Abstract
Venezuelan equine encephalitis virus replicon particles (VRP) without a transgene (null VRP) have been used to adjuvant effective humoral [1], cellular [2], and mucosal [3] immune responses in mice. To assess the adjuvant activity of null VRP in the context of a licensed inactivated influenza virus vaccine, rhesus monkeys were immunized with Fluzone® alone or Fluzone® mixed with null VRP and then challenged with a human seasonal influenza isolate, A/Memphis/7/2001 (H1N1). Compared to Fluzone® alone, Fluzone®+null VRP immunized animals had stronger influenza-specific CD4+ T cell responses (4.4 fold) with significantly higher levels of virus-specific IFN-γ (7.6 fold) and IL-2 (5.3 fold) producing CD4+ T cells. Fluzone®+null VRP immunized animals also had significantly higher plasma anti-influenza IgG (p<0.0001, 1.3 log) and IgA (p<0.05, 1.2 log) levels. In fact, the mean plasma anti-influenza IgG titers after one Fluzone®+null VRP immunization was 1.2 log greater (p<0.04) than after two immunizations with Fluzone® alone. After virus challenge, only Fluzone®+null VRP immunized monkeys had a significantly lower level of viral replication (p<0.001) relative to the unimmunized control animals. Although little anti-influenza antibody was detected in the respiratory secretions after immunization, strong anamnestic anti-influenza IgG and IgA responses were present in secretions of the Fluzone®+null VRP immunized monkeys immediately after challenge. There were significant inverse correlations between influenza RNA levels in tracheal lavages and plasma anti-influenza HI and IgG anti-influenza antibody titers prior to challenge. These results demonstrate that null VRP dramatically improve both the immunogenicity and protection elicited by a licensed inactivated influenza vaccine.
Keywords: influenza vaccine, viral adjuvants, animal model, antibody and cellular immunity
1. Introduction
Seasonal influenza A virus infection is a highly contagious, acute respiratory tract disease of humans that causes substantial morbidity and mortality, particularly among the young, old, and immunocompromised [4]. Since the 1940s, inactivated or “split product” influenza vaccines have been developed for the control of seasonal influenza. Despite the relative success of these vaccines, the relentless antigenic drift of seasonal influenza A viruses requires annual reformulation of the vaccines, a lengthy and costly process that is not always successful and that can lead to critical shortages of vaccine [5]. The current inactivated seasonal influenza A virus vaccines contain 7.5 μg of hemagglutinin (HA) purified from each of 2 influenza A viruses (H1N1 and H3N2). If the amount of antigen per dose could be reduced, and the immunogenicity maintained or improved through the use of adjuvants then the limited supplies of vaccine could be extended to more individuals. Similarly, adjuvants could also improve the efficacy of vaccines against pandemic influenza, extending limited supplies and perhaps reducing vaccine cost.
The inactivated influenza vaccines protect 60%–90% of children and adults <65 years of age and 50%–60% of older adults from laboratory-confirmed influenza illness [6]. While this level of protection provides a significant public health and personal benefit, there is considerable opportunity to improve the efficacy of the vaccines through both qualitative and quantitative improvement in induced immune responses. Addition of an adjuvant to the vaccine could provide these benefits [7]. A number of adjuvants have been tested in preclinical studies of influenza vaccines and variable levels of improved immunogenicity and effectiveness have been reported [8–12].
Because a safe and effective adjuvant could significantly improve influenza vaccination through both dose sparing and increased immunogenicity, a new generation of adjuvants is being developed. These adjuvants are designed to stimulate various aspects of the innate immune response, because a strong innate response is thought to initiate a strong adaptive immune response. Virus infection is a highly efficient means of activating innate immunity, and vertebrate organisms have evolved over millions of years to detect and respond to virus infection. Adaptive immune responses to acute virus infection are typically strong, include T and B cell responses, and are long lasting. Therefore, we explored the use of a modified replication deficient virus to induce innate responses and improve the immunogenicity and efficacy of a commercial seasonal influenza A virus vaccine.
Alphaviruses are small RNA viruses that have been modified as vaccine expression vectors [13]. The complete alphavirus positive sense RNA genome is approximately 11.5 kb in length with the 5′ 2/3 of the genome comprising the replicase cassette. The three structural proteins of the virus, the capsid and two glycoproteins, are encoded in the 3′ 1/3 of the genome and are translated from a subgenomic mRNA transcribed from a subgenomic promoter in infected cells. In the context of an expression or vaccine vector, the structural proteins are replaced by a transgene, and the resulting replicon RNA is packaged into a replicon particle by transcomplementation with the structural protein genes. After intradermal immunization Venezuelan equine encephalitis virus (VEE) replicon particles (VRP) infect dendritic cells that travel to the draining lymph nodes of mice [14] and macaques (West et al., unpublished results). VRP preparations expressing HIV (Chulay JD et al., unpublished results), influenza [15] or human cytomegalovirus [16] immunogens have been utilized safely in phase I vaccine trials in humans.
Immunity to human influenza viruses is often studied in mice and ferrets. Human influenza viruses normally replicate efficiently in mice only after adaptation [17] but ferrets are highly susceptible to infection with human influenza viruses and appear to better recapitulate human innate immunity, disease severity and transmissibility than mice [18–20]. Guinea pigs are also susceptible to human influenza infection and they have been used to study human influenza A virus transmission [21]. Nonhuman primate models are less often used in influenza research but they are commonly employed in AIDS research and are excellent models of the human immune and respiratory systems due to their relatively close phylogenetic relationship with people. Human seasonal influenza A viruses infect and replicate in the respiratory tract of macaques causing either asymptomatic or mild clinical infections [22, 23]. However, the pandemic avian H5N1 [24] and 1918 H1N1 viruses [25] cause acute respiratory distress syndrome in macaques that is very similar to humans.
We engineered modified VRP, designated null VRP, which do not express the viral structural proteins or any heterologous transgene. As with VRP, these null VRP particles infect cells and program intracellular RNA replication, but they are incapable of propagating progeny virions. Immunization with a simple aqueous mixture of antigen and null VRP demonstrated strong systemic adjuvant activity compared with antigen alone [1, 26]. Null VRP also have the unusual property of inducing mucosal responses in mice even when delivery of the adjuvant and immunogen are by systemic routes of immunization [1, 3]. The goal of this study was to determine if null VRP could function as a systemic and mucosal adjuvant in rhesus macaques and to determine whether null VRP could improve the effectiveness of Fluzone® in the rhesus macaque model of human influenza A virus infection.
2. Materials and Methods
2.1. Animals
All animals used in this study were adult rhesus macaques (Macaca mulatta) that were housed at the California National Primate Research Center (Davis, CA) in accordance with the regulations of the Association for Assessment and Accreditation of Laboratory Animal Care International standards. The Institutional Animal Use and Care Committee of the University of California, Davis, approved these experiments. Animals with pre-challenge hemagglutinin inhibition (HI) titers to A/Memphis/7/2001 greater than 1/8 were excluded from the study. The heart rate, temperature, body weight, and respiratory rate of the animals were regularly monitored. For blood collection, animals were anesthetized with ketamine hydrochloride (10 mg/kg body weight; Parke-Davis) or 0.7-mg/kg tiletamine HCl and zolazepam (Telazol; Fort Dodge Animal Health) injected i.m. For virus inoculation and respiratory secretion sample collection animals were additionally anesthetized with 15–30 μg/kg medetomadine HCl (Domitor; Orion Pharma) injected i.m and anesthesia was reversed with 0.07–0.15 mg/kg atipamezole HCl (Antisedan; Pfizer Animal Health) injected i.m.
