Abstract
Placental immune response and its tropism for specific viruses and pathogens affect the outcome of the pregnant woman’s susceptibility to and severity of certain infectious diseases. The generalization of pregnancy as a condition of immune suppression or increased risk is misleading and prevents the determination of adequate guidelines for treating pregnant women during pandemics. There is a need to evaluate the interaction of each specific pathogen with the fetal/placental unit and its responses to design the adequate prophylaxis or therapy. The complexity of the immunology of pregnancy and the focus, for many years, on the concept of immunology of pregnancy as an organ transplantation have complicated the field and delayed the development of new guidelines with clinical implications that could help to answer these and other relevant questions. Our challenge as scientists and clinicians interested in the field of reproductive immunology is to evaluate many of the ‘classical concepts’ to define new approaches for a better understanding of the immunology of pregnancy that will benefit mothers and fetuses in different clinical scenarios.
Keywords: Cytokines, inflammation, macrophages, placenta, pregnancy, TLR
Introduction
Viral or bacterial pandemics threaten the general population; however, there are special populations, such as children and pregnant women, which may be at a higher risk and more susceptible to or more severely affected by infectious diseases. Pregnant women are considered to be a special population group due to their specific susceptibility to some infectious diseases because of the unique ‘immunological’ condition caused by pregnancy. Therefore, pregnancy presents many challenges for making decisions on how to approach, prevent and treat infectious diseases. The most challenging questions include the following: (1) are pregnant women more susceptible to infectious disease threats?, (2) how does a viral infection affect the fetus and the pregnancy outcome?, (3) are prophylaxis and treatment appropriate and beneficial for pregnant women?
The complexity of the immunology of pregnancy and the focus, for many years, on the concept of immunology of pregnancy as an organ transplantation have complicated the field and delayed the development of new guidelines with clinical implications that could help to answer these and other relevant questions.
Our challenge as scientists and clinicians interested in the field of reproductive immunology is to evaluate many of the ‘classical concepts’ to define new approaches for a better understanding of the immunology of pregnancy that will benefit mothers and fetuses in different clinical scenarios.
Are Pregnant Women More Susceptible to Infectious Disease Threats?
The concept that pregnancy is associated with immune suppression has created a myth of pregnancy as a state of immunological weakness and therefore of increased susceptibility to infectious diseases. To discuss this question we will first review some fundamental concepts associated with the immune system and pregnancy.
A fundamental feature of the immune system is to protect the host from pathogens. This function depends upon the innate immune system’s capacity to coordinate cell migration for surveillance and to recognize and respond to invading microorganisms. During normal pregnancy, the human decidua contains a high number of immune cells, such as macrophages, natural killer (NK) cells and regulatory T cells (Treg).1–3 Seventy percent of decidual leukocytes are NK cells, 20–25% are macrophages and 1.7% are dendritic cells.2,4,5 From the adaptive immune system, B cells are absent, but T lymphocytes constitute about 3–10% of the decidual immune cells.6 During the first trimester, NK cells, dendritic cells and macrophages infiltrate the decidua and accumulate around the invading trophoblast cells.7,8 Deletion of either macrophages, NK cells or dendritic cells (DC) has deleterious effects.9–14 Elegant studies have shown that in the absence of NK cells, trophoblast cells are not able to reach the endometrial vascularity leading to termination of the pregnancy.12 These studies suggest that uNK cells are critical for trophoblast invasion in the uterus. Similarly, depletion of DCs prevented blastocyst implantation and decidual formation.15 Indeed, this study suggests that uDC are necessary for decidual formation and may affect the angiogenic response by inhibiting blood vessel maturation.15
More recently, Collins et al. demonstrate that uDC association with T cell responses to the fetal ‘allograft’ starkly contrast with their prominent role in organ transplant rejection.16 These data further support the idea that the fetal–maternal immune interaction is more complex than the comparison to transplant allograft.
Consequently, the presence of immune cells at the implantation site is not associated with a response to the ‘foreign’ fetus but to facilitate and protect the pregnancy. Therefore, the immune system at the implantation site is not suppressed, on the contrary it is active, functional and is carefully controlled.
