Abstract
Background/Aims
The zebrafish is an emerging model system for the study of cardiac electrophysiology and human arrhythmias. High resolution imaging techniques are powerful tools for the study of zebrafish cardiac electrophysiology, but these methods require the complete absence of cardiac contraction. Many pharmacological agents that uncouple cardiac contraction also markedly alter the cardiac action potential (AP). In this study, we compared the effects two uncoupling agents, 2,3- Butanedione monoxime (BDM) and blebbistatin, on contractility and AP parameters in embryonic zebrafish heart.
Methods
Zebrafish hearts were explanted (48 hpf) and superfused with either BDM (15 mM) or blebbistatin (1, 5 or 10 μM), while recording atrial or ventricular APs with the disrupted patch technique. Calcium transients were recorded with a high-speed confocal scanning microscope in hearts loaded intracellularly with 10 μM fluo-4 and superfused with 10 μM blebbistatin.
Results
Despite abolishing cardiac contractility, BDM altered ventricular AP morphology and inhibited spontaneous APs. In contrast, blebbistatin (10 μM) abolished contractility without significantly altering AP morphology or generation of spontaneous APs. Blebbistatin allowed for high fidelity measurements of atrial and ventricular calcium transients.
Conclusion
Blebbistatin is a potent and effective excitation-contraction uncoupling agent in embryonic zebrafish heart.
Key Words: Zebrafish, Heart, Action potential, Electrophysiology, Excitation-contraction coupling
Introduction
The zebrafish, Danio rerio, is a well-established vertebrate model for the study of cardiac development [1] and is an emerging model system for the study of cardiac electrophysiology [2, 3, 4]. High resolution optical recordings using fluorescent dyes [2] and constitutively-expressed fluorescent calcium indicator proteins [3, 4] allow for the simultaneous recording of action potentials (AP) or calcium transients in various regions of the embryonic zebrafish heart. However, these high resolution optical techniques are critically dependent upon the complete absence of cardiac contraction. The primary method to reduce motion artifacts involves the use of pharmacological agents that uncouple cardiac contraction. To date, studies in zebrafish have utilized 2,3-Butanedione monoxime (BDM) as an excitation-contraction uncoupling agent in millimolar concentration [2, 3]. However, at concentrations that impair contractility, BDM is known to alter potassium and calcium currents with unintended consequences on the morphology of the cardiac AP [5, 6]. The effect of BDM on directly measured transmembrane potential (Vm) in zebrafish heart has not been reported.
Blebbistatin is a small synthetic molecule that inhibits cell myosin cycling by preferentially binding to the ADP-Pi complex of myosin II [7, 8, 9]. Recently, blebbistatin was demonstrated to be a potent and effective excitation- contraction uncoupling agent in rat and rabbit cardiac tissue, without altering action potential morphology, ECG parameters or intracellular calcium transients [5, 6]. In this study, we compared the effects of BDM and blebbistatin on contractility and AP parameters in embryonic zebrafish heart.
Methods
All experiments were performed in accordance with the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. Wild-type (WT) adult zebrafish and larvae were raised under standard laboratory conditions at 28°C.
Action potential recordings from embryonic heart
Four-eight hour-post-fertilization (hpf) larvae were dechorionated and anesthetized with 0.02% tricaine for 1 to 2 minutes. The heart was dissected from the thorax en bloc by using fine forceps and transferred to the recording chamber. Only spontaneously beating whole hearts were studied. All experiments were performed at 22°C to 24°C. The recording chamber was superfused with external control solution containing (in mmol/L) NaCl 140, KCl 4, CaCl2 1.8, MgCl2 1, glucose 10, and HEPES 10 (pH 7.4). Suction pipettes were made from borosilicate capillary tubes (8250 glass; A-M Systems) and fire-polished to obtain resistances of 6-9 MΩ when filled with solution containing (in mmol/L) KCl 120, EGTA 5, K2ATP 5, MgCl2 5, and HEPES 10 (pH 7.2).
Vm was measured by using an AxoClamp 2A amplifier (Molecular Devices) in the bridge mode with the disrupted patch technique. The pipette was positioned adjacent to the heart and a seal was formed by application of minimal suction. Using this technique, stable spontaneous APs were recordable for up to 3 hours. Vm was filtered at 10 kHz and digitized at a sampling frequency of 20 kHz with a 12-bit analog-to-digital converter (Digidata 1322A Interface, Molecular Devices). Action potential duration (APD) from a series of five APs was calculated as the time interval between the peak maximum upstroke velocity (phase 0) and the time at 10% (APD10), 50% (APD50) and 90% of repolarization (APD90). To determine the effective refractory period (ERP), the heart was paced by injecting pulses of depolarizing current (1.5x threshold, 3 msec duration) at a cycle length (CL) of 350 msec for the atrium and 400 msec for the ventricle. Ten consecutive stimuli were followed by a single premature stimulus at progressively shorter coupling intervals.
