Abstract
Problem
Apoptosis is a normal constituent of trophoblast turnover in the placenta; however in some cases, this process is related to pregnancy complications such as preeclampsia. Recognition and engulfment of these apoptotic trophoblast cells is important for clearance of dying cells. The aim of this study was to show the cross talk between human endometrial endothelial cells (HEECs) and apoptotic trophoblast cells in an in vitro coculture model and its effect on cytokine production by HEECs.
Method of study
Fluorescent-labeled HEECs were cocultured with fluorescent-labeled apoptotic human trophoblast cells. Confocal microscopy and flowcytometry were used to show the interaction between these two types of cells. Cytokine profiles were determined using multiplex analysis.
Results
HEECs are capable to phagocytose apoptotic trophoblasts. This activity is inhibited by the phagocytosis inhibitor cytochalasin B. Phagocytosis of apoptotic trophoblast cells induced the secretion of the proinflammatory cytokines interleukin-6 and monocyte chemoattractant protein-1 by HEECs.
Conclusion
This study provides the first evidence that HEECs have an ability to phagocytose apoptotic trophoblasts. Furthermore, we demonstrated an inflammatory response of HEECs after phagocytosing the apoptotic trophoblast cells. This event may contribute to the inflammatory response in both normal pregnancy and pathologic pregnancy such as preeclampsia.
Keywords: Endometrium, interleukin-6, inflammation, monocyte chemoattractant protein-1, preeclampsia, pregnancy
Introduction
The normal placental development requires appropriate trophoblasts differentiation and invasion into the maternal decidua. As a developing organ, the placenta undergoes constant tissue remodeling, which is characterized by the functional loss of trophoblast cells by apoptosis. Apoptosis, or programmed cell death, is a physiological process associated with embryonic development, metamorphosis, and maturation of the cell turnover and has been implicated in the maintenance of tissue homeostasis.1,2 Therefore, an appropriate control of apoptosis in the placenta is critical for the establishment of a successful pregnancy where a proper tissue remodeling is crucial.3
At the maternal–fetal interface, apoptotic trophoblasts are quickly removed by phagocytosis, mainly by macrophages, which are present during the early stages of pregnancy. The quick and effective removal of apoptotic cells by phagocytes is an essential process which prevents the lysis and release of self-antigens, and in the case of pregnancy, paternal alloantigens.2,4,5 If apoptotic trophoblasts are not efficiently cleaned by phagocytes, their progressive cell death will interfere with normal placental function.
In addition to macrophages that are considered to be `professional phagocytic cells', other type of cells also known as `non-professional phagocytes' have been shown to possess the capacity to clear apoptotic cells.5,6 The role of macrophages at the maternal–fetal interface has been well characterized by us and others;4 however, the contribution of `non-professional phagocytes' has not been described.
Non-professional phagocytes are one of the well-characterized endothelial cells.6,7 During pregnancy, human endometrial endothelial cells (HEECs) are in close contact with the invading trophoblasts, especially during the process associated with the transformation of the spiral arteries. This process is characterized by increased numbers of apoptotic cells, including trophoblast cells. The role of HEECs as potential phagocytic cells during trophoblast invasion has not been evaluated. We hypothesized that HEECs have the capacity to phagocytose apoptotic trophoblast cells; therefore preventing the release of paternal antigens. In this study, we demonstrate that endothelial cells are highly efficient in removing apoptotic trophoblast cells and the phagocytic process activates HEEC to produce pro-inflammatory cytokines.
Material and method
Reagents
PKH67 green fluorescent cell linker kit (PKH67GL) and PKH26 red fluorescent cell linker kit (PKH26GL) were purchased from Sigma-Aldrich (Sigma, St Louis, MO, USA). Multiplex beads for interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) were purchased from Bio-Rad Laboratories (Hercules, CA, USA). The phagocytosis inhibitor, cytochalasin B, was purchased from Sigma-Aldrich.
