Abstract
Imbalances in cancer cell redox homeostasis provide a platform for new opportunities in the development of anticancer drugs. The control of severe dose-limiting toxicities associated with redox regulation, including myelosuppression and immunosuppression, remains a challenge. Recent evidence implicates a critical role for redox regulation and thiol balance in pathways that control myeloproliferation, hematopoietic progenitor cell mobilization, and immune response. Hematopoietic stem cell (HSC) self-renewal and differentiation is dependent upon levels of intracellular reactive oxygen species (ROS) and niche microenvironments. Redox status and the equilibrium of free thiol:disulfide couples is important in modulating immune response and lymphocyte activation, proliferation and differentiation. This subject matter is the focus of the present review. The potential of redox modulating chemotherapeutics as myeloproliferative and immunomodulatory agents is also covered.
Keywords: Redox signaling, Glutathione (GHS), Thiol, Myeloproliferation, Immune response, Cancer
1. Introduction
Key to the development of any successful therapeutic lies the understanding of disease etiology and progression. Redox (reduction/oxidation) regulation is critical in cellular homeostasis, enzyme activation, gene expression, DNA synthesis and cell cycle regulation (Trachootham et al., 2008). Redox status is controlled by free and protein incorporated thiols that exist in both oxidized and reduced states. Thiol status may be defined by the equilibrium of free thiol :disulfide bonded. This includes levels of cysteine (Cys): cystine (CySS), reduced glutathione (GSH): oxidized glutathione (GSSG), and the post-translational modification of redox sensitive cysteines (e.g. S-glutathionylation) The redox state of these cysteinyl residues is determined by redox environment and disulfide reductases, including thioredoxin (Trx; for Cys:CySS), glutathione reductase (for GSH:GSSG), and the glutaredoxin (Grx)/sulfiredoxin (Srx) systems. Under oxidizing conditions, protein thiols can form intra- and inter-molecular disulfides whereas a reducing environment will favor “free” thiols. The disruption of cellular redox homeostasis is characteristic of a number of disease pathologies and as such, presents therapeutic opportunity.
Glutathione (GSH) is the hallmark redox buffer in living cellular systems. It is not surprising that enzymes involved in GSH synthesis are altered in many human pathologies (Townsend et al., 2003b). GSH is a tripeptide of glutamic acid, cysteine and glycine and is the predominant non-protein thiol in biological systems. In addition to an oxidized or reduced state, GSH may also exist as a mixed disulfide. Extra- and intracellular balance of the GSH:GSSG ratio is critical in maintaining redox homeostasis. In association with functioning as a redox cofactor for catalysis by glutathione S-tranferases (GST) and glutathione peroxidases (GPx), GSH plays critical roles in metabolism, signal transduction, apoptosis and protein and gene expression via thiol:disulfide exchange reactions (Townsend et al., 2003b). Glutathione-dependent redox signaling may also be mediated through post-translational modifications involving covalent binding of GSH to protein cysteine residues (e.g. S-glutathionylation). For example, S-glutathionylation of PKA, PKC, ASK1, and NFκB impacts key signaling pathways involved in cancer cell proliferation and survival (Brigelius-Flohe, 2006; Townsend, 2007). Chronic GSH deficiency is associated with immune disorders, accelerated pathogenesis of viral disease and increased incidence of malignancies. Furthermore, some cancer drugs may exacerbate disease pathologies by reducing plasma and hepatic GSH concentrations. However, many tumors show elevated levels of GSH emphasizing the involvement of dysregulation of GSH homeostasis in the disease.
Oxidative stress and redox dysregulation have direct consequences in the initiation and progression of cancer causing aberrant energy metabolism, deregulation of antioxidant enzymes and signaling cascades involving pathways of survival, proliferation, angiogenesis, and metastasis (Giles, 2006; Gius and Spitz, 2006; Szatrowski and Nathan, 1991). Constitutively elevated intrinsic levels of oxidative stress in malignant cells and primary cancer tissues has been linked with increases in the generation and accumulation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Konstantinov et al., 1987; Szatrowski and Nathan, 1991; Zhou et al., 2003), disruption of thiol and non-radical circuits, and the disruption in the expression of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase, glutathione S-transferases (GST) and peroxiredoxins (Hu et al., 2005; Oberley and Oberley, 1997; Saydam et al., 1997; Tew, 1994). Antioxidant deregulation may further influence cancer progression by contributing to metastasis and drug resistance. Chronic oxidative stress can activate redox regulated transcription factors (nuclear factor κB (NF-κB), nuclear factor-like 2 (Nrf2)), oncogenes and signaling pathways. These may further act to alter the expression of antioxidants, survival factors, such as B-cell lymphoma 2 (BCL2) and myeloid leukemia cell differentiation protein 1 (MCL1), and inhibit the expression of pro-apoptotic pathways involving caspases. In addition, genetic instability caused by ROS/RNS-induced mutagenesis may provide cancer cells with additional opportunities to adapt to oxidative stress. Cancer progression can occur via regulation of redox dependent gene expression in pathways involving cellular proliferation, senescence, metastasis, and angiogenesis.
As key mediators of cellular oxidative stress and redox state, ROS and RNS also provide plausible platforms for drug design. In fact, the efficacy of a number of established anticancer drugs, may be linked to the induction of ROS and subsequent redox dysregulation (Davis et al., 2001). In addition, the importance of non-radicals and thiols in redox metabolism has recently been emphasized in the definition of oxidative stress (Jones, 2008). The utility of GSH in antiviral therapies has been shown (Fraternale et al., 2009). Thus, non-radical based redox therapies represent an opportunity in the development of cancer drugs.
A role for redox signaling in the expression of a number of oncogenes and tumor suppressors has recently been described. Constitutive up-regulation of Ras is associated with elevated ROS production and cellular oxidative stress, as is the chimeric BCR-ABL tyrosine kinase (Nair et al., 2010; Trachootham et al., 2008). Similarly, p53 has a critical role in ROS homeostasis and regulates a number of genes associated with oxidative stress, including SOD and glutathione peroxidase (Drane et al., 2001; Tan et al., 1999). p53 inactivation increases oxidative stress and enhances tumor growth, both of which can be suppressed via antioxidant supplementation (Sablina et al., 2005). During cancer initiation S-glutathionylation of p53 prevents DNA binding (Velu et al., 2007). Redox sensitive cysteines may be considered redox switches that mediate cell-signaling events. Further study of the role of non-radicals and thiol:disulfide reactions in redox signaling may serve to identify specific therapeutically-useful thiol targets.
Redox targeted drugs, including those that manipulate pro- and antioxidant levels and that may act to modulate cellular redox state, have recently been reviewed (Wondrak, 2009). Wondrak cites studies indicating that a strength of such therapeutics may lie in diminished systemic toxicities and off-target effects, in addition to synthetic lethality which confines drug cytotoxicity to cancer cells with specific loss of function mutations in tumor suppressor genes or oncogenes. While advances in drug selectivity are crucial, improving therapeutic activity, overcoming drug resistance, and maintaining quality of life remain important components of drug development. Recent evidence has identified a possible role for blood GSH levels in myeloproliferation, hematopoetic progenitor cell mobilization and immune response. Since myelosuppression is a dose limiting toxicity of many cancer drugs, redox chemotherapeutics that enhance hematologic and immune recovery could be of great value. Subsequent sections focus on the roles of redox modulating chemotherapeutics in myeloproliferation and immune regulation.
2. Redox and the bone marrow environment
2.1 Redox and homeostasis of the immune system
Patients receiving chemotherapy and/or ionizing radiation (IR) often develop acute and residual bone marrow injury that ultimately limits treatment success in addition to adversely affecting quality of life. Dose reduction due to toxic side-effects compromises treatment outcomes of curable malignancies often as a result of the narrow therapeutic index of cytotoxic agents (Daniel and Crawford, 2006). Myelosuppression is the most common dose-limiting toxicity associated with many current chemotherapy regimens. Toxicity may result from leucopenia, a decrease in the number of white blood cells (leukocytes), which leaves the patient immunocompromised and at risk for infection. Alternatively, or in addition, thrombocytopenia, a decrease in blood platelets, or anemia, a deficiency in red blood cells, may also occur. Acute myelosuppression may result from high rates of apoptosis in rapidly proliferating hematopoietic progenitor cells (HPCs) as compared to the relatively quiescent hematopoietic stem cells (HSCs). Treatment with hematopoietic colony-stimulating factors is sometimes necessary. Severe myelosuppression, resulting from the destruction and induced senescence of HSCs, may require bone marrow transplantation.
Despite drug-design efforts towards site-specific inactivation and synthetic lethality (Kaelin, 2005), the inability of drugs to differentiate between malignant and normal cell populations remains a primary hurdle in treatment efficacy. Inducing oxidative stress may be counter-productive and interfere with the ability of chemotherapy drugs to cause cancer death (Shacter et al., 2000). Anti-cancer drugs often augment the formation of ROS. Peripheral polymorphonuclear leukocytes from patients receiving chemotherapy for hematologic and solid malignancies produce elevated levels of hydrogen peroxide and superoxide anions (Sangeetha et al., 1990). These patients also have increased levels of lipid hydroperoxides and thiobarbituric acid-reactive substances (Ladner et al., 1989). While ROS formation can mediate apoptosis it is also associated with bone marrow cytotoxicity, myelosuppression and immunosuppression. In general, chemotherapy and radiation therapies result in increased ROS, depletion of critical plasma and tissue antioxidants and the disruption of cellular thiol redox homeostastis. Analyses of light-density bone marrow derived colony forming units (CFUs) in treated mice suggest that platinum therapies specifically target HSCs (Evans et al., 1984; Su et al., 2000). HSC toxicity may be caused by DNA damage and/or the dysregulation of redox homeostasis and associated redox-mediated signaling events. Recent studies have suggests that ROS cause residual bone marrow injury by selectively inducing HSC senescence through redox-dependent activation of the p38 mitogen-activated protein kinase (p38)-p16Ink4a (p16) pathway (Ito et al., 2006).
Thiol balance is key to maintaining normal activity of the immune system. A number of immune cells, including B and T lymphocytes, require exogenous cysteine to maintain functions such as activation, proliferation, and differentiation. However, T lymphocytes can take up cysteine but not cystine (Ishii et al., 1987). During antigen presentation, dendritic cells (DC) increase cystine uptake via cysteine-exchanger antiporters. Intracellular reduction of cystine to cysteine, mediated by Trx, and subsequent cysteine release allows T-cell activation. DCs secrete Trx upon cognate T-cell recognition (Angelini et al., 2002). Thus, inhibition of either DC cystine uptake or Trx serves to block T lymphocyte proliferation. Schwertassek et al. showed that extracellular Trx1 selectively targets CD30 on activated lymphocytes, resulting in disulfide reduction and changes in the conformation and functional properties of the CD30 ectodomain (Schwertassek et al., 2007). Studies using GSH and the synthetic thiol, N-acetyl cysteine (NAC) confirmed the importance of cysteine in the regeneration of cellular immunity, lymphocyte cell proliferation, interleukin (IL)-2 production and synthesis and turnover of the IL-2 receptor (Cetinkale et al., 1999; Eylar et al., 1993; Hadzic et al., 2005). GSH depletion using BSO and thiol oxidation results in the suppression of T cell clone formation and cell cycle disruption (Hamilos et al., 1989; Levy et al., 1992).
T cells differentiate into two subsets: T helper (Th)-1 and Th2, the differentiation of which is characterized by different cytokine production profiles (Figure 1). Th1/Th2 imbalance is the basis for a number of immune associated pathologies. Thiol balance has been shown to modulate Th1/Th2 lymphokine production by T cells (Jeannin et al., 1995; Monick et al., 2003). Augmentation of intracellular thiol pools in stimulated naïve splenocytes using NAC showed GSH independent inhibition of STAT4 and IFN-γ (a Th1-specific cytokine) induction by αCD3/IL-12. However, splenocyte production of the Th2-specific cytokine, IL-4, was increased (Monick et al., 2003). NAC and GSH treatment restores redox balance, leading to down-regulation in the expression of CD30 by activated B and T cells (Giordani et al., 2002). CD30 has roles in diseases that are characterized by elevated CD4 lymphocytes that produce Th2 cytokines, such as HIV and autoimmune disease. Furthermore, defective antigen processing is concomitant with low levels of intra-macrophage GSH (Collins et al., 1991; Short et al., 1996). Short et al. proposed that GSH depletion results in the inability of antigen presenting cells to reduce antigen disulfide bonds required prior to antigen processing and to decrease the activity of thiol proteases also required for processing and cleaving of the invariant chain from major histocompatability complex II (Short et al., 1996).
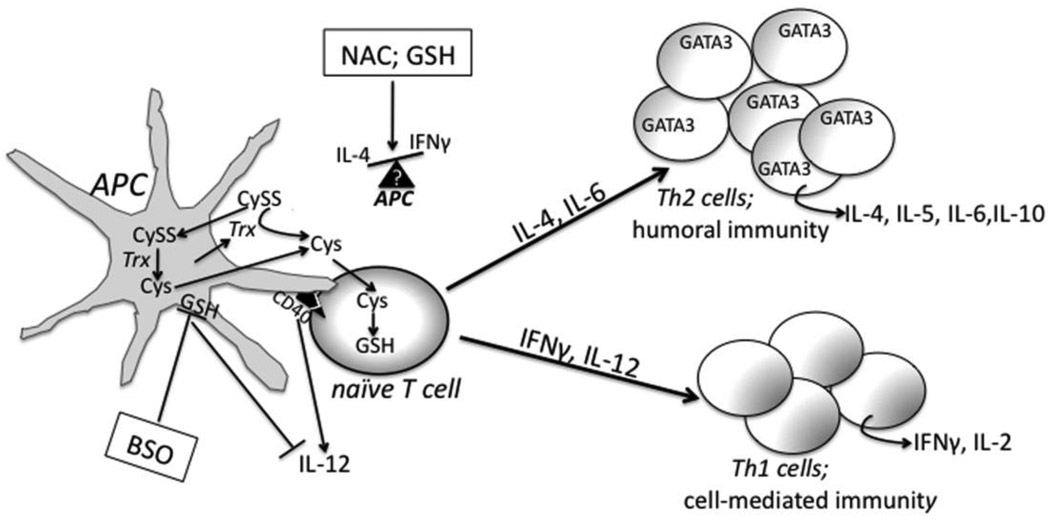
Figure 1.
