Abstract
Coplanar polychlorinated biphenyls (PCBs) may facilitate development of atherosclerosis by stimulating pro-inflammatory pathways in the vascular endothelium. Nutrition, including fish oil-derived long-chain omega-3 fatty acids, such as docosahexaenoic acid (DHA, 22:6ω-3), can reduce inflammation and thus the risk of atherosclerosis. We tested the hypothesis that cyclopentenone metabolites produced by oxidation of DHA can protect against PCB-induced endothelial cell dysfunction. Oxidized DHA (oxDHA) was prepared by incubation of the fatty acid with the free radical generator 2,2-azo-bis(2-amidinopropane) dihydrochloride (AAPH). Cellular pretreatment with oxDHA prevented production of superoxide induced by PCB77, and subsequent activation of nuclear factor-κB (NF-κB). A4/J4-neuroprostanes (NPs) were identified and quantitated using HPLC ESI tandem mass spectrometry. Levels of these NPs were markedly increased after DHA oxidation with AAPH.. The protective actions of oxDHA were reversed by treatment with sodium borohydride (NaBH4), which concurrently abrogated A4/J4-NP formation. Up-regulation of monocyte chemoattractant protein-1 (MCP-1)by PCB77 was markedly reduced by oxDHA, but not by un-oxidized DHA. These protective effects were proportional to the abundance of A4/J4NPs in the oxidized DHA sample. Treatment of cells with oxidized eicosapentaenoic acid (EPA, 20:5ω-3) also reduced MCP-1 expression, but less than oxDHA. Treatment with DHA-derived cyclopentenones also increased DNA binding of NF-E2-related factor-2 (Nrf2)and downstream expression of NAD(P)H:quinone oxidoreductase (NQO1), similarly to the Nrf-2 activator sulforaphane. Furthermore, sulforaphane prevented PCB77-induced MCP-1 expression, suggesting that activation of Nrf-2 mediates the observed protection against PCB77 toxicity. Our data implicate A4/J4-NPs as mediators of omega-3 fatty acid-mediated protection against the endothelial toxicity of coplanar PCBs.
Keywords: Polychlorinated biphenyls(PCBs), endothelial cells, docosahexaenoic acid (DHA), monocyte chemoattractant protein-1 (MCP-1), NF-E2-related factor-2 (Nrf2), oxidative stress
Introduction
Chronic exposure to persistent organic pollutants, such aspolychlorinated biphenyls (PCBs), contributes to the development of cardiovascular diseases in humans (Hennig et al., 2007). It has been well established that inflammation is an important mechanism contributing to the pathology of atherosclerosis, an underlying cause in the majority of cardiovascular deaths (Wilson, 2008). Coplanar PCBs can exacerbate early development of atherosclerosis by increasing production of inflammatory mediators, such as monocyte chemattractant protein-1 (MCP-1), in the vascular endothelium (Hennig et al., 2002; Majkova et al., 2009).
Changing the composition of dietary lipids is a promising strategy to prevent negative outcomes of exposure to environmental chemicals(Wang et al., 2008). There is a substantial number of epidemiological studies demonstrating that fish-derived omega-3 polyunsaturated fatty acids (PUFAs) can reduce cardiovascular morbidity and mortality(Wang et al., 2006; Marik and Varon, 2009). Docosahexaenoic acid (DHA, 22:6ω-3), and eicosapentaenoic acid (EPA, 20:5ω-3)are the major components of fish oil, and their anti-inflammatory properties contribute to the cardioprotective effects of fish oil(Mori and Beilin, 2004).
Long-chain PUFAs in the body are subject to free radical-initiated oxidation, leading to the production of prostaglandin-like compounds called isoprostanes (IsoPs)(Morrow et al., 1992). This reaction proceeds through the formation of an unstable endoperoxide intermediate, which can then be be reduced to generate IsoPs containing F-type prostane rings(F -IsoPs) (Roberts et al., 1998). Alternatively these intermediates can undergo isomerization to form molecules with E-type and D-type prostane rings( E/D -IsoPs) (Reich et al., 2000). E/D-IsoPs are subsequently dehydrated resulting in A -type and J-type compounds (A/J-IsoPs) (Fam et al., 2002). Oxidation of DHA specifically leads to formation of neuroprostanes (NPs) which are IsoP-like compounds found commonly in DHA-rich tissues, in particular brain (Roberts et al., 1998; Musiek et al., 2008). A4/J4-NPsare cyclopentenone metabolites of DHA, that contain electrophilic α,β-unsaturated carbonyl moieties, which allow them to form Michael adducts with nucleophiles, including thiol groups in signaling proteins(Musiek et al., 2008). As a result, they can inhibit inflammatory responses, for example by binding to IκB kinase β (IKKβ), thus inhibiting transcription factor nuclear factor -κB (NF-κB) (Musiek et al., 2008).
Reactive oxygen species (ROSs)are critical mediators of PCB -induced endothelial inflammation (Slim et al., 1999). Redox imbalance leads to activation of oxidative stress-sensitive kinases and transcription factors, including NF-κB, and increased production of inflammatory cytokines and adhesion molecules(Gloire and Piette, 2009). Nuclear factor erythroid 2-related factor 2 (Nrf2) plays a major role in cellular response to oxidative stress by binding to its cognate antioxidant response element (ARE) in promoters of genes encoding cytoprotective proteins, including glutathione synthesis and metabolism enzymes, or NAD(P)H:quinone oxidoreductase (NQO1) (Kensler et al., 2007). Nrf2 is present in aortic endothelial cells, where its activation inhibits inflammatory signaling(Zakkar et al., 2009). Several naturally occurring chemoprotective compounds can activate Nrf-2 and stimulate antioxidant responses(Mann et al., 2009). Interestingly, DHA-derived cyclopentenones increased Nrf -2 transcriptional activity by direct binding to sulfhydryl groups on Keap1, a negative regulator of Nrf2 (Gao et al., 2007). In this report we test the hypothesis that cyclopentenone products of DHA oxidation prevent PCB toxicity in endothelial cells by activation of antioxidant responses and inhibition of PCB-induced oxidative stress.
Materials and Methods
Materials and chemicals
PCB77 was a generous gift from Dr. Larry W. Robertson, University of Iowa, Iowa City, IA. All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). DHA and EPA (>99% pure by gas-liquid chromatography) were obtained from Nu-Chek Prep (Elysian, MN). All cell culture reagents were purchased from Invitrogen (Carlsbad, CA), and all other chemicals from Sigma-Aldrich Corporation(St. Louis, MO), unless otherwise specified.
Cell culture
Primary endothelial cells were isolated from porcine pulmonary arteries as described previously (Hennig et al., 1984). The basic culture media consisted of medium 199 (M199) containing 10% fetal bovine serum (FBS). At confluency, cells were incubated overnight with treatment media, followed by an exposure to tested compounds in treatment media (M199 with 0.5% FBS for parent fatty acids, and M199 with 5% FBS for oxidized fatty acids, respectively). PCB77 was solubilized in dimethyl sulfoxide( DMSO), and subsequently diluted in cell culture media to 5 μM. Similar PCB levels were found in human serum after acute exposure to PCBs (Wassermann et al., 1979; Jensen, 1987; Vaman Rao and Banerji, 1989).
