Abstract
Polyelectrolyte multilayers (PEMs) based on the combinations poly(diallyldimethylammonium chloride)∕poly(acrylic acid) (PDADMAC∕PAA) and poly(allylamine hydrochloride)∕PAA (PAH∕PAA) were adsorbed on poly(dimethylsiloxane) (PDMS) and tested for nonspecific surface attachment of hydrophobic yeast cells using a parallel plate flow chamber. A custom-made graft copolymer containing poly(ethylene glycol) (PEG) side chains (PAA-g-PEG) was additionally adsorbed on the PEMs as a terminal layer. A suitable PEM modification effectively decreased the adhesion strength of Saccharomyces cerevisiae DSM 2155 to the channel walls. However, a further decrease in initial cell attachment and adhesion strength was observed after adsorption of PAA-g-PEG copolymer onto PEMs from aqueous solution. The results demonstrate that a facile layer-by-layer surface functionalization from aqueous solutions can be successfully applied to reduce cell adhesion strength of S. cerevisiae by at least two orders of magnitude compared to bare PDMS. Therefore, this method is potentially suitable to promote planktonic growth inside capped PDMS-based microfluidic devices if the PEM deposition is completed by a dynamic flow-through process.
INTRODUCTION
At present, there exists a great interest in high-throughput screening methods in chemistry and materials research as well as in process development of biological systems, making use of innovative cultivation techniques. Advances in miniaturization enable a global reduction of experimental costs for process development by taking advantage of parallelization and intensified mass transfer in microbioreactors. However, at the microscale level, completely different parameter dependencies and new phenomena may occur. Primarily, direct interactions between microorganisms and bioreactor walls become much more important due to the increased surface-to-volume ratio. Since these interactions are usually not considered or even completely neglected on a macroscale, new challenges in the manufacture and specific functionalization of microdevices arise.
In general, for high-throughput screening as well as for scale-up or scale-down purposes, current microfluidic devices used for cultivation of microorganisms must be prepared from biocompatible and inert materials that allow cultivation in planktonic form.1 However, most of the manufacturing materials that meet the demands for biocompatibility and are therefore widely used in microproduction, especially silicones such as poly(dimethylsiloxane) (PDMS) and related materials, exhibit surface properties that favor unspecific cell adhesion owing to their strong hydrophobicity.
The objective of the present work was therefore to provide a process allowing the “lining” of already sealed PDMS-based microfluidic devices with coatings exhibiting a minimized nonspecific adhesive interaction with hydrophobic microorganisms or particles. In the present work the cell adhesion strength on coated PDMS model surfaces was investigated using the yeast strainS. cerevisiae DSM 2155. Due to its hydrophobicity,2 this strain has a strong tendency to flotation and to adhesive growth on hydrophobic material surfaces, impairing the biological screening process in PDMS-based microbioreactors with non-modified surfaces.
The most common surface hydrophilization methods for PDMS microfluidic devices described in the literature are either based on physisorption of hydrophilic molecules or on covalent modification.3, 4, 5 Covalent modification methods for hydrophilization involving different “grafting from” and “grafting to” techniques,6 grafting of poly(ethylene glycol) (PEG)-silanes7 or PEG-methacrylate,8 usually should be combined with preceding physicochemical surface activation by O2 plasma, UV∕ozone, or corona treatment to create suitable anchoring groups. Alternatively to covalent coupling, swelling of PDMS in organic solvents can also be used to modify the PDMS surface by diffusion of amphiphilic block copolymers into the PDMS matrix.9
Unfortunately, due to a number of boundary conditions dictated by the special geometries of microbioreactors and the multitude of materials generally involved, the choice of feasible processes for the present work is substantially narrowed down. Plasma treatment at 1 bar pressure is in principle possible in sealed microfluidic devices consisting only of dielectric materials;10 but microfluidic devices are usually hybrid systems consisting not only of PDMS but many other materials (glass, steel, gold, and other polymers) which have to be functionalized simultaneously. The presence of metallic parts such as electrodes, fluorescence sensors, or connectors generally precludes the application of gas discharges. Extensive use of nonpolar organic solvents for swelling and subsequent incorporation of amphiphiles is also generally not possible due to the risks of debonding and destroying the device. Radical polymerization in situ processes, as they are involved in “grafting from” approaches, are practically precluded because of the difficult deaeration of the microdevice.
Therefore, a very feasible and realistic method to achieve the objectives of this work would be an adsorption process from aqueous solutions. In the literature the adsorption of amphiphilic block copolymer such as Pluronic™ (BASF, Germany) has frequently been used to achieve protein repellency.4 These copolymers are anchored to the PDMS surface by a hydrophobic interaction which is quite strong in water. However, in the present case, owing to the necessity to assure monoseptic culture conditions, the microbioreactor must be rinsed with ethanol for disinfection. Therefore it was preferred not to rely on hydrophobic interaction and it was decided to use layer-by-layer (lbl) deposition of polyelectrolyte multilayers (PEMs) after a gentle pretreatment of the PDMS surface by aqueous HCl∕H2O2 at room temperature, providing a silanol-covered surface11 which is expected to furnish a more stable adhesion of the PEMs.
PEMs represent versatile tools for surface modification for various applications12 as they can be assembled by alternate adsorption of oppositely charged polyelectrolytes from aqueous solution and, using a dynamic flow-through method,13 can even form coatings in sealed microsystems like microbioreactors. The deposition of PEMs is applicable to virtually any material and shape. So far, different kinds of PEMs were successfully applied to control, i.e., either promote or inhibit, adhesion of several mammalian cell types14, 15, 16 or bacteria17 on different materials. Less attention, however, has been paid to hydrophobic yeast cell adhesion on PEMs with a special focus on capped microdevices.