Redox, cytokines, and T lymphocyte differentiation. Naïve T cells require cysteine (Cys) for proliferation and differentation but cannot take up cystine (CySS). Antigen presenting cells (APC) provide Cys for T lymphocytes by secreting thioredoxin, which reduces CySS to Cys, or by actively taking up CySS and intracellulary reducing CySS to Cys. A number of different cytokines mediate T cell differentation into Th1 or Th2 cells. IFNγ and IL-12 mediate a Th1 response while IL-4, Il-5, IL-6, and Il-10 are associated with a Th2 immune response. Th2 cells are characterized by GATA3 expression. The interaction of CD40 on APC with the CD40 ligand on T-cells mediates IL-12 production. Buthionine sulfoxime (BSO) inhibits GSH synthesis and results in IL-12 supression and a Th2 response. N-acetyl-cysteine (NAC) or glutathione (GSH) treatment effects on cytokine production and Th1/Th2 differentiation and proliferation depend on APC type and cytokine milieu. See text for additional details.
Antigen presenting cells (APC) are central in the initial determination of mechanisms in Th1 or Th2 –mediated immune response patterns. GSH depletion in APCs decreases IL-12 secretion and biases cytokine patterns towards Th2 cell production. Alternatively, macrophages with increased GSH levels actively produce IL-12 leading to Th1 polarization (Murata et al., 2002; Peterson et al., 1998). Utsugi et al. linked macrophage IL-12 production to the negative regulation of JNK and the activation of p38 (Utsugi et al., 2003; Utsugi et al., 2002). The interaction of CD40 on DC with CD40 ligand of T cells mediates the DC production of IL-12. Giordani et al showed that NAC administration to B-cell down-regulated cell surface expression of CD40 and CD27 and attenuated IL-4 production (Giordani et al., 2002). Studies using NAC treatment indicate differential involvement of different types of APCs and cytokine milieus in the outcome of a Th1 or Th2 immune response. Additional research using antioxidants without an SH group such as L-carnitine, catalase, ascorbic acid, and SOD and molecules lacking a free SH indicate that free SH groups are critical in redox-mediated lymphokine production (Jeannin et al., 1995). These studies emphasize the potential impact and therapeutic value of redox targeted therapeutics in immunommodulatory processes.
The modulation of transcription factors by redox modulating drugs may also contribute to an altered immune response. Stress response transcription factors, such as NF-κB and AP-1, incorporate “redox switches” and are influenced by redox state, enabling the regulation of the expression of a number of downstream target genes with antioxidant roles. Both NF-κB and AP-1 have critical roles in IL-2 transcription, an obligatory step in T cell proliferation (Hadzic et al., 2005; Jain et al., 1995). Drugs such as Tavocept and amifostine (see discussion in Section 3.5 and 3.6) have roles in inducing expression of both NF-κB and AP-1, suggesting a potential role in immunomodulation. S-glutathionylation of redox sensitive cysteines provides additional potential to modulate the activity of key transcription factors and signaling pathways. Using two dimensional gel electrophoresis and mass spectrometry techniques, Fratelli et al. reported that a number of proteins are S-glutathionylated in oxidatively stressed human T lymphocytes (Fratelli et al., 2002). Increased protein S-glutathionylation has been proposed to play a role in GSH depletion associated with T lymphocytes in HIV patients (van der Ven et al., 1998). These data suggest the S-glutathionylation induced by agents such as glutathione disulfide mimetics, like NOV-002, and GSTP inhibitors, such as Telintra (see Sections 3.3 and 3.4), may be directly involved in modulating T lymphocyte behavior.
2.2. Human Stem Cells in the bone marrow microenvironment
The bone marrow compartment is responsible for the production of all differentiated hematopoietic cells in the peripheral blood. Within the bone marrow environment HSCs, HPC’s, and mature plasma cells have their own environmental niches associated with a number of factors that maintain and influence HSC number and destiny. Committed progenitors tend to localize to the bone marrow center (Lo Celso et al., 2009).
By definition, HSCs are self-renewing and able to give rise to cells of all hematopoietic lineages. However, the majority of these cells remain quiescent. It has been estimated that ~75% of HSC are in the G0 phase of the cell cycle at any given time (Cheshier et al., 1999). HSCs may reside at the bone-bone marrow interface (osteoblastic niche), where the microenvironment encourages HSC maintenance and quiescence, or around the blood vessels (vascular niche), where the microenvironment favors HSC proliferation and differentiation (Calvi et al., 2003; Iwasaki and Suda, 2009; Lo Celso et al., 2009; Nilsson et al., 2001; Wilson et al., 2008) (Figure 2). Each population is defined by the expression of adhesive molecules, cytokines and chemokine signaling, all of which aid in maintaining a “hold” in a certain niche. The chemokine CXCL12 regulates HSC migration to the vascular niche. Depletion of CXCR4 results in HSC reduction in the vascular niche, emphasizing the importance of CXCL12-CSCR4 interaction in niche maintenance (Arai et al., 2009; Sugiyama et al., 2006). Osteoclast and osteoblast-mediated bone remodeling results in a Ca2+ gradient in the endosteum, enabling calcium-sensitive HSCs to sense and migrate appropriately (Adams et al., 2006).
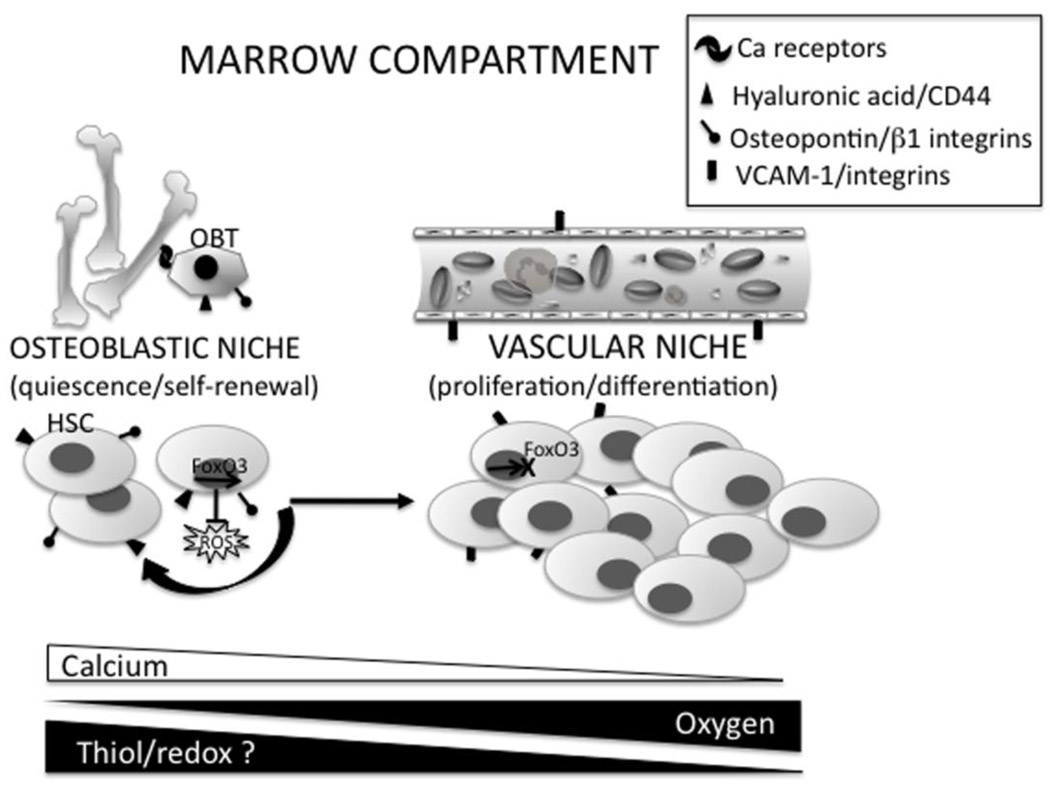
Figure 2.
Redox state and hematopoietic stem cell bone marrow niche microenvironment. Depending on the expression of adhesive molecules, cytokines, and chemokine signaling, hematopoietic stem cells (HSCs) are located at the osteoblastic niche, with the osteoblats (OBT), or at the vascular niche. HSCs in the osteoblastic niche maintain self-renewal and quiescent capabilities while migration to the vascular niche induces proliferation and differentiation. Movement to one niche to another represents a microenvironment gradient of calcium, oxygen and potentially thiol/redox state. In quiescent/self-renewing HSC cells in the osteoblastic niche FoxO3 represses ROS accumulation. In the vascular niche FoxO3 is cytoplasmic and repressed in hematopoietic progenitor cells, indicating an important role for FoxO3 in HSC differentiation. See text for additional details.
As a tissue, the bone marrow is relatively hypoxic (1% to 2% O2) (Cipolleschi et al., 1993). However, as might be expected, oxygen levels are higher closer to the vascular niche, presenting an oxygen gradient. Iwasaki et al., propose that the hypoxic osteoblastic environment encourages quiescence in HSCs and movement to the more oxygenated vascular niche promotes HSC differentiation, thus providing myeloid and lymphoid hematopoietic cells to the peripheral blood supply (Iwasaki and Suda, 2009). While both oxygen and calcium gradients have been considered, the possibility that a redox gradient may influence HSC sub-population migration and differentiation does not seem unreasonable. Older mice show accumulation of HSCs more distantly in the endosteum as well as increased levels of endogenous DNA damage in their HSCs (Kohler et al., 2009; Rossi et al., 2007). This is correlated with an increasing number, but decreasing function of aged HSCs (Chambers et al., 2007) and perhaps may be explained by the accumulation of oxidative stress associated with the aging process. The distinct features of niche microenvironment and HSC sub-populations may present opportunities for the development of targeted therapeutics in addition to personalized medicine.
Studies in the 1950s indicated a critical role for cysteines and thiols in bone marrow cell proliferation (Baldini and Sacchetti, 1953). The role of ROS and the balanced redox environment in regulating the overall fate, function, and lifespan of HSCs is of consequence. Long-term, self-renewing HSCs have low levels of intracellular ROS. However, high ROS, which can occur during chemotherapy, may result in senescence, apoptosis and a failure to self renew. Mice deficient in ROS regulating genes have HSCs that cannot maintain quiescence or self-renewal capacities (Naka et al., 2007). Based on intracellular ROS content, two subpopulations of HSC have been indentified. While both populations showed identical cell surface markers, ROSlow HSCs retained self-renewal capabilities in serial transplantation assays, whereas this capacity was diminished in the transplanted ROShigh HSCs. NAC mediated thiol antioxidant treatment was shown to rescue HSC self renewal (Jang and Sharkis, 2007). Correlation of these subpopulations to migrating tendencies, niche location, and differentiation potential may be of great interest.
The pathways behind HSC intracellular ROS regulation are currently under investigation. Studies using HSCs from Nrf2null mice indicate an increase in in vivo sensitivity to oxidative stress in Nrf2 deficient cells implying a role for this transcription factor in the redox-sensitive regulation of HSC function. Studies using chemoprotective agents have indicated that increasing the activity of Nrf2 reduces tumor incidence via the regulation of genes that both metabolize carcinogens and defend against oxidative stress (Fahey et al., 2002; Ramos-Gomez et al., 2001). Expression of Id1, a helix-loop-helix transcription factor with critical roles in myeloid differentiation, is mediated through redox-dependent mechanisms (Tanaka et al., 1998). The forkhead O (FoxO) family of transcription factors protect quiescent HSC cells from oxidative stress via the up-regulation of ROS detoxifying genes such as MnSOD, catalase, and GADD45. FoxOs are expressed commensurate with the transition of HSCs to myeloid progenitors. Conditional knockout of FoxO results in increased ROS and defects in the cell cycle and repopulating capacities of HSC. Treatment with the antioxidant NAC restored all defects, in addition to the FoxO transcriptional program (Tothova et al., 2007). Studies using FoxO3 germ-line knockout animals indicated a role for p38 MAPK in these pathways (Miyamoto et al., 2007). Such studies suggest that there are complex numbers of transcriptional programs at play that may be exclusive to each subpopulation of HSC. The role ROS in HSC function has been reviewed elsewhere (Naka et al., 2008). Because of the documented involvement of thiol oxidation/reduction in cell signaling (Giles, 2006; Trachootham et al., 2008), and the post-translational modification of proteins via redox sensitive cysteines, it is reasonable to speculate that differences in ROS in myeloid progenitor and quiescent HSCs may be important intracellular signaling events that drive HSC differentiation. Future research may show that post-translational modifications such as S-glutathionylation, may be key in regulating these events.
2.3 Redox and cytokines
The regulated expression of growth factors and cytokines has important consequences in numerous pathways involved in hematopoiesis, stem cell mobilization, and immune system modulation. Cytokines such as IL-3, IL-8, IL-11, fms-like tyrosine (Flt)-3, and stem cell factor (SCF) have known roles in CD34+ mobilization (Laterveer et al., 1996; Maurer et al., 2000). As discussed, IL-12, IL-4, and IL-6 have important roles in maintaining Th1 and Th2 balance and differentiation. Granulocyte colony stimulation factor (G-CSF) and granulocyte macrophage CSF (GM-CSF) have critical roles in the differentiation of progenitor cells into granulocytes and monocytes (Gazitt, 2001). The activation of signal transduction pathways and transcription factors that lead to cytokine expression are redox regulated. Thiols and the modulation of redox equilibrium influence the transcription of IL-1, IL-4, IL-6, IL-7, IL-8 and TNF-α (Jeannin, et al. 1995; Rovin, et al. 1997; Gosset, et al. 1999; Haddad 2002; (DeForge et al., 1992). Studies using NAC and GSH indicate that antioxidant and GSH precursors down-regulate cytokine activation and synthesis, suggesting that disruption of glutathione homeostasis has the potential to impact inflammatory disease (Barrett et al., 1999; Haddad et al., 2001). Alternatively, GSH depletion using BSO results in the upregulation of ROS and enhances cytokine secretion, particularly TNF-α, IL-6, and IL-8 (Gosset et al., 1999). Modulation of the pO2 environment results in a dose-dependent release of cytokines in alveolar epithelial cells (Barrett et al., 1999; Haddad et al., 2001). These studies imply a potentially important link between cytokine expression profiles and redox sensitive microenvironments, such as may occur in bone marrow niches.