Drugs
BDM (Fluka Chemika) was dissolved in external control solution to a concentration of 15 mM. Blebbistatin (Sigma) was dissolved in dimethyl sulfoxide (DMSO, Fisher Scientific) for a stock concentration of 17 mM and further diluted with external control solution to concentrations of 1, 5 or 10 μM. Final concentrations of DMSO were < 0.06%. Care was taken to minimize light exposure of blebbistatin solutions. Each explanted heart was tested against the various concentrations of blebbistatin in random order.
Calcium transient current monitored with confocal microscopy
Calcium transients were recorded in spontaneously beating isolated hearts at 48 hpf with fluo-4 (Molecular Probes) using a high-speed confocal scanning microscope. Hearts were first incubated in the external control solution containing 10 μM fluo 4-AM, 0.35 mM probenicid and 10 μM blebbistatin at 25°C for 2 minutes. Probenicid was included to help retard fluo- 4 loss from the cells. The heart was then continuously bathed in control external solution containing probenicid. High speed scanning at 63 frames/sec was achieved with a confocal microscope (Zeiss 5-Live, excitation 489 nm, emission LP 505, 40x oil).
Statistical analysis
Atrial and ventricular AP parameters, including maximum upstroke velocity, APD, maximum diastolic membrane potential (MDP), CL and ERP were independently analyzed with Student's paired t-test, with a value of p<0.05 considered as statistically significant. Values are given as mean ± SEM.
Results
Action potential recordings in BDM and blebbistatin
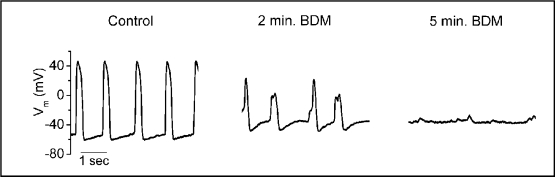
Superfusion of an explanted embryonic heart with 15 mM BDM completely abolished cardiac contractility by visual inspection. However, BDM markedly altered ventricular action potential morphology (Fig. 1). Within 2 minutes of superfusion, BDM decreased MPD from −60 mV to −48 mV and the morphology of the ventricular action potential was profoundly altered. By 5 minutes MDP further depolarized to −40 mV and spontaneous AP generation ceased. The effects of BDM on contractility and AP morphology were reversed after superfusing with external control solution. Similar findings were observed in 4 other hearts tested with BDM.
Fig. 1.
Spontaneous ventricular action potentials in embryonic zebrafish were completely abolished during superfusion with 15 mM 2,3-butanedione monoximine (BDM).
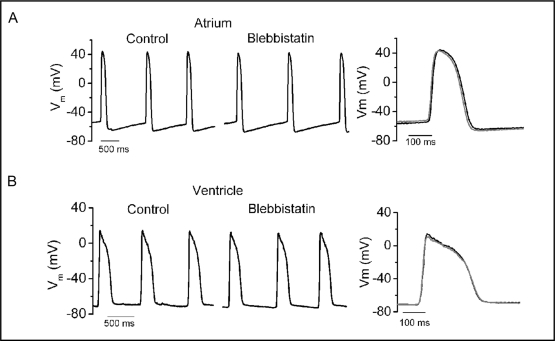
Low concentration of blebbistatin (1 μM) mildly decreased atrial and ventricular contractility by visual inspection. At higher concentration (10 μM), atrial and ventricular contractions were nearly abolished. The effects of blebbistatin on contractility were reversible after superfusing with external control solution.
The effects of 1, 5 and 10 μM blebbistatin on atrial and ventricular AP parameters are listed in Table 1 and Table 2, respectively. Blebbistatin did not alter the CL of spontaneously firing, the upstroke velocity, MDP, ERP or duration of repolarization at the various concentrations of blebbistatin in either the atrium or ventricle. In addition, the morphology of atrial and ventricular AP was not altered by blebbistatin, as noted by the ability to superimpose APs before and after treatment (Fig. 2).
Table 1.