In order to compare the cell repellency of PEM surfaces with PEGylated surfaces, a number of PDMS∕PEM samples were provided with an adsorbed top layer of a poly(acrylic acid)-graft-poly(ethylene glycol) (PAA-g-PEG) graft copolymer. PEG is commonly considered the material of choice for preventing bioadhesion in general18 and has been shown to reduce protein or bacterial adhesion on surfaces effectively.19, 20, 21, 22, 23 Several research groups have treated the adsorption of graft copolymers with PEG side chains attached to positive polyelectrolytes such as poly(ethylene imine) or poly-L-lysine.24, 25, 26, 27 Adsorption of PAA-g-PEG on PEM surfaces and cell repellency of thus modified surfaces has to date not yet been reported.
In this study, the strength of cell adhesion on the PEM- or PEM-PEG-modified PDMS surfaces was investigated using parallel plate flow chamber experiments.28, 29, 30 In addition, a comparison was made with the cell repellent capabilities of a PEG-silane modified PDMS. For this method, plasma pretreatment was used, as already reported in the literature.7
MATERIALS AND METHODS
Polyelectrolytes and other chemicals
Poly(diallyldimethylammonium chloride) (PDADMAC)(Mw=250–300 kg∕mol), 1-hydroxybenzotriazole (HOBT), N,N′-diisopropylcarbodiimide (DIC), N,N-dimethyl-formamide (DMF), hydrochloric acid (HCl), sodium hydroxide (NaOH), and sodium chloride (NaCl) were obtained from Sigma-Aldrich (Germany). Poly(allylamine hydrochloride) (PAH) (Mw=60 kg∕mol)and PAA (Mw=200 kg∕mol) were provided from Polysciences Europe GmbH (Eppelheim, Germany). A methoxy-terminated PEG-amine (mPEG- NH2) (Mw=5000 g∕mol) was purchased from Rapp-Polymere (Tübingen, Germany). Hydrogen peroxide came from Roth (Karlsruhe, Germany). 2-[methoxy(polyethylene-oxy)propyl]-trimethoxysilane (90%) (PEG-silane) was obtained from ABCR GmbH (Karlsruhe, Germany) and toluene and ethanol from J. T. Baker (Griesheim, Germany). All chemicals were used without further purification.
All polyelectrolyte solutions were prepared with ultrapure water (TKA MicroPure purification stage, Niederelbert, Germany) using a polyelectrolyte concentration of 0.01 mol∕l with respect to the monomer unit. Directly before the coating process, the polyelectrolyte solutions of PAH and PAA were carefully adjusted to a fixed pH using dilute NaOH or dilute HCl. The pH of the PDADMAC solution was not adjusted, but in this case 0.1 mol∕l NaCl was added to the solution.
Synthesis of PAA-g-PEG copolymer
To obtain a grafting ratio of g=5, as used in this study (g is the number of acrylic acid monomers divided by the number of PEG side chains), 19 mg DIC (0.15 mmol) and 20 mg HOBT (0.15 mmol) were added to 5 ml of a 0.02M (based on AA mononer units) PAA solution (0.1 mmol AA units) in DMF and reacted at 40 °C for 1 h. After addition of 100 mg mPEG- NH2 (0.02 mmol) the solution was kept at 40 °C for additional 20 h. The DMF was subsequently removed in a rotary evaporator and the resulting nominal PAA(200)-5-PEG(5) copolymer (the expressions in brackets refer to the molecular weights of the polymers, given in kg∕mol) was dissolved in 5 ml water and dialysed against water for one week, using a 100 kDa molecular weight cutoff filter (Spectra∕Por® CE Float-A-Lyzer® G2, Spectrum Laboratories, purchased from Sigma-Aldrich, Germany).
Preparation of PDMS and steel surfaces
PDMS base and curing agent (Sylgard 184 elastomer kit, Dow Corning, Midland, MI, USA) were mixed manually in the standard ratio of 10:1 for 5 min. After degassing, 7.85 ml of the still liquid PDMS was poured on a planar glass using a viscous transfer pipette, leveled, and finally heated to 70 °C for 1 h. After the crosslinking of the PDMS had terminated, the flexible sheet (1 mm in thickness) was peeled off from the glass and cut to the size of 26×76 mm2. The PDMS pieces were mounted void-free on a glass slide and washed in ethanol and ultrapure water. In a second step, directly prior to the coating with PEMs, the PDMS was treated for 5 min in a solution containing HCl (37%), H2O2 (30%), and H2O in a volume ratio of 1:1:5, afterwards it was rinsed with ultrapure water for a minimum time of 10 min. For infrared (IR) analysis stainless steel foils (d=50 μm, Georg Martin GmbH, Germany) were mirror polished manually using water based diamond suspension (MetaDi®, Buehler, Germany) with particle size from 3 μm (polishing time 12 min) down to 1 μm (polishing time 5 min) and cleaned by wiping with an acetone-soaked tissue.