Intracellular macrophage GSH levels affect macrophage cytokine propensities and Th1/Th2 balance (Murata et al., 2002). Increases in cell surface thiols are directly correlated with IL-2 production in T-cells (Gelderman et al., 2006). Furthermore, secreted Trx can act as a potent cytokine that induces IL-12 production and a Th1 mediated immune response (Pekkari et al., 2001). Redox-sensitive transcription factors and cofactors also have key roles in the maintenance of cytokine balance. The expression of HIF-1α and NFκB are tightly regulated by GSH/GSSG equilibrium and are involved in the induction of a number of cytokines and growth factors relevant to hematopoiesis and immune responses (Haddad, 2002; Haddad et al., 2000). These include the regulation on erythropoietin (Epo), vascular endothelial growth factor (VEGF), and a host of genes involved in T-cell development, maturation and proliferation. Further evaluation of the effects of redox modulating chemotherapeutics on the expression profiles of cytokines and growth factors in myeloproliferative and immune responses may be informative. However, such studies are likely to be complicated by the pleiotropic effects of many cytokines when placed in a specialized environment. (Gosset et al., 1999; Haddad, 2002; Jeannin et al., 1995; Rovin et al., 1997)
3. Redox modulating chemoprotective agents
3.1 Glutathione
The utility of agents such as NAC, NOV-002, and free thiol containing drugs in directing T cell and APC cytokine production has potential therapeutic utility in patients undergoing immunosuppressive treatments. Recent in vivo studies suggest the plasticity of the extracellular redox environment in lymphoid tissues may be a crucial component in controlling the behavior of different cell populations during immune response. Specifically, the local lymphoid microenvironment is reductive and free thiol content becomes elevated during an immune response (Castellani et al., 2008). The immunomodulatory value of GSH is evident in the treatment of viral infections and specific Th1 and Th2-mediated pathologies, such as autoimmune and allergic diseases. HIV patients supplemented with α-lipoic acid, a GSH-augmenting disulfide, showed restoration of total GSH levels and improved proliferative response of T-lymphocytes (Jariwalla et al., 2008). Administration of γ-glutamyl cysteinyl ethyl ester (γ-GCE), a membrane-permeating GSH precursor, in a mouse asthma model increased the GSH/GSSG ratio in addition to reducing levels of IL-4, IL-5, and IL-10, enhancing levels of IL-12 and IFN-γ, and suppressing eosinophil infiltration (Koike et al., 2007). These data indicate that GSH alters Th1/Th2 balance through APC IL-12 production and suggest that redox-mediated suppression of chemokine production and eosinophil migration could contribute to the amelioration of disease pathologies.
There is evidence that enzymes involved in GSH synthesis and homeostasis are altered in disease states (Townsend and Tew, 2003). Buthionine sulfoximine (BSO) is an irreversible inhibitor of γ-glutamylcysteine synthetase and interferes with de novo GSH synthesis reducing GSH levels and can sensitize some tumors to alkylating agents (Friedman et al., 1989). However, clinical trials with BSO produced severe leukopenia and thrombocytopenia that limited its therapeutic value (Bailey et al., 1994; O'Dwyer et al., 1996). The following sections summarize some of the agents that have been developed in cancer therapy to manipulate GSH and the general redox environment.
3.2 N-Acetyl Cysteine
De novo synthesis of GSH involves the formation of γ-glutamylcysteine followed by addition of glycine, where cysteine is the rate-limiting amino acid. N-Acetyl Cysteine (NAC) is a synthetic thiol that is deacetylated in tissues/cells to form cysteine, stimulating GSH synthesis (Zafarullah et al., 2003). In addition, NAC, is itself an ROS-scavenger, yielding NAC-disulfide end products (Zhang et al., 1995). Bioavailability of free GSH is limited since passage through cell membranes requires the initial breakdown of GSH into dipeptides and amino acids, followed by transport of these precursors into the cell. NAC penetrates cell membranes and has essentially no toxicity. Furthermore, NAC inhibits TNF-α release, the activation of pro-inflammatory cytokines and cellular apoptosis (Malorni et al., 1993; Sagara et al., 1996).
By restoring GSH levels in hepatic cells, NAC has been used as a protective agent against liver ischemia and reperfusion (Kelly, 1998). Alteration of intracellular redox balance associated with severe glutathione depletion is a hallmark of many viral diseases. Several studies have shown NAC to be a viable therapeutic in the treatment of HIV and other viral infections (Kelly, 1998). NAC usage in HIV positive patients restores GSH levels, prevents NFκB activation and HIV viral replication, and inhibits CD4 D2 mediated viral entry (Matthias et al., 2002; Nakamura et al., 2002). Mechanisms proposed for this activity include the enhancement of the antibody-dependent cellular cytoxicity of neutrophils (Roberts et al., 1995) and the ability to attenuate CD4+ lymphocyte decline (Akerlund et al., 1996).
Ongoing clinical studies use NAC as a chemopreventive and chemoprotectant in cancer treatment. While there is little evidence that NAC has direct anti-tumor capabilities, NAC administration has been shown to prevent cancer development in tumor-prone mouse models, including ROS-susceptible Atm- and p53-deficient mice (Reliene and Schiestl, 2006; Sablina et al., 2005). NAC also suppresses the activation and function of tissue invasion-associated matrix metalloproteinase-9 (Pei et al., 2006). Neuwelt et al. showed that NAC successfully protected bone marrow toxicity without compromising treatment in a rat brain tumor model involving the use of three chemotherapeutics (Neuwelt et al., 2004). NAC also has a protective effect against Carmustine (BCNU) induced myelotoxicity (El-Sayed el et al.). Clinically, BCNU treatment is associated with oxidative stress-mediated apoptosis and delayed bone marrow toxicity. Helal et al. reported that BCNU inactivates glutathione reductase (GR); a NAPDH-dependent antioxidant enzyme involved in the regeneration of GSH and protein thiols from disulfides (Helal and Helal, 2009). This mechanism has been linked to the carbamoylation of GR active site cysteines (Ahmad and Frischer, 1985). NAC may act to neutralize free radicals, increases GSH synthesis, inhibit TNF-α and potentially prevent GR inactivation, all of which may serve to prevent myelotoxicity, and perhaps enhance myeloproliferation. NAC treatment can restore the ability of HSC to self renew (Tothova and Gilliland, 2007). This may be mediated through NAC-mediated ROS scavenging and concomitant regulation of p38 MAPK (Jang and Sharkis, 2007).
3.3 NOV-002
NOV-002 is a complex of GSSG with cisplatin at an approximate ratio of 1000:1, where GSSG is the active component. NOV-002 administration results in elevated serum and tissue levels of stable GSSG (Townsend et al., 2008a; Townsend et al., 2008b). GSSG can act as proximal donor in S-glutathionylation, explaining the increases in S-glutathionylated cellular and serum proteins in response to NOV-002 treatment (Uys et al.) Preclinical and clinical studies indicate that non-small cell lung cancer patients receiving NOV-002 in association carboplatin and paclitaxel show increased chemotherapy tolerance, improved hematologic and immune parameters, improved quality of life, increased tumor response and survival (Pazoles, 2010 AACR abstract #17021; Townsend et al., 2008a). These initial preclinical studies suggest a role for NOV-002 in the production of regulatory cytokines and growth factors that serve to enhance hematologic and immune recovery after chemosuppression (Bailey et al., 1997). When added to monocyte cultures, NOV-002 induced a dose-dependent increase in interleukins IL-1β, IL-2, IL-3, and Il-6 as well as in TNFα, TNFγ and IFNα. Similar cytokine induction was seen in NOV-002 treatment of chemo-suppressed animals (Novelos, unpublished data:Cohen Independent Research Group, Novelos report Sept 2005). Increasing levels of circulating lymphocytes, monocytes, T-cells and natural killer cells indicated that NOV-002 has myeloproliferative properties (Townsend et al., 2008b). Treatment of the pre-myeloid HL-60 cell line enhanced protein S-glutathionylation and activated AKT, JAK2, and STAT5 kinase pathways, all involved in the regulation of hematopoiesis/myeloproliferation (O'Shea et al., 2002; Townsend et al., 2008a). Time and concentration dependent increases in JNK, p38, and ERK phosphorylation were also observed. These kinases have direct roles in cell proliferation and in the self-renewal capacity of HSCs (Ito et al., 2006).
Since GSSG cannot traverse cell membranes, NOV-002 may exert it’s effects by altering cell surface redox status and transmembrane potential through oxidation of cell surface protein thiols. NOV-002 treatment results in the decrease in cell surface sulfhydryl content (Townsend et al., 2008a). Furthermore, S-glutathionylation of trans-membrane protein cysteine residues may mediate intracellular cell growth and signaling cascades. The addition of exogenous GSSG can result in the disruption of intracellular GSH levels by shifting the equilibrium towards mixed disulfide formation (Droge et al., 1994). Alternatively, interaction with membrane-associated enzymes, such as γ-glutamyl transpeptidase (GGT), may stimulate GSH synthesis and H202 production (Dominici et al., 1999). Specifically, GGT can hydrolyze GSH to glutamic acid, and cysteine-glycine. Additional metabolism by dipeptidases results in the release of free cysteine available for cellular uptake and salvage GSH synthesis. Redox-based modulation of surface proteins has the potential to influence a number of pathways that regulate cell function (Jordan and Gibbins, 2006). For example, the sesquiterpene lactone parthenolide’s anti-lymphoma activity is partially derived though the modification of critical exofacial thiols (Skalska et al., 2009). This may explain why, in vitro, NOV-002 treatment of HL-60 cells alters cellular redox balance in addition to stimulating cell proliferation without changing cell differentiation markers (Townsend et al., 2008a).
The immunomodulatory properties of NOV-002 have been observed in ovarian cancer models where treated mice showed an increase in the infiltration of tumors and spleen by memory T cells. Splenocytes from these mice also showed a higher level of immune reactivity associated with elevated IFN-γ when challenged with tumor antigens (Righi et al., 2008 AACR abstract). Additional studies showed that NOV-002 was able to reverse the T-cell suppressive effects of myeloid derived suppressor cells generated after chemotherapy treatment (Diaz-Montero et al., 2010 AACR abstract #5620). Furthermore, NOV-002 treatment of non-small cell lung cancer patients resulted in increased circulating T-lymphocyte subsets (CD4+, CD8+, NK-T lymphocytes) as compared to chemotherapy alone. These data are supported by NOV-002’s ability to induce cell-specific proliferation in myeloid cell lines but not cancer cell lines (Bowers et al., 2010 AACR, abstract #1615). Myeloid cell GGT expression may provide selective delivery of cellular Cys. It seems probable that NOV-002 alters cellular redox balance and mediates post-translational events and that these events contribute to its myeloproliferative properties.
3.4 Targeting GSTP: Telintra
Although many electrophiles spontaneously react with GSH, glutathione S-tranferases (GSTs) can sometimes catalyze their thioether conjugation. In mammals there are six different cytosolic GST isoforms: α, μ, π, θ, ω, and ζ (Townsend and Tew, 2003). GST polymorphisms leading to altered catalytic activity have been linked to cancer susceptibility and prognosis (Kellen et al., 2007; Kraggerud et al., 2009; Raimondi et al., 2006; Townsend and Tew, 2003; Voso et al., 2008). GSTP (π) is of particular interest with regards to cancer because many tumors and cancer cell lines are characterized by high GSTP expression. Furthermore, increased expression of GSTP has also been linked to acquired chemotherapy resistance (Tew, 1994).
GST isozymes have additional regulatory roles via kinase interactions and subsequent downstream control of cellular stress response, apoptosis, and proliferation pathways. For example, GSTP interacts with the mitogen-activated protein kinase (MAPK) c-jun NH2-terminal kinase (JNK) complex and is subject to further regulation by changes in redox conditions. Oxidative stress can destabilize the GSTP:JNK complex and cause an activation of the kinase cascade (Tew, 2007) (Figure 3). Thus, GSTP serves as a sensor of intracellular changes in redox potential and has the potential to directly regulate kinase pathways, perhaps contributing to the GSTP over-expressing drug–resistance phenotype. JNK phosphorylation and subsequent trans-activation of c-Jun transcription factors have been linked to cell proliferation (Shaulian and Karin, 2001). GSTP also has a role in modulating ERK and p38 activation (Yin et al., 2000). Interestingly, the regulation of p38 is important in the maintenance of HSC self-renewal capacity and hematopoiesis (Jang and Sharkis, 2007).
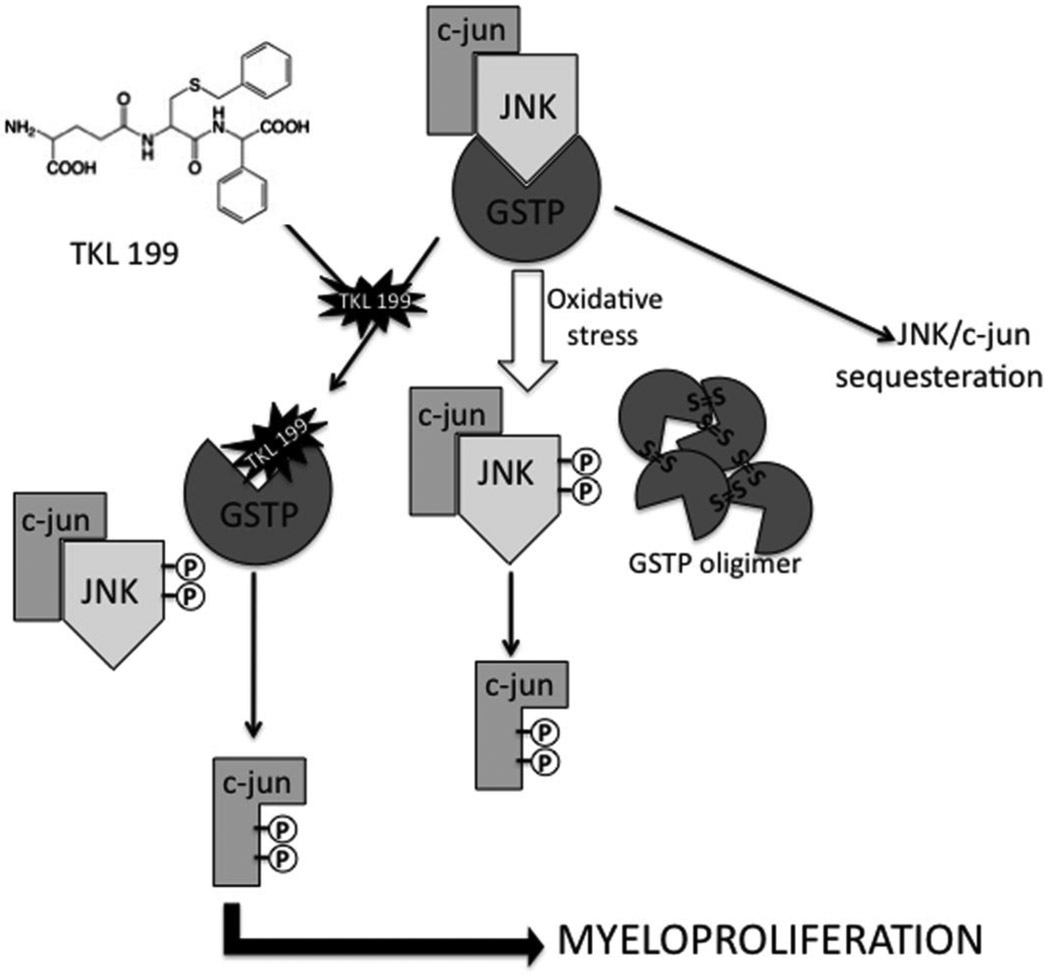
Figure 3.
