Abstract
Selective somatostatin receptor subtype agonists have been proposed as a means to mitigate learning and memory loss associated with Alzheimer's disease. The first aim of this study evaluated blood-to-brain transport and regional brain distribution of NNC 26-9100, a selective somatostatin subtype-4 (sst4) receptor agonist. The entry rate of 131I-NNC 26-9100 was Ki = 0.25 μl/g min, with a ∼93% association with the parenchymal component. The second goal of this study was to evaluate the effect of chronic NNC 26-9100 administration (i.p) on learning and memory, brain Aβx-42 levels, and protein expression of sst4 receptor and amyloid precursor protein (APP) in the senescence-accelerated mouse p8 (SAMP8) model of Alzheimer's disease. Mice chronically treated with NNC 26-9100 showed improved learning (day-21) and memory (day-28) using the T-maze paradigm (20 and 200 μg). Ex vivo tissue analyses showed a decline in Aβx-42 levels at the 20μg dose, while no alterations were observed in sst4 receptor or APP protein expression compared to vehicle controls. These findings indicate NNC 26-9100 is taken up into key brain regions associated with learning and memory. Furthermore, chronic administration of NNC 26-9100 improved learning and memory and decreased Aβx-42 brain levels. These results suggest sst4 receptor agonists may provide a viable therapy in the treatment of Alzheimer's disease and other forms of cognitive impairment.
Keywords: somatostatin subtype-4 receptor agonist, NNC 26-9100, Alzheimer's disease, learning, memory
1. Introduction
Alzheimer's disease is a chronic neurodegenerative disorder associated with a progressive loss in memory and cognitive abilities. Currently, there is no approved drug with actual disease modifying activity for the treatment of Alzheimer's disease (Sano et al., 2008). Nevertheless, a growing number of studies have identified critical elements as to the progression of Alzheimer's disease, bringing new avenues for drug therapy. In this regard, the use of selective somatostatin-based therapeutics has been proposed as a manner by which to mitigate Alzheimer's disease (Iwata et al., 2005; Saito et al., 2005).
Decreases in the levels of somatostatin (somatotropin release-inhibiting factor, SRIF) have been shown in the cerebral cortex and cerebrospinal fluid of Alzheimer's disease patients (Davies et al., 1980; Strittmatter et al., 1997). Decreases in brain somatostatin levels have significant implications in Alzheimer's disease, as somatostatin is involved in learning and memory processes (Epelbaum et al., 2009; Tallent, 2007) and is a key regulator of the beta amyloid (Aβ) degrading enzyme neprilysin (Iwata et al., 2005; Saito et al., 2005). Increased levels of Aβ, and specifically Aβ1-42, is a consistent hallmark of Alzheimer's disease (Selkoe, 2008).
Somatostatin produces its effects through five receptor subtypes (sst1-5) (Cervia and Bagnoli, 2007). The sst4 receptor is expressed in relatively high levels in neocortex and hippocampus (Bruno et al., 1992; Moller et al., 2003), critical areas identified with learning and memory formation, Aβ accumulation, and Alzheimer's disease pathogenesis (Yasojima et al., 2001a; Yasojima et al., 2001b). Furthermore, recent investigations have identified sst4 receptor agonists dose-dependently enhanced cued memory (Gastambide et al., 2009a; Gastambide et al., 2009b). Thus, a selective and stable sst4 receptor agonist may prove to be an effective treatment for Alzheimer's disease.
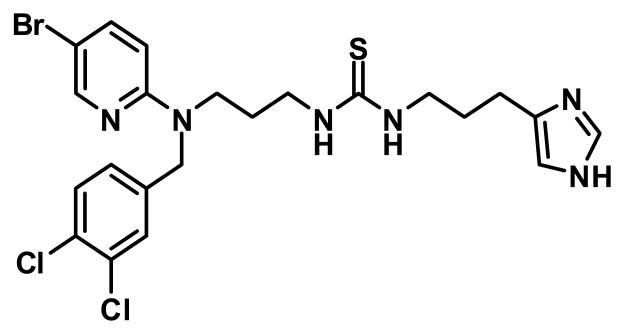
The stable non-peptide drug NNC 26-9100 (Fig. 1) is a highly selective and high affinity sst4 receptor agonist, with >100-fold selectivity for sst4 over all other somatostatin receptor subtypes (Ki = 6 nM) (Ankersen et al., 1998; Crider et al., 2004). To determine the ability of NNC 26-9100 to appropriately distribute to the brain we assessed brain uptake of radioactively labeled NNC 26-9100 via jugular vein injection (mouse), as well as regional distribution to critical brain areas associated with Alzheimer's disease. Subsequently, we evaluated the effect of chronic NNC 26-9100 administration (i.p.) on learning and memory effects in 12-month old senescence-accelerated mouse-p8 (SAMP8) mice. The SAMP8 model exhibits age-dependent learning and memory deficits with increased amounts of amyloid precursor protein (APP) and Aβ in brain tissue, which is similar to Alzheimer's disease progression (Kumar et al., 2000; Morley, 2002; Morley et al., 2002; Poon et al., 2004). Following learning and memory evaluations, ex vivo cortical brain Aβx-42 levels along with sst4 receptor and APP protein expression levels were assessed to determine if any alterations in key drug targets occurred subsequent to chronic drug treatment.
Fig. 1.
NNC 26-9100 is a non-peptide selective sst4 receptor agonist, with a Ki = 6nM at SSTR4 and an EC50 = 26 ± 6 nM (Ankersen et al., 1998; Crider et al., 2004).
2. Materials and Methods
2.1. Reagents
NNC 26-9100 was synthesized, purified, and confirmed via NMR by Dr. A.M. Crider per previously established protocols (Ankersen et al., 1998; Crider et al., 2004). All other chemicals and reagents, unless otherwise stated, were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Animals
Twelve-month old male SAMP8 mice were used for all behavioral and post-treatment molecular assessments. Male CD-1 mice were used for brain uptake and distribution evaluations (Banks et al., 2007). Mice were housed in rooms with a 12 h light/dark cycle (20–22°C) with water and food available ad libitum. All experiments were conducted in accordance with the institutional approval of the animal use subcommittee, which subscribes to the NIH Guide for Care and Use of Laboratory Animals. SAMP8 mice were obtained from the breeding colony at the Veterans Affairs Medical Center - VA hospital (St. Louis, MO). SAMP8 colony was derived from siblings generously provided by Dr. Takeda (Kyoto University, Japan).
