Abstract
Cancer stem cells (CSC) are a very small subset of all cancer cells and possess characteristics very similar to normal stem cells, in particular, the capacity for self-renewal, multipotency and relative quiescence. These chemo- and radiation resistant cells are responsible for maintaining tumor volume leading to therapy failure and recurrence. In glioblastoma multiforme (GBM), the most common primary intracranial malignancy, glioma stem cells have been implicated as one of the key players in treatment failure. Many novel treatment modalities are being investigated to specifically target this small group of cells. In this review, we shed light on one such targeted therapy, specifically, oncolytic virotherapy, and review the literature to highlight the advances and challenges in designing effective oncolytic virotherapy for glioma stem cells.
Keywords: Malignant glioma, Glioblastoma multiforme, Stem cells, Oncolytic therapy, Conditionally replicative virus, Adenovirus
Introduction
Accumulation of genetic and epigenetic alterations affecting cellular machinery controlling cell division, cell proliferation, DNA damage, and signal transduction pathways leads to carcinogenesis by activating proto-oncogenes and inactivating tumor suppressor genes [1]. Stem cells, a long-lived small population of self-renewing and differentiating cells, are preferential targets of these genetic alterations since they are exposed to more genotoxic stress compared to their shorter lived differentiated progeny [2].
Malignant gliomas are a subset of glial cell derived malignancies known as gliomas, the most common primary intracranial malignancy in adults. Even though clonally derived, they are characterized by histological heterogeneity; expressing both differentiated and undifferentiated neural markers. Malignant gliomas (WHO grade III and IV) exhibit great genomic instability and are subject to constant genotypic and phenotypic alterations leading to treatment resistance and failure. For practical purposes, this group of tumors is incurable and resistant to current standard of care, which includes gross total resection followed by adjuvant temozolomide (TMZ) and radiation. Even though we have made progress in terms of increasing survival (Refer to Table 1), the current median survival from the time of diagnosis is only approximately 14 months [3].
Table 1. Advances in glioma therapy.
| Year | Type of trial | Treatment Strategy | Conclusion | Reference |
|---|---|---|---|---|
| 1991 | 2:1 randomized trial | Gross total resection plus radiation at 45 Gy Vs 60 Gy | Median survival increases from 9 months to 12 months | [104] |
| 2002 | Meta-analysis | Gross total resection plus radiation vs. radiation plus chemotherapy | Addition of chemo led to absolute improvement of 6% at 1 year and 5% at 2 years leading to increased overall survival from 40 to 46% and 15 to 20%, respectively | [105] |
| 2003 | Phase III trial | Gross total resection plus placebo wafer vs. gliadel wafer plus radiation | Median survival increases from 11.6 months to 13.9 months | [106] |
| 2005 | Phase III trial | Gross total resection plus radiation +/− TMZ | Median survival increases from 12.1 months to 14.6 months | [3] |
| 2005 | Phase III trial | Analysis of MGMT methylation status in Gross total resection plus radiation +/− TMZ | Median survival increases from 15.3 to 21.7 with TMZ in the group with methylated MGMT promoter | [30] |
Deeper understanding of glioma biology, gliomagenesis and scrutiny of the pattern of therapy failure has opened up many different avenues for tailoring therapy of this ever evolving and devastating disease. One of the many upcoming and promising strategies for treating gliomas consists of oncolytic therapy against cancer stem cells.
