Abstract
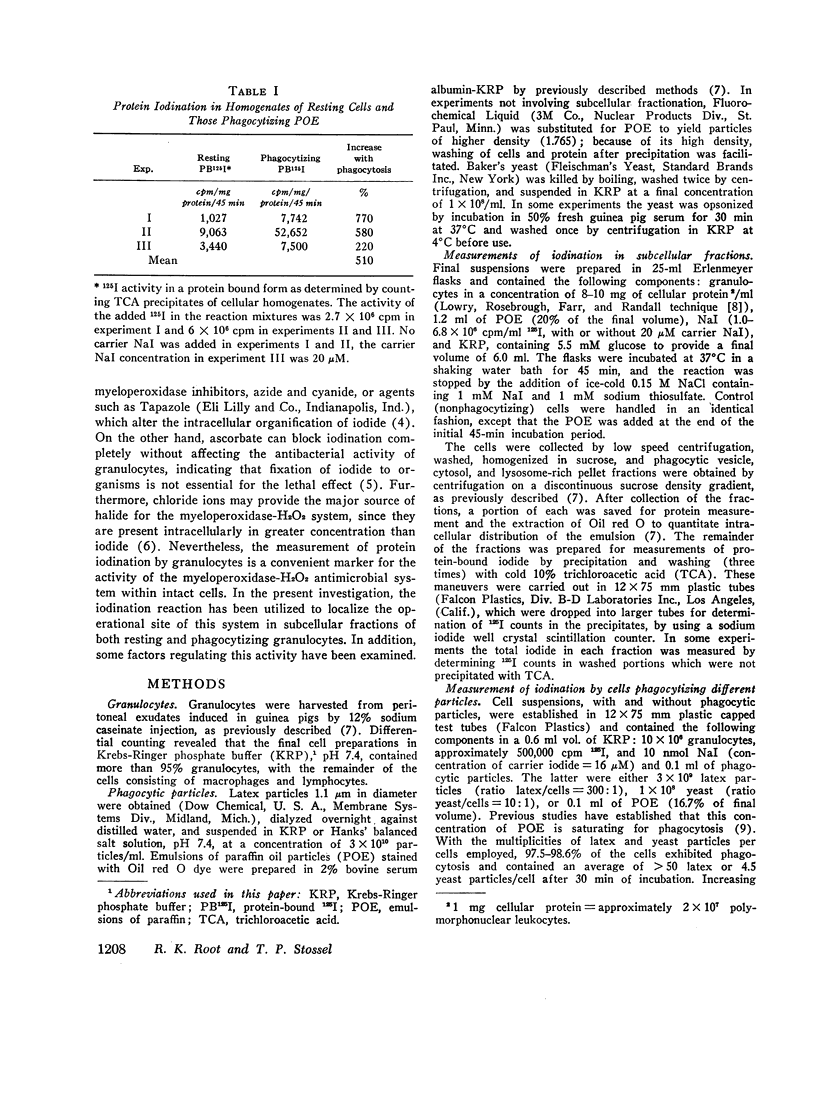
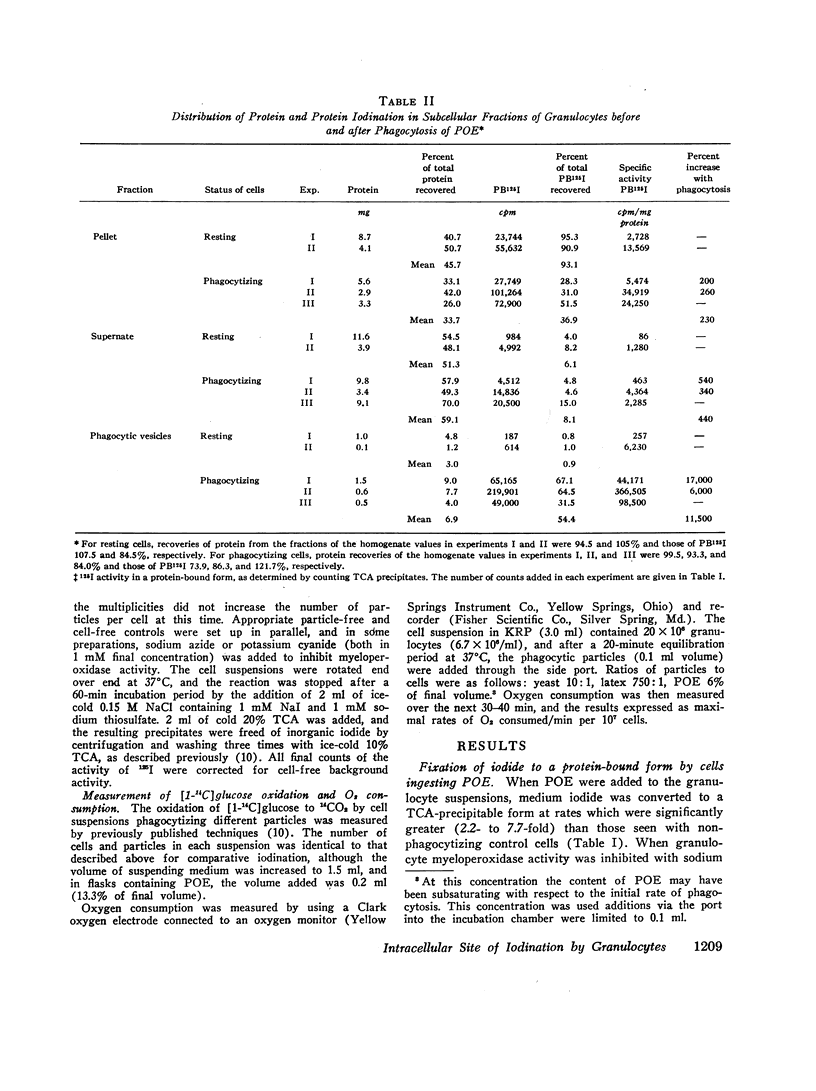
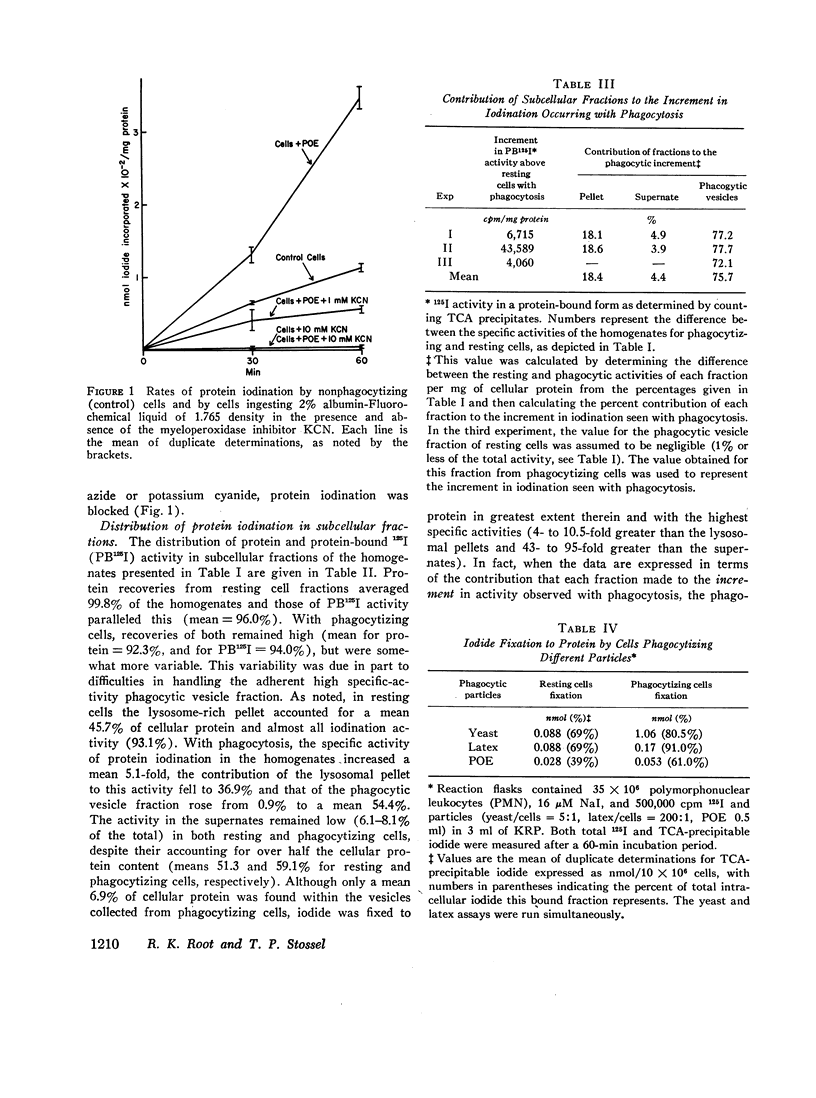
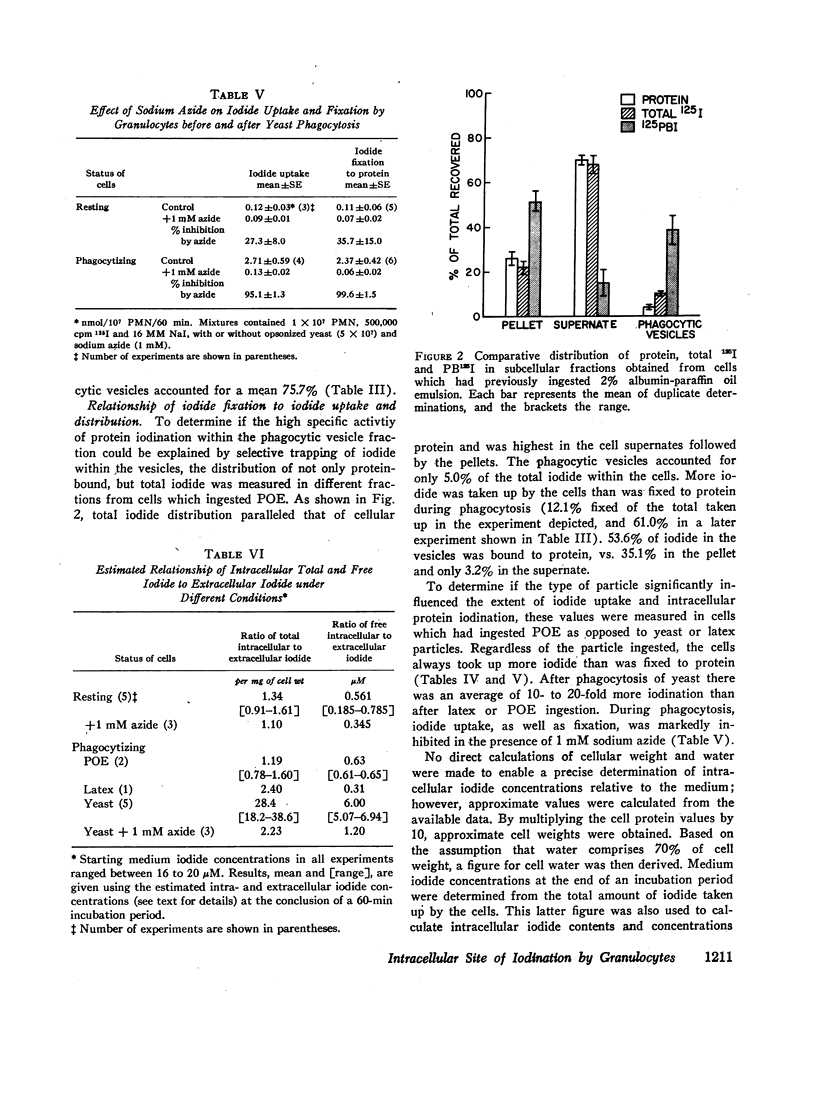
The intracellular site of operation of the myeloperoxidase-H2O2-halide antibacterial system of granulocytes has been determined by utilizing measurements of the fixation of iodide to trichloracetic acid (TCA) precipitates of subcellular fractions, including intact phagocytic vesicles. Na125I was added to suspensions of guinea pig granulocytes in Krebs-Ringer phosphate buffer, and they were then permitted to phagocytize different particles. Phagocytic vesicles were formed by allowing cells to ingest a paraffin oil emulsion (POE) and collected by flotation on sucrose after homogenization. Measurement of 125I bound to TCA precipitates of the different fractions and the homogenates disclosed that the lysosome-rich fraction obtained by centrifugation from control (nonphagocytizing) cells accounted for a mean 93.1% of the total cellular activity. With phagocytosis of POE, TCA-precipitable iodination increased two- to sevenfold, and