Abstract
Lymphomas represent common hematological malignancies with increasing incidence in recent years. The major site of extranodal non-Hodgkin lymphoma is the gastrointestinal tract. Involvement of the large intestine is rare in comparison to the stomach or small bowel. The disease appears later in life, predominantly in the male population. Complaints are nonspecific, requiring a high index of suspicion in order to establish the diagnosis. The treatment varies from chemotherapy alone to multimodal therapies combining surgery, chemotherapy and radiotherapy. The small number of patients with various histological subtypes and different stage at presentation results in unclear protocol for the treatment of primary colorectal lymphoma. The purpose of this paper is to review current data on primary lymphoma of the colon and rectum while analyzing reported case series and published material on the subject.
Keywords: Lymphoma non-Hodgkin, Extranodal lymphoma, Large intestine, Colorectal surgery, Chemotherapy
INTRODUCTION
Thomas Hodgkin was the first to introduce lymphomas into medical science in 1832. Subsequent to his report, two decades later, Virchow divided leukemia into the leukemic and “aleukemic” types, employing the term “lymphosarcoma”. At about the same time, a probable case of acute leukemia was published by Cohnheim in 1865 under the term “pseudoleukemia”. Pseudoleukemia was to become a catch-all for a variety of conditions with lymphadenopathy and splenomegaly in common. In 1893, Kundrat and colleagues again used the term lymphosarcoma. The first one to clearly describe follicular or nodular lymphomas was Brill et al in 1925 followed by Symmers in 1927. In 1942, Gall and Mallory introduced a lymphoma classification based on clinicopathological criteria, the first systematic attempt to bring order into the non-Hodgkin’s lymphoma (NHL) situation[1]. Over years, various classification systems have been used to differentiate lymphomas including the Rappaport Classification (used until the 70s)[2], Kiel System[3], Working Formulation[4] and Revised European-American Classification of Lymphoid Neoplasms (REAL)[5]. The latest WHO classification[6] recognizes 5 histological subtypes of B cell lineage: Extranodal marginal lymphoma (MALT lymphoma), follicular lymphoma, mantle cell lymphoma, diffuse large B-cell lymphoma and Burkitt’s lymphoma. According to REAL classification, intestinal T cell lymphomas were subdivided into enteropathy-associated T cell lymphoma and non-enteropathy associated T cell lymphoma that are now considered peripheral T cell derived lymphomas by the WHO classification[7]. All histological subtypes of nodal lymphomas may arise in the gastrointestinal tract but the major two appearing in more than 90% of cases are diffuse large B-cell NHL and mucosa-associated lymphoid tissue (MALT) NHL[8].
Lymphoma is the sixth most common cause of cancer death in the United States with increasing incidence in recent years[9]. It is a common malignancy affecting more than 58 000 patients in the United States[10]. In 40% of cases, the major site of extranodal NHL is the gastrointestinal tract. Involvement of the large intestine is rare (10%-20% of all gastrointestinal lymphomas) in comparison to the stomach or small bowel[11]. Primary NHL accounts for 0.1%-0.5% of all malignant tumors of the colon and rectum which makes it the third most common large bowel malignancy after adenocarcinoma and carcinoid[12].
DEFINITION, TYPES AND ETIOLOGY
Dawson et al[13] established criteria for the diagnosis of primary colorectal lymphomas in 1961. These are: (1) no enlarged superficial lymph nodes when the patient is first seen; (2) chest radiographs without obvious enlargement of the mediastinal nodes; (3) the white blood cell counts, both total and differential, are within normal range and bone marrow biopsy is also normal; (4) at laparotomy only regional nodes are affected by disease; and (5) the liver and spleen seem free of tumor. In the modern era, these criteria have been expanded to new diagnostic tools. Krol et al[14] in a paper from 2003 described three alternative definitions of primary nodal and extranodal NHL, exploring their effect on the percentage of the patients considered to have primary extranodal disease, their treatment outcome and survival in a population-based cohort of NHL patients. Their analysis showed that in nearly 10% of patients, a distinction between nodal or extranodal origin could not be made. When using a strict definition of extranodal lymphomas, better response rates and overall survival are found due to patient selection. With inclusion of disseminated disease in the extra nodal group, one can find no differences between response rate and overall survival in patients with nodal lymphomas compared to extranodal lymphomas. They proposed a more liberal definition of primary extranodal NHL that includes all patients who present with NHL that apparently originated from an extranodal site, even in the presence of disseminated disease, as long as the extranodal component is clinically dominant[14].
