Abstract
Apoptosis, programmed cell death, is an essential feature of normal placental development but is exaggerated in association with placental disease. Placental development relies upon effective implantation and invasion of the maternal decidua by the placental trophoblast. In normal pregnancy, trophoblast apoptosis increases with placental growth and advancing gestation. However, apoptosis is notably exaggerated in the pregnancy complications, hydatidiform mole, pre-eclampsia, and intra-uterine growth restriction (IUGR). Placental apoptosis may be initiated by a variety of stimuli, including hypoxia and oxidative stress. In common with other cell-types, trophoblast apoptosis follows the extrinsic or intrinsic pathways culminating in the activation of caspases. In contrast, the formation of apoptotic bodies is less clearly identified, but postulated by some to involve the clustering of apoptotic nuclei and liberation of this material into the maternal circulation. In addition to promoting a favorable maternal immune response, the release of this placental-derived material is thought to provoke the endothelial dysfunction of pre-eclampsia. Widespread apoptosis of the syncytiotrophoblast may also impair trophoblast function leading to the reduction in nutrient transport seen in IUGR. A clearer understanding of placental apoptosis and its regulation may provide new insights into placental pathologies, potentially suggesting therapeutic targets.
Keywords: Apoptosis, IUGR, pre-eclampsia, trophoblast
Introduction
Pre-eclampsia and intra-uterine growth restriction (IUGR) are major pregnancy complications, resulting in significant perinatal mortality and morbidity. While their precise etiology is unknown, it is hypothesized that placental dysfunction is central to their development. A common feature of the placenta in pre-eclampsia, IUGR and molar pregnancies, i.e. hydatidiform mole (trophoblast hyperplasia), is exaggerated placental apoptosis. As a result of these observations, apoptosis is suggested to be a key mechanism in placental dysfunction. This review describes the evidence for the presence of apoptosis in normal placental development, its alteration in placental dysfunction and development of placental diseases, such as pre-eclampsia.
Apoptosis
Apoptosis is evident in both physiological and pathological circumstances and was first described by Kerr and Wylie in 1972.1 Apoptosis (Greek: apo – from, ptosis – falling) is a form of programmed cell death characterized by the condensation of cell cytoplasm and organelles into membrane covered dense apoptotic bodies. During nuclear condensation, the nuclear lamina is dissembled, allowing the cleavage of DNA into 200 base pair fragments.2,3 Moreover, the cell membrane undergoes extensive alterations with loss of asymmetry, externalizing phosphatidylserine, promoting phagocytosis.4 In contrast to necrotic cell death, apoptosis represents a series of energy-dependent events, removing unwanted cell material while avoiding an immune response and damage to surrounding tissues. Apoptosis is initiated via the extrinsic or intrinsic pathway. Both pathways rely upon a cascade of protein interactions orchestrated by a family of 14 cysteine proteases, caspases, which are able to cleave structural proteins producing the morphological appearances typical of apoptosis. In addition, active caspases potentiate the apoptotic signal by activating a variety of pro-apoptotic proteins.
The extrinsic pathway is controlled by members of the tumour necrosis factor (TNF) death receptor family. There are eight members of this family with Fas (CD95/APO-1), TNF-R1 (CD120a), and TNF-related apoptosis inducing ligand (TRAIL), being the most studied.5 Binding of an external ligand to the death receptor allows protein–protein interactions between the receptor and a cytoplasmic death domain, such as Fas-associated death domain (FADD) or TNF-R-associated death domain.6 Binding of FADD to the death receptor recruits procaspase-8 or procaspase-10 via death effector domains.7–9 The combination of these proteins forms the death-inducing signaling complex which cleaves procaspase-8 and procaspase-10 to their active forms, initiating the caspase cascade.10 Sometimes this signal is further amplified by cleavage of Bid by caspase 8, which also activates the intrinsic pathway.11
The intrinsic pathway is initiated by cellular stress; such as DNA damage, reactive oxygen species, the unfolded protein response, or removal of growth factor support. Activation of the intrinsic pathway leads to alteration in mitochondrial membrane permeability because of an imbalance in the relationship of pro- and anti-apoptotic Bcl-2 proteins.12 Increased mitochondrial permeability causes membrane pore formation and leakage of cytochrome c into the cytosol.13 In the cytosol, cytochrome c is bound by apoptosis protease activating factor-1 (APAF-1) forming the apoptosome.14 The apoptosome cleaves procaspase-9 activating the terminal pathway of apoptosis. During apoptosis, other mitochondrial contents such as smac/Diablo are also released from the mitochondria, antagonizing anti-apoptotic inhibitor of apoptosis proteins.15,16 Both pathways culminate in a terminal pathway involving the cleavage and activation of caspase-3, 6, and 7 initiating cell destruction by activating DNAses and cleaving DNA repair enzymes such as PARP.17,18
Apoptotic cells can be identified in a variety of ways. The initial description of cell morphology was based upon observations of ultrastructure by electron microscopy including increased nuclear cytoplasmic ratio, cytoplasmic condensation, and deposition of euchromatin around the nuclear periphery. Subsequently, some of these changes in cell morphology particularly increased nuclear/cytoplasmic ratio have been observed by light microscopy. 19 Biochemical measures have also been utilized to confirm the presence of cleaved DNA into 200 bp fragments by the presence of DNA ladders (Fig. 1).20 More recently, combined histological and biochemical approaches have led to the development of staining techniques that recognize cleaved DNA by terminal deoxy-uridine nick-end labeling (TUNEL), 21 by externalization of phosphatidylserine or by cytoskeletal cleavage products indicative of caspase activity e.g. cytokeratin M30 neoepitope22 (Fig. 2). Often studies will combine multiple measures to ensure apoptotic specificity.
Fig. 1.