Targeting the GSTP complex. During homeostasis GSTP is in a complex with JNK1 and c-jun, resulting in the sequestration of downstream JNK/c-jun mediated cellular events. Under conditions of oxidative or chemical stress GSTP disassociates from the complex and undergoes oligerimerization. JNK and c-jun are subsequently phosphorylated and take part in a variety of downstream processes depending on cell type, cell environment etc. TLK-199 (Telintra) is a selective inhibitor of GSTP that binds at the G-site and disassociates the GSTP:JNK complex, resulting in JNK and c-jun phosphorylation and downstream myeloproliferative events in bone marrow.
The involvement of GSTP in oncogenesis, tumor progression and drug resistance inspired the design and development of a peptidomimetic inhibitor of GSTP, TLK 199 [γ-glutamyl-S-(benzyl)-cysteinyl-R-(−) phenyl glycine diethyl ester], now named Telintra. While therapeutic utility initially focused on overcoming GSTP-associated drug resistance, preclinical studies in mice revealed the drug also caused increased circulating blood cells of all lineages (Ruscoe et al., 2001). Telintra increased peripheral white blood cell number in wild type mice as compared to GSTP-deficient mice. Furthermore, GSTP-null animals exhibited an increase in myeloid cell differentiation and proliferation, evidenced by elevated numbers of circulating leukocytes. Data suggest that this phenotype is associated with an increase in bone marrow progenitor cells that populate circulating mature blood cells (Gate et al., 2004). This is consistent with the ability of Telintra to dissociate GSTP from JNK, allowing kinase phosphorylation and downstream myeloproliferative effects. Additionally, the drug’s myeloproliferative effects could be mechanistically linked with activation of STAT proteins in GSTP-deficient mice. The importance of other GST isozymes in myeloproliferative events may be of additional interest. GSTA1 has been shown to suppress stress-induced activation of JNK signaling, suggesting that GST binding may be promiscuous (Romero et al., 2006). Recent evidence indicates a role for GSTP in the catalysis of S-glutathionylation (Townsend et al., 2009). Thus, GSTP has the potential to mediate the S-glutathionylation of a number of proteins that may be involved in myeloproliferative events (e.g. JNK and SHP-1,-2 etc.).
Telintra has recently shown positive results in an ongoing Phase 2 clinical trial for myelodysplastic syndrome (MDS) (online company reference: http://www.telik.com/pr/2010/pr_2010_0608.html), a stem cell disorder characterized by ineffective blood cell production and an increased risk for transformation to acute leukemia. Telintra treated patients with low to intermediate-1 risk MDS demonstrated multilineage hematologic improvement including decreased requirements for red blood cell, platelet, and growth factor support. Additional clinical trials are focusing on the use of Telintra in the treatment of chronic idiopathic neutropenia and additional blood disorders.
3.5 Amifostine (WR-2721; Ethyol)
Amifostine (WR-2721) has been used with traditional anticancer drugs as a cytoprotective chemoprotectant with success in Phase III clinical trials targeting ovarian cancer, head and neck cancer, and lung cancer (Culy and Spencer, 2001). Amifostine is a phosphorylated aminothiol compound originally developed by the Walter Reed Army Institute as a radioprotective agent for military personnel (Capizzi, 1999). Pharmacodynamic studies have identified that dephosphorylation of amifostine by the membrane-bound enzyme alkaline phosphatase converts it to the active metabolite, the free thiol WR-1065 (Korst et al., 1997). Because normal tissues generally have higher levels of alkaline phosphatase, better vascularization and higher pH, the active species of amifostine (WR-1065) preferentially locates there. Chemoprotective activity is linked to the ability of WR-1065 to prevent or repair oxidative stress-induced DNA damage by scavenging free radicals, donating hydrogen ions to free radicals and direct binding and inactivation of cytotoxic drugs.
Studies also indicated that amifostine stimulates HSCs. Pretreatment with amifostine enhanced formation to hematopoietic colonies by up to 7-fold in healthy bone marrow (List et al., 1996). Similar HSC stimulation was seen in MDS patients (List et al., 1997). Furthermore, amifostine induced apoptosis in MDS cell lines (Ribizzi et al., 2000) and selectively protected normal, but not malignant, hematopoietic progenitor cells against cytotoxic drugs. This targeted protection may be explained by the inhibition of stress-induced apoptosis in normal cells via activation of NF-κB/Rel transcription factors (Romano et al., 1999). These features have inspired a number of ongoing clinical uses of amifostine in MDS patients and in patients receiving HSC transplants.
The ability of amifostine to protect selectively hematopoeitic progenitor cells suggests that during cytokine deprivation, amifostine has trophic effects similar to those of hematopoietic cytokines. The free thiol group of WR-1065 most likely impacts a number of intra- and extra-cellular redox-mediated events including those involving redox sensitive transcription factors and cell signaling. Amifostine has recently been identified as a potent hypoxia-mimetic that induces p53, cyclin-dependent kinase inhibitor p21 and JNK expression (Koukourakis et al., 2004; Pluquet et al., 2003). The bone marrow niches are defined by a gradient of oxygen tensions, becoming more oxygenated closer to the vascular niche. The activation of hypoxia-sensitive genes may be required to promote events such as HSC mobilization and differentiation. Furthermore, p53 has numerous roles in pathways that control cell cycle progression and apoptosis as well as genes involved in redox metabolism. Amifostine-binding to NF-kB, AP-1, and p53 result in the enhanced binding of these proteins to target regulatory sequences and subsequent transactivation of downstream genes (Shen et al., 2001). Recently, amifostine has been shown to activate a number of genes considered to be hallmarks of the unfolded protein response (UPR) in cancer cell lines (Dedieu et al.). While studies have linked these genes to the general effects of amifostine on apoptosis, cell cycle progression, and cytoprotection, few have sought to extend these observations to the myeloproliferative effects of the drug.
3.6 Tavocept (BNP77987)
A number of compounds with sulfur-containing nucleophiles have been investigated for utility as chemoprotectants and subsequently were found to have myeloproliferative potential. A few of these, including sodium thiosulfate, diethyldithiocarbonate, and mesna showed high levels of chemical reactivity with platinum based chemotherapeutics, thus limiting their use in such combinations (Verschraagen et al., 2003).
BNP77987 (disodium, 2,2’-dithio-bis-ethane sulfonate; Tavocept) is currently undergoing development as a redox chemoprotectant in combination with ciplastin and taxane therapies. Tavocept is the disulfide form of mesna and does not contain a free thiol group that would interfere with the antitumor effects of redox modulating agents. Unlike the intracellular environment, the plasma has an oxidative environment where reducing enzymes that target disulfides are lacking. It has been hypothesized that water-soluble disulfide drugs, such as Tavocept may reduce toxicity and unfavorable drug-drug interactions when compared to free thiol containing drugs. Verschraagen et al. showed that Tavocept is selectively taken up by the kidneys where endogenous cysteine, glutathione or glutaredoxin (Grx)/thioredoxin (Trx) systems reduce Tavocept into the free thiol form : 2-meraptoethane sulfonate WR-1065 (Verschraagen et al., 2004). Similar reactions may be anticipated in neuronal cells and bone marrow, thus offering other potential clinical utility.
Tavocept was initially targeted to reduce the incidence and severity of cisplatin-induced nephrotoxicity, caused by the binding of cisplatin to GSH via a GGT-dependent pathway in the proximal tubules (Townsend et al., 2003a). By acting as a competitive substrate for GGT, compounds such as GSH or NOV-002, restore GSH content and prevent nephrotocixity (Jenderny et al.) Further research revealed that Tavocept also protected against myelosuppression in rats and dogs (Hausheer et al., 1998). This function is most likely linked to the ability to cause the reversible modulation of plasma thiol and disulfide levels (Pendyala et al., 2003). Furthermore WR-1065 has a net charge of +2, which attracts it to negatively charged DNA where it can directly prevent oxidative damage (Grdina et al., 2002). WR-1065/mesna mixed disulfides serve as substrates for the Trx and Grx systems. It is possible that a Tavocept-mediated shift towards oxidized Trx and Grx may contribute to the chemoprotectant and myeloproliferative activities associated with the drug. The Trx system can be quite catalytically diverse and has a key role in controlling redox homeostasis and the redox regulation of multiple cellular processes, including proliferation and apoptosis. Circulatory Trx-1 has cytokine-like properties and acts as a chemoattractant for monocytes, neutrophils, and lymphocytes in addition to inhibiting neutrophil migration into inflammatory sites (Nakamura et al., 2001) (Bertini et al., 1999). The fact that many human tumors and tumor cell lines over-express Trx-1 has inspired the rational design of a number of agents that target Trx or thioredoxin reductase (Powis and Kirkpatrick, 2007). While additional studies that examine the mechanistic role of the Trx and Grx systems in myeloproliferative events are needed, it is plausible that the modulation of the intracellular balance of the oxidized and reduced forms may have therapeutic significance.
Trx levels may also influence Nf-κB, AP-1, p53, glucocorticoid and HIF1α protein expression (Harper et al., 2001; Hwang et al., 2004; Makino et al., 1999; Seemann and Hainaut, 2005). Jeong et al. showed that the thioredoxin-interacting protein regulates HSC quiescence and mobilization (Jeong et al., 2009). Thioredoxin-interacting protein acts to inhibit Trx activity or limit its bioavailability. It is reasonable to speculate that Tavocept may have a similar role. Furthermore, Trx is subject to S-glutathionylation. This suggests that drugs that induce S-glutathionylation, such as NOV-002, may influence Trx activity and downstream HSC self-renewal and myeloproliferative events. Grx can reverse S-glutathionylation and restore sulfhydryl functionality (Shelton et al., 2005). Under conditions of oxidative stress Grx may catalyze the forward S-glutathionylation reaction (Berndt et al., 2007). Tavocept-mediated disruption of Grx-mediated deglutathionylation may contribute to pathways involved in myeloid progenitor cell mobilization. Interference with the Trx and Grx systems is currently believed to be one of the major mechanisms involved in the increased therapeutic outcome in Tavocept-treated lung cancer patients. While clinical trials are currently ongoing, limited research has been conducted on the myeloproliferative role of Tavocept. Such studies have the potential to reveal the role of the Trx and Grx systems in pathways that mediate HSC self-renewal, mobilization, and differentiation.
3.7 Miscellaneous agents
Squalene is a naturally occurring dietary isoprenoid antioxidant that has been shown to protect mouse bone marrow progenitor cells against cisplatin and carboplatin-induced cytotoxicities (Das et al., 2008). This was concomitant with decreased ROS levels and increased GSH and glutathione peroxidase/GST levels.
Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl) is a redox-cycling nitroxide antioxidant and superoxide dismutase (SOD) mimetic that has been studied extensively in animal models of oxidative stress (Wilcox). Tempol reduces oxidative DNA damage and delays the onset of tumorigenesis in mice (Mitchell et al., 2003; Schubert et al., 2004). Using cancer-prone p53-deficient mice, Erker et al. further linked tempol’s chemopreventive actions to redox-mediated cell signaling events as a result of enhanced p53 phosphorylation at serine 18 and induced p21 protein expression (Erker et al., 2005). SOD mimetics used in association with chemotherapeutics may enhance their pro-oxidant activity based on data that indicate SOD-derived H2O2 preferentially targets rapidly dividing cancer cells with elevated oxidative stress (Alexandre et al., 2006; Laurent et al., 2005). Effects of tempol treatment on myeloproliferative events have not been published. However, the ability of tempol to promote ROS metabolism, improve nitric oxide bioavailability, act as a SOD mimetic, and mediate redox-mediated cell signaling implies a plausible role in HSC maintenance, differentiation and migration.
8-hydroxyquinoline derivatives have therapeutic utility in HIV, malaria, inflammatory and neurodegenerative diseases such as Alzheimer’s (Fakhfakh et al., 2003; Mekouar et al., 1998; Novak and Kovac, 2004; Sawada et al., 2004; Zheng et al., 2005). Recently, Wang et al. investigated the antioxidant potential of novel 2-vinyl-8-hydroxyquinoline derivatives (Wang et al.). Testing of fifteen derivatives indicated variable degrees of antioxidant activity in H202-induced oxidative stress due to a phenolic hydroxyl group capable of reacting with free radicals. Furthermore, certain compounds not only protected bone mesenchymal stem cells (BMSCs) from the effects of oxidative stress but also induced proliferation. This study highlights the potential of synthetic antioxidant compounds as redox chemoprotectants and possible myelostimulators.
Polysaccharide-Kureha (PSK, krestin) is a fungus derived protein-bound polysaccharide that has shown promise as an immune adjuvant cancer therapy. In addition to showing anticancer activity PSK appears to increase leukocyte activation and response through cytokine up-regulation. In vitro and in vivo PSK studies show both natural killer and lymphocyte-activated killer cell activation concomitant with increases in cytokine expression (Kariya et al., 1992; Pedrinaci et al., 1999). The mechanisms that underlie PSK immunomodulatory activity have yet to be deciphered. However, PSK has also been shown to have antioxidant activity, and to be redox active (Liu et al., 1997; Ooi and Liu, 2000). PSK activates natural killer cells peerhaps via mechanisms similar to IL-2. However differences between PSK and IL-2 mediated cellular responses exist. PSK decreases PKCα expression, increases ERK3 expression and enhances CRE binding activity (Garcia-Lora et al., 2003; Garcia-Lora et al., 2001; Jimenez-Medina et al., 2008). NOV-002 and Telintra (via GSTP) have roles in HSC cell proliferation and self-renewal that may be partially mediated through ERK activation and phosphorylation (Townsend et al., 2008a; Yin et al., 2000). Investigation into the role of redox-mediated signaling in PSK-immunomodulatory activity, cytokine activation and in the pathways that control killer cells would be interesting.