The most common histological subtype occurring in the colon and rectum is diffuse large B-cell lymphoma with frequency ranging from 47%-81% depending on the geographical location[12,15,16]. In the study conducted in 4 continents with 1378 patients, Anderson et al[17] have shown that the distribution of NHL subtypes differs in various geographical areas and suggested that this could be a reflection of differences in etiological factors or the host response to these factors. Unlike western countries, frequency of T cell lymphoma in the East is reported to be 17.9%-42%[18,19]. Primary colorectal lymphoma differs from its gastric counterpart. The stomach is the most frequent site of origin of primary gastrointestinal lymphomas. The most common histological type found is MALT lymphoma[20]. Gastric MALT associated lymphoma can be successfully treated by Helicobacter pylori (H. Pylori) eradication. The same histological subtype localized in the large intestine does not have the same connection with H. pylori infection[21].
Staging of the lymphomas is also going through some changes. The widely used Ann Arbor classification for extranodal lymphomas modified by Musshoff[22] has its limitations in terms of tailoring the treatment of each specific lymphoma entity. Several modifications have been developed[23]. The European Gastro-Intestinal Lymphoma Study Group (EGILS) proposed a modified TNM staging system adjusted for gastrointestinal lymphoma, taking into consideration the histopathological characteristics of extranodal B and T-cell lymphomas[24].
Etiological factors involved in the development of primary colorectal lymphoma are unknown. However, high frequency has been observed in conditions of immunosuppression such as inflammatory bowel disease-ulcerative colitis, HIV infection and conditions following organ transplantation[12]. In small series conducted by Doolabh et al[25], 3 out of 7 patients were HIV positive. Since the incidence of infection continues to rise, it would seem that HIV serology in all patients presenting with primary lymphoma of colon is warranted[25].
For the purposes of this overview, the data from the published studies of the primary colorectal lymphoma were analyzed (Table 1).
Table 1.
Published studies on primary colorectal lymphoma
| Author | Patients | Male:female | Analyzed period |
Stage |
Therapy |
Follow up in months (mo) | Overall survival | |||||
| I | II | III | IV | Mult. | Chem. | Surg. | ||||||
| Cai et al[15] | 43 | 29:14 | 1973-2005 | 4 | 10 | 4 | 25 | 26 | 13 | 3 | Mean 64.8 | Rate 42% |
| Bairey et al[16] | 17 | 12:5 | 13 yr | 5 | 2 | 0 | 10 | 9 | 6 | 2 | Median 75 | Median 44 mo |
| Kim et al[18] | 95 | 64:31 | 1986-2002 | 34 | 54 | 0 | 7 | 57 | 23 | 9 | Mean 29.5 | 5 yr rate 55.2% |
| Doolabh et al[25] | 7 | 4:3 | 1989-1998 | 1 | 6 | 0 | 0 | 6 | 0 | 1 | - | - |
| Stanojević et al[27] | 24 | 20:14 | 1991-2005 | 0 | 11 | 12 | 1 | 24 | 0 | 4 | Mean 30.3 | Mean 41.9 mo |
| Fan et al[28] | 37 | 22:15 | 1980-1996 | 9 | 23 | 0 | 5 | 22 | 2 | 13 | Median 50.4 | Median 24 mo |
| Cho et al[29] | 23 | 17:6 | 1946-1993 | 15 | 7 | 0 | 1 | 14 | 4 | 3 | Median 144 | 10 yr rate 61% |
| Gonzalez et al[30] | 15 | 5:10 | 1990-2002 | 15 | 0 | 0 | 0 | 15 | 0 | 3 | Median 28 | Median 60 mo |
| Wong et al[31] | 14 | 13:1 | 1989-1999 | 0 | 5 | 7 | 2 | 11 | 0 | 3 | Median 20 | Rate 57.1% |
| Busch et al[32] | 19 | 16:3 | 1972-1988 | 5 | 6 | 0 | 8 | 14 | 3 | 1 | - | Median 45 mo |
Mult.: Multimodal therapy (surgery combined with chemotherapy and/or radiotherapy); Chem.: Only chemotherapy; Surg.: Only surgery.
