Abstract
Primary carnitine deficiency is caused by impaired activity of the Na+-dependent OCTN2 carnitine/organic cation transporter. Carnitine is essential for entry of long-chain fatty acids into mitochondria and its deficiency impairs fatty acid oxidation. Most missense mutations identified in patients with primary carnitine deficiency affect putative transmembrane or intracellular domains of the transporter. Exceptions are the substitutions P46S and R83L located in an extracellular loop close to putative glycosylation sites (N57, N64, and N91) of OCTN2. P46S and R83L impaired glycosylation and maturation of OCTN2 transporters to the plasma membrane. We tested whether glycosylation was essential for the maturation of OCTN2 transporters to the plasma membrane. Substitution of each of the 3 asparagine (N) glycosylation sites with glutamine (Q) decreased carnitine transport. Substitution of two sites at a time caused a further decline in carnitine transport that was fully abolished when all three glycosylation sites were substituted by glutamine (N57Q/N64Q/N91Q). Kinetic analysis of carnitine and sodium-stimulated carnitine transport indicated that all substitutions decreased the Vmax for carnitine transport, but N64Q/N91Q also significantly increased the Km toward carnitine, indicating that these two substitutions affected regions of the transporter important for substrate recognition. Western blot analysis confirmed increased mobility of OCTN2 transporters with progressive substitutions of asparagines 57, 64 and/or 91 with glutamine. Confocal microscopy indicated that glutamine substitutions caused progressive retention of OCTN2 transporters in the cytoplasm, up to full retention (such as that observed with R83L) when all 3 glycosylation sites were substituted. Tunicamycin prevented OCTN2 glycosylation, but it did not impair maturation to the plasma membrane. These results indicate that OCTN2 is physiologically glycosylated and that the P46S and R83L substitutions impair this process. Glycosylation does not affect maturation of OCTN2 transporters to the plasma membrane, but the 3 asparagines that are normally glycosylated are located in a region important for substrate recognition and turnover rate.
Keywords: Primary carnitine deficiency, Fatty acid oxidation, OCTN2, SLC22A5, Organic cation transporter, Carnitine transport, Glycosylation
1. Introduction
Primary carnitine deficiency (OMIM # 212140) is a recessively inherited disorder of the carnitine cycle that impairs fatty acid oxidation [1]. Carnitine is essential for entry of long-chain fatty acids into mitochondria to allow subsequent β-oxidation [1]. Carnitine deficiency can result in fasting-induced hypoketotic hypoglycemia, hepatic encephalopathy, sudden death, skeletal or cardiac myopathy [2]. The gene for primary carnitine deficiency, SLC22A5, encodes the carnitine transporter OCTN2 [3, 4]. Heterogeneous mutations have been identified in affected patients (reviewed in refs. [1, 5]). OCTN2 is a novel organic cation transporter and operates a sodium-dependent transport of carnitine [3, 4, 6] and a sodium-independent uptake of organic cations [6–9].
The OCTN2 carnitine transporter is composed of 557 amino acids with 12 predicted transmembrane spanning domains [3, 4]. As for other organic cation transporters, both the amino and carboxy terminus are predicted to face the cytoplasm [3, 4]. The study of natural mutations and chimeric transporters obtained by fusing specific domains of the OCTN2 transporter with the OCTN1 transporter (that does not transport carnitine) has identified multiple domains of OCTN2 essential for function. The C-terminus of OCTN2 is required for an efficient transmembrane transfer of the Na+/carnitine complex with an essential role played by tyrosine residues in the intracellular loop between transmembrane domains 10 and 11 [10, 11]. The N-terminus and residues in transmembrane domains 7, 9 and 10 have important roles in carnitine recognition [11]. Other mutations such as S467C and P478L in transmembrane domain 11 can impair carnitine transport without affecting cation transfer [8, 9].
All organic anion transporters (OATs) and cation organic transporters (OCTs) encoded by the SLC22 family share a large extracellular loop between transmembrane domains 1 and 2 [12]. The structure of this loop is highly conserved among OCT family members suggesting an essential role in transporter function [12]. This long extracellular loop contains 2–5 putative glycosylation sites, depending on the specific transporter [12]. Substitution of asparagines with glutamines at N-glycosylation sites of the human organic anion transporter 1 (hOAT1) indicated that one specific residue (N39) plays an important role in substrate recognition, while other residues could be substituted without compromising transporter function [13]. However, simultaneous replacement of all glycosylation sites impaired maturation of the organic anion transporter to the plasma membrane and abolished its function [13]. Similarly, complete inhibition of glycosylation of OAT4 prevents membrane insertion of transporters, while inhibition of specific glycosylation steps results in defective transporters with decreased affinity towards the substrate [14]. In the organic cation transporter 2 (OCT2), substitution of all glycosylation sites retains transporters in the cytoplasm, while substitution of single sites decreases the transporter turnover rate, but can increase affinity towards the substrate [15].
The OCTN2 carnitine transporter contains 3 putative glycosylation sites: N57, N64 and N91 [3, 4], yet there are no detailed studies on the effect of glycosylation on OCTN2 function. OCTN2VT, a splice variant of OCTN2 retaining 24 additional amino acids between residues E131 and W132 in the first extracellular loop, has defective glycosylation, maturation to the plasma membrane and carnitine transport activity [16]. It is unclear whether the inhibition of glycosylation is directly related to the failed maturation to the plasma membrane. A single glycosylation mutant (N91Q) of OCTN2 reduced transporter abundance, but retained carnitine transport activity when corrected for the level of expression [16].
We and others have identified several mutations in the SLC22A5 gene in patients with primary carnitine deficiency [1]. Most substitutions affect putative intracellular or transmembrane domains of the OCTN2 transporter [1]. However, two of these mutations (P46S and R83L) are located close to putative glycosylation sites of OCTN2 [2, 5]. In this paper we show that OCTN2 is physiologically glycosylated and study the effect of natural (P46S and R83L) and artificial mutations on OCTN2 glycosylation and function.
2. Materials and Methods
2.1. Patients
The R83L substitution in the OCTN2 carnitine transporter was originally found in a patient with primary carnitine deficiency identified after an episode of severe hypoglycemia and in her asymptomatic sister [17]. Subsequently, we identified another homozygous male patient who presented with hepatic encephalopathy. This mutation was also identified in a compound heterozygous state with other mutations (G15W and A214V) in an infant and his mother (both affected with primary carnitine deficiency). The latter were identified presymptomatically by expanded newborn screening in the child. Finally, it was found in an unrelated asymptomatic mother in combination with A214V as a result of abnormal newborn screening in an unaffected (carrier) child. As in most other cases of maternal carnitine deficiency [5], these mothers had normal physical examination and no symptoms related to primary carnitine deficiency.