Is the systemic immunity of the mother suppressed? Although we can find numerous studies describing the factors inducing immune suppression (including progesterone, defined as the natural immune suppressor), medical and evolutionary aspects are against the concept of immune suppression. Pregnancy represents the most important period for the conservation of the species, therefore it is fundamental to strengthen all the means to protect the mother and the offspring. The immune system is one of the most important systems protecting the mother against the environment and preventing damage to the fetus. It is during pregnancy when the maternal immune system is characterized by a reinforced network of recognition, communication, trafficking and repair; it is able to raise the alarm, if necessary, to maintain the well-being of the mother and the fetus. On the other side is the fetus that, without any doubt, provides a developing active immune system that will modify the way the mother responds to the environment, providing the uniqueness of the immune system during pregnancy. Therefore, it is appropriate to refer to pregnancy as a unique immune condition that is modulated, but not suppressed.
This unique behavior explains why pregnant women respond differently to the presence of microorganisms or its products. Therefore, pregnancy should not imply more susceptibility to infectious diseases, instead there is a modulation of the immune system which leads to differential responses depending not only on the microorganisms, but on the stages of the pregnancy.
The Allograft Paradigm: Transplantation Versus Implantation
Over 50 years ago, Sir Peter Medawar proposed the paradigm of why the fetus, as a semi-allograft, is not rejected by the maternal immune system17,18 and the presence of the maternal immune system at the implantation site was used as evidence to support this.19 As a result, investigators pursued the mechanisms by which the fetus might escape maternal immune surveillance and varied hypotheses have been proposed.20 Medawar’s observation was based on the assumption that the placenta is an allograft expressing paternal proteins and, therefore, under normal immunological conditions, should be rejected. However, as our knowledge of placental biology has significantly increased over the last 50 years, we can appreciate that the placenta is more than a transplanted organ. Based on the data discussed here and elsewhere, we suggest that, while there may be an active mechanism preventing a maternal immune response against paternal antigens, the trophoblast and the maternal immune system have evolved and established a cooperative status, helping each other for the success of the pregnancy.21,22 This cooperative work involves many tasks, some of which we are just starting to unveil.
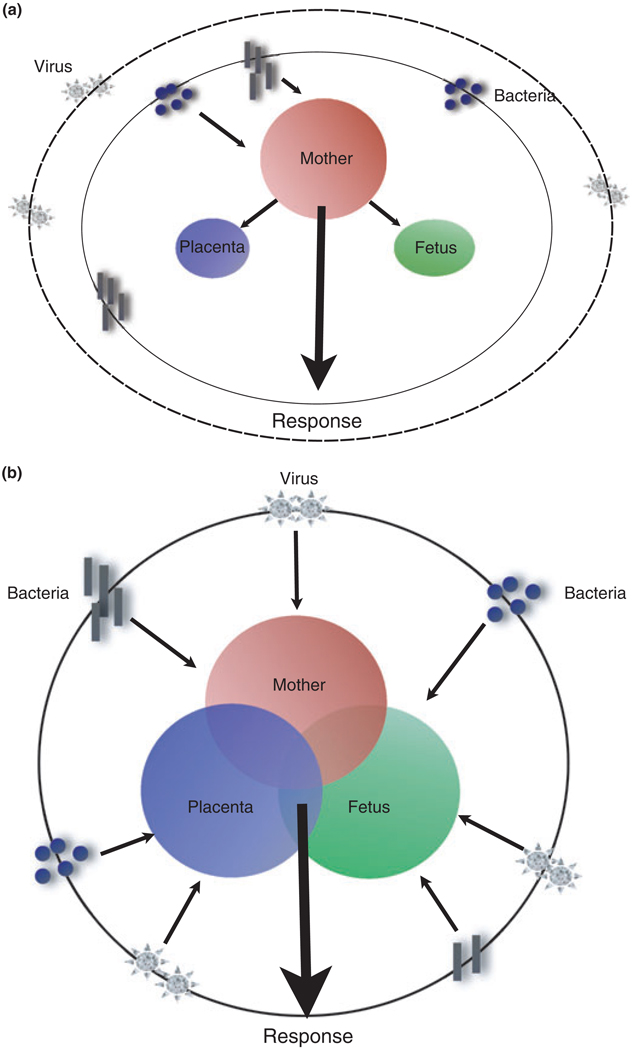
We propose a new paradigm in terms of the immunological response of the mother to microorganisms which will be determined and influenced by the presence and responses from the fetal/placental unit. In other words, the immunology of pregnancy is the result of the combination of signals and responses originated from the maternal immune system and the fetal–placental immune system. The signals originated in the placenta will modulate the way the maternal immune system will behave in the presence of potential dangerous signals (Fig. 1a,b).
