Abstract
Purpose
Fibroblast cell proliferation is major reason for recurrence of pterygia. In the present study, we investigated if small interfering RNA (siRNA)-mediated gene silencing of S phase-kinase-interacting protein 2 (Skp2) can be employed to inhibit protein 27 kinase inhibition protein 1 (p27kip1) down-regulation in pterygium fibroblast cells (PFC) in vitro and in vivo.
Methods
A plasmid containing transgenes encoding Skp2 siRNA was used to decreasing the high constitutive levels of Skp2 protein in PFC and normal fibrboblast cells (NFC) in vitro and in vivo which can lead to consequent degradation of p27kip1. Cell proliferation and viability were investigated using cell counts, 59-bromodeoxyuridine incorporation (BrdU assay) and tetrazolium reduction (MTT assay).
Results
Infection of PFC and NFC with Skp2 siRNA resulted in significant inhibition of cell proliferation and metabolic activity in vitro. Immunoflurescence showed decreased levels of Skp2 and increased levels of p27kip1 in pSkp2 siRNA infected cells, but not in plasmid and uninfected cells.
Conclusions
Skp2 siRNA inhibited the cell proliferation of PFC in vitro and in vivo.
Introduction
Pterygium is a common proliferative disorder involving epithelial hyperplasia and fibrovascular proliferation. In the advanced stages, pterygium can necessitate complex surgery for full visual rehabilitation and recurrences, and ocular complications are not infrequent [1-3]. Ultraviolet (UV) light has long been regarded as the etiologic agent of pterygium [4-6].
The etiology and physiopathology of pterygium have not been completely defined. However, it is well known from epidemiologic and histologic studies that the most important factor in its appearance and development is sunlight exposure. This causes proliferation of fibrovascular tissue which invades the cornea from the exposed conjunctiva.
Although the pathogenesis of pterygium is yet undetermined, tumor-like histologic characteristics, ranging from mild dysplasia to carcinoma in situ and local invasiveness, have been described by different authors [7-9]. Moreover, pterygium fibroblasts (PFs) exhibit characteristics of the transformed phenotype [10], microsatellite instability, and loss of heterozygosity, all reported to be common findings in neoplastic tissue.
However, the mechanisms in the regulation of lens cell proliferation are still unclear.
S phase-kinase-interacting protein 2 (Skp2) was identified as the E3 ubiquitin ligase that targets protein 27 kinase inhibition protein 1 (p27kip1) for ubiquitination [11-13]. Skp-cullin-F (SCF) complexes represent an evolutionarily conserved class of E3 enzymes containing four subunits: Skp1, cullin 1 (Cul1), one of many F box proteins, and ring box protein 1 (Rbx1) [14]. Skp2, an F box protein, is required for the ubiquitination and consequent degradation of p27 both in vivo and in vitro. Skp2 is specifically required for p27 ubiquitination and that Skp2 is a rate-limiting component of the machinery that ubiquitinates and degrades phosphorylated p27. Skp2 is frequently overexpressed in tumor cell lines, and forced expression of Skp2 in quiescent fibroblasts induces DNA synthesis [15].
We showed that expression of Skp2 can be detected in rabbit tenon’s fibroblast cells and confirmed that transfection of Skp2 siRNA can effectively inhibit the proliferation of rabbit tenon’s fibroblast cells after glaucoma surgery [16].
In this study, we examined the expression of Skp2 in pterygium fibroblast cells (PFC) and investigated if small interfering RNA (siRNA)-mediated gene silencing of Skp2 can be employed to inhibit protein 27 kinase inhibition protein 1 (p27kip1) down-regulation in PFC and the effects of down-regulation of p27kip1 on PFC proliferation.
Methods
Patient selection
We recruited eight patients with primary pterygium (age range, 42–66 years; mean, 55.32±2.3 years) and eight eyes with normal conjunctiva (age range, 45–65 years; mean, 56.25±1.5 years) which were donated. Diagnosis of pterygium was based on clinical history and evaluation of signs and symptoms. All patients with pterygium (five men and three women) had at least a five-year history of a slow-growing lesion, with a corneal extension of at least 4 mm, as measured with a caliper, from the limbus to the corneal vertex. Pterygia morphology was clinically graded based on the assessment of pterygium translucency: atrophic (T1), intermediate (T2), or fleshy (T3) pterygium. All pterygia collected in this study were T3. No subject in the control group had any inflammatory signs or symptoms. The research adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all the patients before tissues were collected. This study and all the procedures ere approved by the Ethics Committee of the University of Harbin Medical University. All tissue samples were obtained as previously described [5]. In summary, at the time of surgery, tissue was excised from the inner canthus with microforceps. As a rule, within 1 h of excision, samples were placed in sterile boxes containing a preservative solution and were transferred to the cell culture room.