2.3. Radioactive labeling
The chloramine-T method was used to radioactively label NNC 26-9100 (Banks et al., 2009). Reaction was initiated by adding 5 μg of NNC 26-9100 to 10 μl of chloramine-T at a concentration of 0.25 M in phosphate buffer (pH 7.5). After 1 min reaction time, the mixture was added to a column of G-10 Sephadex and the purified radioactively labeled NNC 26-9100 eluted with phosphate buffer and 100 μl fractions collected.
2.4. Brain influx rate and serum clearance
Mice were anesthetized with an i.p. injection of urethane (40% solution). The right carotid artery and left jugular vein were exposed. Mice were injected with 300,000 cpm labeled 131I -NNC 26-9100 in a volume of 200 μl lactated Ringer's solution containing 1% bovine serum albumin by injection into the jugular vein. Mice were maintained under a heat lamp before and after the i.v. injection and monitored for respiratory difficulty. At 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 30, or 45 min after the jugular injection, blood was collected from the right carotid artery, and the whole brain (WBr) was removed and weighed. Blood was centrifuged at 5400 × g for 10 min (4°C), and the level of radioactivity was measured from 50 μl of the resulting serum. The level of radioactivity in the WBr was also determined in a gamma counter. Brain/serum ratios (μl/g) were determined and plotted against exposure time (Expt).
The brain/serum ratio (μl/g) was calculated as follows:
and
Expt in minutes was calculated as follows:
where Cp is cpm per ml of serum, t is time in minutes and Cpt is the cpm in the serum at time t. When the brain/serum ratio of radioactivity of 131I-NNC 26-9100 is plotted against the exposure time, the slope of the linear regression line represents the unidirectional influx rate (Ki) of 131I-NNC 26-9100 from blood-to-brain (Patlak et al., 1983).
To evaluate clearance from serum, results (cpm in serum) from the brain influx study above were expressed as the percent of the injected dose in each milliliter of serum (% Inj/ml). These values were plotted on a graph against their respective time points (min). The % Inj/ml was calculated from the following formula:
The percent of the i.v. injected dose taken up per g of brain (%Inj/g) was calculated for each time point by the equation:
2.5. Capillary depletion
Capillary depletion as modified for use in the mouse (Banks et al., 2009; Triguero et al., 1990) was used to determine the degree to which 131I-NNC 26-9100 was sequestered and retained by the vascular bed of the brain. Mice were anesthetized with urethane and given an injection into the jugular vein of 0.2 ml saline containing 750,000 cpm of 131I-NNC-26-9100. A single optimized time point (8 min) was used. Arterial blood was obtained, with remaining blood washed-out of the brain by injecting 20 ml of lactated Ringer's solution into the left ventricle of the heart over a 60 s period. Brain was removed and the cerebral cortex was emulsified in homogenizer at 4°C in 0.8 ml of physiological buffer (10 mM HEPES, 141 mM NaCl, 4 mM KCl, 2.8 mM CaCl2, 1 mM MgSO4, 1 mM NaH2PO4, and 10 mM D-glucose adjusted to pH 7.4). Dextran solution (1.6 ml) was added to the homogenate to a final concentration of 15.5%. An aliquot was centrifuged at 5400 × g for 15 min at 4°C in a swinging bucket rotor. The pellet containing the brain microvessels and the supernatant containing the brain parenchyma were carefully separated. Results were expressed as capillary/serum and parenchyma/serum ratios.
2.6. Regional distribution
For regional brain tissue distribution, mice were anesthetized and injected with 131I-NNC 26-9100, with blood collection followed by decapitation as addressed above. A single optimized time point (8 min) was used. The brain was dissected into regions: frontal cortex, parietal cortex, temporal cortex, occipital cortex, pons medulla, occipital cortex, hippocampus, thalamus, striatum, and cerebellum. The brain region/serum ratio was calculated for each brain region in units of μl/g, as addressed above.
2.7. Drug dosing and testing
Measurement of the effects of NNC 26-9100 on acquisition learning and retention was performed following chronic i.p. administration in 12-month-old male SAMP8 mice. NNC 26-9100 dosing range for i.p. administration (0.2-200 μg) was evaluated against vehicle (20% ethanol/saline) control. Respective doses were given once a day over a period of 28 days. Learning and memory assessment was assessed by the T-maze model. Individual performing test was blind to respective dosing. Learning evaluations were conducted after three weeks of treatment on day 21, while memory retention of the learned task was assessed after one additional week of treatment on day 28. Body weights were evaluated weekly, with no animals in the study exhibiting weight loss or abnormal behaviors.
The T-maze avoidance apparatus training and testing procedures have been previously described, and shown as an effective means to assess learning and memory in SAMP8 mice (Farr et al., 2000; Flood and Morley, 1993). The T-maze consisted of a black plastic alley with a start box at one end and two goal boxes at the other. The start box was separated from the alley by a plastic guillotine door, which prevented movement down the alley until training began. An electrifiable stainless steel rod floor ran throughout the maze to deliver scrambled foot-shock. Mice were trained and tested between 07:00 and 15:00 h. Mice were not permitted to explore the maze prior to training. A training trial began when a mouse was placed into the start box. The guillotine door was raised and the buzzer sounded simultaneously. After 5 s, footshock was applied. The goal box the mouse first entered on the first trial was designated as ‘incorrect’. Footshock was continued until the mouse entered the other goal box, which on all subsequent trials was designated ‘correct’ for that particular mouse. At the end of each trial, the mouse was removed from the goal box and returned to its home cage. A new trial began by placing the mouse in the start box, sounding the buzzer, and raising the guillotine door. Footshock was applied 5 s later if the mouse did not leave the start box or failed to enter the correct goal box. The mean trials to first avoidance represents acquisition learning, the retention of the learned task (1 week later, day-28) was reported as the mean trails to criterion. At the end of the evaluations mice were decapitated and the brains flash frozen and stored at -80°C for subsequent analyses.