the lysosomal contribution fell to a mean 36.9% of the total. The appearance of activity within phagocytic vesicles accounted for almost the entire increase seen with phagocytosis (a mean 75.7%), and iodide was bound within these structures with high specific activity. More iodide was taken up by cells than fixed, regardless of iodide concentration, and was distributed widely throughout the cell rather than selectively trapped within the vesicles.
The amount of iodide taken up and fixed varied considerably with the phagocytic particle employed. Yeast particles were found to stimulate iodination to a far greater degree than the ingestion of POE or latex. Such observations are consistent with the concept that the ingested particle is a major recipient of the iodination process. Measurements of metabolic activities related to the formation and utilization of peroxide by cells phagocytizing different particles were made and correlated with iodination. The findings suggest that mechanisms must exist within granulocytes to collect or perhaps even synthesize H2O2 within phagocytic vesicles to serve as substrate for myeloperoxidase. The simultaneous stimulation of other metabolic pathways for peroxide disposal and its release into the medium by phagocytizing cells is consistent with the high diffusability of this important bactericidal substance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Gilman N., Karnovsky M. L. Respiration and glucose oxidation in human and guinea pig leukocytes: comparative studies. J Clin Invest. 1970 Apr;49(4):692–700. doi: 10.1172/JCI106281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. J., Karnovsky M. L. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Jan;48(1):187–192. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loschen G., Flohé L., Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett. 1971 Nov 1;18(2):261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- Paul B. B., Jacobs A. A., Strauss R. R., Sbarra A. J. Role of the Phagocyte in Host-Parasite Interactions XXIV. Aldehyde Generation by the Myeloperoxidase-H(2)O(2)-Chloride Antimicrobial System: a Possible In Vivo Mechanism of Action. Infect Immun. 1970 Oct;2(4):414–418. doi: 10.1128/iai.2.4.414-418.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus S. H., Klebanoff S. J. Quantitative leukocyte iodination. N Engl J Med. 1971 Apr 8;284(14):744–750. doi: 10.1056/NEJM197104082841402. [DOI] [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons S. R., Karnovsky M. L. Iodinating ability of various leukocytes and their bactericidal activity. J Exp Med. 1973 Jul 1;138(1):44–63. doi: 10.1084/jem.138.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolc V. Stimulation of iodoproteins and thyroxine formation in human leukocytes by phagocytosis. Biochem Biophys Res Commun. 1971 Oct 1;45(1):159–166. doi: 10.1016/0006-291x(71)90064-7. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Hartwig J., Vaughan M. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972 Mar;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Pollard T. D., Mason R. J., Vaughan M. Isolation and properties of phagocytic vesicles from polymorphonuclear leukocytes. J Clin Invest. 1971 Aug;50(8):1745–1747. doi: 10.1172/JCI106664. [DOI] [PMC free article] [PubMed] [Google Scholar]



