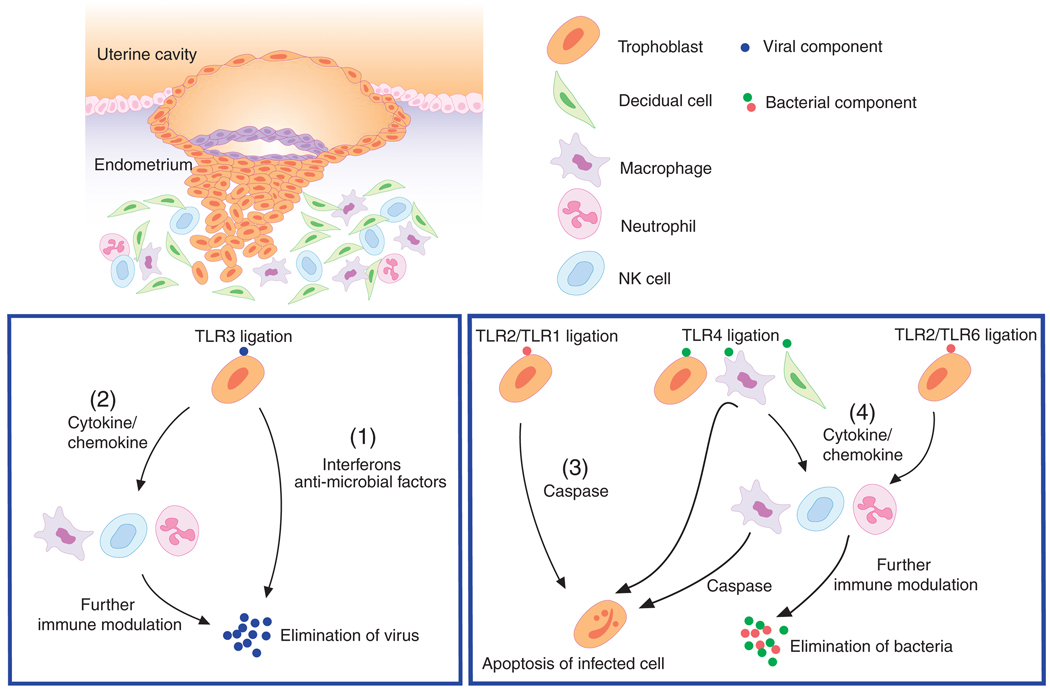
The placenta may become exposed not only to bacteria but also to virus, which may pose a substantial threat to the fetus. The trophoblast has unique characteristics for responding to viral infections. TLR3, a receptor known to mediate immune responses toward viral dsRNA,21 is expressed by first-trimester trophoblasts.38 As a result of poly(I:C) (a synthetic dsRNA) stimulation, trophoblasts secrete pro-inflammatory cytokines as well as anti-microbial products. Using first-trimester trophoblast, we described the production of interferon-β (IFN-β) following poly(I:C) treatment.47 The production of IFN-β is critical to mount an anti-viral response; therefore suggesting that the trophoblast upon recognition of a virus may initiate a classical anti-viral reaction. In addition to interferon, the trophoblast has an ability to produce anti-microbial factors such as secretory leukocyte protease inhibitor (SLPI), 2′, 5′-oligoadenylate synthetase (OAS), Myxovirus resistance A (MxA) and apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G(APOBEC3G). These all have a direct effect on viral activity.47 These findings suggested that the placenta and especially trophoblasts function as an active barrier preventing the transmission of certain viral infections to the fetus.38,50 In summary, all these studies suggest that trophoblasts are able to recognize bacterial or viral products through TLRs and induce differential responses (Fig. 2). The factor(s) associated with the type of response may determine the final outcome and be associated with pregnancy disorders such as preterm labor, pre-eclampsia or IUGR.
Fig. 2.
Trophoblasts recognize viral component via TLR3 and respond by producing interferons and anti-microbial factors to control the viral attack (1). In addition, trophoblast produces cytokines and chemokines that will have a modulatory effect on the maternal immune system (2). Following bacterial infection, TLR2/TLR1 ligation in the trophoblast causes apoptosis, (3) while TLR2/TLR6 ligation or TLR4 ligation promotes cytokine production by trophoblasts (4). The inflammatory response initiated by trophoblast activates macrophages, NK cells and neutrophils for further immune modulation. (Modified from Koga and Mor 2008 Reproductive Sciences)
TLR Signaling Modulate Immune Cell Function
Recently, we proposed that trophoblast cells are potentially able to modulate the immune system at the maternal–fetal interface, by regulating various immune cell functions.38 Our earlier studies demonstrated that first-trimester trophoblasts constitutively secrete cytokines/chemokines such as, GRO-α, MCP-1 and IL8; and that these trophoblasts are also able to recruit monocytes/macrophages, NK cells and neutrophils. 38,51 This cytokine/chemokine expression in trophoblasts is further enhanced upon ligation by TLR4 or TLR3 agonists, followed by a significant increase in the recruitment of immune cells.38 Moreover, the factors produced by trophoblasts have a potent modulatory effect on the maternal immune cells by determining their differentiation and state of activation. For example, monocytes/macrophages incubated in the presence of trophoblasts or their condition media become less sensitive to LPS stimulation.51
Based on these observations, we propose that the trophoblast is able to ‘educate’ immune cells, where signals originated from trophoblast could determine the subsequent immune cell behavior. This proper trophoblast–immune cell cross-talk may be essential for a normal pregnancy, and changes or defects in this interaction may lead to pregnancy complications.