2.8. Evaluation of Aβx-42
Cortical brain levels of Aβx-42 were evaluated ex vivo, from flash frozen whole tissue following respective behavioral analyses, using ultra-centrifugation and solid-phase extraction methods (Lanz and Schachter, 2006; Zupa-Fernandez et al., 2007), coupled with Enzyme-linked immunosorbent assay (ELISA) analysis (Beta-Mark Aβx-42, Covance, Dedham, MA). Based on supplier information the Aβx-42 ELISA has significant reactivity for rodent Aβ1-42 and negligible reactivity for Aβ1-40. Half of the brain tissue was dedicated to Western blot examinations with remainder used for Aβx-42 evaluation. Brain tissue was homogenized using chilled dounce homogenizers using cold 1 mL 50 mM NaCl, 0.4% diethylamine (DEA), pH=10, containing protease inhibitor (Roche, Indianapolis, IN). Samples were then sonicated at 30% for 30 sec and incubated for 3 hr. Following the 3 hr incubation, samples were centrifuged at 355,000 × g for 30 min (4°C) and the supernatant was collected. The supernatant then underwent solid phase extraction using Oasis HLB 3cc columns (Waters, Milford, MA). Oasis columns were activated with 2 mL methanol (MeOH), followed by 2 mL diH2O. Brain homogenates were loaded in 1 mL increments. Samples were then washed sequentially with 1 mL volumes of 5% and 30% MeOH, then eluted with 1 mL 2% NH4OH in 90% MeOH. Eluted samples were collected and vacuum-centrifuged at 1400 rpm, 60°C for 90-120 min until reaching dryness. Once samples were dried completely, they were stored at −80°C until assay. Samples were reconstituted and analyzed in duplicate according to ELISA assay directions via luminometer (Lumistar Optima, BMG Labtech, Durham, NC). The Aβx-42 levels were calculated from linear-regression curve in pg/ml and then set to gram weight of brain tissue and normalized to respective controls.
2.9. Protein expression
Protein expression of cortical brain tissues were evaluated ex vivo, from flash frozen whole brain tissue following respective behavioral analyses, by Western blot analyses for sst4 receptor and APP. Tissues were homogenized in RIPA buffer containing protease inhibitor (Roche), transferred into tubes and spun at 10,000 rpm for 20 min (4°C). The supernatant was taken and a protein assay was used to determine the amount of protein present. Samples (100 μg) were separated using an electrophoretic field on Biorad (Bio-Rad, Hercules, CA) Tris-HCl gels (10%) at 175 V for 70 min following heating at 95°C for 5 min. GelCode Blue Stain (Pierce, Rockford, IL) was used to confirm appropriate protein loading. The proteins were then transferred to nitrocellulose membranes with 240 mA at for 45 min (4°C). The membranes were then blocked using 5% nonfat milk-Tris-buffered saline (20 mM Tris base, 137 mM NaCl, pH 7.6) with 0.1% Tween-20 for 4 hrs. Primary antibodies for the sst4 receptor (Sigma-Aldrich) and APP (22C11) (Millipore, Temecula, CA) were then incubated overnight at 4°C in PBS-0.5% BSA. The membranes were then washed with 5% nonfat milk-Tris-buffered saline buffer before incubation with the respective secondary antibody (in PBS-0.5% BSA) for 60 min at room temperature. Blots were developed using the enhanced chemiluminescence method (ECL+) (Amersham, Springfield, IL), and protein bands visualized on X-ray film. Optical densities were measured by densitometer (Biorad) and evaluated against respective controls. After each initial protein expression assessment, membranes were then stripped and re-probed with actin (AC-40) (Sigma-Aldrich).
2.10. Statistical analyses
Blood-to-brain uptake and clearance regression lines were calculated by the least-squares method (Prism 5.0, GraphPad Software Inc. San Diego, CA). Comparisons of brain distribution, learning acquisition, memory retention, and respective molecular analyses were made using one-way ANOVA and Newman-Keuls post-hoc analysis, with data expressed as means ± S.E.M.
3. Results
3.1. Brain influx rate and serum clearance
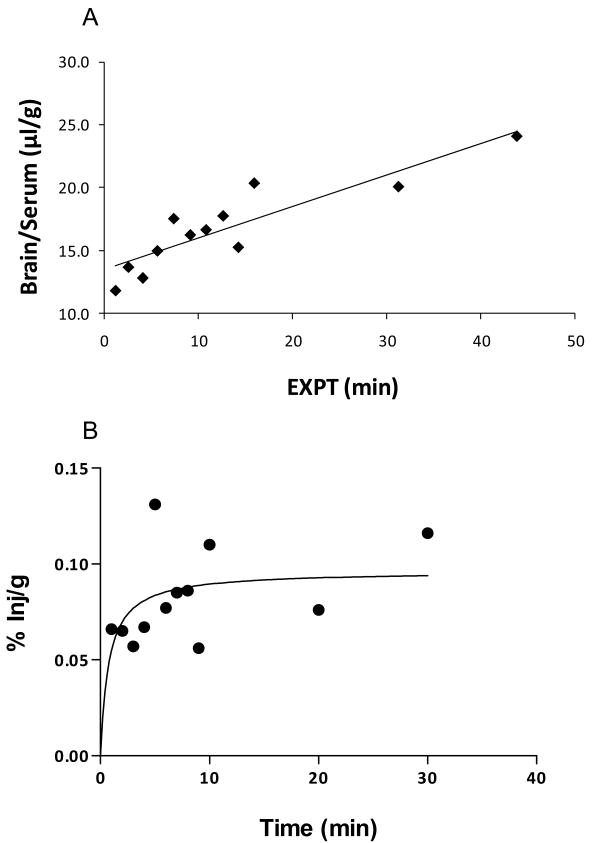
131I-NNC 26-9100 was injected i.v. and evaluated for brain uptake (n = 12). Fig 2A shows the relation between the brain/serum ratios (μl/g) and exposure time (min). The slope of the line represents the rate of influx from blood to brain with a Ki (unidirectional influx rate) = 0.25 μl/g min (r = 0.899, p < 0.0001) (Fig. 2A). The y-intercept representing the initial volume of distribution (Vi) in the brain at time zero = 13.46 ± 0.69 μl/g, approximating the vascular space of the brain. The percent of the i.v. dose taken up per g of brain showed a hyperbolic relation with a maximal value calculated as 0.096 % Inj/g (Fig 2B).
