Abstract
In this longitudinal study of prenatal cocaine exposure (PCE), school-age physical and cognitive development and behavioral characteristics were examined, while controlling for other factors that affect child development. At this follow-up phase, children were on average 7.2 years old, and their caregivers were 33.7 years old, had 12.5 years of education, and 48% were African American. During the first trimester, 20% of the women were frequent cocaine users (≥ 1 line/day). First trimester cocaine exposure predicted decreased weight and height at 7 years. There was no significant relationship between PCE and the cognitive and neuropsychological measures. Third trimester cocaine use predicted more total and externalizing behavior problems on the Child Behavior Checklist [3] and the Teacher Report Form [4], and increased activity, inattention, and impulsivity on the Routh Activity [67] and SNAP scales [55]. Children who were exposed to cocaine throughout pregnancy had more mother- and teacher-rated behavior problems compared to children of women who stopped using early in pregnancy or who never used cocaine prenatally. These detrimental effects of PCE on behavior are consistent with other reports in the literature and with the hypothesis that PCE affects development through changes in neurotransmitter systems. These school-age behaviors may be precursors of later adolescent behavior problems.
Keywords: prenatal cocaine exposure, school age, growth, cognitive development, behavior problems
1. Introduction
Over the last decade, we have seen a group of well-designed studies, many of which are represented in this Special Issue, tackle the important public health topic of the effects of prenatal cocaine exposure (PCE). Previously, we reported that PCE was associated with infant temperament and motor development [61], and with preschool behavior problems, poorer short-term memory, and decreased head circumference [62]. Our longitudinal study and others have continued to follow cohorts into early elementary school (defined for this review as 6 to 8 years old or first through third grade), investigating the effects of PCE on the domains of growth, cognitive development, and behavior.
Most researchers have reported that PCE was not associated with growth at school age [5,29,37,40,41,50]. By contrast, PCE was found to be associated with decreased height at 7 years, but only for those whose mothers were older than 30 at the time of delivery [21], and with decreased height and weight/height z-scores in 6-year-olds [52].
There have been reports of relationships between PCE and specific areas of cognitive or neuropsychological development and school functioning in 6- to 8-year-olds, including language development [8,9,11,46], abstract-visual reasoning [12], visual-motor performance [5], learning disabilities [53], and increased frequency of Individualized Education Plans [45]. Eyler et al. [29] found that PCE was associated with executive function tasks at 7 years, but the effects were mediated by the effects of PCE on birth head circumference. In general, PCE has not been found to be associated with deficits in global cognitive development, as measured by scales such as the Stanford-Binet Intelligence Scale (SBIS) [77], the Wechsler Preschool and Primary Scale of Intelligence-Revised [81], the Wechsler Intelligence Scale for Children [82], and the Differential Abilities Scale [28] [5,8,37,40,41,53], although Bennett et al. [12] reported that PCE predicted lower SBIS composite scores for exposed boys only.
The literature is also inconsistent in the behavior domain. PCE has been reported to be associated with increased caregiver- and teacher-reported behavior problems, particularly externalizing behavior [6,26,40] and aggression [73], and with poorer attention and processing on laboratory measures [1,51]. Nordstrom-Bailey [54] also found an effect of PCE on aggression, but only in girls who had no prenatal alcohol exposure. Other researchers have reported that there were no significant relations between PCE and caregiver’s ratings of behavior problems [2,41,48].
The research presented here is from a longitudinal study of prenatal cocaine use that was designed to address the relations between PCE and physical, cognitive, and behavioral development in a sample of women recruited from a prenatal care clinic. We will address the timing of PCE because multiple interviews were conducted to obtain trimester-specific substance use data. Given the longitudinal design of the study, we will evaluate whether earlier effects of PCE mediate later outcomes. We will also test whether there are any moderating effects of maternal age, home environment, or child gender because of previous reports of interactions between PCE and these variables [11,12,15,21,26,73,85]. Based on our findings at the 1- and 3-year follow-ups [61,62] and on those in the literature, we hypothesize that the strongest associations will be with PCE and behavior problems at 7 years of age, and that this relation will remain significant after adjusting for covariates of cocaine use.
2. Methods
2.1. Study Design
The women and children in this sample are participants in a longitudinal investigation of the effects of PCE. Written consent was obtained according to the guidelines of the University of Pittsburgh's Institutional Review Board and the Research Review and Human Experimentation Committee of Magee-Womens Hospital (MWH). A Department of Health and Human Services’ Confidentiality Certificate assured participants that their responses could not be subpoenaed.
Women ≥ 18 years of age were initially interviewed in the MWH prenatal clinic during their fourth or fifth prenatal month. Women were not enrolled if they did not initiate prenatal care by the fifth month of pregnancy. During the first interview, the women were asked about cocaine, crack, alcohol, tobacco, marijuana, and other illicit drug use in the year prior to pregnancy and during the first trimester. All women who reported any cocaine or crack use during the first trimester were enrolled. The next woman interviewed who reported no cocaine or crack use during both the year prior to pregnancy and the first trimester was also enrolled. The selected sample was interviewed during the seventh prenatal month and at 24 hours post-delivery about substance use during their second and third trimesters, respectively. All infants were examined at delivery by study nurses who were unaware of prenatal exposure status. At 1 year postpartum, growth, mental and motor development, and temperament were assessed [61]. At 3 years postpartum, growth, cognitive development, and behavior were assessed [62].
At the 7-year follow-up, mothers and children were seen in our research offices. The child’s growth was measured by trained research staff and his/her medical history was obtained from the mother. Cognitive development was assessed with the Stanford-Binet Intelligence Scale – 4th Edition (SBIS) [77], which consists of the following scales: verbal reasoning (VR), abstract/visual reasoning (AVR), quantitative reasoning (QR), short-term memory (STM), and the composite score. Academic achievement was assessed with the Wide Range Achievement Test – Revised (WRAT-3) [83], which yields reading, spelling, and arithmetic scores. The screening version of the Wide Range Assessment of Memory and Learning (WRAML) [70] was used to examine visual and verbal memory and verbal learning. The Grooved Pegboard, in which the child is required to place notched pegs into a board of 25 grooved holes, was used as a test of psychomotor speed and eye-hand coordination [66]. The Progressive Figures Test (PFT) was administered to provide an indication of mental flexibility and impulsivity [57]. The PFT is conceptually similar to the Trail Making Test, but is more appropriate for 7-year-olds because it uses shapes rather than numbers and letters. To measure attention and impulsivity, the Continuous Performance Test – Shapes (CPT-3) [49] was administered. The CPT-3 presents various shapes in different colors and requires the child to respond to a target stimulus. Data include the rate of responding and errors of omission and commission.
The examiners were bachelor’s or master’s level research staff who had extensive experience administering standardized child assessments. They were trained to reliability and supervised by a developmental psychologist (GAR). All examiners were blind to prenatal and current substance use status. Periodic reliability checks were conducted in order to maintain consistent administration and scoring.
At 7 years, trained interviewers asked the mothers structured questions about their substance use during the last year and about their demographic and psychological characteristics, household composition, and social support (how often have contact with friends and relatives; someone to turn to in times of need; support received in role as a mother; satisfaction with help received). The Center for Epidemiological Studies - Depression Scale (CES-D) [56] was used to assess maternal depression and the Spielberger State-Trait Anxiety Inventory (STAI) [74] was used to measure anxiety and hostility. The interview version of the HOME [7] was used to measure aspects of the home environment that correlate with cognitive development, such as the availability of reading materials, the frequency of television viewing and family meals, and types of discipline tactics. A total score is obtained with higher scores indicating more supportive home environments. An estimate of maternal intelligence was obtained by administering the two-subtest version (block design and vocabulary) [14] of the Wechsler Adult Intelligence Scale - Revised (WAIS-R) [80].
The mother's view of the child's temperament was measured using the EAS Scale [17], in which the mother rates her child in terms of emotionality or distress, degree of activity, sociability, and shyness. Cronbach’s alphas for the current sample were 0.79, 0.68, 0.55, and 0.58 for the four dimensions, respectively. The sociability and shyness scales were eliminated from the analyses because of the low alphas. The mothers also completed the Routh Activity Scale [67], an assessment of the child’s activity levels in daily situations such as mealtime, bedtime, and playtime, and the SNAP [55], a rating of activity, attention, impulsivity, and peer relations. The alphas in the current sample for the Routh and the four SNAP subscales were 0.91, 0.84, 0.88, 0.85, and 0.66, respectively. As a measure of behavior problems, the Child Behavior Checklist/4–18 (CBCL) [3] was completed by the caregivers at 7 years. In addition, the child’s primary teacher completed the Teacher Report Form [4], which parallels the questions in the CBCL.
