Abstract
Background
The extracellular signal-regulated kinase-1 and 2 (ERK1/2) proteins play an important role in cancer cell proliferation and survival. ERK1/2 proteins also are important for normal cell functions. Thus, anti-cancer therapies that block all ERK1/2 signaling may result in undesirable toxicity to normal cells. As an alternative, we have used computational and biological approaches to identify low-molecular weight compounds that have the potential to interact with unique ERK1/2 docking sites and selectively inhibit interactions with substrates involved in promoting cell proliferation.
Methods
Colony formation and water soluble tetrazolium salt (WST) assays were used to determine the effects of test compounds on cell proliferation. Changes in phosphorylation and protein expression in response to test compound treatment were examined by immunoblotting and in vitro kinase assays. Apoptosis was determined with immunoblotting and caspase activity assays.
Results
In silico modeling was used to identify compounds that were structurally similar to a previously identified parent compound, called 76. From this screen, several compounds, termed 76.2, 76.3, and 76.4 sharing a common thiazolidinedione core with an aminoethyl side group, inhibited proliferation and induced apoptosis of HeLa cells. However, the active compounds were less effective in inhibiting proliferation or inducing apoptosis in non-transformed epithelial cells. Induction of HeLa cell apoptosis appeared to be through intrinsic mechanisms involving caspase-9 activation and decreased phosphorylation of the pro-apoptotic Bad protein. Cell-based and in vitro kinase assays indicated that compounds 76.3 and 76.4 directly inhibited ERK-mediated phosphorylation of caspase-9 and the p90Rsk-1 kinase, which phosphorylates and inhibits Bad, more effectively than the parent compound 76. Further examination of the test compound's mechanism of action showed little effects on related MAP kinases or other cell survival proteins.
Conclusion
These findings support the identification of a class of ERK-targeted molecules that can induce apoptosis in transformed cells by inhibiting ERK-mediated phosphorylation and inactivation of pro-apoptotic proteins.
Background
The extracellular signal-regulated kinases-1 and 2 (ERK1/2) proteins are members of the mitogen activated protein (MAP) kinase superfamily that regulate cell proliferation and survival. ERK1/2-mediated cell survival occurs through protection against apoptosis by inactivating pro-apoptotic proteins. For example, ERK proteins promote cell survival by inhibiting caspase-9 [1,2] or Bim (Bcl-2-interacting mediator of cell death) through direct phosphorylation [3]. Indirect inhibition of apoptosis occurs through ERK phosphorylation and activation of p90Rsk-1, which phosphorylates the pro-apoptotic Bad (Bcl-xL/Bcl-2 associated death promoter) protein and causes 14-3-3-mediated sequestering that prevents interactions with the pro-survival protein Bcl-2 [4,5]. Thus, constitutive activation of the ERK1/2 pathway through mutations in upstream receptors, Ras G-proteins, and kinases, such as B-Raf, provides transformed cancer cells with a survival advantage [6-8].
Significant effort has gone into developing molecules that inhibit proteins in the ERK1/2 pathway [9,10]. These drug discovery efforts include monoclonal antibodies and small molecules that inhibit receptor tyrosine kinases, Ras G-proteins, Raf, or MEK proteins [9,11-13]. Although some of these therapies have shown promising clinical results, toxicity to skin, cardiac, and gastrointestinal tissue has been reported [14,15]. The toxicity associated with upstream inhibition of ERK1/2 signaling is likely due to the effects on the ERK pathway in normal tissue and the various ERK1/2 substrates that regulate cellular functions [6,16]. Thus, inhibition of specific ERK functions, such as regulation of pro-apoptotic proteins, may be an alternative approach to alleviating toxic side effects resulting from complete inhibition of ERK signaling by compounds targeting upstream proteins. To test this, we have identified molecules that act independent of the ATP binding site and are predicted to be selective for ERK1/2 substrate docking domains [17,18]. By developing compounds that are substrate selective, our goal is to inhibit ERK functions that are associated with cancer cell survival but preserve ERK functions in normal non-cancerous cells.
ERK1/2 are proline-directed serine/threonine kinases that phosphorylate substrate protein sequences containing, at minimum, a proline in the +1 position (S/TP site). Proline in the -2 position (PXS/TP sequence) may also determine phosphorylation specificity [19]. While this consensus sequence is shared by the other MAP kinases proteins, including p38 MAP kinases, c-Jun N-terminal kinases (JNKs), and ERK5, each MAP kinase retains substrate specificity suggesting that other determinants of kinase-substrate interactions are involved. Currently, two distinct docking domains on substrates have been identified to mediate interactions between protein substrates and MAP kinases [19-22]. The D-domain or DEJL site (docking site for ERK or JNK, LXL), consists of two or more basic residues, a short peptide linker, and a cluster of hydrophobic residues. ERK1/2 substrates containing D-domains include ELK-1, p90Rsk-1, MKP-3, and caspase-9 [1,23,24]. D-domains have been found on substrates for ERK, JNK, and p38 MAP kinases [25,26]. MAP kinase substrates may also contain an F-site or DEF (docking site for ERK, FXF) motif, which contains the consensus FXFP motif. The F-site is 6-20 amino acids C-terminal to the phosphorylation site [19] is also found on ELK-1 as well as substrates like KSR and nucleoporins [27].
Specific residues on MAP kinases form docking domains that determine binding specificity with substrate proteins. ERK1/2 and other MAP kinases contain a common docking (CD) domain, which includes aspartate residues 316 and 319 (labeled for ERK2) that are located on the side opposite of the TXY activation loop [25] and mediates interactions with the substrate D-domains [27,28]. While the CD domain shares common features among MAP kinases, differences in the CD domains and adjacent residues of ERK1/2 and p38 MAP kinases may be responsible for determining the specificity of substrate interactions [29]. The F-site containing substrates are thought to form hydrophobic interactions with ERK1/2 on a F-site recruitment site (FRS), which consists of leucines 198, 232, 235 and tyrosines 231 and 261 residues (labeled for ERK2) located near the TXY activation site [27].
We have previously utilized computer aided drug design (CADD) to identify low-molecular weight (LMW) compounds that are predicted to interact near the ERK2 CD domain and selectively disrupt ERK2 interactions with substrate proteins [17,18]. These studies identified several compounds that inhibited ERK1/2's ability to phosphorylate selected substrate proteins but not affect the overall activity of the ERK enzyme supporting the goal of using small molecules to inhibit productive protein-protein interactions. Moreover, some of the compounds tested did not appear to affect the ability for the related p38 MAP kinase to phosphorylate substrate proteins [18]. In addition to inhibiting ERK substrate phosphorylation, several compounds identified inhibited cell proliferation. In the current studies, we have identified a class of structurally similar compounds that inhibit cell proliferation and cause rapid induction of apoptosis in transformed cancer cell lines. Apoptosis appeared to occur through an intrinsic mechanism involving the prevention of ERK2-mediated inhibition of caspase-9 and p90Rsk-1 phosphorylation of the pro-apoptotic protein Bad. In addition, transformed cells appeared more sensitive to the growth inhibitory effects of the test compounds as compared to non-transformed epithelial cells. These findings support the identification of novel LMW compounds that target ERK1/2 proteins and restore apoptosis responses in cancer cells by preventing phosphorylation events that inactivate pro-apoptotic proteins.