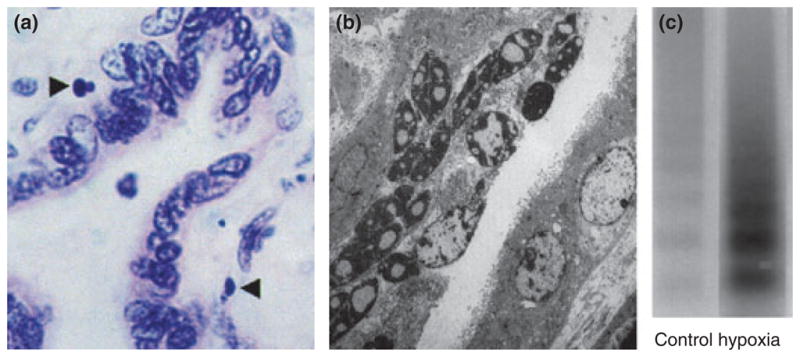
Apoptosis in the villous placenta. (a) Light micrograph (oil immersion, H & E stained) of 4 μm section of term villous placenta. Apoptotic nuclei of trophoblast highlighted (arrows). Magnification × 1000. (b) Electron micrograph showing pyknotic and apoptotic nuclei within the syncytiotrophoblast (original magnification × 4250). Smith et al. 1997, reproduced with permission. (c) Increased DNA fragmentation (laddering) in term trophoblast under hypoxic conditions. Reproduced from Levy et al. 2000 with permission.
Fig. 2.
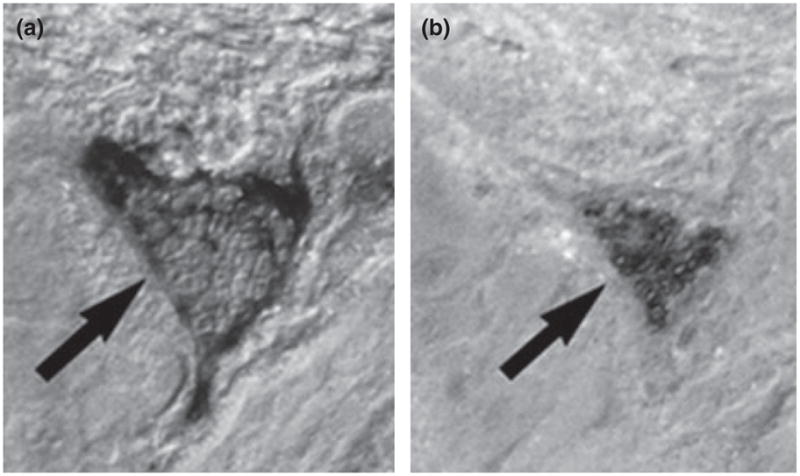
Apoptotic multi-nucleated giant cell from the third trimester placental bed (arrow). (a) Stained for cleaved cytokeratin 18 (M30) and (b) TUNEL. Reproduced from Kadyrov et al. 2001 with permission.
Apoptosis and implantation
Normal placental development relies upon the invasion of the maternal decidua by extravillous trophoblast, and the subsequent remodeling of maternal spiral arteries to provide stability to the placenta and efficient utero-placental blood flow. Extravillous trophoblast invades the maternal decidua as far as the proximal third of the myometrium, with a greater depth observed centrally.23 A subgroup of extravillous trophoblast invades the maternal spiral arteries replacing the endothelium and remodeling the maternal vasculature. Initially this process is trophoblast independent,24 although eventually requiring trophoblast for completion.25 Trophoblast disrupts the endothelium causing the loss of underlying smooth muscle allowing further trophoblast invasion of the decidua.26 Recently, it has been suggested that vascular remodeling may be indirectly controlled by intravascular trophoblast that stimulates endothelial cells to secrete chemokines. These chemokines attract decidual leukocytes, particularly uterine natural killer cells and macrophages, leading to vascular smooth muscle cell apoptosis.27 A suggested mechanism for endothelial cell destruction is via the Fas/FasL system, which is present on endothelial and vascular smooth muscle cells of the uterine spiral arteries.25
The remodeling of maternal spiral arteries is again not uniform throughout the decidua, with a higher incidence in the center region compared to the periphery.23,28 In addition to remodeling, trophoblast has been suggested to block maternal vessels allowing the fetus to develop initially in a hypoxic environment, protected from reactive oxygen species until around 10 weeks gestation, when placental blood flow is restored.29
In pre-eclampsia and IUGR, there may be a reduction in the number of trophoblast cells within the spiral arteries, which has been associated with increased apoptosis and a reduced luminal size.30,31 Others have found a reduction in the extent of trophoblast invasion in severe pre-eclampsia, both in the spiral arteries and the myometrium.32 Poor trophoblast invasion and remodeling of uterine spiral arteries have been suggested to lead to the development of a high pressure placental blood supply, which may in turn damage the developing villous tree causing a change in placental structure.33,34 It has been suggested that this damage leads to hypoxia and impaired blood flow, as determined by aberrant Doppler ultrasound waveforms in IUGR and severe pre-eclampsia.35
Apoptosis in placental villi
After 10 weeks gestation, the human villous placenta develops within a lake of maternal blood, with the tree-like structure becoming progressively more branched and convoluted to form the terminal villi. These terminal villi consist of stroma, containing fetal capillaries, beneath a layer of progenitor cytotrophoblast cells, which are in turn covered by a continuous multinucleated syncytium called the syncytiotrophoblast. The syncytiotrophoblast forms a barrier between the fetal and maternal circulations and is essential for the normal immunological, endocrine, and nutritional functions of the placenta. Fusion between the cytotrophoblasts and the syncytiotrophoblast has been suggested as a means of replenishing the syncytiotrophoblast, although the exact physiological function is unknown. Fusion of the villous cytotrophoblast with the syncytiotrophoblast is associated with the presence of GCM-1,36 Syncytin-1 & -237 and caspase-8.38
The amount of apoptosis in placental villi changes throughout normal pregnancy, being lowest in the first trimester, increasing in the third, and markedly accelerating beyond 40 weeks gestation.39,40 Interest has been stimulated by the observation that increased levels of villous trophoblast apoptosis have been identified in placental pathologies (Fig. 3), including early pregnancy loss,41,42 pre-eclampsia,43–46 IUGR,45,47–49 and gestational trophoblastic disease, including partial and complete hydatidiform mole and choriocarcinoma.50,51 Systemic disease can also impact upon the human placenta, as maternal diabetes is similarly associated with increased placental apoptosis.52 Moreover, the finding of increased apoptotic maternal is not confined to the placenta. In pre-eclampsia, increased levels of syncytial derived cytokeratin M30 neoepitope can be detected in maternal serum.53
Fig. 3.