Fig. 2.
Multiple-time regression analysis of 131I-NNC 26-9100 following intravenous injection. (A) Slope of the line shows unidirectional uptake of blood-to-brain, plotted as brain/ratio versus exposure time (EXPT). (B) Percent of injected dose of 131I-NNC 26-9100 per gram of brain tissue over time.
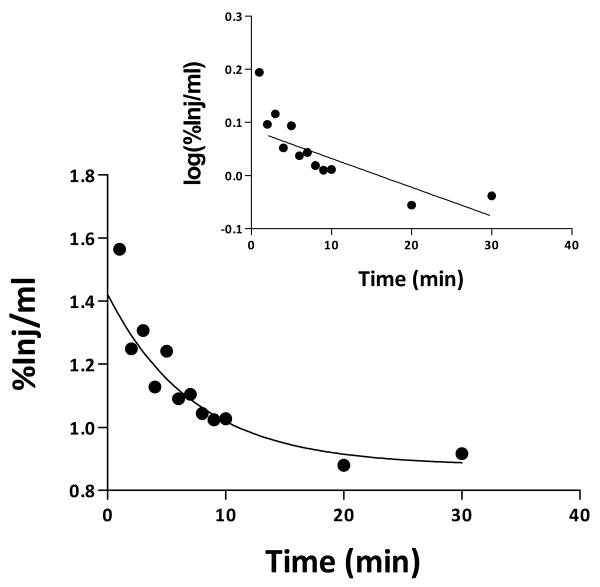
The early phase of clearance from serum after i.v. injection for 131I-NNC 26-9100 followed first-order kinetics. The relation between the log of levels of radioactivity in arterial serum was expressed as the %Inj/ml versus time after i.v. injection (Fig 3). The value for 1 min is shown in figure 1, but was not used in the calculation of half-time as it likely represent the early distribution phase. Linear regression analysis showed a statistically significant relation between log(% Inj/ml) and time (min) (r = 0.841, P < 0.01) (Fig. 3- inset). The half-time disappearance rate from serum was 55.5 min.
Fig. 3.
Kinetics of blood-to-brain transport of 131I-NNC 26-9100 after i.v. administration. Clearance of 131I-NNC 26-9100 from blood after i.v. injection. Inset shows the initial distribution phase was linear.
3.2. Regional distribution
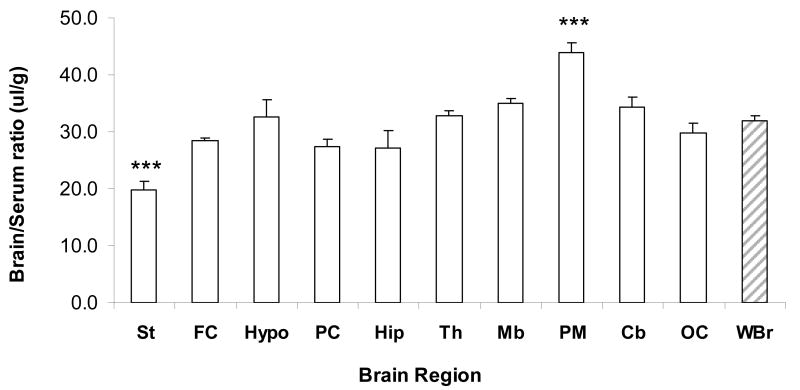
Regional brain uptake evaluation of 131I -NNC 26-9100 was determined at 8 min (period of optimal brain uptake) following i.v. injection (n = 5). Data identified 131I -NNC 26-9100 to have consistent uptake across brain regions, with a significant increase (P < 0.001) in the pons medulla and a decrease (P < 0.001) in the striatum as compared to whole brain levels (brain/serum ratio in μl/g) (Fig. 4).
Fig. 4.
Regional variation in brain/serum ratio determination in mouse brain uptake at 8 min post i.v. injection of radiolabled I-NNC 26-9100 (n = 5/region). Striatum (St), frontal cortex (FC), hypothalamus (Hypo), parietal cortex (PC), hippocampus (Hip), thalamus (Th), midbrain (Mb), pons medulla (PM), cerebellum (Cb), occipital cortex (OC), whole brain (WBr). *** P < 0.001, as compared to whole brain, one-way ANOVA.
3.3. Capillary depletion
Capillary depletion analysis was conducted to evaluate the proportion of 131I -NNC 26-9100 associated with the brain capillaries (n = 5). Results shown 7.2% of 131I-NNC 26-9100 was associated with the brain capillaries with washout of vascular space at 8 min after i.v. injection.