The P46S substitution was initially reported in mothers with primary carnitine deficiency identified by expanded newborn screening in their child [5]. To date, we have found this substitution in 7 mothers and two children, always in combination with a variety of other mutations (N32S, A42S/R488H, T232M, R254X, R282X, P398L, and T520fs521X). All patients with the P46S mutations were identified presymptomatically and family studies identified two additional adults compound heterozygous with the same substitution (total of 9 adults with the P46S substitution). In our series of patients with primary carnitine deficiency, P46S is the mutation most frequently encountered in asymptomatic or minimally symptomatic (easy fatigability, muscle pain with exercise, fasting intolerance) adults.
2.2. DNA analysis
DNA studies were approved by the Institutional Review Board of the University of Utah. Informed consent or parental consent was obtained prior to all DNA studies. Genomic DNA was extracted from fibroblasts or peripheral blood by standard methods and amplified using PCR and primers flanking each of the 10 exons of the SLC22A5 gene [2, 5]. PCR products were visualized by agarose gel electrophoresis, purified by Qiagen column, and sequenced directly using an ABI automated DNA sequencer. Mutations were confirmed by sequencing in both directions in two independent PCR products.
2.3. Cell culture and carnitine transport
Chinese Hamster Ovary (CHO) cells were grown in Ham F12 medium supplemented with 6% fetal bovine serum. Carnitine transport was measured at 37 C with the cluster-tray method as described previously [10, 11]. HEK-293 cells were grown in Dulbecco’s modified Eagle’s Medium supplemented with 10% fetal bovine serum. For transport measurement, cells were grown to confluence in 24-well plates (Costar) and depleted of intracellular amino acids by incubation for 90 min in Earle’s balanced salt solution containing 5.5 mM D-glucose and supplemented with 0.1% bovine serum albumin. Carnitine (0.5 µM, 0.5 µCi/ml) was then added to the cells for 30 min. Nonsaturable carnitine transport was measured in the presence of 2 mM cold substrate. The transport reaction was stopped by rapidly washing the cells 3 times with ice-cold 0.1 M MgCl2. Intracellular carnitine was extracted from the cells with 0.5 ml of ice-cold ethanol and added to 5 ml of scintillation fluid for counting. Intracellular carnitine was normalized for the protein content and intracellular water of each well and expressed as nmol/ml cell water [10, 11]. Saturable carnitine transport was calculated by subtracting transport in the presence of excess cold substrate from total transport and values are reported as means ± SE of 3–6 independent determinations. Initial experiments on CHO cells transfected or not with OCTN2 indicated that carnitine (0.5 µM) accumulation was linear up to 60 min (not shown).
Kinetic constants for carnitine (0.5–100 µM) transport were determined by nonlinear regression analysis according to a Michaelis-Menten equation [10, 11]. Nonsaturable carnitine transport, measured in the presence of 2 mM carnitine, was subtracted from total transport to obtain saturable carnitine transport prior to nonlinear regression analysis [10, 11]. Carnitine transport in the absence of sodium was measured substituting methylglucamine for sodium, so that the sum of methylglucamine and sodium remained constant at 150 mM [18]. Nonlinear parameters are expressed in the text and figures as means ± SD.
In previous studies, we used multiple carnitine concentrations to define the Km of OCTN2 toward sodium (KNa) [18, 19]. However, we noted that the value obtained from the intercept of multiple regressions was identical to the value obtained at the lowest concentration of carnitine (0.5 µM, refs. [18, 19]). This is because, in a random bireactant system, such as the carnitine/Na cotransporter, the apparent Km toward the co-substrate (KNa) approaches the true Km (KNa) as the concentration of the substrate (carnitine) decreases to near zero [20]. Since our previous studies indicated experimentally that a concentration of carnitine of 0.5 µM provided values of apparent KNa indistinguishable from those calculated from the intersection of multiple curves, in this study we used an even lower concentration of carnitine (0.1 µM) to obtain an apparent KNa that approaches closely true KNa.
2.4. OCTN2 expression studies
The vector containing the OCTN2 cDNA fused in frame with the green fluorescent protein was previously described [18]. Mutant OCTN2 cDNAs were created by site-directed mutagenesis using the Quick Change system (Stratagene) following the manufacturer’s instructions. All final plasmids were sequenced to confirm the presence of the mutation and the absence of PCR artifacts. Plasmids were transfected into CHO cells by lipofectamine according to the manufacturer’s instructions (Invitrogen). Cells were selected with 0.8 mg/ml G418 (Gibco-BRL) for two weeks and a mass culture composed of several independent clones of transfected cells was isolated. HEK 293 cells were transiently transfected using calcium chloride with the same vectors and used 2 days after transfection.
2.5. Western blot analysis
CHO cells stably transfected with normal and mutant OCTN2 transporters were washed twice with phosphate buffered saline solution (PBS), scraped from plates and re-suspended in homogenization buffer (20mM Tris pH 7.4, 1mM EDTA, 0.25M sucrose and Complete protease inhibitors (Roche)). Cells were homogenized with a Dounce Teflon homogenizer with 3 strokes of 20 seconds each on ice. Homogenates were transferred to centrifuge tubes and centrifuged for 10 min at 500 g to sediment the nuclei fraction. Supernatants were transferred to clean tubes and centrifuged for 20 min at 5000 g to remove mitochondria. Supernatants were transferred to clean tubes and the membrane fractions were sedimented at 38,000 g for 60 min, followed by a wash in homogenization buffer and re-centrifugation at 38,000 g for 15 min. 20 mg native membrane fractions were solubilized in sample buffer (without boiling) and separated by SDS-PAGE. Proteins were transferred to Immobilon-P membranes (Millipore) by semi-dry Western blot. Membranes were probed with a 1:1000 dilution of a rabbit anti-GFP antibody (Invitrogen) followed by a 1:5000 goat anti-rabbit HRP (Santa Cruz). Immunoreactive bands were visualized using the ECL plus chemiluminescence kit (Amersham).