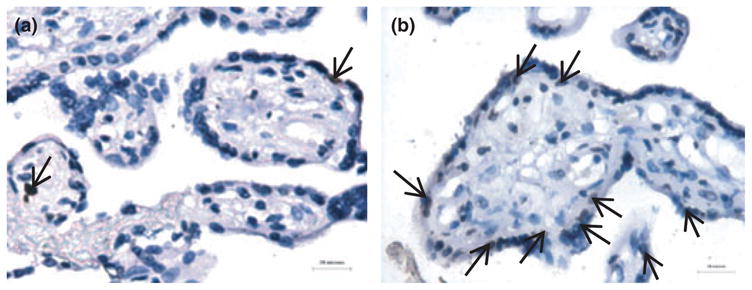
Exaggerated trophoblast apoptosis in pre-eclampsia. (a) occasional TUNEL positive nuclei (arrows) in the syncytiotrophoblast in normal pregnancy. (b) increased apoptotic events in pre-eclampsia (brown stained nuclei). Reproduced from Heazell et al. 2008 with permission.
Apoptosis within the villus is predominantly localized to the syncytiotrophoblast with virtually no events within term cytotrophoblast and a very low incidence in first trimester cytotrophoblast.19,54–56 In support, the syncytiotrophoblast demonstrates features consistent with apoptosis, such as externalization of phosphatidylserine, caspase-8 and caspase-9 activation, cytokeratin-18 cleavage, and DNAse activity.38,56–58 Importantly, apoptotic pathways may be involved in the maintenance of the syncytiotrophoblast. This stems from two observations, first that caspase 8 is involved in the fusion of cytotrophoblast with syncytiotrophoblast, and second that some syncytiotrophoblast nuclei exhibit morphological features of apoptosis with peripheral chromatin condensation and gradual pyknosis.
Within the syncytiotrophoblast, syncytial knots are aggregations of pyknotic nuclei which accumulate at the syncytial surface before being lost into the maternal circulation as membrane bound entities. The presence of such material has been identified within the maternal venous circulation prior to destruction by pulmonary macrophages.59,60 It is the release of this apoptotic material into the maternal circulation that has been suggested as a mechanism for maternal endothelial disruption in pre-eclampsia.61 However, the theory that syncytial knots consist of truly apoptotic nuclei has recently been challenged.62 Nevertheless, apoptosis occurs in discrete areas within the syncytiotrophoblast, particularly those associated with damage e.g. fibrin deposition. This is unexpected as apoptosis in other organs and tissues is confined to individual cells,63 and an apoptotic signal would be expected to spread throughout the syncytiotrophoblast.
The origin of exaggerated apoptosis in pregnancy complications is not clear. Although the extrinsic pathway is active in trophoblast,64,65 the in vivo association with abnormal conversion of the uterine spiral arteries, more readily implicates hypoxia and/or oxidative stress. In support, exaggerated apoptosis can be reproduced in trophoblast in vitro by exposure to hypoxia66 and reactive oxygen species.67 Interestingly, villous trophoblast from placentas of pregnancies complicated by IUGR or pre-eclampsia demonstrate increased susceptibility to apoptosis, an inherent change rendering these cells more vulnerable to oxidative damage.43–45,47,48 In molar pregnancies, increased apoptosis is thought to reflect the cell fate of some trophoblast resulting from uncontrolled hyperplasia. This is confirmed by greater levels of apoptosis in more invasive and proliferative disease.50
The interplay of pro and anti-apoptotic regulators is crucial for the control of apoptosis and the expression of these in villous trophoblast has been the subject of significant study (reviewed in detail by Heazell and Crocker, 2008).68 It is well recognized that both cytotrophoblast and syncytiotrophoblast express TNF receptors, Fas and Fas ligand, and TRAIL69,70 and its death and decoy receptors, each may play an important role, not only in apoptosis but also in immune regulation (comprehensively reviewed by Straszewski-Chavez et al., 2005). Other important regulators of cell fate include proteins such as the transcription factor p53 and members of the Bcl-2 family. p53 has been identified in cytotrophoblasts but is only rarely observed in the syncytiotrophoblast.71,72 Placental p53 is present in a non-mutated, wild-type form in both normal tissue and gestational trophoblast disease.73,74 By contrast, the natural inhibitor of p53, Mdm2, is expressed within both the cytotrophoblast and syncytiotrophoblast in the first trimester and at reduced levels in third-trimester tissue.74–76 A variety of p53 target proteins are also located in the villous placenta including the cell cycle regulator p21, which is strongly expressed in first trimester cytotrophoblasts and more weakly expressed in the syncytiotrophoblast and third trimester tissue.71,75,77
The regulation of apoptosis in placental disease
In general, more dramatic morphological and developmental changes occur in the villous placenta in cases of IUGR and early-onset pre-eclampsia, i.e. before 32 weeks gestation. These changes predominantly relate to impoverished villus development and reduced capillary growth.78 In pre-eclampsia, apoptosis has been associated with a reduction in syncytiotrophoblast, a response not seen in idiopathic IUGR,79 and an observation supportive of irregular syncytiotrophoblast formation or excessive syncytial loss.
Alterations in a variety of pro- and anti-apoptotic proteins have been observed in pre-eclampsia and IUGR (Table 1). For example, p5380 and the pro-apoptotic isoform of Mcl-181 are increased in pre-eclampsia and Bcl-245 and syncytin decreased.81 Others have found p53 to be increased in severe pre-eclampsia with HELLP syndrome.82 In IUGR, the pattern is similar with increased staining observed for p53,47 caspase-3,48 and p21.83 However, not all proteins are affected equally with most observers finding no effect upon Bax43,47 and conflicting results reported for Bcl-2.45,47
Table I.