Cell Culture
HEECs were isolated from endometrial specimens treated with 1 mg/mL of biotinylated UEA-1 (Ulex europaeus) lectin and immortalized by telomerase-mediated transformation as previously described.8,9 HEECs were maintained in EMB-2 growth media purchased from Cambrex (East Rutherford, NJ, USA) supplemented with an additional 10% fetal bovine serum (FBS) purchased from Gemini Bio-Products (Woodland, CA, USA) and 800 ng/mL puromycin. The first-trimester human extravillous trophoblast cell line HTR-8/SVneo, which from now on will be referred to as H8, was a gift from Dr. Charles Graham (Queens University, Kingston, ON, Canada).10 H8 cell lines were maintained in Roswell Park Memorial Institute (RPMI) 1640 media purchased from Gibco (Gaithersburg, MD, USA) supplemented with 10% FBS. RPMI media were supplemented with 10 mM HEPES, 0.1 mM minimum essential medium non-essential amino acids, 1 mM sodium pyruvate, and 100 IU/mL penicillin/streptomycin all purchased from Gibco. All cell lines were maintained in 5% CO2 at 37°C. To induce apoptosis, H8 cells were cultured with 800 ng/mL of puromycin. Induction of apoptosis, but not necrosis, was confirmed by the increase in caspase 3 and negative staining of propidium iodide.
Fluorescent Linker Dye PKH26/PKH67 Labeling of Cells
Cells were washed twice in phosphate buffered saline (PBS), and then labeled using the PKH26-GL/PKH67-GL kit, according to the manufacturer's instructions. Briefly, 5 × 105 cells were incubated with either PKH26 or PKH67 dye in 200 μL diluent C (supplied with the kit) for 2 min at room temperature with gentle mixing. The final concentrations of the dye varied among experiments and will be indicated later. The reaction was stopped with 200 μL of FBS and incubating for 1 min at room temperature, then diluted with 2.0 mL of PBS, and centrifuged at 800 × g for 10 min. The supernatant was removed, and the labeled cell pellet was resuspended with media and plated.
Coculture of Endothelial Cells with Trophoblast for Confocal Microscope Observation
HEECs (7 × 104) labeled with green fluorescent linker dye PKH67 (final concentration: 2 × 10−5 M) were plated at 60-mm tissue culture dish in EMB-2 growth media supplemented with an additional 10% FBS and 800 ng/mL puromycin. When they reached 70% confluence, red fluorescent linker dye PKH26 (final concentration: 2 × 10−5 M) labeled H8 cells (1 × 105) were overlaid on HEECs and incubated over a period of 6–24 hr in 5% CO2 at 37°C. Imaging of the cells was performed using an Olympus FluoView FV300 laser scanning confocal microscope (Olympus Inc., Tokyo, Japan) equipped with lasers providing 488 and 543 nm laser lines for PKH67 and PKH26 visualization, respectively. Prior to each individual observation, we adjusted the photomultiplier voltage using single stained cells to avoid color spillover. z-stack pictures were also obtained. For this experiment, a series of images were taken at every 1 μm in the Z axis to display a three-dimensional image.
Flowcytometric Analysis of Endothelial Cell and Trophoblast Coculture
H8 cells labeled with red fluorescent linker dye PKH26 (final concentration: 4 × 10−7 M) were overlaid on 70% confluent HEECs that had been labeled with the green fluorescent linker dye PKH67 (final concentration: 2 × 10−6 M) in a six-well tissue culture plate in EBM-2 supplemented with 10% FBS and 800 ng/mL puromycin. Cocultured cells were harvested at 2, 4, 6, 8, and 24 hr by treatment with 0.02% trypsin–ethylenediaminetetraacetic acid. Detached cells were collected, washed with PBS, and fixed with 2% paraformaldehyde. Labeled cells were analyzed on a flowcytometer (BD LSRII Green; Becton Dickinson, San Jose, CA, USA) using 488-nm blue laser for PKH67 and 532 green laser for PKH26. The negative control sample was prepared using green HEECs and red H8 cells that had been cultured separately and mixed just prior to flowcytometric analysis. A total of 10,000 events were recorded for each sample. The PKH67 and PKH26 double positive population was defined as HEECs containing phagocytosed H8. For phagocytosis inhibition studies, HEECs and H8 cells were cocultured in the presence of 10 μg/mL cytochalasin B for 24 hr.