Hek293 cells were washed twice with PBS, lysed in lysis buffer (20mM Tris pH 7.5, 100mM NaCl, 0.5% NP-40, 0.5mM EDTA). Samples (20 µg) were treated with Peptide:N-Glycosidase F (PNGase F, New England Biolabs) at 37 C for 1 h using the manufacturer’s protocol with the exception of omitting the denaturing steps prior to the enzymatic digest. The treated samples were separated on SDS-PAGE and probed as above.
2.6. Confocal microscopy
Subcellular distribution of normal and mutant OCTN2 carnitine transporters conjugated with the green fluorescent protein was analyzed with confocal microscopy (Olympus FVX laser scanning confocal IX70 microscope using a 60X 1.4NA PLANAPO oil objective). Cells were seeded on glass bottom (No. 0 coverglass) microwell dishes (P35G-0-14-C, MatTek Corporation, Ashland, MA, USA, www.mattek.com), covered with medium and evaluated in vivo after labeling with Bodipy-ceramide (Molecular Probes, Eugene, OR, USA, http://probes.invitrogen.com/) to visualize the Golgi/endoplasmic reticulum (ER) in red. Images of the cells were obtained at 1 µm sections. Contrast microscopy was used on the same cells to define cell borders and intracellular structures. Images were then digitally superimposed using Adobe Photoshop [2]. Multiple cells were scanned and representative cells are shown.
For quantitative studies, images were analyzed using the MetaMorph® Imaging System (Molecular Devices, Sunnyvale, CA). Images obtained for the green dye (carnitine transporters) were co-localized with those obtained for the red dye (Golgi/endoplasmic reticulum). Pixels not co-localizing were expressed as percent of total green pixels. The data obtained were averaged and analyzed using SigmaPlot.
3. Results
The SLC22A5 gene encoding the OCTN2 carnitine transporter was sequenced in patients with primary carnitine deficiency. The P46S and R83L substitutions were identified in several patients (see Patients in Experimental Procedures). Both mutations markedly impaired carnitine transport when expressed in CHO cells (Fig. 1), although P46S retained transport activity significantly above that measured in untransfected CHO cells (p<0.01 using analysis of variance).
Fig. 1. Carnitine transport in CHO cells expressing normal and mutant carnitine transporters.
CHO cells were transfected with normal and mutant OCTN2 cDNAs and selected for resistance to G418 (0.8 mg/ml). Carnitine (0.5 µM) transport was measured for 30 min in stably transfected cells and corrected for nonsaturable uptake (measured in the presence of 2 mM cold carnitine). Points are averages ± SE of 3–5 independent experiments (each in triplicate). * p<0.01 versus CHO-K1, R83L, N57/64/91Q. **p<0.01 versus P46S and N64/91Q; ***p<0.01 versus OCTN2, N57/64Q, N57/91Q using analysis of variance.
Analysis of P46S- and R83L-GFP-tagged transporters by confocal microscopy indicated that both mutations retained the majority of the protein in the cytoplasm when expressed in CHO cells (Fig. 2B). The P46S and R83L substitutions are located close to the three putative glycosylation sites of OCTN2 (N57, N64 and N91) (Fig. 2A). We tested whether substitution of these putative glycosylation sites with glutamine (Q) impaired carnitine transport and membrane localization of OCTN2. As an added control, we generated an additional mutant, N133Q, as asparagine 133 is not predicted to be glycosylated. Carnitine transport decreased significantly in CHO cells expressing N57Q, N64Q, N91Q and N133Q as compared to the normal OCTN2 (Fig. 1). Carnitine transport decreased further with the simultaneous substitution of two putative glycosylation sites (N57/64Q, N57/91Q, N64/91Q), with the N64/91Q double substitution being the most effective in reducing carnitine transport. The increase in carnitine transport above that measured in untransfected cells (CHO-K1) was abolished when all three glycosylation sites were substituted by glutamine (N57/64/91Q) (Fig. 1).
Fig. 2.
A. Schematic of the carnitine transporter with putative glycosylation sites (branching). The natural mutations (P46S and R83L) and the artificial substitution N133Q are also shown. Putative function of different domains of the transporter were determined in previous studies [10, 11, 22]. B. Subcellular distribution of normal and mutant OCTN2 carnitine transporters. The wild-type (panel A), P46S- (panel B) and R83L-mutant (panel C) OCTN2 transporters were tagged with the green fluorescent protein and stably expressed in CHO cells. Cells were labeled in vivo with Bodipy-ceramide to visualize the Golgi. Phase contrast microscopy was used to define the cell borders and intracellular structures. Images were digitally overlayed to show the location of green transporters compared to the red Golgi and plasma membrane. Co-localization of membrane transporters with the Golgi is seen as a yellow signal.
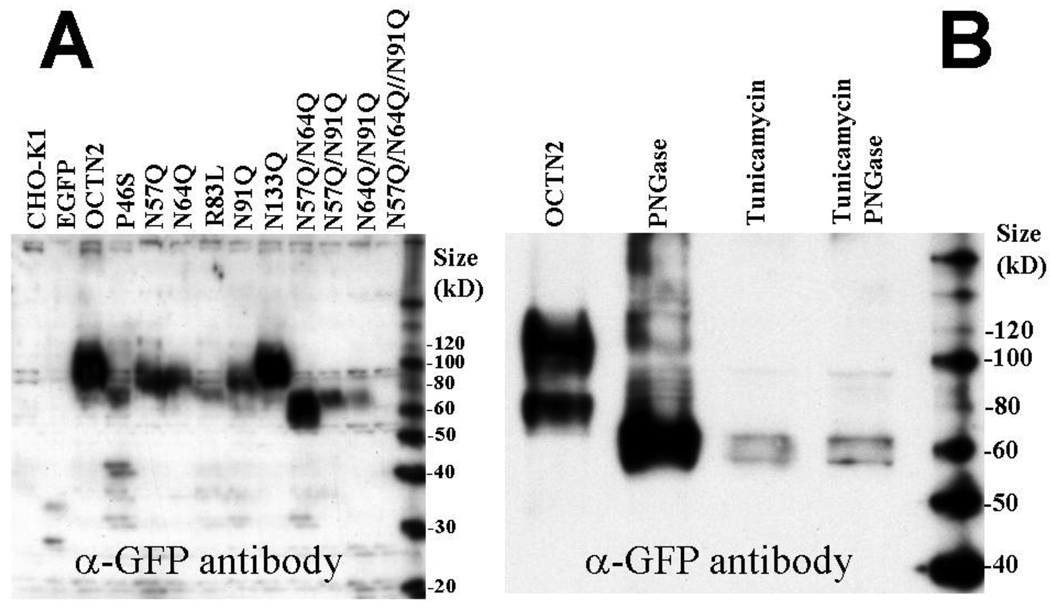
Western-blot analysis of native proteins (boiling markedly decreased protein reactivity and created multiple artificial bands) from CHO lysates indicated that P46S- and R83L-OCTN2 had decreased abundance and increased mobility as compared to wild-type OCTN2 (Fig. 3A). Substitution of putative glycosylation sites caused a progressive increased mobility of the OCTN2 transporter as more asparagines were substituted by glutamine (Fig. 3A). Although estimation of protein size on native gels is only approximate, OCTN2 transporters substituted at more than one glycosylation site had an apparent mobility close to the 60 kD standard (the predicted size of unglycosylated OCTN2 is 62,751). The substitution of glycosylation sites also decreased the intensity of the OCTN2-GFP signal, that was the most reduced with N64/N91Q double substitution and with the N57/64/91Q triple substitution in which only a shadow was seen (Fig. 3A). By contrast, substitution of N133 (not a glycosylation site) with glutamine did not affect mobility or intensity of the OCTN2 signal, confirming that this residue is not physiologically glycosylated.