PlasmidSuppressor (pSuppressor) containing Skp2 siRNA and control pSuppressor was purchased from Imgenex (San Diego, CA). Synthetic oligonucleotide primers was purchased from Qiagen (Valencia, CA). Skp2 antibody and the Steptavidin-Alkaline Phosphatase Complex (SABC) kit were purchased from the Boster Company (Wuhan, China). Enhanced chemiluminescence kit, p27kip1 antibody, and PCNA antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Fluorescein-conjugated anti-sheep IgG was purchased from Zhongshan Biotechnology (Beijing, China). Vectashield mounting medium was purchased from Vector laboratories (Burlington, CA). Dulbecco’s modified Eagle’s medium and F12 medium (DMEM F12) was purchased from Gibco (Burlington, VT). Streptomycin, penicillin, fetal calf serum and Trizol™ were purchased from Invitrogen (Carlsbad, CA). Hanks solution was purchased from Hyclone (Logan, UT). Poly-lysine was purchased from Sigma (St. louis, MO). Culture plate was purchased from BD Biosciences (San Jose, CA). Hiperfect transfection reagent was purchased from Qiagen. Methylthiazolyltetrazolium (MTT) was purchased from Sigma. MTT cell proliferation kit was purchased from the ATCC (Manassas, VA). The Cell Counter was purchased from Coulter Z1 (Coulter, FL). Metafectene Pro reagent was purchased from Biontex (Martinstried, Germany).
Equipment
The fluorescence microscope (IX70; Olympus, Tokyo, Japan), optic microscope (Olympus, Tokyo, Japan), CO2 incubator (BB16HF: Heal Force, Hong Kong, China), ultraclean work Table (D8C-010; Heal Force), and incubation plate (Costar Corporation, Cambridge, MA).
Cell culture of human pterygium fibroblasts (hPF)
For fibroblast isolation, pterygium and normal conjunctiva tissues were dissected into three to four tissue fragments (1 mm2). The planted tissue was attached to the bottom of a six-well plate with a sterile coverslip and overlaid with DMEM medium. All culture media were supplemented with streptomycin (50 mg and penicillin 500,000 U/l) . To ensure fibroblast cell proliferation, the media was also supplemented with fetal calf serum (FCS; 10% of final volume). Cells from passage 3–5 were used for all experiments. All cells were grown at 37 °C with 5% CO2 ventilation.
Plasmids and transfection
Vector pSuppressorNeo generates biologically active siRNAs from the U6promoter. Synthetic oligonucleotide primers (5’-tcg aGG GAG UGA CAA AGA CUU UGg agu acu gCA AAG UCU UUG UCA CUC CCU UUU U-3’ and 5’-cta gAA AAA GGG AGU GAC AAA GAC UUU Gca gua cuc CAA AGU CUU UGU CAC UCC C-3’) containing XhoI and XbaI overhangs were annealed and then were introduced into pSuppressor Neovector. Oligo-nucleotide sequences correspond to a 19-nucleotide sequence from Skp2 (nucleotides114–133), which is separated by an 8-nucleotide linker from the reverse complement of the same 19-nucleotide sequence. A transcriptional termination (UUUUU) was added to the end of oligonucleotide. We used circular control plasmid, which contains a scrambled sequence as a control. Human ptergium fibroblasts (hPF) transfected with pSuppressor containing Skp2 siRNA, pSuppressor only, and medium served as experimental group, vehicle control group, and blank control group, respectively. Transfection was performed in 60 mm plates using 2 μg of vector in 10 μl of Metafectene Pro reagent. After 48 h of transfection, cells were treated with G418 for 2 weeks for positive clone selection. After G418 treatment, we cloned several stably transfectant cells. Each clone was screened for expression of Skp2 by western blot analysis.
Immunocytochemistry
Cells cultured on coverslips were fixed with 4% paraformaldehyde for 10 min at room temperature, permeabilized, and blocked with 0.1% Triton X-100 and 5% goat serum for 30 min. Cells were incubated with rabbit anti-Vimentin antibody (1:500) at 4 °C overnight, followed by goat-anti-rabbit IgG (1:500) for 1 h at room temperature. Then, cells were incubated with strep-avidin-biotin complex (SABC) at 37 °C for 30 min and colored with DAB. After coloration with DAB, dehydration, and dimethyl benzene treatment, slides were mounted. Controls were stained by omitting the primary antibody.
Cells were incubated with Skp2 monoclonal antibody (1:500 dilution), p27kip1 antibody (1:500 dilution), PCNA antibody (1:1,000 dilution) at 4 °C overnight, followed by secondary antibody for immunoflurescence staining, respectively. Controls were stained by omitting the primary antibody.
(3-(4,5-dimethylthiazolyl-2-)-2,5-diphenyltetrazoliumbromide, MTT) cell viability assay
Cell viability was examined by the MTT cell proliferation kit following the instructions of the manufacturer. The assay is based on measuring the reduction of yellow tetrazolium to a purple for mazanas facilitated by dehydrogenases of metabolically active cells. The intracellular for mazan can be solubilized and quantified by spectrophotometric means. Quadruple samples of hPFC were grown on 96 well plates and were infected with 100 mol of either of the two vectors, or weren’t infected. After 2, 4, 6, 8, and 14 days, wells were incubated in a medium containing yellow tetrazolium for 20 h.