PEM coating and PEGylation
The deposition of PEM films was done by an automated dipping robot (Riegler & Kirstein GmbH, Berlin, Germany). PEMs were assembled by exposing the pretreated PDMS or the stainless steel foil alternately to the two polymer solutions for 2 min with three rinsing steps (2 min, 1 min, and 1 min) in between. During one such a cycle a polyelectrolyte double layer (DL) is created on the surface. On several PEM films terminated with apolycation, the negatively charged PAA(200)-5-PEG(5) copolymer was then adsorbed for 10 min (5 mM solution based on the concentration of acrylic acid monomers, pH ≈3.5), followed by 10 min rinsing in water under agitation.
For PEGylation with PEG-silane, the PDMS surfaces were exposed to oxygen plasma (75 ccm O2) in a barrel etcher type 308 PC (Surface Technology Systems, Newport, UK) for 30 s at 85 W and 140 mTorr. Directly afterwards, the activated samples were immersed in a solution of 0.1M PEG-silane in toluene and stored for 30 min under continuous stirring. Subsequently, the samples were rinsed twice with fresh ethanol for 20 min and finally dried in a vacuum oven at 70 °C for 2 h.
To determine the effects of (a) different types of PEM coatings, (b) terminal polyelectrolyte layer (surface charge), (c) additional surface PEGylation of PEMs, and (d) a different PEGylation method (PEG-silanization) on the adhesion strength of the hydrophobic yeast S. cerevisiae on hydrophobic PDMS, the film combinations summarized in Table 1 were used.
Table 1.
Coatings on PDMS used for adhesion experiments with yeast S. cerevisiae DSM 2155.
| No. | Film compositiona | Terminal layerb |
|---|---|---|
| 1 | PDMS, uncoated reference | Bare PDMS |
| 2 | (PAH2.0∕PAA2.0)33 | PAA (−) |
| 3 | (PAH2.0∕PAA2.0)32.5_PAA(200)-5-PEG(5) | PEG |
| 4 | (PAH6.5∕PAA6.5)33 | PAA (−) |
| 5 | (PAH6.5∕PAA6.5)32.5_PAA(200)-5-PEG(5) | PEG |
| 6 | (PAH7.5∕PAA3.5)11 | PAA (−) |
| 7 | (PAH7.5∕PAA3.5)10.5 | PAH (+) |
| 8 | (PAH7.5∕PAA3.5)10.5_PAA(200)-5-PEG(5) | PEG |
| 9 | (PDADMAC@0.1M NaCl∕PAA3.5)11 | PAA (−) |
| 10 | (PDADMAC@0.1M NaCl∕PAA3.5)10.5 | PDADMAC (+) |
| 11 | (PDADMAC@0.1M NaCl∕PAA3.5)10.5_PAA(200)-5-PEG(5) | PEG |
| 12 | PEG-silane (adsorbed from 0.1M PEG-silane in toluene) | PEG |
The expression behind the polyelectrolyte name refers to the pH of the solution at assembly (for PAH and PAA) or in the case of PDADMAC to the salt content of the solution. The number behind the brackets refers to the number of adsorbed polyelectrolyte double layers (DL), with one DL being a combination of one polycation and one polyanion layer.
Terminal layer: (−)=polyanion(PE−), (+)=polycation(PE+), and PEG=poly (ethylene glycol).
Considering the different growing behavior of the films,14, 31, 32 a different number of double layers was assembled. As determined via ellipsometry (SE 850, Sentech GmbH, Berlin, Germany) on stainless steel surfaces, films based on PDADMAC∕PAA and PAH7.5∕PAA3.5 had a thickness of approximately 120–150 nm, whereas for PAH∕PAA coatings, assembled at solution pH of 2.0∕2.0 and 6.5∕6.5, respectively, the number of double layers was chosen with respect to a reasonable coating time and therefore multiplied by three, resulting in approximately 30 nm thick layers for 6.5∕6.5. Compared to literature data,14 the (PAH2.0∕PAA2.0)33 PEM turned out to be unexpectedly thin as can be derived from Fig. 2a (confirmed by independent measurements). The same system also showed greater scatter in water contact angles, see below.
Figure 2.
(a) FTIR-ATR spectra of the investigated PEM coatings on stainless steel before PEGylation (curves are arranged according to the order of the labeling). (b) FTIR-ATR spectra of PEM coatings on stainless steel after adsorption of PAA(200)-5-PEG(5) copolymer (spectra of PEMs on stainless steel foil were subtracted).
Surface characterization
In order to determine the extent of PEGylation highly sensitive Fourier transform infrared spectroscopy in the attenuated total reflection mode (FTIR-ATR) was performed on the stainless steel foil and not on PDMS itself as many IR signals of PEG are superimposed by strong vibrations of the PDMS in the interesting regions. ATR measurements were completed using a Nicolet 5700 FT-IR spectrometer equipped with a DuraSamplIR single-reflection 45° diamond ATR crystal using parallel polarized light (with respect to the plane of incidence).
Additionally, the hydrophilicity of coated and uncoated PDMS, respectively, was characterized by dynamic measurements of advancing contact angles with water at a dosing rate of 0.06 μl∕s (contact angle measuring instrument OCA 20, Dataphysics, Filderstadt, Germany). Between the preparation of the coatings and the contact angle measurements the samples were stored in air under ambient conditions for a maximum time of 4 h.
Yeast cultivation
20 ml of the inoculum culture was cultivated in 100 ml shake flasks at 30 °C for 12 h. The medium was the modified mineral VERDUYN medium set to a pH of 4.5. The preparation of the medium has already been explained in detail by Edlich et al.33 For each parallel plate flow chamber experiment, an individual shake flask was precultured. Since a cell adhesion experiment in the microfluidic flow chamber lasted approximately 2 h, the inoculation was carried out in intervals of this time period to obtain the same cell growing conditions for each subsequent test. Cells were harvested in exponential growth phase after 12 h and the optical density (OD) of the cell suspension was set to an OD600 nm of 0.2 (spectrophotometer Helios, Spectronic Unicam, Cambridge, UK) directly before each experiment.