Selected Studies of Apoptosis in Spiral Artery Remodeling and in the Placental Pathologies, Pre-eclampsia, and Intrauterine Growth Restriction (IUGR)
| Author | Year | Tissue | Method | Main findings |
|---|---|---|---|---|
| IUGR | ||||
| Smith et al. | 1997 | Villous TB | IHC, EM | TUNEL staining demonstrates an increase in apoptosis in IUGR |
| Ishihara et al. | 2002 | Villous TB | IHC, EM | Increased TUNEL-positive nuclei in pre-eclampsia in both ST and CT. BcL-2 reduced in severe pre-eclampsia and IUGR. No difference in Fas |
| Levy et al. | 2002 | Villous TB | IHC | Increased TUNEL-positive nuclei in IUGR. Increased p53 in CT of IUGR but no difference in BcL-2 family proteins |
| Crocker et al. | 2003 | Villous TB | IHC | Enhanced apoptosis in IUGR and pre-eclampsia after treatment with 3%O2 or TNFa |
| Crocker et al. | 2004 | Villous TB | IHC | Increased apoptosis in placentas from women with pre-eclampsia or IUGR exposed to TNFa or 3%O2 |
| Daayana et al. | 2004 | Villous TB | Microscopy | Reduced syncytial area in IUGR. Reduction in syncytial/villous area ratio in pre-eclampsia but not in IUGR |
| Endo et al. | 2005 | Villous TB | IHC, EM | TUNEL staining and activated caspase-3 showed increased apoptosis in IUGR vs normal. No difference in p53 or Bax |
| Kadyrov et al. | 2006 | EVT | IHC | Severely impaired trophoblast invasion in pre-eclampsia and IUGR. Increased EVT apoptosis |
| Davy et al. | 2009 | Villous TB | Southern analysis | Increase in cell senescence regulators p21, p16, and EF-1 alpha in FGR placentas |
| Pre-eclampsia | ||||
| Difederico et al. | 1999 | EVT | IHC | 15–50% EVT apoptosis in pre-eclampsia, virtually zero in control. Lack of Bcl-2 staining in preeclamptic EVT |
| Allaire et al. | 2000 | Villous TB | IHC | Increased TUNEL-positive nuclei, increased Fas and reduced FasL in the villous TB of preeclamptic patients vs controls |
| Leung et al. | 2001 | Villous TB | EM, microscopy | Increased apoptosis in placentas from women with pre-eclampsia |
| Ishihara et al. | 2002 | Villous TB | IHC, EM | Increased TUNEL-positive nuclei in pre-eclampsia in both ST and CT. BcL-2 reduced in severe pre-eclampsia and IUGR. No difference in Fas |
| Crocker et al. | 2003 | Villous TB | IHC | Enhanced apoptosis in IUGR and pre-eclampsia after treatment with 3%O2 or TNFa |
| Crocker et al. | 2004 | Villous TB | IHC | Increased apoptosis in placentas from women with pre-eclampsia or IUGR exposed to TNFa or 3%O2 |
| Daayana et al. | 2004 | Villous TB | Microscopy | Reduced syncytial area in IUGR. Reduction in syncytial/villous area ratio in pre-eclampsia but not in IUGR |
| Heazell et al. | 2005 | Villous TB | IHC | Increased expression of p53 in ST nuclei and ST cytoplasm in placentas from women with pre-eclampsia. Reduction in Mdm2 in pre-eclampsia |
| Jeschke et al. | 2006 | Villous TB | IHC, IF | p53 and ki67 elevated in HELLP syndrome but not in pre-eclampsia. p53 reduced in CT from IUGR placentas, no effect upon proliferation |
| Kadyrov et al. | 2006 | EVT | IHC | Severely impaired trophoblast invasion in pre-eclampsia and IUGR. Increased EVT apoptosis |
| De Falco et al. | 2007 | Villous TB | IHC | p21 is expressed by CT and ST in pre-eclampsia |
| Cobellis et al. | 2007 | Villous TB | IHC | Increased Bax expression in miscarriage vs termination. Reduced Bax in Cesarean section vs normal birth. Also increased Bax in pre-eclampsia |
| Vascular remodelling | ||||
| Craven et al. | 1998 | EVT | IHC | Initial spiral artery changes, such as VCAM-1 expression and smooth muscle disruption are independent of trophoblast. |
| Difederico et al. | 1999 | EVT | IHC | 15–50% EVT apoptosis in pre-eclampsia, virtually zero in control. Lack of Bcl-2 staining in preeclamptic EVT |
| Dunk et al. | 2003 | EVT | IHC | EVTs penetrate the decidua and stimulate endothelial and smooth muscle disruption. Not seen in vessels cultured in the absence of EVT |
| Ashton et al. | 2005 | EVT | IHC, WB | Endothelial cells and VSMC express Fas and FasL. Trophoblast induced apoptosis in cultured endothelial cells |
| Kadyrov et al. | 2006 | EVT | IHC | Severely impaired trophoblast invasion in pre-eclampsia and IUGR. Increased EVT apoptosis |
| Smith et al. | 2009 | EVT | IHC | 4-stage model of trophoblast remodelling of spiral arteries. Transient role for uNK cells and macrophages in VSMC apoptosis |
ST, syncytiotrophoblast; CT, cytotrophoblast; EVT, extravillous trophoblast; FGR, fetal growth restricted; TB, trophoblast; VSMC, vascular smooth muscle cells; uNK, uterine natural killer.
Apoptosis and the maternal vasculature
Pre-eclampsia and IUGR are associated not only with apoptosis but also with excessive syncytial knot formation. With 10–30% of normal terminal villi containing knots compared to virtually all terminal villi in pre-eclampsia.62,84,85 Small microparticles of syncytiotrophoblast microvillous membrane (STBMs), are found in the maternal circulation from the second trimester and in increasing amounts with gestation and are thought to represent apoptotic material released as part of normal syncytiotrophoblast turnover. 60 Exaggerated levels of this material are associated with pre-eclampsia, but not IUGR.86 This reconciles with greater syncytiotrophoblast loss and damage.60,87,88 This finding is reproducible under hypoxic conditions in vitro, further supporting hypoxia and reactive oxygen species as the underlying pathophysiological trigger in the formation of syncytial debris.89,90 Other smaller fragments, including cell-free fetal DNA, are also released and may also be associated with severe pre-eclampsia.91
Syncytial debris and systemic inflammatory response
The presence of STBMs, and newly identified nano-particles 92 within the maternal circulation are associated with alterations in immunological response, specifically neutrophil activation93,94 and the release of superoxide radicals.95 Neutrophil activation may be further exaggerated in pre-eclampsia as shown by an increased incidence of DNA lattices (NETS) in placentae from these pregnancies.94 The culture of syncytiotrophoblast-derived particles with non-pregnant peripheral blood monocytes stimulates the release of the cytokines, TNF-alpha, IL-1beta, IL-6, IL-8, IL12p70, IL-18, with an additional increase in adhesion molecule CD54.96,97 These findings suggest a systemic inflammatory response in pregnancy, one further exaggerated in pre-eclampsia. In support, maternal levels of monocyte derived IL-1β, IL-6, and IL-8 are increased in pre-eclampsia.98 Furthermore, STBMs are able to disrupt endothelial cells in vitro, again promoting a link between placental apoptosis, syncytial microparticle liberation and the maternal vascular complications, characteristic of the pre-eclamptic syndrome.99,100
Conclusion
In conclusion, apoptosis is a feature of villous trophoblast throughout pregnancy and is an essential feature of placental invasion, cytotrophoblast fusion, and syncytiotrophoblast function as well as potentially playing a role in maternal immune tolerance. This process is not uncontrolled or haphazard in nature. In fact, the many key proteins and cytokines involved in presenting foreign material to the placenta and controlling the response of this tissue to external stimuli have only been partially explored. We do know that alterations to placental function by external factors such as hypoxia and reactive oxygen species can lead to significant increases in placental apoptosis, which may be the underlying cause in the pathophysiology of pre-eclampsia and IUGR. A growing body of evidence also suggests that abnormal placental apoptosis has effects beyond the placenta upon maternal vascular endothelial behavior and immune tolerance. Our understanding of the role of key protein pathways involved in regulating placental apoptosis is constantly expanding and it is at this mechanistic level the future therapeutic strategies may be derived.