Methods
Cells and reagents
HeLa S3 (human cervical carcinoma) and retinal pigment epithelial cells that stably express human telomerase (hTERT-RPE) were purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained in complete medium consisting of Dulbecco's modified Eagle medium (DMEM) plus 10% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA) and antibiotics (Penicillin, 100 U/ml; Streptomycin, 100 μg/ml) (Invitrogen, Carlsbad, CA). Hygromycin B, 0.01 mg/mL, (Roche, Indianapolis, IN) was used to maintain selection for the hTERT-RPE cells. Epidermal growth factor (EGF) and etoposide were purchased from Sigma (St. Louis, MO) and used at final concentrations of 25 ng/ml and 50 μM, respectively. The ERK (pT183/pY185) and α-tubulin antibodies were purchased from Sigma. Antibodies against phosphorylated Bad (pS112 or pS136), total Bad, phosphorylated Rsk-1 (pT573), phosphorylated Akt substrates (RXRXXpS/T), Mcl-1, phosphorylated caspase-9 (pT125), and total caspase-9 were purchased from Cell Signaling (Beverly, MA). Antibodies against poly ADP-ribose polymerase (PARP), total Rsk-1, and ERK5 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). U0126, SB203580, and Akt Inhibitor I were purchased from Calbiochem and used at final concentrations of 10 μM, 20 μM, and 25 μM, respectively. LY294002 was purchased from Cell Signaling and used at a final concentration of 25 μM. Test compounds were purchased from Chembridge (San Diego, CA) and stored as 25 mM stock solutions in DMSO. The general caspase inhibitor, Z-VAD-FMK, was purchased from BD Biosciences (San Jose, CA) and used at a final concentration of 20 μM.
Protein expression
Plasmids for mammalian and bacterial expression of caspase-9 (catalytically inactive C287A mutant) were purchased from Addgene (catalog #11819 and 11830). The His6-tagged caspase-9 (C287A) was purified from BL21(DE3) cells as described [30]. Briefly, BL21(DE3) cells were induced with 0.2 mM isopropyl β-D-1-thiogalactopyranoside for 4 hours and harvested with BugBuster protein extraction reagent (EMD Biosciences, San Diego, CA). Lysates were loaded onto a Talon Co2+- IMAC affinity resin column (BD Biosciences, San Jose, CA) and eluted with imidazole. GST-p90Rsk-1 was expressed and purified as previously described [31]. His6-tagged ERK was expressed and purified as previously described [17]. The plasmid for Bad was provided by Dr. Michael Greenberg (Harvard University). Transient expression of Bad and caspase 9 (C287A) in HeLa cells was done using Lipofectamine (Invitrogen).
Immunoblotting
Cells were washed with cold phosphate buffered saline (PBS, pH 7.2; Invitrogen) and protein lysates were collected with 2× SDS-PAGE sample buffer (4% SDS, 5.7 M β-mercaptoethanol, 0.2 M Tris pH 6.8, 20% glycerol, 5 mM EDTA) or cold tissue lysis buffer (TLB; 20 mM Tris-HCl pH 7.4, 137 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.1% SDS, 25 mM β-glycerophosphate, 2 mM sodium pyrophosphate, 10% glycerol, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine). Lysates collected in TLB were centrifuged at 20,000 (× g) to remove insoluble material and then diluted with an equal volume of 2× SDS-sample buffer. Proteins were separated by SDS-PAGE and analyzed by immunoblotting using enhanced chemiluminesence (ECL, GE Healthcare, United Kingdom). The relative protein levels were determined by densitometry scanning (Alpha Innotech), keeping the pixel intensity within the linear range of detection.
Fluorescence Quenching Assay
Fluorescence quenching of 1 μM ERK2 was evaluated by spectra analysis (SpectraMax5, Molecular Devices) utilizing an excitation wavelength of 295 nm and the emission spectra was monitored from 300 to 500 nm in the presence or absence of 25 μM of the indicated test compound. Relative changes in ERK2 fluorescence intensity in presence of test compounds were compared to ERK2 fluorescence in the presence of DMSO vehicle only.
Cell proliferation assays
Cell proliferation was evaluated by colony formation or by water soluble tetrazolium-1 (WST-1) assays. For colony formation, cells (~250 cells/mL) were plated and allowed to recover for 24 hours before treatment with test compounds. Cells were grown for 10-14 days and colonies that formed (approximately 40 cells or more) were fixed for 10 minutes in 4% paraformaldehyde and stained with 0.2% crystal violet in 20% methanol for 1-2 minutes. The number of colonies in the treated samples was expressed as a percentage of the controls, which consisted of 75, 146, or 154 colonies from 3 separate experiments, respectively. WST-1 assay was done according to manufacturer's instructions and cleavage of WST-1 to formazan by cellular mitochondrial dehydrogenases is used as an indicator of viable cells. Briefly, cells (~250/mL) were seeded in 96 well plates and allowed to recover overnight followed by test compound treatment for 7 days. WST-1 reagent was added and absorbance was read at 450 nm with background readings taken at 650 nm. After background subtraction, values were normalized to the control (DMSO only) cells.
Kinase assays
Active ERK2 (2 ng, New England BioLabs, Ipswich, MA) was incubated with 0.5 μg His6-tagged caspase 9 (C287A) or p90RSK-1 for 60 minutes at 30°C in 50 mM Tris-HCl, 10 mM MgCl2, 1 mM EGTA, 2 mM DTT, 0.01% Brij 35 (pH 7.5) containing 20 μM ATP and 2 μCi γ-32P-ATP. Reactions were stopped with an equal volume of 2× SDS-PAGE sample buffer and the proteins were resolved by SDS-PAGE. The gels were stained with coomassie blue, dried, and 32P incorporation into substrate was determined by phosphoimager analysis.
Caspase activity assays
HeLa cells were seeded at equal density and treated with indicated compound for 5 hours. Cells were harvested and frozen at -20°C. Fluorometric kits (Calbiochem) were used according to manufacturer's instructions to determine the activity of caspases-8 and 9 in cell lysates. Briefly, cell pellets were resuspended with 100 μL sample buffer and incubated on ice for 10 minutes. Samples were centrifuged at 10,000 × g for 10 minutes at 4°C; 50 μL of clarified lysate was transferred to a black walled 96 well plate and 50 μL of assay buffer was added. Fluorescent caspase substrate was added and a baseline reading was taken utilizing 400 nm/505 nm excitation/emission filters. The plates were incubated at 37°C for two hours and read again utilizing the same filters as baseline. Data shown reflects the mean difference ± SEM of relative fluorescence units (RFU) for three independent experiments.