3.4. Learning and memory effect
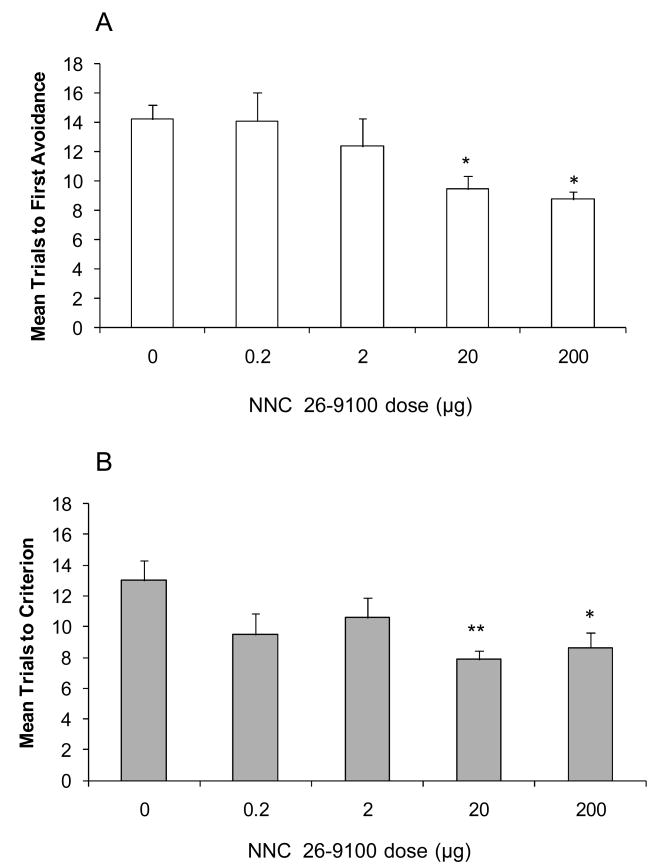
The T-maze was used to test for the effects of chronic NNC 26-9100 on learned acquisition and retention in 12-month old SAMP8 mice. On day 21 of treatment, there was a dose dependent decrease in the mean trials to first avoidance. Both the 20 and 200 μg daily doses showed a significantly lower number of mean trials to first avoidance (P < 0.05) compared to vehicle control, demonstrating an improvement in learning (Fig. 5A). Memory was subsequently evaluated in the same animals seven days later (on treatment day 28). Mice treated with either the 20 μg (P < 0.01) or 200 μg (P < 0.05) dose of NNC 26-9100 demonstrated significantly lower mean trials to criterion compared to vehicle treated animals, demonstrating both these doses improved memory (Fig. 5B).
Fig. 5.
(A) Acquisition learning at day-21, and (B) retention memory at day-28 of 12-month old SAMP8 mice following i.p. injection of NNC 26-9100 (0.2-200 μg/day) or vehicle control (0 μg/day) (n = 8-10/group). *P < 0.05; **P < 0.01 as compared to vehicle, one-way ANOVA.
3.5. Drug effect on cortical on Aβx-42 levels
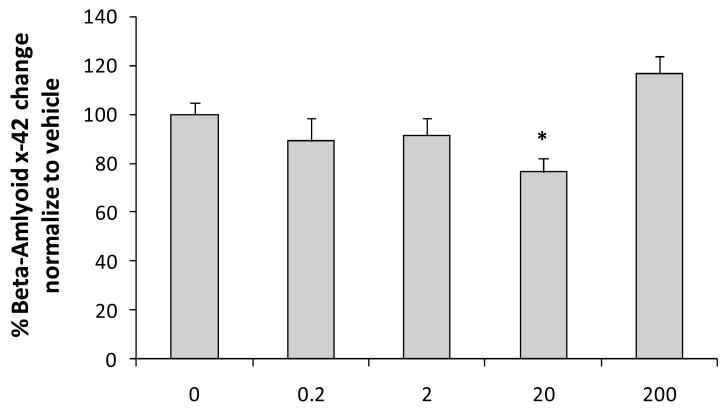
To determine the impact of chronic NNC 26-9100 treatment on Aβx-42 levels, ELISA analysis of brain tissues from animals used in the learning and memory experiments were performed. Animals treated with 20 μg of NNC 26-9100 showed a significant decrease (P < 0.05) in Aβx-42 when compared to vehicle treated animals (Fig. 6). However, no significant changes were observed with any other dose of NNC 26-9100 when compared to vehicle control.
Fig. 6.
ELISA analysis of Aβx-42 performed and calculated as pg/ml/gram of brain tissue and normalized to vehicle (n = 6/group). Evaluation performed from SAMP8 mice brain tissue following NNC 26-9100 memory retention evaluation. *P < 0.05 (0ug is vehicle control), oneway ANOVA.
3.6. Drug effect on cortical protein expression
To determine the impact of chronic NNC 26-9100 treatment on expression of the sst4 receptor and APP, Western blot analyses were performed using brain tissues from animals used in the learning acquisition and retention experiments. No significant changes were observed in the expression of the sst4 receptor or APP with any dose of NNC 26-9100 when compared to vehicle control (Fig. 7). Actin expression levels were consistent across all samplings within respective evaluation sets.
Fig. 7.
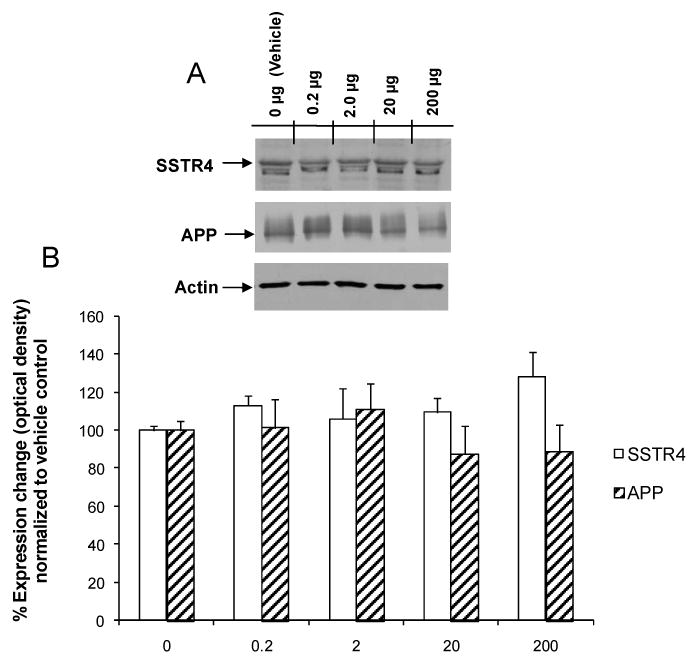
(A) Representative Western blot. (B) Western blot expression analysis of SSTR4 and APP performed with optical densities normalized to vehicle (0 μg) animals (n = 5-6/group). No significant shown compared to vehicle, one-way ANOVA.