Cancer Stem Cell Hypothesis
Solid tumors are a caricature of their normal tissue counterpart. Just like normal tissue, solid tumors consist of heterogeneous mix of cells and structures, such as neoplastic cells, stromal cells, inflammatory cells and vascular structures. There also exists a cellular hierarchy in the tumor where a small group of slowly replicating cells with unique property of self-renewal and differentiation carry the burden of maintaining tumor volume. Although tumor heterogeneity can be explained by continuous and ongoing mutagenesis, the observation that only a small group of cells isolated from the tumor are capable of growing in vitro and can reproduce the original tumor with all the complexity and features of the original tumor when transplanted into an immunodeficient host [4] led to the evolution of cancer stem cell (CSC) theory (Fig. 1). Normal tissues contain a small population of stem cells, which are responsible for tissue maintenance and are characterized by their ability to self-renew and differentiate into mature phenotype. Drawing from the same concept, the CSC hypothesis implicates that neoplastic clones are exclusively maintained by a very small fraction of transformed stem cells or progenitor cells with acquired stem cell like properties such as self-renewal and differentiation [2]. This small group of CSC defines tumor behavior like proliferation, infiltration, progression and response to therapy [5]. A major limitation of current cancer treatment modalities is the fact that most therapies treat tumors as a homogeneous entity. However, evolution of CSC theory has led to a paradigm shift in our thinking strategy for designing effective anti-tumor treatment modalities.
Fig. 1.
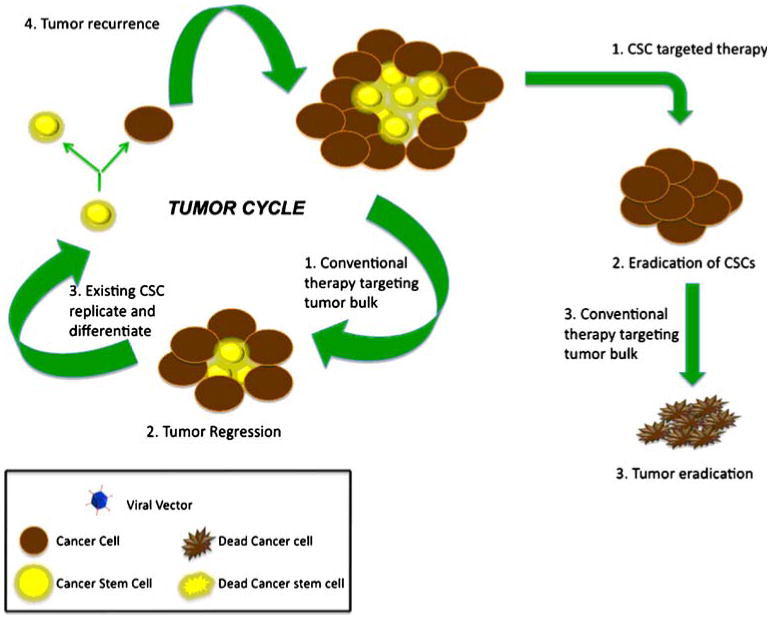
Cancer Stem Cell Hypothesis. Solid tumors are maintained by a small group of chemo and radiation resistant cell. These cells possess stem cell like properties such as self-replication and differentiation. Conventional therapy helps with debulking the tumor, however, presence of CSCs in the residual tumor leads to recurrence. Goal of novel stem cell targeted therapy is to eradicate the population of CSC and the absence of tumor sustaining CSCs will lead to tumor eradication
Prospective Isolation of Cancer Stem Cells
As early as 1960s, a well designed experiment by McCulloch et al., where intravenous injection of bone marrow cells in fully irradiated host led to colony formation of proliferating cells in the spleen, provided the essential framework for defining and isolating normal stem cells [6]. Just like normal stem cells, CSCs were first defined in the context of hematological malignancies [7] and Bonnet et al., first prospectively isolated a small group of CD34+ CD38-hematopoietic stem cells in acute myeloid leukemia (AML). These cells were capable of initiating human AML in non-obese diabetic mice with severe combined immunodeficiency disease (NOD/SCID mice). Similar to normal hematopoietic stem cells, these CD34+ CD38- cells exhibited differentiative and proliferative capacities, and the potential for self-renewal as all the cells derived from these CD34+ CD38- cells were not CD34+ CD38- but a heterogeneous mix of differentiated and undifferentiated cell types [8]. In breast cancer xenograft model Al-Hajj et al., prospectively identified and isolated a small population of CD44(+)CD24 (−/low)Lineage(−) tumorigenic cells that possess the capacity of self renewal and the ability to differentiate into mature phenotype and were able to generate original tumor in immunocompromised mice when injected in small number [9]. Li et al., isolated pancreatic adenocarcenoma cells of CD44(+)CD24(+)ESA(+) phenotype with stem cell like properties of self-renewal, the ability to produce differentiated progeny, and increased expression of the developmental signaling molecule sonic hedgehog [10]. In head and neck squamous cell carcinoma (HNSCC), Prince et al., identified tumorigenic CD44+ cells, with primitive morphology that costained with the basal cell marker Cytoker-atin 5/14 and expressed high levels of nuclear BMI1, which has been demonstrated to play a role in self-renewal in other stem cell types and to be involved in tumorigenesis [11]. Using similar techniques, a small population of tumorigenic cells with stem cell like properties has been prospectively identified in bladder cancer, melanoma and ovarian cancer [12–14].