Acknowledgments
Andrew Sharp is funded by a research training fellowship from The Wellcome Trust
References
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazebnik YA, Cole S, Cooke CA, Nelson WG, Earnshaw WC. Nuclear events of apoptosis in vitro in cell-free mitotic extracts: a model system for analysis of the active phase of apoptosis. J Cell Biol. 1993;123:7–22. doi: 10.1083/jcb.123.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arends MJ, Wyllie AH. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- 4.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 5.Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26:877–897. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- 6.Chinnaiyan AM, Tepper CG, Seldin MF, O’Rourke K, Kischkel FC, Hellbardt S, Krammer PH, Peter ME, Dixit VM. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 7.Varfolomeev EE, Boldin MP, Goncharov TM, Wallach D. A potential mechanism of “cross-talk” between the p55 tumor necrosis factor receptor and Fas/APO1: proteins binding to the death domains of the two receptors also bind to each other. J Exp Med. 1996;183:1271–1275. doi: 10.1084/jem.183.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death–inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ. Caspase-10 is an initiator caspase in death receptor signaling. Proc Natl Acad Sci U S A. 2001;98:13884–13888. doi: 10.1073/pnas.241358198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Zhu H, Xu C-j, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 12.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 13.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 14.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saelens X, Festjens N, Walle LV, Gurp Mv, Loo Gv, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 16.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 17.Tewari M, Quan LT, O’Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32[beta], a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 18.Koh DW, Dawson TM, Dawson VL. Mediation of cell death by poly(ADP-ribose) polymerase-1. Pharmacol Res. 2005;52:5–14. doi: 10.1016/j.phrs.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Smith S, Baker PN, Symonds EM. Placental apoptosis in normal human pregnancy. Am J Obstet Gynecol. 1997;177:57–61. doi: 10.1016/s0002-9378(97)70438-1. [DOI] [PubMed] [Google Scholar]
- 20.Levy R, Smith SD, Chandler K, Sadovsky Y, Nelson DM. Apoptosis in human cultured trophoblasts is enhanced by hypoxia and diminished by epidermal growth factor. Am J Physiol Cell Physiol. 2000;278:C982–C988. doi: 10.1152/ajpcell.2000.278.5.C982. [DOI] [PubMed] [Google Scholar]
- 21.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, Bjorklund P, Ramaekers FC, Bjorklund B, Nap M, Jornvall H, Schutte B. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Pijnenborg R, Bland JM, Robertson WB, Dixon G, Brosens I. The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy. Placenta. 1981;2:303–316. doi: 10.1016/s0143-4004(81)80027-6. [DOI] [PubMed] [Google Scholar]
- 24.Craven CM, Morgan T, Baker PN, Cartwright JE. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta. 1998;19:241–252. doi: 10.1016/s0143-4004(98)90055-8. [DOI] [PubMed] [Google Scholar]
- 25.Ashton SV, Whitley GSJ, Dash PR, Wareing M, Crocker IP, Baker PN, Cartwright JE. Uterine spiral artery remodeling involves endothelial apoptosis induced by extravillous trophoblasts through Fas/FasL interactions. Arterioscler Thromb Vasc Biol. 2005;25:102–108. doi: 10.1161/01.ATV.0000148547.70187.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunk C, Petkovic L, Baczyk D, Rossant J, Winterhager E, Lye S. A novel in vitro model of trophoblast-mediated decidual blood vessel remodeling. Lab Invest. 2003;83:1821–1828. doi: 10.1097/01.lab.0000101730.69754.5a. [DOI] [PubMed] [Google Scholar]
- 27.Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol. 2009;174:1959–1971. doi: 10.2353/ajpath.2009.080995. Epub 2009 April 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meekins JW, Luckas MJ, Pijnenborg R, McFadyen IR. Histological study of decidual spiral arteries and the presence of maternal erythrocytes in the intervillous space during the first trimester of normal human pregnancy. Placenta. 1997;18:459–464. doi: 10.1016/s0143-4004(97)80048-3. [DOI] [PubMed] [Google Scholar]
- 29.Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am J Pathol. 2003;162:115–125. doi: 10.1016/S0002-9440(10)63803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadyrov M, Kingdom JCP, Huppertz B. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am J Obstet Gynecol. 2006;194:557–563. doi: 10.1016/j.ajog.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 31.DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155:293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meekins JW, Pijnenborg R. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. BJOG. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 33.Burton GJ, Jauniaux E, Charnock-Jones DS. The influence of the intrauterine environment on human placental development. Int J Dev Biol. 2009;54:303–312. doi: 10.1387/ijdb.082764gb. [DOI] [PubMed] [Google Scholar]
- 34.Hutchinson ES, Brownbill P, Jones NW, Abrahams VM, Baker PN, Sibley CP, Crocker IP. Utero-placental haemodynamics in the pathogenesis of pre-eclampsia. Placenta. 2009;30:634–641. doi: 10.1016/j.placenta.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Aardema MW, Oosterhof H, Timmer A, van Rooy I, Aarnoudse JG. Uterine artery doppler flow and uteroplacental vascular pathology in normal pregnancies and pregnancies complicated by pre-eclampsia and small for gestational age fetuses. Placenta. 2001;22:405–411. doi: 10.1053/plac.2001.0676. [DOI] [PubMed] [Google Scholar]