Cytokine Analysis
Coculture of HEECs and H8 cells was performed as described earlier, without cell labeling. Supernatant from coculture or control single culture was collected at 2, 6, 8, and 24 hr and centrifuged. Cell-free supernatant was then transferred to a fresh tube and stored at −80°C until further use. Cytokine production was determined using cytokine multiplex assays from Bio-Rad Laboratories. Briefly, wells of a 96-well filter plate were loaded with either 50 μL of prepared standard solution or 50 μL of cell-free supernatant and incubated on an orbital shaker at ±800 × g for 2 hr in the dark at room temperature. Wells were then vacuum-washed three times with 100 μL wash buffer. Samples were then incubated with 25 μL of biotinylated detection antibody at ±800 × g for 30 min at room temperature in the dark. After three washes, 50 μL of streptavidin-Phycoerythrin was added to each well and incubated for 10 min at ±800 × g at room temperature in the dark. After a final wash, the beads were resuspended in 125 μL of assay buffer for measurement using the Bio-Plex 200 (Bio-Rad Laboratories).
Statistical Analysis
Data were analyzed using ANOVA or student's t-test as appropriate. Results were considered to be statistically significant if P < 0.05.
Results
Phagocytosis of Apoptotic Trophoblast Cell by HEECs
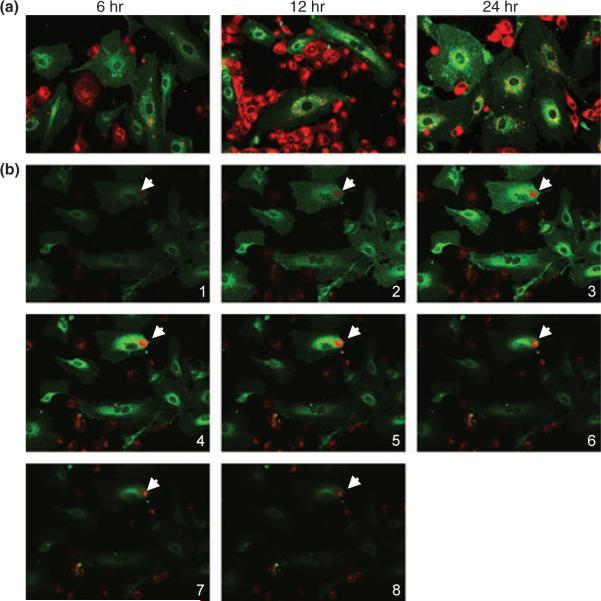
To determine the capacity of HEECs to phagocytose apoptotic trophoblast cells, we overlaid the first-trimester trophoblast H8 cells labeled with red fluorescent linker dye PKH26 to 70% confluent HEECs that had been labeled with green fluorescent linker dye PKH67 in EBM-2. Prior coculture trophoblast cells were treated with 800 ng/mL puromycin for 2 hr, which we found to be effective on initiating apoptosis. The coculture was monitored under a confocal microscope for a period of 24 hr. After 6 hr of coculture, we observed the presence of red fluorescent particles from apoptotic trophoblast cells clearly located in the cytoplasm of green HEECs, which indicated that HEECs phagocytosed H8 cells (Fig. 1a). Further increase in the number of trophoblast apoptotic particles was observed at 12 and 24 hr. Interestingly, we observed increasing number of red fluorescent particles in lysosomes around the nucleus, indicating lysosome digestion of trophoblast fragment (Fig. 1a). To further confirm that trophoblast particles locate inside the cytoplasm of HEECs but not simply sit on the surface of HEECs, we used z-stack imaging. As shown in Fig. 1b, the trophoblast-derived particles certainly do locate inside the endothelial cell but not on its surface.
Fig. 1.