Annexin-V/Propidium Iodide Staining
HeLa cells were plated at equal density and treated with 50 μM of indicated test compound. Cells were washed and stained according to manufacturers protocol (Calbiochem). Briefly, ~5×105 cells were incubated for 15 minutes with AnnexinV-FITC. Cells were centrifuged at 1,000 × g for 5 minutes and resuspended in 0.5 mL of cold 1× binding buffer. Propidium Iodide (PI) was added and samples were immediately analyzed by flow cytometry (FACScan Analyser; Becton Dickinson, Franklin Lakes, NJ)
Statistical Analysis
Comparisons between control or EGF and treated samples were performed utilizing an unpaired students t-test with equal variance using KaleidaGraph software (Synergy Software, Reading, PA). Comparisons within treated groups were performed utilizing a one-way analysis of variance (ANOVA) followed by a Tukey's post hoc analysis with an α-value of 0.05. Statistical significance was indicated with an asterisk (*) if the p value was equal to or less than 0.05 or a number sign (#) if the p value was equal to or less than 0.01.
Results
ERK docking domain inhibitors inhibit cell proliferation
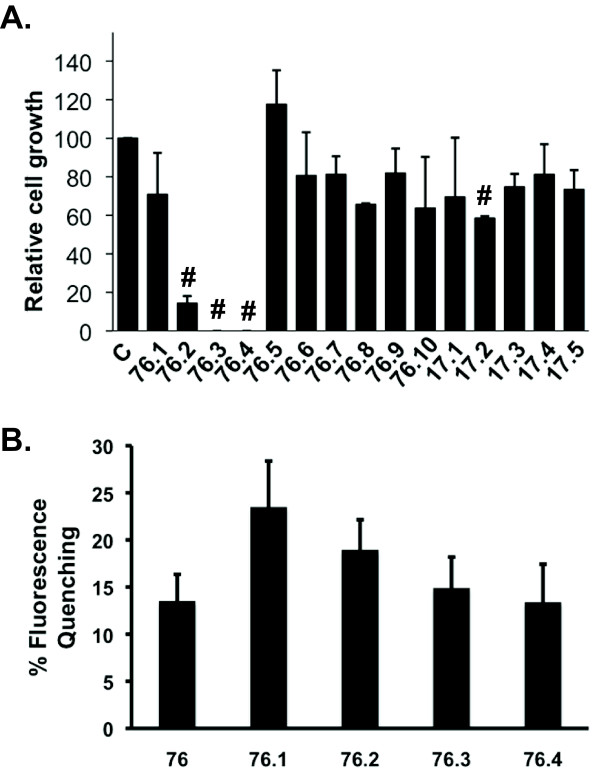
We have previously used computational and cell-based assays to identify LMW compounds that interact with ERK2, inhibit phosphorylation of ERK substrates, and inhibit cell proliferation [17,18,32]. The current studies used a computational search to identify additional compounds that share chemical features with two previously identified active compounds, referred to as 17 and 76 [17]. The search was based on the chemical similarity using the MAC-BITS fingerprints in conjunction with the Tanimoto Similarity index to search a virtual database of over 1 million commercially available compounds as described [33]. From this computational search, 5 compounds similar to 17 and 10 compounds similar to 76 were selected (Figure 1) and tested for inhibition of cell proliferation. Similar to 76, test compounds 76.2, 76.3, and 76.4 inhibited proliferation of HeLa cells at 100 μM (Figure 2A) and other transformed cell lines from pancreatic, melanoma, colon, and breast cancer tissue (Table 1). However, compounds similar to 17 had little effect on cell proliferation and were not pursued further in the current studies. Each of the compounds that inhibited cell proliferation showed interactions with ERK2 (Figure 2B) using fluorescence quenching assays that we previously described for the parent compound 76 [17]. Inhibition of HeLa cell proliferation was dose dependent for compounds 76.2, 76.3, and 76.4 (Table 2). Importantly, non-transformed epithelial cells that have been immortalized by stable expression of human telomerase reverse transcriptase (TERT) were less sensitive to the growth inhibitory effects of 76.3 or 76.4 (Table 2). TERT cells are useful cell culture controls for drug discovery as they retain relatively normal geno- and phenotypes and do not acquire cancer cell characteristics [34]. However, compounds 76 and 76.2 had similar potencies for inhibiting HeLa or TERT cell proliferation. Although the common chemical features of compounds 76.2, 76.3, 76.4 and the parent compound 76 include a thiazolidinedione core with an aminoethyl side group (Figure 1), the effects on cell proliferation suggest that the test compounds may have differences in their mechanisms of action.
Figure 1.
Structure of test compounds used in these studies. The area circled represents the thiazolidinedione core with aminoethyl side group that is common to compounds showing similar biological activity
Figure 2.
Effects of ERK targeted docking domain inhibitors on cell proliferation. (A) HeLa cells were seeded at 250 cells/mL in the presence or absence of 100 uM of the indicated test compound. After 10-14 days, cell colonies were fixed with formaldehyde and stained with crystal violet. Colonies were counted and normalized to the controls for each experiment. Data represent mean ± SD from 3 experiments. # indicates statistical significance compared to untreated controls p ≤ 0.01. C, Control. (B) Fluorescence quenching of ERK2 in the presence of test compounds. Data show the mean ± SD for percent fluorescence quenching as compared to ERK2 fluorescence in the presence of vehicle (DMSO) from 3 independent experiments.
Table 1.
Effects of ERK targeted docking domain inhibitors on proliferation of cancer cell lines.
| Compound | HeLa | SUM159 | HCT-116 | SKMEL-28 | Panc-1 |
|---|---|---|---|---|---|
| 76 | ++ | ++ | ND | ++ | ++ |
| 76.1 | - | ++ | - | - | ND |
| 76.2 | ++ | ++ | ++ | ++ | ND |
| 76.3 | ++ | ++ | ++ | ++ | ND |
| 76.4 | ++ | ++ | ++ | + | - |
| 76.5 | - | - | - | - | ND |
| 76.6 | - | - | - | - | ND |
| 76.7 | - | - | - | - | ND |
| 76.8 | - | - | - | - | ND |
| 76.9 | + | - | ++ | + | ND |
| 76.10 | - | - | - | - | ND |
| 17 | + | ND | ND | - | ND |
| 17.1 | - | - | - | - | ND |
| 17.2 | + | ++ | ++ | + | ND |
| 17.3 | - | - | - | - | ND |
| 17.4 | - | - | - | - | ND |
| 17.5 | - | - | - | - | ND |
Transformed cell lines from cervical (HeLa), breast (SUM159), colon (HCT-116), skin (SKMEL-28), or pancreatic (Panc-1) tissue were treated for 7 days with 100 μM of the indicated test compound. The proliferation in treated cells is shown relative to control (DMSO only) following analysis using the colony formation assay. (-) ≤ 25%, (+) 25-75%, and (++) ≥75% inhibition; ND, No data
Table 2.