Fatty acid treatments
Fatty acids were diluted in EtOH (50 mg/ml), aliquoted, and stored at −80°C. Treatment with parent fatty acids was performed as described previously (Mattos et al., 2003). Briefly, the ethanol was evaporated with nitrogen gas, and the fatty acids were diluted to 1 mM in M199 cell culture medium containing 33 mg/ml of fatty acid -free bovine serum albumin (BSA) to achieve a molar fatty acid to BSA ratio of 2:1. This solution was incubated for 2 h at 37°C to allow binding of the FA to BSA, and then further diluted in treatment media to final treatment concentrations. For the experiments with oxidized fatty acids, ethanol stock solutions were diluted to 1 mM in phosphate-buffered saline (PBS), containing 2 mM of a free radical generator free radical generator 2,2-azo-bis(2-amidinopropane) dihydrochloride (AAPH; Cayman Chemical, Ann Arbor, MI), a methods that was reported to produce cyclopentenone IsoPs (Musiek et al., 2008). The solutions were incubated at 37°Cfor 16 h, unless otherwise indicated, diluted in treatment media, filtered, and exposed to the cells. Sodium borohydride (NaBH 4) reduction of oxidized DHA (oxDHA) was performed as before (Musiek et al., 2008)with minor modifications. Oneml of 18% (w/w) NaBH4 in water was added to 0.66 mg of previously oxidized DHA in 2 mM AAPH/PBS, vortexed, and incubated on ice for 30 min. Then, a molar excess of HCl was added to neutralize NaBH4, and lipids were extracted into the chloroform phase by the addition of chloroform and methanol (a final ratio of 1:1:0.9 of chloroform:methanol:acidic aqueous phase), followed by vortexing and centrifugation for 5 min each. The lower phase was dried under nitrogen, and the lipid extracts were re -dissolved in EtOH/PBS/treatment media, and exposed to the cells. Both of these resulted in comparable levels of albumin in the experimental media (5% FBS or 30 μM of albumin for parent fatty acids and 20 μM albumin for delivery of oxidized fatty acid), which is the critical predictor of fatty acid availability (Hostmark, 1995).
Assessment of superoxide (O2−·) levels
Cells were grown to confluence in 4-chamber culture slides (BDB iosciences, Bedford, MA). After treatments, the cells were rinsed 2x with Krebs-Ringer buffer (KRB; 118 mM NaCl, 4.7 mM KCl, 1.3 mM CaCl2, 12 mM MgCl2, 1 mM NaH2PO4, 25 mM NaHCO3, and 11 mM glucose, pH = 7.4), followed by incubation with 5 μM dihydroethidium (DHE) or KRB (blank) at 37°C for 30 min. Cells were then rinsed with KRB, fixed with 10% buffered formalin, and washed with PBS. Slides were mounted with ProLong Gold Antifade reagent containing 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA) to visualize the nuclei. The slides were evaluated under an Olympus BX61W1 fluorescence microscope and the images were captured digitally using a Retiga-EXi camera and QCapture Pro 5.1.1.14 sotware (QImaging, Surrey, BC, Canada). Mean fluorescence intensity was quantified using ImageJ 1.42q (NIH, Bethesda, MD).
Electrophoretic mobility shift assay (EMSA)
After treatments, nuclear extracts were prepared as described previously (Oesterling et al., 2008). DNA-binding activities of NF-κB and Nrf2 were assessed using LightShift Chemiluminescent EMSA Kit (Pierce, Rockford, IL) and binding reactions were carried out as published before (Sauzeau et al., 2003), with 8 μg of antibodies against p65 (NF-κB subunit) or Nrf2 used to confirm band specificity. Synthetic 5′-biotinylated complementary oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). The cognate DNA sequence for NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was described previously (Lim et al., 2007), and antioxidant response element (ARE) from porcine NQO1 promoter (5′-TAGTCACAGTGACTCAGCGAGATTC-3′) was identified based on conserved ARE sequence (Wasserman and Fahl, 1997).
Analysis of A4/J4-NPs
Analysis of neuroprostanes and DHA was carried out using a Shimadzu UFLC coupled with an ABI 4000-Qtrap hybrid linear ion trap triple quadrupole mass spectrometer in multiple reaction monitoring (MRM) mode. DHA and neuroprostanes were separated using a Zorbax Eclipse XDB C8 column, 5 um, 4.6 × 150 mm (Agilent). The mobile phase consisted of 63/37/0.5 v/v/v: Water/Acetonitrile/Formic acid as solvent A and 50/50 v/v: Acetonitrile/IPA as solvent B. For the analysis of DHA and neuroprostanes the separation was achieved using a gradient of 100 to 0 % solvent B in 6 min and maintaining at 0 % B for the next 9 min and equilibrated back to the initial conditions in 3 min. The flow rate was 0.5 mL/min with a column temperature of 30 C. The sample injection volume was 10 uL. The mass spectrometer was operated in the negative electrospray ionization mode with optimal ion source settings with a declustering potential of −80 V, entrance potential of −10 V, collision energy of −14 V, collision cell exit potential of −11 V, curtain gas of 20 psi, ion spray voltage of −4500 V, ion source gas1/gas2 of 40 psi and temperature of 550 C. MRM transitions monitored were as follows: Neuroprostanes - m/z 357/339, m/z 357/295, m/z 357/313, m/z 357/161, m/z 357/175, and for DHA – m/z 327/283, m/z 327/229, m/z 327/177, m/z 327/191, m/z 327/249. D4 -isoprostane was used as an internal recovery standard and a surrogate calibrator to quantitate NPs and DHA. The MRM transitions used to quantitate d4-isoprostane were m/z 333.5/314.6, m/z 333.5/296.6.
Measurement of MCP-1 mRNA and protein levels
MCP-1 mRNA expression was assessed using real-time PCR (RT-PCR), and MCP-1 protein levels were measured in cell culture media using Quantikine ELISA kit (R&D Systems, Minneapolis, MN) as described previously (Majkova et al., 2009).
Measurements of thiobarbituric acid reactive substances (TBARS)
TBARS formation in oxidized fatty acid solutions before cell treatments was assessed using the TBARS Assay Kit (Cayman Chemical) according to manufacturer’s instruction.
Western blotting
NQO1 protein levels were assessed by Western Blotting as described previously (Han et al., 2010). Rabbit polyclonal anti NQO1 antibody was incubated overnight at 4 °C in blocking buffer (5% non-fat milk in Tris-buffered saline containing 0.05% Tween 20).
Statistical analysis
Values are reported as means ± SE obtained from of at least three independent experiments. Comparisons were made by one-way, two-way, or three-way analysis of variance (ANOVA), followed by post-hoc Fisher’s least significant difference (LSD)test, using SigmaStat 2.0 software (Systat Software, Point Richmond, CA). Statistical probability of p < 0.05 was considered significant.