Phagocytosis of apoptotic trophoblast cell by endothelial cell in vitro. PKH26-labeled H8 cells (red) were overlaid on 70% confluent PKH67-labeled human endometrial endothelial cells (HEECs) (green) in EBM-2 supplemented with 10% fetal bovine serum and 800 ng/mL puromycin and observed under confocal microscope at different time points (magnification: 400×). (a) Coculture of HEECs with apoptotic H8 cells for 6 hr results in engulfment of trophoblast fragment by endothelial cell. More phagocytosed particles appear in the lysosome around the nucleus after 12 and 24 hr of coculture. (b) Serial z-stack images (from top to bottom, 1 μm apart) of HEECs coculture with apoptotic H8 cells. Note trophoblast-derived particle locates inside the endothelial cells (arrows).
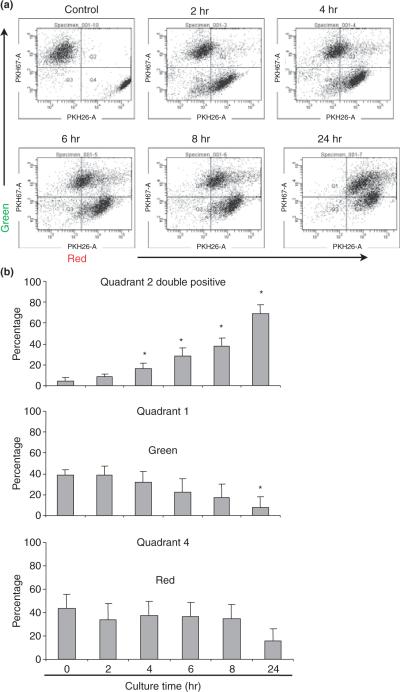
To quantify the process of phagocytosis, we cocultured HEECs with apoptotic H8 cells for 2, 4, 6, 8, and 24 hr and performed fluorescence-activated cell sorter analysis. In Fig. 2a, the population of PKH26 (red) and PKH67 (green) double positive (upper quadrant; Quadrant 2) indicates HEECs (green labeled) that have engulfed apoptotic H8 cells (red labeled). The control sample that is a mixture of HEECs and non-apoptotic H8 cells had negligible amount of double positive cells (4.6%). When we looked at individual quadrants quantitatively, the percentage of double positive cells at 2, 4, 6, 8, and 24 hr of coculture increased from 8.2 to 15.9, 28.6, 37.6, and 69.0%, respectively (Fig. 2b, Quadrant 2). This increase was statistically significant from 4 hr and onwards. Moreover, the percentage of single stained green HEECs, which indicates the HEECs that have not engulfed H8 cells, decreased gradually over time. Therefore, the number of cells became significantly lower in 24 hr (Fig. 2b, Quadrant 1).
Fig. 2.
Flowcytometric analyze of trophoblast cell and endothelial cells co culture in vitro. PKH26-labeled H8 cells (red) were overlaid on 70% confluent PKH67-labeled human endometrial endothelial cells (HEECs) (green) in EBM-2 supplemented with 10% fetal bovine serum and 800 ng/mL puromycin. Cells were harvested and analyzed using flowcytometry after 2, 4, 6, 8, and 24 hr of coculture. Negative control was prepared by mixing HEECs with H8 cells just before flowcytometry. (a) Control and 2, 4, 6, 8, and 24 hr of HEECs and H8 cells coculture. Quadrant 1 (upper left): green fluorescent HEECs only; Quadrant 2 (upper right): double fluorescent HEECs phagocytosed apoptotic H8 cells; Quadrant 4 (lower left): red fluorescent H8 cells only. (b) Bar chart shows the average percentage of HEECs phagocytosed apoptotic H8 cells (Quadrant 2), HEECs (Quadrant 1) and H8 cells (Quadrant 4) at different time point of coculture. Data are presented as mean ± S.D.; *P < 0.05 relative to control.