Effects of ERK targeted docking domain inhibitors on cell proliferation.
| 76 | 76.2 | 76.3 | 76.4 | |||||
|---|---|---|---|---|---|---|---|---|
| HeLa | TERT | HeLa | TERT | HeLa | TERT | HeLa | TERT | |
| 25 μM | 20.1 ± 5.2# | 21.7 ± 3.6# | 73.7 ± 12.6 | 81.0 ± 8.1 | 58.2 ± 7.4# | 112.9 ± 19.1 | 53.7 ± 6.0# | 120.0 ± 26.5 |
| 50 μM | 20.4 ± 4.1# | 23.5 ± 12.0* | 31.2 ± 12.9# | 16.2 ± 4.0# | 40.1 ± 8.0# | 127.8 ± 12.8 | 36.3 ± 7.4# | 48.1 ± 11.7# |
| 100 μM | 20.5 ± 5.9# | 29.3 ± 12.1* | 17.3 ± 7.1# | 19.9 ± 12.5# | 17.1 ± 6.1# | 53.6 ± 6.8# | 21.0 ± 6.3# | 45.4 ± 12.6# |
HeLa or non-transformed retinal pigment epithelial cells that stably express human telomerase reverse transcriptase (TERT) were seeded at 250 cells/mL and then treated for 7 days with 25-100 uM of the indicated test compound. The proliferation in treated cells is shown as a percentage of control cells (DMSO only set at 100%) following analysis using the WST-1 assay. Data represent mean ± SEM from 3 experiments. * and # indicates statistical significance compared to untreated controls, p ≤ 0.05 and p ≤ 0.01, respectively
Activation of intrinsic apoptosis by ERK inhibitors
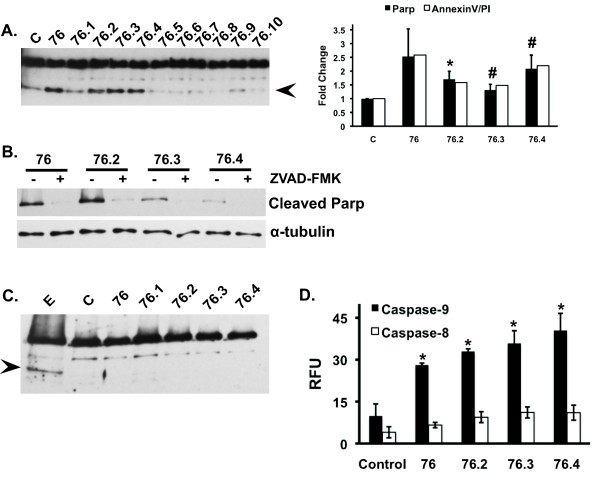
To evaluate whether the changes in proliferation were linked to an apoptotic response, cells were treated with test compounds at 50 μM, which resulted in at least 50% inhibition of HeLa cell proliferation (Table 2), and examined for cleavage of poly-ADP ribose polymerase (PARP) as a marker of apoptosis. Compounds 76, 76.2, 76.3, and 76.4 induced PARP cleavage and annexin V/PI staining after 5 hours exposure (Figure 3A) and this response could be blocked with a general caspase inhibitor, Z-VAD-FMK (Figure 3B). In contrast, PARP cleavage was not observed in TERT cells even after longer (16 hour) exposures to the test compounds (Figure 3C). To determine whether the test compounds induced apoptosis through extrinsic or intrinsic mechanisms, caspase-8 and 9 activities were examined. Compounds that induced PARP cleavage also induced caspase-9 activity but had little effect on caspase-8 activity indicating that ERK-targeted test compounds activate an intrinsic apoptosis pathway (Figure 3D).
Figure 3.
Test compounds induce the intrinsic apoptosis pathway. HeLa (A) or TERT (C) cells were treated in the absence or presence of test compounds (50 uM) for 5 hours (HeLa) or 16 hours (TERT). Cell lysates were immunoblotted for total and cleaved PARP (arrowhead). Graph on the right shows densitometry quantification of cleaved PARP to α-tubulin ratios and annexinV/PI staining relative control. PARP data represents the mean ± SEM from four independent experiments; annexinV/PI data are representative of two independent experiments. (B) HeLa cells were treated with 50 uM of the indicated compound for 5 hours and assayed for cleaved PARP in the presence and absence of the general caspase inhibitor ZVAD-FMK. (D) HeLa cells were treated in the absence or presence of 50 uM of the indicated compound for 5 hours and assayed for caspase 8 or 9 activity. Data represent the mean ± SEM from 3 independent experiments. * and # indicates statistical significance compared to untreated controls, p ≤ 0.05 and p ≤ 0.01, respectively. C, Control; E, Etoposide
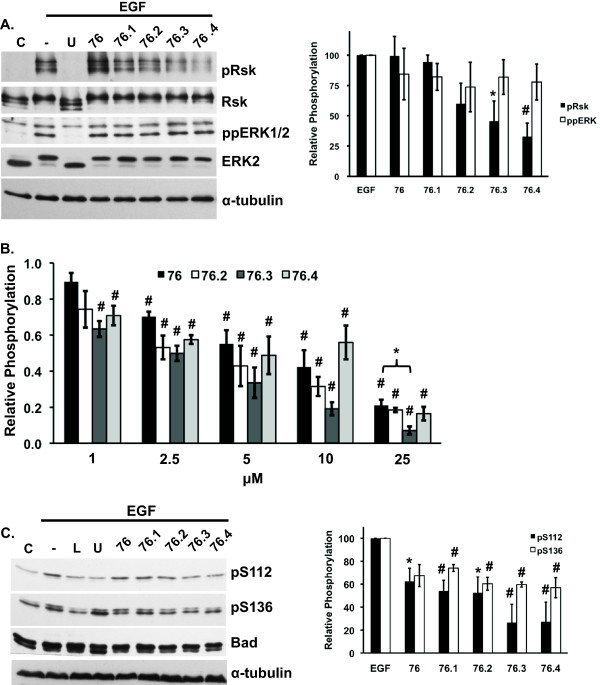
Test compounds inhibit ERK-mediated phosphorylation of p90 Rsk-1 and Bad proteins
ERK proteins provide a survival advantage by directly or indirectly phosphorylating and inactivating the Bcl-2 family members of pro-apoptotic proteins [3,4]. Bad is a pro-apoptotic Bcl-2 family member that is inactivated by phosphorylation at S112 by p90RSK-1 and S136 by Akt [4,35]. The intrinsic mechanisms of apoptosis induction by the test compounds were first examined by evaluating ERK-mediated phosphorylation of p90Rsk-1. Phosphorylation of p90Rsk-1 was inhibited by 40, 54, and 67% in the presence of 50 μM of test compound 76.2, 76.3, and 76.4, respectively (Figure 4A). As expected, 76.1, which was less effective in inhibiting cell proliferation, did not inhibit p90Rsk-1 phosphorylation (Figure 4A). It was also somewhat surprising that 76 did not affect p90Rsk-1 phosphorylation at this dose although 2 fold higher doses did inhibit p90Rsk-1 phosphorylation by 50% in our previous studies [17]. None of the compounds caused a statistically significant inhibition of ERK1/2 activation. These compounds are predicted to target ERK2 near the CD domain and may affect ERK activation by upstream MEK1/2 proteins, which reportedly interact with ERK through the CD domain [36]. Nonetheless, compounds 76.3, and 76.4 appeared to show selectivity for inhibiting ERK-mediated phosphorylation of p90Rsk-1 without inhibiting ERK activation. Similarly, 76.3 was the most potent inhibitor of phosphate incorporation into p90Rsk-1 as measured using in vitro kinase assays and statistically more potent than the parent compound 76 at 25 μM (Figure 4B).