PRESENTATION AND DIAGNOSIS
The mean age at diagnosis is 55 years. Men are affected twice as often as women[16,26-28]. The most common symptoms in more than half of patients are abdominal pain and weight loss or changing in bowel habits[16,27,28]. Lower gastrointestinal bleeding can be found in 13%-82% of patients[15,28,29]. Infrequency of these complaints suggests that, despite of the large size of the lymphoma, mucosal ulceration does not usually occur[28].
The cecum is the most frequent location for primary colorectal lymphoma[16,27,28,30], probably due to the larger amount of lymphoid tissue in this region[31]. In different series, the data on the frequency in the rectum varies from 8.3% to 35%[27,29,31,32].
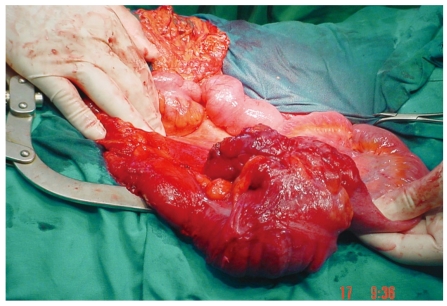
The lack of specific complaints makes the diagnosis hard to establish. Unfortunately, for some patients, a surgical procedure is the only diagnostic tool. As a rare lesion, it requires a high index of suspicion. Due to a delay in diagnosis in 33%-65% of patients, operative procedure is either urgent or emergent[12,15,16]. In more than half of patients, lymphoma is a bulky disease (Figure 1), reaching over 5 cm in diameter[16,27,28]. These bulky masses can usually be palpated by simple physical examination and can be viewed by ultrasonography[16].
Figure 1.
Primary lymphoma of the cecum.
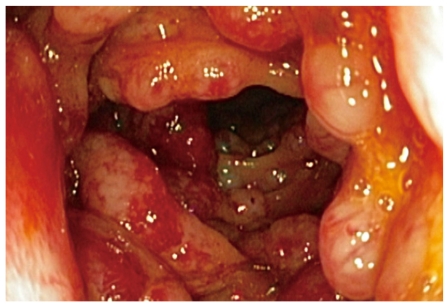
Colonoscopy is valuable in the diagnosis of primary lymphoma of the colon in a non-urgent setting but it is not always possible to determine this type of tumor due to an inadequate biopsy and the need for time consuming immunohistochemical staining. The growth patterns that can be seen in the colon vary. Usually colorectal lymphomas are large, polypoid lesions but mucosal ulceration can also be found (Figure 2). Lymphomatous polyposis can appear similar to familial adenomatous polyposis[33]. Plain radiographs of the abdomen are not helpful. Barium enema can be useful but it cannot distinguish adenocarcinoma or polyposis from lymphoma (Figure 3).
Figure 2.
Colonoscopy findings of mantle cell lymphoma.
Figure 3.
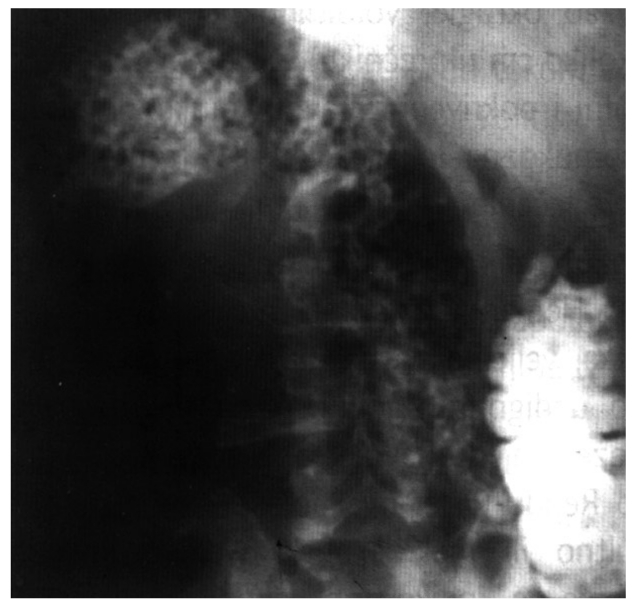
Barium enema showing lymphomatous polyposis.