Toll-like Receptor in Pregnancy Complications
The importance of TLR’s role in various pregnancy disorders, such as abortion, preterm labor, pre-eclampsia, and even fetal disorder has been demonstrated by either animal models or clinical observations. First, we will review studies of animal models followed by clinical studies.
TLR Animal Models for Pregnancy Complications
Preterm labor
TLR-2 and TLR-4 response and preterm labor
It has been established that gram-negative bacteria trigger preterm labor in various animal models, and many attempts have been made to clarify this mechanism. Wang and Hirsch reported that TLR4 is essential for normal susceptibility to preterm delivery induced by gram-negative bacteria using TLR4 mutant mice model. They injected heat-killed Escherichia coli (HKE) into the uterus of pregnant mice at gestational day 14 and showed that HKE-induced preterm delivery in 100% of TLR4 normal mice but in 0% of C3H/HeJ mice, which have a spontaneous mutation in TLR4.52 Similar results were obtained independently by another group using LPS injection model.53
To elucidate the mechanism by which TLR4 signaling induced preterm delivery, Wang and Hirsch, using the same mice model, examined the prostaglandin pathway in the injected uterus. They showed that ligation of TLR4 with LPS down-regulates the expression of 15-hydroxyprostaglandin dehydrogenase, a prostaglandin-catabolizing enzyme, in fetal and maternal tissue. The authors hypothesized that TLR4 mediates bacterially induced preterm labor via downregulation of prostaglandin degradation.52
LPS administration is also shown to change the cytokine profile by increasing maternal serum concentration of TNF-α and IL6, as well as placental expression of TNF-α, IL6 and IL1-α.54 Besides the cytokine profile, LPS treatment markedly changed the profile of immune cells; up-regulated the percentages of blood CD45(+)CD86(+), CD3(+) CD69(+), CD49b(+)CD69(+) cells, and placenta CD45(+)CD86(+), CD45(+)CD49b(+), CD49b(+)CD69(+) cells.55 These observations may imply that systemic and local inflammatory responses followed by LPS administration cause preterm labor.
Gram-positive bacterial components have been associated with preterm labor as well. For example, in rodents, LTA was shown to induce preterm delivery following cervical ripening and placental abruption. 56 These effects on pregnancy seems to be TLR mediated as shown by a recent study where either PDG or LTA, both TLR2 ligands, induced preterm delivery in mice when injected intra uterus.57 In terms of the mechanism, contrary to the effects of TLR4 ligation, TLR2 ligation does not seem to induce inflammatory responses. The expression of TNF-α and IL1-β was examined in uterine tissues, but no up-regulation was found in PDG-treated mice.57 We also recently established a novel mouse model, injected PDG intraperitoneally on gestational day 6 and observed uterine cytokine production, NK cells activation and apoptosis on day 12. In this model, no change in cytokine production or NK cell activation was found in PDG-treated uterus,48 in contrast to the findings in LPS-treated mice where cytokine up-regulation and NK cell activation were observed.58 On the other hand, a significant increase in apoptotic trophoblasts were observed in PDG-treated mice,48 which is consistent with the in vitro studies showing that PDG treatment to trophoblasts induced TLR2-mediated apoptosis.39 These results suggested that the mechanism underlying preterm labor triggered by PDG is not the result of an inflammatory reaction but apoptosis of the trophoblast.
TLR3 response and preterm labor
Administration of poly(I:C) which is a synthetic dsRNA mimicking viral RNA during late pregnancy also has detrimental effects on pregnancy as shown by a study using intrauterine injection model. When administrated on gestational day 15.5, poly(I:C) induced preterm labor in 31% of the animals along with the induction of interferon-β and RANTES expression in uterine tissues.57
Our animal study further demonstrated that intraperitoneal administration of poly(I:C) induced cytokine/chemokine production in the placenta, and as a consequence, immune cells such as macrophage and NK cells were attracted toward the placenta.59 These results are consistent with our previous in vitro results that the placenta, and more specifically the trophoblast, plays an active role on the response to poly(I:C).47 We further demonstrated that these responses are mediated by TLR3 in trophobalsts, since poly(I:C) effects are not observed in TLR3 KO mice.59
Antagonizing TLRs as a therapeutic strategy for preterm labor
Given that bacterial and viral infections induce preterm labor by provoking inflammatory response through TLRs, an idea came up that the TLRs system could be a target for therapeutic strategy for preterm labor. Administration of fusobacterium nucleatum, a gram-negative anaerobe, is known to induce preterm birth and fetal death in mice. Using this model, Liu et al. demonstrated that TLR4 antagonist reduced the fetal death and decidual necrosis. Interestingly, TLR4 antagonist did not affect the bacterial colonization in the placentas, indicating that antagonizing TLRs has no bactericidal activity but control inflammatory response.42 Adams Waldorf et al.60 further showed with their rhesus monkey model that the administration of TLR4 antagonist together with antibiotics was able to inhibit the LPS-induced preterm labor.