4. Other therapeutic strategies
4.1 Bone marrow priming
In 1975, Millar and colleagues showed that animals treated with a sufficiently high dose of busulphan died ~14 days later from bone marrow failure. A single, appropriately timed injection of cyclophosphamide saved these mice. They suggested that the nature of this protection was cyclophosphamide induced elaboration of a humoral factor(s) which stimulated hematopoietic recovery (Millar et al., 1975). This effect could be extended to other alkylating drugs and radiation (Millar and Hudspith, 1976). Patients given a priming dose of cyclophosphamide 7 days before a high dose of melphalan showed faster recovery of marrow function than those receiving melphalan alone (Hedley et al., 1978). Moreover, in a small trial of 19 patients a pre-treatment dose of cyclophosphamide was capable of significantly reducing the abnormalities in intestinal permeability that resulted from subsequent high dose melphalan. While these early studies did not translate into full-scale clinical utility, they did illustrate the mechanistic importance of priming normal tissues through exposure to sub-therapeutic doses of standard alkylating agents. The implications for the involvement of stress response pathways in these split dose protocols is apparent and may have importance to thiol response pathways in bone marrow.
In a similar manner, temporal scheduling of chemoprotective compounds can also influence clinical outcomes. For example, chemotherapeutic treatment in a brain cancer rat model in coordination with the delivery of NAC in to the descending aorta offered targeted myeloprotection with limited delivery of NAC to the brain (Neuwelt et al., 2001; Neuwelt et al., 2004). This served to enhance the therapeutic index of the approach. Alternatively, chemoprotection could be achieved through administration prior to, or interspersed with, standard drug treatments. In such an instance, priming can be defined as the administration of a potential chemoprotectant or sensitizing agent prior to chemotherapy. Neuwult et al found that optimum protective benefits without impacting antitumor efficacy were obtained when NAC was administered prior to tri-drug chemotherapy treatment in a rat intra-cerebral xenograft model (Neuwelt et al., 2004). As a further example, preclinical studies indicated that Telintra administered prior to anticancer drugs potentiated their cytotoxicity in different tumor cell lines. Telintra pretreatment also enhanced the in vivo sensitivity of a number of mouse xenograft models with elevated GST activities (Morgan et al., 1996; O'Brien et al., 1999).
4.2. Biomarkers
A number of extracellular and intracellular oxidative stress markers have shown potential as redox biomarkers and targets for redox therapeutics (Baker et al., 2006; Cha et al., 2009; Katahira et al., 2004; Miyazaki et al., 1998; Nakamura et al., 2000; Pawlowicz et al., 1991; Tew, 1994). As discussed in Section 3.4, GSTP is over-expressed in tumors and cancer cell lines and has been linked to acquired chemotherapy resistance, offering potential to GST inhibitors such as Telintra (Tew, 1994). Furthermore, genetic differences in expression patterns within the different human GST isozymes may play a role in cancer susceptibility and treatment (Townsend and Tew, 2003). Telintra has also been shown to effectively inhibit the multidrug resistance associated protein 1 (MRP-1) transporter and permit reversal of MRP-1 resistance in transfected NIH3T3 cells (O'Brien et al., 1999). Advances in techniques that aim to measure tumor antioxidant capacity and phenotypic profiling have proved useful in predicting paclitaxel sensitivity and the success of some hypoxia-activated drugs (Marcu and Olver, 2006; Ramanathan et al., 2005). Future work might focus on which biomarkers may be predictive of marrow response to myeloproliferative treatments. In this case, amelioration of side effects could have a significant therapeutic benefit. To this end, our lab has demonstrated that members of serine protease inhibitor (SERPIN) family, specifically Serpin A1 and A3, are S-glutathionylated in NOV-002 treated serum (Townsend et al., 2009, Grek et al., 2010 AACR abstract #430) Accumulating evidence indicates a role for serpin family members in myeloproliferation and hematopoietic progenitor cell mobilization (van Pel et al., 2006; Winkler et al., 2005). Winkler et al. showed that the down-regulation of serpins A1 and A3 in bone marrow occurs during progenitor cell mobilization and influences the marrow microenvironment and migratory behavior of hematopoietic precursor cells. It is plausible that S-glutathionylation of serpin family members following NOV-002 treatment may regulate serpin enzymatic activity and subsequent myeloproliferation. Recently, bomapin has been identified as a redox-sensitive serpin that directly affects the responsiveness of myeloid progenitor cells to their microenvironment. Under different growth factor conditions, a ‘mutant’ reduced form of bomapin interfered with myeloid cell proliferation, indicating how important the disulfide formation is to this process (Przygodzka et al.). The authors conclude that the redox state of bomapin is likely a sensor in a pathway that serves to sensitize myeloid progenitor cells to their growth environment.
4.3. Targeting Cancer Stem Cells
Although controversial, it is generally accepted that the growth and propagation of cancer depends in part on a relatively small population of stem cells, originally identified in leukemia (Bonnet and Dick, 1997; Huntly and Gilliland, 2004). While generally quiescent, these cells are characterized by a limitless capacity to proliferate. Furthermore, cancer stem cells locate to areas of low oxygen tension, suggesting that redox signals may be important in the maintenance of their “stemness.” This carries the implication that unique redox signaling pathways may be relevant to the resistance of these cells to chemo/radiotherapy.
The link between ROS and self-renewal of HSC was further indicated by the fact that treatment of Fox03-null animals with NAC created a cause:effect relationship for ROS and myeloproliferation, confirming an important regulatory role for FoxO3 in hematopoietic stem and progenitor cell proliferation (Yalcin et al., 2008). Thus, preclinically NAC has a niche in the management of hematapoietic malignancies and perhaps suggests possible extended opportunities to targeting redox based molecular pathways in cancer stem cells. Studies that cause cancer stem cells to differentiate and/or lose their “stemness” have also been pursued (Gil et al., 2008). Investigating those pathways required for cancer stem cell self-renewal and differentiation may strengthen the case for redox based therapeutic intervention.
5. Conclusions
Therapeutic potential in the manipulation of redox signaling is evidenced by the plethora of redox modulating chemotherapeutics that are now in use or in clinical trials. Most of these agents achieve success through the targeted disruption of redox signaling in malignant cells. Fewer studies have focused on their potential as myeloproliferatives or immunomodulators. A growing body of evidence indicates that thiol balance and redox conditions are critical to pathways that regulate myeloid differentiation and proliferation and regulate immune response. Manipulation of GSH is now used to treat immunosupression in HIV patients and specific conditions associated with Th1 and Th2-mediated responses. However, a better understanding of how these approaches manipulate cytokine balance and downstream immune response is still needed. Indications are that marrow microenvironment can determine cellular redox state and proliferative capacity, particularly of HSC sub-populations. Moreover, redox-dependent post-translational modifications such as S-glutathionylation may control some of the protease enzymes that initiate cell migration into the general circulation. Historically, drug combinations and bone marrow priming also lend themselves to a redox based mechanistic explanation. For the future, identification of extra- and intra-cellular redox active biomarkers may enable the optimized design of treatment approaches for cancer patients where bone marrow and immune complications are dose limiting. The redox platform still provides a number of targets that are ripe for small molecule design.
Abbreviations
- APC
antigen presenting cell
- BSO
buthionine sulfoxime
- Cys
cysteine
- CySS
cystine
- FoxO
forkhead O
- DC
dendritic cell
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- GPx
glutathione peroxidase
- GR
glutathione reductase
- Grx
glutaredoxin
- GST
glutathione S-transferase
- HPCs
hematopoeiteic progentitor cells
- HSC
hematopoietic stem cell
- IR
ionizing radiation
- JNK
c-jun NH2-terminal kinase
- MAPK
mitogen-activated protein kinase
- NAC
N-acetyl-cysteine
- NF-κB
nuclear factor κB
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- Th1
T helper cell type 1
- Th2
T helper cell type 2
- Trx
thioredoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- Ahmad T, Frischer H. Active site-specific inhibition by 1,3-bis(2-chloroethyl)-1-nitrosourea of two genetically homologous flavoenzymes: glutathione reductase and lipoamide dehydrogenase. J Lab Clin Med. 1985;105(4):464–471. [PubMed] [Google Scholar]
- Akerlund B, Jarstrand C, Lindeke B, Sonnerborg A, Akerblad AC, Rasool O. Effect of N-acetylcysteine(NAC) treatment on HIV-1 infection: a double-blind placebo-controlled trial. Eur J Clin Pharmacol. 1996;50(6):457–461. doi: 10.1007/s002280050140. [DOI] [PubMed] [Google Scholar]
- Alexandre J, Nicco C, Chereau C, Laurent A, Weill B, Goldwasser F, Batteux F. Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. J Natl Cancer Inst. 2006;98(4):236–244. doi: 10.1093/jnci/djj049. [DOI] [PubMed] [Google Scholar]
- Angelini G, Gardella S, Ardy M, Ciriolo MR, Filomeni G, Di Trapani G, Clarke F, Sitia R, Rubartelli A. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci U S A. 2002;99(3):1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai F, Yoshihara H, Hosokawa K, Nakamura Y, Gomei Y, Iwasaki H, Suda T. Niche regulation of hematopoietic stem cells in the endosteum. Ann N Y Acad Sci. 2009;1176:36–46. doi: 10.1111/j.1749-6632.2009.04561.x. [DOI] [PubMed] [Google Scholar]
- Bailey HH, Mulcahy RT, Tutsch KD, Arzoomanian RZ, Alberti D, Tombes MB, Wilding G, Pomplun M, Spriggs DR. Phase I clinical trial of intravenous L-buthionine sulfoximine and melphalan: an attempt at modulation of glutathione. J Clin Oncol. 1994;12(1):194–205. doi: 10.1200/JCO.1994.12.1.194. [DOI] [PubMed] [Google Scholar]
- Bailey HH, Ripple G, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Mahvi D, Schink J, Pomplun M, Mulcahy RT, Wilding G. Phase I study of continuous-infusion L-S,R-buthionine sulfoximine with intravenous melphalan. J Natl Cancer Inst. 1997;89(23):1789–1796. doi: 10.1093/jnci/89.23.1789. [DOI] [PubMed] [Google Scholar]
- Baker AF, Dragovich T, Tate WR, Ramanathan RK, Roe D, Hsu CH, Kirkpatrick DL, Powis G. The antitumor thioredoxin-1 inhibitor PX-12 (1-methylpropyl 2-imidazolyl disulfide) decreases thioredoxin-1 and VEGF levels in cancer patient plasma. J Lab Clin Med. 2006;147(2):83–90. doi: 10.1016/j.lab.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini M, Sacchetti C. [Effect of cystine and cysteine on human bone marrow cultured in medium deficient in amino acids.] Rev Hematol. 1953;8(1):3–19. [PubMed] [Google Scholar]
- Barrett EG, Johnston C, Oberdorster G, Finkelstein JN. Antioxidant treatment attenuates cytokine and chemokine levels in murine macrophages following silica exposure. Toxicol Appl Pharmacol. 1999;158(3):211–220. doi: 10.1006/taap.1999.8716. [DOI] [PubMed] [Google Scholar]
- Berndt C, Lillig CH, Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2007;292(3):H1227–H1236. doi: 10.1152/ajpheart.01162.2006. [DOI] [PubMed] [Google Scholar]
- Bertini R, Howard OM, Dong HF, Oppenheim JJ, Bizzarri C, Sergi R, Caselli G, Pagliei S, Romines B, Wilshire JA, Mengozzi M, Nakamura H, Yodoi J, Pekkari K, Gurunath R, Holmgren A, Herzenberg LA, Ghezzi P. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J Exp Med. 1999;189(11):1783–1789. doi: 10.1084/jem.189.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Bowers RR, Townsend D, Manevich Y, Garret T, Pazoles CJ, Tew KD. The redox modulator NOV-002 inhibits proliferation of ovarian tumor cells but increases proliferation of myeloid cells [abstract 1615]; American Association for Cancer Research Annual Meeting; April 17–21; Washington DC. 2010. [Google Scholar]
- Brigelius-Flohe R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem. 2006;387(10–11):1329–1335. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Capizzi RL. The preclinical basis for broad-spectrum selective cytoprotection of normal tissues from cytotoxic therapies by amifostine. Semin Oncol. 1999;26(2) Suppl 7:3–21. [PubMed] [Google Scholar]
- Castellani P, Angelini G, Delfino L, Matucci A, Rubartelli A. The thiol redox state of lymphoid organs is modified by immunization: role of different immune cell populations. Eur J Immunol. 2008;38(9):2419–2425. doi: 10.1002/eji.200838439. [DOI] [PubMed] [Google Scholar]
- Cetinkale O, Senel O, Bulan R. The effect of antioxidant therapy on cell-mediated immunity following burn injury in an animal model. Burns. 1999;25(2):113–118. doi: 10.1016/s0305-4179(98)00124-7. [DOI] [PubMed] [Google Scholar]