Radiographical findings associated with colorectal lymphoma can be localized or diffuse. Computerized tomography (CT) shows the focal form of NHL characterized by infiltrative spread rising from the submucosa, resulting in uniform thickening of the intestinal wall, usually without associated desmoplastic reaction. Infiltration of the muscularis propria and the autonomic plexus may result in atonic, aneurismal dilatation of the lumen. Sometimes, lymphoma may form an annular napkin-ring lesion that mimics carcinoma[34]. When CT reveals the presence of an infiltrative process accompanied by enlarge lymph nodes in the abdomen or pelvis, lymphoma should be the primary consideration in the differential diagnosis and must be excluded by endoscopic biopsy. However, without the presence of enlarged lymph nodes it might be difficult to distinguish this type of tumor from a primary adenocarcinoma. This difficulty arises predominantly in cases with solitary mass lesions[35]. The role of PET (positron emission tomography) in the diagnosis and follow up of patients with lymphoma is yet to be established[36].
Disease stage at the time of diagnosis varies in publications. In 2008, in a retrospective study of 15 patients with colorectal lymphoma, Gonzales et al[30] reported all stage IE. Similar results were published in a German study of 371 patients by Koch et al[26] from 2001. On the contrary, other papers reported that than 54.2% of patients were treated in stage IIIE and IVE[15,27]. In a recent paper by Bairey et al[16], seven patients out of seventeen were reported to have stage I or II at diagnosis whereas all others had stage IV. In a study by Fan et al[28], 13.5% of the patients were stage IVE while 24.3% were stage IE and 62.3% were stage IIE.
MANAGEMENT
Although the role of surgery in the treatment of gastrointestinal lymphoma is debatable, the majority of the reported patients were operated on irrespective of the disease stage. There are no controlled randomized trials due to the low incidence of the disease. According to some series, since the rate of spontaneous perforation is high (5 out of 17 patients), it is beneficial to perform a hemicolectomy in order to prevent this complication[16]. Some authors argue that early diagnosis and timely onset of chemotherapy might do the job for such patients, thus avoiding a surgical procedure. There is tacit agreement that in the absence of disseminated disease, surgical resection is generally performed[25,27,28,30,37]. It is believed that surgery may provide important prognostic information, including history, tumor extent and stage, could prevent complications and may offer a chance for cure with or without chemotherapy[28].
In the published studies, treatment varies from chemotherapy alone to multimodal therapies combining surgery, chemotherapy and radiotherapy. Chemotherapy remains the basis of treatment for the rapidly proliferating aggressive lymphomas because these malignancies almost always extend beyond local fields, encompassed by surgery or radiation. The CHOP chemotherapeutic regimen (cyclophosphamide, doxorubicin, vincristine and prednisone) remains the first line therapy for all moderate and high-grade B-cell lymphomas. Several prospective trials have shown that adding rituximab to standard CHOP regiment (R-CHOP) resulted in higher response rates and better progression-free, event-free, disease-free and overall survival[38]. Fan et al[28] found significant improvement in the survival of patients with stage IIE who received adjuvant chemotherapy. However, the analysis of the subgroup of patients with high grade lymphoma in stage I and II showed that adding chemotherapy did not significantly impact survival. In 2005, Bilsel et al[35] reported the case of primary lymphoma of the rectum with complete clinical response after combination of radiation and chemotherapy. Similar results were published by other authors[39]. In case series published in 2002, Pricolo et al[40] treated patients with surgical resection and adjuvant chemo and radiotherapy, while Shimono et al[41] from Japan suggested preoperative radiotherapy. Some authors proposed the so called preventive chemotherapy in patients with stage IE, reaching five year survival of 80%[42]. In cases of indolent lymphomas (mantle cell, follicular cell and T cell lymphomas), complete surgical resection and radiation are recommended because of decreased chemoresponsiveness[43]. The median survival in reported series is generally low. Patient with stage IV disease demonstrates poor survival (up to 8 mo)[16].