2.2. Sample Characteristics
Recruitment occurred between March 1988 and December 1992 and 90% of the women who were approached agreed to participate. Only 5% of a random sample of women who refused to participate had a history of drug use during the current pregnancy, according to a medical chart review. A total of 320 women met the inclusion criteria and were enrolled into the study. Between enrollment at the 4th or 5th month of pregnancy and delivery, 20 subjects were eliminated for the following reasons: home delivery, miscarriage/abortion/fetal death, moved, lost to follow-up, and refused. Thus, delivery assessments were completed on 300 women. Four pairs of twins and one child with Trisomy 21 were excluded from additional follow-up, resulting in a birth cohort of 295 mothers and infants.
By 7 years, 51 subjects were lost to follow-up: 5 children died, 5 mothers lost custody and the children could not be traced, 15 families moved out of state, 14 mothers refused to participate, and 12 were missed. The 244 subjects interviewed at the 7-year follow-up represented 83% of the birth cohort. Ten percent of the children were not in maternal custody at 7 years, in which case the current caretaker was interviewed. The majority of these caregivers were relatives of the child (father, grandparent, aunt).
One child with a severe disability (Alagille Syndrome) was excluded from the analyses, resulting in an analysis cohort of 243 mothers and children. The mothers who were not included in the 7-year analysis (N=52) were younger (23.2 vs. 25.1 years, p < .01) than those who were included in the analysis (N=243). There were no other significant differences in maternal sociodemographic, prenatal substance use, or infant birth characteristics between those who were and were not included in this analysis. The TRF was completed for 211 of the 243 subjects (87%).
The median child age at assessment was 7.2 years (mean = 7.4 years; SD = 0.5; range = 6.6 – 9.0). Eighty-nine percent were seen by 8.0 years of age. Fifty-three percent of the children were male. The average weight was 60.2 pounds (range = 35 – 140), height was 49.3 inches (range = 43 – 57), and head circumference was 525 mm (range = 480 – 585). The mean SBIS scores were: VR - 96; AVR - 88; QR - 94; STM - 91; and composite - 91 (range = 49 – 137). The mean WRAT-3 reading, spelling, and arithmetic scores were 95, 95, and 94, respectively, and the mean WRAML screener composite score was 94 (range = 56 – 126).
At the 7-year phase, the women were, on average, 33.7 years old (range = 21 – 71), 52% were Caucasian, and 48% were African American. Women had a median family income of $1,200 per month (range = $172 – $8,000/month) and an average educational level of 12.5 years (range = 8 – 18). Thirty-one percent were married, 50% had a man living in the household (includes husband, boyfriend, father or stepfather of the child), and 58% worked or went to school. The mean estimated maternal IQ score was 90 (SD = 12; range = 61 – 126).
2.3. Data Analysis
Cocaine and crack use were reported in lines, rocks, or grams, or in cost at 7 years if the woman could not report quantity. For analytic purposes, first trimester cocaine use was defined as use/no use and as frequent use (≥ 1 line/day) versus non-frequent use (< 1 line/day). Cocaine use was dichotomized into use/no use for the second and third trimesters and for the 7-year postpartum phase because of the small number of women who used during those time periods.
The alcohol and marijuana variables were average number of drinks or joints per day, respectively, and were log transformed to reduce skewness. Tobacco use was analyzed as number of cigarettes per day. The alcohol, marijuana, and tobacco variables were ascertained separately for each trimester of pregnancy, as well as at 7 years postpartum, and were used as continuous variables in the analyses. Detailed information about calculation of the first trimester substance use variables has been previously published [24,61].
The growth outcomes were weight, height, and head circumference. The cognitive and neuropsychological outcomes were: SBIS VR, AVR, QR, STM, and composite scores; WRAML story, picture, verbal, design subscales and composite screener; WRAT-3 reading, spelling, and arithmetic scores; PFT time to complete (log-transformed) and number of errors (0/1 vs. 2+); Grooved Pegboard dominant and non-dominant hand time to insert and time to remove (log-transformed) and number of pegs dropped (0/1 vs. 2+); and CPT number of omission and commission errors. The behavior outcomes were: EAS emotionality and activity; Routh total score; and SNAP activity, attention, impulsivity, and peer relations. To reduce Type I error rate due to multiple tests on the same outcome, the first set of regression analyses on the CBCL and TRF was with the total, internalizing, and externalizing scales. If PCE was a significant predictor of these three scales, then further analyses were conducted with the withdrawn, somatic complaints, anxious/depressed, social problems, thought problems, attention problems, delinquent behavior, and aggressive behavior scales. T scores were used for all of the CBCL and TRF scales.
Significant covariates for the final model were selected using stepwise multiple regression analyses. To explore the stability of the variables entering the model, the initial significance level for entry and removal was 0.10. Variables that were significant at an alpha of 0.05 were retained for the final models. Child characteristics, maternal sociodemographic, psychosocial and environmental characteristics, and prenatal and 7-year substance use were considered as covariates because of their associations with the outcomes and/or PCE in initial bivariate analyses and based on the literature and prior analyses of these data. Table 1 lists potential covariates and shows those that were retained for the final models. All regressions were run separately by trimester to assess the effects of exposure during each time period.
Table 1.
Variables considered for inclusion as covariates
| 7-Year Child Characteristics |
| Age at assessmenta (for growth, Progressive Figures, Pegboard, and CPT analyses only) |
| Gendera (not used for CBCL and TRF analyses) |
| Number of illnesses |
| Number of injuries |
| Number of hospitalizations |
| Number of siblingsa |
| Stanford-Binet composite scorea (for TRF analyses only) |
| 7-Year Maternal Demographic and Environmental Characteristics |
| Adult male in householda |
| Agea |
| Custody (maternal vs. non-maternal) |
| Depressiona [51] |
| Educationa |
| Family income |
| Heighta (for growth analyses only) |
| HOMEa [7] |
| Hostilitya [74] |
| IQa [14] (not used for growth analyses) |
| Marital status |
| Racea |
| Social supporta |
| Work statusa |
| 7-Year Maternal Substance Use |
| Alcohola |
| Cocainea |
| Marijuanaa |
| Other illicit drugs |
| Tobaccoa |
| Prenatal Substance Use (for each trimester) |
| Alcohola |
| Cocainea |
| Marijuanaa |
| Other illicit drugs |
| Tobaccoa |
Variable was retained in final model
Residuals and the modified Cook’s statistic [20] were used to identify possible outliers and influential points. Only those relations with PCE that remained stable after removal of the influential cases are included in this report. There were 4 influential points for weight and 2 for head circumference, 2 for SBIS, 7 for WRAML, 4 for WRAT, 2 for PFT, 3 for Pegboard, 1 for omission and 2 for commission errors on the CPT, 2 for Routh, 2 for EAS, and 1 for TRF total score. The tolerance of each predictor was examined to assure that the estimated regression slopes were not unstable due to multicollinearity.
A descriptive group analysis was conducted to investigate the impact of duration of cocaine use and three groups were defined: women who abstained from cocaine throughout pregnancy (“Never”) (N=130); those who used first trimester only (“Stopped”) (N=60); and those who used both first and third trimesters (“Continued”) (N=27). Analysis of covariance was then used in order to adjust the outcomes for significant covariates from the regression analyses.
We tested whether there were interactions between PCE and maternal age, home environment, and child gender by including these interaction terms in the regression models. We also evaluated whether there were any mediating effects of birth weight on 7-year weight and of 3-year behavior problems on the 7-year outcomes. Mediation was evaluated through path analysis using the product of coefficients [72] and by examining the significance of the direct effect after inclusion of the mediating effect in the model. If the direct effect remained significant, we considered the mediation to be partial. Parameters were estimated using Lisrel 8.5 [38].