Parallel plate flow chamber experiments
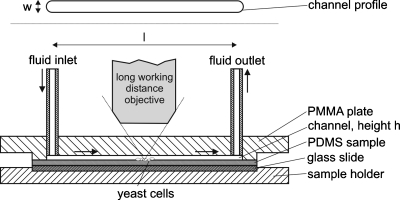
Cell adhesion experiments were carried out in a parallel, home built rectangular flow chamber of 64 mm length (l)×4 mm width (w) and approximately 120 μm height (h) (see Fig. 1). The whole bottom plate consists of the PEM-coated PDMS sample and the upper transparent poly(methyl methacrylate) plate contains a channel (in the above described dimensions) in which the fluid inlet and outlet are mounted. The fluid inlet is connected to a syringe pump (neMESYS Cetoni, Korbußen, Germany) for application of low volumetric flow rates and to a low-pulse peristaltic pump (Watson-Marlow, Great Britain, 520U, 505L pump head) for high volumetric flow rates. Assuming a fully developed laminar flow in the middle of the channel, the wall shear stress (τwin N∕m2=Pa) acting on the upper and bottom plate of the channel can be estimated by
| (1) |
where η is the dynamic viscosity of the fluid in kg∕(ms) (for VERDUYN medium it was assumed to be the same as for water) and Q is the volumetric flow rate in m3∕s.
Figure 1.
Scheme of the parallel flow channel used in this study (bottom: sectional view; top: channel profile).
As PDMS is a flexible material and might be compressed while fixing the chamber, the correct channel height was determined prior to each adhesion experiment by measuring the pressure drop between fluid inlet and fluid outlet for four different known volumetric flow rates of water. Subsequently, the whole chamber was disinfected with a solution of 70% ethanol in water for three minutes, rinsed with ultra pure water and flooded with the modified VERDUYN medium which contained 1 g∕l glucose. The chamber was then inoculated manually with 10 ml of a S. cerevisiae cell suspension by syringe. The cells were allowed to settle onto the bottom plate for 20 min. In order to achieve steady-state conditions before the adhesion experiments (e.g., removing cells that did not adhere on the channel walls) the whole volume of the parallel plate flow chamber as well as the inlet tube was exchanged at least three times at τw=0.1 Pa with fresh medium. After that procedure, the number of cells remaining in an approximately 460×620 μm2 wide area situated in the middle of the channel on the bottom plate was taken as the 100% reference value.
Similar to the method used by Guillemot et al.,30 the volumetric flow rate of the VERDUYN medium (e.g., the wall shear stress) was then increased stepwise every 3 min up to a value of 128 N∕m2, taking a microscope picture (Nikon, Japan, Eclipse LV150, Fig. 1) of the selected area after each step. The adhering cells were counted out manually and for each coating the experiment was repeated at least three times unless otherwise stated.
RESULTS AND DISCUSSION
FTIR analysis
Figures 2a, 2b show FTIR-ATR spectra obtained for several PEMs on stainless steel before and after adsorption of PAA(200)-5-PEG(5). The height of the PEG peaks in Fig. 2b gives an indication of the amount of PAA-g-PEG adsorbed as well as a hint toward a pronounced vertical PEG chain orientation on the surface.34 Interestingly, the amount of adsorbed PAA-g-PEG copolymer depends on the type of PEM used as a base layer. In general, PAH∕PAA-PEMs assembled under acid conditions (e.g., at PAH∕PAA solution pHs of 2.0∕2.0) show low binding affinity for PAA(200)-5-PEG(5), whereas a high PEGylation degree, i.e., a high amount of adsorbed PAA(200)-5-PEG(5), can be achieved for PAH∕PAA-PEMs deposited at pH of 7.5∕3.5 as well as for PDADMAC∕PAA-PEMs. The change in film configuration due to adsorption of a single monolayer (e.g., the change in surface charge) is pictured in Fig. 2a. After deposition of PAA at pH 2.0 or pH 3.5 (11 and 33 DL, respectively; PAA not fully charged) an increase in peak (shoulder) height between 1712 and 1720 cm−1 can be seen which is assigned to the adsorption of protonated acid groups (COOH). Deposition at pH 6.5, however, where PAA is near its fully charged state, leads to a predominant increase of the carboxylate peak (COO−) at 1561–1570 cm−1. In general, after deposition of positively charged polyelectrolytes (PAH and PDADMAC; 10.5 DL∕32.5 DL and 10.5 DL, respectively) which are in an almost fully or completely charged state, a decrease of the COOH signal, usually together with an increase in COO− signal, is observed showing that some of the formerly protonated PAA groups become ionized within the film, confirming that the positively charged polyelectrolyte is deposited.
Contact angle measurements
To determine hydrophilicity of the PEM and PEG coatings, measurements of dynamic contact angle Θ were performed at about 38%–42% relative humidity. For all PEM∕PEM-PEG films and the PEG-silane coating hydrophilicity was enhanced compared to bare PDMS (equilibrium contact angleΘ≈115°), indicating successful deposition. The contact angles of the coatings were generally in the range between 12° and 50°.