Fig. 3. Western blot analysis of normal and mutant OCTN2 transporters tagged with GFP.
(A) Cell lysates from CHO cells expressing normal and mutant OCTN2 transporters were separated by native gel electrophoresis and recognized by an anti-GFP polyclonal antibody. CHO cells expressing the green fluorescent protein alone (EGFP) were included as an internal control. (B) Cells expressing the normal OCTN2 transporter were preincubated for 24 h in the absence or presence of tunicamycin (1 µg/ml). Cell extracts were digested with Peptide: N-Glycosidase F (PNGase) prior to separation by native gel electrophoresis where indicated.
Glycosylation can be studied with inhibitors or with digestion of previously glycosylated proteins. Unfortunately, the amount of OCTN2 protein produced by stably transfected CHO cells was not sufficient for this type of analysis. HEK-293 cells produced increased amounts of OCTN2 protein after transfection in comparison to CHO cells and were used for this type of study. Digestion of extracts from HEK-293 cells overexpressing the OCTN2 carnitine transporter with Peptide: N-Glycosidase F (PNGase) or preincubation with tunicamycin, a glycosylation inhibitor, reduced the apparent size of the OCTN2 transporter to a size similar to that seen with multiple asparagine to glutamine substitutions (Fig. 3B). No additional size reduction was observed in extracts from tunicamycin-treated cells that were subsequently treated with PNGase, indicating that both treatments completely removed all N-linked glycosylation.
Since P46S- and R83L-mutant carnitine transporters failed to mature to the plasma membrane, we tested whether glycosylation mutants were also retained in the cytoplasm (Fig. 4). There was a progressive increase in the retention of OCTN2 transporters in the cytoplasm when more asparagine glycosylation sites were substituted by glutamine. N133Q also reduced membrane localization of the OCTN2 transporter. As shown in Fig. 4, normal OCTN2 transporters reaching the plasma membrane (green) did not colocalize with markers for the Golgi/Endoplasmic reticulum (red). For this reason, we measured the percent of total OCTN2 transporters reaching the plasma membrane as those not colocalizing with the red signal using MetaMorph. There was a significant correlation between membrane transport activity and percent of OCTN2 transporters localized on the plasma membrane (Fig. 5), indicating that cytoplasmic retention was an important factor in reducing carnitine transport activity by the substitutions evaluated.
Fig. 4. Subcellular distribution of normal and mutant carnitine transporters tagged with the green fluorescent protein.
CHO cells stably transfected with normal and mutant OCTN2-GFP were seeded on glass-bottom dishes. Live cells were visualized by confocal microscopy at 1 µm sections. Cells were labeled in vivo with Bodipy-ceramide to visualize the Golgi. Phase contrast microscopy was used to define the cell borders and intracellular structures. Images were digitally overlayed to show the location of green transporters compared to the red Golgi and plasma membrane. Co-localization of membrane transporters with the Golgi is seen as a yellow signal.
Fig. 5. Correlation between carnitine transport and membrane localization of mutant OCTN2-carnitine transporters.
Confocal microscope images were analyzed using the MetaMorph® Imaging System (Molecular Devices, Sunnyvale, CA). Images obtained for the green dye (carnitine transporters) were co-localized with those obtained for the red dye (Golgi/endoplasmic reticulum). Pixels not co-localizing were expressed as percent of total green pixels. The data obtained were averaged and analyzed using SigmaPlot. Membrane-localized transporters were then correlated with carnitine transport. The line represents a linear regression of data. Significance of the regression was obtained using analysis of variance. Points are averages of quadruplicates for imaging and 3–5 samples for transport activity. Standard deviations are indicated in both directions and are not visible when smaller than the symbol.