Results
Oxidized DHA prevents up -regulation of superoxide by PCB77 in endothelial cells
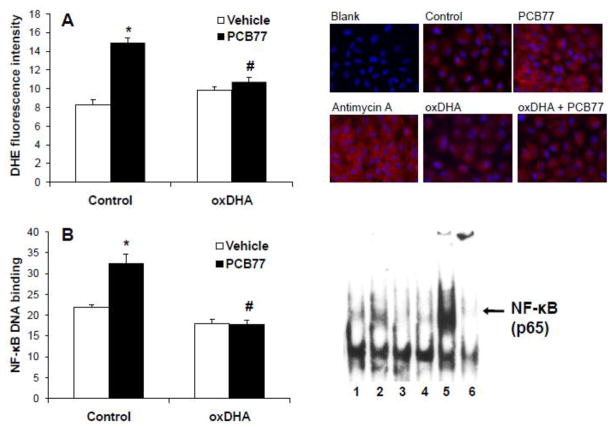
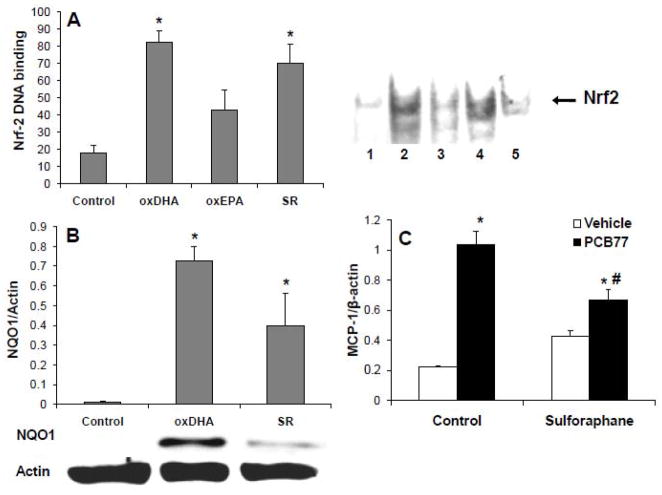
Oxidative stress is a key component of endothelial activation by coplanar PCBs (Slim et al., 1999). Cyclopentenone metabolites of omega-3 PUFAs can activate antioxidant defenses in the cell (Gao et al., 2007), which could provide a protection from PCB toxicity. To test this hypothesis, endothelial cells were pretreated with oxDHA, produced by free radical-initiated oxidation with AAPH, followed by exposure to vehicle or PCB77. Pre-treatments with oxDHA were performed for 4 h. This time-point was selected based on a previously published study (Gao et al., 2007)that reported Nrf2 stimulation by oxDHA after 4 h exposure. Production of superoxide was assessed using dihydroethidium(D HE), a cell-permeable compound that can be oxidized by O2−· into a fluorescent product (Tarpey et al., 2004). Antimycin A, a mitochondrial electron transport inhibitor (Piskernik et al., 2008), was used as a positive control. O2−· levels were assessed by fluorescent microscopy (Figure 1A)and fluorescence intensity was quantified by ImageJ 1.42q software. PCB77 increased superoxide production and pre-treatment with oxDHA prevented this, demonstrating that oxDHA can protect endothelial cells form PCB-induced oxidative stress.
Figure 1.
Oxidized DHA prevents PCB77-induced superoxide production and activation of NF-κB. (A) Oxidized DHA (oxDHA) was generated via oxidation for 16 h in 2 mM AAPH. Cells were pre-treated with control or oxDHA (40 μM) for 4 h; followed by exposure to vehicle control (DMSO) or PCB77 (5 μM) for 8 h, or antimycin A (50 μM) for 6 h. Cells were then stained with DHE, and red fluorescence was assessed using microscope and quantified by ImageJ 1.42q. (B) Cells were pre-treated with control or oxDHA (40 μM) for 4 h, followed by exposure to control or PCB77 (5 μM) for 6 h. TNF-α (5 ng/mL) treatment for 2 h was used as a positive control. NF-κB DNA-binding was assessed by EMSA. 1, control; 2, PCB77; 3, oxDHA; 4, oxDHA + PCB77; 5, TNF-α; 6, p65 supershift. Data represent mean ± SEM of 3–4 independent experiments. Two-way ANOVA revealed a statistically significant interaction between oxDHA and PCB77. *Significantly different compared to vehicle control (p<0.05). #Significantly different compared to PCB77-treated control without oxDHA (p<0.05).
Activation of NF-κB by PCB77 is inhibited by oxidized DHA
ROS production is known to enhance nuclear translocation and transcriptional activity NF -κB (Gloire and Piette, 2009) a central regulator of endothelial inflammatory responses (Shin et al., 2002). DNA-binding activity of NF-κB after exposure to PCB77 was measured by EMSA. Tumor necrosis factor-α (TNF-α) was used as a positive control, and supershift with antibody against NF-κB subunit p65 confirmed the band identity. In agreement with our previous studies (Lim et al., 2007), PCB77 increased NF-κB activity after 6 h. Pre -treatment with oxDHA completely abolished NF-κB activation(Figure 1B), a likely result of a decreased inflammatory ROS production in the vascular endothelium to PCB77 after exposure to oxDHA.
Oxidation of DHA in vitro leads to the production of A4/J4-NPs
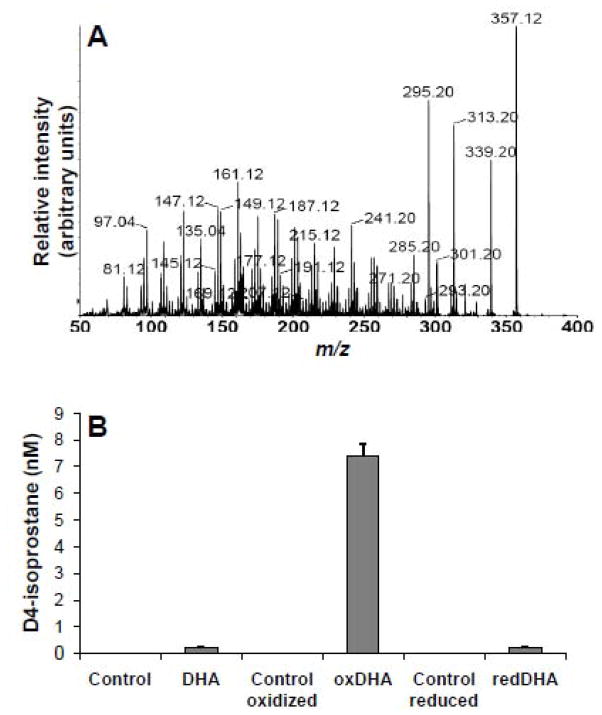
Cyclopentenone metabolites of DHA, i.e. A4/J4-NPs, are uniquely active due to their ability to interact with sulfhydryl groups of signaling proteins (Gao et al., 2007; Musiek et al., 2008). We examined our oxDHA preparations, generated by treatment with AAPH, for the presence of A4/J4-NPs by infusion mode tandem mass spectrometry. We identified an ion with the predicted m/z ratio for the M -H-ion of J 4/A4NPs ( m/z 357) in these preparations. A product ion spectrum obtained after collisional dissociation of this m/z 357 species correlated well with that reported previously for J4/A4-NP (Fam et al., 2002)(Figure 2A). Although the unavailability of synthetic standards for these molecules makes unambiguous assignment of the product ion spectrum challenging as observed by (Fam et al., 2002), some of the abundant product ions derived from the m/z 357 species, for example m/z 339 ([M − H] − H2O)−, m/z 313 ([M − H] − CO2)−, and m/z 295 ([M − H] − H2O CO2)−, represent commonly observed transitions for this category of oxidized fatty acids. We used several of these precursor product ion pairs to establish selective reaction monitoring mode HPLC ESI MS/MS methods for quantitation of A4/J4-NPs in our oxidized DHA samples. We determined that, as also observed previously, untreated DHA contains low levels of A4/J4-NPs (presumably the result of auto oxidation) but levels of these compounds were increased markedly by AAPH-initiated oxidation (Figure 2B), and abrogated after reduction with NaBH4(Figure 2B). These data demonstrate that oxDHA contains substantially elevated levels of A4/J4-NPs, which can be reduced using NaBH4.
Figure 2.
Analysis of A4/J4-NPs by tandem mass spectrometry.(A) Oxidized DHA sample was infused into the ion source of our instrument, a species of m/z 357 generated in negative mode ESI was subjected to CID, and daughter ions identified from m/z 50 to 400. (B) A 4/J4-NP levels were quantitated in samples containing 40 μM of controls, unoxidized DHA, AAPH-oxidized DHA (oxDHA), or sodium borohydride ( NaBH4)-reduced DHA (redDHA). Qunatification was performed by HPLC ESI MS/MS using d4 -isoprostane as a surrogate calibration standard.