Early pregnancy loss
TLR stimulation is also known to induce fetal resorption when it occurs in early pregnancy. Administration of Poly(I:C) induces fetal loss when injected during early pregnancy in various mating pairs such as ‘resorption-prone’ mating (male DBA/2J with female CBA/J),61 syngeneic mating (male BALB/c with female BALB/c) and allogeneic mating (male BALB/c with female C57BL/6).62 Li et al. demonstrated that poly(I:C) induces resorption in pregnant mice through TLR3, because injection of a neutralizing antibody for TLR3 abrogated the effects of poly(I:C).62 In addition, they demonstrated that ligation of TLR3 with poly(I:C) on gestational day 7 induced IL2 and inhibited IL10 expression in CD45+ cells isolated from the placenta.62 The same authors further demonstrated that poly(I:C) injection in early pregnancy induced uNK cells activation and speculated that this is the cause of poly(I:C)-induced embryo resorption.62 Zhang and coworkers63 showed that poly(I:C) treatment impaired uterine vascular remodeling through endometrial TNF-α up-regulation and suggested that this induced fetal loss.
Pre-eclampsia
In 1994, Faas et al.64 developed an animal model for pre-eclampsia by injecting ultra-low dose of LPS into pregnant rat on day 14 of gestation, although at that time, the role of TLR4 was completely unknown. Recently, Tinsley et al.65 tested the effect of TLR3 activation on the development of pre-eclampsia-like symptoms in rats. They inject poly(I:C) intra peritoneal from days 10, 12, 14, 16, 18 of gestation and found that the treated rats showed pre-eclampsia-like symptom such as proteinuria and hypertension. These findings suggest that the activation of TLRs in pregnancy causes not only preterm labor and pregnancy loss, but also pre-eclampsia.
Fetal development
The fetus is not indifferent to a viral or bacterial infection, and the immunological responses by the maternal immune system or the placenta or fetal immune system may have important consequences on the normal development and survival of the fetus. In the following section, we will discuss some studies related to the long-term effects of TLR ligation in the offspring.
Administration of LPS to pregnant mice was shown to cause acute fetal cardiovascular depression, 54 and inhibit structural development of the distal fetal mouse lung in a TLR4-dependent manner.66 Similarly, cerebral white matter damage, which is one of the biggest problems seen in preterm neonates because of its strong association with their lifetime adverse outcome, is also believed to be caused by TLR4 activation in the fetus.67 It is worthy to mention that low-dose LPS, which has no adverse effects on pregnancy outcome, dramatically increases brain injury to subsequent hypoxic–ischemic challenge in a newborn rat animal model.67 These findings are compatible with clinical findings showing that maternal exposure to bacteria not only causes preterm labor, but also contributes to long-term adverse outcome in the offspring such as cerebral white matter damage.
Adverse effects of maternal TLR3 activations were also found in fetuses in various animal models. Maternal poly(I:C) or virus exposure cause marked behavioral changes in the offspring mouse,68 which is relevant to many epidemiological studies showing that maternal exposure to virus causes not only abortion or preterm birth but also fetal schizophrenia and autism.69,70 Offspring of poly(I:C)-treated pregnant mice were shown to have less expression of brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF) and TNF-α in their placenta, liver/spleen and brain, which may represent a potential mechanism through which maternal viral infection increases a risk of such neurodevelopmental disorders.71
In contrast to the ‘adverse’ effects of maternal infection on fetus, there is a notion called ‘hygiene hypothesis’, that is, ‘adequate’ maternal microbial exposure has protective effects on neonatal allergic disease. Very recently, it was demonstrated that TLR system is also involved in this effect. Conrad et al.72 showed that an administration of non-pathogenic microbe Acinetobacter Iwoffii F78 to pregnant mice has a protective effect on postnatal asthma, and the effect was completely abolished in TLR2/3/4/7/9/null mice.
Given that maternal infections are detrimental to offspring long-term health, the next question is whether these effects of maternal infections on the fetus are due to a direct effect of the pathogen (virus, bacteria) or secondary effect through maternal response (inflammation, cytokines). In this context, Kim and coworkers 73 demonstrated the expression of TLR2 and TLR4 in skin samples obtained from preterm delivered babies by immunohistochemistry. As for function of TLR in fetus, studies of mouse and human fetal cells show stimulation of fetal intestinal cells or fetal monocyte with LPS results in production of chemokines and cytokines.74,75 These findings indicate that fetal cells are also capable of recognizing microbial products and participate in innate immune defense in the case of microbial invasion of the amniotic cavity; although the expressions of other PRRs in various fetal tissues/organs still need to be elucidated.