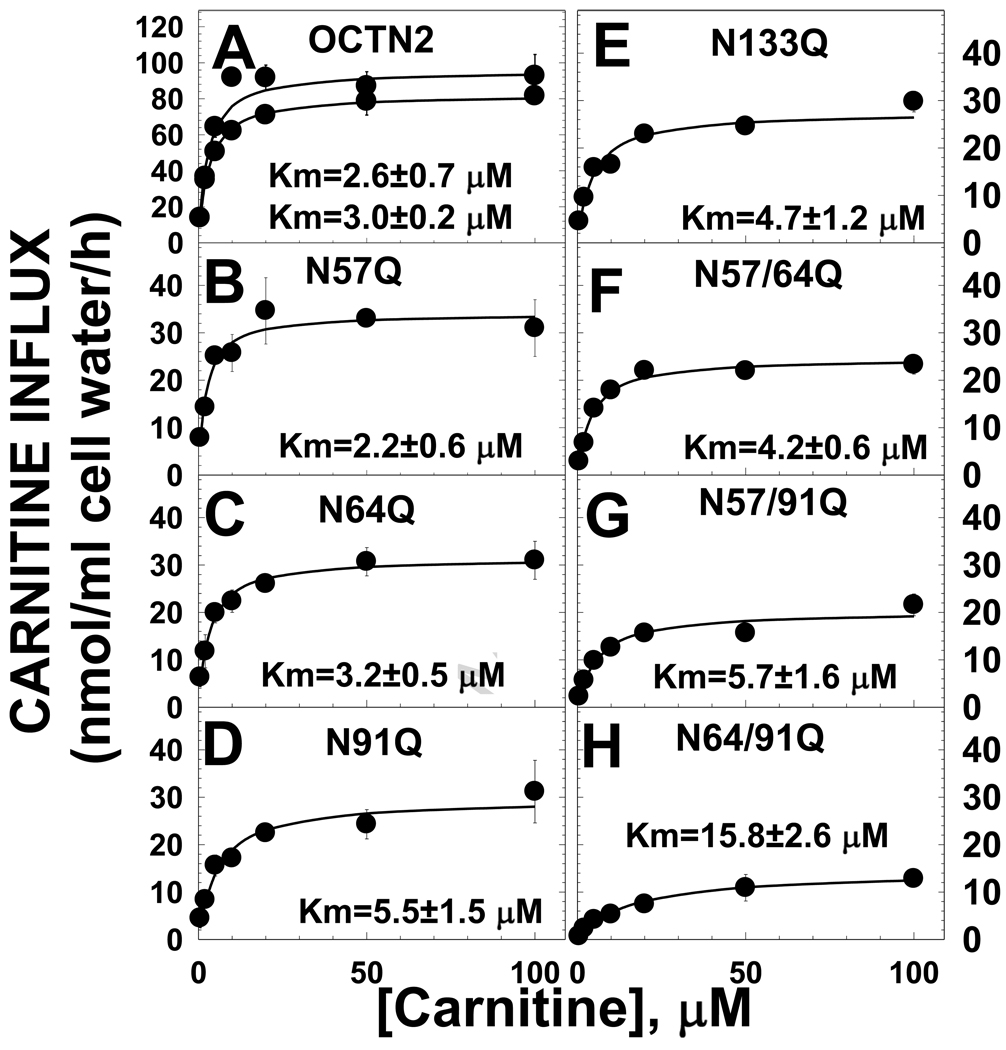
Since the N-terminus of the OCTN2 carnitine transporter is involved in substrate and co-substrate recognition [11], we evaluated whether the glycosylation mutants affected the kinetic constants for carnitine transport. All glycosylation substitutions decreased the Vmax for carnitine transport, the effect being more marked for the combination N64/91Q (Fig. 6). The N64/91Q substitution also caused a significant (p<0.01 versus OCTN2 using confidence intervals) increase in the Km toward carnitine, indicating that N64 and N91 may be part or close to a site important for substrate recognition.
Fig. 6. Kinetic analysis of transport by normal and mutant OCTN2.
Carnitine (0.5–100 µM) transport was measured for 30 min in CHO cells stably transfected with normal and mutant OCTN2 expression vectors. Non-saturable carnitine transport, measured in the presence of 2 mM cold carnitine, was subtracted from each point prior to calculating kinetic constants. Each point is the mean of triplicates ± SE. Lines represent the best fit of data to a Michaelis-Menten equation. Kinetic parameters, obtained by nonlinear regression analysis, are expressed as means ± SD. Two different mass cultures expressing the normal OCTN2 transporter were evaluated in each experiment and both are shown.
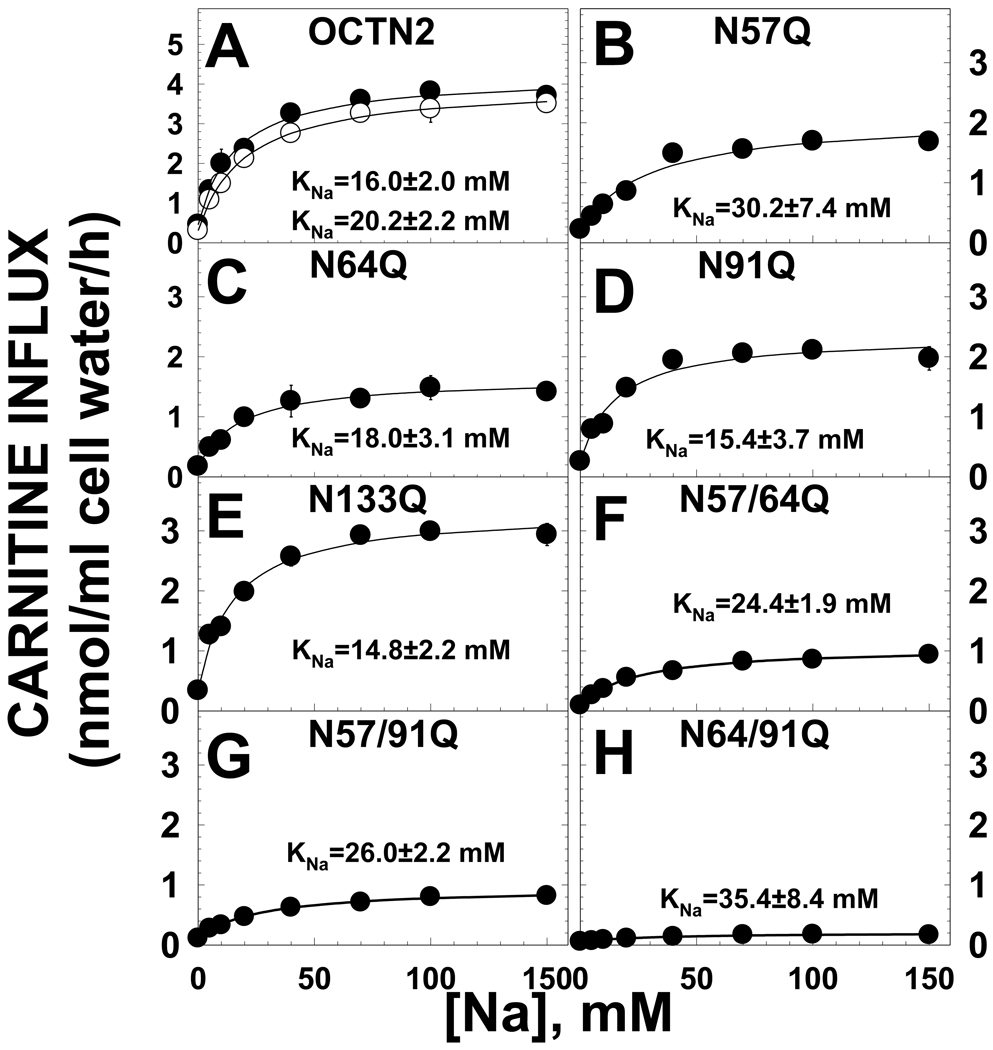
The OCTN2 carnitine transporter is energized by the sodium electrochemical potential. We previously found that the C-terminus of OCTN2 is involved in the transfer of the Na/carnitine complex inside the cell [10, 18]. We evaluated whether substitutions in the first extracellular loop also affected sodium stimulation of carnitine transport. Despite a marked decline in the amount of carnitine transport that could be stimulated by sodium, there was no significant difference in the concentration of sodium at which half-maximal stimulation of transport by sodium was observed (KNa) between the normal OCTN2 and any of the mutants (Fig. 7).