Fig. 1.
Integrational view of the immune system during pregnancy. a) The old model conceives the maternal immune system as the major player in response to the fetus and microorganisms. Fetal responses (fetus and placenta) are considered limited. b) New integrational model where the fetal–placental immune response and the maternal immune system are integrated. (see text for detail).
Cytokine Shift
The definition of pregnancy as a ‘Th-2’ or anti-inflammatory state was enthusiastically embraced, and numerous studies attempted to prove and support this hypothesis. This theory postulates that pregnancy is an anti-inflammatory condition23–25 and a shift in the type of cytokines produced would lead to abortion or pregnancy complications. While many studies confirmed this hypothesis, a similar number of studies argued against this notion.19 The reason for these contradictory results may be owing to oversimplification of disparate observations made during pregnancy. In the aforementioned studies, pregnancy was evaluated as a single event, when in reality it has three distinct immunological phases that are characterized by distinct biological processes and can be symbolized by how the pregnant woman feels.22,26
Implantation, placentation and the first and early second trimester of pregnancy resemble ‘an open wound’ that requires a strong inflammatory response. During this first stage, the blastocyst has to break through the epithelial lining of the uterus to implant, damage the endometrial tissue to invade; followed by the trophoblast replacement of the endothelium and vascular smooth muscle of the maternal blood vessels to secure an adequate placental–fetal blood supply.27 All these activities create a veritable ‘battleground’ of invading cells, dying cells and repairing cells. An inflammatory environment is required to secure the adequate repair of the uterine epithelium and the removal of cellular debris. Mean-while, the mother’s well-being is clinically affected: she feels sick because her whole body is struggling to adapt to the presence of the fetus (in addition to hormonal changes and other factors, this inflammatory response is responsible for ‘morning sickness’). Thus, the first trimester of pregnancy is a proinflammatory phase.28
The second immunological phase of pregnancy is, in many ways, the optimal time for the mother. This is a period of rapid fetal growth and development. The mother, placenta and fetus are symbiotic, and the predominant immunological feature is induction of an anti-inflammatory state. The woman no longer suffers from nausea and fever as she did in the first stage, in part because the immune response is no longer the predominant endocrine feature.
Finally, during the last immunological phase of pregnancy, the fetus has completed its development; all the organs are functional and prepared for the external world. Now the mother needs to deliver the baby; this is achieved through renewed inflammation. Parturition is characterized by an influx of immune cells into the myometrium to promote recrudescence of an inflammatory process.29,30 This pro-inflammatory environment promotes the contraction of the uterus, expulsion of the baby and rejection of the placenta. In conclusion, pregnancy is a pro-inflammatory and anti-inflammatory condition, depending upon the stage of gestation.31,32
These differences in cytokines may also reflect the sensitivity to infectious diseases. Pregnant women in malaria-endemic regions are more susceptible to malaria infection during the first half of the pregnancy and this risk gradually declines during the second half.33 Lassa fever, caused by infection with a arenavirus, showed a higher rate of case-fatality in pregnant women particularly in the third trimester.34 However, those are not the rule and may even be the exception; in general, pregnant women are resistant to viral infections including HIV.
Thus, the obvious question is why pregnant women are more susceptible to some viruses or to some specific microorganisms than non-pregnant women? Is the presence of the placenta affecting the sensitivity to specific infections?