Defining Cancer Stem Cells
Even though many cell surface markers have been used for identification and prospective isolation of CSCs, like CD44, CD24, CD20, epithelial cell adhesion molecule (EpCAM), THY1, ATP binding cassette B5 (ABCB5) and Hoechst 33342 [15], the CD133 antigen, a pentaspan membrane glycoprotein and a marker of normal hematopoietic stem cells, has been shown to be present on stem cells identified from many solid tumors like colon [16], pancreas [17], lung [18], osteosarcoma [19], and brain tumors [20]. In these solid tumors, CD133+ cells represent a small subpopulation of cells with capabilities to recapitulate the original tumor when serially transplanted in SCID mice and ability to self-renew and differentiate into mature phenotype lacking stem cell like properties. Evidence suggests that these cells are not only involved in tumor progression but also metastasis [17]. These CSCs bare many similarities with normal stem cells such as self-renewal, multipotency, relative quiescence and cytoprotective mechanisms like activation of DNA repair mechanisms and expression of drug transporters. Presence of these cytoprotective mechanisms renders immunity to CSCs from cytotoxic therapy, making these CSCs very attractive targets for future cancer therapy.
Prospective Isolation of Glioma Stem Cells
Nestin is an intermediate filament cytoskeletal protein found in neuroepithelial stem cells and progenitor cells [21]. It has been shown that glial derived neoplasms also express nestin, and the level of expression is elevated in high grade gliomas compared to low grade tumors [22]. Using the neurosphere assay, Ignatova et al., prospectively isolated nestin expressing cells capable of forming clones and the expression of neural lineage-specific proteins [23]. Subsequently, when Singh et al., cultured glioma cells in serum free medium, it was observed that a small percentage of all glioma cells ranging from 0.3% to 25.1%, were capable of self-replication, formed non-adherent neurospheres and maintained tumor culture overtime via multiple passages [20]. These self-renewing and tumor culture maintaining cells not only stained positive for undifferentiated neural stem cell marker nestin, they also stained positive for CD133, a hematopoietic stem cell marker present on normal human neural stem cell [24, 25]. However, they lacked expression of beta tubulin III and GFAP markers for differentiated neuronal lineage. In stark contrast to this small group of CD133+ cells, the majority of the glioma cells were CD133- and were incapable of forming self-sustaining neurospheres. As a general observation, the fraction of CD133+ glioma stem cells (GSC) increased with the grade of the tumor as well as GSCs from more aggressive tumors exhibited increased self renewal capacity compared to less aggressive tumors. In the presence of differentiating conditions, GSCs lost the expression of primitive markers like CD133 and nestin and instead expressed differentiated markers for the cell of origin. When serially transplanted in the brain of NOD-SCID (non-obese diabetic, severe combined immunodeficient) mice, these GSCs were able to produce exact phenocopy of the patient's original tumor with all the histopathological features and cell surface markers of the original tumor. Immunohistological staining of these GBM xenografts demonstrating differential staining for CD133 and GFAP underlines the fact that GSCs can differentiate into mature progeny. Even though the presence of GSCs can clearly account for inherent heterogeneous nature of gliomas, cellular and genetic analysis of GSCs showed that these cells were genetically transformed with enhanced self-renewal properties and possessed abnormal karyotype, which was not only limited to CD133+ cells, but was present in both CD133+ and CD133- cells, suggesting that all the cancer cells were clonally derived. [26].