Fig. 7. Sodium stimulation of carnitine transport in CHO cells expressing normal and mutant OCTN2 transporters.
Carnitine (0.1 µM) transport was measured for 30 min at the indicated concentrations of sodium (0–150 mM). Cells were washed three times with a sodium-free solution prior to the transport experiment. Sodium-independent carnitine transport, measured in the absence of extracellular sodium, was subtracted from all points prior to plotting. Points are averages ± SE of triplicates. The data obtained were subjected to nonlinear regression analysis according to a Michaelis-Menten equation to obtain the concentration of sodium at which half-maximal stimulation was obtained (KNa, see “Methods”). Parameters are reported as averages ± SD. Two different mass cultures expressing the normal OCTN2 transporter were evaluated in each experiment and both are shown.
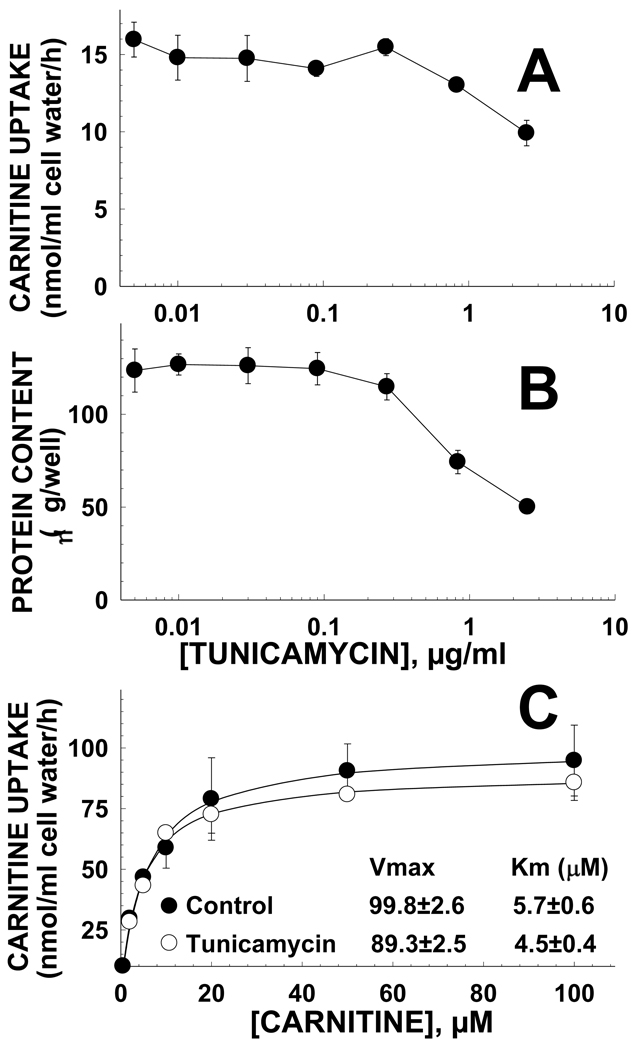
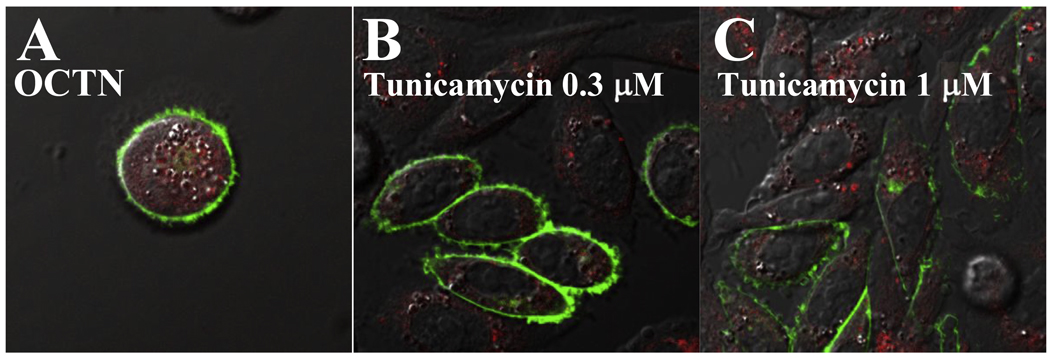
To determine whether the structural change of the first extracellular loop caused by the amino acidic substitution, rather than glycosylation affected transporter activity, carnitine transport was measured after preincubation of cells with tunicamycin. Tunicamycin effectively prevented OCTN2 glycosylation (Fig. 3B), but had no effect on carnitine transport (Fig. 8A) until a toxic effect was observed at about 1 µg/ml as indicated by decreased amounts of cellular proteins at the end of the incubation period (Fig. 8B). After preincubation with tunicamycin (0.5 µg/ml) for 16 hours, no significant changes in the kinetic parameters for carnitine transport were observed in tunicamycin-treated cells (Fig. 8C). Finally, we checked whether incubation with tunicamycin affected maturation of the transporters to the plasma membrane. OCTN2 carnitine transporters had normal membrane distribution whether or not incubated for 16 h with tunicamycin (0.3–1 µg/ml) (Fig. 9). Analysis of data with MetaMorph indicated that more than 95% of the green signal (OCTN2 transporters tagged with GFP) did not co-localize with the red signal (Golgi and ER stained with Bodipy-ceramide) in cells treated with tunicamycin 0.3 or 1 µg/ml, a value similar to cells not exposed to tunicamycin (Fig. 9A).
Fig. 8. Tunicamycin effect on carnitine transport (A), cell protein content (B), and kinetic constants for carnitine transport (C) in CHO cells overexpressing the OCTN2 carnitine transporter.