3. Results
3.1. Descriptive Analyses
During the first trimester, 19.8% of the women were frequent users (≥ 1 line of powder cocaine per day, or the equivalent in crack). By the third trimester, 6.7% of the women were frequent users (Table 2). Only 13% of the women who used first trimester also used second and third trimester. All women who used cocaine during the second and third trimesters also used cocaine during the first trimester. The prevalence of frequent cocaine use did not change significantly during the first and third postpartum years from that of the third trimester, but was significantly lower by 7 years postpartum. The mean level of cocaine use for the women who used during the first trimester was 0.26 g/day, or ~9 lines/day (range = < 1 line/month – 4 g/day). The mean level of use for the second and third trimester users was 0.17 g/day (~6 lines/day) (range = < 1 line/month – 1 g/day) and 0.14 g/day (~5 lines/day) (range = < 1 line/month – 2 g/day), respectively. At the 7-year follow-up, the mean level of cocaine use for the women who used was 0.20 g/day, or ~7 lines/day (range = < 1 line/month – 1.4 g/day).
Table 2.
Prevalence of Cocaine Use (%)
| Level of Use | |||
|---|---|---|---|
| Time Period | None | Occasionala | Frequentb |
| Year prior to pregnancy (N = 241) | 55.2 | 23.2 | 21.6 |
| First trimester (N = 243) | 57.2 | 23.0 | 19.8 |
| Second trimester (N = 218) | 91.7 | 3.2 | 5.0 |
| Third trimester (N = 240) | 88.7 | 4.6 | 6.7 |
| One-year follow-up (N = 231) | 84.4 | 7.8 | 7.8 |
| Three-year follow-up (N = 234) | 87.2 | 6.4 | 6.4 |
| Seven-year follow-up (N = 243) | 91.4 | 5.8 | 2.9 |
Occasional: > 0 and < 1 line/day of cocaine or gram equivalent of crack
Frequent: ≥ 1 line/day of cocaine or gram equivalent of crack
We reported previously that women who used ≥ 1 line of cocaine/day during the first trimester were significantly more likely to be older, African American, single, and to have lower family incomes than the women who did not use first trimester [62]. Frequent first trimester cocaine users also used more alcohol, cigarettes, marijuana, and other illicit drugs than did first trimester non-cocaine users [62].
These same characteristics also describe women who continued to use cocaine throughout pregnancy: They were more likely to be African American, single, to have lower family incomes, and to use more alcohol, cigarettes, and marijuana than women who stopped or never used during pregnancy (Table 3).
Table 3.
Maternal Characteristics Associated with Duration of Prenatal Cocaine Use
| Never Useda | Stoppedb | Continuedc | |
|---|---|---|---|
| Third Trimester | n = 130 | n = 60 | n = 27 |
| Maternal Characteristics | |||
| Mean (SD) Education (yrs) | 12.1 (1.3) | 12.1 (1.2) | 11.9 (1.0) |
| % Caucasian | 60.0 | 61.7 | 7.4*** |
| % Married | 32.3 | 11.7 | 7.4*** |
| % Work/Attend School | 16.2 | 15.0 | 7.4 |
| % Family Income < $500/mo | 44.4 | 63.3 | 81.5*** |
| % Drink Alcohol | 31.5 | 53.3 | 63.0*** |
| Mean (SD) # Drinks/Day | .05 (0.2) | .14 (0.3) | 1.2 (1.9)*** |
| % Smoke Cigarettes | 36.7 | 68.3 | 80.8*** |
| Mean (SD) # Cigarettes/Day | 5.4 (8.7) | 9.1 (9.5) | 8.2 (6.4)* |
| % Use Marijuana | 6.9 | 15.0 | 25.9* |
| Mean (SD) # Joints/Day | .02 (0.1) | .11 (0.4) | .03 (0.1) |
Did not use cocaine during any trimester
Used cocaine first trimester only
Used cocaine both first and third trimesters
p < .05
p < .01
p < .001: Overall significance using F test for continuous variables and Chi-square test for dichotomous variables.
We also looked at the characteristics of women who used cocaine/crack at the 7-year phase. Current users were more likely to be Caucasian, and to report higher hostility scores and more alcohol, tobacco, marijuana, and other illicit drug use than women who did not use cocaine at 7 years (Table 4).
Table 4.
Current Caregiver Characteristics Associated with Cocaine Use at 7-Year Follow-Up
| No Cocaine Use | Cocaine Use | |
|---|---|---|
| at 7 Years | at 7 Years | |
| N= 222 | N= 21 | |
| Mean (SD) Age (yrs) | 33.9 (6.7) | 31.2 (4.2) |
| Mean (SD) Education (yrs) | 12.5 (1.5) | 12.0 (1.7) |
| % Caucasian | 49.6 | 76.2* |
| % Married | 31.1 | 33.3 |
| % Work/Attend School | 57.7 | 57.1 |
| Mean (SD) Family Income/Mo ($) | 1407 (945) | 1835 (1777) |
| % Male in Household | 50.5 | 42.9 |
| % in Maternal Custody | 10.4 | 9.5 |
| Mean (SD) Maternal IQ [14] | 89.6 (11.8) | 90.5 (12.8) |
| Mean (SD) Depression (total CES-D score) [56] | 18.5 (9.1) | 23.4 (11.4) |
| Mean (SD) Hostility (total STAI score) [74] | 15.7 (3.9) | 19.1 (6.3)* |
| Mean (SD) HOME [7] | 12.4 (2.8) | 11.7 (2.5) |
| % Drink Alcohol | 77 | 95* |
| Mean (SD) # Drinks/Day | 0.73 (1.3) | 2.3 (2.9)* |
| % Smoke Cigarettes | 55 | 91*** |
| Mean (SD) # Cigarettes/Day | 7.9 (9.8) | 15.1 (10.9)*** |
| % Use Marijuana | 16 | 67*** |
| Mean (SD) # Joints/Day | 0.09 (0.1) | 0.06 (0.5)*** |
| % Use Other Drugs (excluding cocaine) | 1.3 | 38.1*** |
p < .05
p < .01
p < .001: Overall significance using t-test for continuous variables and Chi-square test for dichotomous variables.
3.2. Regression Analyses
Exposure to cocaine during the first trimester (defined as use/no use) predicted decreased weight and height at 7 years (Table 5). The average weight for children of first trimester users was 4.3 pounds smaller than that of children of women who were not cocaine users during the first trimester. The effect size for height was 0.63 inches. There were no effects of PCE on 7-year head circumference.
Table 5.
Significant Predictors of Growtha
| Raw Beta | Standardized Regression Coefficient | p value | |
|---|---|---|---|
| Weight (Total R2 = .16) | |||
| Child age at assessment | 7.72 | 0.29 | .00 |
| First trimester cocaine use (use vs. no use) | −4.31 | −0.17 | .00 |
| Number of siblings | −1.58 | −0.15 | .02 |
| Maternal height | .67 | 0.15 | .02 |
| Height (Total R2 = .36) | |||
| Child age at assessment | 2.56 | 0.51 | .00 |
| Raceb | −1.01 | −0.21 | .00 |
| Maternal height | .18 | 0.20 | .00 |
| Number of siblings | −0.25 | −0.12 | .02 |
| First trimester cocaine use (use vs. no use) | −0.63 | −0.11 | .02 |
| Head Circumferencec (Total R2 = .12) | |||
| Genderd | 8.24 | 0.28 | .00 |
| Maternal height | 0.99 | −0.19 | .00 |
| Current tobacco use | −0.19 | −0.13 | .05 |
Listed in order of standardized regression coefficient, an indication of the magnitude of the effect.
0=African American; 1=Caucasian
Measured in millimeters
0=female; 1=male
PCE was not a significant predictor of any of the cognitive or neuropsychological assessments, which included the SBIS, WRAML, WRAT, Grooved Pegboard, PFT, or CPT (data not shown). Significant predictors of better performance on these assessments were higher maternal IQ, Caucasian race, and higher home environment scores. First trimester marijuana use was significantly associated with poorer performance on the WRAML (beta = −7.52, p < .05).
Third trimester cocaine exposure (defined as use/no use) was a significant predictor of the CBCL total, externalizing, attention problems, and aggressive behavior scales (Table 6). The effect sizes were about 5 to 6 points. There were no significant relations between PCE and the CBCL internalizing, withdrawn, somatic, anxious/depressed, social, thought, or delinquent behavior scales. Increased maternal hostility, less social support, and younger maternal age were significant predictors of more maternal-rated behavior problems. Third trimester cocaine use was also a significant predictor of increased activity scores (Routh and SNAP), as well as of increased inattention, impulsivity, and problems with peers (SNAP), with effect sizes ranging from 1.7 to 4.7 points (Table 6).