4. Discussion
Somatostatin mediated processes have been implicated in the age-dependent development of Alzheimer's disease (Epelbaum et al., 2009). The use of selective somatostatin receptor agonist-based therapeutics has been hypothesized to be an avenue in which to mitigate Alzheimer's disease progression (Saito et al., 2005). Such a therapeutic would need to be appropriately stable for chronic use, readily taken up into the brain, and highly selective for receptors within key regions of the brain associated with Alzheimer's disease. Our parent compound NNC 26-9100 is a stable non-peptide sst4 receptor selective based drug (Ankersen et al., 1998; Crider et al., 2004).
The initial goal of this evaluation was to determine the viability of NNC 26-9100 uptake into the brain and regional distribution. Transport of drugs across the blood-brain barrier is essential in determining the clinical feasibility of peripherally administered CNS directed therapeutics (Banks, 2009). The degree by which radioactively (I131) labeled NNC 26-9100 crossed from blood-to-brain was determined using multiple-time regression analysis. The unidirectional influx-rate (Ki = 0.25 μl/g min) identified a moderate degree of uptake. This rate was indicative of non-saturable transport across the blood-brain barrier, which is dependent on molecular weight and lipophilicity (Oldendorf, 1974). The uptake was also consistent with the chemical characteristics of NNC 26-9100 (i.e. MW: 556, cLogP = 5.59). The percent of the i.v. administered dose entering the brain was 0.096 %Inj/ml and is well within the range of appropriate uptake to exert a therapeutic effect. The half-time disappearance from serum of 55.5 min also falls within the viable range for therapeutic use. Additionally, the distribution of 131I-NNC 26-9100 within the capillary and parenchymal compartments of the brain showed that ∼93% of the compound was associated with the parenchyma. This indicates a limited sequestration of the compound within the capillary component, and that the calculated brain uptake was not an artifact of excessive capillary binding. Thus, we conclude that NNC 26-9100 has the appropriate biochemical and molecular characteristics for appropriate brain uptake and would be able to interact with sst4 receptors in the brain.
Regional brain distribution of 131I-NNC 26-9100 demonstrated generally uniform uptake. Exceptions to this included the pons medulla and the striatum, showing a statistical increase and decrease, respectively, in uptake compared to whole brain. Nevertheless, the critical regions associated with memory and learning (i.e. cortex, hippocampus), which correspond to sst4 receptor distribution (Bruno et al., 1992; Moller et al., 2003), show effective uptake. Based on these uptake rates, peripherally administered NNC 26-9100 readily distributes to primary brain regions impacted in Alzheimer's disease further substantiating the viability of the drug.
Evaluations of the effects of chronic NNC 26-9100 administration on learning and memory were then performed. SAMP8 mice at 12-months of age consistently show neurochemical and morphological changes similar to cognitive dementia and Alzheimer's disease (Tomobe and Nomura, 2009). The T-maze test has been consistently shown to be an effective means to assess learning and memory in SAMP8 mice (Banks et al., 2007; Farr et al., 2000; Flood and Morley, 1993; Poon et al., 2004). Acquisition learning via T-maze was conducted on day 21 of treatment with memory retention being subsequently measured on day 28 of treatment. SAMP8 mice chronically treated with NNC 26-9100 demonstrated significantly enhanced learning (20 and 200 μg doses) of the task compared to vehicle controls. On day 28 day the mice showed enhanced memory retention of the task, again at the 20 and 200 μg doses. This is the first study showing that a chronic peripherally administered sst4 receptor agonist directly enhances learning and memory in a model of Alzheimer's disease.
Following learning and memory testing, cortical brain levels of soluble Aβ42 (Aβx-42) were evaluated. The 42-amino acid isoform of Aβ readily aggregates and has been identified as the predominant isoform in plaque formation (Findeis, 2007). The impaired learning and memory exhibited by SAMP8 mice at 12-months of age has shown to be mitigated via antibodies to Aβ (Morley et al., 2002). Additionally, 12-month SAMP8 mice do not express plaques (Morley, 2002; Tomobe and Nomura, 2009), but rather the soluble form of Aβ, shown to be associated with deficits in learning and memory (Selkoe, 2008). Such soluble forms of Aβ may be susceptible to enzymatic degradation. Moreover, emerging research has shown soluble oligomeric forms of Aβ are involved in learning and memory loss associated with mouse models of Alzheimer's disease (Freir et al., 2010; Lesne et al., 2006; Selkoe, 2008). It has been hypothesized that somatostatin-based receptor agonists may induce downstream enzymatic degradation of Aβ and associated oligomers providing a beneficial impact on learning and memory (Iwata et al., 2005; Saito et al., 2005). Such oligomers have been shown to be sensitive to enzymatic degradation via neprilysin (Kanemitsu et al., 2003), a principle regulator of Aβ levels in the brain (Iwata et al., 2001; Iwata et al., 2000). Neprilysin activity has also been shown to be directly mediated through somatostatin (Iwata et al., 2005; Saito et al., 2005). Thus, NNC 26-9100 action at the sst4 receptor may enhance learning and memory effects through degradation of soluble oligomers. Within this examination, cortical tissues of the NNC 26-9100 treated SAMP8 mice identified a decrease in soluble Aβx-42 at the 20 μg dose compared to control, which corresponded to an enhancement in learning and memory. However, we did not observe a similar decrease in Aβx-42 at the 200 μg dose, despite the enhanced learning and memory effect. The Aβx-42 levels shown with the 200 μg dose may be indicative of a dose related degradation of oligomeric Aβ42 isoforms (Kanemitsu et al., 2003), resulting in a subsequent increase in monomeric isoform levels and potentially negating an identifiable degradation observable within our ELISA analyses. Alternatively, NNC 26-9100 activity at the sst4 receptor may impact learning and memory independent of Aβ degradation. While a mechanism has not been elucidated, it has been proposed that hippocampal somatostatinergic interneuron modulation of learning and memory processes are mediated, at least in part, via sst4 activity (Epelbaum et al., 2009; Gastambide et al., 2009b). A recent evaluation of sst4 receptor agonist L-803-087 was shown to enhance cue-based memory formation (Gastambide et al., 2009b). As the Gastambide study was over an acute time frame using direct intrahippocampal injections in wild-type mice (Gastambide et al., 2009b), it strongly implicates a direct receptor mediated action. Others have also demonstrated somatostatin augments long-term potentiation, a primary process in learning and memory (Chen et al., 2009; Matsuoka et al., 1991; Tallent, 2007). In this regard, NNC 26-9100 could directly impact learning and memory in a dose-dependent manner. Nevertheless, this does not negate the potential that NNC 26-9100 could work in more than one manner to produce observed outcomes (i.e. dual mechanisms of action).