2.2. Virus Stock and Strains
The human influenza A virus isolate, A/Memphis/7/2001 (H1N1), used for all animal inoculations in this study has been previously described [22]. Briefly, this isolate was expanded on Madin-Darby Canine Kidney cells (MDCK [American Type Culture Collection]) to produce the virus stock used for animal inoculations. The virus stock has a titer of 106.5 50% tissue culture infectious dose (TCID50) on MDCK cells by the method of Reed and Muench to estimate endpoints [27]. The 2006–2007 trivalent inactivated pediatric influenza vaccine (Fluzone®, Sanofi-Pasteur Inc, Swiftwater PA) used in these studies was generated using three reference strains: A/New Caledonia/20/99(H1N1)-like, A/Wisconsin/67/05(H3N2)-like, and B/Malaysia/2506/2004-like. Using AlignX in Vector NTI Advance 11.0 (Invitrogen Life Technologies), we determined that the amino acid sequences of all 8 genome segments of the A/Memphis/7/01 challenge strain (NCBI Taxon ID 416736) have greater than 98% homology to the A/New Caledonia/20/99 vaccine strain (NCBI Taxon ID 381512).
2.3. VEE Replicon Particles (VRP)
The VRP genomes (replicon RNA) used in these studies consisted of the 5′ untranslated region of VEE, the non-structural protein cassette (nonstructural protein genes 1–4), the 26S promoter, a multiple cloning site, the 3′ untranslated region, and a poly A tail. The null VRP utilized in this study were prepared and packaged as previously described [2]. Briefly, in-vitro-transcribed replicon RNA, along with two defective helper RNAs, which express the viral structural genes in trans, were co-electroporated into BHK-21 cells. Only the replicon RNAs were packaged into particles as the viral-specific packaging signal is absent from the helper RNAs. The replicon used in this study lacks a functional transgene downstream of the 26S promoter creating a null VEE replicon particle with adjuvant properties. All replicon particles were packaged in the wild-type VEE (V3000) envelope. Null VRP were quantitated by diluting the stock and infecting BHK cells on titer slides. Following incubation at 37°C to establish infection, the cells were stained with antibody to one of the replicase proteins (NSP2), and fluorescent cells were counted by microscopy. Null VRP were diluted in PBS to a concentration of 2.57×107 infectious units per 100 μl.
2.4. Animal immunization and inoculation
Eighteen animals were assigned to 3 experimental groups (groups A–C) taking into account age and gender (Table 1). At weeks 0 and 4 group A was immunized with Fluzone® alone and group B was immunized with Fluzone® mixed with null VRP. All intradermal immunizations were in the upper arm and consisted of a total of 22.5ug HA of the 2006–2007 inactivated pediatric influenza virus vaccine (Fluzone®, Sanofi-Pasteur Inc, Swiftwater PA) added to either 0.25 ml PBS alone (group A) or 1×107 null VRP in 0.25 ml PBS (group B). The final vaccine for both groups contained 22.55 μg of total HA in 0.5 ml. Tracheal washes, nasopharyngeal washes, and blood samples were collected on weeks 0, 2, 4, 6, 8, and 12 post immunization (PI). Twelve weeks after the second immunization (day 0 post challenge [PC]) all animals were challenged with the A/Memphis/7/01 virus stock. The inoculum consisted of 1 ml virus stock instilled into the trachea, 1 ml of virus stock dripped intranasally, and a drop of virus stock onto each conjunctiva. Pulmonary and nasopharyngeal secretions were collected on days −6, −4, 1, 2, 3, 7, and 14 days PC from all animals. To collect secretions from the lower respiratory tract, an 8-french pediatric feeding tube (Kendall) was inserted into the trachea with the aid of a laryngoscope, with the tip of the tube placed just cranial to the carina. Twelve milliliters of sterile PBS were instilled into the trachea and a MadaVac aspirator pump (Henry Schein) was used to aspirate the maximum volume of sample. To collect upper respiratory secretions a 5-french pediatric feeding tube was inserted into each nostril and 2 ml PBS was instilled and aspirated with a syringe to recover the maximum volume of sample. The mucosal samples were stored on ice prior to processing (below). Blood samples were collected on days −6, −4, 0, 1, 2, 3, 7, 14, and 28 PC from each animal.
Table 1.
Effect of null VRP on plasma antibody responses and influenza A virus replication in the lower respiratory tract
| Animal Number | Age (years) | Sex | vRNA in tracheal lavage |
HI Titers Post Vaccination |
HI Titers Post Challenge |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak vRNA(log10 copies/ml) | Peak Day of vRNA | Pre | Week 2 | Week 6 | fold increasec | Day 0 | Day 28 | fold increased | |||
| Fluzone®e | |||||||||||
| 34357 | 5.58 | F | 5.5 | 3 | 4 | 4 | 256 | 64 | 64 | 512 | 8 |
| 34615 | 5.00 | F | 5.5 | 3 | 4 | 4 | 16 | 4 | 32 | 256 | 8 |
| 34999 | 4.75 | F | 6.0 | 1 | 4 | 32 | 32 | 8 | 64 | 256 | 4 |
| 35686 | 3.83 | F | 6.5 | 1 | 4 | 4 | 32 | 8 | 64 | 256 | 4 |
| 36378 | 2.83 | F | 5.8 | 1 | 4 | 4 | 16 | 4 | 32 | 64 | 2 |
| 36871 | 2.67 | M | 5.6 | 1 | 4 | 4 | 32 | 8 | 64 | 64 | 1 |
| Meang | 4.11 | - | 5.82a | 2 | 4 | 6b | 36b | 9b | 51 | 181 | 4 |
| Fluzone® + null VRPf | |||||||||||
| 34313 | 5,58 | M | 5.2 | 1 | 4 | 64 | 512 | 128 | 128 | 1024 | 8 |
| 34501 | 5.33 | F | 5.2 | 3 | 4 | 16 | 256 | 64 | 64 | 512 | 8 |
| 34993 | 4.75 | F | 5.1 | 1 | 4 | 32 | 2048 | 512 | 256 | 512 | 2 |
| 35366 | 3.92 | F | 5.1 | 1 | 4 | 32 | 2048 | 512 | 128 | 512 | 4 |
| 36223 | 2.92 | F | 5.3 | 1 | 4 | 128 | 1024 | 256 | 64 | 512 | 8 |
| 36529 | 2.75 | F | 4.9 | 1 | 4 | 4 | 512 | 128 | 128 | 256 | 2 |
| Meang | 4.21 | - | 5.13a | 1 | 4 | 29b | 813b | 203b | 114 | 512 | 4 |
| Unimmunized | |||||||||||
| 35360 | 4.83 | M | 6.0 | 1 | - | - | - | - | 4 | 64 | 16 |
| 36795 | 3.58 | F | 6.2 | 1 | - | - | - | - | 4 | 1024 | 256 |
| 35235 | 5.50 | F | 6.3 | 1 | - | - | - | - | 4 | 256 | 64 |
| 36179 | 3.83 | F | 5.5 | 3 | - | - | - | - | 4 | 256 | 64 |
| 35808 | 4.67 | F | 5.3 | 1 | - | - | - | - | 4 | 256 | 64 |
| 36714 | 3.67 | F | 5.4 | 1 | - | - | - | - | 4 | 128 | 32 |
| Meang | 4.35 | - | 5.78a | 1 | - | - | - | 4 | 228 | 57 | |
Mean value of Fluzone®+null VRP-immunized group are significantly lower than either Fluzone®-immunized group or naïve control group (p<0.05;ANOVATukey)
Mean value of Fluzone®+null VRP-immunized group are significantly higher than Fluzone®-immunized group (p<0.01; T test)
Titer at week 6 PI relative to the pre PV titer
Titer at day 28 PC relative to the day of challenge titer
Two immunizations of 22.5ug HA of the 2006–2007 inactivated pediatric Fluzone®
Two immunizations of 22.5ug HA of the 2006–2007 inactivated pediatric Fluzone® mixed with 1×107 iu null VRP
value is the anti-log of the geometric mean titer
2.5. Tracheal lavage sample processing
The tracheal lavage samples were processed as previously described [22]. Briefly, samples were diluted with a solution (51 μl/ml sample) containing 0.3% BSA, 20x antibiotic-antimycotic solution, and 1 mg/ml gentamicin sulfate. The treated samples were spun at 1,000 g for 10 min, and all but 1 ml of the supernatant was removed and stored at −80°C. The frozen aliquots were subsequently used to determine infectious virus titer. The 1 ml aliquot of fresh supernatant was immediately processed for RNA isolation to assess viral RNA (vRNA) levels.