Table 6.
| Raw Beta | Standardized Regression Coefficient | p value | |
|---|---|---|---|
| CHILD BEHAVIOR CHECKLISTc | |||
| Total (Total R2 = 0.31) | |||
| Hostilityd | 0.56 | 0.24 | .00 |
| Social support | −3.22 | −0.22 | .00 |
| Current tobacco use | 0.20 | 0.20 | .00 |
| Maternal age | −0.37 | −0.18 | .00 |
| Third trimester cocaine use (use vs. no use) | 5.12 | 0.15 | .00 |
| Internalizing (Total R2 = 0.26) | |||
| Depressionf | 0.26 | 0.25 | .00 |
| Hostility | 0.47 | 0.21 | .00 |
| Social support | −2.39 | −0.17 | .02 |
| Male in household | 2.95 | 0.15 | .02 |
| Maternal age | −0.26 | −0.13 | .03 |
| Externalizing (Total R2 = 0.25) | |||
| Maternal age | −0.46 | −0.23 | .00 |
| Hostility | 0.56 | 0.23 | .00 |
| Social support | −3.09 | −0.21 | .00 |
| Third trimester cocaine use (use vs. no use) | 6.42 | 0.20 | .00 |
| Current tobacco use | 0.15 | 0.14 | .02 |
| Attention Problems (Total R2 = 0.27) | |||
| Social support | −2.96 | −0.31 | .00 |
| Maternal age | −0.28 | −0.22 | .00 |
| Hostility | 0.27 | 0.18 | .00 |
| Second trimester (use vs. no use) | 2.84 | 0.12 | .05 |
| Third trimester (use vs. no use) | 4.88 | 0.23 | .00 |
| Current tobacco use | 0.08 | 0.13 | .03 |
| Aggressive Behavior (Total R2 = 0.26) | |||
| Social support | −2.46 | −0.26 | .00 |
| Maternal age | −0.34 | −0.25 | .00 |
| Current tobacco use | 0.15 | 0.22 | .00 |
| Third trimester cocaine use (use vs. no use) | 4.45 | 0.21 | .00 |
| Hostility | 0.18 | 0.12 | .05 |
| ROUTH ACTIVITY SCALE (Total R2 = 0.17) | |||
| Social support | −3.49 | −0.30 | .00 |
| Third trimester cocaine use (use vs. no use) | 4.73 | 0.18 | .00 |
| Maternal age | −0.26 | −0.17 | .01 |
| Hostility | 0.26 | 0.13 | .04 |
| SNAP SCALE | |||
| Activity (Total R2 = 0.20) | |||
| Genderg | 1.69 | 0.24 | .00 |
| Social support | −1.08 | −0.21 | .00 |
| Raceh | −1.28 | −0.18 | .00 |
| Third trimester cocaine use (use vs. no use) | 1.81 | 0.16 | .02 |
| Hostility | 0.10 | 0.12 | .05 |
| Inattention (Total R2 = 0.23) | |||
| Third trimester cocaine use (use vs. no use) | 1.98 | 0.21 | .00 |
| Maternal age | −0.11 | −0.19 | .00 |
| Social support | −0.76 | −0.18 | .00 |
| Current tobacco use | 0.05 | 0.18 | .00 |
| Home environmenti | −0.16 | −0.15 | .02 |
| Gender | 0.81 | 0.14 | .02 |
| Race | −0.79 | −0.14 | .03 |
| Impulsivity (Total R2 = 0.19) | |||
| Third trimester cocaine use (use vs. no use) | 1.65 | 0.16 | .01 |
| Gender | 1.00 | 0.16 | .02 |
| Social support | −0.73 | −0.16 | .02 |
| Home environment | −0.15 | −0.13 | .05 |
| Hostility | 0.10 | 0.13 | .05 |
| Peer Interactions (Total R2 = 0.25) | |||
| Current tobacco use | 0.07 | 0.19 | .00 |
| Hostility | 0.16 | 0.18 | .00 |
| Third trimester cocaine use (use vs. no use) | 2.00 | 0.17 | .00 |
| Gender | 1.14 | 0.15 | .01 |
| Social support | −0.79 | −0.15 | .03 |
| Race | −1.02 | −0.14 | .03 |
| Home environment | −0.17 | −0.13 | .05 |
| Maternal age | −0.09 | −0.12 | .05 |
Listed in order of standardized regression coefficient, an indication of the magnitude of the effect.
Each trimester was analyzed separately but all are presented together for convenience. In cases where more than one trimester was significant, the betas for the other variables were essentially the same across models. If more than one trimester was significant, the values presented are those for the third trimester model.
Achenbach [3]
Spielberger [74]
Frequent=≥ 1 line/day; not frequent=< 1 line/day
Radloff [56]
0=female; 1=male
0=African American; 1 = Caucasian
Baker & Mott [7]
First trimester cocaine exposure significantly predicted higher TRF total, withdrawn, thought problems, and aggressive behavior scores (Table 7). Third trimester cocaine exposure was a significant predictor of the TRF total, externalizing, anxious/depressed, social, thought, and aggressive behavior scales. The effect sizes for third trimester use ranged from 2.9 to 7.1 points. There were no significant relations between PCE and the TRF internalizing, somatic, attention, or delinquent behavior scales. Increased maternal hostility, younger maternal age, and lower SBIS composite scores were significant predictors of more teacher-rated behavior problems.
Table 7.
| Raw Beta | Standardized Regression Coefficient | p value | |
|---|---|---|---|
| TEACHER REPORT FORMc | |||
| Total (Total R2 = 0.20) | |||
| Stanford-Binet composite score | −0.20 | −0.29 | .00 |
| First trimester (frequentd vs. not frequent) | 4.14 | 0.15 | .03 |
| Third trimester (use vs. no use) | 7.06 | 0.20 | .00 |
| Hostilitye | 0.38 | 0.15 | .02 |
| Maternal age | −0.29 | −0.14 | .04 |
| Internalizing (Total R2 = 0.10) | |||
| Stanford-Binet composite score | −0.18 | −0.29 | .00 |
| Hostility | 0.37 | 0.17 | .02 |
| Externalizing (Total R2 = 0.16) | |||
| Home environmentf | −0.74 | −0.19 | .00 |
| Current marijuana use | 10.38 | 0.18 | .00 |
| Third trimester cocaine use (use vs. no use) | 5.70 | 0.16 | .03 |
| Raceg | −3.20 | −0.15 | .04 |
| Withdrawn (Total R2 = 0.07) | |||
| Stanford-Binet composite score | −0.09 | −0.21 | .00 |
| First trimester cocaine use (frequent vs. not frequent) | 2.27 | 0.14 | .04 |
| Anxious/Depressed (Total R2 = 0.09) | |||
| Stanford-Binet composite score | −0.07 | −0.18 | .00 |
| Third trimester (use vs. no use) | 2.92 | 0.16 | .03 |
| Hostility | 0.20 | 0.15 | .03 |
| Social Problems (Total R2 = 0.10) | |||
| Third trimester cocaine use (use vs. no use) | 4.39 | 0.20 | .00 |
| Stanford-Binet composite score | −0.06 | −0.13 | .05 |
| Thought Problems (Total R2 = 0.11) | |||
| First trimester (use vs. no use) | 1.41 | 0.17 | .02 |
| First trimester (frequent vs. not frequent) | 2.39 | 0.24 | .00 |
| Second trimester (use vs. no use) | 3.14 | 0.20 | .00 |
| Third trimester (use vs. no use) | 3.56 | 0.30 | .00 |
| Maternal age | −0.10 | −0.15 | .04 |
| Aggressive Behavior (Total R2 = 0.13) | |||
| Race | −2.98 | −0.18 | .02 |
| First trimester (frequent vs. not frequent) | 3.47 | 0.17 | .02 |
| Third trimester (use vs. no use) | 4.78 | 0.18 | .02 |
| Social support | −1.49 | −0.13 | .05 |
| Current marijuana use | 6.37 | 0.13 | .05 |
Listed in order of standardized regression coefficient, an indication of the magnitude of the effect.
Each trimester was analyzed separately but all are presented together for convenience. In cases where more than one trimester was significant, the betas for the other variables were essentially the same across models. If more than one trimester was significant, the values presented are those for the third trimester model.
Achenbach [4]
Frequent=≥ 1 line/day; not frequent=< 1 line/day
Spielberger [74]
Baker & Mott [7]
0=African American; 1 = Caucasian
3.3. Group Analysis
The group analysis for duration of cocaine use (never, stopped, continued) was limited to those outcomes for which PCE was a significant predictor in the regression analyses. As shown in Table 8, children who were exposed throughout pregnancy had significantly more mother- and teacher-rated behavior problems (CBCL externalizing, attention, aggressive; Routh activity; all 4 SNAP scales; TRF total, externalizing, anxious/depressed, social, thought, aggressive) compared to those who stopped and to those with no PCE. In addition, children who were exposed first trimester only had decreased height and weight compared to those with no PCE. These analyses were adjusted for the significant covariates from the regression analyses.