For some PEM∕PEM-PEG coatings Θ was quite reproducible and spatially homogeneous across a single sample: PEGylated PDADMAC∕PAA films, for example, repeatedly gave a contact angle of Θ≈21° in several deposition experiments. A few coatings, on the other hand, showed significant differences between contact angles although they were always prepared under nominally identical conditions: (PAH2.0∕PAA2.0) PEMs with PAA capping was the extreme example with angles of 28° and 90° found in consecutive experiments. Some films exhibited unexpectedly high contact angles in general [e.g., (PAH6.5∕PAA6.5) layers with PAA capping: Θ≈82°]. It has been shown in the literature that contact angles of PEM coatings do not only depend on the composition of the multilayers but also on their history with respect to previous contacts with water and their present state of swelling.35 Therefore, in spite of relatively small time intervals between film preparation and measurements, our observations are possibly to some extent due to this factor. However, it cannot completely be excluded that in case of the less hydrophilic surfaces (Θ>80°) an incomplete coverage of the PDMS surface with these PEMs, possibly due to an inhomogeneous distribution of silanol anchoring groups, could be responsible.
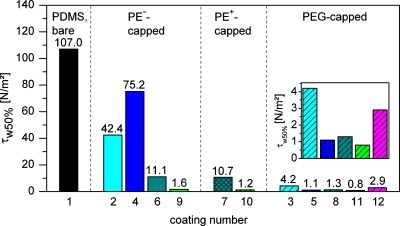
At this point it should be mentioned that a large contact angle did not necessarily correlate with an increase in yeast cell adhesion. Examples are PEGylated (PAH6.5∕PAA6.5) layers which, although exhibiting contact angles in the range ofΘ≈53°–64°, showed very low τw50% values (τw50%: wall shear stress at which 50% of the initially adherent cells have been removed from the surface) comparable to those of other, significantly more hydrophilic, PEM, e.g., PEGylated (PAH7.5∕PAA3.5) layers with Θ≈16°–22° (see Fig. 5).
Figure 5.
Value of τw50% (based on linear interpolation) for all PDMS surface modifications investigated in this study (coating numbers according to Table 1). Same colors refer to the same type of PEM. No hatching: PEMs terminating with negatively charged polyelectrolyte(PE−). Cross-hatched: PEMs terminating with positively charged polyelectrolyte(PE+). Striped: PEMs capped with PAA(200)-5-PEG(5) or PEG-silane.
In our opinion the observed water contact angle can in fact give a hint toward effective film deposition and support interpretation of adhesion data (e.g., large scattering) but, taken alone, it is not sufficient for comprehensive explanation. Other factors such as mechanical properties36 and the state of swelling of the coatings under given environmental conditions are expected to play a major role in yeast cell adhesion.
Detachment experiments using the parallel plate flow chamber
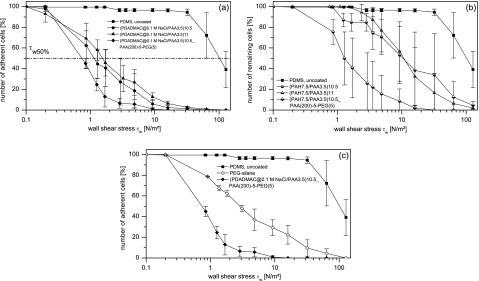
In Figs. 3a, 3b, 3c the relative number of remaining yeast cells, i.e., the ratio of presently adherent to initially adherent yeast cells, is presented with respect to the applied wall shear stress τ for uncoated PDMS (squares) and examples for PEM modified PDMS surfaces terminating with either a polycation (circles), a polyanion (triangles) or a PAA(200)-5-PEG(5) layer (diamonds). In Fig. 3c the detachment profiles of a PEGylated PEM on PDMS and the PEG-silane modified PDMS are compared.
Figure 3.
(a) Relative number of remaining cells as a function of the wall shear stress τw for PDADMAC∕PAA coatings. (b) Relative number of remaining cells as a function of the wall shear stress τw for PEM coatings assembled at PAH∕PAA solution pH of 7.5∕3.5. (c) Relative number of remaining cells as a function of the wall shear stress τw for a PEGylated PEM compared to PEG-silane modified PDMS (in the case of PEG-silane the experiment was repeated twice instead of three times).
As can be seen from Figs. 3a, 3b, 3c, both PEMs and PEM-PEG films, as well as the PEG-silane coating, are able to significantly reduce the adhesion strength of S. cerevisiae with respect to bare PDMS while the efficiency of some coatings is clearly superior in comparison. The efficiency in reduction can be estimated by considering the value ofτw50%, i.e., the value of the wall shear stress at which the relative number of initially adherent cells (see Fig. 4 for absolute number of initially adherent cells) is reduced to 50%.29, 30, 37 The values of τw50% for all the PEM coatings used in this study are summarized in Fig. 5. From the shown data, the following general tendencies can be derived.
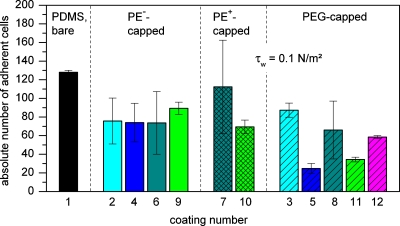
Figure 4.
Absolute number of remaining cells after application of τw=0.1 N∕m2 for all PDMS surface modifications investigated in this study (coating numbers according to Table 1). Same colors refer to the same type of PEM. No hatching: PEMs terminating with negatively charged polyelectrolyte(PE−). Cross-hatched: PEMs terminating with positively charged polyelectrolyte(PE+). Striped: PEMs capped with PAA(200)-5-PEG(5) or PEG-silane.