Oxidation of omega-3 PUFAs is required for the protection from PCB toxicity
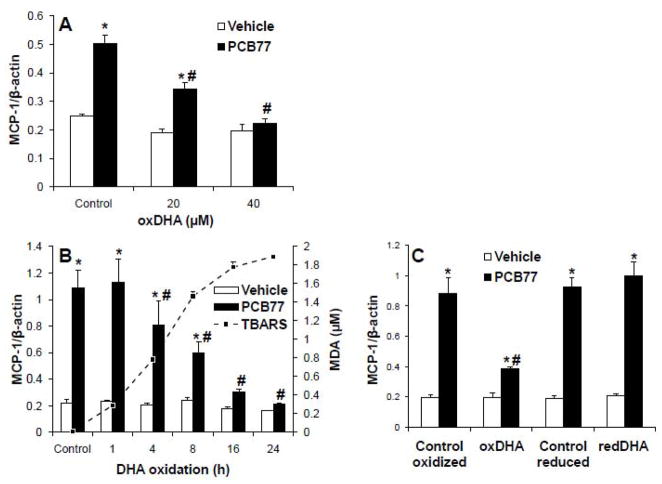
The capacity of parent DHA and EPA to prevent PCB -induced inflammation was tested by measuring the expression levels of MCP-1, a cytokine mediator of monocyte recruitment into endothelium in early stages of atherosclerosis (Shin et al., 2002). DHA and EPA were delivered using fatty acid-free BSA as a vehicle. After pre-treatment with control or fatty acids, cells were treated with PCB77, and mRNA levels of MCP-1 were assessed using RT-PCR. Neither DHA, nor EPA, had any effect on MCP-1 up-regulation by PCB77 (data not shown). By contrast, oxDHA decreased up-regulation of MCP-1 by PCB77 in a dose-dependent manner, with 40 μM being the most effective concentration (Figure 3A). In order to further demonstrate that only the oxidized metabolites are protective, the same concentrations of parent DHA were oxidized for increasing periods of time before treatments. The levels of oxidation are expressed as malondialdehyde (MDA) equivalents using TBARS assay and increased gradually over 24 hours (dashed line in Figure 3B). There was a direct correlation between the level of DHA oxidation measured using this assay and the effectiveness of preventing MCP-1 up-regulation by PCB77 (Figure 3B), supporting the hypothesis that DHA oxidation can result in production of anti -inflammatory compounds.
Figure 3.
Oxidized DHA prevents MCP-1 mRNA up-regulation by PCB77. (A) Cells were pre-treated with control or oxDHA (0–40 μM) for 4 h. After exposure to control or PCB77 (5 μM) for 24 hours, MCP-1 mRNA expression was assessed using RT-PCR. (B) DHA was oxidized for increasing periods of time, and levels of malondialdehyde (MDA) were assessed using TBARS assay (dashed line). Cells were pre-treated with control or oxDHA (40 μM) for 4 h, followed by PCB77 treatment and RT-PCR as described in (A). (C) DHA was oxidized by AAPH and/or reduced using NaBH4 (redDHA). Cells were pre -treated with respective controls, oxDHA, or redDHA for 4 h, followed by PCB77 treatment and RT-PCR as described in (A). Data represent the mean ± SEM of 4-5 independent experiments. Two-way ANOVA revealed a statistically significant interaction between oxDHA and PCB77. *Significantly different compared to vehicle control (p<0.05). #Significantly different compared to PCB77-treated control without oxDHA (p<0.05).
Cyclopenenone metabolites are the anti-inflammatory component of oxDHA
To test the hypothesis that A4/J4-NPs are the active compounds that prevent PCB toxicity, oxDHA was subjected to chemical reduction with NaBH4 in order to reduce the carbonyl moiety on the cyclopentenone ring to a non-reactive alcohol (Mohanazadeh et al., 2005; Musiek et al., 2008). Residual NaBH4 was removed by using chloroform -methanol extraction. This procedure decreased the concentration of A4/J4-NPs to the baseline (Figure 2B). As presented in Figure 3C, oxDHA prevented MCP-1 up-regulation, but after reduction with NaBH4 (red DHA), it had no effect. This demonstrates that NaBH4-sensitive components of our oxDHA preparations which clearly include A4/J4-NPsare responsible for prevention of the PCB-induced inflammatory response.
Oxidized DHA is more protective than oxidized EPA
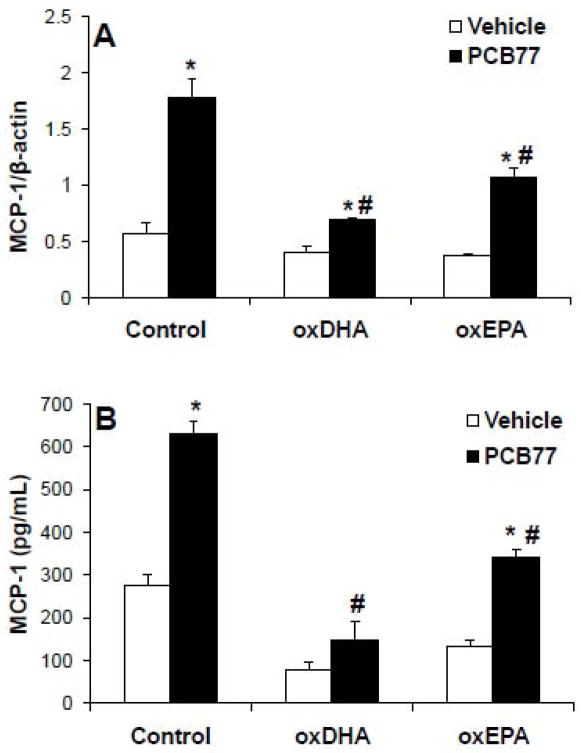
The relative potency of omega -3 PUFAs in cardiovascular prevention varies (Mori and Woodman, 2006; Gorjao et al., 2009). Free radical-induced oxidation of both DHA and EPA yields cyclopentenone metabolites that can exert anti -inflammatory responses (Chaudhary et al., 2004; Musiek et al., 2008). Since DHA is more susceptible to oxidation due to higher number of double bonds (Visioli et al., 1998), we tested the hypothesis that oxDHA is more protective than oxEPA. DHA and EPA were oxidized with 2 mM AAPH for 16 h. Under these conditions, DHA was oxidized to a greater extent than EPA measured using the TBARS assay (1.73±0.02 μM MDA per 40 μM of DHA) than EPA (1.22±0.02 μM MDA per 40 μM of EPA), which is consistent with previous reports(Visioli et al., 1998). Correspondingly, oxEPA significantly inhibited PCB -mediated up-regulation of MCP-1 mRNA (Figure 4A) and protein (Figure 4B), but oxDHA had a more pronounced inhibitory effect (Figures 4A and 4B). This suggests that oxDHA is more protective than oxEPA against PCB toxicity which likely relates to its greater susceptibility to free radical -initiated oxidation and IsoP formation.
Figure 4.
Inhibition of PCB77-induced MCP-1 up-regulation by oxDHA and oxEPA. (A) Cells were pre-exposed to control, oxDHA (40 μM), or oxEPA (40 μM) for 4 h, followed by exposure to control or PCB77 (5 μM) for 24 hours. MCP-1 mRNA expression was assessed using RT-PCR. (B) Cells were treated as in (A) and MCP-1 protein levels in cell culture media were assessed using ELISA. Data represent mean ± SEM of 4 independent experiments. Two-way ANOVA revealed a significant interaction between oxidized fatty acids and PCB77. *Significantly different compared to vehicle control (p<0.05). #Significantly different compared to PCB77-treated control without oxidized fatty acid (p<0.05).