2.6. Influenza virus RNA PCR
To determine the amount of virion-associated RNA in respiratory secretions, fresh tracheal lavages were processed and quantified by RT-PCR as previously described [22]. Briefly, 1 ml of fresh tracheal lavage was centrifuged at 1,000 × g for 10 min. The supernatant was removed and lysed in TRIzol LS (Invitrogen Life Technologies). cDNA was prepared using random hexamer primers (Amersham Biosciences) and SuperScript III reverse transcriptase (Invitrogen Life Technologies). The Influenza A virus matrix gene in the samples was quantified using a real-time RT-PCR assay on a Prism 7900 sequence detection system (Applied Biosystems) and previously described influenza A virus matrix gene-specific PCR primers [28]. The influenza A matrix copy numbers were determined by a modification of a method previously described [29]. Briefly, the copy number of matrix gene was determined by interpolation of the average measured threshold cycle number onto a standard curve produced with a purified plasmid containing a fragment of the M1 gene cloned from the A/Memphis/7/2001 stock. Quantification of the purified plasmid was based on A260 measurements.
2.7. Influenza Antibody ELISA
Titers of anti-influenza antibodies were determined by a modification of a method previously described [22, 30]. Briefly, all plasma, tracheal aspirate, and nasopharyngeal secretion samples were initially tested for anti-A/New Caledonia/20/99 in a screening assay. The screening dilutions for IgG and IgA in plasma were 1:800 and 1:80, respectively, and all secretion screening dilutions were 1:4 for both IgG and IgA. Results of the screening assay were calculated from optical density absorbance units (OD) using the following ratio: change in OD (ΔOD)/cutoff, where ΔOD is defined as the difference between the mean OD of a diluted sample tested in two influenza Ag-coated wells and the mean OD of the same diluted sample tested in two uncoated wells. The cutoff value is the mean ΔOD of two pre-treatment time points of a sample plus 3 SD values. If only one pre-treatment time point was available, the cutoff value is the mean ΔOD of one pre-infection time point run in two independent assays plus 3 SD values. If the ΔOD/cutoff ratio for a sample was >1.0 and the ΔOD>0.10, the sample was considered to be positive and the titer of anti-influenza antibody was determined. To determine anti-influenza A antibody titers in plasma or secretion samples that were positive in the screening assay serial doubling dilutions of the samples were loaded onto a 96-well plate (Nunc-Immuno Maxisorp plate II) with uncoated wells and wells coated with detergent disrupted A/New Caledonia/20/99 influenza A (Biodesign International). Antibody binding was detected with a peroxidase-conjugated goat anti-monkey IgG (Fc) or IgA (Fc) (Accurate Chemicals). For each sample, the endpoint titer of anti-influenza A antibody was defined as the last dilution giving a ΔOD value >0.10.
2.8. HI assay
Titers of anti-H1 antibodies were calculated using the revised World Health Organization HI test of hemagglutinin inhibition as previously described [22, 31]. The viral antigen used in the HI was the A/Memphis/7/2001 stock grown in 10-day-old embroynated chicken eggs (Charles River).
2.9. Intracellular cytokine staining for assessing influenza-specific T cell responses
For intracellular staining to detect influenza-specific T cells in PBMCs, modifications of previously reported methods were used [22, 32, 33]. Cryopreserved cells collected prior to challenge were stimulated with pediatric Fluzone® at 5μg H1/ml.
Data were acquired using a FACSAria flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star) and a Mac Pro computer (Apple). At least 100,000 events in the forward/side scatter lymphocyte gate were acquired. The background level of cytokine staining varied from sample to sample but was typically <0.05% of the unstimulated CD8+ T lymphocytes. The only samples considered positive were those in which, after subtracting the background (media only) control, there were at least five positive events for a single functional marker, three positive events for two or more simultaneous functional markers, and the sum of the different combinations of responses represented at least 10 events. In addition, a sample was not considered positive for a particular combination of functions if the frequency of responding T cells responding with that particular combination of functions was lower than 0.02%. If T cell responses were greater than both the absolute count and relative frequency cutoff, then SPICE v5.1024 and PESTLE v1.6.2 software programs (a gift from M. Roederer, Vaccine Research Center, NIAID/NIH) were used to create pie charts that represent the mean group response after immunization. Additionally, scatter plots depict the number of influenza-specific T cells per ml blood in each animal, where non-responding animals were assigned a number equal to 0.01% of total CD4+ or CD8+ T cells.
2.10. Statistical analysis
Statistics are reported as the mean and the standard error of the mean for each group using Prism 5.0a software (GraphPad Software, San Diego, CA), and data are presented as the probability and test used for analysis. Two groups were compared with a one-tailed unpaired T test (T test) and 3 groups were compared with a one-way ANOVA with a Tukey-Kramer post-hoc test (ANOVA-Tukey). Based on previous data [1], we predicted that Fluzone®+null VRP-immunized animals would have higher antibody titers and lower viral titers compared to the Fluzone®-immunized and non-immunized monkeys, respectively.
3. Results
3.1. Null VRP enhance CD4+ T cell but not CD8+ T cell responses after Fluzone® immunization
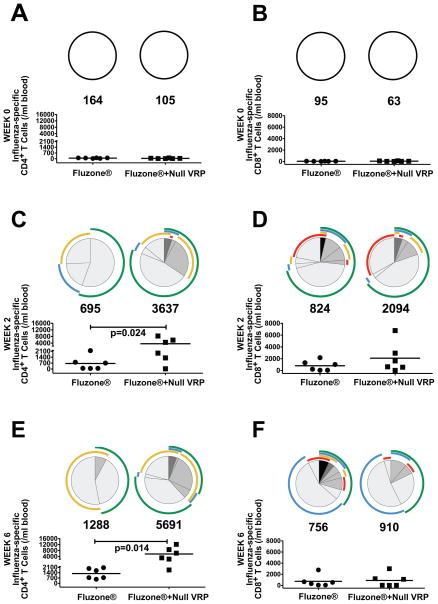
On the day of vaccination, influenza-specific CD4+ or CD8+ T cell responses were below cutoff in all animals (Fig. 1A and 1B). Two weeks post immunization (PI), Influenza-specific CD4+ T cell responses were found in 50% of Fluzone®-immunized animals (3/6) and 83% of Fluzone®+null VRP-immunized animals (5/6), (Fig. 1C). In addition to the larger proportion of responders, the mean number of influenza-specific CD4+ T cell in Fluzone®+null VRP-immunized animals was 5.2 fold higher than in the Fluzone® animals (695 vs. 3637 influenza-specific CD4+ T cells/ml lymphocytes; p=0.024, T test). The number of functions expressed by the influenza-specific CD4+ T cells in Fluzone®+null VRP animals was higher compared to the Fluzone®-immunized animals (Fig. 1C). Further, at week two PI, the Fluzone®+null VRP-immunized monkeys had significantly higher mean numbers of IL-2 (9.6-fold, p<0.02; T test) and TNF (3.6-fold, p<0.05; T test) secreting influenza-specific CD4+ cells when compared to Fluzone®-immunized monkeys. By six weeks PI (2 weeks post boost), 100% of Fluzone®- and Fluzone®+null VRP-immunized animals had influenza-specific CD4+ T cell responses. However, Fluzone®+null VRP-immunized animals had a significantly higher (4.4-fold) mean number of Fluzone®-specific CD4+ T cells than the Fluzone® animals (1288 vs. 5691 CD4+ T cells/ml lymphocytes; p=0.014, T test). The number of cell functions was also higher in the influenza-specific CD4+ T cells from Fluzone®+null VRP animals compared to Fluzone®-immunized animals (Fig. 1E). Further at week 6 PI the Fluzone®+null VRP-immunized monkeys had significantly higher mean numbers of IFN-γ (7.6-fold, p<0.002; T Test), IL-2 (5.3-fold, p<0.006; T Test), and TNF (6.8-fold, p<0.05; T test) secreting influenza-specific CD4+ cells when compared to Fluzone®-immunized monkeys.