Cell proliferation and bromodeoxyuridine (BrdU) incorporation
Cells (5.0×103) were plated onto a 24-well multiwell plate (3047, Falcon; Becton Dickinson, Franklin Lakes, NJ) and allowed to attach for 24 h. The culture medium was then replaced with fresh medium. Then, trypsinized cells were counted suing a cell counter (Coulter Z1; Coulter) at 0, 2, 4, and 6 days. For bromodeoxyuridine (BrdU) incorporation, cells growing on coverslips were incubated with 10 μmol/l BrdU for 3 h. The cells were fixed in cold methanol/acetone 1:1 for 10 min. In brief the cells were sequentially incubated in 1.5 mol/l HCl for 10 min. Then cells were washed thrice with PBS and incubated with the mouse anti-BrdU-primary antibody for 1 h. The cells were washed four times with PBS. The nuclei were simultaneously stained with 10 mg/ml of 4V, 6-diamidino-2-phenylindole. Cells with different BrdU-incorporation patterns were checked and counted with a microscope.
Animals
Male rabbits (2–3 kg) were supplied by the Experimental Animal Center of Harbin Medical University. All animal care and experimental procedures were in accordance with our institutional guidelines for animal care and the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85–23, revised 1996).
Developing the pterygium model in rabbits and pSkp2 siRNA delivery
We transplanted an auricular cartilage fragments (2×2 mm) obtained from the same rabbit’s ear. The fragment was grafted onto the upper limbal area of the rabbit’s eye, positioning it between the conjunctiva and the sclera. The pterygium on the rabbit eye formed after the cartilage fragment transplantion for six weeks.
A hypothesis concerning the origin of pterygium suggests that the lachrymal film’s unequal distribution stimulates its growth. We created an area of irregular distribution of tears by inserting cartiliage into the rabbits’ scleral-corneal limbus to evaluate this hypothesis. A slight to moderate inflammatory reaction was observed during the first days in the surgically affected area. The reaction then disappeared, leaving the cartiliage transplanted in the subconjunctival space visible. An initial inflammation reaction was observed, followed by later formation of fibrous tissue in the injured area. Its macroscopic aspect was similar to that of pterygium. Chronic inflammation, fibrosis, an increased number of fibroblasts, and the presence of immune system ploymorphonuclear cells were found; however, no elastosis or hyalinization of subepithelial connective tissue was observed, these being characteristic signs of pterygium.
Vector (2 µg) in 10 µl of Metafectene was delivered to the pterygium by subconjunctival injection at the superonasal quadrant 14 days before the surgery. The pterygium was cut. The neighboring fibroblast and tenon’s capsule fibroblast was cut after transfection during the surgery.
BrdU incorporation in vivo
After transfection for 14 days, 0.5 ml BrdUrd (10 µmol/l) was injected into the tenon’s capsule fibroblast.
Pathological and histochemistry examinations
The animals were perfused through the heart with heparin saline followed by 4% paraformaldehyde after 14 day transfection. Eyes were fixed overnight, and transferred to a 30% sucrose solution overnight (4 °C). Frozen sections (5 μm) were cut longitudinally on a cryostat, thaw mounted onto coated glass slides, and stained with hematoxylin and eosin [3], skp2 antibody, p27kip1 antibody, PCNA antibody, and anti-Brdu-fluorescein primary antibody. The procedure of histochemistry in vivo was the same as in vitro.
Statistical analysis
Data were analyzed by the two-tailed Student t-test using Origin ver. 6.0 software (OriginLab Corp., Northampton, MA) and a p<0.05 was considered significance.
Results
Downregulation of Skp2 protein by siRNA in hPFC
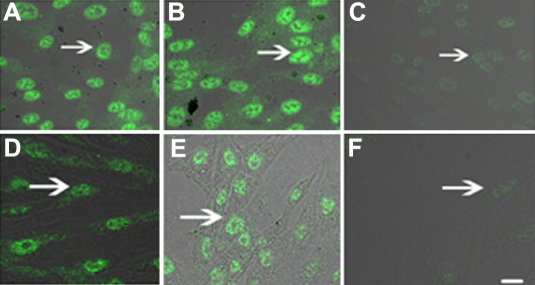
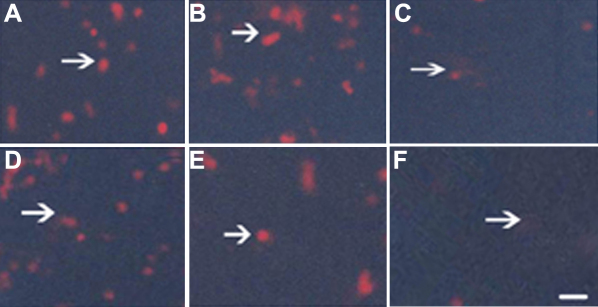
After 48 h of transfection and 2 weeks of treatment with G418, we cloned several stably transfectant cells. Immunoflurescence staining demonstrated high constitutive expression of Skp2 protein in nucleolus of PFC and normal fibroblast cells (NFC) transfected with pSuppressor vehicle (Figure 1A,D and Figure 2A,D) or without transfection (Figure 1B,E and Figure 2B,E). Transfection with Skp2 siRNA dramatically decreased the expression of Skp2 protein in cells of PFC (Figure 1C,F and Figure 2C,F).