Active Protection of the Trophoblast Against Viral Infection
The trophoblast, the cellular unit of the placenta, not only recognizes microorganisms and initiates an immune response as previously described, it may also produce anti-microbial peptides and, therefore, actively protect itself against pathogens. Studies have demonstrated the expression of the antimicrobial human beta defensins 1 and 3 by trophoblast cells.35,36 Secretory leukocyte protease inhibitor (SLPI), which is a potent inhibitor of HIV infection37 and inducer of bacterial lysis,38 has also been found in trophoblast cells.35 The expression of TLR-3, TLR-7, TLR-8 and TLR-9 by trophoblast cells may explain how the placenta regulates the expression of these anti-microbial factors. Stimulation of first trimester trophoblast cells through TLR-3 with Poly (I:C) promotes the production and secretion of SLPI and IFN-β, two important anti-viral factors. These factors provide the first line of defense against viral infections and have the potential to activate multiple intracellular pathways.39 IFN-β and SLPI production by trophoblast cells, in response to a viral infection at the maternal-fetal interface, may represent a potential mechanism by which the placenta prevents transmission of viral infection (e.g. HIV) to the fetus during pregnancy. These data suggest that the placenta represents an active immunological organ, (innate immune system), capable of recognizing and responding to pathogens. However, it also indicates that the placenta is prone to infections from microorganisms, which in its absence (non-pregnant) would never take place.
How a Viral Infection Affects the Fetus and the Pregnancy Outcome
Pregnant women are exposed to many infectious agents that are potentially harmful not only to the mother but also to the fetus. Risk evaluation has been focused on whether there is a maternal viremia or fetal transmission. Viral infections which are able to reach the fetus by crossing the placenta might have a detrimental effect on the pregnancy. It is well accepted that in those cases infection will lead to embryonic and fetal death, induce miscarriage or induce major congenital anomalies.40 However, even in the absence of placental transmission, the fetus could be adversely affected by the maternal response to the infection.
Epidemiological studies have demonstrated an association between viral infections and preterm labor, and fetal congenital anomalies of the central nervous system and the cardiovascular system.41–43 Although some viral infections during pregnancy may be asymptomatic, approximately half of all preterm deliveries are associated with histologic evidence of inflammation of the placenta, termed acute chorioamnionitis (ACA)44 or chronic chorioamnionitis. Despite the high incidence of ACA, only a fraction of fetuses have demonstrable infection. Most viral infections affecting the mother do not cause congenital fetal infection, suggesting that the placenta may play an important role as a potent immune-regulatory interface protecting the fetus from systemic infection.21,44
Recent observations indicate that the placenta functions as a regulator of the trafficking between the fetus and the mother rather than as a barrier.32 Fetal and maternal cells move in the two directions;45,46 similarly, some viruses and bacteria can reach the fetus by transplacental passage with adverse consequences. Although viral infections are common during pregnancy, transplacental passage and fetal infection appear to be the exception rather than the rule.
There is a paucity of evidence that viral infections lead to preterm labor; however, there are several areas of controversy and open questions. For example, what effects do subclinical viral infections of the decidua and/or placenta during early pregnancy have in response to other microorganisms such as bacteria? and what is the effect of a subclinical viral infection of the placenta on the fetus?
Studies from our laboratory suggest that the type of response initiated in the placenta may determine the immunological response of the mother and consequently, the pregnancy outcome. A placental infection that is able to elicit the production of inflammatory cytokines, such as TNFα, INFγ, IL-12 and high levels of IL-6, will activate the maternal immune system and lead to placental damage and abortion or preterm labor.47 On the other hand, a viral infection in the placenta that triggers a mild inflammatory response will not terminate the pregnancy but might be able to activate the immune system, not only from the mother but also from the fetus as well. This activation may have several consequences: (1) sensitize the mother to other microorganisms, and therefore increase the apparent risk of pregnant women to infection; (2) promote an inflammatory response in the fetus, even though there is no viral transmission.