Chemo and Radioresistance of Glioma Stem Cell
Even though the jury is still out on the exact mechanism of gliomagenesis, our new found understanding of the presence of functional hierarchy in glioma makes the slow growing mutated GSC a key player in our understanding of the mechanisms of treatment failure. GSCs are reported to be resistant to a wide variety of chemotherapeutic agents and possess remarkable ability of recovering from cytotoxic therapy [27]. Kang et al., reported that upon exposure of GBM cells to a lethal dose of carmustine (BCNU), a small population of multipotent CD133+ cancer cells survived and proliferated. When transplanted into severe combined immunodeficient (SCID) mouse brain, the original tumor was able to be reproduced [28]. Significantly higher level of CD133+ cells are reported to be present in previously treated GBM when compared with newly diagnosed GBM [29]. Gene profile of CD133+ cells showed high level of expression of antiapoptotic genes and chemotherapy resistance genes like BCRP1, MDR1, MRP1 and MGMT [29–31] rendering these cells resistant to many commonly used chemotherapeutic agents including temozolomide, carboplatin, paclitaxel (Taxol) and etoposide (VP16). In the same tumor, genes like multi drug resistance-associated proteins 1 and 3 were found to be markedly elevated in GSCs when compared to non-GSCs [32] emphasizing their role in chemoresistance. GSCs not only play a crucial role in chemoresistance, but they are vital to the failure of radiation therapy since tumors surviving radiotherapy are found to be enriched in CSCs. In the study by Bao et al., irradiation of in vivo glioma xenograft led to 3–5 fold increase in CD133+ cell population as compared to untreated xenografts, suggesting that IR leads to enrichment of CD133+ cells in the tumor and subsequent formation of more aggressive tumors with decreased latency on serial transplantation [33]. Given the pattern of treatment failure seen with current standard therapy, selectively targeting of this functionally distinct chemo- and radiation resistant group of GSCs might provide better success in treating this deadly disease.
History of Oncolytic Virotherapy
Viruses are strictly intracellular organisms that replicate inside host cell using cellular machinery. Oncolytic virotherapy manipulates this lytic property to achieve tumor cell lysis by intra-neoplastic virus replication. The anti-tumor effect of oncolytic virus is a delicate balance between anti-tumor immune response and anti-viral immune response [34, 35]. A viral vector must possess certain characteristics to achieve effective oncolytic activity such as:
It must selectively target the tumor and have minimal brain and systemic toxicities.
It should reach all neoplastic foci beyond tumor resection border.
The viral vector has to remain active despite evoking an immune response.