PEMs without PEG
For PDADMAC∕PAA-PEMs, despite the interesting observation that the absolute number of initially adhering yeast cells did not considerably differ from that of the other PEMs (Fig. 4), lower adhesion strengths compared to PAH∕PAA-based PEMs were observed. Additionally, the latter films generally showed larger experimental deviations, irrespective of assembly solution pH.
However, for both PDADMAC∕PAA and PAH∕PAA based coatings no distinct influence of the outermost PE layer on yeast cell adhesion strength (i.e., τw50%(9)=1.6 N∕m2∕τw50%(10)=1.2 N∕m2andτw50%(6)=11.1 N∕m2∕τw50%(7)=10.7 N∕m2, respectively) and the initial cell attachment [with respect to the large scattering for PAH capped (PAH7.5∕PAA3.5) films] is visible. These observations indicate that chemical composition of the layers and electrostatic interactions between S. cerevisiae cells and the modified surfaces play only a minor role. With respect to the small Debye length (≈0.7 nm) at the ionic strength of the modified VERDUYN medium, the latter result can clearly be explained by the minor contribution of double layer interactions to cell adhesion. A possible explanation of the superior performance of the PDADMAC∕PAA coatings compared to the one of the PAH∕PAA-PEMs might be their highly pronounced swellability38 in a salt rich aqueous environment, strongly reducing the hydrophobic interaction between the cells of the hydrophobic strain of S. cerevisiae and the channel wall.
In cultivation experiments with NR6WT fibroblasts Mendelsohn et al.14 found that the adhesion of cells to PAH∕PAA coated tissue culture polystyrene was strongly dependent on the pH used for assembly of the polyelectrolytes. Cell adhesion was completely suppressed on PEMs deposited under acid conditions such as (PAH2.0∕PAA2.0)10, it was moderate on (PAH7.5∕PAA3.5)10, and cells were strongly adherent when PAH∕PAA-PEMs were assembled at pH at which the ionic crosslink density in the film was relatively higher and consequently the swellability lower like in (PAH6.5∕PAA6.5)25. However, this behavior could not be directly transferred to the adhesion ofS. cerevisiae onto PDMS observed in this study. A possible reason might be that, although 33 DL were deposited for 2.0∕2.0 and 6.5∕6.5, respectively, the PEM film on the strongly hydrophobic PDMS still is relatively thin, as can be derived from the FTIR spectra in Fig. 2a. Therefore, the hydrophobic PDMS might still partially be in contact with the cell suspension. The tendency with respect to the work of Mendelsohn et al., however, can be seen here as well with the (PAH6.5∕PAA6.5) PEM (coating number 4, see Fig. 5) exhibiting the comparatively largest adhesion strength[τw50%,(4)=75.2 N∕m2].
PAA-g-PEG capped PEMs and PEG-silane
The strongest decrease in adhesion strength can be achieved for PEMs with immobilized PAA-g-PEG as a terminal layer. For example, the coating with the best performance observed in this study, (PDADMAC@0.1M NaCl∕PAA3.5)_PAA(200)-5-PEG(5), reduces the adhesion strength by more than two orders of magnitude compared to unmodified PDMS [τw50%(11)=0.8 N∕m2andτw50%(1)=107.0 N∕m2, respectively] and to approximately half the τw50%-value of its non-PEGylated counterparts. Compared to the PEG-silane coated PDMS which serves as an example of a frequently used PEGylation method the τw50%-value is approximately one-third[τw50%(12)=2.9 N∕m2]. The mentioned (PDADMAC@0.1M NaCl∕PAA3.5) based coatings also showed the best reproducibility of the PEM and PEM-PEG films investigated in this study. On all PEM surfaces that bind a considerable amount of PAA-g-PEG [(PAH7.5∕PAA3.5), (PAH6.5∕PAA6.5), and (PDADMAC@0.1M NaCl∕PAA3.5), see Fig. 2b], the absolute number of initial adhering cells is in general significantly lower in comparison with their non-PEGylated counterparts [with respect to the large variations concerning the (PAH7.5∕PAA3.5) system].
Contrary to what one might expect from the latter observation, the reduction in cell adhesion strength for the PEGylated PEMs compared to their non-PEGylated counterparts is not directly related to the amount of surface bound PAA-g-PEG: The film (PAH6.5∕PAA6.5), e.g., which shows the largest reduction in τw50% after PEGylation [approximately factor 70, compared to factor 9 for (PAH7.5∕PAA3.5)] does not bind the largest amount of PAA-g-PEG (according to the FTIR measurements, see Fig. 2).
This result suggests that not only the absolute amount of absorbed PAA-g-PEG but also other factors like film morphology (flat films∕loop rich films), the homogeneity of the PEGylation and the stability of PAA-g-PEG on a particular PEM (some of the already bound PAA-g-PEG might get lost during the experiment) influence cell adhesion strength. With respect to these factors a certain density of PEG chains seems to be sufficient to reach a level of τw50% around 1.1 to 1.3 N∕m2. Nevertheless, with a PEM that binds a high amount of PAA-g-PEG and also shows a high τw50% reduction in its non-PEGylated state, e.g., the PDADMAC∕PAA-PEMs, the lowest τw50%-value is achieved in this study.