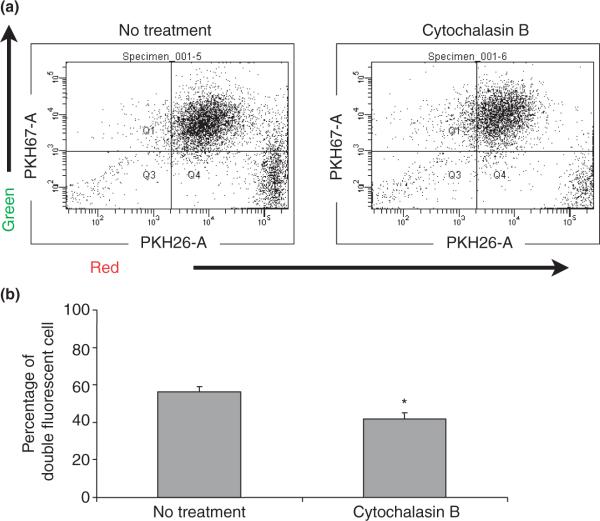
To further confirm that double positive cells in this analysis were representing cells in phagocytosis process, we carried out inhibitory experiments, using phagocytosis inhibitor cytochalasin B. As Fig. 3a,b shows, the percentage of double positive cells was significantly reduced by 24-hr treatment of cytochalasin B, which indicates that this population is representative of cells with phagocytosis.
Fig. 3.
(a) Human endometrial endothelial cells (HEECs) phagocytosed apoptotic H8 cells in the presence or absence of 10 μg/mL cytochalasin B for 24 hr. (b) Bar chart shows the average percentage of double fluorescent HEECs phagocytosed apoptotic H8 cells in the presence or absence of 10 μg/mL cytochalasin B for 24 hr. Data are presented as mean ± S.D.; *P < 0.05.
The Effect of Phagocytosis on HEECs's Cytokine Production
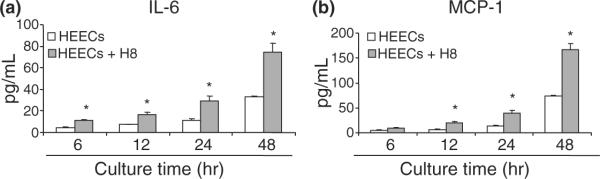
Knowing that the HEECs have the capacity to phagocytose apoptotic trophoblast, we further studied the effect of phagocytosis on the character of endothelial cells, focusing on their cytokine production. Thus, HEECs were incubated in the presence or absence of apoptotic trophoblast; and cytokine production was monitored at 6, 12, 24, and 48 hr. Fig. 4 demonstrates the concentration of IL-6 and MCP-1, in cell-free supernatants from either HEECs single culture or HEECs cocultured with apoptotic H8 cells. The level of IL-6 and MCP-1 in supernatants was significantly higher in HEECs exposed to apoptotic H8 cells compared with control HEECs. The increase followed a time-dependent manner (Fig. 4a,b). These findings suggest that the engulfment of apoptotic trophoblasts stimulates the production of cytokines by HEECs.
Fig. 4.
Cytokine secretion of endothelial cells and apoptotic trophoblast coculture in vitro. H8 cells were cocultured with human endometrial endothelial cells (HEECs) monolayered in EBM-2 supplemented with 10% fetal bovine serum and 800 ng/mL puromycin. After 6, 12, 24, and 48 hr of coculture, cell-free supernatant was collected, and cytokine concentration was measured using LUMINEX (Austin, TX, USA). The cytokine level of HEECs and H8 cells coculture group is minus with the cytokine level of H8 cells single culture control group. Data are presented as mean ± S.D.; *P < 0.05. (a) Interleukin-6; (b) monocyte chemoattractant protein-1.
Discussion
Our study demonstrated that HEECs, which are adjacent to trophoblast cells at the maternal–fetal interface, can recognize and clear apoptotic trophoblast cells.
Trophoblast cells are `carriers' of paternal antigens, which are foreign to the maternal immune system. If those antigens are released, as a result of cell death, it may initiate an immune response with lethal consequences for the fetus. Therefore, a quick and effective removal of apoptotic trophoblast cells by phagocytosis before the release of their intracellular contents is critical for the prevention of placental tissue damage and fetal rejection.4,11,12
We demonstrated by confocal microscopy that apoptotic trophoblasts are phagocytosed by HEECs in vitro within 6 hr. Flowcytometry analysis further confirmed that HEECs phagocytosed apoptotic trophoblast cells in a time-dependent manner and this process was partially inhibited by phagocytosis inhibitor cytochalasin B. This novel function for HEECs implicates that HEECs are not simply a component of the vessel wall but function as `non-professional phagocytes' and contribute to maintain the placental function.