Based on this principle, many different viral vectors have been tested with variable degree of success. In a phase I/II clinical trial Freeman et al., demonstrated that administration of lentogenic NDV-HUJ virus, a single-stranded RNA virus whose natural host is poultry, systemically through peripheral or central line in GBM patients is well tolerated. [36–38]. However the biggest limitation of this viral vector was the non-specificity for cancer cells since analysis of blood, saliva, urine, tumor tissue and tumor cyst fluid from 5 of the 14 patients showed presence of infectious NDV particles. Many glioma cells express PDGFR [39] or EGFR [40], which in turn lead to stimulation of the RAS pathway and as a consequence the inhibition of RNA-activated protein kinase activation. Reovirus, which selectively replicates in cells with activated ras signaling, has been shown to demonstrate tumor regression in intracranial human glioma xenografts in mice [41]. A phase I trial by Forsyth et al., showed that administration of live, replication competent and genetically unmodified reovirus directly into the tumors of patients with malignant glioma is safe and well tolerated with no evidence of clinical encephalitis [42]. HSV naturally targets human brain and causes necrotizing hemorrhagic encephalitis. A genetically engineered HSV vector G207, with deletion of γ34.5 gene at both loci and a disabling insertion of lacZ in UL39, the gene encoding for the large subunit of viral ribonucleotide reductase, was proven to be completely non-virulent even when directly inoculated into CNS in high titers [43, 44]. In phase I and Ib trial, Markert et al., demonstrated that this vector is safe when administered directly to the enhancing portion of glioma [45] and directly into the brain surrounding the tumor [46] and preferentially targeted cancer cells while sparing normal brain. However, the main limitation of this vector was the fact that the mutation that rendered mutant HSV-1 more selective for replication in human gliomas also attenuated the viral progeny, thus decreasing its treatment power [43, 47, 48]. A recombinant replication deficient retrovirus (RV) vector with HSV thymidine kinase transgene insert, when tested in a multicenter randomized controlled phase III trial, showed no significant benefit in the treatment group when compared to untreated group [49]. In vivo, modified measles virus (MV) with insertion of carcinoembryonic antigen (CEA) proved efficacious leading to regression of glioma in nude mice and increased survival; however, its efficacy in humans still needs to be tested [50].
Adenovirus is an attractive candidate for virotherapy because it is not very pathogenic in humans. It does not integrate in host cell genome and can be grown in high titers. To limit viral vector exposure in normal tissue, the initial strategy of developing adenoviral gene therapy vectors involved rendering them replication defective. However, the biggest limitation of this strategy seemed to be the limited biodistribution of these vectors. In a study by Puumalainen et al., a replication deficient adenoviral vector was used to transfer β-galactosidase gene in malignant gliomas and the study reported that transegene expression was unevenly distributed around the injection site, and there were significant differences in the anatomical distribution of the marker gene [51]. Adenovirus vector (Ad-p53, INGN 201) designed to deliver p53 gene locally to glioma cells by intratumoral injection was also tested in a phase I trial and results showed that the cells expressing p53 were only limited to within 5 mm of the injection site [52]. Due to low infectivity and poor therapeutic gene transduction limited to very short distance from the site of injection, replication deficient adenoviral vectors lost their appeal as a desirable viral vector.
Conditionally Replicative Adenoviruses (CRAd)
The challenge imposed by replication deficient viral vectors was addressed by evolution of replication competent viral vectors that have oncolytic potential. Conditionally replicative adenoviruses, or CRAds, are naturally selected or genetically engineered adenoviruses that preferentially replicate in neoplastic cells and release many progenies by lysis of the malignant cells, which in turn infect neighboring neoplastic cells and the process continues [53–56] leading to both widespread infection and tumor lysis. However, one of the biggest challenges for CRAds is to design vectors with high specificity for neoplastic cells in order to limit toxicity to normal brain. The tropism of CRAd's can be improved by mainly modifying three events in the viral replication pathway:
-
Deletion of viral genomic regions that are not required for replication in cancer cell with specific cell cycle checkpoint pathway alterations [57–62]
Rb and p53 are tumor suppressors that play key role in cell cycle regulation by regulating cell entry to S-phase from G1 phase. Transition of a cell from G1 to S phase is crucial for adenovirus replication in a cell. Thus virus genome encodes for proteins like E1A and E1B that interfere with Rb and p53 and induce G1 to S transition (Fig. 2). However, to our advantage, most glioma cells have mutated or altered p53 and Rb function [63, 64]. Hence, deletion of the viral genome responsible for encoding E1A and E1B proteins helps viral vectors to be able to replicate only within cancer cells with disrupted p53 and Rb protein but not in normal cells. One such genetically altered virus is ONYX-015, which replicates more efficiently in cells with defective p53 pathway due to deletion of viral genomic region coding for E1B 55K. In a phase I trial of ONYX-015, Chiocca et al., injected 1010 plaque forming units of ONYX-015 virus into brain tissue adjacent to a freshly excised glioma and showed that it was well tolerated [65]. Ad5-Delta24 is another vector that carries a 24-bp deletion in the Rb binding region of the E1A protein, which leads to removal of Rb inhibition on E2F, and can replicate in and lyse cancer cells with defective Rb function with great efficiency. Most human glioma cells went through cell lysis within 10–14 days after infection with Ad5-Delta24 at 10 PFU/cell. A single dose of the Ad5-Delta24 virus, in vivo, induced a 66.3% inhibition of tumor growth and multiple injections showed an 83.8% inhibition of tumor growth in nude mice with no evidence of infection in normal fibroblasts or cancer cells with restored Rb [59].