Therefore it is critical to take into consideration that during pregnancy it is not only the maternal immune system responding, but also the fetal/placental unit. In the past, we have considered the placenta and fetus as non-active immunological organs which depend only on the action of the maternal immune system. Our data suggest the contrary. The placenta and the fetus represent an additional immunological organ which affects the global response of the mother to microbial infections. This is relevant for making decisions associated with treatment and prevention during pandemics.
Mother–Placenta–Fetus: A Complex Response to Infection
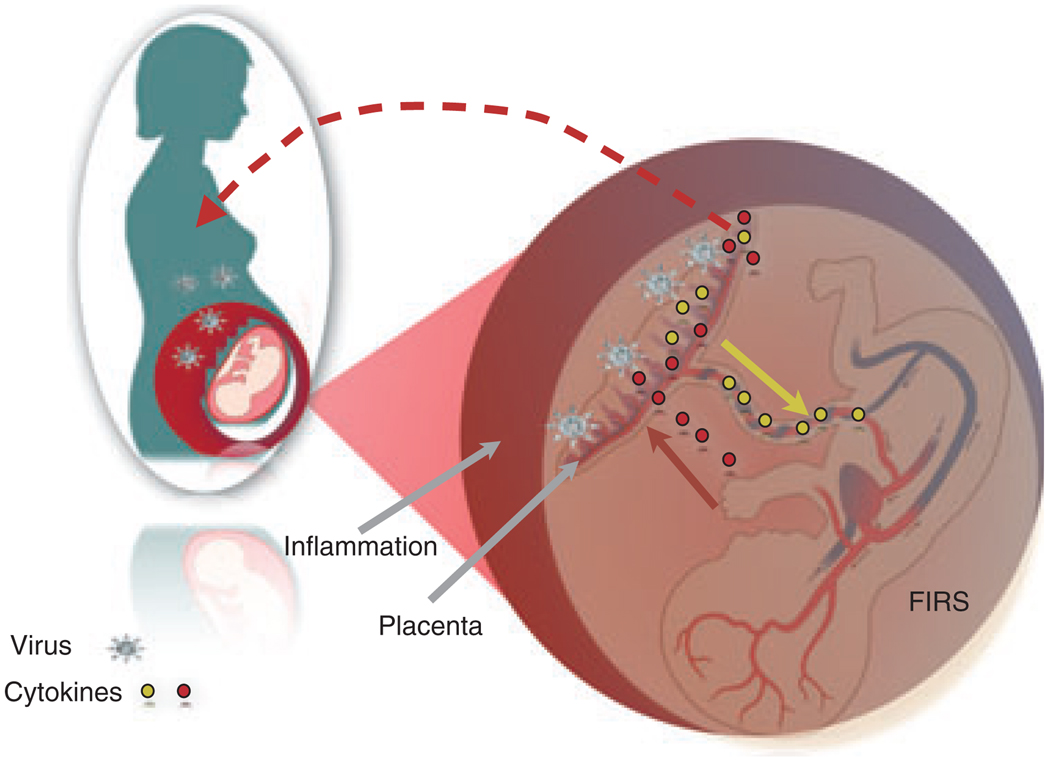
Fetal inflammatory response syndrome (FIRS) is a condition where, despite an absence of cultivable microorganisms, neonates with placental infections have very high circulating levels of inflammatory cytokines, such as IL-1, IL-6, IL-8 and TNF-α.48–50 Studies in our laboratory using an animal model have shown that viral infection of the placenta triggers a fetal inflammatory response similar to the one observed in FIRS, even though the virus is not able to reach the fetus.51 In the case of human FIRS, these cytokines have been shown to affect the CNS and the circulatory system.50,52 Interestingly, we found fetal morphologic abnormalities in the animals, including ventriculomegaly and hemorrhages, which may be caused by fetal pro-inflammatory cytokines such as Il-1, TNFα, MCP-1, MIP1-β and INF-γ. Beyond morphological effects on the fetal brain, the presence of FIRS increases the future risk for autism, schizophrenia, neurosensorial deficits and psychosis induced in the neonatal period.53–55 Moreover, there is evidence that the fetal immune response may predispose to diseases in adulthood.49 Because of this, we propose that an inflammatory response in the placenta, which alters the cytokine balance in the fetus, may affect the normal development of the fetal immune system leading to anomalous responses during childhood or later in life (Fig. 2). One example of this is the differential responses in children to vaccination or the development of allergies. Antenatal infections can have a significant impact on later vaccine responses. We can observe this type of outcome in other conditions associated with placental infection, such as malaria. A few studies suggest that surviving infants with placental malaria may suffer adverse neurodevelopmental sequelae and may have abnormal responses to a later parasitic infection.56 In all these cases the parasite did not reach the placenta, but the inflammatory process in the placenta affected the normal fetal development.57
Fig. 2.