We also found that HEECs that had engulfed apoptotic trophoblast cells secreted high levels of IL-6 and MCP-1, compared with HEECs controls. It has been previously shown that upon engulfment of apoptotic cells, endothelial cells increase expression of proinflammatory chemokines, growth and survival factors and enhance binding of leukocytes to endothelial cells.7,13,14 In other words, endothelial cells, not only eliminate apoptotic cells, but also influence the specific local microenvironment. IL-6 is a multifunctional cytokine that regulates various immune responses, such as acute phase reaction and hematopoiesis. It plays a central role in host defense mechanisms and is implicated in the recruitment of T cells to the site of inflammation. MCP-1 is a chemoattractant for monocytes, and increased MCP-1 expression may result in the adherence of monocytes to the vascular wall.15,16 MCP-1 may activate not only infiltrating leukocytes, but also surrounding structural cells and maintain the inflammatory milieus.17,18 In addition to these immune regulatory functions, IL-6 and MCP-1 are important factors promoting tissue repair and renewal.4
Taken together, our results strongly suggest that endometrial endothelial cells, upon engulfment of apoptotic trophoblast cells, may initiate an inflammatory response and modulate the immune cells present at the maternal–fetal interface.
It has been shown that the first trimester of pregnancy is characterized by mild proinflammatory status both systemically and locally. Indeed, the concentrations of serum MCP-1 levels in the first trimester are higher than other stages of pregnancy,19–21 and local expression of IL6 and MCP-1 is known to be upregulated.22 In this regard, it is tempting to speculate that cytokine/chemokines produced by endometrial endothelial cells that engulfed physiological apoptotic trophoblast cells may contribute to this proinflammatory status.
In contrast to normal pregnancy, which characterized by a mild inflammatory response, preeclampsia, a serious pregnancy related disorder, is characterized by a similar but greater intense inflammatory response.23 Indeed, the expression of primary proinflammatory cytokines like tumour necrosis factor-α, IL-6, and interleukin-8 in the preeclamptic placenta is reported to be increased.24,25 It has been also shown that in preeclampsia, trophoblast apoptosis is exaggerated, and its clearance by macrophages is delayed.26,27 These features of preeclampsia further prompt us to speculate that in preeclampsia, phagocytosis of apoptotic trophoblast by endometrial endothelial cells is exaggerated, and as a result, the inflammatory response may be enhanced and that contributes to the pathogenesis of preeclampsia.
In summary, our study provides the first evidence that HEECs have an ability to engulf and phagocytose apoptotic trophoblast as a `non-professional phagocyte'. Furthermore, we demonstrated that the process of phagocytosis may play a physiological role promoting tissue repair and spiral arteries transformation. Alterations in the balance between apoptosis and cell clearance may lead to abnormal levels of inflammatory cytokine affecting endothelial cells' homeostasis; which then may lead to pregnancy implications such as preeclampsia.
Acknowledgment
This study is in part funded by grants from the National Institute of Health, NICDH P01HD054713.