Table 8.
Child Characteristics Associated with Duration of Prenatal Cocaine Use
| Never Useda | Stoppedb | Continuedc | ||
|---|---|---|---|---|
| n = 130 | n = 60 | n = 27 | ||
| Adjusted Means | ||||
| Weight (lbs) | 62.4 | 56.9 | 58.2* | |
| Height (ins) | 49.6 | 48.9 | 49.4 | |
| CBCL: | Total | 51.7 | 50.6 | 54.8 |
| Externalizing | 51.8 | 51.6 | 57.0* | |
| Attention | 55.2 | 55.1 | 59.1** | |
| Aggressive | 54.9 | 54.4 | 58.6* | |
| Routh Activity Scale | 39.6 | 39.2 | 45.0** | |
| SNAP: | Activity | 10.2 | 10.0 | 12.4** |
| Attention | 8.9 | 8.9 | 11.2*** | |
| Impulsivity | 10.7 | 10.8 | 12.4* | |
| Peer Problems | 11.4 | 11.3 | 13.7** | |
| TRF: | Total | 51.8 | 52.4 | 59.0** |
| Externalizing | 51.8 | 53.3 | 60.7** | |
| Withdrawn | 54.9 | 55.0 | 56.7 | |
| Anxious/Dep | 53.9 | 53.2 | 57.6** | |
| Social Prob. | 54.6 | 54.7 | 58.7* | |
| Thought Prob. | 50.7 | 51.4 | 55.7*** | |
| Aggressive | 55.2 | 56.1 | 62.0** | |
Did not use cocaine during any trimester
Used cocaine first trimester only
Used cocaine both first and third trimesters
p < .05
p < .01
p < .001: Overall significance using F test for continuous variables and Chi-square test for dichotomous variables.
In order to address the issue of clinical significance, we looked at the percent of each group that was ≥ 60 on the TRF, the suggested clinical cutoff score [4]. For example, for TRF total behavior problems, 20% of the “never used” group was above the cutoff score. This is comparable to the 17% who were above the clinical range on TRF total behavior problems in Achenbach’s non-referred sample [4]. The percent of the “continued” group who scored above the TRF total behavior problems cutoff was 50% (significantly different from “never used” group; p < .006), which is similar to the 64% of Achenbach’s [4] referred sample who scored above the cutoff. We also compared our sample means to those reported by Achenbach [4]. For example, the TRF total and externalizing behavior problem means of the “never used” group (~52) (Table 8) are comparable to the TRF normative sample means (~50) [4]. The TRF total and externalizing behavior problem means of the “continued” group (~60) (Table 8) are comparable to the referred group means (~60 – 62) [4]. Thus, the level of behavior problems in the “continued” group is comparable to that in a clinically-referred sample.
3.4. Moderating Analyses
There was no significant interaction between first trimester cocaine use and maternal age, home environment, and child gender for weight, height, or head circumference. We also tested for interactions between third trimester cocaine use and maternal age, home environment, and child gender for the CBCL and TRF total, internalizing, and externalizing scales, Routh Activity Scale, and SNAP attention subscale. We found a significant interaction between third trimester cocaine use and maternal age on the TRF internalizing score (beta = 1.1, p < .01). Children of older women who used cocaine were rated by their teachers as having more internalizing behavior problems compared to those of younger women who used cocaine. For women who did not use cocaine, there was no difference between offspring of younger and older women. Of the 33 interactions that we tested, this was the only significant interaction between PCE and maternal age, home environment, or child gender.
3.5. Mediating Analyses
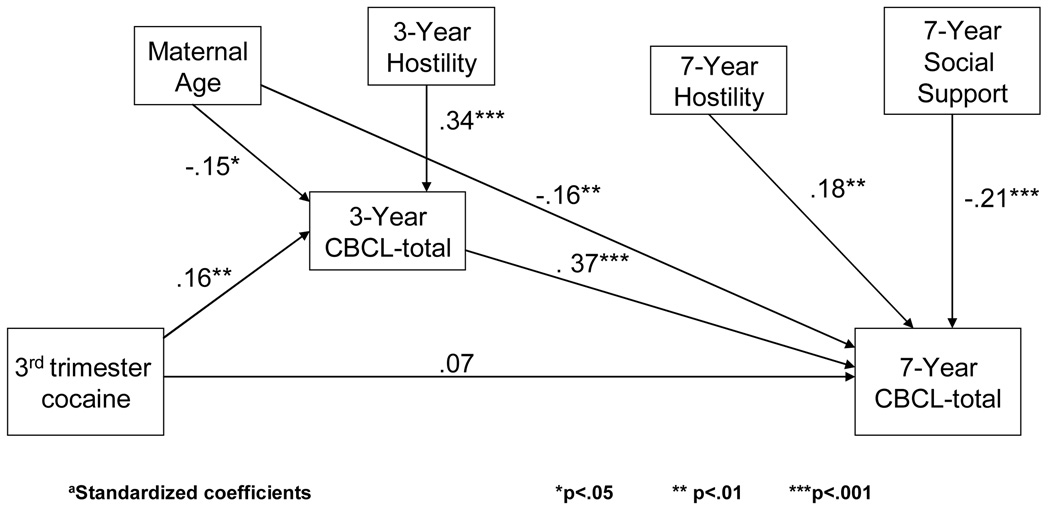
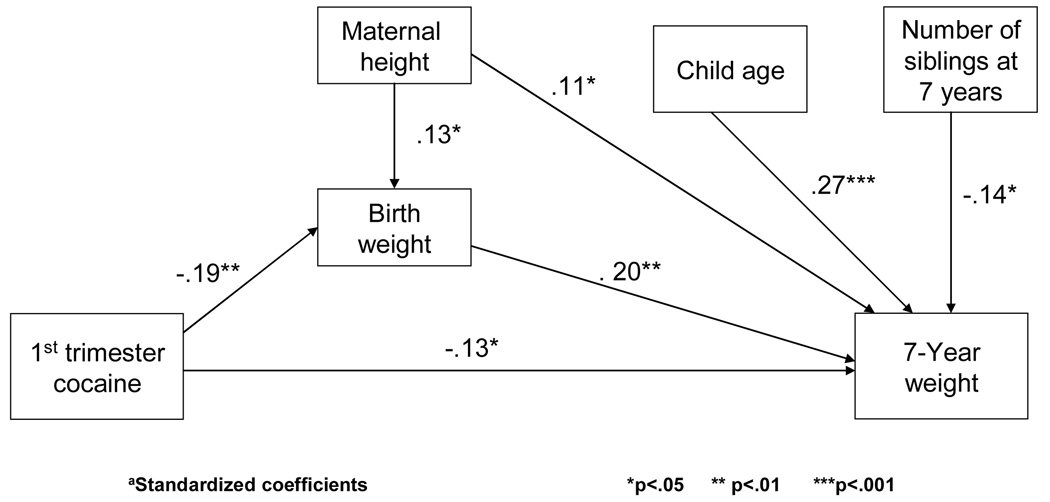
Because we found that PCE was a significant predictor of behavior problems at 3 years of age [62], we tested whether the changes at 3 years of age mediated the effects of PCE on behavior problems that were found at 7 years. Figure 1 shows that when 3-year CBCL total behavior problems were entered into the model, third trimester cocaine use was no longer a significant predictor of 7-year CBCL total behavior problems. The indirect effect measured as the product of coefficients was significant (Sobel z = 2.5, p < .01). That is, the effect of third trimester use on 7-year total behavior problems was due to the effect of third trimester use on 3-year total behavior problems. The other significant effects of PCE on 7-year behavior reported in Tables 6 and 7 were not mediated by 3-year behavior. In addition, the effect of PCE on 3-year temperament [62] did not mediate the effect of PCE on 7-year CBCL total behavior problems. We also tested whether the effect of PCE on birth weight [64] mediated the effect of PCE on 7-year weight and found that there is both an indirect effect of first trimester use and birth weight on 7-year weight (Sobel z = −2.2, p < .05) and a direct effect of first trimester exposure on 7-year weight (t = −2.2, p < .05) (Figure 2).
Figure 1.