Role of the placenta as a modulator of fetal and maternal responses. Inflammation at the placenta has a bi-directional effect: Activates the maternal immune system as well as the fetus by creating an inflammatory environment. FIRS, Fetal inflammatory response syndrome.
Are Prophylaxis and Treatment Appropriate and Beneficial for Pregnant Women?
The number of infectious diseases has increased during the past two decades and will continue to increase as result of the changes in the behavior of the human population.58 As travel to and from different regions of the world increases, the appearance of new pathogens will also increase. The challenge to determine whether each new pathogen represents a major risk for pregnancy will become more and more difficult if our understanding of the immunology of pregnancy does not evolve from where it is today. In addition, when evaluating the maternal responses to the pathogen, it is important to know the placental response to the pathogen; because, as indicated earlier, some microorganisms may not directly affect the pregnancy but could ‘sensitize’ the mother and the fetus to additional pathogens. In those cases, prophylaxis is required, and the earlier the better. The mantra is first do no harm. Therefore, the risk-benefit of vaccination during all stages of pregnancy should be carefully evaluated.
Conclusion
Placental immune response and its tropism for specific viruses and pathogens affect the pregnant woman’s susceptibility to and severity of certain infectious diseases. The generalization of pregnancy as a condition of general immune suppression or increased risk is misleading and prevents the determination of adequate guidelines for treating pregnant women during pandemics. There is a need to evaluate the interaction of each specific pathogen with the fetal/placental unit and its responses to design the adequate prophylaxis or therapy.
In addition, it is essential to evaluate the presence of maternal viral infections prenatally to prevent long-term adverse outcomes for the child and the mother. Future studies are needed to develop useful biomarkers for viral infections during pregnancy even in a subclinical state as a strategy of early detection and prevention of fetal damage and maternal mortality. Furthermore, it is extremely important to take into consideration the possibility of placental infection when determining a response to emerging infectious disease threats.
Acknowledgment
We thank JoAnn Bilyard for editorial work of the manuscript. This study is in part funded by grants from the National Institute of Health, NICDH P01HD054713 and 3N01 HD23342 and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
References
- 1.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 2.Bulmer JN, Pace D, Ritson A. Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Dev. 1988;28:1599–1613. doi: 10.1051/rnd:19881006. [DOI] [PubMed] [Google Scholar]
- 3.Zenclussen AC. CD4(+)CD25 + T regulatory cells in murine pregnancy. J Reprod Immunol. 2005;65:101–110. doi: 10.1016/j.jri.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 4.King A, Loke YW, Chaouat G. NK cells and reproduction. Immunol Today. 1997;18:64–66. doi: 10.1016/s0167-5699(97)01001-3. [DOI] [PubMed] [Google Scholar]
- 5.Mor G, Straszewski-Chavez SL, Abrahams VM. Macrophage–trophoblast interactions. Methods Mol Med. 2006;122:149–163. doi: 10.1385/1-59259-989-3:149. [DOI] [PubMed] [Google Scholar]
- 6.Wicherek L, Basta P, Pitynski K, Marianowski P, Kijowski J, Wiatr J, Majka M. The characterization of the subpopulation of suppressive B7H4(+) macrophages and the subpopulation of CD25(+) CD4(+) and FOXP3(+) regulatory T-cells in decidua during the secretory cycle phase, Arias Stella reaction, and spontaneous abortion – a preliminary report. Am J Reprod Immunol. 2009;61:303–312. doi: 10.1111/j.1600-0897.2009.00696.x. [DOI] [PubMed] [Google Scholar]