Figure 1.
Down-regulation of Skp2 protein by siRNA in PFC in vitro by immunoflurescence. Immunoflurescence staining indicated high constitutive levels of Skp2 protein (arrow indication) in the nucleolus of PFC and NFC transfected with pSuppressor vehicle (A, D) or without transfection (B, E). Transfection with Skp2 siRNA dramatically decreased the expression of Skp2 protein in PFC cells (C, F). The scale bar is equal to 40 μm.
Figure 2.
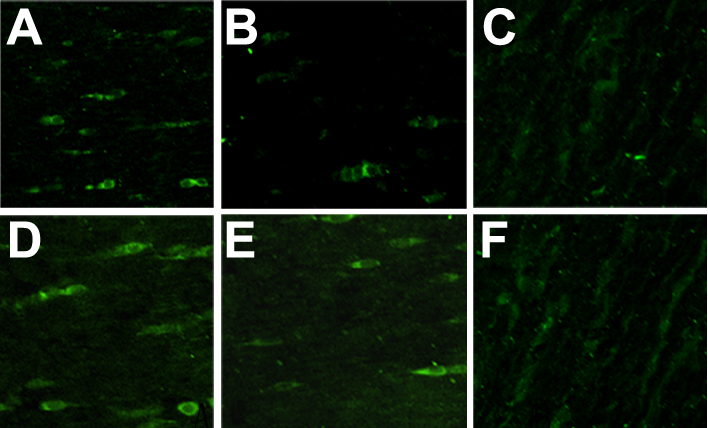
Down-regulation of Skp2 protein by siRNA in PFC in vivo by immunoflurescence. Immunoflurescence staining indicated high constitutive levels of Skp2 protein in the nucleolus of PFC and NFC transfected with pSuppressor vehicle (A, D) or without transfection (B, E). Transfection with Skp2 siRNA dramatically decreased the expression of Skp2 protein in PFC cells (C, F). The scale bar is equal to 40 μm.
Upregulation of p27kip1 protein by siRNA in hPFC
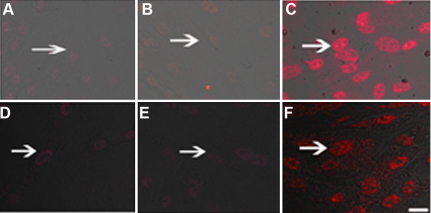
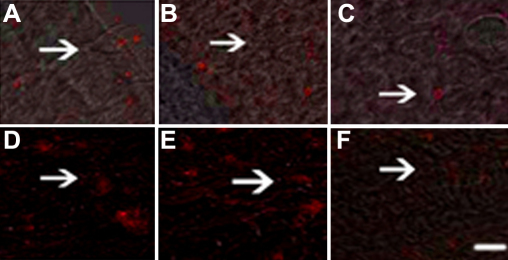
Skp2 is required for the ubiquitination and consequent degradation of p27kip1 [11]. Immunoflurescence staining demonstrated little expression of p27 protein in the PFC transfected with pSuppressor vehicle (Figure 3A,D and Figure 4A,D) or without transfection (Figure 3B,E and Figure 4B,E) in vitro. Transfection with Skp2 siRNA increased the expression of p27kip1 protein in cells of PFC and NFC (Figure 3C,F and Figure 4C,F) in vitro. Upregulation of p27kip1 made it possible for us to investigate the effect of p27kip1 inhibition on the proliferation of PFC and NFC.
Figure 3.
Skp2 siRNA induced p27kip1 accumulationin in PFC in vitro by immunofluorescence. Immunofluorescence staining indicated the expression of p27kip1 dramatically increased in PFC and NFC transfection with Skp2 siRNA (C, F) when compared with PFC transfection with pSuppressor vehicle (A, D) or without transfection (B, E). The scale bar is equal to 40 μm.
Figure 4.
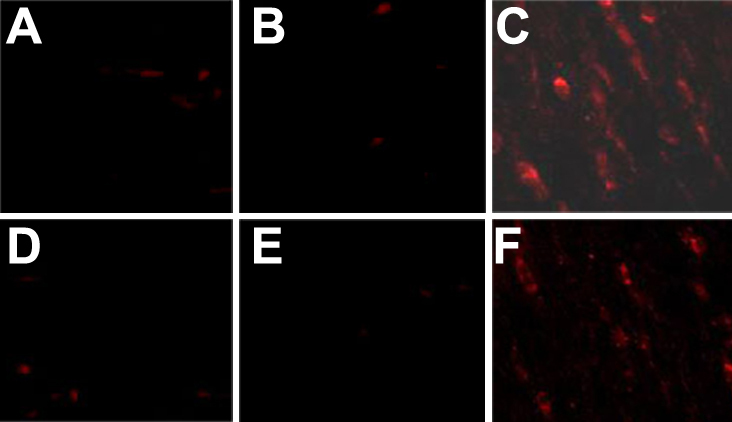
Skp2 siRNA induced p27kip1 accumulationin in PFC in vivo by immunofluorescence. Immunofluorescence staining indicated the expression of p27kip1 dramatically increased in PFC and NFC transfection with Skp2 siRNA (C, F) when compared with PFC transfection with pSuppressor vehicle (A, D) or without transfection (B, E). The scale bar is equal to 40 μm.