Figure 4.
Test compounds inhibit ERK-mediated phosphorylation of p90Rsk-1 and Bad. (A) HeLa cells were pre-treated for one hour in the presence or absence of 50 uM of the indicated test compounds and then stimulated with EGF (25 ng/ml) for 10 minutes. Immunoblots of phosphorylated p90Rsk-1 (pRsk), total Rsk (Rsk), phosphorylated ERK1/2 (ppERK), and total ERK2 (ERK2). α-tubulin was used as a loading control. Graph shows densitometry quantification of pRsk-1 to total Rsk or ppERK2 to ERK2 ratios. Data represents the mean ± SEM from three independent experiments. (B) In vitro kinase assays examining 32P incorporation into p90Rsk-1 following incubation with active ERK2 and γ-32P-ATP for 60 min. in the absence or presence of 1-25 μM of test compounds. Relative phosphate incorporation was quantified by phosphoimager analysis. (C) HeLa cells were serum starved overnight and pre-treated for 1 hr with 50 uM indicated test compounds, 10 mM U0126 (U), or 25 mM LY294002 (L) prior to stimulation with or without EGF (25 ng/ml). Immunoblot analysis of Bad phosphorylated on Ser112 (pS112) or Ser136 (pS136) and total Bad. α-tubulin was used as a loading control. Data represents the mean ± SEM from three independent experiments. * and # indicates statistical significance compared to EGF-only treatment (A and C) or untreated controls (B), p ≤ 0.05 and p ≤ 0.01, respectively. C, untreated control; (-) EGF treated control.
We next examined Bad phosphorylation at S112 and S136 in HeLa cells treated with test compounds. Compounds 76.3 and 76.4 appeared the most potent inhibitors and reduced EGF-induced S112 phosphorylation by ~75-80% (Figure 4C). In contrast, S136 phosphorylation in the presence of these compounds was inhibited by ~40%, which supports a selective inhibition of the ERK/p90Rsk-1 over Akt signaling. As controls, the MEK1/2 inhibitor, U0126, inhibited phosphorylation of S112 but not S136, whereas the PI3K inhibitor, LY294002, inhibited phosphorylation of both S112 and S136 (Figure 4C) consistent with studies suggesting that phosphorylation of S136 induces a conformational change in Bad to promotes access to kinases that phosphorylate other sites [37].
Test compounds affect the expression of pro- and anti-apoptotic proteins Bad and Mcl-1
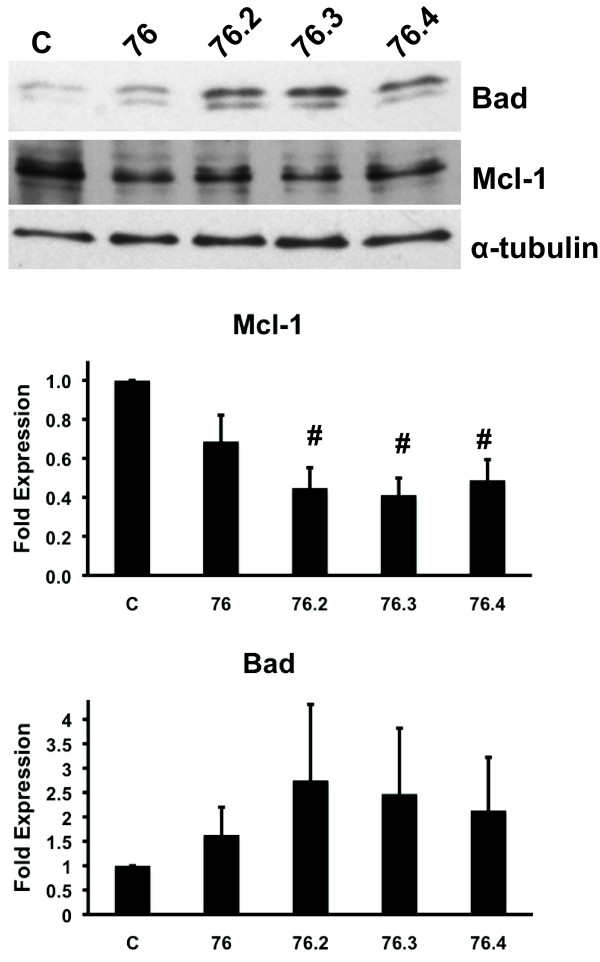
We also examined the test compounds effects on protein expression of Bad as well as Mcl-1, which is stabilized following ERK phosphorylation [38]. Although a two-fold increase in total Bad expression was observed in cells treated for 16 hours with the active test compounds (Figure 5), these data were not statistically significant. Nonetheless, these findings support previous studies showing increased Bad expression following inhibition of the ERK pathway with the MEK1/2 inhibitor, PD98059 [39]. However, the test compounds caused a statistically significant decrease in the expression of the anti-apoptotic protein Mcl-1 (Figure 5). Thus, these data indicate that the test compounds may sensitize cells to undergo apoptosis by increasing the expression of pro-apoptotic proteins and decreasing the expression of anti-apoptotic proteins, which are regulated by the ERK pathway.
Figure 5.