- 7.Shimada S, Nishida R, Takeda M, Iwabuchi K, Kishi R, Onoe K, Minakami H, Yamada H. Natural killer, natural killer T, helper and cytotoxic T cells in the decidua from sporadic miscarriage. Am J Reprod Immunol. 2006;56:193–200. doi: 10.1111/j.1600-0897.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 8.Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwood JD, Minhas K, di Santo JP, Makita M, Kiso Y, Croy BA. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta. 2000;21:693–702. doi: 10.1053/plac.2000.0556. [DOI] [PubMed] [Google Scholar]
- 10.Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G. Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol. 2004;51:275–282. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 11.Le Bouteiller P, Piccinni MP. Human NK cells in pregnant uterus: why there? Am J Reprod Immunol. 2008;59:401–406. doi: 10.1111/j.1600-0897.2008.00597.x. [DOI] [PubMed] [Google Scholar]
- 12.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 13.Manaseki S, Searle RF. Natural killer (NK) cell activity of first trimester human decidua. Cell Immunol. 1989;121:166–173. doi: 10.1016/0008-8749(89)90014-2. [DOI] [PubMed] [Google Scholar]
- 14.Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 15.Birnberg T, Plaks V, Berkutzki T, Mor G, Neeman M, Dekel N, Jung S. Dendritic cells are crucial for decidual development during embryo implantation. Am J Reprod Immunol. 2007;57:342. [Google Scholar]
- 16.Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119:2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medawar PB. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 18.Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1952;7:320–338. [Google Scholar]
- 19.Saito S, Miyazaki S, Sasaki Y. Th1/Th2 Balance of the Implantation Site in Humans. Georgetown, TX: Landes Bioscience/Springer Science; 2006. [Google Scholar]
- 20.Loke YW, King A. Immunology of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:827–837. doi: 10.1053/beog.2000.0122. [DOI] [PubMed] [Google Scholar]
- 21.Mor G, Romero R, Aldo PB, Abrahams VM. Is the trophoblast an immune regulator? The role of toll-like receptors during pregnancy. Crit Rev Immunol. 2005;25:375–388. doi: 10.1615/critrevimmunol.v25.i5.30. [DOI] [PubMed] [Google Scholar]
- 22.Mor G. Pregnancy reconceived. Nat Hist. 2007;116:36–41. [Google Scholar]
- 23.Wegmann T. Fetal protection against abortion: is it immunosupression or immunostimulation? Ann Immunol. 1984;135D:309–312. doi: 10.1016/s0769-2625(84)81196-4. [DOI] [PubMed] [Google Scholar]
- 24.Szekeres-Bartho J, Wegmann TG. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J Reprod Immunol. 1996;31:81–95. doi: 10.1016/0165-0378(96)00964-3. [DOI] [PubMed] [Google Scholar]
- 25.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 26.Mor G, Koga K. Macrophages and pregnancy. Reprod Sci. 2008;15:435–436. doi: 10.1177/1933719108317253. [DOI] [PubMed] [Google Scholar]
- 27.Dekel N, Gnainsky Y, Granot I, Mor G. Inflammation and implantation. Am J Reprod Immunol. 2010;63:17–21. doi: 10.1111/j.1600-0897.2009.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mor G, Abrahams V. Immunology of implantation. In: Arici A, editor. Immunology and Allergy Clinics. Philadelphia: W.B. Saunders Company; 2002. pp. 545–565. [Google Scholar]
- 29.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113 Suppl. 3:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero R. Novel aspects of neutrophil biology in human pregnancy. Am J Reprod Immunol. 2005;53:275. [Google Scholar]
- 32.Mor G. Inflammation and pregnancy: the role of toll-like receptors in trophoblast-immune interaction. Ann N Y Acad Sci. 2008;1127:121–128. doi: 10.1196/annals.1434.006. [DOI] [PubMed] [Google Scholar]
- 33.Okoko BJ, Enwere G, Ota MO. The epidemiology and consequences of maternal malaria: a review of immunological basis. Acta Trop. 2003;87:193–205. doi: 10.1016/s0001-706x(03)00097-4. [DOI] [PubMed] [Google Scholar]