Factors affecting survival differ among authors. Stage at the diagnosis has impact on survival[15,16,27]. According to Fan et al[28], only histological grade seems to be an adverse factor for prognosis. Urgency of the surgical procedure is also found to be an important factor affecting survival[16,27].
CONCLUSION
The vast majority of patients with primary colorectal lymphoma underwent some form of resection although the role of surgery and its extent is yet to be established. After resection, most patients undergo CHOP or other multiagent therapy. The optimal management of primary lymphoma of the colon and rectum has never been determined by randomized trials. The small number of patients with various histological subtypes and different stage at presentation results in unclear treatment protocol. Since all the published studies are retrospective, their scientific validity is partially diminished, imposing the need for conducting prospective multicentric studies. Meanwhile, systematized clinical experiences contribute usefully to the knowledge of clinicians.
Footnotes
Peer reviewers: Tatsuo Kanda, MD, PhD, Division of Digestive and General Surgery, Graduate School of Medical and Dental Sciences, Niigata University, Niigata City 951-8510, Japan; Jaw Yuan Wang, Professor, MD, PhD, Department of Surgery, Kaohsiung Medical University and Hospital, 100, Tzyou 1st Road, Kaohsiung 807, Taiwan, China; Ioannis A Voutsadakis, MD, PhD, Department of Medical Oncology, University Hospital of Larissa, Larissa 41110, Greece
S- Editor Wang JL L- Editor Roemmele A E- Editor Ma WH
References
- 1.Aisenberg AC. Historical review of lymphomas. Br J Haematol. 2000;109:466–476. doi: 10.1046/j.1365-2141.2000.01988.x. [DOI] [PubMed] [Google Scholar]
- 2.Rappaport H. Tumors of the hematopoetic system. In: Atlas of tumor pathology., editor. Washington, DC: Armed Forces Institute of Pathology; 1966. [Google Scholar]
- 3.Stansfeld AG, Diebold J, Noel H, Kapanci Y, Rilke F, Kelényi G, Sundstrom C, Lennert K, van Unnik JA, Mioduszewska O. Updated Kiel classification for lymphomas. Lancet. 1988;1:292–293. doi: 10.1016/s0140-6736(88)90367-4. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute sponsored study of classifications of non-Hodgkin’s lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin’s Lymphoma Pathologic Classification Project. Cancer. 1982;49:2112–2135. doi: 10.1002/1097-0142(19820515)49:10<2112::aid-cncr2820491024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 6.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J. Lymphoma classification--from controversy to consensus: the R.E.A.L. and WHO Classification of lymphoid neoplasms. Ann Oncol. 2000;11 Suppl 1:3–10. [PubMed] [Google Scholar]
- 7.Novakovic BJ, Novakovic S, Frkovic-Grazio S. A single-center report on clinical features and treatment response in patients with intestinal T cell non-Hodgkin's lymphomas. Oncol Rep. 2006;16:191–195. [PubMed] [Google Scholar]
- 8.Liang R, Todd D, Ho FC. Aggressive non-Hodgkin’s lymphoma: T-cell versus B-cell. Hematol Oncol. 1996;14:1–6. doi: 10.1002/(SICI)1099-1069(199603)14:1<1::AID-HON555>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 9.America Cancer Society. Cancer Facts and Figures. Atlanta, GA: American Cancer Society; 2005. [Google Scholar]