Test compounds affect the expression of the pro- and anti-apoptotic proteins, Bad and Mcl-1, respectively. Total Bad and Mcl-1 immunoblots from lysates collected from HeLa cells treated for 16 hour with 50 μM of indicated test compounds (top panel) and fold expression quantified by densitometry (bottom panel). α-tubulin was used as a loading control. Data represent the mean ± SEM of three independent experiments. # indicates statistical significance compared to untreated controls, p ≤ 0.01. C, Control
Test compounds inhibit caspase-9 phosphorylation
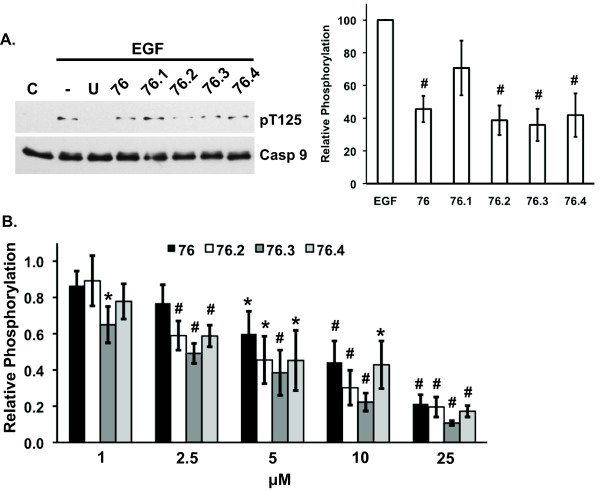
ERK phosphorylation of caspase-9 inhibits caspase-9 proteolytic activity and provides another mechanism by which activated ERK proteins mediate cell survival [2]. The effects of test compounds on ERK-mediated phosphorylation of caspase-9 on T125 were examined by immunoblotting lysates from cells where the ERK pathway was stimulated with EGF. Apoptosis-inducing test compounds 76, 76.2, 76.3, and 76.4, inhibited caspase-9 phosphorylation by 50-60% in treated cells (Figure 6A). Next, active ERK2 was incubated with caspase-9 protein in the absence or presence of test compounds and phosphorylation was examined using in vitro kinase assays. Figure 6B shows that compounds 76, 76.2, 76.3, and 76.4 inhibited ERK-mediated phosphorylation of caspase-9 in a dose dependent manner. While 76.3 appeared to be the more potent inhibitor of caspase-9 phosphorylation, there was no statistical difference between compounds at any of the doses.
Figure 6.
Test compounds inhibit ERK-mediated phosphorylation of caspase-9 in cells and in in vitro kinase assays. (A) HeLa cells transfected with caspase-9 (C287A) were pre-treated for 1 hour with 50 uM of indicated test compound, stimulated with EGF (25 ng/ml), and immunoblotted for phosphorylated caspase-9 (pT125) or total caspase-9. Graph shows the mean ± SEM for relative phosphorylation as determined by densitometry scanning from 3 independent experiments. (B) In vitro kinase assays examining 32P incorporation into caspase-9 following incubation with active ERK2 and γ-32P-ATP for 60 min. in the absence or presence of 1-25 μM of the indicated test compound. Relative phosphate incorporation was quantified by phosphoimager analysis. Data show the mean ± SEM of three independent experiments. * and # indicates statistical significance compared to EGF-only treatment (A) or untreated controls (B), p ≤ 0.05 and p ≤ 0.01, respectively. C, untreated control; (-), EGF treated control; U, U0126
Discussion
The goal of these studies was to characterize a class of novel ERK inhibitor compounds being developed in our laboratory that inhibit cell proliferation. Our findings demonstrate the induction of an apoptotic response by structurally-related compounds that inhibit ERK regulation of pro-apoptotic proteins. Aberrant activation of ERK signaling has been well-documented and provides a survival advantage in a number of cancers by inactivating pro-apoptotic proteins [40]. Thus, selective inhibition of ERK anti-apoptotic functions is a potential approach to sensitize cancer cells to chemotherapeutic agents. One important feature of the active test compounds is that some appear to be more selective for decreasing cell proliferation and inducing apoptosis in transformed cells as compared to non-transformed cells (Table 2 and Figure 3). This finding is in agreement with our previous studies indicating that the test compounds did not affect the life span or cause general toxicity in a C. elegans whole organism model [32]. Interestingly, we also have not observed overt toxicity when treating mice harboring B-cell lymphomas with compound 76 [41]. Coincidentally, these in vivo studies also demonstrated that B-cell lymphoma tumor burden was significantly reduced by 76. However, we predict that the significance of the ERK-targeted inhibitors in reducing tumor burden will best be realized using potentially more potent compounds, such as 76.3, in combination with other chemotherapeutic agents.
Our data support a mechanism by which the ERK-targeted compounds prevent inactivation of caspase-9 and p90Rsk-1 phosphorylation of Bad (Figures 4 and 6). Both caspase-9 and p90Rsk-1 contain D-domains that are thought to form contacts with the CD domain of ERK proteins [1,24]; the region predicted to be targeted by the test compounds. Structural studies underway are determining the site of interactions between the compounds and ERK proteins to better understand the mechanisms of inhibition and identify chemical features that can be modified to improve inhibitor binding and efficacy. A region analogous to the CD domain of ERK can be found on other MAP kinases family members including p38 and JNK [29,42]. The subtle differences between these regions are determinants of substrate selectivity between the MAP kinases. In addition, differences in the residues associated with the D-domains of MAP kinase interacting proteins can confer selectivity. For example, the differences in D-domains on MAP kinase kinase (MKK) proteins appear to be important for determining what MAP kinase the MKK isoform will target [43].
The p90Rsk-1 protein is a major regulator of ERK-mediated cell survival [5] and, like Bad, other p90Rsk-1 substrates may be affected by the test compounds. For example, inhibition of p90Rsk-1 could also restore activity of the death associated protein kinase (DAPK), which is a pro-apoptotic tumor suppressor protein that is phosphorylated and inactivated by p90Rsk-1 [44]. Moreover, it is recognized that other survival proteins may be affected by the test compounds and involved in mediating the apoptotic response observed. While some inhibition of PI3K/Akt signaling may have occurred with the test compounds (data not shown), these effects were not to the extent of inhibition of Akt substrates with the PI3K inhibitor, LY294002. In addition, the activation of other growth related proteins like ERK5 and EGF receptor (data not shown) were not affected, which further supports the mechanism of apoptosis induction by the test compounds involves inhibition of ERK1/2 signaling.
Another objective of this study was to determine whether improvements in potency and selectivity of the parent compound (76) could be achieved by examining structurally similar compounds. Cell based and in vitro kinase assays in figures 4 and 6 suggest that compound 76.3 has higher potency than 76. Thus, these studies have identified a class of structurally similar compounds that have similar biological effects and chemical features that can be modified to improve potency. Studies are now aimed at performing a more detailed structure activity relationship to identify the mode of the compound's interaction with ERK, improve potency, and determine whether other substrate proteins are affected. The present results also indicate the potential utility of using LMW compounds that inhibit protein-protein interactions in a therapeutic setting. While it has traditionally been assumed that small compounds would not be effective inhibitors of protein-protein interactions [45,46], recent studies involving the transcription factor BCL6 indicate that protein-protein interactions can be targeted in vivo with LMW compounds [47]. The efficacy of the present compounds as inhibitors of ERK-substrate interactions and their activity in a xenograft mouse model [41] further indicate that protein-protein interactions are valid therapeutic targets of LMW compounds.