CBCL-Total at 3 years mediates the relation between third trimester cocaine exposure and CBCL-Total at 7 yearsa
Figure 2.
Birth weight partially mediates the relation between first trimester cocaine exposure and weight at 7 yearsa
4. Discussion
This report investigated the relationship between PCE and growth, cognitive development, and behavior at 7 years of age. First trimester cocaine use predicted reduced 7-year weight and height. There was a direct effect between PCE and 7-year weight that was not solely due to the effects of PCE on birth weight [64]. Head circumference was not significantly predicted by PCE in these analyses, although we previously reported that PCE was a predictor of head circumference at 7 years [60]. However, that was a report using longitudinal modeling across four phases and had a different sample size than the current report, which likely accounts for the different findings. Most other research groups have not found effects of PCE on child growth [5,29,37,40,41,50]. However, most of those groups defined PCE as any use during pregnancy. We found that 7-year weight and height were both affected by first trimester use, which is consistent with the idea that symmetric growth retardation results from exposure early in pregnancy [10,79].
Prenatal cocaine use did not predict performance on the cognitive, learning and memory, achievement, or neuropsychological assessments. The lack of a significant association between PCE and global cognitive development is consistent with other reports [5,8,37,40,41,53], and is an indication that the effects of PCE on cognitive function may be subtle and therefore not detectable by global measures. We also did not find specific neuropsychological effects at this age. While there have been some reports of significant effects of PCE on neuropsychological measures, there is no one domain that is consistently affected. It may be that effects in these domains will not become evident until there is development of more advanced cognitive abilities, such as occurs in middle childhood [35,42].
Consistent with our hypothesis, we found associations between PCE and child behavior problems that were evident on both mother and teacher ratings. Third trimester use, which reflects use throughout pregnancy, was associated with significantly more externalizing behavior problems, such as aggressive behavior and attention problems, and with increased activity, inattention, and impulsivity, controlling for prenatal and current maternal substance use, sociodemographic and environmental factors, and child characteristics. Our findings of increased behavior problems are also consistent with other reports [6,26,40,54,73]. There were no interactions between PCE and gender or home environment, although there was an interaction between PCE and maternal age on the TRF internalizing scale. The effect of PCE on total behavior problems at age 7, as measured by the CBCL, was mediated by the effects of PCE on total behavior problems at 3 years, but the effects of PCE on CBCL externalizing behaviors and on TRF total and externalizing behaviors were direct.
At 3 years, we found that first trimester exposure was associated with more mother-rated externalizing behavior problems [62]. At 7 years, third trimester exposure, but not first, predicted the mother’s rating of externalizing problems. Thus, the affected domains remained the same across time, but the time of exposure differed with maturation. There could be several reasons for this: One, there could have been selective subject loss among the children who were affected and exposed only early in gestation. However, we have shown that there was no selective drop-out, so this possibility is not likely. Two, women who used cocaine throughout pregnancy may differ from women who did not on significant characteristics that we did not measure and thus were not able to control for. While we controlled for socioeconomic, psychological, and home environment characteristics, we did not have measures of exposure to violence or community characteristics at the 7-year assessment. We have added these measures to later follow-up phases. Three, children who were exposed in the first trimester only may have been able to compensate for the effects of exposure by age 7. This possibility, particularly in a population of offspring with low to moderate levels of exposure, is supported by literature on plasticity in development [75], and is likely the best explanation.
PCE affects fetal development through a number of mechanisms, including: 1) neurochemical changes in the dopamine, norepinephrine, and serotonin systems; 2) indirect vasoconstrictive effects; and 3) disruptions in fetal programming [44]. In animal models, there are effects of PCE on behaviors that are regulated by the dopamine and norepinephrine neurotransmitter systems, including cognitive abilities, motor performance, reward, mood, and stress reactivity [36]. In humans, PCE effects would be expected to occur in brain regions such as the prefrontal cortex that express dopamine receptors and receive dopaminergic projections from the midbrain [34]. Behaviorally, changes would be expected in domains that rely on the function of the dopamine systems in the brain such as attention, arousal, mood, state regulation, and executive functioning [18,19,22,27]. Our findings and the reports of others show that, during childhood, PCE affects the capacity to control behavior and regulate emotion, reflecting disturbances in the prefrontal cortex as well as other brain regions. The behavioral effects of PCE could be due to direct effects on neurotransmitter systems, consistent with the first mechanism, or they could be indirect effects mediated through changes in the HPA axis, consistent with the third mechanism [13,18,22,30,44].
A potential limitation of the study is that biological measures were not used to document drug use. While it is possible that some women who used drugs denied use and were misclassified, this would reduce the differences between groups and would not affect the significant findings. Moreover, biological screening fails to detect many cocaine users because of the short time period for detection [39,78]. Our interviews identified a higher percentage of users than did urine screening [64], a finding also reported by others [43,86]. Thus, detailed, confidential interviewing is an effective way to identify users and to characterize the quantity, timing, and pattern of use [58,65].
Another issue to consider is whether maternal ratings of behavior reflect the child’s behavior or the mother’s perception of the child’s behavior. Women who use cocaine might be more depressed than women who do not use cocaine and therefore they may perceive their child’s behavior differently. However, PCE and maternal depression were not related in our study or in others [47,68,71]. Maternal hostility was associated with child behavior, but when we controlled for maternal hostility, PCE was still a significant predictor of behavior. In addition, PCE was a significant predictor of teacher-rated behavior problems, a further indication that the reported behavior changes are not a reflection of the mother’s perception.
The strengths of this study include its prospective design, large number of subjects, good follow-up rates, and statistical control for confounding factors, including other drugs and current environmental influences on child development. We can assess the effects of light to moderate levels of PCE and of the timing and duration of exposure on development. Women enrolled in this study received prenatal care by their fourth or fifth month of pregnancy. They were interviewed at defined time points and at frequent intervals to minimize recall bias. We have also established that the instruments were reliable and valid [23,25,59]. This prenatal care sample represents the typical pattern of substance use in a general population of pregnant women and allows us to study the effects of exposure to cocaine early in pregnancy, as well as throughout pregnancy.
In conclusion, this is a unique study of women from a prenatal clinic who used low to moderate amounts of cocaine early in pregnancy. We have shown previously that PCE is a significant predictor of neurobehavioral and neurophysiological changes at birth [63,69], temperament at 1 year [61], and memory, temperament, and behavior at 3 years [62]. We have now documented increased mother- and teacher-rated behavior problems at 7 years. The extent of the teacher-rated problems in the continuously-exposed group was comparable to that in a clinical sample [4]. This continuing pattern of behavior problems associated with PCE, a reflection of behavioral dysregulation, is important because temperament, externalizing behaviors, and inattention are predictors of adolescent psychopathology, including substance use, delinquent behaviors, ADHD, anxiety, and depression [13,16,30–33,44,76,84]. The identification of this pattern will allow targeted early intervention with children who are vulnerable because of their prenatal cocaine exposure in order to disrupt this potential pathway to adolescent psychopathology.
Acknowledgements
We wish to thank Dr. Vincent Smeriglio for his years of support of our research endeavor, not only as our Program Officer, but as a colleague. He had a genuine interest in the research, was always helpful in working through responses to reviews, and provided valuable insight about the direction of the field. Here’s to a relaxing and fulfilling retirement!