A. Cells were incubated in 24-well plates with the indicated concentrations of tunicamycin for 24 h before the transport assay. Carnitine (0.5 µM) transport was measured for 30 min at 37 C. B. Protein content was measured in the same wells in which carnitine transport was measured. Points are averages of 6 observations +/− SE. C. Kinetic constants for carnitine transport in cells incubated for 16 h in the absence or presence of tunicamycin were determined as in Fig. 6. Lines represent the best fit of the data to a Michaelis-Menten equation.
Fig. 9. Subcellular distribution of normal OCTN2 carnitine transporters tagged with the green fluorescent protein.
CHO cells stably transfected with OCTN2-GFP expression vectors were seeded on glass-bottom dishes in normal medium. Cells were incubated for 16 hours with the indicated concentrations of tunicamycin prior to analysis by confocal microscopy. Live cells were visualized by confocal microscopy at 1 µm sections. Cells were labeled in vivo with Bodipy-ceramide to visualize the Golgi. Phase contrast microscopy was used to define the cell borders and intracellular structures. Images were digitally overlayed to show the location of green transporters compared to the red Golgi and plasma membrane. Co-localization of membrane transporters with the Golgi is seen as a yellow signal.
4. DISCUSSION
All membrane transporters encoded by the SLC22 gene family share a highly conserved large extracellular loop between transmembrane domains 1 and 2 suggesting an essential role of this domain in transporter function [12]. Very few natural mutations affect this extracellular loop in patients with primary carnitine deficiency, with the exception of P46S and R83L [1, 2]. This loop contains 3 putative glycosylation sites in the OCTN2 carnitine transporter (Fig. 2A). Substitution of each of these asparagine residues with glutamine reduced carnitine transport (Fig. 1) and affected mobility of the OCTN2 carnitine transporter (Fig. 3). By contrast, substitution of the unrelated asparagine 133 did not affect transporter mobility, although transport activity was still reduced. Protein mobility was further affected when multiple sites were substituted, with the highest mobility seen when all three sites were mutated (Fig. 3). The mobility of the OCTN2 transporter with all three asparagine sites substituted was similar to that of transporters treated with PNGase or to that of cells treated with tunicamycin to prevent glycosylation (Fig. 3). These results indicate that the OCTN2 carnitine transporter is physiologically glycosylated at asparagine in positions 57, 64 and 91 and, consequently, confirm that this loop is located in the extracellular space.
Substitution of asparagine residues with glutamine generally decreased the abundance of the OCTN2 transporter and caused progressive retention of transporters in the cytoplasm (Figs. 4 and 5). Interestingly, mild cytoplasmic retention of membrane transporters was also seen with the N133Q substitution, not affecting a glycosylation site. The natural mutations P46L and R83L identified in patients with primary carnitine deficiency decreased transporter abundance, increased transporter mobility to the levels seen when multiple glycosylation sites were substituted, caused cytoplasmic transporter retention, and abolished carnitine transport (Figs. 1–3). Therefore, these natural mutations induce a conformational change of the OCTN2 transporter and cause a defect in the maturation to the plasma membrane and in glycosylation, although we are not sure whether this latter defect is complete or not. In fact, repeated experiments to determine whether PNGase affected the mobility of these mutant transporters were unsuccessful due to their low abundance.
Glycosylation per se had only limited influence on maturation of the transporters to the plasma membrane since incubation with tunicamycin did not affect membrane transport or maturation to the plasma membrane (Figs. 8,9). Since the N133Q substitution also decreased transport activity, the structural change of the extracellular loop caused by the amino acid substitution, rather than glycosylation itself, affected carnitine transport. This also suggests that this large extracellular loop of the transporter, independently from its glycosylation status, is important for transporter maturation to the plasma membrane and overall function.
Evaluation of kinetic constants of the OCTN2 transporter toward carnitine (Fig. 6) or its co-substrate sodium (Fig. 7) indicated that all asparagine substitutions tested decreased the Vmax for carnitine transport (Fig. 6). It is difficult to normalize transporter activity for the amount of transporter protein (Fig. 3), since the different transporter structure also resulted in decreased maturation of transporters to the plasma membrane (Fig. 5). The N133Q substitution and the double substitution N57Q/N64Q resulted in an abundance of immunoreactive OCTN2 transporters similar to that seen with the wild-type OCTN2 (Fig. 3). Nevertheless, only about 20% of N57/64Q transporters reached the plasma membrane as compared to 70% of N133Q transporters and >90% of the wild type OCTN2 (Fig. 5). This indicates that one of the major effects of the N133Q substitution was to reduce the unitary turnover rate (number of molecules transported inside the cell by each transporter) of the OCTN2 transporter, independently from an effect on glycosylation (Fig. 3) and with only a limited effect on cytoplasmic retention (Fig. 5). By contrast, the effect of glycosylation mutants was more related to the retention of transporters in the cytoplasm, with a significant correlation between the amount of transporters localizing to the plasma membrane and carnitine transport activity (Fig. 5). The double mutant N64/91Q that was farther away from the regression line (Fig. 5) was unique in significantly increasing the Km toward carnitine to about 5 times normal (Fig. 6). None of the mutants, however, affected the recognition of sodium with KNa that were not significantly different from that seen in control cells (Fig. 7). These results indicate that asparagine substitutions affect the unitary turnover rate of the transporter, maturation of the transporters to the plasma membrane and that the region around residues 64 and 91 might be involved in carnitine recognition. This effect on transporter affinity toward the substrate might explain why the R83L natural substitution, located between these two important residues, is completely ineffective in transporting carnitine while P46S, located closer to the other asparagines residue involved in glycosylation, retains residual transport activity and might be responsible for a milder or no overt phenotype [5]. It is still unclear whether carnitine supplements are warranted for patients carrying the P46S mutation, although some patients have experienced benefits (such as capacity to sustain exercise) with initiation of therapy.
Other organic cation and anion transporters part of the SLC22 family share this large extracellular loop between transmembrane domains 1 and 2 and glycosylation has been studied mostly in organic anion transporters [21]. In organic anion transporters, proper glycosylation is important for protein folding, membrane targeting, and substrate binding [14]. Our results in OCTN2 show that the overall structure of this large extracellular domain, rather than glycosylation itself, is the most important determinant for these important transporter functions.