Atrial action potential parameters in response to blebbistatin. C: control; B: blebbistatin; CL: cycle length (msec); MDP: maximum diastolic potential (mV); Pmax: maximum upstroke velocity (mV/msec); AP10, AP50 or AP90: action potential duration to 10%, 50% or 90% of repolarization (msec); ERP: effective refractory period (msec). Values are mean ± SEM.
| luM (n=4) | 5uM (n=4) | 10uM (n=4) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | B | P | C | B | P | C | B | P | |
| CL | 1254 ± 262 | 1366 ± 164 | 0.40 | 1110 ± 127 | 1139 ± 106 | 0.64 | 1040 ± 125 | 1163 ± 181 | 0.18 |
| MDP | −58.9 ± 1.4 | −592 ± 2.6 | 0.81 | −52.6 ± 6.3 | −53.6 ± 5.0 | 0.49 | −56.2 ± 6.2 | −55.7 ± 6.2 | 0.41 |
| Pmax | 5.1 ± 1.4 | 4.6 ± 1.2 | 0.28 | 4.5 ± 0.8 | 4.3 ± 1.6 | 0.23 | 5.6 ± 1.6 | 4.8 ± 1.0 | 0.30 |
| AP10 | 104.3 ± 15.7 | 115.7 ± 17.9 | 0.06 | 90.2 ± 7.2 | 88.8 ± 6.9 | 0.78 | 83.9 ± 7.7 | 81.8 ± 8.0 | 0.16 |
| AP50 | 151.7 ± 20.3 | 129.0 ± 8.3 | 0.79 | 129.0 ± 8.3 | 126.9 ± 7.9 | 0.73 | 121.1 ± 11.8 | 118.6 ± 12.5 | 0.14 |
| AP90 | 195.8 ± 31.4 | 190.9 ± 27.8 | 0.76 | 161.4 ± 15.2 | 159.8 ± 11.1 | 0.83 | 146.2 ± 14.1 | 146.3 ± 14.6 | 0.95 |
| ERP | 298 ± 13.1 | 301 ± 11.6 | 0.22 | ||||||
Table 2.
Ventricular action potential parameters in response to blebbistatin. C: control; B: blebbistatin; CL: cycle length (msec); MDP: maximum diastolic potential (mV); Pmax: maximum upstroke velocity (mV/msec); AP10, AP50 or AP90: action potential duration to 10%, 50% or 90% of repolarization (msec); ERP: effective refractory period (msec). Values are mean ± SEM.
| 1uM (n=4) | 5uM (n=4) | 10uM (n=4) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | B | P | C | B | P | C | B | P | |
| CL | 1235 ± 459 | 1610 ± 616 | 0.11 | 1357 ± 400 | 1450 ± 450 | 0.18 | 1411 ± 459 | 1545 ± 414 | 0.53 |
| MDP | −68.0 ± 1.0 | −68.1 ± 1.6 | 0.96 | −68.0 ± 1.7 | −67.4 ± 1.4 | 0.52 | −63.0 ± 1.7 | −67.3 ± 1.4 | 0.80 |
| Pmax | 5.9 ± 1.1 | 6.2 ± 0.9 | 0.58 | 5.4 ± 1.0 | 4.9 ± 0.4 | 0.61 | 4.7 ± 0.2 | 4.2 ± 0.4 | 0.10 |
| AP10 | 149.7 ± 8.6 | 146.3 ± 20.0 | 0.89 | 147.3 ± 22.8 | 133.1 ± 16.2 | 0.12 | 144.0 ± 20.6 | 140.7 ± 11.4 | 0.77 |
| AP50 | 241.8 ± 10.3 | 247.1 ± 18.8 | 0.79 | 246.9 ± 16.3 | 230.9 ± 9.5 | 0.21 | 238.8 ± 16.3 | 233.9 ± 10.9 | 0.67 |
| AP90 | 275.0 ± 9.7 | 281.2 ± 16.2 | 0.76 | 283.2 ± 12.6 | 268.4 ± 8.8 | 0.24 | 274.9 ± 13.9 | 272.9 ± 10.3 | 0.87 |
| ERP* | 307 ± 11.5 | 320 ± 5.0 | 0.55 | ||||||
Fig. 2.
Spontaneous atrial (a) and ventricular (b) action potentials before and during superfusion with blebbistatin (10 μM). Overlay of action potentials before (—) and during superfusion (—) with blebbistatin is demonstrated in the right panels.
Measurement of calcium transients in embryonic zebrafish heart
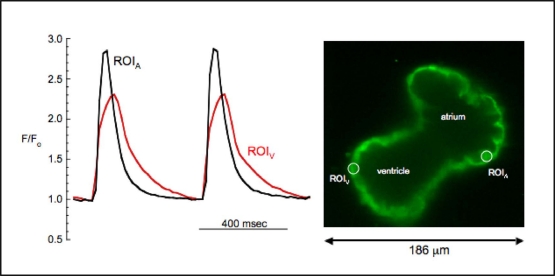
In order to test the practical utility of blebbistatin in an experimental system, we measured atrial and ventricular calcium transients in explanted embryonic zebrafish hearts using the calcium-sensitive fluorescent dye, fluo-4. Spontaneous cardiac contractions were abolished during perfusion with control external solution containing 10 μM blebbistatin. Robust fluorescent signals were recorded simultaneously from atrium and ventricle, with representative atrial and ventricular transients presented in Fig. 3. Atrial calcium transients preceded ventricular transients and were shorter in duration than their ventricular counterparts (Fig. 3). The morphology of atrial and ventricular calcium transients correlated with the time course of atrial and ventricular APs (Fig. 2). This mechanical uncoupling to minimize motion artifacts allows for the optimization of signal to noise ratio and highlights the practical utility of blebbistatin combined with high-speed confocal laser microscopy.