The specific targeting of the ERK1/2 pathway to treat cancer has been best studied using MEK1/2 inhibitors (reviewed in [48]). Although a number of preclinical studies showed very good efficacy of MEK inhibitors for reducing cancer cell proliferation and inducing apoptosis, patient responses in clinical trials have been minimal with these compounds as single agents. Thus, MEK inhibitors in combination with other chemotherapeutic drugs will likely be more effective. A potential problem with targeting MEK for cancer therapy is the development of drug resistance, possibly through compensatory mechanisms involving elevated expression and activity of MEK/ERK proteins [49]. Thus, the approach to completely inhibit MEK/ERK signaling may be counterproductive in the long term. In contrast, the use of compounds that selectively inhibit ERK functions involved in cell survival may have the potential to sensitize cancer cells to other chemotherapeutic agents without inducing the compensatory pathways that lead to acquired drug resistance.
Conclusion
We have identified a class of LMW compounds that are selective for ERK docking domains involved in regulating interactions with substrates that promote cell proliferation and survival. These new compounds inhibit transformed cell proliferation and induce apoptosis, in part, by inhibiting substrate phosphorylation events that regulate pro- and anti-apoptotic proteins. Future optimization of the chemical features of the new lead compounds may provide more potent and selective molecules that can improve the efficacy of existing chemotherapeutic agents by disabling some of ERK1/2 protein functions.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SB participated in study design, performed the experiments and statistical analysis as well as writing the manuscript. RD performed the colony formation assays. UP and AM designed the ERK targeted docking domain inhibitors. SS provided valuable insight and critically reviewed the manuscript. AM and PS participated in the conception and study design. PS coordinated the study and the writing of the manuscript. All authors approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Sarice R Boston, Email: ssmit022@umaryland.edu.
Rahul Deshmukh, Email: rdesh003@umaryland.edu.
Scott Strome, Email: sstrome@smail.umaryland.edu.
U Deva Priyakumar, Email: deva@iiit.ac.in.
Alexander D MacKerell, Jr, Email: amackere@rx.umaryland.edu.
Paul Shapiro, Email: pshapiro@rx.umaryland.edu.
Acknowledgements
The authors would like to thank Dr. Michael Greenberg (Harvard University) for kindly providing the Bad plasmid. The project was supported by R01CA120215 (SB and PS) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- Martin MC, Allan LA, Mancini EJ, Clarke PR. The docking interaction of caspase-9 with ERK2 provides a mechanism for the selective inhibitory phosphorylation of caspase-9 at threonine 125. J Biol Chem. 2008;283:3854–3865. doi: 10.1074/jbc.M705647200. [DOI] [PubMed] [Google Scholar]
- Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 2003;5:647–654. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- Harada H, Quearry B, Ruiz-Vela A, Korsmeyer SJ. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci USA. 2004;101:15313–15317. doi: 10.1073/pnas.0406837101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Yu S, Eder A, Mao M, Bast RC Jr, Boyd D, Mills GB. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene. 1999;18:6635–6640. doi: 10.1038/sj.onc.1203076. [DOI] [PubMed] [Google Scholar]
- Shimamura A, Ballif BA, Richards SA, Blenis J. Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr Biol. 2000;10:127–135. doi: 10.1016/S0960-9822(00)00310-9. [DOI] [PubMed] [Google Scholar]
- Shapiro P. Ras-MAP kinase signaling pathways and control of cell proliferation: relevance to cancer therapy. Crit Rev Clin Lab Sci. 2002;39:285–330. doi: 10.1080/10408360290795538. [DOI] [PubMed] [Google Scholar]
- Burkhard K, Smith S, Deshmukh R, MacKerell AD Jr, Shapiro P. Development of extracellular signal-regulated kinase inhibitors. Curr Top Med Chem. 2009;9:678–689. doi: 10.2174/156802609789044416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C, Brumatti G, Martin SJ. Oncogenic B-RafV600E inhibits apoptosis and promotes ERK-dependent inactivation of Bad and Bim. J Biol Chem. 2008;283:22128–22135. doi: 10.1074/jbc.M800271200. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Milella M, Tafuri A, Martelli AM, Lunghi P, Bonati A, Cervello M, Lee JT, Steelman LS. Targeting the Raf/MEK/ERK pathway with small-molecule inhibitors. Curr Opin Investig Drugs. 2008;9:614–630. [PubMed] [Google Scholar]
- Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006;38:200–211. doi: 10.1080/07853890600551037. [DOI] [PubMed] [Google Scholar]
- Harandi A, Zaidi AS, Stocker AM, Laber DA. Clinical Efficacy and Toxicity of Anti-EGFR Therapy in Common Cancers. J Oncol. 2009;2009:567486. doi: 10.1155/2009/567486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SK. Therapeutic protein kinase inhibitors. Cell Mol Life Sci. 2009;66:1163–1177. doi: 10.1007/s00018-008-8539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace EM, Lyssikatos JP, Yeh T, Winkler JD, Koch K. Progress towards therapeutic small molecule MEK inhibitors for use in cancer therapy. Curr Top Med Chem. 2005;5:215–229. doi: 10.2174/1568026053507723. [DOI] [PubMed] [Google Scholar]
- de Azambuja E, Bedard PL, Suter T, Piccart-Gebhart M. Cardiac toxicity with anti-HER-2 therapies: what have we learned so far? Target Oncol. 2009;4:77–88. doi: 10.1007/s11523-009-0112-2. [DOI] [PubMed] [Google Scholar]
- Al-Dasooqi N, Gibson R, Bowen J, Keefe D. HER2 targeted therapies for cancer and the gastrointestinal tract. Curr Drug Targets. 2009;10:537–542. doi: 10.2174/138945009788488440. [DOI] [PubMed] [Google Scholar]
- Lewis TS, Shapiro PS, Ahn NG. Signal tranduction through MAP Kinase Cascades. Advances in Cancer Research. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. full_text. [DOI] [PubMed] [Google Scholar]
- Hancock CN, Macias A, Lee EK, Yu SY, Mackerell AD Jr, Shapiro P. Identification of novel extracellular signal-regulated kinase docking domain inhibitors. J Med Chem. 2005;48:4586–4595. doi: 10.1021/jm0501174. [DOI] [PubMed] [Google Scholar]