Research Highlights
Natural mutations P46S and R83 L in the OCTN2 transporter affect glycosylation
Identification of natural glycosylation sites of the OCTN2 carnitine transporter
Characterization of the effects of OCTN2 glycosylation on membrane maturation
Characterization of the effects of OCTN2 glycosylation on transport activity
Acknowledgements
This project was supported by Award Number R01-DK053824 from the National Institute of Diabetes And Digestive And Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Longo N, Amat di San Filippo C, Pasquali M. Disorders of carnitine transport and the carnitine cycle. American journal of medical genetics. 2006;42C:77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amat di San Filippo C, Pasquali M, Longo N. Pharmacological rescue of carnitine transport in primary carnitine deficiency. Hum Mutat. 2006;27:513–523. doi: 10.1002/humu.20314. [DOI] [PubMed] [Google Scholar]
- 3.Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. The Journal of biological chemistry. 1998;273:20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- 4.Wu X, Prasad PD, Leibach FH, Ganapathy V. cDNA sequence, transport function, and genomic organization of human OCTN2, a new member of the organic cation transporter family. Biochemical and biophysical research communications. 1998;246:589–595. doi: 10.1006/bbrc.1998.8669. [DOI] [PubMed] [Google Scholar]
- 5.Schimmenti LA, Crombez EA, Schwahn BC, Heese BA, Wood TC, Schroer RJ, Bentler K, Cederbaum S, Sarafoglou K, McCann M, Rinaldo P, Matern D, di San Filippo CA, Pasquali M, Berry SA, Longo N. Expanded newborn screening identifies maternal primary carnitine deficiency. Molecular genetics and metabolism. 2006;90(4):441–445. doi: 10.1016/j.ymgme.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Ohashi R, Tamai I, Nezu Ji J, Nikaido H, Hashimoto N, Oku A, Sai Y, Shimane M, Tsuji A. Molecular and physiological evidence for multifunctionality of carnitine/organic cation transporter OCTN2. Molecular pharmacology. 2001;59:358–366. doi: 10.1124/mol.59.2.358. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Huang W, Prasad PD, Seth P, Rajan DP, Leibach FH, Chen J, Conway SJ, Ganapathy V. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. The Journal of pharmacology and experimental therapeutics. 1999;290:1482–1492. [PubMed] [Google Scholar]
- 8.Seth P, Wu X, Huang W, Leibach FH, Ganapathy V. Mutations in novel organic cation transporter (OCTN2), an organic cation/carnitine transporter, with differential effects on the organic cation transport function and the carnitine transport function. The Journal of biological chemistry. 1999;274:33388–33392. doi: 10.1074/jbc.274.47.33388. [DOI] [PubMed] [Google Scholar]
- 9.Ohashi R, Tamai I, Inano A, Katsura M, Sai Y, Nezu J, Tsuji A. Studies on functional sites of organic cation/carnitine transporter OCTN2 (SLC22A5) using a Ser467Cys mutant protein. The Journal of pharmacology and experimental therapeutics. 2002;302:1286–1294. doi: 10.1124/jpet.102.036004. [DOI] [PubMed] [Google Scholar]
- 10.Amat Di San Filippo C, Longo N. Tyrosine Residues Affecting Sodium Stimulation of Carnitine Transport in the OCTN2 Carnitine/Organic Cation Transporter. The Journal of biological chemistry. 2004;279:7247–7253. doi: 10.1074/jbc.M309171200. [DOI] [PubMed] [Google Scholar]
- 11.Amat di San Filippo C, Wang Y, Longo N. Functional domains in the carnitine transporter OCTN2, defective in primary carnitine deficiency. The Journal of biological chemistry. 2003;278:47776–47784. doi: 10.1074/jbc.M307911200. [DOI] [PubMed] [Google Scholar]
- 12.Burckhardt G, Wolff NA. Structure of renal organic anion and cation transporters. American journal of physiology. 2000;278:F853–F866. doi: 10.1152/ajprenal.2000.278.6.F853. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka K, Xu W, Zhou F, You G. Role of glycosylation in the organic anion transporter 1 (OAT1) The Journal of biological chemistry. 2004;279:14961–14966. doi: 10.1074/jbc.M400197200. [DOI] [PubMed] [Google Scholar]
- 14.Zhou F, Xu W, Hong M, Pan Z, Sinko PJ, Ma J, You G. The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Molecular pharmacology. 2005;67:868–876. doi: 10.1124/mol.104.007583. [DOI] [PubMed] [Google Scholar]
- 15.Pelis RM, Suhre WM, Wright SH. Functional influence of N-glycosylation in OCT2-mediated tetraethylammonium transport. American journal of physiology. 2006;290:F1118–F1126. doi: 10.1152/ajprenal.00462.2005. [DOI] [PubMed] [Google Scholar]
- 16.Maekawa S, Mori D, Nishiya T, Takikawa O, Horinouchi T, Nishimoto A, Kajita E, Miwa S. OCTN2VT, a splice variant of OCTN2, does not transport carnitine because of the retention in the endoplasmic reticulum caused by insertion of 24 amino acids in the first extracellular loop of OCTN2. Biochimica et biophysica acta. 2007;1773:1000–1006. doi: 10.1016/j.bbamcr.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Makhseed N, Vallance HD, Potter M, Waters PJ, Wong LT, Lillquist Y, Pasquali M, Amat di San Filippo C, Longo N. Carnitine transporter defect due to a novel mutation in the SLC22A5 gene presenting with peripheral neuropathy. J Inherit Metab Dis. 2004;27:778–780. doi: 10.1023/b:boli.0000045837.23328.f4. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Meadows TA, Longo N. Abnormal sodium stimulation of carnitine transport in primary carnitine deficiency. The Journal of biological chemistry. 2000;275:20782–20786. doi: 10.1074/jbc.M000194200. [DOI] [PubMed] [Google Scholar]
- 19.Scaglia F, Wang Y, Longo N. Functional characterization of the carnitine transporter defective in primary carnitine deficiency. Arch Biochem Biophys. 1999;364:99–106. doi: 10.1006/abbi.1999.1118. [DOI] [PubMed] [Google Scholar]
- 20.Segel IH. Enzyme Kinetics. New York: John Wiley & Sons, Inc; 1975. [Google Scholar]
- 21.Zhou F, You G. Molecular insights into the structure-function relationship of organic anion transporters OATs. Pharmaceutical research. 2007;24:28–36. doi: 10.1007/s11095-006-9144-9. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Kelly MA, Cowan TM, Longo N. A missense mutation in the OCTN2 gene associated with residual carnitine transport activity. Hum Mutat. 2000;15:238–245. doi: 10.1002/(SICI)1098-1004(200003)15:3<238::AID-HUMU4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]