Stability of the PEM-PEG-coatings
An important property of the functional coatings under study is their stability in the relevant environment. It should therefore be pointed out that all the results described above were obtained after the PEM-PEG films were subject to (1) the procedure of channel height determination in water under shear stresses up to about 0 N∕m2, i.e., at the given channel geometry, mean overflow velocities of about 1 m∕s, (2) disinfection with ethanol (3 min), and (3) the exposure to yeast cells containing VERDUYN media of high ionic strength (during the 20 min settling phase of the cells). Although it cannot be precluded that some of the PEGylated PEM films lose some PAA-g-PEG from the surface during the procedure described above, no delamination of the films could be observed by optical microscopy during the whole duration of each parallel plate flow chamber experiment (about 2 h).
CONCLUSIONS
Different PEM surface modifications of PDMS were performed to reduce attachment and adhesion strength of the hydrophobic yeast S. cerevisiae DSM 2155. As determined by parallel plate flow chamber experiments, the adhesion strength, expressed by the τw50%-value, ofS. cerevisiae DSM 2155 on PDMS can be controlled by the type of PEM used. While all multilayer modifications significantly reduced the adhesion strength compared to that of bare PDMS, some differences in, e.g., efficiency and reproducibility (scattering) of cell adhesion data could be observed. Especially PEMs based on PDADMAC∕PAA and those with surface bound PAA-g-PEG copolymer behaved superior to the others.
For (PDADMAC@0.1M NaCl∕PAA3.5)10.5_PAA(200)-5-PEG(5) the τw50%-value was two orders of magnitude lower compared to bare PDMS and was approximately one third of the value of conventionally PEG-silane modified PDMS. Interestingly, although τw50% differed from coating to coating, the initial number of adherent cells on PEMs without PAA-g-PEG capping or with low binding affinity for the PAA-g-PEG copolymer did not vary to a great extent, but was in general reduced compared to non-modified PDMS. However, a PEM with high binding affinity for PAA-g-PEG also showed a reduction of the initial yeast cell attachment.
The PEM surface charge did not have a significant influence on neither initial attachment nor the adhesion strength under the given environmental conditions. The PEM-PEG coatings on PDMS exhibited their functionality after treatment in different media (water, ethanol, VERDUYN medium) under shear flow and are therefore considered to possess a certain stability.
Conclusively, the lbl modification using the combined properties of PEMs and PAA-g-PEG seems to be a promising method to generate films with defined adhesion behavior toward microorganisms or other particles inside already capped microfluidic devices. The combination of lbl deposition time, the reproducibility of film properties (correlated with scatter in cell adhesion data), and the capability to serve as an anchoring layer to stable adsorption of PAA-g-PEG copolymer determine the practical relevance of a single PEM type for surface modification. In this study, some possibly more suitable PEM coatings and PEM-PEG combinations could be distinguished from others. Future work will focus on long-term stability of PEM-PEG films under different environmental conditions.
ACKNOWLEDGMENTS
The authors thank the German Research Foundation (DFG) for support of this work in the framework of the Collaborative Research Group FOR 856 mikroPART “Microsystems for particulate life-science products” at the Technische Universität Braunschweig, Germany.
References
- Schäpper D., Alam M. N. H. Z., Szita N., Lantz A. E., and Gernaey K. V., Anal. Bioanal. Chem. 395, 679 (2009). 10.1007/s00216-009-2955-x [DOI] [PubMed] [Google Scholar]
- Wang S., Kretzmer G., and Schügerl K., Appl. Microbiol. Biotechnol. 41, 537 (1994). 10.1007/BF00178485 [DOI] [PubMed] [Google Scholar]
- Makamba H., Kim J. H., Lim K., Park N., and Hahn J. H., Electrophoresis 24, 3607 (2003). 10.1002/elps.200305627 [DOI] [PubMed] [Google Scholar]
- Wong I. and Ho C. -M., Microfluid. Nanofluid. 7, 291 (2009). 10.1007/s10404-009-0443-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Ellis A. V., and Voelcker N. H., Electrophoresis 31, 2 (2010). 10.1002/elps.200900475 [DOI] [PubMed] [Google Scholar]
- Wu D., Zhao B., Dai Z., Qin J., and Lin B., Lab Chip 6, 942 (2006). 10.1039/b600765a [DOI] [PubMed] [Google Scholar]
- Sharma V., Dhayal M., Govind, Shivaprasad S. M., and Jain S. C., Vacuum 81, 1094 (2007). 10.1016/j.vacuum.2007.02.004 [DOI] [Google Scholar]
- Hu S., Ren X., Bachman M., Sims Ch. E., Li G. P., and Allbritton N., Anal. Chem. 74, 4117 (2002). 10.1021/ac025700w [DOI] [PubMed] [Google Scholar]
- Yu K. and Han Y., Soft Matter 2, 705 (2006). 10.1039/b602880m [DOI] [PubMed] [Google Scholar]
- Klages C. -P., Hinze A., Lachmann K., Berger C., Borris J., Eichler M., Hausen M. v., Zänker A., and Thomas M., Plasma Processes Polym. 4, 208 (2007). 10.1002/ppap.200600116 [DOI] [Google Scholar]
- Sui G., Wang J., Lee C. -C., Lu W., Lee S. P., Leyton J. V., Wu A. M., and Tseng H. -R., Anal. Chem. 78, 5543 (2006). 10.1021/ac060605z [DOI] [PubMed] [Google Scholar]
- Bertrand P., Jonas A., Laschewsky A., and Legras R., Macromol. Rapid Commun. 21, 319 (2000). [DOI] [Google Scholar]
- Schmolke H., Demming S., Jansen A., and Klages C. -P., Conference of the European Colloid and Interface Society (ECIS), Krakau, Poland, 30 September to 5 August 2008.