- Cha MK, Suh KH, Kim IH. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J Exp Clin Cancer Res. 2009;28:93. doi: 10.1186/1756-9966-28-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5(8):e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96(6):3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82(7):2031–2037. [PubMed] [Google Scholar]
- Collins DS, Unanue ER, Harding CV. Reduction of disulfide bonds within lysosomes is a key step in antigen processing. J Immunol. 1991;147(12):4054–4059. [PubMed] [Google Scholar]
- Culy CR, Spencer CM. Amifostine: an update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome. Drugs. 2001;61(5):641–684. doi: 10.2165/00003495-200161050-00012. [DOI] [PubMed] [Google Scholar]
- Daniel D, Crawford J. Myelotoxicity from chemotherapy. Semin Oncol. 2006;33(1):74–85. doi: 10.1053/j.seminoncol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Das B, Antoon R, Tsuchida R, Lotfi S, Morozova O, Farhat W, Malkin D, Koren G, Yeger H, Baruchel S. Squalene selectively protects mouse bone marrow progenitors against cisplatin and carboplatin-induced cytotoxicity in vivo without protecting tumor growth. Neoplasia. 2008;10(10):1105–1119. doi: 10.1593/neo.08466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W, Jr, Ronai Z, Tew KD. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J Pharmacol Exp Ther. 2001;296(1):1–6. [PubMed] [Google Scholar]
- Dedieu S, Canron X, Rezvani HR, Bouchecareilh M, Mazurier F, Sinisi R, Zanda M, Moenner M, Bikfalvi A, North S. The cytoprotective drug amifostine modifies both expression and activity of the pro-angiogenic factor VEGF-A. BMC Med. 2010;8:19. doi: 10.1186/1741-7015-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForge LE, Fantone JC, Kenney JS, Remick DG. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J Clin Invest. 1992;90(5):2123–2129. doi: 10.1172/JCI116097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Montero CM, Perez A, Zidane S, Pazoles CJ, Montero AJ. Immunomodulatory activity of NOV-002 potentiates the anti-tumor efficacy of cyclophosphamide in the CT26 murine colon cancer model [abstract 5620]; American Association for Cancer Research Annual Meeting; April 17–21; Washington DC. 2010. [Google Scholar]
- Dominici S, Valentini M, Maellaro E, Del Bello B, Paolicchi A, Lorenzini E, Tongiani R, Comporti M, Pompella A. Redox modulation of cell surface protein thiols in U937 lymphoma cells: the role of gamma-glutamyl transpeptidase-dependent H2O2 production and S-thiolation. Free Radic Biol Med. 1999;27(5–6):623–635. doi: 10.1016/s0891-5849(99)00111-2. [DOI] [PubMed] [Google Scholar]
- Drane P, Bravard A, Bouvard V, May E. Reciprocal down-regulation of p53 and SOD2 gene expression-implication in p53 mediated apoptosis. Oncogene. 2001;20(4):430–439. doi: 10.1038/sj.onc.1204101. [DOI] [PubMed] [Google Scholar]
- Droge W, Schulze-Osthoff K, Mihm S, Galter D, Schenk H, Eck HP, Roth S, Gmunder H. Functions of glutathione and glutathione disulfide in immunology and immunopathology. FASEB J. 1994;8(14):1131–1138. [PubMed] [Google Scholar]
- El-Sayed el SM, Abdel-Aziz AA, Helal GK, Saleh S, Saad AS. Protective effect of N-acetylcysteine against carmustine-induced myelotoxicity in rats. Food Chem Toxicol. 2010;48(6):1576–1580. doi: 10.1016/j.fct.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Erker L, Schubert R, Yakushiji H, Barlow C, Larson D, Mitchell JB, Wynshaw-Boris A. Cancer chemoprevention by the antioxidant tempol acts partially via the p53 tumor suppressor. Hum Mol Genet. 2005;14(12):1699–1708. doi: 10.1093/hmg/ddi181. [DOI] [PubMed] [Google Scholar]
- Evans RG, Wheatley C, Engel C, Nielsen J, Ciborowski LJ. Modification of the bone marrow toxicity of cis-diamminedichloroplatinum(II) in mice by diethyldithiocarbamate. Cancer Res. 1984;44(9):3686–3690. [PubMed] [Google Scholar]
- Eylar E, Rivera-Quinones C, Molina C, Baez I, Molina F, Mercado CM. N-acetylcysteine enhances T cell functions and T cell growth in culture. Int Immunol. 1993;5(1):97–101. doi: 10.1093/intimm/5.1.97. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002;99(11):7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhfakh MA, Fournet A, Prina E, Mouscadet JF, Franck X, Hocquemiller R, Figadere B. Synthesis and biological evaluation of substituted quinolines: potential treatment of protozoal and retroviral co-infections. Bioorg Med Chem. 2003;11(23):5013–5023. doi: 10.1016/j.bmc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, Bonetto V, Mengozzi M, Duffieux F, Miclet E, Bachi A, Vandekerckhove J, Gianazza E, Ghezzi P. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci U S A. 2002;99(6):3505–3510. doi: 10.1073/pnas.052592699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraternale A, Paoletti MF, Casabianca A, Nencioni L, Garaci E, Palamara AT, Magnani M. GSH and analogs in antiviral therapy. Mol Aspects Med. 2009;30(1–2):99–110. doi: 10.1016/j.mam.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Colvin OM, Griffith OW, Lippitz B, Elion GB, Schold SC, Jr, Hilton J, Bigner DD. Increased melphalan activity in intracranial human medulloblastoma and glioma xenografts following buthionine sulfoximine-mediated glutathione depletion. J Natl Cancer Inst. 1989;81(7):524–527. doi: 10.1093/jnci/81.7.524. [DOI] [PubMed] [Google Scholar]
- Garcia-Lora A, Martinez M, Pedrinaci S, Garrido F. Different regulation of PKC isoenzymes and MAPK by PSK and IL-2 in the proliferative and cytotoxic activities of the NKL human natural killer cell line. Cancer Immunol Immunother. 2003;52(1):59–64. doi: 10.1007/s00262-002-0336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lora A, Pedrinaci S, Garrido F. Protein-bound polysaccharide K and interleukin-2 regulate different nuclear transcription factors in the NKL human natural killer cell line. Cancer Immunol Immunother. 2001;50(4):191–198. doi: 10.1007/s002620100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate L, Majumdar RS, Lunk A, Tew KD. Increased myeloproliferation in glutathione S-transferase pi-deficient mice is associated with a deregulation of JNK and Janus kinase/STAT pathways. J Biol Chem. 2004;279(10):8608–8616. doi: 10.1074/jbc.M308613200. [DOI] [PubMed] [Google Scholar]
- Gazitt Y. Recent developments in the regulation of peripheral blood stem cell mobilization and engraftment by cytokines, chemokines, and adhesion molecules. J Hematother Stem Cell Res. 2001;10(2):229–236. doi: 10.1089/15258160151134908. [DOI] [PubMed] [Google Scholar]
- Gelderman KA, Hultqvist M, Holmberg J, Olofsson P, Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc Natl Acad Sci U S A. 2006;103(34):12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J, Stembalska A, Pesz KA, Sasiadek MM. Cancer stem cells: the theory and perspectives in cancer therapy. J Appl Genet. 2008;49(2):193–199. doi: 10.1007/BF03195612. [DOI] [PubMed] [Google Scholar]
- Giles GI. The redox regulation of thiol dependent signaling pathways in cancer. Curr Pharm Des. 2006;12(34):4427–4443. doi: 10.2174/138161206779010549. [DOI] [PubMed] [Google Scholar]
- Giordani L, Quaranta MG, Malorni W, Boccanera M, Giacomini E, Viora M. N-acetylcysteine inhibits the induction of an antigen-specific antibody response down-regulating CD40 and CD27 co-stimulatory molecules. Clin Exp Immunol. 2002;129(2):254–264. doi: 10.1046/j.1365-2249.2002.01897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gius D, Spitz DR. Redox signaling in cancer biology. Antioxid Redox Signal. 2006;8(7–8):1249–1252. doi: 10.1089/ars.2006.8.1249. [DOI] [PubMed] [Google Scholar]
- Gosset P, Wallaert B, Tonnel AB, Fourneau C. Thiol regulation of the production of TNF-alpha, IL-6 and IL-8 by human alveolar macrophages. Eur Respir J. 1999;14(1):98–105. doi: 10.1034/j.1399-3003.1999.14a17.x. [DOI] [PubMed] [Google Scholar]
- Grdina DJ, Murley JS, Kataoka Y. Radioprotectants: current status and new directions. Oncology. 2002;63 Suppl 2:2–10. doi: 10.1159/000067146. [DOI] [PubMed] [Google Scholar]
- Grek CL, Townsend DM, Uys JD, Christopher J, Pazoles J, Tew KD. Serpin-A1 and A3 as potential pharmacodynamic biomarkers for NOV-002, a redox modulating anticancer agent. [abstract 430]; American Association for Cancer Research Annual Meeting; April 17–21; Washington DC. 2010. [Google Scholar]
- Haddad JJ. Redox regulation of pro-inflammatory cytokines and IkappaBalpha/NF-kappaB nuclear translocation and activation. Biochem Biophys Res Commun. 2002;296(4):847–856. doi: 10.1016/s0006-291x(02)00947-6. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1alpha and NF-kappa B redox sensitivity. Evidence for inhibition by glutathione oxidation in alveolar epithelial cells. J Biol Chem. 2000;275(28):21130–21139. doi: 10.1074/jbc.M000737200. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Safieh-Garabedian B, Saade NE, Land SC. Thiol regulation of pro-inflammatory cytokines reveals a novel immunopharmacological potential of glutathione in the alveolar epithelium. J Pharmacol Exp Ther. 2001;296(3):996–1005. [PubMed] [Google Scholar]
- Hadzic T, Li L, Cheng N, Walsh SA, Spitz DR, Knudson CM. The role of low molecular weight thiols in T lymphocyte proliferation and IL-2 secretion. J Immunol. 2005;175(12):7965–7972. doi: 10.4049/jimmunol.175.12.7965. [DOI] [PubMed] [Google Scholar]
- Hamilos DL, Zelarney P, Mascali JJ. Lymphocyte proliferation in glutathione-depleted lymphocytes: direct relationship between glutathione availability and the proliferative response. Immunopharmacology. 1989;18(3):223–235. doi: 10.1016/0162-3109(89)90020-9. [DOI] [PubMed] [Google Scholar]
- Harper R, Wu K, Chang MM, Yoneda K, Pan R, Reddy SP, Wu R. Activation of nuclear factor-kappa b transcriptional activity in airway epithelial cells by thioredoxin but not by N-acetyl-cysteine and glutathione. Am J Respir Cell Mol Biol. 2001;25(2):178–185. doi: 10.1165/ajrcmb.25.2.4471. [DOI] [PubMed] [Google Scholar]
- Hausheer FH, Kanter P, Cao S, Haridas K, Seetharamulu P, Reddy D, Petluru P, et al. Modulation of platinum-induced toxicities and therapeutic index: mechanistic insights and first- and second-generation protecting agents. Semin Oncol. 1998;25(5):584–599. [PubMed] [Google Scholar]
- Hedley DW, McElwain TJ, Millar JL, Gordon MY. Acceleration of bone-marrow recovery by pre-treatment with cyclophosphamide in patients receiving high-dose melphalan. Lancet. 1978;2(8097):966–968. doi: 10.1016/s0140-6736(78)92529-1. [DOI] [PubMed] [Google Scholar]
- Helal GK, Helal OK. Metallothionein attenuates carmustine-induced oxidative stress and protects against pulmonary fibrosis in rats. Arch Toxicol. 2009;83(1):87–94. doi: 10.1007/s00204-008-0325-7. [DOI] [PubMed] [Google Scholar]
- Hu Y, Rosen DG, Zhou Y, Feng L, Yang G, Liu J, Huang P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J Biol Chem. 2005;280(47):39485–39492. doi: 10.1074/jbc.M503296200. [DOI] [PubMed] [Google Scholar]
- Huntly BJ, Gilliland DG. Blasts from the past: new lessons in stem cell biology from chronic myelogenous leukemia. Cancer Cell. 2004;6(3):199–201. doi: 10.1016/j.ccr.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Hwang CY, Ryu YS, Chung MS, Kim KD, Park SS, Chae SK, Chae HZ, Kwon KS. Thioredoxin modulates activator protein 1 (AP-1) activity and p27Kip1 degradation through direct interaction with Jab1. Oncogene. 2004;23(55):8868–8875. doi: 10.1038/sj.onc.1208116. [DOI] [PubMed] [Google Scholar]
- Ishii T, Sugita Y, Bannai S. Regulation of glutathione levels in mouse spleen lymphocytes by transport of cysteine. J Cell Physiol. 1987;133(2):330–336. doi: 10.1002/jcp.1041330217. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12(4):446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Suda T. Cancer stem cells and their niche. Cancer Sci. 2009;100(7):1166–1172. doi: 10.1111/j.1349-7006.2009.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7(3):333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110(8):3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwalla RJ, Lalezari J, Cenko D, Mansour SE, Kumar A, Gangapurkar B, Nakamura D. Restoration of blood total glutathione status and lymphocyte function following alpha-lipoic acid supplementation in patients with HIV infection. J Altern Complement Med. 2008;14(2):139–146. doi: 10.1089/acm.2006.6397. [DOI] [PubMed] [Google Scholar]
- Jeannin P, Delneste Y, Lecoanet-Henchoz S, Gauchat JF, Life P, Holmes D, Bonnefoy JY. Thiols decrease human interleukin (IL) 4 production and IL-4-induced immunoglobulin synthesis. J Exp Med. 1995;182(6):1785–1792. doi: 10.1084/jem.182.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenderny S, Lin H, Garrett T, Tew KD, Townsend DM. Protective effects of a glutathione disulfide mimetic (NOV-002) against cisplatin induced kidney toxicity. Biomed Pharmacother. 2010;64(1):73–76. doi: 10.1016/j.biopha.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong M, Piao ZH, Kim MS, Lee SH, Yun S, Sun HN, Yoon SR, Chung JW, Kim TD, Jeon JH, Lee J, Kim HN, Choi JY, Choi I. Thioredoxin-interacting protein regulates hematopoietic stem cell quiescence and mobilization under stress conditions. J Immunol. 2009;183(4):2495–2505. doi: 10.4049/jimmunol.0804221. [DOI] [PubMed] [Google Scholar]