-
Facilitation of viral transduction in malignant cells [57, 66–68]
Viral transduction is the process of virion entry into the host cell and one way of enhancing CRAd tropism for glioma cells is by facilitating this process. Interactions between specific cell surface receptors and viral proteins lead to entry of adenoviral particle into the cell. Neoplastic astrocytes express a variety of receptors like PDGFR, EGFR, or αvβ3 and αvβ5 integrins, which can be targeted for viral transduction. Expression of CAR, an adhesion protein, widely expressed in most tissues but with limited expression in glioma [57, 67, 69–72] is required to achieve transduction of adenovirus serotype 5 into a cell. One way of overcoming this obstacle is to design vectors that are capable of CAR independent transduction. Ad5-Delta24RGD, which was obtained by incorporation of Arg-Gly-Asp (RGD) motif, known to interact with αvβ3 and αvβ5 integrin (abundant expression in glioma), in the HI loop of fiber knob of Delta24 adenovirus [58], showed stronger oncolytic effect than the non-RGD-expressing variant in a broad panel of primary glioma [57]. Intratumoral injections of Ad5-Delta24RGD in glioma xenograft in nude mice resulted in complete tumor regression in 9 of 10 mice and long-term survival in all treated mice [57]. Epidermal growth factor receptor (EGFR) has negligible expression in normal non-proliferating neural tissue but is highly expressed in high grade gliomas and this expression is associated with EGFR gene amplification [40, 73, 74]. Upon binding its ligand, EGFR is internalized and leads to activation of PI3K [75], which is required for adenovirus entry into host cells [76]. Miller et al., used a bispecific antibody conjugate to ablate adenoviral binding to fiber receptors and retargeted binding to the epidermal growth factor receptor (EGFR) to significantly enhance adenoviral gene delivery to established glioma cell lines and cultured primary gliomas in EGFR specific and fiber-fiber receptor independent fashion [67]. Wang et al. demonstrated that gene transfer using FGF2 as a targeting ligand to redirect adenoviral infection to neoplastic cell is effective in gliomas with low or deficient levels of CAR [77].
-
Transcriptional targeting of viral genes or transgenes using tumor specific promoters [78–82]
To enhance tumor growth and invasion, malignant glioma cells express many different promoters, such as tissue specific promoters like GFAP [83] and myelin basic protein (MBP) [80] and tumor specific promoters like nestin [57], human telomerase reverse transcriptase (hTERT) [84], E2F1 [82], CXCR4 [85, 86], midkine [87], and survivin [88]. One strategy of developing successful tumor specific oncolytic viral vectors is to incorporate these tumor specific promoters into viral genome to drive the expression of viral genes and transgenes, thus limiting infection to only neoplastic cells bearing the specific promoter. Using this principle, Post et al., developed a hypoxia/HIF-dependent replicative adenovirus (HYPR-Ad) to target hypoxic glioma cells, which displayed hypoxia-dependent E1A expression and conditional cytolysis of hypoxic but not normoxic cells [89]. Telomerase, an enzyme that adds “TTAGGG” sequence to the 3′ end of DNA strand to stabilize chromosome, renders cells the ability to avoid death and continue infinite number of cell divisions. Telomerase has no expression in normal brain; however, it is heavily expressed in glioma cells [90]. To induce apoptosis in glioma cell lines, Komata et al. constructed an expression vector consisting of the constitutively active caspase-6 (rev-caspase-6) under the hTERT promoter, regulator of telomerase activity, (hTERT/rev-caspase-6) and showed that the hTERT/rev-caspase-6 construct not only induced apoptosis in hTERT-positive malignant glioma cells but also suppressed the growth of subcutaneous tumors in nude mice [84].