- Mendelsohn J. D., Yang S. Y., Hiller J., Hochbaum A. I., and Rubner M. F., Biomacromolecules 4, 96 (2003). 10.1021/bm0256101 [DOI] [PubMed] [Google Scholar]
- Berg M. C., Yang S. Y., Hammond P. T., and Rubner M. F., Langmuir 20, 1362 (2004). 10.1021/la0355489 [DOI] [PubMed] [Google Scholar]
- Picart C., Curr. Med. Chem. 15, 685 (2008). 10.2174/092986708783885219 [DOI] [PubMed] [Google Scholar]
- Lichter J. A., Van Vliet K. J., and Rubner M. F., Macromolecules 42, 8573 (2009). 10.1021/ma901356s [DOI] [Google Scholar]
- Harris J. M. and Zalipsky S., Poly(Ethylene Glycol) Chemistry and Biological Applications, ACS Symposium Series No. 680 (American Chemical Society, Washington, DC, 1997). [Google Scholar]
- Kingshott P., Wei J., Bagge-Ravn D., Gadegaard N., and Gram L., Langmuir 19, 6912 (2003). 10.1021/la034032m [DOI] [Google Scholar]
- Park K. D., Kim Y. S., Han D. K., Kim Y. H., Lee E. H. B., Suh H., and Choi K. S., Biomaterials 19, 851 (1998). 10.1016/S0142-9612(97)00245-7 [DOI] [PubMed] [Google Scholar]
- Pasche S., De Paul S. M., Vörös J., Spencer N. D., and Textor M., Langmuir 19, 9216 (2003). 10.1021/la034111y [DOI] [Google Scholar]
- Roosjen A., Busscher H. J., Norde W., and van der Mei H. C., Microbiology 152, 2673 (2006). 10.1099/mic.0.29005-0 [DOI] [PubMed] [Google Scholar]
- Ngadi N., Abrahamson J., Fee C., and Morison K., PWASET 37, 2070 (2009). [Google Scholar]
- Ngadi N., Abrahamson J., Fee C., and Morison K., Int. J. Nat. Sci. and Eng. 1 (3), 126 (2008). [Google Scholar]
- Kenausis G. L., Vörös J., Elbert D. L., Huang N. -P., Hofer R., Ruiz-Taylor L., Textor M., Hubbell J. A., and Spencer N. D., J. Phys. Chem. B 104, 3298 (2000). 10.1021/jp993359m [DOI] [Google Scholar]
- Huang N. -P., Michel R., Vörös J., Textor M., Hofer R., Rossi A., Elbert D. L., Hubbell J. A., and Spencer N. D., Langmuir 17, 489 (2001). 10.1021/la000736+ [DOI] [Google Scholar]
- Marie R., Beech J. P., Vörös J., Tegenfeldt J. O., and Höök F., Langmuir 22, 10103 (2006). 10.1021/la060198m [DOI] [PubMed] [Google Scholar]
- Busscher H. J. and van der Mei H. C., Clin. Microbiol. Rev. 19, 127 (2006). 10.1128/CMR.19.1.127-141.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot G., Vaca-Medina G., Martin-Yken H., Vernhet A., Schmitz P., and Mercier-Bonin M., Colloids Surf., B 49, 126 (2006). 10.1016/j.colsurfb.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Guillemot G., Lorthois S., Schmitz P., and Mercier-Bonin M., Chem. Eng. Res. Des. 85, 800 (2007). 10.1205/cherd06082 [DOI] [Google Scholar]
- Shiratori S. S. and Rubner M. F., Macromolecules 33, 4213 (2000). 10.1021/ma991645q [DOI] [Google Scholar]
- Bieker P. and Schönhoff M., Macromolecules 43, 5052 (2010). 10.1021/ma1007489 [DOI] [Google Scholar]
- Edlich A., Magdanz V., Rasch D., Demming S., Zadeh S. A., Segura R., Kähler C., Radespiel R., Büttgenbach S., Franco-Lara E., and Krull R., Biotechnol. Prog. 26, 1259 (2010). 10.1002/btpr.449 [DOI] [PubMed] [Google Scholar]
- Klages C. -P., Hartwig S., and Schmolke H., “Adsorption of poly(acrylic acid)-graft-poly)ethylene glycol) on polyelectrolyte multilayers,” Prog. Colloid Polym. Sci. (to be published).
- Köstler S., Delgado A. V., and Ribitsch V., J. Colloid Interface Sci. 286, 339 (2005). 10.1016/j.jcis.2005.01.039 [DOI] [PubMed] [Google Scholar]
- Lichter J. A., Thompson M. T., Delgadillo M., Nishikawa T., Rubner M. F., and Van Vliet K. J., Biomacromolecules 9, 1571 (2008). 10.1021/bm701430y [DOI] [PubMed] [Google Scholar]
- Mercier-Bonin M., Ouazzani K., Schmitz P., and Lorthois S., J. Colloid Interface Sci. 271, 342 (2004). 10.1016/j.jcis.2003.11.045 [DOI] [PubMed] [Google Scholar]
- Dubas S. T. and Schlenoff J. B., Langmuir 17, 7725 (2001). 10.1021/la0112099 [DOI] [Google Scholar]