Recent studies from our laboratory have shown that viral infection of the mouse placenta, which does not induce preterm labor, has a detrimental effect on fetal development.59 A striking finding was the observation of a general inflammatory fetal condition, very similar to those observed in the human condition known as fetal inflammatory response syndrome (FIRS).76 This inflammatory condition was present in the fetus in spite of undetectable viral titers.
Morphologic examination of the fetus reveled changes in the brain, heart and lungs. This data suggests that although the virus may not reach the fetus, an inflammatory process at the placenta will affect the normal development of the fetus, with potential after birth severe consequences.
Role of TLRs in Human Pregnancies: Clinical Observation
Recent clinical studies have linked TLRs to pregnancy disorders. In the following section, we will discuss some of the most relevant observations.
TLR expressions at the maternal–fetal interface in pregnancy disorders
Intrauterine infection and subsequent chorioaminionitis (CAM) are known to be among the most important causes of preterm delivery. 1 We evaluated the expression of TLR2 and TLR4 in chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with CAM. TLR2 and TLR4 mRNA expression were significantly higher in membranes from women at term with spontaneous labor than women not in labor. TLR2 expression in chorioamniotic membranes was significantly higher in patients with CAM than those without CAM. The expression of TLR2 was also restricted to the basal surface of amniotic epithelial cells in non-CAM preterm, labor whereas in CAM cases, diffuse and strong positive staining for the entire cytoplasm of epithelium was observed.39 On the other hand, Rindsjo et al.77 demonstrated that TLR2 expression in trophoblast was decreased in patients with CAM compared to those without CAM. These findings suggest that the response to infection varies in the different parts of the maternal–fetal interface. However, we have to take into consideration the possibility that these variations might be the result of technical variations among study groups.
As for TLR4, Kumazaki et al.41 showed that TLR4 expression in the villous Hofbauer cells was higher in preterm placenta with CAM than preterm placenta without CAM, or term placenta with or without CAM. Recently, TLR4 expression was shown at the amniotic epithelium, and the strongest immunoreactivity for TLR4 was observed at basal membrane in CAM patients. The authors suggested that an infection may induce the translocation of TLR4 from apical to basal membrane to decrease TLR signaling during early infection but allow the amniotic epithelium to remain competent to invasive bacteria.42
In addition to CAM, we also evaluated the involvement of TLRs in the etiology of pre-eclampsia. Thus, TLR4 expression in trophoblast was significantly higher in women with preterm delivery associated with pre-eclampsia than in women with or without CAM preterm delivery. Furthermore, TLR4 expression was co-localized with activated NFκB, TNF-α and M30 (an apoptosis marker specific for epithelial cells), suggesting that inflammatory cytokines can induce TLR4 expression and thereby enhance further trophoblast response to TLR ligands.49 Similarly, Wang and coworkers78 described a correlation between high levels of TLR4 expression in microvessel endothelial cells isolated from placental villi, and placental vascular disease, defined by an abnormal umbilical artery Doppler study. These findings imply that the level of TLR expression in zthe placenta is controlled by certain pathogen per se and/or endogenous molecule produced upon inflammation, as a feedback mechanism to enhance or inhibit further immune responses, although precise mechanisms are not clarified yet.
A new aspect on TLR function is related to its ability to recognize not only microbial ligands but also host products, also know as ‘danger signals’ released by injured cells,79 suggesting that TLRs might be involved not only in infection but also in non-infection-related conditions associated with pregnancy. For instance, Holmlund et al.80 demonstrated that HMGB1, a ligand for TLR4, is highly expressed in decidua from pre-eclamptic patients. Anti-phospholipid antibodies, which is known to be involved in the pathology of recurrent miscarriage, pre-eclampsia and preterm labor, was also shown to induce a pro-inflammatory response in first-trimester trophoblast via TLR4 pathway.81
TLR polymorphisms and pregnancy disorders
Given that the TLR system is involved in many pregnancy disorders, it is possible that the TLR polymorphisms affect on the susceptibility to pregnancy disorders. Indeed, a number of studies evaluated whether polymorphisms in TLR are associated with pregnancy disorder. As for preterm labor, most of the studies are focusing on polymorphism in TLR2 and TLR4. Interestingly, not only polymorphism in the mother, but also that in the infant was analyzed and proved associations between fetal polymorphism and susceptibility to preterm labor. These findings imply that not only the immune system in the mother, but also that in the fetus or placenta contributes the innate immune response in preventing adverse outcomes in pregnancy.82 One study evaluated infants’ genomic DNA and showed that infants who carried two polymorphic TLR2 alleles (-16934TA/AA and 2258GA/AA) had significantly shorter gestational ages.83 Another study conducted within Finnish population found that Gly in TLR4 299, in both infants and mothers, was associated with preterm labor,84 and same trend was observed in a study in Uruguay.85
Bacterial vaginosis (BV), known to induce preterm birth, is also reported to be associated with TLR4 polymorphisms. One study found Thr for TLR4 399 was significantly less common in women with BV compared with women without BV.86 Another study showed Gly for TLR4 299, which is known to impair responses to LPS, was associated with an increase in vaginal pH, Gardnerella vaginalis levels and concentration of anaerobic gram-negative rods.87
TLRs polymorphisms also affect on the susceptibility to pre-eclampsia. Recently, van Rijn et al.88 suggested that maternal TLR4 polymorphisms alter susceptibility to early-onset pre-eclampsia and elevated liver enzymes and low platelets (HELLP) syndrome. Hirschfeld et al. also found that the presence of TLR2 Arg753Gln and two TLR4 SNPs (Asp299Gly and Thr399Ile) was associated with normal pregnancy controls.89
These clinical observations indicate an important role for the TLR systems in pregnancy disorders, although further investigations are required to determine the specific mechanism underlying in each condition.