Nrf2 activation is involved in protection against PCB77 toxicity
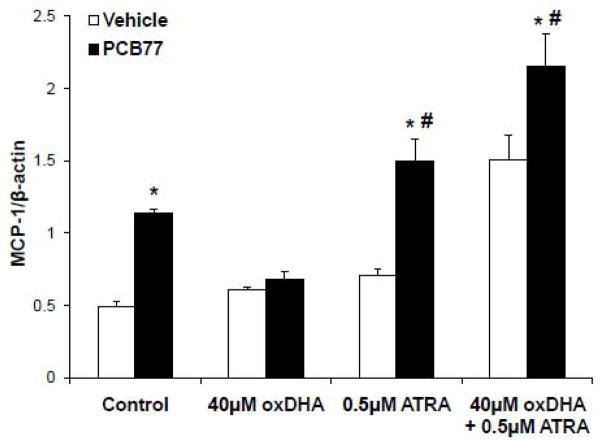
Nrf2 is a key regulator of antioxidant defenses in cells (Kensler et al., 2007), and an important anti-inflammatory mediator in vascular endothelium (Zakkar et al., 2009). Previous studies suggested that A4/J4-NPs can activate Nrf2 by binding to sulfhydryl groups in Keap1 (Gao et al., 2007). Since coplanar PCBs cause endothelial dysfunction by increasing oxidative stress, an increase inNrf2 transcriptional could prevent PCB toxicity. Nrf2 activity was assessed by EMSA using DNA sequence corresponding to Nrf2 -binding site in the promoter of NQO1, aNrf2 -responsive antioxidant enzyme (Nioi and Hayes, 2004). Nrf2 activity was significantly induced by cell exposure to oxDHA (Figure 5A). OxEPA tended to increase Nrf2 activity, but this increase was not significant. The Nrf2 data corresponded to a lesser degree of oxidation of EPA compared with DHA (1.18±0.03 μM MDA per 40 μM of EPA versus 1.93±0.07 μM MDA for DHA, respectively). Nrf2 stimulation by oxDHA was comparable to that observed in cells treated with suforaphane (SR) (Figure 5A), a dietary isothiocyanate known to stimulate Nrf2 transcriptional activity (Ahn et al., 2010). The ability of oxDHA to increase protein levels of NQO1 was tested by Western Blot (Figure 5B). NQO1 was up-regulated markedly by oxDHA, and similar but less pronounced effect s were obtained by exposure to SR (Figure 5B). In order to confirm that up-regulation of Nrf2 activity can lead to protection against PCB toxicity, the ability of SR to prevent MCP-1 up-regulation by PCB77 was tested. Cells were pretreated with SR, followed by exposure to PCB77. MCP-1 mRNA expression induced by PCB77 was prevented by SR treatment, suggesting that induction of Nrf2 can protect against PCB-mediated endothelial cell activation. Additional experiments were conducted to demonstrate the importance of Nrf2 in cellular protection against PCB toxicity. Figure 6 illustrates that inhibiting Nrf2 with all-trans retinoic acid (ATRA) (Wang et al., 2007) can further increase PCB-induced MCP -1 expression. Most importantly, inhibiting Nrf2 totally negated any protective properties of oxDHA, suggesting that functional Nrf2 is required for oxDHA to protect against PCB-induced induction of MCP-1.
Figure 5.
Nrf2 activation in endothelial cells. (A) Cells were treated with control, oxDHA (40 μM), oxEPA (40 μM), or sulforaphane (SR, 10 μM), for 4 h; followed by an assessment of Nrf2 DNA-binding activity using EMSA. 1, control; 2, oxDHA; 3. oxEPA; 4, SR; 5, Nrf2 supershift. (B) Cells were treated for 24 h with control, oxDHA (40 μM), or SR (10 μM); and protein levels of NQO1 were measured by Western Blot. (C) Cells were pre-treated with SR (5 μM) for 4 h, followed by exposure to control or PCB77 (5 μM) for 24 hours. MCP-1 mRNA expression was assessed using RT-PCR. Data represent mean ± SEM of 3–5 independent experiments. One-way ANOVA revealed a statistically significant treatment effect (A and B), and two-way ANOVA revealed an interaction between SR and PCB77 (C). *Significantly different compared to vehicle control (p<0.05). #Significantly different compared to PCB77-treated control without SR (p<0.05).
Figure 6.
Inhibiting Nrf2 negates protection by oxDHA against PCB77 -mediated induction of MCP-1. Cells were pre-treated with the Nrf2 inhibitor all-trans retinoic acid (ATRA, 0.5 μM) (Wang et al., 2007)prior to exposure to oxDHA (40 μM) and PCB77 (5 μM). Data represent mean ± SEM of 3 independent experiments.*Significantly different compared to respective vehicle controls(p<0.05). #Significantly different compared to PCB77 -treated control (p<0.05).
Discussion
Humans are constantly exposed to complex mixtures of environmental chemicals with potentially deleterious effects. Diet modifications are viable means for preventing adverse outcomes of these exposures. This current work demonstrates that oxidized metabolites of long chain omega-3 polyunsaturated fatty acids, and in particular cyclopentenone NPs formed by free radical-initiated oxidation of DHA, can prevent endothelial dysfunction induced by coplanar PCBs. PCBs are ubiquitous environmental pollutants, and significant levels are present in human tissues (Zamir et al., 2009). Furthermore, increased exposure to PCBs can contribute to cardiovascular mortality (Gustavsson and Hogstedt, 1997; Rylander et al., 2009), and coplanar PCBs facilitate atherosclerotic lesion formation in vivo (Arsenescu et al., 2008). Increased endothelial expression of adhesion molecules and cytokines, such as MCP-1, augments monocyte recruitment in early stages of atherosclerosis, and contributes to plaque formation (Aiello et al., 1999). We have previously demonstrated that coplanar PCBs can induce MCP-1 expression in endothelial cells and that this effect is mediated via the aryl hydrocarbon receptor (AhR) (Majkova et al., 2009).
Oxidative stress is a central mediator of PCB-induced endothelial dysfunction. Coplanar PCBs increase ROS production by up -regulation and uncoupling of cytochrome P450 monoxygenases (Schlezinger et al., 2006)and/or activation of endothelial nitric oxide synthase (Lim et al, 2007), while non-coplanar PCBs can affect endothelial NADPH oxidase (Eum et al., 2009). PCB-induced ROS can activate the transcription factor NF -κB (Hennig et al., 2002; Lee et al., 2003), an integral mediator of inflammatory responses in the vascular endothelium (Ding et al., 2009). In particular, ROS stimulate IKKβ-independent phosphorylation of IκB and nuclear translocation NF -κB(Gloire and Piette, 2009), resulting in an increased production of inflammatory mediators, including MCP-1 (Shin et al., 2002). Coplanar PCBs and other dioxin-like chemicals up-regulate MCP-1 release by endothelial cells, thus contributing to monocyte recruitment and plaque formation (Majkova et al., 2009).