Another gene that is commonly mutated in glioma cells is Rb. The ICOVIR-5 adenovirus, which encompasses a delta-24 mutation in the retinoblastoma (Rb) protein-binding CR2 region of E1A, substitution of the E1A promoter for E2F-responsive elements, and an RGD-4C peptide modification of the fiber HI loop to enhance adenoviral tropism, showed a potent antiglioma effect [91]. Chemokine C-X-C motif receptor 4 (CXCR4), the inhibitor of apoptosis protein (IAP) survivin, and the heparin binding growth factor midkine are tumor specific promoters that play important role in tumor growth and survival and are up-regulated in majority of all gliomas [85–88]. A midkine promoter-based conditionally replicating adenovirus (Ad-MK) showed strong oncolytic effects in midkine-positive glioma cells but did not exhibit cytotoxicity in midkine-negative primary normal brain cells, and in vivo completely eradicated midkine-positive glioma xenografts [81]. We compared the feasibility of transcriptional targeting of glioma cells by examining the activity of survivin, midkine and CXCR4 and showed that of the three promoters, only survivin exhibits 10,000-fold increased expression in tumor cells compared to normal tissue. We tested the cytolytic activity of CRAd-Survivin-pk7, a chimeric vector containing a pk7 fiber modification and a survivin promoter driving E1A replication, and showed that it effectively replicates in many glioma cell lines like U-87MG, U-251MG, A172, Kings-1, No. 10 and U-118MG and leads to tumor oncolysis in these cell lines with minimal viral replication and toxicity in normal human brain. Intratumoral injection of CRAd-S-pk7 in U-87MG glioma xenograft showed inhibition of tumor growth by more than 300% with 67% of the mice surviving long term (>120 days).
Fig. 2.
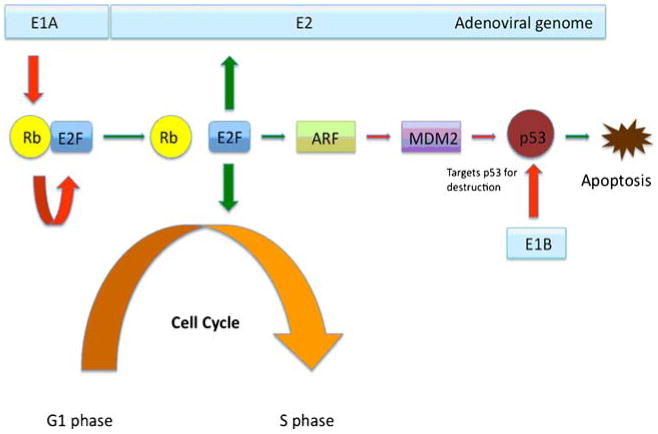
p53 and Rb alteration by adenoviral genome. Adenoviral E1A protein binds to Rb and releases Rb inhibition of E2F. E2F starts G1 to S transition of the cell, directly activates E2 viral gene and activates p14ARF, an inhibitor of the oncoprotein MDM2. MDM2 targets tumor suppressor p53 for destruction. Adenovirus E1B targets p53 for destruction, because in the presence of p53, viral replication cannot occur
Oncolyitc Virotherapy Against Glioma Stem Cells
With better understanding of tumor sustaining glioma stem cells, there are many new emerging therapeutic options for GBM that specifically target this small group of slow growing cells instead of the entire tumor bulk. One such therapeutic strategy has shown promising results in recent studies (Fig. 3). As discussed earlier, one of the major limitations of oncolytic viral vectors is limited biodistribution, poor replication and poor transduction of neighboring tumor cells following intracranial injection. E1B in ONYX-015 and deletion of γ34.5 (RL1) in G207 resulted in decreased viral replication [92]; consequently clinical trials of these vectors failed to show much efficacy [45, 65]. One of the ways to circumvent around this problem is to design vectors that can specifically target the small population of chemo- and radiation resistant GSCs instead of the entire tumor mass. This can be achieved by use of GSC specific promoters or by modification of viral capsids to specifically target GSC surface receptors to enhance viral transduction.