Figure 1. Influenza-specific CD4+ and CD8+ T cell responses after immunization.
Day of immunization (A and B), 2 weeks PI (C and D), and 6 weeks PI (two weeks post-boost; E and F). Both CD4+ T cell responses (A, C, E) and CD8+ T cell responses (B, D, F) are shown. The scatter plots indicate the number of influenza-specific T cells/ml of blood in each animal and the number below each circle indicates the mean number of influenza-specific T cells/ml blood in each group. Only results from responding animals are included in the pie charts, which indicate the functional capacity of the influenza-specific T cells. The shaded portions of the pie indicate the percentage of influenza-specific T cells that responded to viral antigens with one ( ), two (
), two ( ), three (
), three ( ), or four (■) functions and the colored arcs around the pie show the function or combination of functions of in the responding T cells. Arranged in increasing arc radius: CD107 (
), or four (■) functions and the colored arcs around the pie show the function or combination of functions of in the responding T cells. Arranged in increasing arc radius: CD107 ( ), IL-2, interleukin 2 (
), IL-2, interleukin 2 ( ), IFN-γ, interferon γ (
), IFN-γ, interferon γ ( ), TNF, tumor necrosis factor (
), TNF, tumor necrosis factor ( ). Fluzone®-immunized animals ●; Fluzone®+null VRP-immunized animals ■.
). Fluzone®-immunized animals ●; Fluzone®+null VRP-immunized animals ■.
By 2 weeks PI, influenza-specific CD8+ T cell responses were detected in 67% of Fluzone® and 83% of Fluzone®+null VRP-immunized monkeys (Fig. 1D). The mean strength of the influenza-specific CD8+ T cell responses (824 vs. 2094 influenza-specific CD8+ T cells/ml lymphocytes) and number of functions in the positive CD8+ T cells were similar in the two groups (Fig. 1D). By 6 weeks PI (2 weeks post boost), only 67% of Fluzone®-immunized animals and 50% Fluzone®+null VRP-immunized animals had detectable Fluzone®-specific CD8+ T cell responses. There was relatively little change in the mean strength of the response from week 2 to week 6-post immunization; however IFN-γ secreting T cells were more prevalent at week 6 (Fig. 1F).
3.2. Null VRP enhance anti-influenza antibody responses after Fluzone® immunization
To determine the effect of the co-administration of null VRP on Fluzone®-induced antibody levels, plasma and mucosal whole anti-influenza IgG and IgA titers, as well as, hemagglutinin inhibiting (HI) antibody titers were determined from weeks 0 to 16 post-immunization. The overall strength of the antibody response was assessed by converting the longitudinal data from each animal into an area under the curve (AUC) value and mean AUC values for each group were compared. In addition, the mean antibody levels at weeks 2 and 8 PI and the peak PI titers in the groups were compared.
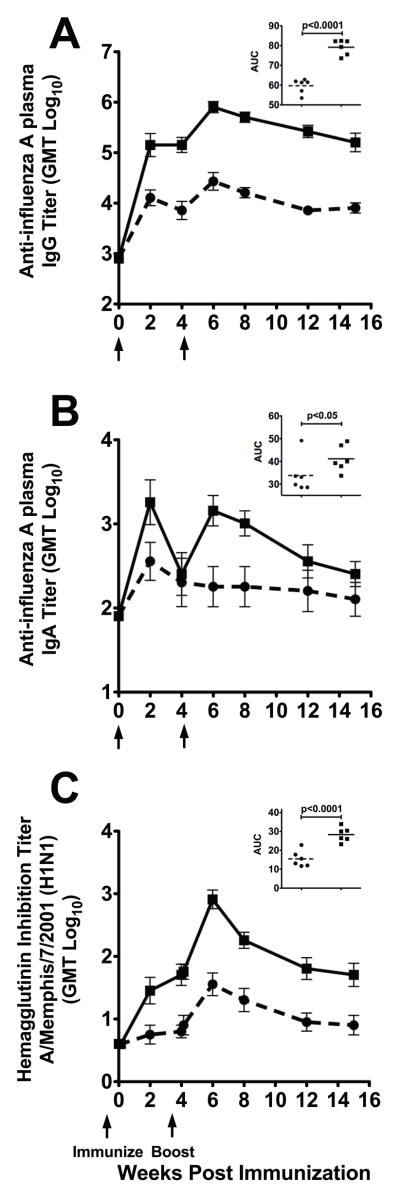
Co-administration of null VRP with Fluzone® resulted in a 1.3 log increase in the mean AUC values of plasma anti-influenza IgG antibodies when compared to Fluzone® alone (p<0.0001; T test) (Fig. 2A). Further, the addition of null VRP to Fluzone® immunization significantly increased the mean plasma anti-influenza IgG titers at week 2 (1.3 log, p<0.02; T test), week 8 (1.4 log, p<0.0001; T test), and at the maximum PI titer (1.3 log, p<0.0001; T test) when compared to animals immunized with Fluzone® alone. Of note, the mean plasma anti-influenza IgG level at week 2 PI (1 immunization) in the Fluzone®+null VRP animals was significantly higher (1.2 log, p=0.031; Two-tailed T test) than the mean IgG levels at week 6 PI (2 immunizations) in the Fluzone® animals. Thus, the addition of null VRP to Fluzone® increased the anti-influenza IgG antibody titer and decreased the time and number of immunizations needed to achieve strong anti-influenza plasma IgG antibody responses.
Figure 2. Plasma anti-influenza antibody responses to Fluzone® immunization.
(A and B) Mean plasma influenza specific IgG antibody and IgA antibody ELISA titers. (C) Average plasma hemagglutinin inhibition antibody titers against anti-A/Memphis/7/2001. Arrows indicate timing of immunizations. ●, Fluzone®-immunized (n=6); ■, Fluzone®+null VRP-immunized (n=6).
Fluzone®+null VRP-immunized animals also had a 1.2 log increase in mean plasma anti-influenza IgA antibody AUC compared to the Fluzone® animals (p<0.05; T test) (Fig. 2B). When individual time points were compared, null VRP+Fluzone® immunization significantly increased the mean plasma IgA titers at week 2 (1.3 log, p=0.036; T test), week 8 (1.3 log, p=0.012), and the maximum PI titer (1.4 log, p=0.006) when compared to animals immunized with Fluzone® alone. Similar to anti-influenza IgG antibody levels, the mean plasma anti-influenza IgA levels at week 2 PI in the Fluzone®+null VRP animals was significantly higher (1.5 log, p<0.02; Two-tailed T test) than the mean IgA levels at week 6 PI in the Fluzone® animals.