Diets rich in fish oil-derived omega-3 PUFAs are associated with lower rates of cardiovascular mortality (Marik and Varon, 2009), partially due to the anti-inflammatory properties of DHA and EPA (Mori and Beilin, 2004). Dietary intervention with omega-3 PUFAs also leads to reduced oxidative stress in vivo (Yin et al., 2009). Recent evidence suggests that metabolites of omega-3 PUFAs contribute to the inhibition of an inflammatory response (Musiek et al., 2008; Tian et al., 2009). Most importantly, free radical-mediated oxidation of DHA and EPA in tissues has been reported to result in production of biologically active IsoPs (Musiek et al., 2008; Yin et al., 2009). To test the hypothesis that omega-3 PUFAs-derived IsoPs can alleviate PCB toxicity in endothelial cells, oxidized metabolites were produced by incubation of fatty acids with the free radical generator AAPH. Since increased ROS production is required for endothelial toxicity of coplanar PCBs (Slim et al., 1999), the capacity of oxDHA to prevent PCB77-induced rise in O2−· was assessed using DHE fluorescence. Pre -treatment with oxDHA abolished PCB77-induced ROS production. The observed protection could be caused by activation of Nrf2, a transcription factor that regulates a variety of genes involved in antioxidant defenses (Kensler et al., 2007). Indeed, IsoPs produced by oxidation of omega -3 PUFAs can specifically activate Nrf2 by covalently binding its regulator protein Keap1 (Gao et al., 2007), and Nrf2 activation has recently been implicated in prevention of PCB toxicity (Park et al., 2010).
Subsequent experiments from this study showed that oxDHA increases Nrf2 DNA-binding activity, and also protein levels of NQO1, a Nrf2-regulated enzyme involved in the detoxification of reactive quinones and replenishing antioxidants (Nioi and Hayes, 2004). Because PCB metabolism results in the formation of toxic quinones (Song et al., 2008), Nrf2 activation and NQO1 induction could explain the decrease in PCB77-induced superoxide formation observed after oxDHA pre-treatment. As a result of the decreased ROS formation, oxDHA prevented PCB77 -induced activation of NF-κB. It has been shown that DHA-derived A4/J4-NPs can induce NF-κB directly by binding to IκB kinase β ( IKKβ ) (Musiek et al., 2008). This mechanism, however, is not likely to counteract PCB toxicity, because coplanar PCBs induce NF-κB through oxidative stress signaling, i.e. downstream from IKKβ (Gloire and Piette, 2009). Rather, Nrf2-mediated induction of antioxidant enzymes probably resulted in rapid detoxification of PCB77, and prevented the ROS build-up and NF-κB activation. Another possibility would be NF-κB inhibition by Nrf2 cross-talk (Kensler et al., 2010). In our study, a well established Nrf2 inducer, dietary isothiocyanate sulforaphane (Ahn et al., 2010), also activated Nrf2 and NQO1 expression, and subsequently prevented MCP-1 up-regulation by PCB77. This suggests that Nrf2 activation by dietary compounds can prevent environmental insult caused by coplanar PCBs in the vascular endothelium. It is quite possible that other Nrf2 targets than NQO1 would reduce PCB toxicity in endothelial cells. Nrf2 also mediates induction of various enzymes involved in cellular anti-oxidant defense, e.g., those involved in the synthesis of glutathione (Osburn and Kensler, 2008). Other genes that are regulated by Nrf2 include multiple UDP-glucuronosyl transferases (Osburn and Kensler, 2008), which can play a role in phase II metabolism and removal of PCBs from a cell (Daidoji et al., 2005).
Oxidized DHA prevented MCP-1 up-regulation by PCB77 in a dose-dependent manner. Both oxDHA and oxEPA decreased MCP -1 up-regulation, but oxDHA was more potent. This was associated with a more pronounced oxidation of DHA (assessed using the TBARS assay), potentially resulting in a higher concentration of the active metabolites. Oxidation of DHA for increasing periods of time led to larger TBARS levels, and more effective inhibition of MCP-1 up-regulation. Because EPA is less susceptible to oxidation than DHA, higher levels of the parent fatty acid may be needed to produce desired levels of active metabolites. Interestingly, the parent long-chain fatty acids (DHA and EPA) had no effect on MCP -1 up-regulation by PCB77. These data support the notion that oxidized omega -3 fatty acid metabolites are uniquely protective in endothelial cells, findings consistent with a previous report where only oxidized omega-3 PUFAs prevented cytokine production (Mishra et al., 2004).
Oxidation of long-chain PUFAs leads to formation of a complex mixture of metabolites. Prostaglandin-like products of free radical-mediated oxidation include F -type IsoPs with hydroxylated cyclopentane ring, or carbonyl-containing D/E-IsoPs that subsequently get dehydrated to form cyclopentenone-containing compounds (A/J -IsoPs)(Roberts and Milne, 2009). Certain factors, including oxygen tension and glutathione concentrations, affect the relative levels of different IsoPs (Morrow et al., 1998). Prostaglandins and IsoPs containing cyclopentenone rings are particularly effective in reducing an inflammatory response (Musiek et al., 2008). For example, arachidonic acid-derived 15-deoxyΔ12,14-PGJ2(15d -PGJ2) inhibited KF -κB activation (Rossi et al., 2000)and MCP -1 production(Rovin et al., 2001), while prostaglandins lacking a cyclopentenone group were ineffective in these studies. Also, DHA-and EPA-derived cyclopentenones specifically activated Nrf2 (Gao et al., 2007). In the current study, we found that inhibition of Nrf2 with the antagonist ATRA further increased MCP-1 as induced by PCB77. Furthermore, inhibiting Nrf2 totally negated any protective properties of oxDHA, suggesting that functional Nrf2 is required for oxDHA to protect against PCB-induced induction of MCP-1. The limitations associated with non-specific inhibition of Nrf2 by ATRA should also be considered. It has been shown that ATRA decreases inducible Nrf2 mediated ARE activity in vitro, probably through direct interaction of RARα with Nrf2 (Wang et al., 2007). Thus, the pleiotropic effects of ATRA treatment to inhibit Nrf2 invites the possibility of other mechanisms by which ATRA may prevent oxDHA dependent protection against PCB induced MCP-1 expression.
Omega-3 PUFAs-derived IsoPs (NPs) are present in various tissues (Fam et al., 2002); and their concentrations increase after dietary supplementation with fish oil (Yin et al., 2009). In order to find out whether cyclepentenone NPs are the protective metabolites of oxDHA, A 4/J4-NPs in oxDHA were identified using tandem mass spectrometry approaches. A species of m/z 357, corresponding to the predicted m/z of the M -H- ion of A4/J4NPs was detected and its product ion spectrum revealed the presence of daughter ions consistent with the behavior of A4/J4-NPs reported previously (Fam et al., 2002). Relative levels of A4/J4-NPs increased markedly after oxidation by AAPH, consistent with the observation that parent omega-3 PUFAs were ineffective in preventing MCP -1 up-regulation. Subsequently, cyclopentonone groups were reduced using NaBH 4, which resulted in loss of protection against PCB-induced MCP -1 up-regulation. Taken together, our data show for the first time that only oxidized DHA can counteract PCB toxicity and that cyclopentenone NPs are likely the major active DHA oxidation metabolites.
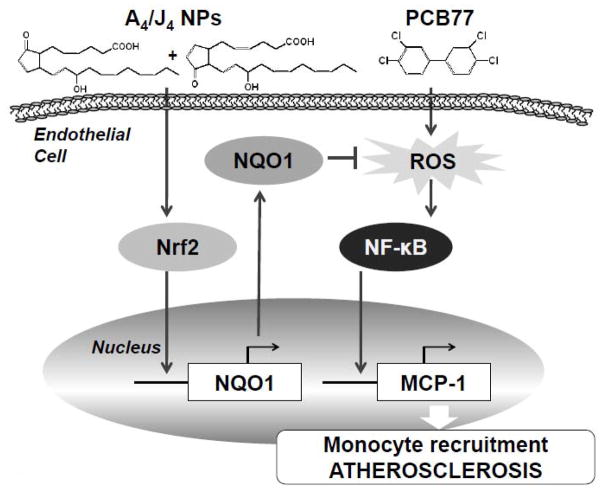
Our study shows that components of oxidized DHA, most likely A4/J4-NPs, can alleviate endothelial dysfunction caused by coplanar PCB77. This is likely mediated by activation of Nrf2 and and cellular antioxidant defenses, resulting in reduced ROS formation and decreased production of inflammatory chemokine MCP-1 (Figure 7). These data imply that dietary supplementation with omega -3 PUFAs, and in particular DHA, might prevent toxicity resulting from environmental exposure to PCBs.