- 10.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 11.Crump M, Gospodarowicz M, Shepherd FA. Lymphoma of the gastrointestinal tract. Semin Oncol. 1999;26:324–337. [PubMed] [Google Scholar]
- 12.Stanojević G, Stojanović M, Jovanović M, Stojanović M, Jeremić M, Branko B, Ignjatović N, Katić VV. [Primary colorectal lymphomas] Vojnosanit Pregl. 2009;66:295–301. doi: 10.2298/vsp0904295s. [DOI] [PubMed] [Google Scholar]
- 13.Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961;49:80–89. doi: 10.1002/bjs.18004921319. [DOI] [PubMed] [Google Scholar]
- 14.Krol AD, le Cessie S, Snijder S, Kluin-Nelemans JC, Kluin PM, Noordijk EM. Primary extranodal non-Hodgkin’s lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West population-based NHL registry. Ann Oncol. 2003;14:131–139. doi: 10.1093/annonc/mdg004. [DOI] [PubMed] [Google Scholar]
- 15.Cai S, Cannizzo F Jr, Bullard Dunn KM, Gibbs JF, Czuczman M, Rajput A. The role of surgical intervention in non-Hodgkin’s lymphoma of the colon and rectum. Am J Surg. 2007;193:409–412; discussion 412. doi: 10.1016/j.amjsurg.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Bairey O, Ruchlemer R, Shpilberg O. Non-Hodgkin’s lymphomas of the colon. Isr Med Assoc J. 2006;8:832–835. [PubMed] [Google Scholar]
- 17.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin’s lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 1998;9:717–720. doi: 10.1023/a:1008265532487. [DOI] [PubMed] [Google Scholar]
- 18.Kim YH, Lee JH, Yang SK, Kim TI, Kim JS, Kim HJ, Kim JI, Kim SW, Kim JO, Jung IK, et al. Primary colon lymphoma in Korea: a KASID (Korean Association for the Study of Intestinal Diseases) Study. Dig Dis Sci. 2005;50:2243–2247. doi: 10.1007/s10620-005-3041-7. [DOI] [PubMed] [Google Scholar]
- 19.Wang MH, Wong JM, Lien HC, Lin CW, Wang CY. Colonoscopic manifestations of primary colorectal lymphoma. Endoscopy. 2001;33:605–609. doi: 10.1055/s-2001-15320. [DOI] [PubMed] [Google Scholar]
- 20.Mendelson RM, Fermoyle S. Primary gastrointestinal lymphomas: a radiological-pathological review. Part 2: Small intestine. Australas Radiol. 2006;50:102–113. doi: 10.1111/j.1440-1673.2006.01539.x. [DOI] [PubMed] [Google Scholar]
- 21.Zighelboim J, Larson MV. Primary colonic lymphoma. Clinical presentation, histopathologic features, and outcome with combination chemotherapy. J Clin Gastroenterol. 1994;18:291–297. [PubMed] [Google Scholar]
- 22.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31:1860–1861. [PubMed] [Google Scholar]
- 23.Rohatiner A, d’Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem P, Shipp M. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5:397–400. doi: 10.1093/oxfordjournals.annonc.a058869. [DOI] [PubMed] [Google Scholar]
- 24.Ruskoné-Fourmestraux A, Dragosics B, Morgner A, Wotherspoon A, De Jong D. Paris staging system for primary gastrointestinal lymphomas. Gut. 2003;52:912–913. doi: 10.1136/gut.52.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doolabh N, Anthony T, Simmang C, Bieligk S, Lee E, Huber P, Hughes R, Turnage R. Primary colonic lymphoma. J Surg Oncol. 2000;74:257–262. doi: 10.1002/1096-9098(200008)74:4<257::aid-jso3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 26.Koch P, del Valle F, Berdel WE, Willich NA, Reers B, Hiddemann W, Grothaus-Pinke B, Reinartz G, Brockmann J, Temmesfeld A, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001;19:3861–3873. doi: 10.1200/JCO.2001.19.18.3861. [DOI] [PubMed] [Google Scholar]