Co-administration of null VRP with Fluzone® immunization resulted in a 1.8 log increase in mean plasma HI antibodies AUC (p<0.0001; T test) when compared to Fluzone® alone (Fig. 2C). Further, null VRP+Fluzone® immunization significantly increased the mean HI antibody titer at week 2 (1.9 log, p=0.011; T test), week 8 (1.7 log, p<0.001; T test), and the maximum PI titer (1.9 log, p<0.0001; T test) when compared to animals immunized with Fluzone® alone. The current policy for licensing influenza vaccines requires the induction of a serum HI titer of ≥40 (≥4 fold titer increase) to the vaccine virus in the majority of the vaccines [34]. A 4-fold increase in HI titer was detected in 83% and 100% of Fluzone®+null VRP immunized animals by week 2 and 6 PI, respectively. However, only 17% and 67% of Fluzone® immunized animals had a 4-fold increase in HI titers by weeks 2 and 6 PI, respectively. Thus, after a single dose, addition of null VRP to Fluzone® dramatically increases the number of animals with a “protective” HI response.
3.3. Anti-influenza IgA and IgG responses were detected at low levels in the mucosal secretions of the respiratory tract
Fluzone®+null VRP immunization significantly increased the mean anti-influenza IgG titers in upper respiratory (URT) secretions at week 8 (2.3 log; p<0.03; T test) and the maximum PI titer (1.5 log; p<0.01; T Test) compared to animals immunized with Fluzone® alone (data not shown). More importantly, tracheal anti-influenza IgG antibody titers at week 15 PI (1 week prior to virus challenge), Fluzone®+null VRP immunized animals were 7.6-fold higher compared to the Fluzone® alone animals (p<0.001; T test) (Table 2). Further, anti-influenza IgG titers in URT secretions at week 15 of Fluzone®+null VRP immunized animals were 1.8-fold increase higher compared to Fluzone® alone (p<0.001; T test). Although mucosal anti-influenza responses were moderate in all animals, addition of null VRP to Fluzone® immunization increased the number of animals generating a mucosal anti-influenza igG antibody responses.
Table 2.
Effect of null VRP on respiratory tract mucosal antibody responses
| Animal Number | Tracheal Secretions | Nasal Secretions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-influenza IgG |
Anti-influenza IgA |
Anti-influenza IgG |
Anti-influenza IgA |
|||||||||
| Pre | Week 15 | fold increased | Pre | Week 15 | fold increased | Pre | Week 15 | fold increased | Pre | Week 15 | fold increased | |
| Fluzone®e | ||||||||||||
| 34357 | 4 | 32 | 8 | 4 | 128 | 32 | 4 | 4 | 1 | 4 | 4 | 1 |
| 34615 | 4 | 4 | 1 | 4 | 32 | 8 | 4 | 4 | 1 | 4 | 4 | 1 |
| 34999 | 4 | 4 | 1 | 4 | 32 | 8 | 4 | 4 | 1 | 4 | 4 | 1 |
| 35686 | 4 | 4 | 1 | 4 | 64 | 16 | 4 | 4 | 1 | 4 | 64 | 16 |
| 36378 | 4 | 4 | 1 | 4 | 16 | 4 | 4 | 4 | 1 | 4 | 4 | 1 |
| 36871 | 4 | 4 | 1 | 4 | 4 | 1 | 4 | 4 | 1 | 4 | 4 | 1 |
| Meanc | 4.0 | 5.7a | 1.4b | 4.0 | 28.5 | 7.1 | 4.0 | 4.0b | 1.5b | 4.0 | 6.4 | 1.6 |
| Fluzone® + null VRPf | ||||||||||||
| 34313 | 4 | 40 | 10 | 4 | 64 | 16 | 4 | 4 | 1 | 4 | 4 | 1 |
| 34501 | 4 | 16 | 4 | 4 | 16 | 4 | 4 | 16 | 4 | 4 | 64 | 16 |
| 34993 | 4 | 80 | 20 | 4 | 32 | 8 | 4 | 8 | 2 | 4 | 8 | 2 |
| 35366 | 4 | 80 | 20 | 4 | 16 | 4 | 4 | 8 | 2 | 4 | 8 | 2 |
| 36223 | 4 | 40 | 10 | 4 | 4 | 1 | 4 | 8 | 2 | 4 | 4 | 1 |
| 36529 | 4 | 40 | 10 | 4 | 16 | 4 | 4 | 4 | 1 | 4 | 4 | 1 |
| Meanc | 4.0 | 43.3a | 10.8b | 4.0 | 18.0 | 4.5 | 4.0 | 7.1b | 1.9b | 4.0 | 8.0 | 2.0 |
Mean value of Fluzone®+null VRP-immunized group are significantly higher than Fluzone®-immunized group (p<0.001; T test)
Mean value of Fluzone®+null VRP-immunized group are significantly higher than Fluzone®-immunized group (p<0.01; Ttest)
value is the anti-log of the geometric mean titer
Titer at week 15 PI relative to the pre PI titer
Two immunizations of 22.5ug HA of the 2006–2007 inactivated pediatric Fluzone®
Two immunizations of 22.5ug HA of the 2006–2007 inactivated pediatric Fluzone® mixed with 1×107 iu null VRP
3.4. Null VRP reduces viral replication after challenge of Fluzone® immunized monkeys
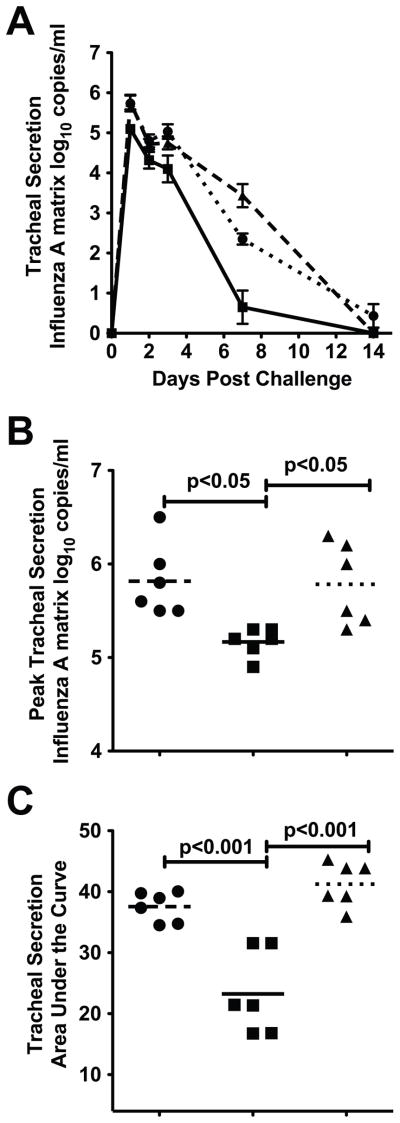
To determine effectiveness, monkeys immunized with Fluzone® or Fluzone®+null VRP were challenged with A/Memphis/7/01 (H1N1) as previously described [22], and the level of virus replication post-challenge (PC) was compared among the animal groups. Influenza RNA was detectable in the tracheal lavage samples of all inoculated animals on days 1, 2, 3 and 7 PC (Fig. 3A). Fluzone®+null VRP-immunized monkeys had significantly lower mean peak vRNA (p<0.05; ANOVA Tukey) and mean vRNA AUC (p<0.001; ANOVA Tukey) in tracheal secretions than naive control animals (Fig. 3B, 3C, and Table I). In addition, the mean peak vRNA (p<0.01; ANOVA Tukey) and mean vRNA AUC (p<0.001; ANOVA Tukey) in tracheal secretions of Fluzone®+null VRP-immunized monkeys was significantly lower than in Fluzone®-immunized monkeys (Fig. 3B). Although there was a trend toward lower peak and AUC vRNA levels in the tracheal secretions of Fluzone®-immunized animals compared to the naïve control monkeys, this difference was not significant. By day 7 PC, the level of vRNA in the tracheal secretions of the Fluzone®+null VRP-immunized animals was significantly lower than the naïve control animals (p<0.001; ANOVA Tukey) and Fluzone®-immunized animals (p<0.001; ANOVA Tukey). Thus, in the rhesus macaque model, mixing the null VRP adjuvant with Fluzone® produced significant protection from virus challenge.