Aβ peptides are formed via sequential enzymatic processing of APP by beta and gamma secretase. Alterations in APP correspond to shifts in Aβ levels and associated deposits (Burgos-Ramos et al., 2008). Previous studies have identified APP protein expression and mRNA to increase in the brains of SAMP8 mice at 12-months of age, which correlated with decreased acquisition (learning) and retention (memory) measurements (Morley et al., 2000; Poon et al., 2004). The cortical tissue examinations of the NNC 26-9100 treated SAMP8 mice (post-memory testing) identified no significant changes in protein expression for APP across the dosing range compared to the vehicle. This would support an NNC 26-9100 mechanism of action independent of APP alteration. As to sst4 receptor expression, again no change was observed across the dosing range as compared to the vehicle. This is also an important observation, given the potential for receptor down-regulation over a chronic period of drug administration. Thus, the viability of chronic NNC 26-9100 treatment is maintained.
5. Conclusion
In summary, our study shows that the selective high-affinity sst4 receptor agonist NNC 26-9100 can cross the BBB, with limited capillary sequestration. Moreover, sufficient uptake occurs to the primary areas within the brain associated with learning, memory and Alzheimer's disease pathology. Chronic i.p. administration induced a discernable enhancement of learning and memory in an established model of cognitive decline associated with increased Aβ levels. While additional examinations are required to fully elucidate the mode of action, these findings indicate that NNC 26-9100 is viable as a peripherally administered drug, capable of enhancing learning and memory. Thus, agonists-directed at sst4 receptor subtype may provide a therapy in the treatment of Alzheimer's disease and/or other forms of cognitive impairment.
Acknowledgments
This work was supported by the Alzheimer's Drug Discovery Foundation (Grant: 261105.01), VA merit review, and the National Institutes of Health National Institute on Aging (Grant: R21AG029318).
Footnotes
Disclosure Statement: A patent is being sought for the drug being investigated within this examination by the university (SIUE), with no other disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ankersen M, Crider AM, Liu S, Ho B, Andersen HS, Stidsen CE. Discovery of a Novel Non-Peptide Somatostatin Agonist with SST4 Selectivity. Journal of the American Chemical Society. 1998;120:1368–1373. [Google Scholar]
- Banks WA. Characteristics of compounds that cross the blood-brain barrier. BMC neurology. 2009;9 1:S3. doi: 10.1186/1471-2377-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE, Wolf KM, Geylis V, Steinitz M. Anti-amyloid beta protein antibody passage across the blood-brain barrier in the SAMP8 mouse model of Alzheimer's disease: an age-related selective uptake with reversal of learning impairment. Exp Neurol. 2007;206:248–256. doi: 10.1016/j.expneurol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Robinson SM, Diaz-Espinoza R, Urayama A, Soto C. Transport of prion protein across the blood-brain barrier. Exp Neurol. 2009;218:162–167. doi: 10.1016/j.expneurol.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JF, Xu Y, Song J, Berelowitz M. Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc Natl Acad Sci U S A. 1992;89:11151–11155. doi: 10.1073/pnas.89.23.11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Ramos E, Hervas-Aguilar A, Aguado-Llera D, Puebla-Jimenez L, Hernandez-Pinto AM, Barrios V, Arilla-Ferreiro E. Somatostatin and Alzheimer's disease. Molecular and cellular endocrinology. 2008;286:104–111. doi: 10.1016/j.mce.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Cervia D, Bagnoli P. An update on somatostatin receptor signaling in native systems and new insights on their pathophysiology. Pharmacol Ther. 2007;116:322–341. doi: 10.1016/j.pharmthera.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Chen HX, Jiang M, Akakin D, Roper SN. Long-term potentiation of excitatory synapses on neocortical somatostatin-expressing interneurons. Journal of neurophysiology. 2009;102:3251–3259. doi: 10.1152/jn.00641.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider AM, Liu S, Li T, Mahajan S, Ankersen M, Stidsen CE. Somatostatin receptor subtype 4 (sst4) ligands: Synthesis and evaluation of indol-3-yl- and 2-pyridyl-thioureas. Letters in Drug Design & Discovery. 2004;1:84–87. [Google Scholar]
- Davies P, Katzman R, Terry RD. Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementa. Nature. 1980;288:279–280. doi: 10.1038/288279a0. [DOI] [PubMed] [Google Scholar]
- Epelbaum J, Guillou JL, Gastambide F, Hoyer D, Duron E, Viollet C. Somatostatin, Alzheimer's disease and cognition: An old story coming of age? Prog Neurobiol. 2009;89:153–161. doi: 10.1016/j.pneurobio.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, La Scola ME, Flood JF, Morley JE. Permanent and temporary inactivation of the hippocampus impairs T-maze footshock avoidance acquisition and retention. Brain Res. 2000;872:242–249. doi: 10.1016/s0006-8993(00)02495-1. [DOI] [PubMed] [Google Scholar]
- Findeis MA. The role of amyloid beta peptide 42 in Alzheimer's disease. Pharmacol Ther. 2007;116:266–286. doi: 10.1016/j.pharmthera.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE. Age-related changes in footshock avoidance acquisition and retention in senescence accelerated mouse (SAM) Neurobiol Aging. 1993;14:153–157. doi: 10.1016/0197-4580(93)90091-o. [DOI] [PubMed] [Google Scholar]