- 36.Janatpour MJ, Utset MF, Cross JC, Rossant J, Dong J, Israel MA, Fisher SJ. A repertoire of differentially expressed transcription factors that offers insight into mechanisms of human cytotrophoblast differentiation. Dev Genet. 1999;25:146–157. doi: 10.1002/(SICI)1520-6408(1999)25:2<146::AID-DVG9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Huppertz B, Bartz C, Kokozidou M. Trophoblast fusion: fusogenic proteins, syncytins and ADAMs, and other prerequisites for syncytial fusion. Micron. 2006;37:509–517. doi: 10.1016/j.micron.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Black S, Kadyrov M, Kaufmann P, Ugele B, Emans N, Huppertz B. Syncytial fusion of human trophoblast depends on caspase 8. Cell Death Differ. 2003;11:90–98. doi: 10.1038/sj.cdd.4401307. [DOI] [PubMed] [Google Scholar]
- 39.Smith SC, Baker PN. Placental apoptosis is increased in post-term pregnancies. Br J Obstet Gynaecol. 1999;106:861–862. doi: 10.1111/j.1471-0528.1999.tb08410.x. [DOI] [PubMed] [Google Scholar]
- 40.Athapathu H, Jayawardana MA, Senanayaka L. A study of the incidence of apoptosis in the human placental cells in the last weeks of pregnancy. J Obstet Gynaecol. 2003;23:515–517. doi: 10.1080/0144361031000153756. [DOI] [PubMed] [Google Scholar]
- 41.Hempstock J, Jauniaux E, Greenwold N, Burton GJ. The contribution of placental oxidative stress to early pregnancy failure. Hum Pathol. 2003;34:1265–1275. doi: 10.1016/j.humpath.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Cobellis L, De Falco M, Torella M, Trabucco E, Caprio F, Federico E, Manente L, Coppola G, Laforgia V, Cassandro R, Colacurci N, De Luca A. Modulation of Bax expression in physiological and pathological human placentas throughout pregnancy. In Vivo. 2007;21:777–783. [PubMed] [Google Scholar]
- 43.Allaire AD, Ballenger KA, Wells SR, Mcmahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol. 2000;96:271–276. doi: 10.1016/s0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- 44.Leung DN, Smith SC, To KF, Sahota DS, Baker PN. Increased placental apoptosis in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2001;184:1249–1250. doi: 10.1067/mob.2001.112906. [DOI] [PubMed] [Google Scholar]
- 45.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 46.Heazell AE, Buttle HR, Baker PN, Crocker IP. Altered expression of regulators of caspase activity within trophoblast of normal pregnancies and pregnancies complicated by preeclampsia. Reprod Sci. 2008;15:1034–1043. doi: 10.1177/1933719108322438. [DOI] [PubMed] [Google Scholar]
- 47.Levy R, Smith SD, Yusuf K, Huettner PC, Kraus FT, Sadovsky Y, Nelson DM. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am J Obstet Gynecol. 2002;186:1056–1061. doi: 10.1067/mob.2002.122250. [DOI] [PubMed] [Google Scholar]
- 48.Endo H, Okamoto A, Yamada K, Nikaido T, Tanaka T. Frequent apoptosis in placental villi from pregnancies complicated with intrauterine growth restriction and without maternal symptoms. Int J Mol Med. 2005;16:79–84. [PubMed] [Google Scholar]
- 49.Smith SC, Baker PN, Symonds EM. Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol. 1997;177:1395–1401. doi: 10.1016/s0002-9378(97)70081-4. [DOI] [PubMed] [Google Scholar]
- 50.Wong SY, Ngan HY, Chan CC, Cheung AN. Apoptosis in gestational trophoblastic disease is correlated with clinical outcome and Bcl-2 expression but not Bax expression. Mod Pathol. 1999;12:1025–1033. [PubMed] [Google Scholar]
- 51.Chiu PM, Ngan YS, Khoo US, Cheung AN. Apoptotic activity in gestational trophoblastic disease correlates with clinical outcome: assessment by the caspaserelated M30 CytoDeath antibody. Histopathology. 2001;38:243–249. doi: 10.1046/j.1365-2559.2001.01065.x. [DOI] [PubMed] [Google Scholar]
- 52.Sgarbosa F, Barbisan LF, Brasil MA, Costa E, Calderon IM, Goncalves CR, Bevilacqua E, Rudge MV. Changes in apoptosis and Bcl-2 expression in human hyperglycemic, term placental trophoblast. Diabetes Res Clin Pract. 2006;73:143–149. doi: 10.1016/j.diabres.2005.12.014. Epub 2006 March 24. [DOI] [PubMed] [Google Scholar]
- 53.Hamad RR, Bremme K, Kallner A, Sten-Linder M. Increased levels of an apoptotic product in the sera from women with pre-eclampsia. Scand J Clin Lab Invest. 2009;69:204–208. doi: 10.1080/00365510802474384. [DOI] [PubMed] [Google Scholar]
- 54.Burton GJ, Skepper JN, Hempstock J, Cindrova T, Jones CJP, Jauniaux E. A reappraisal of the contrasting morphological appearances of villous cytotrophoblast cells during early human pregnancy; evidence for both apoptosis and primary necrosis. Placenta. 2003;24:297–305. doi: 10.1053/plac.2002.0882. [DOI] [PubMed] [Google Scholar]
- 55.Smith SC, Leung TN, To KF, Baker PN. Apoptosis is a rare event in first-trimester placental tissue. Am J Obstet Gynecol. 2000;183:697–699. doi: 10.1067/mob.2000.106555. [DOI] [PubMed] [Google Scholar]
- 56.De Falco M, Fedele V, Cobellis L, Mastrogiacomo A, Leone S, Giraldi D, De Luca B, Laforgia V, De Luca A. Immunohistochemical distribution of proteins belonging to the receptor-mediated and the mitochondrial apoptotic pathways in human placenta during gestation. Cell Tissue Res. 2004;318:599–608. doi: 10.1007/s00441-004-0969-4. Epub 2004 October 2. [DOI] [PubMed] [Google Scholar]