Figure 3. Virus replication in the lower respiratory tract after influenza A virus challenge.
(A) Mean vRNA copy number in tracheal lavages (Log10 copies/ml). (B) Mean tracheal lavage peak vRNA (C) vRNA levels from days 1–7 PI as AUC. Indicated P values generated using ANOVA with Tukey-Kramer post hoc test. ● Immunized with Fluzone® (n=6); ■ Immunized with Fluzone® and null VRP (n=6); ▲ naïve controls (n=6).
3.5. Correlates of protection in Fluzone® immunized animals
To understand the nature of the immune responses responsible for protection after Fluzone® immunization, Spearman’s correlation was used to compare PI antibody titers to peak vRNA titers and vRNA AUCs. Week 6 HI, IgG, and IgA anti-influenza antibody titers inversely correlated with peak vRNA titers and vRNA AUCs (all points tested at least p<0.02; Spearman’s, data not shown). Week 6 IgG and IgA antibody titers in respiratory secretions did not correlate with peak vRNA levels. Three days prior to challenge plasma HI, IgG, and IgA anti-influenza antibody titers also inversely correlated with peak vRNA levels and vRNA AUCs (all points at least p<0.05; Spearman’s, data not shown). Further, IgG anti-influenza antibody titers in tracheal secretions 3 days prior to challenge also inversely correlated with vRNA AUCs (p<0.01; Spearman’s, data not shown).
3.6. Null VRP enhanced anamnestic antibody responses after influenza virus challenge
To determine the effect of null VRP on anamnestic anti-influenza antibody responses following a homologous H1N1 challenge, systemic and mucosal anti-influenza IgG, IgA, and, HI antibody levels were determined from days 1–28 PC. Fluzone® and Fluzone®+null VRP immunized animals had a rapid rise in plasma IgG, IgA, and HI titers after virus challenge consistent with an anamnestic immune response (Fig. 4). Further, compared to the non-immunized group, mean plasma anti-influenza IgG titers were significantly higher in both the Fluzone®+null VRP (1.4 fold, p<0.001; Tukey) and Fluzone® only (1.3 fold, p<0.001; Tukey) immunized animals (Fig. 4A insert). Similarly, mean HI antibody were significantly higher in both Fluzone®+null VRP (1.7 fold, p<0.001; Tukey) and Fluzone® only (1.4 fold, p<0.01; Tukey) when compared to non-immunized animals (Fig. 4C insert).
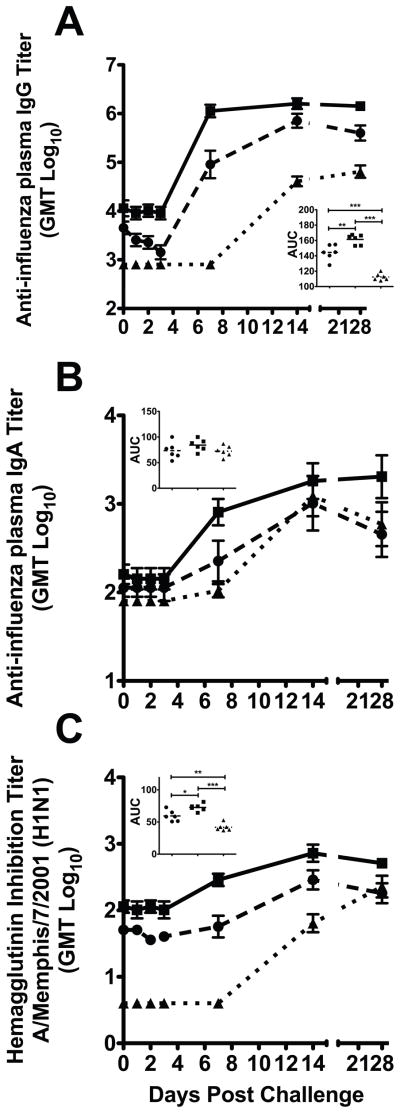
Figure 4. Anti-influenza IgG and IgA antibody responses in plasma after influenza A virus (A/Memphis/7/2001) challenge.
(A and B) Mean plasma anti-influenza IgG and IgA antibody titers. (C) Mean plasma HI antibody titers against A/Memphis/7/2001. (A, B, and C insets) Comparison of the mean anti-influenza antibody AUC in plasma, P values generated using ANOVA with a Tukey-Kramer post hoc test; (*) p<0.05, (**) p<0.01, (***) p<0.001. ● Fluzone®-immunized (n=6); ■ Fluzone®+null VRP-immunized (n=6) ▲ naïve controls (n=6)
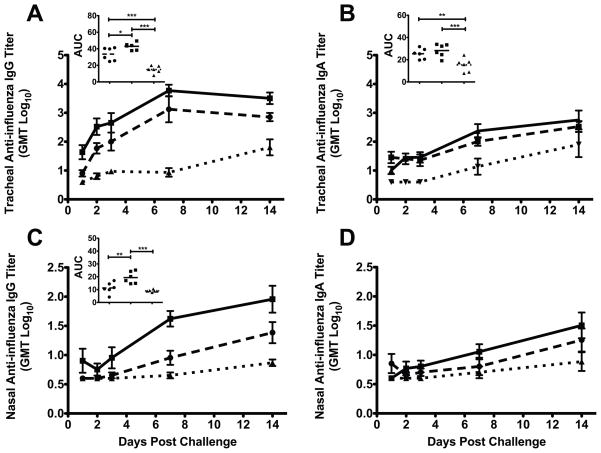
There were rapid increases in anti-influenza IgG and IgA antibodies in the lower respiratory tract (LRT) secretions of both the Fluzone®+null VRP and Fluzone® immunized monkeys after challenge (Fig. 5A and 5B). Compared to the non-immunized animals, the mean AUC of LRT IgG antibody levels was significantly higher in both the Fluzone®+null VRP (2.9 fold, p<0.001; Tukey) and Fluzone® alone (2.2 fold, p<0.001; Tukey) animals (Fig. 5A). Similarly, the mean AUC of LRT IgA antibody was significantly higher in both Fluzone®+null VRP (1.9 fold, p<0.001; Tukey) and Fluzone® only (1.7 fold, p<0.01; Tukey) when compared to non-immunized animals (Fig. 5B insert). Although anti-influenza IgG and IgA responses were difficult to detect in LRT secretions after vaccination, the rapid increase in mucosal antibodies after virus challenge is evidence that local mucosal immune responses were primed in the immunized animals.
Figure 5. Anti-influenza IgG and IgA antibody responses in the respiratory tract after influenza virus A (A/Memphis/7/2001) challenge.
(A and B) Mean anti-influenza IgG and IgA antibody titers in tracheal secretions. (C and D) Mean anti-influenza IgG and IgA antibody titers in nasal secretions. (A, B, and C insets) Comparison of the mean anti-influenza Ig antibody AUCs in secretions, P values generated using ANOVA with a Tukey-Kramer post hoc test; (*) p<0.05, (**) p<0.01, (***) p<0.001. ● Fluzone®-immunized (n=6); ■ Fluzone®+null VRP-immunized (n=6) ▲ naïve controls (n=6)
Both Fluzone® and Fluzone®+null VRP immunized monkeys had detectable anti-influenza IgG and IgA antibodies in the URT before day 7 PC (Fig. 5C and 5D). No responses were detectable in control monkeys at this point. Further, the Fluzone®+null VRP immunized monkeys, but not Fluzone® only animals, had significantly higher mean AUC IgG antibody levels after virus challenge (2.1 fold, p<0.001; ANOVA Tukey) in URT secretions compared to the non-immunized animals (Fig. 5C inset). Although the mean AUC levels were not significantly higher, more Fluzone®+null VRP monkeys had anti-influenza IgA responses after challenge than the unimmunized controls (Fig. 5D).