Summary
The spatial and temporal pattern of TLR expression at the maternal–fetal interface has been described in physiological and pathological conditions. There is growing evidence that these TLRs recognize pathogens and react to them, not only in immune cells but also in non-immune cells such as the trophoblast. This implies clinical applications in pregnancy disorders, i.e., using TLR agonists as a therapeutic and/or prophylactic treatment, or detection of TLR expression as a diagnostic tool.
There are several points that still need to be elucidated. While we have recognized the importance of the TLRs in the defense against pathogens, the role of these receptors in establishing tolerance to the growing fetus is still unknown. It is intriguing to speculate that TLRs at the maternal–fetal interface may play a role in establishing normal pregnancy, given the fact that commensal bacteria, which may potentially be bound to the TLRs, are present in the reproductive tract, although further studies are required to elucidate this hypothesis. It is also still unclear what regulates the expression pattern and functional activity of TLRs during pregnancy, either in physiological or in pathological conditions. Addressing this question may also help develop clinical applications.
Recent research in the field of TLR shows that these receptors play so many important roles in various areas. Further studies on TLRs at the maternal–fetal interface will shed light on how the balance between tolerance to allergenic fetus and host defense against possible pathogens is maintained.
Acknowledgments
The authors thank Mrs. JoAnn Bilyard for her assistance with the manuscript. This study is in part funded by grants from the National Institute of Health, NICDH P01HD054713 and 3N01 HD23342 and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
References
- 1.Aldo PB, Krikun G, Visintin I, Lockwood C, Romero R, Mor G. A novel three-dimensional in vitro system to study trophoblast-endothelium cell interactions. Am J Reprod Immunol. 2007;58:98–110. doi: 10.1111/j.1600-0897.2007.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 3.Arechavaleta-Velasco F, Gomez L, Ma Y, Zhao J, McGrath CM, Sammel MD, Nelson DB, Parry S. Adverse reproductive outcomes in urban women with adeno-associated virus-2 infections in early pregnancy. Hum Reprod. 2008;23:29–36. doi: 10.1093/humrep/dem360. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 5.Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991;6:791–798. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- 6.Bulmer JN, Pace D, Ritson A. Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Dev. 1988;28:1599–1613. doi: 10.1051/rnd:19881006. [DOI] [PubMed] [Google Scholar]
- 7.Kammerer U, Schoppet M, McLellan AD, Kapp M, Huppertz HI, Kampgen E, Dietl J. Human decidua contains potent immunostimulatory CD83(+) dendritic cells. Am J Pathol. 2000;157:159–169. doi: 10.1016/S0002-9440(10)64527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kammerer U, Eggert AO, Kapp M, McLellan AD, Geijtenbeek TB, Dietl J, van Kooyk Y, Kampgen E. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol. 2003;162:887–896. doi: 10.1016/S0002-9440(10)63884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 10.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Akira S. Roles of Toll-like receptors in innate immune responses. Genes Cells. 2001;6:733–742. doi: 10.1046/j.1365-2443.2001.00458.x. [DOI] [PubMed] [Google Scholar]
- 12.See RH, Caday-Malcolm RA, Singaraja RR, Zhou S, Silverston A, Huber MT, Moran J, James ER, Janoo R, Savill JM, Rigot V, Zhang LH, Wang M, Chimini G, Wellington CL, Tafuri SR, Hayden MR. Protein kinase A site-specific phosphorylation regulates ATP-binding cassette A1 (ABCA1)-mediated phospholipid efflux. J Biol Chem. 2002;277:41835–41842. doi: 10.1074/jbc.M204923200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 14.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 15.Poltorak A, He X, Smirnova I, Liu M-Y, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 16.Aliprantis AO, Yang R-B, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 17.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by toll-like receptor-2. EMBO J. 2000;19:3325–3336. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 19.Krutzik SR, Ochoa MT, Sieling PA, Uematsu S, Ng YW, Legaspi A, Liu PT, Cole ST, Godowski PJ, Maeda Y, Sarno EN, Norgard MV, Brennan PJ, Akira S, Rea TH, Modlin RL. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med. 2003;9:525–532. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- 20.Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 21.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 22.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 23.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 25.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem. 2001;276:5197–5203. doi: 10.1074/jbc.M009160200. [DOI] [PubMed] [Google Scholar]