- 34.Price ME, Fisher-Hoch SP, Craven RB, McCormick JB. A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy. BMJ. 1988;297:584–587. doi: 10.1136/bmj.297.6648.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abrahams VM, Schaefer TM, Fahey JV, Visintin I, Wright JA, Aldo PB, Romero R, Wira CR, Mor G. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I : C) Hum Reprod. 2006;21:2432–2439. doi: 10.1093/humrep/del178. [DOI] [PubMed] [Google Scholar]
- 36.King AE, Critchley HO, Kelly RW. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol Hum Reprod. 2000;6:191–196. doi: 10.1093/molehr/6.2.191. [DOI] [PubMed] [Google Scholar]
- 37.McNeely TB, Shugars DC, Rosendahl M, Tucker C, Eisenberg SP, Wahl SM. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997;90:1141–1149. [PubMed] [Google Scholar]
- 38.Sallenave JM. Antimicrobial activity of antiproteinases. Biochem Soc Trans. 2002;30:111–115. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 39.Koga K, Mor G. Expression and function of toll-like receptors at the maternal-fetal interface. Reprod Sci. 2008;15:231–242. doi: 10.1177/1933719108316391. [DOI] [PubMed] [Google Scholar]
- 40.Srinivas SK, Ma Y, Sammel MD, Chou D, McGrath C, Parry S, Elovitz MA. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am J Obstet Gynecol. 2006;195:797–802. doi: 10.1016/j.ajog.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 41.Han YW, Ikegami A, Bissada NF, Herbst M, Redline RW, Ashmead GG. Transmission of an uncultivated Bergeyella strain from the oral cavity to amniotic fluid in a case of preterm birth. J Clin Microbiol. 2006;44:1475–1483. doi: 10.1128/JCM.44.4.1475-1483.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seubert DE, Maymon E, Pacora P, Gervasi MT, Berry SM, Torry DS, Romero R. A study of the relationship between placenta growth factor and gestational age, parturition, rupture of membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;182:1633–1637. doi: 10.1067/mob.2000.107437. [DOI] [PubMed] [Google Scholar]
- 43.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 44.Mel’nikova VF, Aksenov OA. Infectious placentitis and characterization of the placenta as an immune barrier. Arkh Patol. 1993;55:78–81. [PubMed] [Google Scholar]
- 45.Stevens AM, McDonnell WM, Mullarkey ME, Pang JM, Leisenring W, Nelson JL. Liver biopsies from human females contain male hepatocytes in the absence of transplantation. Lab Invest. 2004;84:1603–1609. doi: 10.1038/labinvest.3700193. [DOI] [PubMed] [Google Scholar]
- 46.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koga K, Cardenas I, Aldo P, Abrahams VM, Peng B, Fill S, Romero R, Mor G. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61:196–212. doi: 10.1111/j.1600-0897.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies JK, Shikes RH, Sze CI, Leslie KK, McDuffie RS, Jr, Romero R, Gibbs RS. Histologic inflammation in the maternal and fetal compartments in a rabbit model of acute intra-amniotic infection. Am J Obstet Gynecol. 2000;183:1088–1093. doi: 10.1067/mob.2000.108888. [DOI] [PubMed] [Google Scholar]
- 49.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 50.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, Kim JS, Edwin SS, Gomez R, Draghici S. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63:73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cardenas I, Aldo P, Koga K, Means R, Lang SH, Mor G. Subclinical viral infection in pregnancy lead to inflammatory process at the placenta with non-lethal fetal damage. Am J Reprod Immunol. 2009;61:397. [Google Scholar]
- 52.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golan HM, Lev V, Hallak M, Sorokin Y, Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48:903–917. doi: 10.1016/j.neuropharm.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 56.Labeaud AD, Malhotra I, King MJ, King CL, King CH. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl Trop Dis. 2009;3:e442. doi: 10.1371/journal.pntd.0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 58.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006;12:1638–1643. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]