Skp2 siRNA inhibited cell proliferation of PFC in vitro
MTT
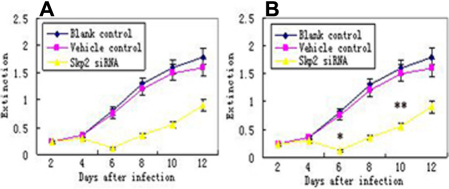
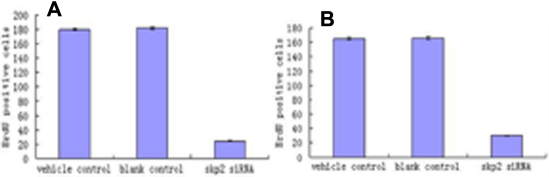
After transfection, the number of cells increased less in Skp2 siRNA transfectant hPFC and hNFC than vehicle control and blank control groups (Figure 5A,B).
Figure 5.
Cell viability assay by MTT. After 6 to 12 days transfection with Skp2 siRNA, cell viability was significantly decreased in cultured hPFC cells compared with vehicle control and blank control epsecially on the 6th day after transfection. *p<0.01 versus vehicle and blank control; **p<0.05 versus vehicle and blank control.
Brdu incorporation
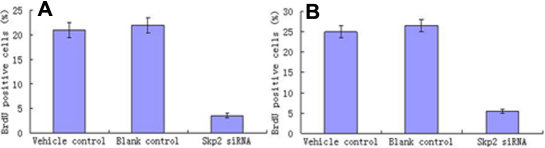
For BrdU incorporation assay, cells growing on coverslips were incubated with BrdU. Statically analysis (Figure 6A,B) after cell counting showed that Brdu positive cells in hPF and hNFC transfected with Skp2 siRNA significantly decreased compared with control cells (p<0.01 versus vehicle and blank control; Figure 7A,B).
Figure 6.
Skp2 siRNA inhibited the cell proliferation of PFC in vitro (Brdu). For Brdu incorporation, cells growing on coverslips were incubated with Brdu. Incorporated Brdu was detected with antibodies as described in the Methods. Statistical analysis after cell counting showed that Brdu positive cells decreased after hPFC transfection with Skp2 siRNA compared with control cells. **p<0.01 versus vehicle and blank control.
Figure 7.
Skp2 siRNA inhibited the cell proliferation of PFC in vivo (Brdu). For Brdu incorporation, ptergium tissue were incubated with Brdu. Incorporated Brdu was detected with antibodies as described in the Methods. Statistical analysis after cell counting showed that Brdu positive cells decreased after hPFC transfection with Skp2 siRNA compared with control cells. **p<0.01 versus vehicle and blank control.
Proliferating cell nuclear antigen (PCNA) protein expression in PFC after transfection with Skp2 siRNA
PCNA is a marker that indicates the proliferation potential of cells. Immunofluorescence staining demonstrated expression of PCNA protein in the hPFC and hNFC transfected with pSuppressor vehicle (Figure 8A,D and Figure 9A,D) or without transfection (Figure 8B,E and Figure 9B,E) in vitro. Transfection with Skp2 siRNA increased the expression of PCNA protein in cells of hPFC (Figure 8C,F and Figure 9C,F) in vitro.
Figure 8.
Down-regulation of PCNA protein by siRNA in PFC in vitro and in vivo. Immunofluorescence staining indicated the expression of PCNA decreased in PFC and NFC transfection with Skp2 siRNA (C, F) when compared with hPFC and hNFC transfection with pSuppressor vehicle (A, D) or without transfection (B, E). The scale bar is equal to 40 μm.
Figure 9.
Down-regulation of PCNA protein by siRNA in PFC in vitro and in vivo. Immunofluorescence staining indicated the expression of PCNA decreased in PFC and NFC transfection with Skp2 siRNA (C, F) when compared with hPFC and hNFC transfection with pSuppressor vehicle (A, D) or without transfection (B, E). The scale bar is equal to 40 μm.
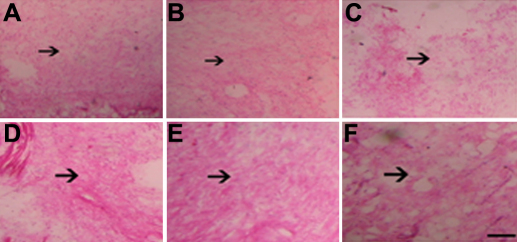
Histological observation for inhibition of PFC and NFC proliferation
High level of PFC and NFC proliferation can be detected in vehicle control (Figure 10A,D) and blank control (Figure 10B,E). However, PFC and NFC proliferation decreased in Skp2 siRNA transfectant group (Figure 10C,F).