- 27.Collins Y, Tan DF, Pejovic T, Mor G, Qian F, Rutherford T, Varma R, McQuaid D, Driscoll D, Jiang M, Deeb G, Lele S, Nowak N, Odunsi K. Identification of differentially expressed genes in clinically distinct groups of serous ovarian carcinomas using cDNA microarray. Int J Mol Med. 2004;14:43–53. [PubMed] [Google Scholar]
- 28.Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M. Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves toll-like receptor 4. J Immunol. 2002;168:5989–5992. doi: 10.4049/jimmunol.168.12.5989. [DOI] [PubMed] [Google Scholar]
- 29.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 30.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., III The extra domain A of fibronectin activates toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 31.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 34.Akira S. Toll-like receptor signaling. J Biol Chem. 2003;278:38105–38108. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 36.Klaffenbach D, Rascher W, Rollinghoff M, Dotsch J, Meissner U, Schnare M. Regulation and signal transduction of toll-like receptors in human chorioncarcinoma cell lines. Am J Reprod Immunol. 2005;53:77–84. doi: 10.1111/j.1600-0897.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 37.Mitsunari M, Yoshida S, Shoji T, Tsukihara S, Iwabe T, Harada T, Terakawa N. Macrophage-activating lipopeptide-2 induces cyclooxygenase-2 and prostaglandin E(2) via toll-like receptor 2 in human placental trophoblast cells. J Reprod Immunol. 2006;72:46–59. doi: 10.1016/j.jri.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Abrahams VM, Fahey JV, Schaefer TM, Wright JA, Wira CR, Mor G. Stimulation of first trimester trophoblast cells with Poly(I:C) induces SLPI secretion. Am J Reprod Immunol. 2005;53:280. ASRI205-204. [Google Scholar]
- 39.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 40.Beijar EC, Mallard C, Powell TL. Expression and subcellular localization of TLR-4 in term and first trimester human placenta. Placenta. 2006;27:322–326. doi: 10.1016/j.placenta.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Kumazaki K, Nakayama M, Yanagihara I, Suehara N, Wada Y. Immunohistochemical distribution of Toll-like receptor 4 in term and preterm human placentas from normal and complicated pregnancy including chorioamnionitis. Hum Pathol. 2004;35:47–54. doi: 10.1016/j.humpath.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y, Mor G, Abrahams VM, Buhimschi IA, Buhimschi CS, Guller S. Alterations in syncytiotrophoblast cytokine expression following treatment with lipopolysaccharide. Am J Reprod Immunol. 2006;55:12–18. doi: 10.1111/j.1600-0897.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 43.Canavan TP, Simhan HN. Innate immune function of the human decidual cell at the maternal-fetal interface. J Reprod Immunol. 2007;74:46–52. doi: 10.1016/j.jri.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Dulay AT, Buhimschi CS, Zhao G, Oliver EA, Mbele A, Jing S, Buhimschi IA. Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. J Immunol. 2009;182:7244–7253. doi: 10.4049/jimmunol.0803517. [DOI] [PubMed] [Google Scholar]
- 45.Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekstrom ES, Scheynius A. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002;107:145–151. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Equils O, Lu D, Gatter M, Witkin SS, Bertolotto C, Arditi M, McGregor JA, Simmons CF, Hobel CJ. Chlamydia heat shock protein 60 induces trophoblast apoptosis through TLR4. J Immunol. 2006;177:1257–1263. doi: 10.4049/jimmunol.177.2.1257. [DOI] [PubMed] [Google Scholar]
- 47.Abrahams VM, Schaefer TM, Fahey JV, Visintin I, Wright JA, Aldo PB, Romero R, Wira CR, Mor G. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I : C) Human Reprod. 2006;21:2432–2439. doi: 10.1093/humrep/del178. [DOI] [PubMed] [Google Scholar]