- Freir DB, Fedriani R, Scully D, Smith IM, Selkoe DJ, Walsh DM, Regan CM. Abeta oligomers inhibit synapse remodelling necessary for memory consolidation Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.001. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastambide F, Lepousez G, Viollet C, Loudes C, Epelbaum J, Guillou JL. Cooperation between hippocampal somatostatin receptor subtypes 4 and 2: Functional relevance in interactive memory systems. Hippocampus. 2009a;20:745–757. doi: 10.1002/hipo.20680. [DOI] [PubMed] [Google Scholar]
- Gastambide F, Viollet C, Lepousez G, Epelbaum J, Guillou JL. Hippocampal SSTR4 somatostatin receptors control the selection of memory strategies. Psychopharmacology. 2009b;202:153–163. doi: 10.1007/s00213-008-1204-x. [DOI] [PubMed] [Google Scholar]
- Iwata N, Higuchi M, Saido TC. Metabolism of amyloid-beta peptide and Alzheimer's disease. Pharmacol Ther. 2005;108:129–148. doi: 10.1016/j.pharmthera.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- Kanemitsu H, Tomiyama T, Mori H. Human neprilysin is capable of degrading amyloid beta peptide not only in the monomeric form but also the pathological oligomeric form. Neurosci Lett. 2003;350:113–116. doi: 10.1016/s0304-3940(03)00898-x. [DOI] [PubMed] [Google Scholar]
- Kumar VB, Farr SA, Flood JF, Kamlesh V, Franko M, Banks WA, Morley JE. Site-directed antisense oligonucleotide decreases the expression of amyloid precursor protein and reverses deficits in learning and memory in aged SAMP8 mice. Peptides. 2000;21:1769–1775. doi: 10.1016/s0196-9781(00)00339-9. [DOI] [PubMed] [Google Scholar]
- Lanz TA, Schachter JB. Demonstration of a common artifact in immunosorbent assays of brain extracts: development of a solid-phase extraction protocol to enable measurement of amyloid-beta from wild-type rodent brain. J Neurosci Methods. 2006;157:71–81. doi: 10.1016/j.jneumeth.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Matsuoka N, Kaneko S, Satoh M. Somatostatin augments long-term potentiation of the mossy fiber-CA3 system in guinea-pig hippocampal slices. Brain Res. 1991;553:188–194. doi: 10.1016/0006-8993(91)90823-e. [DOI] [PubMed] [Google Scholar]
- Moller LN, Stidsen CE, Hartmann B, Holst JJ. Somatostatin receptors. Biochim Biophys Acta. 2003;1616:1–84. doi: 10.1016/s0005-2736(03)00235-9. [DOI] [PubMed] [Google Scholar]
- Morley JE. The SAMP8 mouse: a model of Alzheimer disease? Biogerontology. 2002;3:57–60. doi: 10.1023/a:1015207429786. [DOI] [PubMed] [Google Scholar]
- Morley JE, Farr SA, Flood JF. Antibody to amyloid beta protein alleviates impaired acquisition, retention, and memory processing in SAMP8 mice. Neurobiol Learn Mem. 2002;78:125–138. doi: 10.1006/nlme.2001.4047. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kumar VB, Bernardo AE, Farr SA, Uezu K, Tumosa N, Flood JF. Beta-amyloid precursor polypeptide in SAMP8 mice affects learning and memory. Peptides. 2000;21:1761–1767. doi: 10.1016/s0196-9781(00)00342-9. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH. Proceedings of the Society for Experimental Biology and Medicine. Vol. 147. Society for Experimental Biology and Medicine; New York, N.Y: 1974. Lipid solubility and drug penetration of the blood brain barrier; pp. 813–815. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Poon HF, Joshi G, Sultana R, Farr SA, Banks WA, Morley JE, Calabrese V, Butterfield DA. Antisense directed at the Abeta region of APP decreases brain oxidative markers in aged senescence accelerated mice. Brain Res. 2004;1018:86–96. doi: 10.1016/j.brainres.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Saito T, Iwata N, Tsubuki S, Takaki Y, Takano J, Huang SM, Suemoto T, Higuchi M, Saido TC. Somatostatin regulates brain amyloid beta peptide Abeta42 through modulation of proteolytic degradation. Nat Med. 2005;11:434–439. doi: 10.1038/nm1206. [DOI] [PubMed] [Google Scholar]
- Sano M, Grossman H, Van Dyk K. Preventing Alzheimer's disease : separating fact from fiction. CNS drugs. 2008;22:887–902. doi: 10.2165/00023210-200822110-00001. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter M, Cramer H, Reuner C, Strubel D, Hamann G, Schimrigk K. Molecular forms of somatostatin-like immunoreactivity in the cerebrospinal fluid of patients with senile dementia of the Alzheimer type. Biological psychiatry. 1997;41:1124–1130. doi: 10.1016/S0006-3223(96)00211-9. [DOI] [PubMed] [Google Scholar]
- Tallent MK. Somatostatin in the dentate gyrus. Progress in brain research. 2007;163:265–284. doi: 10.1016/S0079-6123(07)63016-7. [DOI] [PubMed] [Google Scholar]
- Tomobe K, Nomura Y. Neurochemistry, neuropathology, and heredity in SAMP8: a mouse model of senescence. Neurochemical research. 2009;34:660–669. doi: 10.1007/s11064-009-9923-x. [DOI] [PubMed] [Google Scholar]
- Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem. 1990;54:1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Yasojima K, Akiyama H, McGeer EG, McGeer PL. Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of beta-amyloid peptide. Neurosci Lett. 2001a;297:97–100. doi: 10.1016/s0304-3940(00)01675-x. [DOI] [PubMed] [Google Scholar]
- Yasojima K, McGeer EG, McGeer PL. Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 2001b;919:115–121. doi: 10.1016/s0006-8993(01)03008-6. [DOI] [PubMed] [Google Scholar]
- Zupa-Fernandez A, Rozzi AM, Arnold HM, Felsenstein KM, Rowley A, Treton G, Yohrling G. Optimization of soluble amyloid beta extraction from non-transgenic rodents to facilitate the testing of gamma-secretase modulators; Society for Neuroscience, 37th; San Diego. 2007. [Google Scholar]