4. Discussion
Use of null VRP as an adjuvant strengthened and altered the immune response of rhesus macaques to Fluzone®, a licensed inactivated influenza vaccine. Most importantly, mixing null VRP with Fluzone® reduced viral replication after influenza virus challenge, while Fluzone® alone had only a marginal effect. While both immunized groups generated strong anamnestic anti-influenza antibody responses, the level of viral replication in the LRT of immunized animals inversely correlated with the titer of anti-influenza antibodies prior to challenge. In fact, inverse correlations with viral replication and anti-influenza IgG, IgA, and HI antibody titers in plasma and LRT IgG levels were found, strongly suggesting that these anti-influenza IgG and IgA antibody responses mediate protection in Fluzone® immunized rhesus macaques. Further, the addition of null VRP to Fluzone® strengthened the CD4+ T cell response in immunized animals and the influenza-specific CD4+ T cells producing IL-2, TNF, and IFN-γ. Thus macaques accurately model human responses to influenza immunization as serum antibody levels in people vaccinated with inactivated influenza vaccines correlate with protection from disease [35].
As influenza virus enters the body by a mucosal route, vaccines that induce mucosal immunity could be especially effective [36]. Early mouse studies suggested that the mucosal surfaces share a common set of mucosal lymphocytes, that home to the various mucosal sites by detecting site-specific extravasation, migration, and retention signals. Mucosal immune responses have been reliably generated in mice by delivering antigen to mucosal surfaces (reviewed in [37, 38]); however, a growing body of literature suggests that it is also possible to generate mucosal immune responses after parenteral immunization [39–43].
Null VRP efficiently infect dendritic cells (DC) resulting in IFN-α production [14, 44] and enhanced mucosal immunity to systemically delivered antigens in mice [1, 2, 45]. IFN-α is a powerful polyclonal B-cell activator that induces a strong primary humoral immune response characterized by Ig isotype switching [46, 47]. Thus, footpad immunization of mice with null VRP as an adjuvant produced strong mucosal secretory IgA responses and strong systemic IgG responses [1, 3]. In contrast, while co-administration of null VRP with Fluzone® produced much stronger plasma anti-influenza antibody responses in rhesus macaques, mucosal antibody responses to immunization were only marginally improved with the addition of null VRP. Despite the relatively weak mucosal IgG and IgA responses in macaques, it is clear that anamnestic mucosal antibody responses were primed by null VRP immunization. These results highlight the differences in the mucosal immune system of mice compared to primates.
Targeting DCs during immunization may enhance the DC primed CD4+ T cell differentiation into at least four different early effector lineages (T helper type 1 [TH1], TH2, TH17, and inducible regulatory [iTreg] cells) (reviewed in [48, 49]). However, the presence of cognate B cells at the time of DC CD4+ T cell priming results in CD4+ differentiation into follicular helper T cells (TFH), TFH produce cytokines, particularly IL-21, that are important stimulators of B cell proliferation and the formation of germinal centers, memory B, and long-lived plasma cells (reviewed in [48, 49]). Thus, the increased influenza-specific CD4+ T cell responses in conjunction with the increased anti-influenza antibody responses, suggests the addition of null VRP to the Fluzone® immunization increased both CD4+ effector and TFH activity.
This report shows that mixing null VRP with Fluzone® results in stronger CD4+ T cell responses, higher serum IgG and IgA anti-influenza antibody levels, and enhanced control of influenza A virus replication in the lower respiratory tract after challenge. The level of protection correlated with the level of plasma anti-influenza antibodies that were induced in the immunized animals. This demonstration in a primate model that null VRPs enhance the immunogenicity and effectiveness of Fluzone® vaccination suggests that this adjuvant could be used to significantly improve the efficacy, extend the supply, and/or reduce the cost of inactivated influenza vaccines. Should null VRP demonstrate in humans the advantageous characteristics that it has demonstrated in experimental animals, then considerable future work will be required on to develop production processes to scale up manufacture of null VRPs.
Acknowledgments
This work was supported by Public Health Service grants P51RR00169 from the National Center for Research Resources and U01AI074512 from the National Institute of Allergy and Infectious Diseases. The authors thank Martha Collier at the Carolina Vaccine Institute, University of North Carolina at Chapel Hill, the Primate Services Unit at the CNPRC, and Ding Lu, Jun Li, Tracy Rourke, Lili Guo, and Joseph Dutra for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thompson JM, Whitmore AC, Konopka JL, Collier ML, Richmond EM, Davis NL, et al. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3722–7. doi: 10.1073/pnas.0600287103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson JM, Whitmore AC, Staats HF, Johnston R. The contribution of type I interferon signaling to immunity induced by alphavirus replicon vaccines. Vaccine. 2008 Sep 15;26(39):4998–5003. doi: 10.1016/j.vaccine.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson JM, Nicholson MG, Whitmore AC, Zamora M, West A, Iwasaki A, et al. Nonmucosal alphavirus vaccination stimulates a mucosal inductive environment in the peripheral draining lymph node. J Immunol. 2008 Jul 1;181(1):574–85. doi: 10.4049/jimmunol.181.1.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Wolters Kluwer | Lippincott Williams & Wilkins; 2007. pp. 1691–40. [Google Scholar]
- 5.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004 Nov 25;351(22):2295–301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 6.Palache AM, Beyer WE, Osterhaus AD. Influenza vaccine dosages. Vaccine. 2008 May 2;26(19):2305–6. doi: 10.1016/j.vaccine.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Kemble G, Greenberg H. Novel generations of influenza vaccines. Vaccine. 2003 May 1;21(16):1789–95. doi: 10.1016/s0264-410x(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 8.Cooper CL, Davis HL, Morris ML, Efler SM, Krieg AM, Li Y, et al. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine. 2004 Aug 13;22(23–24):3136–43. doi: 10.1016/j.vaccine.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi M, Satou E, Ueki R, Yano M, Miyano-Kurosaki N, Fujii M, et al. Resistance to influenza A virus infection by antigen-conjugated CpG oligonucleotides, a novel antigen-specific immunomodulator. Biochemical and biophysical research communications. 2005 Apr 1;329(1):230–6. doi: 10.1016/j.bbrc.2005.01.116. [DOI] [PubMed] [Google Scholar]
- 10.Lay M, Callejo B, Chang S, Hong DK, Lewis DB, Carroll TD, et al. Cationic lipid/DNA complexes (JVRS-100) combined with influenza vaccine (Fluzone((R))) increases antibody response, cellular immunity, and antigenically drifted protection. Vaccine. 2009 May 8; doi: 10.1016/j.vaccine.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine. 2001 Mar 21;19(17–19):2673–80. doi: 10.1016/s0264-410x(00)00499-0. [DOI] [PubMed] [Google Scholar]
- 12.Rimmelzwaan GF, Claas EC, van Amerongen G, de Jong JC, Osterhaus AD. ISCOM vaccine induced protection against a lethal challenge with a human H5N1 influenza virus. Vaccine. 1999 Mar 17;17(11–12):1355–8. doi: 10.1016/s0264-410x(98)00390-9. [DOI] [PubMed] [Google Scholar]