Figure 7.
Scheme illustrating the inhibition of PCB77-induced endothelial inflammatory response by A4/J4-NPs. A4/J4-NPs bind Keap1 in the cytoplasm thus increasing Nrf2 nuclear translocation and DNA-binding activity. This leads to induction of antioxidant defense genes, including NQO1. Antioxidant enzymes (NQO1) inhibit ROS production by PCB77, leading to inhibition ofPCB77 -induced NF-κB activation and expression of pro-inflammatory genes, such as MCP-1.
Acknowledgments
We thank A. Stromberg for assistance with statistical analysis. This work was supported by NIH/NIEHS grant P42ES007380 NIH GM50388 and P20RR021954 and with funds from the University of Kentucky Agricultural Experiment Station.
Footnotes
Conflict of Interest Statement
The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn YH, Hwang Y, Liu H, Wang XJ, Zhang Y, Stephenson KK, Boronina TN, Cole RN, Dinkova-Kostova AT, Talalay P, Cole PA. Electrophilic tuning of the chemoprotective natural product sulforaphane. Proc Natl Acad Sci U S A. 2010;107:9590–9595. doi: 10.1073/pnas.1004104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello RJ, Bourassa PA, Lindsey S, Weng W, Natoli E, Rollins BJ, Milos PM. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–1525. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116:761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary A, Mishra A, Sethi S. Oxidized omega-3 fatty acids inhibit pro-inflammatory responses in glomerular endothelial cells. Nephron Exp Nephrol. 2004;97:e136–145. doi: 10.1159/000079178. [DOI] [PubMed] [Google Scholar]
- Daidoji T, Gozu K, Iwano H, Inoue H, Yokota H. UDP-glucuronosyltransferase isoforms catalyzing glucuronidation of hydroxy-polychlorinated biphenyls in rat. Drug Metab Dispos. 2005;33:1466–1476. doi: 10.1124/dmd.105.004416. [DOI] [PubMed] [Google Scholar]
- Ding J, Song D, Ye X, Liu SF. A pivotal role of endothelial-specific NF-kappaB signaling in the pathogenesis of septic shock and septic vascular dysfunction. J Immunol. 2009;183:4031–4038. doi: 10.4049/jimmunol.0900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum SY, Andras I, Hennig B, Toborek M. NADPH oxidase and lipid raft-associated redox signaling are required for PCB153-induced upregulation of cell adhesion molecules in human brain endothelial cells. Toxicol Appl Pharmacol. 2009;240:299–305. doi: 10.1016/j.taap.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam SS, Murphey LJ, Terry ES, Zackert WE, Chen Y, Gao L, Pandalai S, Milne GL, Roberts LJ, Porter NA, Montine TJ, Morrow JD. Formation of highly reactive A-ring and J-ring isoprostane-like compounds (A4/J4-neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 2002;277:36076–36084. doi: 10.1074/jbc.M205638200. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang J, Sekhar KR, Yin H, Yared NF, Schneider SN, Sasi S, Dalton TP, Anderson ME, Chan JY, Morrow JD, Freeman ML. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J Biol Chem. 2007;282:2529–2537. doi: 10.1074/jbc.M607622200. [DOI] [PubMed] [Google Scholar]
- Gloire G, Piette J. Redox regulation of nuclear post-translational modifications during NF-kappaB activation. Antioxid Redox Signal. 2009;11:2209–2222. doi: 10.1089/ars.2009.2463. [DOI] [PubMed] [Google Scholar]
- Gorjao R, Azevedo-Martins AK, Rodrigues HG, Abdulkader F, Arcisio-Miranda M, Procopio J, Curi R. Comparative effects of DHA and EPA on cell function. Pharmacol Ther. 2009;122:56–64. doi: 10.1016/j.pharmthera.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Am J Ind Med. 1997;32:234–239. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Han SG, Eum SY, Toborek M, Smart E, Hennig B. Polychlorinated biphenyl-induced VCAM-1 expression is attenuated in aortic endothelial cells isolated from caveolin-1 deficient mice. Toxicol Appl Pharmacol. 2010 doi: 10.1016/j.taap.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, Robertson LW. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002;181:174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Hennig B, Oesterling E, Toborek M. Environmental toxicity, nutrition, and gene interactions in the development of atherosclerosis. Nutr Metab Cardiovasc Dis. 2007;17:162–169. doi: 10.1016/j.numecd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Hennig B, Shasby DM, Fulton AB, Spector AA. Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis. 1984;4:489–497. doi: 10.1161/01.atv.4.5.489. [DOI] [PubMed] [Google Scholar]
- Hostmark AT. Serum fatty acid/albumin molar ratio and the risk of diseases. Med Hypotheses. 1995;44:539–541. doi: 10.1016/0306-9877(95)90520-0. [DOI] [PubMed] [Google Scholar]
- Jensen AA. Polychlorobiphenyls (PCBs), polychlorodibenzo-p-dioxins (PCDDs) and polychlorodibenzofurans (PCDFs) in human milk, blood and adipose tissue. Sci Total Environ. 1987;64:259–293. doi: 10.1016/0048-9697(87)90250-6. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Slocum SL, Skoko JJ, Shin S. When Nrf2 Talks, Who’s Listening? Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YW, Park HJ, Son KW, Hennig B, Robertson LW, Toborek M. 2,2′,4,6,6′-pentachlorobiphenyl (PCB 104) induces apoptosis of human microvascular endothelial cells through the caspase-dependent activation of CREB. Toxicol Appl Pharmacol. 2003;189:1–10. doi: 10.1016/s0041-008x(03)00084-x. [DOI] [PubMed] [Google Scholar]
- Lim EJ, Smart EJ, Toborek M, Hennig B. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H3340–3347. doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- Majkova Z, Smart E, Toborek M, Hennig B. Up-regulation of endothelial monocyte chemoattractant protein-1 by coplanar PCB77 is caveolin-1-dependent. Toxicol Appl Pharmacol. 2009;237:1–7. doi: 10.1016/j.taap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann GE, Bonacasa B, Ishii T, Siow RC. Targeting the redox sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: protection afforded by dietary isoflavones. Curr Opin Pharmacol. 2009;9:139–145. doi: 10.1016/j.coph.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Marik PE, Varon J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32:365–372. doi: 10.1002/clc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos R, Guzeloglu A, Badinga L, Staples CR, Thatcher WW. Polyunsaturated fatty acids and bovine interferon-tau modify phorbol ester-induced secretion of prostaglandin F2 alpha and expression of prostaglandin endoperoxide synthase-2 and phospholipase-A2 in bovine endometrial cells. Biol Reprod. 2003;69:780–787. doi: 10.1095/biolreprod.102.015057. [DOI] [PubMed] [Google Scholar]
- Mishra A, Chaudhary A, Sethi S. Oxidized omega-3 fatty acids inhibit NF-kappaB activation via a PPAR alpha-dependent pathway. Arterioscler Thromb Vasc Biol. 2004;24:1621–1627. doi: 10.1161/01.ATV.0000137191.02577.86. [DOI] [PubMed] [Google Scholar]
- Mohanazadeh F, Hosini M, Tajbakhsh M. Sodium borohydride -ammonium carbonate: An effective reducing system for aldehydes and ketones. Monatshefte Fur Chemie. 2005;136:2041–2043. [Google Scholar]
- Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6:461–467. doi: 10.1007/s11883-004-0087-5. [DOI] [PubMed] [Google Scholar]
- Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104. doi: 10.1097/01.mco.0000214566.67439.58. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ., 2nd Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci U S A. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ, Daniel VC, Awad JA, Mirochnitchenko O, Swift LL, Burk RF. Comparison of formation of D2/E2-isoprostanes and F2-isoprostanes in vitro and in vivo--effects of oxygen tension and glutathione. Arch Biochem Biophys. 1998;353:160–171. doi: 10.1006/abbi.1998.0645. [DOI] [PubMed] [Google Scholar]
- Musiek ES, Brooks JD, Joo M, Brunoldi E, Porta A, Zanoni G, Vidari G, Blackwell TS, Montine TJ, Milne GL, McLaughlin B, Morrow JD. Electrophilic cyclopentenone neuroprostanes are anti-inflammatory mediators formed from the peroxidation of the omega-3 polyunsaturated fatty acid docosahexaenoic acid. J Biol Chem. 2008;283:19927–19935. doi: 10.1074/jbc.M803625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nioi P, Hayes JD. Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat Res. 2004;555:149–171. doi: 10.1016/j.mrfmmm.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Oesterling E, Toborek M, Hennig B. Benzo[a]pyrene induces intercellular adhesion molecule-1 through a caveolae and aryl hydrocarbon receptor mediated pathway. Toxicol Appl Pharmacol. 2008;232:309–316. doi: 10.1016/j.taap.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburn WO, Kensler TW. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutat Res. 2008;659:31–39. doi: 10.1016/j.mrrev.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Jang JH, Chen CY, Na HK, Surh YJ. A formulated red ginseng extract rescues PC12 cells from PCB-induced oxidative cell death through Nrf2-mediated upregulation of heme oxygenase-1 and glutamate cysteine ligase. Toxicology. 2010 doi: 10.1016/j.tox.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Piskernik C, Haindl S, Behling T, Gerald Z, Kehrer I, Redl H, Kozlov AV. Antimycin A and lipopolysaccharide cause the leakage of superoxide radicals from rat liver mitochondria. Biochim Biophys Acta. 2008;1782:280–285. doi: 10.1016/j.bbadis.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Reich EE, Zackert WE, Brame CJ, Chen Y, Roberts LJ, 2nd, Hachey DL, Montine TJ, Morrow JD. Formation of novel D-ring and E-ring isoprostane-like compounds (D4/E4-neuroprostanes) in vivo from docosahexaenoic acid. Biochemistry. 2000;39:2376–2383. doi: 10.1021/bi992000l. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, 2nd, Milne GL. Isoprostanes. J Lipid Res. 2009;50(Suppl):S219–223. doi: 10.1194/jlr.R800037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LJ, 2nd, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, Dettbarn WD, Morrow JD. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–13612. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- Rovin BH, Lu L, Cosio A. Cyclopentenone prostaglandins inhibit cytokine-induced nf-kappab activation and chemokine production by human mesangial cells. J Am Soc Nephrol. 2001;12:1659–1667. doi: 10.1681/ASN.V1281659. [DOI] [PubMed] [Google Scholar]
- Rylander C, Sandanger TM, Brustad M. Associations between marine food consumption and plasma concentrations of POPs in a Norwegian coastal population. J Environ Monit. 2009;11:370–376. doi: 10.1039/b811868j. [DOI] [PubMed] [Google Scholar]
- Sauzeau V, Rolli-Derkinderen M, Marionneau C, Loirand G, Pacaud P. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J Biol Chem. 2003;278:9472–9480. doi: 10.1074/jbc.M212776200. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Struntz WD, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquat Toxicol. 2006;77:422–432. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Shin WS, Szuba A, Rockson SG. The role of chemokines in human cardiovascular pathology: enhanced biological insights. Atherosclerosis. 2002;160:91–102. doi: 10.1016/s0021-9150(01)00571-8. [DOI] [PubMed] [Google Scholar]
- Slim R, Toborek M, Robertson LW, Hennig B. Antioxidant protection against PCB-mediated endothelial cell activation. Toxicol Sci. 1999;52:232–239. doi: 10.1093/toxsci/52.2.232. [DOI] [PubMed] [Google Scholar]
- Song Y, Buettner GR, Parkin S, Wagner BA, Robertson LW, Lehmler HJ. Chlorination increases the persistence of semiquinone free radicals derived from polychlorinated biphenyl hydroquinones and quinones. J Org Chem. 2008;73:8296–8304. doi: 10.1021/jo801397g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004;286:R431–444. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- Tian H, Lu Y, Sherwood AM, Hongqian D, Hong S. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest Ophthalmol Vis Sci. 2009;50:3613–3620. doi: 10.1167/iovs.08-3146. [DOI] [PubMed] [Google Scholar]
- Vaman Rao C, Banerji S. Polychlorinated biphenyls in human blood samples of Bombay. Bulletin of Environmental Contamination and Toxicology. 1989;43:656–659. doi: 10.1007/BF01701983. [DOI] [PubMed] [Google Scholar]
- Visioli F, Colombo C, Galli C. Oxidation of individual fatty acids yields different profiles of oxidation markers. Biochem Biophys Res Commun. 1998;245:487–489. doi: 10.1006/bbrc.1998.8463. [DOI] [PubMed] [Google Scholar]
- Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary-and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- Wang L, Reiterer G, Toborek M, Hennig B. Changing ratios of omega-6 to omega-3 fatty acids can differentially modulate polychlorinated biphenyl toxicity in endothelial cells. Chem Biol Interact. 2008;172:27–38. doi: 10.1016/j.cbi.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Hayes JD, Henderson CJ, Wolf CR. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc Natl Acad Sci U S A. 2007;104:19589–19594. doi: 10.1073/pnas.0709483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci U S A. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann M, Wassermann D, Cucos S, Miller HJ. World PCBs map: storage and effects in man and his biologic environment in the 1970s. Ann N Y Acad Sci. 1979;320:69–124. doi: 10.1111/j.1749-6632.1979.tb13137.x. [DOI] [PubMed] [Google Scholar]
- Wilson PW. Evidence of systemic inflammation and estimation of coronary artery disease risk: a population perspective. Am J Med. 2008;121:S15–20. doi: 10.1016/j.amjmed.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Yin H, Liu W, Goleniewska K, Porter NA, Morrow JD, Peebles RS., Jr Dietary supplementation of omega-3 fatty acid-containing fish oil suppresses F2-isoprostanes but enhances inflammatory cytokine response in a mouse model of ovalbumin-induced allergic lung inflammation. Free Radic Biol Med. 2009;47:622–628. doi: 10.1016/j.freeradbiomed.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakkar M, Van der Heiden K, Luong le A, Chaudhury H, Cuhlmann S, Hamdulay SS, Krams R, Edirisinghe I, Rahman I, Carlsen H, Haskard DO, Mason JC, Evans PC. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 2009;29:1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- Zamir R, Athanasiadou M, Nahar N, Mamun MI, Mosihuzzaman M, Bergman A. Persistent organohalogen contaminants in plasma from groups of humans with different occupations in Bangladesh. Chemosphere. 2009;74:453–459. doi: 10.1016/j.chemosphere.2008.09.043. [DOI] [PubMed] [Google Scholar]