Figure 10.
Skp2 siRNA inhibited the proliferation of PFC and NFC in vivo. Hematoxylin and eosin staining was performed to examine the histological changes 14 days after transfection. Obvious PFC and NFC proliferation was detected in pSuppressor vehicle group or without transfection (A, D, B, E). There was little PFC and NFC proliferation in Skp2 siRNA transfection group (C, F). Scale bar is equal to 20 μm.
Discussion
Pterygium is a condition characterized by epithelial overgrowth of the cornea, usually bilateral, and occupying a nasal interpalpebral location. It has a characteristic wing-shaped appearance. The pterygium epithelium that centripetally invades the cornea display squamous metaplasia and goblet cell hyperplasia. There is also an underlying breakdown of Bowman’s layer. A stromal overgrowth of fibroblasts and blood vessels is accompanied by an inflammatory cell infiltrate and an abnormal extracellular matrix accumulation composed of elastin and collagen [17-22].
Recently, we demonstrated expression of matrix metalloproteinases (MMPs) in resected pterygium specimens [23,24] and localized these enzymes at the advancing edges of the lesions [25]. In addition, data suggests that proinflammatory cytokines [26] can modify the expression of these extracellular matrix denaturing enzymes. These investigations imply that MMPs may play a significant role in tissue remodeling and invasion and in the dissolution of Bowman’s layer associated with pterygia.
Although MMPs may be important effective molecules in the pathogenesis of pterygia, the roles of cytokines and growth factors have yet to be established. Several studies have documented the expression of cytokines, such as tumor necrosis factor (TNF), basic fibroblast growth factor (bFGF), transforming growth factor (TGF), and platelet-derived growth factor (PDGF) in pterygia and cultured pterygium cells.
In addition, the localization of vascular endothelial growth factor (VEGF) in the pterygium epithelium and vascular endothelium and the presence of intraepithelial capillaries in pterygia suggest a role for angiogenic cytokines in this disease.
Cellular proliferation is regulated primarily by cell cycle regulation. Cell cycle progression is regulated by a combination of positive and negative regulators. Cyclin-dependent kinase (CDKs) inhibitors (CKI) negatively regulate progression of the cell cycle by inhibiting the activity of cyclin-CDK complexes. p27kip1, a member of the CKI family, plays a pivotal role in the control of cell proliferation [27-29]. The level of p27kip1 is high during the G0 phase but decreases rapidly upon reentry of the cells into the G1 phase. Rapid removal of p27kip1 at the G0/G1 transition is required for effective progression of the cell cycle to S phase [30-32]. According to our study, a low level of p27 kip1 expression was correlated with high proliferative and migratory capacity, whereas nuclear accumulation of the Cyclin-dependent kinases inhibition (CKI) was associated with a quiesecnet and static phenotype. In previous studies, Yoshida et al. suggested that the disappearance of p27kip1 was correlated with cell proliferation in the corneal epithelium after injury [33].
The level of p27kip1 is regulated by Skp2. Skp2 is specifically required for p27kip1 ubiquitination and is a rate-limiting component of the machinery that degrades phosphorylated p27kip1. Skp2 is constitutively expressed in normal skin tissue and scar tissue. High expression of Skp2 and decreased expression of p27kip1 with fibroblast from pathological scar tissue [30]. There is negative correlation between expression of Skp2 and p27kip1 in fibroblasts from pathological scar tissue. However, there are no previous studies involving the role of Skp2 in hPFC proliferation after cataract surgery. After 48 h of transfection and 2 weeks of treatment with G418, transfection with Skp2 siRNA decreased the expression of Skp2 protein in cells of hPFC by immunoflurescence staining and western blot. The above indicated that transfection with Skp2 siRNA can inhibit expression of Skp2 in hPFC in vitro and vivo. Then upregulation of p27kip1 of hPFC in vitro and in vivo was investigated by immunoflurescence staining and western blot. After transfection, we confirmed that the number of cells increased less in Skp2 siRNA transfectant hPFC than vehicle control and blank control groups by MTT especially on 6th day. For BrdUrd incorporation assay, high level staining of BrdU can be detected in hLEC of vehicle control and blank control, but decreased in Skp2 siRNA transfectant cells. Furthermore, for the first time, we showed that inhibition of Skp2 expression by siRNA enhanced p27kip1 protein levels and prevented hPFC proliferation.
Approaches to inhibition the hPFC proliferation afer surgery by gene transfer have been reported by different research groups. Nelly et al. [34] showed that Trefoil Factor 1 can inhibit the proliferation of hPFC. Timothy et al. [20] confirmed that heparin-binding epidermal growth factor–like growth factor has the potential proliferative function for PFC. Juan et al. [35] showed that P. peruviana fruit juice can inhibition proliferation of PFC.