- 13.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997 Dec 22;239(2):389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald GH, Johnston RE. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J Virol. 2000 Jan;74(2):914–22. doi: 10.1128/jvi.74.2.914-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubby B, Talarico T, Maughan M, Reap EA, Berglund P, Kamrud KI, et al. Development and preclinical evaluation of an alphavirus replicon vaccine for influenza. Vaccine. 2007 Nov 23;25(48):8180–9. doi: 10.1016/j.vaccine.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein DI, Reap EA, Katen K, Watson A, Smith K, Norberg P, et al. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine. 2009 Dec 11;28(2):484–93. doi: 10.1016/j.vaccine.2009.09.135. [DOI] [PubMed] [Google Scholar]
- 17.Hartley CA, Reading PC, Ward AC, Anders EM. Changes in the hemagglutinin molecule of influenza type A (H3N2) virus associated with increased virulence for mice. Arch Virol. 1997;142(1):75–88. doi: 10.1007/s007050050060. [DOI] [PubMed] [Google Scholar]
- 18.Kugel D, Kochs G, Obojes K, Roth J, Kobinger GP, Kobasa D, et al. Intranasal administration of alpha interferon reduces seasonal influenza A virus morbidity in ferrets. J Virol. 2009 Apr;83(8):3843–51. doi: 10.1128/JVI.02453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, et al. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science. 2009 Jul 24;325(5939):484–7. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svitek N, Rudd PA, Obojes K, Pillet S, von Messling V. Severe seasonal influenza in ferrets correlates with reduced interferon and increased IL-6 induction. Virology. 2008 Jun 20;376(1):53–9. doi: 10.1016/j.virol.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 21.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS pathogens. 2007 Oct 19;3(10):1470–6. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll TD, Matzinger SR, Genesca M, Fritts L, Colon R, McChesney MB, et al. Interferon-induced expression of MxA in the respiratory tract of rhesus macaques is suppressed by influenza virus replication. J Immunol. 2008 Feb 15;180(4):2385–95. doi: 10.4049/jimmunol.180.4.2385. [DOI] [PubMed] [Google Scholar]
- 23.Rimmelzwaan GF, Baars M, van Beek R, van Amerongen G, Lovgren-Bengtsson K, Claas EC, et al. Induction of protective immunity against influenza virus in a macaque model: comparison of conventional and iscom vaccines. J Gen Virol. 1997 Apr;78( Pt 4):757–65. doi: 10.1099/0022-1317-78-4-757. [DOI] [PubMed] [Google Scholar]
- 24.Rimmelzwaan GF, Kuiken T, van Amerongen G, Bestebroer TM, Fouchier RA, Osterhaus AD. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J Virol. 2001 Jul;75(14):6687–91. doi: 10.1128/JVI.75.14.6687-6691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007 Jan 18;445(7125):319–23. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 26.LoBue AD, Thompson JM, Lindesmith L, Johnston RE, Baric RS. Alphavirus-adjuvanted norovirus-like particle vaccines: heterologous, humoral, and mucosal immune responses protect against murine norovirus challenge. J Virol. 2009 Apr;83(7):3212–27. doi: 10.1128/JVI.01650-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed LJ, Muench H. A simple method for estimating fifty percent endpoints. Am J Hyg. 1938;27(3):493–7. [Google Scholar]
- 28.Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, et al. Development of a Real-Time Reverse Transcriptase PCR Assay for Type A Influenza Virus and the Avian H5 and H7 Hemagglutinin Subtypes. Journal of Clinical Microbiology. 2002 September;40(9):3256–60. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lifson JD, Rossio JL, Piatak M, Jr, Parks T, Li L, Kiser R, et al. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol. 2001 Nov;75(21):10187–99. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller CJ, McChesney MB, Lu X, Dailey PJ, Chutkowski C, Lu D, et al. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997 Mar;71(3):1911–21. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webster RG, Cox N, Stöhr K. Response CDSa. WHO Manual on Animal Influenza Diagnosis and Surveillance. 1. World Health Organization; 2002. pp. 28–36. [Google Scholar]
- 32.Genesca M, Rourke T, Li J, Bost K, Chohan B, McChesney MB, et al. Live attenuated lentivirus infection elicits polyfunctional simian immunodeficiency virus Gag-specific CD8+ T cells with reduced apoptotic susceptibility in rhesus macaques that control virus replication after challenge with pathogenic SIVmac239. J Immunol. 2007 Oct 1;179(7):4732–40. doi: 10.4049/jimmunol.179.7.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genesca M, Skinner PJ, Bost KM, Lu D, Wang Y, Rourke TL, et al. Protective attenuated lentivirus immunization induces SIV-specific T cells in the genital tract of rhesus monkeys. Mucosal Immunol. 2008 May;1(3):219–28. doi: 10.1038/mi.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Developments in biologicals. 2003;115:63–73. [PubMed] [Google Scholar]
- 35.Clements ML, Snyder MH, Buckler-White AJ, Tierney EL, London WT, Murphy BR. Evaluation of avian-human reassortant influenza A/Washington/897/80 x A/Pintail/119/79 virus in monkeys and adult volunteers. J Clin Microbiol. 1986 Jul;24(1):47–51. doi: 10.1128/jcm.24.1.47-51.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006 Feb;6(2):148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 37.Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008 Jan;1(1):31–7. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- 38.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nature medicine. 2005 Apr;11(4 Suppl):S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 39.Belyakov IM, Hammond SA, Ahlers JD, Glenn GM, Berzofsky JA. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J Clin Invest. 2004 Apr;113(7):998–1007. doi: 10.1172/JCI20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egan MA, Chong SY, Hagen M, Megati S, Schadeck EB, Piacente P, et al. A comparative evaluation of nasal and parenteral vaccine adjuvants to elicit systemic and mucosal HIV-1 peptide-specific humoral immune responses in cynomolgus macaques. Vaccine. 2004 Sep 9;22(27–28):3774–88. doi: 10.1016/j.vaccine.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Enioutina EY, Visic D, McGee ZA, Daynes RA. The induction of systemic and mucosal immune responses following the subcutaneous immunization of mature adult mice: characterization of the antibodies in mucosal secretions of animals immunized with antigen formulations containing a vitamin D3 adjuvant. Vaccine. 1999 Aug 6;17(23–24):3050–64. doi: 10.1016/s0264-410x(99)00147-4. [DOI] [PubMed] [Google Scholar]
- 42.Gockel CM, Bao S, Beagley KW. Transcutaneous immunization induces mucosal and systemic immunity: a potent method for targeting immunity to the female reproductive tract. Molecular immunology. 2000 Jun;37(9):537–44. doi: 10.1016/s0161-5890(00)00074-2. [DOI] [PubMed] [Google Scholar]
- 43.McCluskie MJ, Weeratna RD, Payette PJ, Davis HL. Parenteral and mucosal prime-boost immunization strategies in mice with hepatitis B surface antigen and CpG DNA. FEMS Immunol Med Microbiol. 2002 Feb 18;32(3):179–85. doi: 10.1111/j.1574-695X.2002.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 44.Moran TP, Burgents JE, Long B, Ferrer I, Jaffee EM, Tisch RM, et al. Alphaviral vector-transduced dendritic cells are successful therapeutic vaccines against neu-overexpressing tumors in wild-type mice. Vaccine. 2007 Sep 4;25(36):6604–12. doi: 10.1016/j.vaccine.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leitner WW, Hwang LN, deVeer MJ, Zhou A, Silverman RH, Williams BR, et al. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nature medicine. 2003 Jan;9(1):33–9. doi: 10.1038/nmxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001 Apr;14(4):461–70. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 47.Tovey MG, Lallemand C, Meritet JF, Maury C. Adjuvant activity of interferon alpha: mechanism(s) of action. Vaccine. 2006 Apr 12;24(Suppl 2):S2-46–7. doi: 10.1016/j.vaccine.2005.01.117. [DOI] [PubMed] [Google Scholar]
- 48.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010 Feb;11(2):114–20. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–66. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]