References
- 1.Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Fadeel B. Programmed cell clearance. Cell Mol Life Sci. 2003;60:2575–2585. doi: 10.1007/s00018-003-3145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26:877–897. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- 4.Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G. Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol. 2004;51:275–282. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 5.Savill J, Gregory C, Haslett C. Cell biology. Eat me or die. Science (New York, NY) 2003;302:1516–1517. doi: 10.1126/science.1092533. [DOI] [PubMed] [Google Scholar]
- 6.Hess KL, Tudor KS, Johnson JD, Osati-Ashtiani F, Askew DS, Cook-Mills JM. Human and murine high endothelial venule cells phagocytose apoptotic leukocytes. Exp Cell Res. 1997;236:404–411. doi: 10.1006/excr.1997.3745. [DOI] [PubMed] [Google Scholar]
- 7.Kirsch T, Woywodt A, Beese M, Wyss K, Park JK, Erdbruegger U, Hertel B, Haller H, Haubitz M. Engulfment of apoptotic cells by microvascular endothelial cells induces proinflammatory responses. Blood. 2007;109:2854–2862. doi: 10.1182/blood-2006-06-026187. [DOI] [PubMed] [Google Scholar]
- 8.Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145:2291–2296. doi: 10.1210/en.2003-1606. [DOI] [PubMed] [Google Scholar]
- 9.Krikun G, Mor G, Huang J, Schatz F, Lockwood CJ. Metalloproteinase expression by control and telomerase immortalized human endometrial endothelial cells. Histol Histopathol. 2005;20:719–724. doi: 10.14670/HH-20.719. [DOI] [PubMed] [Google Scholar]
- 10.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 11.Dini L, Lentini A, Diez GD, Rocha M, Falasca L, Serafino L, Vidal-Vanaclocha F. Phagocytosis of apoptotic bodies by liver endothelial cells. J Cell Sci. 1995;108(Pt 3):967–973. doi: 10.1242/jcs.108.3.967. [DOI] [PubMed] [Google Scholar]
- 12.Hall SE, Savill JS, Henson PM, Haslett C. Apoptotic neutrophils are phagocytosed by fibroblasts with participation of the fibroblast vitronectin receptor and involvement of a mannose/fucose – specific lectin. J Immunol. 1994;153:3218–3227. [PubMed] [Google Scholar]
- 13.Maderna P, Godson C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim Biophys Acta. 2003;1639:141–151. doi: 10.1016/j.bbadis.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, Henson PM, Voelkel NF. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J. 2004;18:1716–1718. doi: 10.1096/fj.04-1853fje. [DOI] [PubMed] [Google Scholar]
- 15.Watson C, Whittaker S, Smith N, Vora AJ, Dumonde DC, Brown KA. IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin Exp Immunol. 1996;105:112–119. doi: 10.1046/j.1365-2249.1996.d01-717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaouat G, Dubanchet S, Ledee N. Cytokines: important for implantation? J Assist Reprod Genet. 2007;24:491–505. doi: 10.1007/s10815-007-9142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 18.Wu D, Koga T, Martin KR, Meydani M. Effect of vitamin E on human aortic endothelial cell production of chemokines and adhesion to monocytes. Atherosclerosis. 1999;147:297–307. doi: 10.1016/s0021-9150(99)00199-9. [DOI] [PubMed] [Google Scholar]
- 19.Mor G. Inflammation and pregnancy: the role of toll-like receptors in trophoblast-immune interaction. Ann NY Acad Sci. 2008;1127:121–128. doi: 10.1196/annals.1434.006. [DOI] [PubMed] [Google Scholar]
- 20.Mor G, Romero R, Aldo PB, Abrahams VM. Is the trophoblast an immune regulator? The role of Toll-like receptors during pregnancy. Crit Rev Immunol. 2005;25:375–388. doi: 10.1615/critrevimmunol.v25.i5.30. [DOI] [PubMed] [Google Scholar]
- 21.Koga K, Aldo PB, Mor G. Toll-like receptors and pregnancy: trophoblast as modulators of the immune response. J Obstet Gynaecol Res. 2009;35:191–202. doi: 10.1111/j.1447-0756.2008.00963.x. [DOI] [PubMed] [Google Scholar]
- 22.Engert S, Rieger L, Kapp M, Becker JC, Dietl J, Kammerer U. Profiling chemokines, cytokines and growth factors in human early pregnancy decidua by protein array. Am J Reprod Immunol. 2007;58:129–137. doi: 10.1111/j.1600-0897.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- 23.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 24.Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. 2007;58:21–30. doi: 10.1111/j.1600-0897.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 25.Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin immunopathol. 2007;29:151–162. doi: 10.1007/s00281-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 26.Levy R. The role of apoptosis in preeclampsia. Isr Med Assoc J. 2005;7:178–181. [PubMed] [Google Scholar]
- 27.Crocker IP, Cooper S, Ong SC, Baker PN. Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol. 2003;162:637–643. doi: 10.1016/S0002-9440(10)63857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]