This research was supported by the National Institute on Drug Abuse grants DA05460, DA06839, and DA08916 (G. Richardson, Principal Investigator).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Accornero V, Amado A, Morrow C, Xue L, Anthony J, Bandstra E. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J Dev Behav Pediatr. 2007;28:195–205. doi: 10.1097/01.DBP.0000268560.72580.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accornero V, Anthony J, Morrow C, Xue L, Bandstra E. Prenatal cocaine exposure: an examination of childhood externalizing and internalizing behavior problems at age 7 years. Epidemiologia E Psichiatria Sociale. 2006;15:20–29. [PMC free article] [PubMed] [Google Scholar]
- 3.Achenbach T. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 4.Achenbach T. Manual for the Teacher's Report Form and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 5.Arendt R, Short E, Singer L, Minnes S, Hewitt J, Flynn S, Carlson L, Min M, Klein N, Flannery D. Children prenatally exposed to cocaine: developmental outcomes and environmental risks at seven years of age. Dev Behav Pediatr. 2004;25:83–90. doi: 10.1097/00004703-200404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bada H, Das A, Bauer C, Shankaran S, Lester B, LaGasse L, Hammond J, Wright L, Higgins R. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:e348–e359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- 7.Baker P, Mott F. National Longitudinal Study of Youth Child Handbook, Center for Human Resource Research. Columbus, OH: Ohio State University; 1989. [Google Scholar]
- 8.Bandstra E, Morrow C, Vogel A, Fifer R, Ofir A, Dausa A, Xue L, Anthony J. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotoxicol Teratol. 2002;24:297–308. doi: 10.1016/s0892-0362(02)00192-7. [DOI] [PubMed] [Google Scholar]
- 9.Bandstra E, Vogel A, Morrow C, Xue L, Anthony J. Severity of prenatal cocaine exposure and child language functioning through age seven years: a longitudinal latent growth curve analysis. Subst Use Misuse. 2004;39:25–59. doi: 10.1081/JA-120027765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayer S, Altman J, Russo R, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 11.Beeghly M, Martin B, Rose-Jacobs R, Cabral H, Heeren T, Augustyn M, Bellinger D, Frank D. Prenatal cocaine exposure and children's language functioning at 6 and 9.5 years: moderating effects of child age, birth weight, and gender. J Pediatr Psychol. 2006;31:98–115. doi: 10.1093/jpepsy/jsj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett D, Bendersky M, Lewis M. Children's cognitive ability from 4 to 9 years old as a function of prenatal cocaine exposure, environmental risk, and maternal verbal intelligence. Dev Psychol. 2008;44:919–928. doi: 10.1037/0012-1649.44.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brady K, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- 14.Brooker B, Cyr J. Tables for clinicians to use to convert WAIS-R short forms. J Clin Psychol. 1986;42:982–986. [Google Scholar]
- 15.Brown J, Bakeman R, Coles C, Platzman K, Lynch M. Prenatal cocaine exposure: a comparison of 2-year-old children in parental and nonparental care. Child Dev. 2004;75:1282–1295. doi: 10.1111/j.1467-8624.2004.00739.x. [DOI] [PubMed] [Google Scholar]
- 16.Burke J, Loeber R, White H, Stouthamer-Loeber M, Pardini D. Inattention as a key predictor of tobacco use in adolescence. J Abnorm Psychol. 2007;116:249–259. doi: 10.1037/0021-843X.116.2.249. [DOI] [PubMed] [Google Scholar]
- 17.Buss A, Plomin R. In: Temperament: Early Developing Personality Traits. Erlbaum L, editor. Hillsdale, NJ: L. Erlbaum, Publishers; 1984. [Google Scholar]
- 18.Casey B, Castellano F, Giedd J, Marsh W, Hamburger S, Schubert A, Vauss Y, Vaituzis A, Dickstein D, Sarfatti S, Rapoport J. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Chiriboga C, Starr D, Kuhn L, Wasserman G. Prenatal cocaine exposure and prolonged focus attention. Poor infant information processing ability or precocious maturation of attentional systems? Dev Neurosci. 2009;31:149–158. doi: 10.1159/000207502. [DOI] [PubMed] [Google Scholar]
- 20.Cook R, Weisberg S. Residuals and Influence in Regression. New York: Chapman and Hall; 1982. [Google Scholar]
- 21.Covington C, Nordstrom-Klee B, Ager J, Sokol R, Delaney-Black V. Birth to age 7 growth of children prenatally exposed to drugs: a prospective cohort study. Neurotoxicol Teratol. 2002;24:489–496. doi: 10.1016/s0892-0362(02)00233-7. [DOI] [PubMed] [Google Scholar]
- 22.Dahl R. The regulation of sleep and arousal: Development and psychopathology. Dev Psychopathol. 1996;8:3–27. [Google Scholar]
- 23.Day N, Jasperse D, Richardson GA, Robles N, Sambamoorthi U, Taylor P, Scher M, Stoffer D, Cornelius M. Prenatal exposure to alcohol: effect on infant growth and morphologic characteristics. Pediatrics. 1989;84:536–541. [PubMed] [Google Scholar]
- 24.Day N, Robles N. Methodological issues in the measurement of substance use. Ann N Y Acad Sci. 1989;562:8–13. doi: 10.1111/j.1749-6632.1989.tb21002.x. [DOI] [PubMed] [Google Scholar]
- 25.Day N, Sambamoorthi U, Taylor P, Richardson GA, Robles N, Jhon Y, Scher M, Stoffer D, Cornelius M, Jasperse D. Prenatal marijuana use and neonatal outcome. Neurotoxicol Teratol. 1991;13:329–334. doi: 10.1016/0892-0362(91)90079-c. [DOI] [PubMed] [Google Scholar]
- 26.Delaney-Black V, Covington C, Nordstrom B, Ager J, Janisse J, Hannigan J, Chiodo L, Sokol R. Prenatal cocaine: quantity of exposure and gender moderation. Dev Behav Pediatr. 2004;25:254–263. doi: 10.1097/00004703-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Dev Psychol. 2006;42:688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott C. Differential Ability Scales. San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- 29.Eyler F, Warner T, Behnke M, Hou W, Wobie K, Garavan C. Executive functioning at ages 5 and 7 years in children with prenatal cocaine exposure. Dev Neurosci. 2009;31:121–136. doi: 10.1159/000207500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fumagelli F, Molteni R, Racagni G, Riva M. Stress during development: impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol. 2007;81:197–217. doi: 10.1016/j.pneurobio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Gau S, Chong M, Yang P, Yen C, Liang K, Cheng A. Psychiatric and psychosocial predictors of substance use disorders among adolescents. Br J Psychiatry. 2007;190:42–48. doi: 10.1192/bjp.bp.106.022871. [DOI] [PubMed] [Google Scholar]
- 32.Giancola P, Martin C, Tarter R, Pelham W, Moss H. Executive cognitive functioning and aggressive behavior in preadolescent boys at high risk for substance abuse/dependence. J Studies Alc. 1996;57:352–359. doi: 10.15288/jsa.1996.57.352. [DOI] [PubMed] [Google Scholar]
- 33.Glantz M, Chambers J. Prenatal drug exposure effects on subsequent vulnerability to drug abuse. Dev Psychopathol. 2006;18:893–922. doi: 10.1017/s0954579406060445. [DOI] [PubMed] [Google Scholar]
- 34.Goldman-Rakic P, Lidow M, Gallager D. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harold G, Hay D. Normal development in middle childhood. Psychiatry. 2005;4:3–5. [Google Scholar]
- 36.Harvey J. Cocaine effects on the developing brain: current status. Neurosci Biobehav Rev. 2004;27:751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Hurt H, Giannetta J, Brodsky N, Malmud E, Pelham T. Are there neurologic correlates of in utero cocaine exposure at age 6 years? J Pediatr. 2001;138:911–913. doi: 10.1067/mpd.2001.113709. [DOI] [PubMed] [Google Scholar]
- 38.Jöreskog K, Sörbom D. LISREL 8.5 for Windows [Computer software] Lincolnwood, IL: Scientific Software International, Inc; 2001. [Google Scholar]
- 39.Julien R. A Primer of Drug Action. Seventh Edition. New York: W.H. Freeman Company; 1995. [Google Scholar]
- 40.Kable J, Coles C, Lynch M, Platzman K. Physiological responses to social and cognitive challenges in 8-year olds with a history of prenatal cocaine exposure. Dev Psychobiol. 2008;50:251–265. doi: 10.1002/dev.20285. [DOI] [PubMed] [Google Scholar]
- 41.Kilbride H, Castor C, Fuger K. School-age outcome of children with prenatal cocaine exposure following early case management. Dev Behav Pediatri. 2006;27:181–187. doi: 10.1097/00004703-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Lerner R. Early Adolescence: Perspectives on Research, Policy, and Intervention. Hillsdale, NJ: L. Erlbaum Associates, Publishers; 1993. [Google Scholar]