- Jimenez-Medina E, Berruguilla E, Romero I, Algarra I, Collado A, Garrido F, Garcia-Lora A. The immunomodulator PSK induces in vitro cytotoxic activity in tumour cell lines via arrest of cell cycle and induction of apoptosis. BMC Cancer. 2008;8:78. doi: 10.1186/1471-2407-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295(4):C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan PA, Gibbins JM. Extracellular disulfide exchange and the regulation of cellular function. Antioxid Redox Signal. 2006;8(3–4):312–324. doi: 10.1089/ars.2006.8.312. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5(9):689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- Kariya Y, Inoue N, Kihara T, Okamoto N, Sugie K, Mori T, Uchida A. Activation of human natural killer cells by the protein-bound polysaccharide PSK independently of interferon and interleukin 2. Immunol Lett. 1992;31(3):241–245. doi: 10.1016/0165-2478(92)90121-4. [DOI] [PubMed] [Google Scholar]
- Katahira T, Takayama T, Miyanishi K, Hayashi T, Ikeda T, Takahashi Y, Takimoto R, Matsunaga T, Kato J, Niitsu Y. Plasma glutathione S-Transferase P1-1 as a prognostic factor in patients with advanced non-Hodgkin's lymphoma (stages III and IV) Clin Cancer Res. 2004;10(23):7934–7940. doi: 10.1158/1078-0432.CCR-03-0679. [DOI] [PubMed] [Google Scholar]
- Kellen E, Hemelt M, Broberg K, Golka K, Kristensen VN, Hung RJ, et al. Pooled analysis and meta-analysis of the glutathione S-transferase P1 Ile 105Val polymorphism and bladder cancer: a HuGE-GSEC review. Am J Epidemiol. 2007;165(11):1221–1230. doi: 10.1093/aje/kwm003. [DOI] [PubMed] [Google Scholar]
- Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3(2):114–127. [PubMed] [Google Scholar]
- Kohler A, Schmithorst V, Filippi MD, Ryan MA, Daria D, Gunzer M, Geiger H. Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood. 2009;114(2):290–298. doi: 10.1182/blood-2008-12-195644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike Y, Hisada T, Utsugi M, Ishizuka T, Shimizu Y, Ono A, Murata Y, Hamuro J, Mori M, Dobashi K. Glutathione redox regulates airway hyperresponsiveness and airway inflammation in mice. Am J Respir Cell Mol Biol. 2007;37(3):322–329. doi: 10.1165/rcmb.2006-0423OC. [DOI] [PubMed] [Google Scholar]
- Konstantinov AA, Peskin AV, Popova E, Khomutov GB, Ruuge EK. Superoxide generation by the respiratory chain of tumor mitochondria. Biochim Biophys Acta. 1987;894(1):1–10. doi: 10.1016/0005-2728(87)90206-4. [DOI] [PubMed] [Google Scholar]
- Korst AE, Eeltink CM, Vermorken JB, van der Vijgh WJ. Pharmacokinetics of amifostine and its metabolites in patients. Eur J Cancer. 1997;33(9):1425–1429. doi: 10.1016/s0959-8049(97)00138-x. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Chong W, Simopoulos C, Polychronidis A, Sivridis E, Harris AL. Amifostine induces anaerobic metabolism and hypoxia-inducible factor 1 alpha. Cancer Chemother Pharmacol. 2004;53(1):8–14. doi: 10.1007/s00280-003-0691-z. [DOI] [PubMed] [Google Scholar]
- Kraggerud SM, Oldenburg J, Alnaes GI, Berg M, Kristensen VN, Fossa SD, Lothe RA. Functional glutathione S-transferase genotypes among testicular germ cell tumor survivors: associations with primary and post-chemotherapy tumor histology. Pharmacogenet Genomics. 2009;19(10):751–759. doi: 10.1097/FPC.0b013e3283304253. [DOI] [PubMed] [Google Scholar]
- Ladner C, Ehninger G, Gey KF, Clemens MR. Effect of etoposide (VP16-213) on lipid peroxidation and antioxidant status in a high-dose radiochemotherapy regimen. Cancer Chemother Pharmacol. 1989;25(3):210–212. doi: 10.1007/BF00689585. [DOI] [PubMed] [Google Scholar]
- Laterveer L, Lindley IJ, Heemskerk DP, Camps JA, Pauwels EK, Willemze R, Fibbe WE. Rapid mobilization of hematopoietic progenitor cells in rhesus monkeys by a single intravenous injection of interleukin-8. Blood. 1996;87(2):781–788. [PubMed] [Google Scholar]
- Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, Levy E, Goldwasser F, Panis Y, Soubrane O, Weill B, Batteux F. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65(3):948–956. [PubMed] [Google Scholar]
- Levy EM, Wu J, Salibian M, Black PH. The effect of changes in thiol subcompartments on T-cell colony formation and cell cycle progression: relevance to AIDS. Cell Immunol. 1992;140(2):370–380. doi: 10.1016/0008-8749(92)90203-2. [DOI] [PubMed] [Google Scholar]
- List AF, Brasfield F, Heaton R, Glinsmann-Gibson B, Crook L, Taetle R, Capizzi R. Stimulation of hematopoiesis by amifostine in patients with myelodysplastic syndrome. Blood. 1997;90(9):3364–3369. [PubMed] [Google Scholar]
- List AF, Heaton R, Glinsmann-Gibson B, Capizzi RL. Amifostine protects primitive hematopoietic progenitors against chemotherapy cytotoxicity. Semin Oncol. 1996;23(4) Suppl 8:58–63. [PubMed] [Google Scholar]
- Liu F, Ooi VE, Chang ST. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997;60(10):763–771. doi: 10.1016/s0024-3205(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lo Celso C, Wu JW, Lin CP. In vivo imaging of hematopoietic stem cells and their microenvironment. J Biophotonics. 2009;2(11):619–631. doi: 10.1002/jbio.200910072. [DOI] [PubMed] [Google Scholar]
- Makino Y, Yoshikawa N, Okamoto K, Hirota K, Yodoi J, Makino I, Tanaka H. Direct association with thioredoxin allows redox regulation of glucocorticoid receptor function. J Biol Chem. 1999;274(5):3182–3188. doi: 10.1074/jbc.274.5.3182. [DOI] [PubMed] [Google Scholar]
- Malorni W, Rivabene R, Santini MT, Donelli G. N-acetylcysteine inhibits apoptosis and decreases viral particles in HIV-chronically infected U937 cells. FEBS Lett. 1993;327(1):75–78. doi: 10.1016/0014-5793(93)81043-y. [DOI] [PubMed] [Google Scholar]
- Marcu L, Olver I. Tirapazamine: from bench to clinical trials. Curr Clin Pharmacol. 2006;1(1):71–79. doi: 10.2174/157488406775268192. [DOI] [PubMed] [Google Scholar]
- Matthias LJ, Yam PT, Jiang XM, Vandegraaff N, Li P, Poumbourios P, Donoghue N, Hogg PJ. Disulfide exchange in domain 2 of CD4 is required for entry of HIV-1. Nat Immunol. 2002;3(8):727–732. doi: 10.1038/ni815. [DOI] [PubMed] [Google Scholar]
- Maurer AM, Liu Y, Caen JP, Han ZC. Ex vivo expansion of megakaryocytic cells. Int J Hematol. 2000;71(3):203–210. [PubMed] [Google Scholar]
- Mekouar K, Mouscadet JF, Desmaele D, Subra F, Leh H, Savoure D, Auclair C, d'Angelo J. Styrylquinoline derivatives: a new class of potent HIV-1 integrase inhibitors that block HIV-1 replication in CEM cells. J Med Chem. 1998;41(15):2846–2857. doi: 10.1021/jm980043e. [DOI] [PubMed] [Google Scholar]
- Millar JL, Hudspith BN. Sparing effect of cyclophosphamide (NSC-26271) pretreatment on animals lethally treated with gamma-irradiation. Cancer Treat Rep. 1976;60(4):409–414. [PubMed] [Google Scholar]
- Millar JL, Hudspith BN, Blackett NM. Reduced lethality in mice receiving a combined dose of cyclophosphamide and busulphan. Br J Cancer. 1975;32(2):193–198. doi: 10.1038/bjc.1975.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JB, Xavier S, DeLuca AM, Sowers AL, Cook JA, Krishna MC, Hahn SM, Russo A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic Biol Med. 2003;34(1):93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1(1):101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Noda N, Okada S, Hagiwara Y, Miyata M, Sakurabayashi I, et al. Elevated serum level of thioredoxin in patients with hepatocellular carcinoma. Biotherapy. 1998;11(4):277–288. doi: 10.1023/a:1008032703468. Y. [DOI] [PubMed] [Google Scholar]
- Monick MM, Samavati L, Butler NS, Mohning M, Powers LS, Yarovinsky T, Spitz DR, Hunninghake GW. Intracellular thiols contribute to Th2 function via a positive role in IL-4 production. J Immunol. 2003;171(10):5107–5115. doi: 10.4049/jimmunol.171.10.5107. [DOI] [PubMed] [Google Scholar]
- Morgan AS, Ciaccio PJ, Tew KD, Kauvar LM. Isozyme-specific glutathione S-transferase inhibitors potentiate drug sensitivity in cultured human tumor cell lines. Cancer Chemother Pharmacol. 1996;37(4):363–370. doi: 10.1007/s002800050398. [DOI] [PubMed] [Google Scholar]
- Murata Y, Shimamura T, Hamuro J. The polarization of T(h)1/T(h)2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int Immunol. 2002;14(2):201–212. doi: 10.1093/intimm/14.2.201. [DOI] [PubMed] [Google Scholar]
- Nair RR, Tolentino J, Hazlehurst LA. The bone marrow microenvironment as a sanctuary for minimal residual disease in CML. Biochem Pharmacol. 2010 doi: 10.1016/j.bcp.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K, Muraguchi T, Hoshii T, Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal. 2008;10(11):1883–1894. doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- Naka K, Ohmura M, Hirao A. Regulation of the self-renewal ability of tissue stem cells by tumor-related genes. Cancer Biomark. 2007;3(4–5):193–201. doi: 10.3233/cbm-2007-34-504. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Bai J, Nishinaka Y, Ueda S, Sasada T, Ohshio G, Imamura M, Takabayashi A, Yamaoka Y, Yodoi J. Expression of thioredoxin and glutaredoxin, redox-regulating proteins, in pancreatic cancer. Cancer Detect Prev. 2000;24(1):53–60. [PubMed] [Google Scholar]
- Nakamura H, Herzenberg LA, Bai J, Araya S, Kondo N, Nishinaka Y, Yodoi J. Circulating thioredoxin suppresses lipopolysaccharide-induced neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2001;98(26):15143–15148. doi: 10.1073/pnas.191498798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Masutani H, Yodoi J. Redox imbalance and its control in HIV infection. Antioxid Redox Signal. 2002;4(3):455–464. doi: 10.1089/15230860260196245. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Pagel MA, Hasler BP, Deloughery TG, Muldoon LL. Therapeutic efficacy of aortic administration of N-acetylcysteine as a chemoprotectant against bone marrow toxicity after intracarotid administration of alkylators, with or without glutathione depletion in a rat model. Cancer Res. 2001;61(21):7868–7874. [PubMed] [Google Scholar]
- Neuwelt EA, Pagel MA, Kraemer DF, Peterson DR, Muldoon LL. Bone marrow chemoprotection without compromise of chemotherapy efficacy in a rat brain tumor model. J Pharmacol Exp Ther. 2004;309(2):594–599. doi: 10.1124/jpet.103.063347. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97(8):2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- Novak I, Kovac B. UV photoelectron spectroscopic study of substituent effects in quinoline derivatives. J Org Chem. 2004;69(15):5005–5010. doi: 10.1021/jo040154n. [DOI] [PubMed] [Google Scholar]
- O'Brien ML, Vulevic B, Freer S, Boyd J, Shen H, Tew KD. Glutathione peptidomimetic drug modulator of multidrug resistance-associated protein. J Pharmacol Exp Ther. 1999;291(3):1348–1355. [PubMed] [Google Scholar]
- O'Dwyer PJ, Hamilton TC, LaCreta FP, Gallo JM, Kilpatrick D, Halbherr T, Brennan J, Bookman MA, Hoffman J, Young RC, Comis RL, Ozols RF. Phase I trial of buthionine sulfoximine in combination with melphalan in patients with cancer. J Clin Oncol. 1996;14(1):249–256. doi: 10.1200/JCO.1996.14.1.249. [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109 Suppl:S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- Oberley TD, Oberley LW. Antioxidant enzyme levels in cancer. Histol Histopathol. 1997;12(2):525–535. [PubMed] [Google Scholar]
- Ooi VE, Liu F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr Med Chem. 2000;7(7):715–729. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
- Pawlowicz Z, Zachara BA, Trafikowska U, Maciag A, Marchaluk E, Nowicki A. Blood selenium concentrations and glutathione peroxidase activities in patients with breast cancer and with advanced gastrointestinal cancer. J Trace Elem Electrolytes Health Dis. 1991;5(4):275–277. [PubMed] [Google Scholar]
- Pazoles CJ, Gernstein H. NOV-002, a chemoprotectant/immunomodulator, added to first line carboplatin/paclitaxel in advanced non-small cell lung cancer (NSCLC): a randomized phase 1/2, open label, controlled study [abstract 17021]. Journal of Clinical Oncology; ASCO Annual Meeting Proceedings Part I; 2006. p. 17021. (June 20 Supplement) [Google Scholar]
- Pedrinaci S, Algarra I, Garrido F. Protein-bound polysaccharide (PSK) induces cytotoxic activity in the NKL human natural killer cell line. Int J Clin Lab Res. 1999;29(4):135–140. doi: 10.1007/s005990050079. [DOI] [PubMed] [Google Scholar]
- Pei P, Horan MP, Hille R, Hemann CF, Schwendeman SP, Mallery SR. Reduced nonprotein thiols inhibit activation and function of MMP-9: implications for chemoprevention. Free Radic Biol Med. 2006;41(8):1315–1324. doi: 10.1016/j.freeradbiomed.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkari K, Avila-Carino J, Bengtsson A, Gurunath R, Scheynius A, Holmgren A. Truncated thioredoxin (Trx80) induces production of interleukin-12 and enhances CD14 expression in human monocytes. Blood. 2001;97(10):3184–3190. doi: 10.1182/blood.v97.10.3184. [DOI] [PubMed] [Google Scholar]
- Pendyala L, Schwartz G, Smith P, Zdanowicz J, Murphy M, Hausheer F. Modulation of plasma thiols and mixed disulfides by BNP7787 in patients receiving paclitaxel/cisplatin therapy. Cancer Chemother Pharmacol. 2003;51(5):376–384. doi: 10.1007/s00280-003-0587-y. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998;95(6):3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluquet O, North S, Bhoumik A, Dimas K, Ronai Z, Hainaut P. The cytoprotective aminothiol WR1065 activates p53 through a non-genotoxic signaling pathway involving c-Jun N-terminal kinase. J Biol Chem. 2003;278(14):11879–11887. doi: 10.1074/jbc.M207396200. [DOI] [PubMed] [Google Scholar]
- Powis G, Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr Opin Pharmacol. 2007;7(4):392–397. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Przygodzka P, Ramstedt B, Tengel T, Larsson G, Wilczynska M. Bomapin is a redox-sensitive nuclear serpin that affects responsiveness of myeloid progenitor cells to growth environment. BMC Cell Biol. 11:30. doi: 10.1186/1471-2121-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S, Paracchini V, Autrup H, Barros-Dios JM, Benhamou S, Boffetta P, et al. Meta- and pooled analysis of GSTT1 and lung cancer: a HuGE-GSEC review. Am J Epidemiol. 2006;164(11):1027–1042. doi: 10.1093/aje/kwj321. [DOI] [PubMed] [Google Scholar]