- 27.Stanojević GZ, Stojanović MP, Stojanović MM, Krivokapić Z, Jovanović MM, Katić VV, Jeremić MM, Branković BR. Non-Hodgkin’s lymphomas of the large bowel-clinical characteristics, prognostic factors and survival. Acta Chir Iugosl. 2008;55:109–114. doi: 10.2298/aci0803109s. [DOI] [PubMed] [Google Scholar]
- 28.Fan CW, Changchien CR, Wang JY, Chen JS, Hsu KC, Tang R, Chiang JM. Primary colorectal lymphoma. Dis Colon Rectum. 2000;43:1277–1282. doi: 10.1007/BF02237436. [DOI] [PubMed] [Google Scholar]
- 29.Cho MJ, Ha CS, Allen PK, Fuller LM, Cabanillas F, Cox JD. Primary non-Hodgkin lymphoma of the large bowel. Radiology. 1997;205:535–539. doi: 10.1148/radiology.205.2.9356641. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez QH, Heslin MJ, Dávila-Cervantes A, Alvarez-Tostado J, de los Monteros AE, Shore G, Vickers SM. Primary colonic lymphoma. Am Surg. 2008;74:214–216. [PubMed] [Google Scholar]
- 31.Wong MT, Eu KW. Primary colorectal lymphomas. Colorectal Dis. 2006;8:586–591. doi: 10.1111/j.1463-1318.2006.01021.x. [DOI] [PubMed] [Google Scholar]
- 32.Busch E, Rodriguez-Bigas M, Mamounas E, Barcos M, Petrelli NJ. Primary colorectal non-Hodgkin’s lymphoma. Ann Surg Oncol. 1994;1:222–228. doi: 10.1007/BF02303527. [DOI] [PubMed] [Google Scholar]
- 33.Quayle FJ, Lowney JK. Colorectal lymphoma. Clin Colon Rectal Surg. 2006;19:49–53. doi: 10.1055/s-2006-942344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montgomery M, Chew FS. Primary lymphoma of the colon. AJR Am J Roentgenol. 1997;168:688. doi: 10.2214/ajr.168.3.9057516. [DOI] [PubMed] [Google Scholar]
- 35.Bilsel Y, Balik E, Yamaner S, Bugra D. Clinical and therapeutic considerations of rectal lymphoma: a case report and literature review. World J Gastroenterol. 2005;11:460–461. doi: 10.3748/wjg.v11.i3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305–332. doi: 10.1148/radiol.2312021185. [DOI] [PubMed] [Google Scholar]
- 37.Avilés A, Neri N, Huerta-Guzmán J. Large bowel lymphoma: an analysis of prognostic factors and therapy in 53 patients. J Surg Oncol. 2002;80:111–115. doi: 10.1002/jso.10103. [DOI] [PubMed] [Google Scholar]
- 38.Morrison VA. Evolution of R-CHOP therapy for older patients with diffuse large B-cell lymphoma. Expert Rev Anticancer Ther. 2008;8:1651–1658. doi: 10.1586/14737140.8.10.1651. [DOI] [PubMed] [Google Scholar]
- 39.Guney N, Basaran M, Aksakalli N, Bavbek S, Erseven G. Primary non-Hodgkin’s lymphoma of the rectum. Onkologie. 2007;30:385–387. doi: 10.1159/000103590. [DOI] [PubMed] [Google Scholar]
- 40.Pricolo R, Parziale A, Filosa M, Voltolini F, Zangrandi A. [Primary lymphoma of the rectum: a case report and review of the literature] Chir Ital. 2002;54:549–554. [PubMed] [Google Scholar]
- 41.Shimono R, Mori M, Kido A, Adachi Y, Sugimachi K. Malignant lymphoma of the rectum treated preoperatively with hyperthermia and radiation. Eur J Surg Oncol. 1995;21:83–84. doi: 10.1016/s0748-7983(05)80074-5. [DOI] [PubMed] [Google Scholar]
- 42.Devine R, Brand M. Miscellaneous Neoplasms. In: Wolff B, Fleshman J, Beck D, Pemberton J, Wexner S, editors. The ASCRS textbook of colon and rectal surgery. Springer; 2007. pp. 515–524. [Google Scholar]
- 43.Koniaris LG, Drugas G, Katzman PJ, Salloum R. Management of gastrointestinal lymphoma. J Am Coll Surg. 2003;197:127–141. doi: 10.1016/S1072-7515(03)00002-4. [DOI] [PubMed] [Google Scholar]