- 57.Huppertz B, Frank HG, Reister F, Kingdom J, Korr H, Kaufmann P. Apoptosis cascade progresses during turnover of human trophoblast: analysis of villous cytotrophoblast and syncytial fragments in vitro. Lab Invest. 1999;79:1687–1702. [PubMed] [Google Scholar]
- 58.Kadyrov M, Kaufmann P, Huppertz B. Expression of a cytokeratin 18 neo-epitope is a specific marker for trophoblast apoptosis in human placenta. Placenta. 2001;22:44–48. doi: 10.1053/plac.2000.0616. [DOI] [PubMed] [Google Scholar]
- 59.Huppertz B, Kadyrov M, Kingdom JCP. Apoptosis and its role in the trophoblast. Am J Obstet Gynecol. 2006;195:29–39. doi: 10.1016/j.ajog.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 60.Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105:632–640. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 61.Redman CWG, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 62.Burton GJ, Jones CJ. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan J Obstet Gynecol. 2009;48:28–37. doi: 10.1016/S1028-4559(09)60032-2. [DOI] [PubMed] [Google Scholar]
- 63.Ratts VS, Tao X-J, Webster CB, Swanson PE, Smith SD, Brownbill P, Krajewski S, Reed JC, Tilly JL, Nelson DM. Expression of BCL-2, BAX and BAK in the trophoblast layer of the term human placenta: a unique model of apoptosis within a syncytium. Placenta. 2000;21:361–366. doi: 10.1053/plac.1999.0486. [DOI] [PubMed] [Google Scholar]
- 64.Crocker IP, Tansinda DM, Jones CJ, Baker PN. The influence of oxygen and tumor necrosis factor-alpha on the cellular kinetics of term placental villous explants in culture. J Histochem Cytochem. 2004;52:749–757. doi: 10.1369/jhc.3A6176.2004. [DOI] [PubMed] [Google Scholar]
- 65.Crocker IP, Cooper S, Ong SC, Baker PN. Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol. 2003;162:637–643. doi: 10.1016/S0002-9440(10)63857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crocker IP, Tansinda DM, Baker PN. Altered cell kinetics in cultured placental villous explants in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. J Pathol. 2004;204:11–18. doi: 10.1002/path.1610. [DOI] [PubMed] [Google Scholar]
- 67.Moll SJ, Jones CJ, Crocker IP, Baker PN, Heazell AE. Epidermal growth factor rescues trophoblast apoptosis induced by reactive oxygen species. Apoptosis. 2007;12:1611–1622. doi: 10.1007/s10495-007-0092-6. [DOI] [PubMed] [Google Scholar]
- 68.Heazell AEP, Crocker IP. Live and let die – regulation of villous trophoblast apoptosis in normal and abnormal pregnancies. Placenta. 2008;29:772–783. doi: 10.1016/j.placenta.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod. 2004;10:55–63. doi: 10.1093/molehr/gah006. [DOI] [PubMed] [Google Scholar]
- 70.Bai X, Williams JL, Greenwood SL, Baker PN, Aplin JD, Crocker IP. A placental protective role for trophoblast-derived TNF-related apoptosis-inducing ligand (TRAIL) Placenta. 2009;30:855–860. doi: 10.1016/j.placenta.2009.07.006. Epub 2009 August 11. [DOI] [PubMed] [Google Scholar]
- 71.Quenby S, Brazeau C, Drakeley A, Lewis-Jones D, Vince G. Oncogene and tumour suppressor gene products during trophoblast differentiation in the first trimester. Mol Hum Reprod. 1998;4:477–481. doi: 10.1093/molehr/4.5.477. [DOI] [PubMed] [Google Scholar]
- 72.Haidacher S, Blaschitz A, Desoye G, Dohr G. Cell proliferation and apoptosis: Immunohistochemical evidence of p53 protein in human placenta and choriocarcinoma cell lines. Hum Reprod. 1995;10:983–988. doi: 10.1093/oxfordjournals.humrep.a136082. [DOI] [PubMed] [Google Scholar]
- 73.Shi YF, Xie X, Zhao CL, Ye DF, Lu SM, Hor JJ, Pao CC. Lack of mutation in tumour-suppressor gene p53 in gestational trophoblastic tumours. Br J Cancer. 1996;73:1216–1219. doi: 10.1038/bjc.1996.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheung AN, Shen DH, Khoo US, Chiu MP, Tin VP, Chung LP, Ngan HY. Immunohistochemical and mutational analysis of p53 tumor suppressor gene in gestational trophoblastic disease: correlation with mdm2, proliferation index, and clinicopathologic parameters. Int J Gynecol Cancer. 1999;9:123–130. doi: 10.1046/j.1525-1438.1999.09904.x. [DOI] [PubMed] [Google Scholar]
- 75.Fulop V, Mok SC, Genest DR, Gati I, Doszpod J, Berkowitz RS. p53, p21, Rb and mdm2 oncoproteins. Expression in normal placenta, partial and complete mole, and choriocarcinoma. J Reprod Med. 1998;43:119–127. [PubMed] [Google Scholar]
- 76.Heazell AEP, Lacey HA, Jones CJP, Huppertz B, Baker PN, Crocker IP. Effects of oxygen on cell turnover and expression of regulators of apoptosis in human placental trophoblast. Placenta. 2008;29:175–186. doi: 10.1016/j.placenta.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 77.De Falco M, Cobellis L, Giraldi D, Mastrogiacomo A, Perna A, Colacurci N, Miele L, De Luca A. Expression and distribution of notch protein members in human placenta throughout pregnancy. Placenta. 2007;3:118–126. doi: 10.1016/j.placenta.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 78.Mayhew TM, Wijesekara J, Baker PN, Ong SS. Morphometric evidence that villous development and fetoplacental angiogenesis are compromised by intrauterine growth restriction but not by pre-eclampsia. Placenta. 2004;25:829–833. doi: 10.1016/j.placenta.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 79.Daayana S, Baker P, Crocker I. An image analysis technique for the investigation of variations in placental morphology in pregnancies complicated by preeclampsia with and without intrauterine growth restriction. J Soc Gynecol Investig. 2004;11:545–552. doi: 10.1016/j.jsgi.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 80.Heazell AEP, Brown LM, Baker P, Crocker I. Expression of oncoproteins p53 and Mdm2 within trophoblast of normal and pre-eclamptic pregnancies. J Soc Gynecol Investig. 2005;12:362A. [Google Scholar]