Finally, the number of cases included in this study is small and the result maybe has its limitation. In view of the growing interest in Skp2 and p27kip1 as a target for drug development in inhibition of hPFC proliferation after surgery, it is our hope that the data presented here will help to promote going efforts to decrease the recurrence of pterygium after surgery.
Acknowlegements
We would like to thank Professor Yasusei Kudo at the Hiroshima University of Japan, for providing us with pSuppressor containing Skp2 siRNA and control pSuppressor. This work was supported by the grant of nature science foundation of Heilongjiang province of China (No. D2007–80), Scientific foundation of education ministry of China (20092307120003), and Postdoctral foundation of China (20080430137, 200902418). Dr. Feng Wang and Dr. Hao Cui contributed equally to the work in this publication and can be considered as co-corresponding authors.
References
- 1.Hirst LW. The treatment of pterygium. Surv Ophthalmol. 2003;48:145–80. doi: 10.1016/s0039-6257(02)00463-0. [DOI] [PubMed] [Google Scholar]
- 2.Ang LPK, Tan DTH, Cajucom-Uy H, Beuerman RW. Autologous cultivated conjunctival transplantation for pterygium surgery. Am J Ophthalmol. 2005;139:611–9. doi: 10.1016/j.ajo.2004.10.056. [DOI] [PubMed] [Google Scholar]
- 3.Tosi G-M, Massaro-Giordano M, Caporossi A, Toti P. Amniotic membrane transplantation in ocular surface disorders. J Cell Physiol. 2005;202:849–51. doi: 10.1002/jcp.20181. [DOI] [PubMed] [Google Scholar]
- 4.Coroneo MT, DiGirolamo N, Wakefield D. The pathogenesis of pterygia. Curr Opin Ophthalmol. 1999;10:282–8. doi: 10.1097/00055735-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Threlfall TJ, English DR. Sun exposure and pterygium of the eye: a dose-response curve. Am J Ophthalmol. 1999;128:280–7. doi: 10.1016/s0002-9394(99)00161-0. [DOI] [PubMed] [Google Scholar]
- 6.Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis. Arch Ophthalmol. 2001;119:695–706. doi: 10.1001/archopht.119.5.695. [DOI] [PubMed] [Google Scholar]
- 7.Clear AS, Chirambo MC, Hutt MSR. Solar keratosis, pterygium and squamous cell carcinoma of the conjunctiva in Malawi. Br J Ophthalmol. 1979;63:102–9. doi: 10.1136/bjo.63.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan DT, Liu YP, Sun L. Flow cytometry measurements of DNA content in primary and recurrent pterygia. Invest Ophthalmol Vis Sci. 2000;41:1684–6. [PubMed] [Google Scholar]
- 9.Chan CM, Liu YP, Tan DT. Ocular surface changes in pterygium. Cornea. 2002;21:38–42. doi: 10.1097/00003226-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Chen J-K, Tsai RJF, Lin S-S. Fibroblasts isolated from human pterygia exhibit transformed cell characteristics. In Vitro Cell Dev Biol. 1994;30A:243–8. doi: 10.1007/BF02632046. [DOI] [PubMed] [Google Scholar]
- 11.Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A. The cell cycle regulatory protein Ckst is required for SCF (Skp2) mediated ubiquitinylation of P27. Nat Cell Biol. 2001;3:321–4. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- 12.Akimoto M, Miyahara T, Arai J, Akimoto A, Hamada H, Yoshida Y, Yoshimura N. A new delivery system for 5-fluorouracil using prodrug and converting enzyme. Br J Ophthalmol. 2002;86:581–6. doi: 10.1136/bjo.86.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson KTM, Rodicker F, Heise K.Heinz C, Steuhl KP, Pützer BM, Hudde TAdenoviral p53 gene transfer inhibits human Tenon’s capsule fibroblast proliferation. Br J Ophthalmol 200589508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–9. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 15.Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Müller U, Krek W. p45SKP2 promotes p27 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–14. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Qi L, Su Y, Yan QH, Teng Y. S phase kinase-interacting protein 2 (Skip2) targeting Small interfering RNA inhibits cell proliferation of tenon’s capsule fibroblast by increasing p27 protein level. Invest Ophthalmol Vis Sci. 2010;51:1475–82. doi: 10.1167/iovs.09-4363. [DOI] [PubMed] [Google Scholar]
- 17.Tseng SH, Chen YT, Cheng HC, Huang FC, Lee SC, Chen FK. Impression cytology study of conjunctival epithelial phenotypes on the healing ocular surface after pterygium excision. Cornea. 2001;20:244–50. doi: 10.1097/00003226-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Wang IJ, Lai WT, Liou SW, Chiu CZ, Hu FR, Kao WW, Hung PT. Impression cytology of pterygium. J Ocul Pharmacol Ther. 2000;16:519–28. doi: 10.1089/jop.2000.16.519. [DOI] [PubMed] [Google Scholar]