- Chen F, Hancock CN, Macias AT, Joh J, Still K, Zhong S, MacKerell AD Jr, Shapiro P. Characterization of ATP-independent ERK inhibitors identified through in silico analysis of the active ERK2 structure. Bioorg Med Chem Lett. 2006;16:6281–6287. doi: 10.1016/j.bmcl.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan DL, Kong Y, Parker SA, Dalby KN, Turk BE. Substrate Discrimination among Mitogen-activated Protein Kinases through Distinct Docking Sequence Motifs. J Biol Chem. 2008;283:19511–19520. doi: 10.1074/jbc.M801074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D, Glossip D, Xing H, Muslin AJ, Kornfeld K. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 1999;13:163–175. doi: 10.1101/gad.13.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantz DA, Jacobs D, Glossip D, Kornfeld K. Docking sites on substrate proteins direct extracellular signal-regulated kinase to phosphorylate specific residues. J Biol Chem. 2001;276:27256–27265. doi: 10.1074/jbc.M102512200. [DOI] [PubMed] [Google Scholar]
- Yang SH, Whitmarsh AJ, Davis RJ, Sharrocks AD. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. Embo J. 1998;17:1740–1749. doi: 10.1093/emboj/17.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks AD, Yang SH, Galanis A. Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem Sci. 2000;25:448–453. doi: 10.1016/S0968-0004(00)01627-3. [DOI] [PubMed] [Google Scholar]
- Dimitri CA, Dowdle W, MacKeigan JP, Blenis J, Murphy LO. Spatially separate docking sites on ERK2 regulate distinct signaling events in vivo. Curr Biol. 2005;15:1319–1324. doi: 10.1016/j.cub.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- Yang SH, Galanis A, Sharrocks AD. Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Mol Cell Biol. 1999;19:4028–4038. doi: 10.1128/mcb.19.6.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Hoofnagle AN, Kabuyama Y, Stroud J, Min X, Goldsmith EJ, Chen L, Resing KA, Ahn NG. Docking motif interactions in MAP kinases revealed by hydrogen exchange mass spectrometry. Mol Cell. 2004;14:43–55. doi: 10.1016/S1097-2765(04)00161-3. [DOI] [PubMed] [Google Scholar]
- Zhou T, Sun L, Humphreys J, Goldsmith EJ. Docking interactions induce exposure of activation loop in the MAP kinase ERK2. Structure. 2006;14:1011–1019. doi: 10.1016/j.str.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Maeda R, Adachi M, Nishida E. Identification of a docking groove on ERK and p38 MAP kinases that regulates the specificity of docking interactions. Embo J. 2001;20:466–479. doi: 10.1093/emboj/20.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. Embo J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher TL, Blenis J. Evidence for two catalytically active kinase domains in pp90rsk. Mol Cell Biol. 1996;16:1212–1219. doi: 10.1128/mcb.16.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Mackerell AD Jr, Luo Y, Shapiro P. Using Caenorhabditis elegans as a model organism for evaluating extracellular signal-regulated kinase docking domain inhibitors. J Cell Commun Signal. 2008;2:81–92. doi: 10.1007/s12079-008-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias AT, Mia MY, Xia G, Hayashi J, MacKerell AD. Lead validation and SAR development via chemical similarity searching; application to compounds targeting the pY+3 site of the SH2 domain of p56lck. J Chem Inf Model. 2005;45:1759–1766. doi: 10.1021/ci050225z. [DOI] [PubMed] [Google Scholar]
- Lee KM, Choi KH, Ouellette MM. Use of exogenous hTERT to immortalize primary human cells. Cytotechnology. 2004;45:33–38. doi: 10.1007/10.1007/s10616-004-5123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Robinson FL, Whitehurst AW, Raman M, Cobb MH. Identification of novel point mutations in ERK2 that selectively disrupt binding to MEK1. J Biol Chem. 2002;277:14844–14852. doi: 10.1074/jbc.M107776200. [DOI] [PubMed] [Google Scholar]
- Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6:41–51. doi: 10.1016/S1097-2765(00)00006-X. [DOI] [PubMed] [Google Scholar]
- Domina AM, Vrana JA, Gregory MA, Hann SR, Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23:5301–5315. doi: 10.1038/sj.onc.1207692. [DOI] [PubMed] [Google Scholar]
- Boucher MJ, Morisset J, Vachon PH, Reed JC, Laine J, Rivard N. MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J Cell Biochem. 2000;79:355–369. doi: 10.1002/1097-4644(20001201)79:3<355::AID-JCB20>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Chang F, Steelman LS, Shelton JG, Lee JT, Navolanic PM, Blalock WL, Franklin R, McCubrey JA. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review) Int J Oncol. 2003;22:469–480. [PubMed] [Google Scholar]
- Dai B, Zhao XF, Hagner P, Shapiro P, Mazan-Mamczarz K, Zhao S, Natkunam Y, Gartenhaus RB. Extracellular signal-regulated kinase positively regulates the oncogenic activity of MCT-1 in diffuse large B-cell lymphoma. Cancer Res. 2009;69:7835–7843. doi: 10.1158/0008-5472.CAN-09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo YS, Kim SK, Seo CI, Kim YK, Sung BJ, Lee HS, Lee JI, Park SY, Kim JH, Hwang KY. et al. Structural basis for the selective inhibition of JNK1 by the scaffolding protein JIP1 and SP600125. Embo J. 2004;23:2185–2195. doi: 10.1038/sj.emboj.7600212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell AJ, Frankson E, Bardwell L. Selectivity of docking sites in MAPK kinases. J Biol Chem. 2009;284:13165–13173. doi: 10.1074/jbc.M900080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum R, Roux PP, Ballif BA, Gygi SP, Blenis J. The tumor suppressor DAP kinase is a target of RSK-mediated survival signaling. Curr Biol. 2005;15:1762–1767. doi: 10.1016/j.cub.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Astriab-Fisher A, Falke D. Macromolecular therapeutics: emerging strategies for drug discovery in the postgenome era. Mol Interv. 2001;1:40–53. [PubMed] [Google Scholar]
- Kuntz ID. Structure-based strategies for drug design and discovery. Science. 1992;257:1078–1082. doi: 10.1126/science.257.5073.1078. [DOI] [PubMed] [Google Scholar]
- Cerchietti LC, Ghetu AF, Zhu X, Da Silva GF, Zhong S, Matthews M, Bunting KL, Polo JM, Fares C, Arrowsmith CH. et al. A small-molecule inhibitor of BCL6 kills DLBCL cells in vitro and in vivo. Cancer Cell. 2010;17:400–411. doi: 10.1016/j.ccr.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14:342–346. doi: 10.1158/1078-0432.CCR-07-4790. [DOI] [PubMed] [Google Scholar]
- Yip-Schneider MT, Klein PJ, Wentz SC, Zeni A, Menze A, Schmidt CM. Resistance to mitogen-activated protein kinase kinase (MEK) inhibitors correlates with up-regulation of the MEK/extracellular signal-regulated kinase pathway in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2009;329:1063–1070. doi: 10.1124/jpet.108.147306. [DOI] [PubMed] [Google Scholar]