- 48.Abrahams VM, Aldo PB, Murphy SP, Visintin I, Koga K, Wilson G, Romero R, Sharma S, Mor G. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180:6035–6043. doi: 10.4049/jimmunol.180.9.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, Gomez R, Kuivaniemi H. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18:405–416. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirata T, Osuga Y, Hamasaki K, Hirota Y, Nose E, Morimoto C, Harada M, Takemura Y, Koga K, Yoshino O, Tajima T, Hasegawa A, Yano T, Taketani Y. Expression of toll-like receptors 2, 3, 4, and 9 genes in the human endometrium during the menstrual cycle. J Reprod Immunol. 2007;74:53–60. doi: 10.1016/j.jri.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Fest S, Aldo PB, Abrahams VM, Visintin I, Alvero A, Chen R, Chavez SL, Romero R, Mor G. Trophoblast-macrophage interactions: a regulatory network for the protection of pregnancy. Am J Reprod Immunol. 2007;57:55–66. doi: 10.1111/j.1600-0897.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed N, Oliva K, Wang Y, Quinn M, Rice G. Downregulation of urokinase plasminogen activator receptor expression inhibits Erk signalling with concomitant suppression of invasiveness due to loss of uPAR-beta1 integrin complex in colon cancer cells. Br J Cancer. 2003;89:374–384. doi: 10.1038/sj.bjc.6601098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rounioja S, Rasanen J, Ojaniemi M, Glumoff V, Autio-Harmainen H, Hallman M. Mechanism of acute fetal cardiovascular depression after maternal inflammatory challenge in mouse. Am J Pathol. 2005;166:1585–1592. doi: 10.1016/S0002-9440(10)62469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agiostratidou G, Li M, Suyama K, Badano I, Keren R, Chung S, Anzovino A, Hulit J, Qian B, Bouzahzah B, Eugenin E, Loudig O, Phillips GR, Locker J, Hazan RB. Loss of retinal cadherin facilitates mammary tumor progression and metastasis. Cancer Res. 2009;69:5030–5038. doi: 10.1158/0008-5472.CAN-08-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kajikawa S, Kaga N, Futamura Y, Kakinuma C, Shibutani Y. Lipoteichoic acid induces preterm delivery in mice. J Pharmacol Toxicol Methods. 1998;39:147–154. doi: 10.1016/s1056-8719(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 57.Ilievski V, Lu SJ, Hirsch E. Activation of toll-like receptors 2 or 3 and preterm delivery in the mouse. Reprod Sci. 2007;14:315–320. doi: 10.1177/1933719107302959. [DOI] [PubMed] [Google Scholar]
- 58.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK Cells Mediate Inflammation-Induced Fetal Demise in IL-10-Null Mice. J Immunol. 2005;175:4084–4090. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- 59.Cardenas I, Aldo P, Koga K, Means R, Lang SH, Mor G. Subclinicla viral infection in pregnancy lead to inflammatoruy process at the placenta with non-lethal fetal damage. Am J Reprod Immunol. 2009;61:397. Abstract #S312. [Google Scholar]
- 60.Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci. 2008;15:121–127. doi: 10.1177/1933719107310992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimada S, Iwabuchi K, Watano K, Shimizu H, Yamada H, Minakami H, Onoe K. Expression of allograft inflammatory factor-1 in mouse uterus and poly(I:C)-induced fetal resorption. Am J Reprod Immunol. 2003;50:104–112. doi: 10.1034/j.1600-0897.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 62.Li L, Kang J, Lei W. Role of Toll-like receptor 4 in inflammation-induced preterm delivery. Mol Hum Reprod. 2010;16:267–272. doi: 10.1093/molehr/gap106. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Wei H, Wu D, Tian Z. Toll-like receptor 3 agonist induces impairment of uterine vascular remodeling and fetal losses in CBA × DBA/2 mice. J Reprod Immunol. 2007;74:61–67. doi: 10.1016/j.jri.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Faas MM, Schuiling GA, Baller JF, Visscher CA, Bakker WW. A new animal model for human preeclampsia: ultra-low-dose endotoxin infusion in pregnant rats. Am J Obstet Gynecol. 1994;171:158–164. doi: 10.1016/0002-9378(94)90463-4. [DOI] [PubMed] [Google Scholar]
- 65.Tinsley JH, Chiasson VL, Mahajan A, Young KJ, Mitchell BM. Toll-like receptor 3 activation during pregnancy elicits preeclampsia-like symptoms in rats. Am J Hypertens. 2009;22:1314–1319. doi: 10.1038/ajh.2009.185. [DOI] [PubMed] [Google Scholar]
- 66.Prince LS, Dieperink HI, Okoh VO, Fierro-Perez GA, Lallone RL. Toll-like receptor signaling inhibits structural development of the distal fetal mouse lung. Dev Dyn. 2005;233:553–561. doi: 10.1002/dvdy.20362. [DOI] [PubMed] [Google Scholar]
- 67.Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- 68.Akira S, Hoshino K. Myeloid differentiation factor 88-dependent and -independent pathways in toll-like receptor signaling. J Infect Dis. 2003;187 Suppl. 2:S356–S363. doi: 10.1086/374749. [DOI] [PubMed] [Google Scholar]