- 19.Chui J, Di Girolamo N, Coroneo MT. Wakefield. The role of substance P in the pathogenesis of pterygia. Invest Ophthalmol Vis Sci. 2007;48:4482–9. doi: 10.1167/iovs.07-0123. [DOI] [PubMed] [Google Scholar]
- 20.Peiretti E, Dessi S, Mulas C, Abete C, Norfo C, Putzolu M, Fossarello M. Modulation of cholesterol homeostasis by antiproliferative drugs in human pterygium fibroblasts. Invest Ophthalmol Vis Sci. 2007;48:3450–8. doi: 10.1167/iovs.06-1054. [DOI] [PubMed] [Google Scholar]
- 21.Nolan TM, Di Girolamo N, Coroneo MT, Wakefield D. Proliferative effects of heparin-binding epidermal growth factor–like growth factor on pterygium epithelial cells and fibroblasts. Invest Ophthalmol Vis Sci. 2004;45:110–3. doi: 10.1167/iovs.03-0046. [DOI] [PubMed] [Google Scholar]
- 22.Solomon A, Grueterich M, Li DQ, Meller D, Lee SB, Tseng SC. Overexpression of insulin-like growth factor–binding protein-2 in pterygium body fibroblasts. Invest Ophthalmol Vis Sci. 2003;44:573–80. doi: 10.1167/iovs.01-1185. [DOI] [PubMed] [Google Scholar]
- 23.Di Girolamo N, McCluskey PJ, Lloyd A, Coroneo MT, Wakefield D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:671–9. [PubMed] [Google Scholar]
- 24.Di Girolamo N, Coroneo MT, Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci. 2001;42:1963–8. [PubMed] [Google Scholar]
- 25.Di Girolamo N, Wakefield D, Coroneo MT. Differential expression of matrix metalloproteinases and their tissue inhibitors at the advancing pterygium head. Invest Ophthalmol Vis Sci. 2000;41:4142–9. [PubMed] [Google Scholar]
- 26.Kria L, Ohira A, Amemiya T. Growth factors in cultured pterygium fibroblasts: immunohistochemical and ELISA analysis. Graefes Arch Clin Exp Ophthalmol. 1998;236:702–8. doi: 10.1007/s004170050144. [DOI] [PubMed] [Google Scholar]
- 27.Nelsen CJ, Hansen LK, Rickheim DG, Chen C, Stanley MW, Krek W, Albrecht JH. Induction of hepatocyte proliferation and liver hyperplasia by the targeted expression of cyclin E and skp2. Oncogene. 2001;20:1825–31. doi: 10.1038/sj.onc.1204248. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama K, Nagahama H, Minamishima Y, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, Kitagawa M, Nakayama K, Hatakeyama S. Targeted disruption of Skp2 results in accumulation of cyclinE and p27, polyploidy and centrosome over duplication. EMBO J. 2000;19:2069–81. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–8. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- 30.Ezoe S, Matsumura I, Nakata S, Gale K, Ishihara K, Minegishi N, Machii T, Kitamura T, Yamamoto M, Enver T, Kanakura Y. GATA2 estrogen receptor chimer are gulates cytokine dependent growth of hematopoietic cells through accumulation of p21 (WAF1) and p27 (Kip1) proteins. Blood. 2002;100:3512–20. doi: 10.1182/blood-2002-04-1177. [DOI] [PubMed] [Google Scholar]
- 31.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene P27kip1 is haplo insufficient for tumour suppression. Nature. 1998;396:177–80. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kudo Y, Takata T, Ogawa I, Kaneda T, Sato S, Takekoshi T, Zhao M, Miyauchi M, Nikai H. p27 accumulation by inhibition of proteasome function induces apoptosis in oral squamous cell carcinoma cells. Clin Cancer Res. 2000;6:916–23. [PubMed] [Google Scholar]
- 33.Yoshida K, Nakayama K, Nagahama H, Harada T, Harada C, Imaki J, Matsuda A, Yamamoto K, Ito M, Ohno S, Nakayama K. Involvement of p27(KIP1) degradation by Skp2 in the regulation of proliferation in response to wounding of corneal epithelium. Invest Ophthalmol Vis Sci. 2002;43:364–70. [PubMed] [Google Scholar]
- 34.Buron N, Guery L, Creuzot-Garcher C, Lafontaine PO, Bron A, Rio MC, Solary E. Trefoil Factor TFF1-Induced Protection of Conjunctival Cells from Apoptosis at Premitochondrial and Postmitochondrial Levels. Invest Ophthalmol Vis Sci. 2008;49:3790–8. doi: 10.1167/iovs.07-1270. [DOI] [PubMed] [Google Scholar]
- 35.Pardo JM, Fontanilla MR, Ospina LF, Espinosa L. Determining the pharmacological activity of physalis peruviana fruit juice on rabbit eyes and fibroblast primary cultures. Invest Ophthalmol Vis Sci. 2008;49:3074–9. doi: 10.1167/iovs.07-0633. [DOI] [PubMed] [Google Scholar]