- 43.Lester B, ElSohly M, Wright L, Smeriglio V, Verter J, Bauer C, Shankaran S, Bada H, Walls H, Huestis M, Finnegan L, Maza P. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 44.Lester B, Padbury J. Third pathophysiology of prenatal cocaine exposure. Dev Neurosci. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- 45.Levine T, Liu J, Das A, Lester B, LaGasse L, Shankaran S, Bada H, Bauer C, Higgins R. Effects of prenatal cocaine exposure on special education in school-aged children. Pediatrics. 2008;122:e83–e91. doi: 10.1542/peds.2007-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis B, Kirchner H, Short E, Minnes S, Weishampel P, Satayathum S, Singer L. Prenatal cocaine and tobacco effects on children's language trajectories. Pediatrics. 2007;120:e78–e85. doi: 10.1542/peds.2006-2563. [DOI] [PubMed] [Google Scholar]
- 47.Lewis M, Misra S, Johnson H, Rosen T. Neurological and developmental outcomes of prenatally cocaine-exposed offspring from 12 to 36 months. Am J Drug Alcohol Abuse. 2004;30:299–320. doi: 10.1081/ada-120037380. [DOI] [PubMed] [Google Scholar]
- 48.Linares T, Singer L, Kirchner L, Short E, Min M, Hussey P, Minnes S. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31:85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindgren S, Lyons D. Pediatric Assessment of Cognitive Efficiency (PACE) Iowa City: University of Iowa, Department of Pediatrics; 1984. [Google Scholar]
- 50.Lumeng J, Cabral H, Gannon K, Heeren T, Frank D. Pre-natal exposures to cocaine and alcohol and physical growth patterns to age 8 years. Neurotoxicol Teratol. 2007;29:446–457. doi: 10.1016/j.ntt.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayes L, Molfese D, Key A, Hunter N. Event-related potentials in cocaine-exposed children during a Stroop task. Neurotoxicol Teratol. 2005;27:797–813. doi: 10.1016/j.ntt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 52.Minnes S, Robin N, Alt A, Kirchner H, Satayathum S, Salbert B, Ellison L, Singer L. Dysmorphic and anthropometric outcomes in 6-year-old prenatally cocaine-exposed children. Neurotoxicol Teratol. 2006;28:28–38. doi: 10.1016/j.ntt.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrow C, Culbertson J, Accornero V, Xue L, Anthony J, Bandstra E. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30:905–931. doi: 10.1207/s15326942dn3003_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nordstrom-Bailey B, Sood B, Sokol R, Ager J, Janisse J, Hannigan J, Covington C, Delaney-Black V. Gender and alcohol moderate prenatal cocaine effects on teacher-report of child behavior. Neurotoxicol Teratol. 2005;27:181–189. doi: 10.1016/j.ntt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Pelham W, Bender M. Peer relationships in hyperactive children: description and treatment. Adv in Learning Behav Disabilities. 1982;1:365–436. [Google Scholar]
- 56.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 57.Reitan R. Progressive Figures Test. Tucson, AZ: Reitan Neuropsychology Laboratory; 1979. [Google Scholar]
- 58.Richardson GA, Day N, McGauhey P. The impact of prenatal marijuana and cocaine use on the infant and child. Clin Obstet Gynecol. 1993;36:302–318. doi: 10.1097/00003081-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 59.Richardson GA, Day N, Taylor P. The effect of prenatal alcohol, marijuana, and tobacco exposure on neonatal behavior. Infant Behav Dev. 1989;12:199–209. [Google Scholar]
- 60.Richardson GA, Goldschmidt L, Larkby C. Effects of prenatal cocaine exposure on growth: a longitudinal analysis. Pediatrics. 2007;120:1017–1027. doi: 10.1542/peds.2006-3482. [DOI] [PubMed] [Google Scholar]
- 61.Richardson GA, Goldschmidt L, Willford J. The effects of prenatal cocaine use on infant development. Neurotoxicol Teratol. 2008;30:96–106. doi: 10.1016/j.ntt.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richardson GA, Goldschmidt L, Willford J. Continued effects of prenatal cocaine use: preschool development. Neurotoxicol Teratol. 2009;31:325–333. doi: 10.1016/j.ntt.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richardson GA, Hamel S, Goldschmidt L, Day N. Effects of prenatal cocaine use on neonatal neurobehavioral status. Neurotoxicol Teratol. 1996;18:519–528. doi: 10.1016/0892-0362(96)00062-1. [DOI] [PubMed] [Google Scholar]
- 64.Richardson GA, Hamel S, Goldschmidt L, Day N. Growth of infants prenatally exposed to cocaine/crack: a comparison of a prenatal care and a no prenatal care sample. Pediatrics. 1999;104:e18. doi: 10.1542/peds.104.2.e18. [DOI] [PubMed] [Google Scholar]
- 65.Richardson GA, Huestis M, Day N. Assessing in utero exposure to cannabis and cocaine. In: Bellinger DC, editor. Human Developmental Neurotoxicology. New York: Taylor & Francis Group; 2006. pp. 287–302. [Google Scholar]
- 66.Rourke B, Yanni D, MacDonald G, Young G. Neuropsychological significance of lateralized deficits on the Grooved Pegboard Test for older children with learning disabilities. J Consult Clin Psychol. 1973;41:128–134. doi: 10.1037/h0035613. [DOI] [PubMed] [Google Scholar]
- 67.Routh D, Schroeder C, O'Tuama L. Development of activity level in children. Dev Psychol. 1974;10:163–168. [Google Scholar]
- 68.Salisbury A, Lester B, Seifer R, LaGasse L, Bauer C, Shankaran S, Bada H, Wright L, Liu J, Poole K. Prenatal cocaine use and maternal depression: effects on infant neurobehavior. Neurotoxicol Teratol. 2007;29:331–340. doi: 10.1016/j.ntt.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scher M, Richardson GA, Day N. Effects of prenatal cocaine/crack exposure on EEG-sleep studies at birth and one year. Pediatrics. 2000;105:39–48. doi: 10.1542/peds.105.1.39. [DOI] [PubMed] [Google Scholar]
- 70.Sheslow D, Adams W. Wide Range Assessment of Memory and Learning. Wilmington, DE: Jastak Associates; 1990. [Google Scholar]
- 71.Singer L, Arendt R, Farkas K, Minnes S, Huang J, Yamashita T. Relationship of prenatal cocaine exposure and maternal postpartum psychological distress to child developmental outcome. Dev Psychopathol. 1997;9:473–489. doi: 10.1017/s0954579497001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sobel M. Direct and indirect effects in linear structural equation models. Sociological Methods & Research. 1987;16:155–176. [Google Scholar]
- 73.Sood B, Nordstrom-Bailey B, Covington C, Sokol R, Ager J, Janisse J, Hannigan J, Delaney-Black V. Gender and alcohol moderate caregiver reported child behavior after prenatal cocaine. Neurotoxicol Teratol. 2005;27:191–201. doi: 10.1016/j.ntt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 74.Spielberger C, Gorsuch R, Lushene R. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc.; 1970. [Google Scholar]
- 75.Stiles J. Neural plasticity and cognitive development. Dev Neuropsychol. 2000;18:237–272. doi: 10.1207/S15326942DN1802_5. [DOI] [PubMed] [Google Scholar]
- 76.Tarter R, Kirisci L, Mezzich A, Cornelius J, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- 77.Thorndike R, Hagen E, Sattler J. The Stanford-Binet Intelligence Scale. Fourth Edition. Chicago, IL: Riverside Publishing Company; 1986. [Google Scholar]
- 78.Verebey K. Cocaine abuse detection by laboratory methods. In: Washton AM, Gold MS, editors. Cocaine - A Clinician's Handbook. New York: Guilford Press; 1987. pp. 214–228. [Google Scholar]
- 79.Villar J, Belizan J. The timing factor in the pathophysiology of the intrauterine growth retardation syndrome. Obstet Gynecol Surv. 1982;37:499–506. doi: 10.1097/00006254-198208000-00001. [DOI] [PubMed] [Google Scholar]
- 80.Wechsler D. Wechsler Adult Intelligence Scale - Revised. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- 81.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Revised. Psychological Corporation: San Antonio, TX; 1989. [Google Scholar]
- 82.Wechsler D. Wechsler Intelligence Scale for Children – Third Edition. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 83.Wilkinson G. The Wide Range Achievement Test Administration Manual. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- 84.Wilson J, Levin F. Attention deficit hyperactivity disorder (ADHD) and substance use disorders. Curr Psychiatry Rep. 2001;3:497–506. doi: 10.1007/s11920-001-0044-8. [DOI] [PubMed] [Google Scholar]
- 85.Yumoto C, Jacobson S, Jacobson J. Fetal substance exposure and cumulative environmental risk in an African American cohort. Child Dev. 2008;79:1761–1776. doi: 10.1111/j.1467-8624.2008.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zuckerman B, Frank D, Hingson R, Amaro H, Levenson S, Kayne H, Parker S, Vinci R, Aboagye K, Fried L, Cabral H, Timperi R, Bauchner H. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med. 1989;320:762–768. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]