- 69.Patterson PH. Neuroscience. Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- 70.Ciaranello AL, Ciaranello RD. The neurobiology of infantile autism. Annu Rev Neurosci. 1995;18:101–128. doi: 10.1146/annurev.ne.18.030195.000533. [DOI] [PubMed] [Google Scholar]
- 71.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Conrad ML, Ferstl R, Teich R, Brand S, Blumer N, Yildirim AO, Patrascan CC, Hanuszkiewicz A, Akira S, Wagner H, Holst O, von Mutius E, Pfefferle PI, Kirschning CJ, Garn H, Renz H. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med. 2009;206:2869–2877. doi: 10.1084/jem.20090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Espinoza J, Romero R, Mee Kim Y, Kusanovic JP, Hassan S, Erez O, Gotsch F, Gabor Than N, Papp Z, Jai Kim C. Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med. 2006;34:447–458. doi: 10.1515/JPM.2006.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manimtim WM, Hasday JD, Hester L, Fairchild KD, Lovchik JC, Viscardi RM. Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect Immun. 2001;69:3906–3915. doi: 10.1128/IAI.69.6.3906-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, Kim JS, Edwin SS, Gomez R, Draghici S. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63:73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rindsjo E, Holmlund U, Sverremark-Ekstrom E, Papadogiannakis N, Scheynius A. Toll-like receptor-2 expression in normal and pathologic human placenta. Hum Pathol. 2007;38:468–473. doi: 10.1016/j.humpath.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 78.Rahman MA, Li M, Li P, Wang H, Dey SK, Das SK. Hoxa-10 deficiency alters region-specific gene expression and perturbs differentiation of natural killer cells during decidualization. Dev Biol. 2006;290:105–117. doi: 10.1016/j.ydbio.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 80.Holmlund U, Wahamaa H, Bachmayer N, Bremme K, Sverremark-Ekstrom E, Palmblad K. The novel inflammatory cytokine high mobility group box protein 1 (HMGB1) is expressed by human term placenta. Immunology. 2007;122:430–437. doi: 10.1111/j.1365-2567.2007.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mulla MJ, Brosens JJ, Chamley LW, Giles I, Pericleous C, Rahman A, Joyce SK, Panda B, Paidas MJ, Abrahams VM. Antiphospholipid antibodies induce a pro-inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am J Reprod Immunol. 2009;62:96–111. doi: 10.1111/j.1600-0897.2009.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63 doi: 10.1111/j.1600-0897.2010.00836.x. doi:10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krediet TG, Wiertsema SP, Vossers MJ, Hoeks SB, Fleer A, Ruven HJ, Rijkers GT. Toll-like receptor 2 polymorphism is associated with preterm birth. Pediatr Res. 2007;62:474–476. doi: 10.1203/PDR.0b013e31813c9401. [DOI] [PubMed] [Google Scholar]
- 84.Lorenz E, Hallman M, Marttila R, Haataja R, Schwartz DA. Association between the Asp299Gly polymorphisms in the Toll-like receptor 4 and premature births in the Finnish population. Pediatr Res. 2002;52:373–376. doi: 10.1203/00006450-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 85.El Costa H, Casemayou A, Aguerre-Girr M, Rabot M, Berrebi A, Parant O, Clouet-Delannoy M, Lombardelli L, Jabrane-Ferrat N, Rukavina D, Bensussan A, Piccinni MP, Le Bouteiller P, Tabiasco J. Critical and differential roles of NKp46- and NKp30-activating receptors expressed by uterine NK cells in early pregnancy. J Immunol. 2008;181:3009–3017. doi: 10.4049/jimmunol.181.5.3009. [DOI] [PubMed] [Google Scholar]
- 86.Goepfert AR, Varner M, Ward K, Macpherson C, Klebanoff M, Goldenberg RL, Mercer B, Meis P, Iams J, Moawad A, Carey JC, Leveno K, Wapner R, Caritis SN, Miodovnik M, Sorokin Y, O’Sullivan MJ, Van Dorsten JP, Langer O. Differences in inflammatory cytokine and Toll-like receptor genes and bacterial vaginosis in pregnancy. Am J Obstet Gynecol. 2005;193:1478–1485. doi: 10.1016/j.ajog.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 87.Bryant AE, Genc M, Hurtado RM, Chen KT. Pulmonary Kaposi’s sarcoma in pregnancy. Am J Perinatol. 2004;21:355–363. doi: 10.1055/s-2004-831880. [DOI] [PubMed] [Google Scholar]
- 88.van Rijn BB, Franx A, Steegers EA, de Groot CJ, Bertina RM, Pasterkamp G, Voorbij HA, Bruinse HW, Roest M. Maternal TLR4 and NOD2 gene variants, pro-inflammatory phenotype and susceptibility to early-onset preeclampsia and HELLP syndrome. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0001865. E1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirschfeld AF, Jiang R, Robinson WP, McFadden DE, Turvey SE. Toll-like receptor 4 polymorphisms and idiopathic chromosomally normal miscarriage. Hum Reprod. 2007;22:440–443. doi: 10.1093/humrep/del377. [DOI] [PubMed] [Google Scholar]