Fig. 3.
High-speed confocal imaging of calcium transients in a spontaneously beating embryonic zebrafish heart bathed in 10 μM blebbistatin. The signals were simultaneously recorded from regions of interest in the atrium and ventricle (ROIA, ROIV).
Discussion
Cutting-edge technologies, such as high-resolution optical imaging using fluorescent dyes or genetically encoded calcium indicator proteins, allow for detailed, temporal-spatial characterization of electrical activity in the developing heart. However, the elimination of motion artifacts is crucial for high-fidelity recordings of membrane voltage and intracellular calcium transients. Genetic modification of cardiac contraction in zebrafish, by knocking down troponin T (tnnt2) expression, is an attractive approach to uncouple excitation-contraction in early embryonic heart [4]. However, tnnt2 mutations disrupt formation of the fast conduction system and thereby limit the role of tnnt2 mutations or anti-sense morpholinos for the study of cardiac electrophysiology at later developmental time points [3]. Thus, pharmacological uncoupling of cardiac contraction remains an appealing methodology to minimize motion artifacts. While BDM has been widely used as a pharmacological method of excitation-contraction uncoupling, significant distortion of AP properties has been reported in some species [5, 6]. In this study, we found that BDM depolarized MDP and markedly altered the morphology of ventricular APs that result in the eventual abolishment of spontaneous APs in zebrafish embryonic heart.
By contrast, at concentrations that inhibited cardiac contraction, blebbistatin was effective at reducing cardiac contractility without altering basic properties of the cardiac action potential including spontaneous CL, upstroke velocity, amplitude and MDP. Atrial and ventricular repolarization trajectory and ERP were unaffected by concentrations of blebbistatin up to 10 μM. Our results indicate that blebbistatin is an effective agent for excitation-contraction uncoupling in embryonic zebrafish heart and thus allows for high-fidelity measurements of atrial and ventricular calcium transients in embryonic zebrafish heart.
Acknowledgements
This work was supported in part by NIHT-32 training grant to CJJ, grants from American Heart Association Western States Affiliate to MTF and the National Heart, Lung, and Blood Institute Grant R37-HL-42873 to KWS. Special thanks to Children's Health Research Center at University of Utah and the Nora Eccles Treadwell Foundation.
References
- 1.Stainier DY. Zebrafish genetics and vertebrate heart formation. Nat Rev Genet. 2001;2:39–48. doi: 10.1038/35047564. [DOI] [PubMed] [Google Scholar]
- 2.Milan DJ, Kim AM, Winterfield, Jones IL, Pfeufer A, Sanna S, Arking DE, Amsterdam AH, Sabeh KM, Mably JD, Rosenbaum DS, Peterson RT, Chakravarti A, Kaab S, Roden DM, MacRae CA. Drug-sensitized zebrafish screen identifies multiple genes, including gins3, as regulators of myocardial repolarization. Circulation. 2009;120:553–559. doi: 10.1161/CIRCULATIONAHA.108.821082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi NC, Shaw RM, Jungblut B, Huisken J, Ferrer T, Arnaout R, Scott I, Beis D, Xiao T, Baier H, Jan LY, Tristani-Firouzi M, Stainier DY. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 2008;6:e109. doi: 10.1371/journal.pbio.0060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnaout R, Ferrer T, Huisken J, Spitzer K, Stainier DY, Tristani-Firouzi M, Chi NC. Zebrafish model for human long qt syndrome. Proc Natl Acad Sci U S A. 2007;104:11316–11321. doi: 10.1073/pnas.0702724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li D, Nattel S. Pharmacological elimination of motion artifacts during optical imaging of cardiac tissues: Is blebbistatin the answer? Heart Rhythm. 2007;4:627–628. doi: 10.1016/j.hrthm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Kettlewell S, Walker NL, Cobbe SM, Burton FL, Smith GL. The electrophysiological and mechanical effects of 2,3-butane-dione monoxime and cytochalasin-d in the langendorff perfused rabbit heart. Exp Physiol. 2004;89:163–172. doi: 10.1113/expphysiol.2003.026732. [DOI] [PubMed] [Google Scholar]
- 7.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin ii inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers Mechanism of blebbistatin inhibition of myosin ii. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 9.Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin ii. Nat Struct Mol Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]