- Ramanathan B, Jan KY, Chen CH, Hour TC, Yu HJ, Pu YS. Resistance to paclitaxel is proportional to cellular total antioxidant capacity. Cancer Res. 2005;65(18):8455–8460. doi: 10.1158/0008-5472.CAN-05-1162. [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98(6):3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reliene R, Schiestl RH. Antioxidant N-acetyl cysteine reduces incidence and multiplicity of lymphoma in Atm deficient mice. DNA Repair (Amst) 2006;5(7):852–859. doi: 10.1016/j.dnarep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Ribizzi I, Darnowski JW, Goulette FA, Sertoli MR, Calabresi P. Amifostine cytotoxicity and induction of apoptosis in a human myelodysplastic cell line. Leuk Res. 2000;24(6):519–525. doi: 10.1016/s0145-2126(00)00007-2. [DOI] [PubMed] [Google Scholar]
- Righi E, Lindstrom V, Linton C, Leblanc P, Santosuosso M, Pazoles C, et al. Positive immunomodulatory effects of NOV-002, an oxidized glutathione mimetic, in a murine model of ovarian cancer; AACR Conference on Tumor Immunology; December 2; Miami, FL. 2008. [Google Scholar]
- Roberts RL, Aroda VR, Ank BJ. N-acetylcysteine enhances antibody-dependent cellular cytotoxicity in neutrophils and mononuclear cells from healthy adults and human immunodeficiency virus-infected patients. J Infect Dis. 1995;172(6):1492–1502. doi: 10.1093/infdis/172.6.1492. [DOI] [PubMed] [Google Scholar]
- Romano MF, Lamberti A, Bisogni R, Garbi C, Pagnano AM, Auletta P, Tassone P, Turco MC, Venuta S. Amifostine inhibits hematopoietic progenitor cell apoptosis by activating NF-kappaB/Rel transcription factors. Blood. 1999;94(12):4060–4066. [PubMed] [Google Scholar]
- Romero L, Andrews K, Ng L, O'Rourke K, Maslen A, Kirby G. Human GSTA1-1 reduces c-Jun N-terminal kinase signalling and apoptosis in Caco-2 cells. Biochem J. 2006;400(1):135–141. doi: 10.1042/BJ20060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Rovin BH, Dickerson JA, Tan LC, Fassler J. Modulation of IL-1-induced chemokine expression in human mesangial cells through alterations in redox status. Cytokine. 1997;9(3):178–186. doi: 10.1006/cyto.1996.0152. [DOI] [PubMed] [Google Scholar]
- Ruscoe JE, Rosario LA, Wang T, Gate L, Arifoglu P, Wolf CR, Henderson CJ, Ronai Z, Tew KD. Pharmacologic or genetic manipulation of glutathione S-transferase P1-1 (GSTpi) influences cell proliferation pathways. J Pharmacol Exp Ther. 2001;298(1):339–345. [PubMed] [Google Scholar]
- Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11(12):1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara M, Satoh J, Wada R, Yagihashi S, Takahashi K, Fukuzawa M, Muto G, Muto Y, Toyota T. Inhibition of development of peripheral neuropathy in streptozotocin-induced diabetic rats with N-acetylcysteine. Diabetologia. 1996;39(3):263–269. doi: 10.1007/BF00418340. [DOI] [PubMed] [Google Scholar]
- Sangeetha P, Das UN, Koratkar R, Suryaprabha P. Increase in free radical generation and lipid peroxidation following chemotherapy in patients with cancer. Free Radic Biol Med. 1990;8(1):15–19. doi: 10.1016/0891-5849(90)90139-a. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Kayakiri H, Abe Y, Mizutani T, Inamura N, Asano M, Hatori C, Aramori I, Oku T, Tanaka H. Discovery of the first non-peptide full agonists for the human bradykinin B(2) receptor incorporating 4-(2-picolyloxy)quinoline and 1-(2-picolyl)benzimidazole frameworks. J Med Chem. 2004;47(11):2853–2863. doi: 10.1021/jm030468n. [DOI] [PubMed] [Google Scholar]
- Saydam N, Kirb A, Demir O, Hazan E, Oto O, Saydam O, Guner G. Determination of glutathione, glutathione reductase, glutathione peroxidase and glutathione S-transferase levels in human lung cancer tissues. Cancer Lett. 1997;119(1):13–19. doi: 10.1016/s0304-3835(97)00245-0. [DOI] [PubMed] [Google Scholar]
- Schubert R, Erker L, Barlow C, Yakushiji H, Larson D, Russo A, Mitchell JB, Wynshaw-Boris A. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum Mol Genet. 2004;13(16):1793–1802. doi: 10.1093/hmg/ddh189. [DOI] [PubMed] [Google Scholar]
- Schwertassek U, Balmer Y, Gutscher M, Weingarten L, Preuss M, Engelhard J, Winkler M, Dick TP. Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin-1. EMBO J. 2007;26(13):3086–3097. doi: 10.1038/sj.emboj.7601746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann S, Hainaut P. Roles of thioredoxin reductase 1 and APE/Ref-1 in the control of basal p53 stability and activity. Oncogene. 2005;24(24):3853–3863. doi: 10.1038/sj.onc.1208549. [DOI] [PubMed] [Google Scholar]
- Shacter E, Williams JA, Hinson RM, Senturker S, Lee YJ. Oxidative stress interferes with cancer chemotherapy: inhibition of lymphoma cell apoptosis and phagocytosis. Blood. 2000;96(1):307–313. [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20(19):2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005;7(3–4):348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- Shen H, Chen ZJ, Zilfou JT, Hopper E, Murphy M, Tew KD. Binding of the aminothiol WR-1065 to transcription factors influences cellular response to anticancer drugs. J Pharmacol Exp Ther. 2001;297(3):1067–1073. [PubMed] [Google Scholar]
- Short S, Merkel BJ, Caffrey R, McCoy KL. Defective antigen processing correlates with a low level of intracellular glutathione. Eur J Immunol. 1996;26(12):3015–3020. doi: 10.1002/eji.1830261229. [DOI] [PubMed] [Google Scholar]
- Skalska J, Brookes PS, Nadtochiy SM, Hilchey SP, Jordan CT, Guzman ML, Maggirwar SB, Briehl MM, Bernstein SH. Modulation of cell surface protein free thiols: a potential novel mechanism of action of the sesquiterpene lactone parthenolide. PLoS One. 2009;4(12):e8115. doi: 10.1371/journal.pone.0008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su WC, Chang SL, Chen TY, Chen JS, Tsao CJ. Comparison of in vitro growth-inhibitory activity of carboplatin and cisplatin on leukemic cells and hematopoietic progenitors: the myelosuppressive activity of carboplatin may be greater than its antileukemic effect. Jpn J Clin Oncol. 2000;30(12):562–567. doi: 10.1093/jjco/hyd137. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51(3):794–798. [PubMed] [Google Scholar]
- Tan M, Li S, Swaroop M, Guan K, Oberley LW, Sun Y. Transcriptional activation of the human glutathione peroxidase promoter by p53. J Biol Chem. 1999;274(17):12061–12066. doi: 10.1074/jbc.274.17.12061. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Pracyk JB, Takeda K, Yu ZX, Ferrans VJ, Deshpande SS, Ozaki M, Hwang PM, Lowenstein CJ, Irani K, Finkel T. Expression of Id1 results in apoptosis of cardiac myocytes through a redox-dependent mechanism. J Biol Chem. 1998;273(40):25922–25928. doi: 10.1074/jbc.273.40.25922. [DOI] [PubMed] [Google Scholar]
- Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54(16):4313–4320. [PubMed] [Google Scholar]
- Tew KD. Redox in redux: Emergent roles for glutathione S-transferase P (GSTP) in regulation of cell signaling and S-glutathionylation. Biochem Pharmacol. 2007;73(9):1257–1269. doi: 10.1016/j.bcp.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1(2):140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128(2):325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Townsend D, Tew K. Cancer drugs, genetic variation and the glutathione-S-transferase gene family. Am J Pharmacogenomics. 2003;3(3):157–172. doi: 10.2165/00129785-200303030-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM. S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol Interv. 2007;7(6):313–324. doi: 10.1124/mi.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM, Deng M, Zhang L, Lapus MG, Hanigan MH. Metabolism of Cisplatin to a nephrotoxin in proximal tubule cells. J Am Soc Nephrol. 2003a;14(1):1–10. doi: 10.1097/01.asn.0000042803.28024.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM, He L, Hutchens S, Garrett TE, Pazoles CJ, Tew KD. NOV-002, a glutathione disulfide mimetic, as a modulator of cellular redox balance. Cancer Res. 2008a;68(8):2870–2877. doi: 10.1158/0008-5472.CAN-07-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, Tew KD. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem. 2009;284(1):436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM, Pazoles CJ, Tew KD. NOV-002, a mimetic of glutathione disulfide. Expert Opin Investig Drugs. 2008b;17(7):1075–1083. doi: 10.1517/13543784.17.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003b;57(3–4):145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10(8):1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugi M, Dobashi K, Ishizuka T, Endou K, Hamuro J, Murata Y, Nakazawa T, Mori M. c-Jun N-terminal kinase negatively regulates lipopolysaccharide-induced IL-12 production in human macrophages: role of mitogen-activated protein kinase in glutathione redox regulation of IL-12 production. J Immunol. 2003;171(2):628–635. doi: 10.4049/jimmunol.171.2.628. [DOI] [PubMed] [Google Scholar]
- Utsugi M, Dobashi K, Koga Y, Shimizu Y, Ishizuka T, Iizuka K, et al. Glutathione redox regulates lipopolysaccharide-induced IL-12 production through p38 mitogen-activated protein kinase activation in human monocytes: role of glutathione redox in IFN-gamma priming of IL-12 production. J Leukoc Biol. 2002;71(2):339–347. [PubMed] [Google Scholar]
- Uys JD, Manevich Y, Devane LC, He L, Garret TE, Pazoles CJ, Tew KD, Townsend DM. Preclinical pharmacokinetic analysis of NOV-002, a glutathione disulfide mimetic. Biomed Pharmacother. 2010 doi: 10.1016/j.biopha.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ven AJ, Blom HJ, Peters W, Jacobs LE, Verver TJ, Koopmans PP, Demacker P, van der Meer JW. Glutathione homeostasis is disturbed in CD4-positive lymphocytes of HIV-seropositive individuals. Eur J Clin Invest. 1998;28(3):187–193. doi: 10.1046/j.1365-2362.1998.00267.x. [DOI] [PubMed] [Google Scholar]
- van Pel M, van Os R, Velders GA, Hagoort H, Heegaard PM, Lindley IJ, Willemze R, Fibbe WE. Serpina1 is a potent inhibitor of IL-8-induced hematopoietic stem cell mobilization. Proc Natl Acad Sci U S A. 2006;103(5):1469–1474. doi: 10.1073/pnas.0510192103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu CS, Niture SK, Doneanu CE, Pattabiraman N, Srivenugopal KS. Human p53 is inhibited by glutathionylation of cysteines present in the proximal DNA-binding domain during oxidative stress. Biochemistry. 2007;46(26):7765–7780. doi: 10.1021/bi700425y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschraagen M, Boven E, Torun E, Hausheer FH, Bast A, van der Vijgh WJ. Possible (enzymatic) routes and biological sites for metabolic reduction of BNP7787, a new protector against cisplatin-induced side-effects. Biochem Pharmacol. 2004;68(3):493–502. doi: 10.1016/j.bcp.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Verschraagen M, Kedde MA, Hausheer FH, Van Der Vijgh WJ. The chemical reactivity of BNP7787 and its metabolite mesna with the cytostatic agent cisplatin: comparison with the nucleophiles thiosulfate, DDTC, glutathione and its disulfide GSSG. Cancer Chemother Pharmacol. 2003;51(6):499–504. doi: 10.1007/s00280-003-0610-3. [DOI] [PubMed] [Google Scholar]
- Voso MT, Hohaus S, Guidi F, Fabiani E, D'Alo F, Groner S, Spath D, Doehner K, Leone G, Doehner H, Schlenk RF. Prognostic role of glutathione S-transferase polymorphisms in acute myeloid leukemia. Leukemia. 2008;22(9):1685–1691. doi: 10.1038/leu.2008.169. [DOI] [PubMed] [Google Scholar]
- Wang TT, Zeng GC, Li XC, Zeng HP. In vitro studies on the antioxidant and protective effect of 2-substituted -8-hydroxyquinoline derivatives against H(2)O(2)-induced oxidative stress in BMSCs. Chem Biol Drug Des. 75(2):214–222. doi: 10.1111/j.1747-0285.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther. 126(2):119–145. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Winkler IG, Hendy J, Coughlin P, Horvath A, Levesque JP. Serine protease inhibitors serpina1 and serpina3 are down-regulated in bone marrow during hematopoietic progenitor mobilization. J Exp Med. 2005;201(7):1077–1088. doi: 10.1084/jem.20042299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondrak GT. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid Redox Signal. 2009;11(12):3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, Vercherat C, et al. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem. 2008;283(37):25692–25705. doi: 10.1074/jbc.M800517200. [DOI] [PubMed] [Google Scholar]
- Yin Z, Ivanov VN, Habelhah H, Tew K, Ronai Z. Glutathione S-transferase p elicits protection against H2O2-induced cell death via coordinated regulation of stress kinases. Cancer Res. 2000;60(15):4053–4057. [PubMed] [Google Scholar]
- Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60(1):6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Spapen H, Nguyen DN, Rogiers P, Bakker J, Vincent JL. Effects of N-acetyl-L-cysteine on regional blood flow during endotoxic shock. Eur Surg Res. 1995;27(5):292–300. doi: 10.1159/000129412. [DOI] [PubMed] [Google Scholar]
- Zheng H, Weiner LM, Bar-Am O, Epsztejn S, Cabantchik ZI, Warshawsky A, Youdim MB, Fridkin M. Design, synthesis, and evaluation of novel bifunctional iron-chelators as potential agents for neuroprotection in Alzheimer's, Parkinson's, and other neurodegenerative diseases. Bioorg Med Chem. 2005;13(3):773–783. doi: 10.1016/j.bmc.2004.10.037. [DOI] [PubMed] [Google Scholar]