- 81.Soleymanlou N, Jurisicova A, Wu Y, Chijiiwa M, Ray JE, Detmar J, Todros T, Zamudio S, Post M, Caniggia I. Hypoxic switch in mitochondrial myeloid cell leukemia factor-1/Mtd apoptotic rheostat contributes to human trophoblast cell death in preeclampsia. Am J Pathol. 2007;171:496–506. doi: 10.2353/ajpath.2007.070094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeschke U, Schiessl B, Mylonas I, Kunze S, Kuhn C, Schulze S, Friese K, Mayr D. Expression of the proliferation marker Ki-67 and of p53 tumor protein in trophoblastic tissue of preeclamptic, HELLP, and intrauterine growth-restricted pregnancies. Int J Gynecol Pathol. 2006;25:354–360. doi: 10.1097/01.pgp.0000225838.29127.6. [DOI] [PubMed] [Google Scholar]
- 83.Davy P, Nagata M, Bullard P, Fogelson NS, Allsopp R. Fetal growth restriction is associated with accelerated telomere shortening and increased expression of cell senescence markers in the placenta. Placenta. 2009;30:539–542. doi: 10.1016/j.placenta.2009.03.005. Epub 2009 April 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fox H. The significance of villous syncytial knots in the human placenta. J Obstet Gynaecol Br Commonw. 1965;72:347–355. doi: 10.1111/j.1471-0528.1965.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 85.Heazell AEP, Moll SJ, Jones CJP, Baker PN, Crocker IP. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007;28(Suppl 1):S33–S40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 86.Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, von Dadelszen P. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. Epub 2005 January 25. [DOI] [PubMed] [Google Scholar]
- 87.Orozco AF, Jorgez CJ, Ramos-Perez WD, Popek EJ, Yu X, Kozinetz CA, Bischoff FZ, Lewis DE. Placental release of distinct DNA-associated micro-particles into maternal circulation: reflective of gestation time and preeclampsia. Placenta. 2009;30:891–897. doi: 10.1016/j.placenta.2009.06.012. Epub 2009 August 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong XY, Holzgreve W, Hahn S. The levels of circulatory cell free fetal DNA in maternal plasma are elevated prior to the onset of preeclampsia. Hypertens Pregnancy. 2002;21:77–83. doi: 10.1081/PRG-120002911. [DOI] [PubMed] [Google Scholar]
- 89.Orozco AF, Bischoff FZ, Horne C, Popek E, Simpson JL, Lewis DE. Hypoxia-induced membrane-bound apoptotic DNA particles: potential mechanism of fetal DNA in maternal plasma. Ann N Y Acad Sci. 2006;1075:57–62. doi: 10.1196/annals.1368.007. [DOI] [PubMed] [Google Scholar]
- 90.Tjoa ML, Cindrova-Davies T, Spasic-Boskovic O, Bianchi DW, Burton GJ. Trophoblastic oxidative stress and the release of cell-free feto-placental DNA. Am J Pathol. 2006;169:400–404. doi: 10.2353/ajpath.2006.060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sifakis S, Zaravinos A, Maiz N, Spandidos DA, Nicolaides KH. First-trimester maternal plasma cell-free fetal DNA and preeclampsia. Am J Obstet Gynecol. 2009;201:472. doi: 10.1016/j.ajog.2009.05.025. Epub 2009 July 24. [DOI] [PubMed] [Google Scholar]
- 92.Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A, Ohkuchi A, Matsubara S, Takeshita T. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81:717–729. doi: 10.1095/biolreprod.108.075481. Epub 2009 June 3. [DOI] [PubMed] [Google Scholar]
- 93.von Dadelszen P, Hurst G, Redman CW. Supernatants from co-cultured endothelial cells and syncytiotrophoblast microvillous membranes activate peripheral blood leukocytes in vitro. Hum Reprod. 1999;14:919–924. doi: 10.1093/humrep/14.4.919. [DOI] [PubMed] [Google Scholar]
- 94.Gupta A, Hasler P, Gebhardt S, Holzgreve W, Hahn S. Occurrence of neutrophil extracellular DNA traps (NETs) in pre-eclampsia: a link with elevated levels of cell-free DNA? Ann N Y Acad Sci. 2006;1075:118–122. doi: 10.1196/annals.1368.015. [DOI] [PubMed] [Google Scholar]
- 95.Aly AS, Khandelwal M, Zhao J, Mehmet AH, Sammel MD, Parry S. Neutrophils are stimulated by syncytiotrophoblast microvillous membranes to generate superoxide radicals in women with preeclampsia. Am J Obstet Gynecol. 2004;190:252–258. doi: 10.1016/j.ajog.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 96.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178:5949–5956. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 97.Messerli M, May K, Hansson SR, Schneider H, Holzgreve W, Hahn S, Rusterholz C. Feto-maternal interactions in pregnancies: placental microparticles activate peripheral blood monocytes. Placenta. 2010;31:106–112. doi: 10.1016/j.placenta.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 98.Luppi P, DeLoia JA. Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin Immunol. 2006;118:268–275. doi: 10.1016/j.clim.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Cockell AP, Learmont JG, Smarason AK, Redman CW, Sargent IL, Poston L. Human placental syncytiotrophoblast microvillous membranes impair maternal vascular endothelial function. Br J Obstet Gynaecol. 1997;104:235–240. doi: 10.1111/j.1471-0528.1997.tb11052.x. [DOI] [PubMed] [Google Scholar]
- 100.Hoegh AM, Tannetta D, Sargent I, Borup R, Nielsen FC, Redman C, Sorensen S, Hviid TV. Effect of syncytiotrophoblast microvillous membrane treatment on gene expression in human umbilical vein endothelial cells. BJOG. 2006;113:1270–1279. doi: 10.1111/j.1471-0528.2006.01061.x. [DOI] [PubMed] [Google Scholar]