Fig. 3.
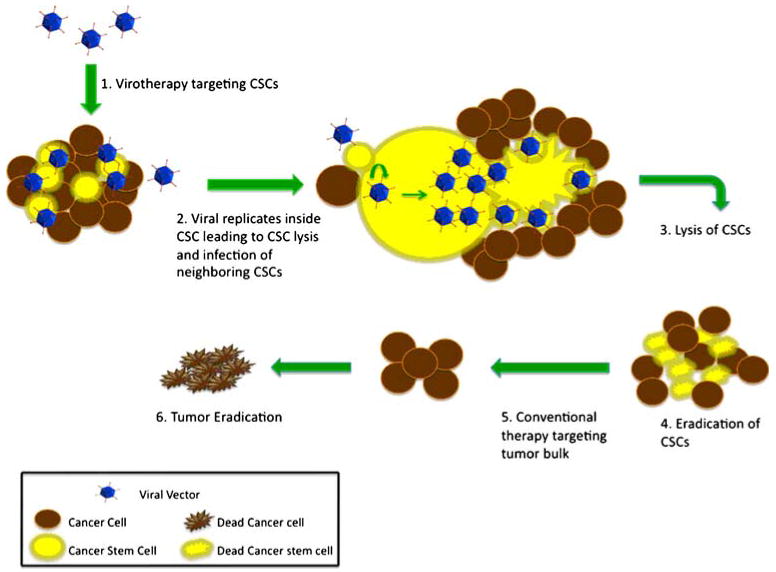
Stem cell targeted virotherapy. Adenoviral vectors are genetically modified to recognize and multiply only in CSCs. Viral replication in CSCs leads to destruction of CSCs and release of viral progeny, which in turn further infect neighboring stem cells. Repetition of this cycle leads to eradication CSCs. Thus targeted therapy in addition to conventional therapy can lead to eradication of the tumor
Marcato et al., treated immunocompromised nude mice with breast cancer xenograft with oncolytic reovirus and showed that CSC population was equally reduced and was as susceptible to reovirus treatment as the non-CSC population [93]. In the setting of esophageal cancer, Zhang et al., established preferential CSC targeting with a viral vector by treating mice bearing stem cell enriched, radioresistant esophageal cancer xenograft with a telomerase-specific oncolytic adenoviral vector carrying apoptotic tumor necrosis factor-related apoptosis-inducing ligand and E1A gene (Ad/TRAIL-E1) and showed significant tumor growth suppression and longer survival with no significant toxicity [94].
In the setting of malignant glioma, Fueyo at al., used Delta-24-RGD, an oncolytic adenovirus with enhanced tropism for GSCs and selective replication in cells with abnormal p16INK4/Rb pathway, to target GSCs. He showed for the first time that brain tumor stem cells are susceptible to adenovirus mediated cell death, both in vivo and in vitro, via autophagy as evident by presence of cytoplasmic autophagic vacuoles and remarkable induction of endogenous ATG5, a key molecule in the conversion of LC3-I to −II and essential for autophagosome formation and autophagic cell death [95]. Bao et al., used lentiviral-mediated short hairpin RNA (shRNA) interference to target L1CAM, a neuronal cell adhesion molecule, in CD133(+) glioma cells and showed potent disruption of neurosphere formation, induction of apoptosis, and inhibition of growth specifically in glioma stem cells. L1CAM knockdown decreased expression of the basic helix-loop-helix transcription factor Olig2 and up-regulated the p21(WAF1/CIP1) tumor suppressor in CD133(+) glioma cells. When tested in vivo, the viral vector showed tumor growth suppression and increased survival of tumor-bearing animals [96]. Wakimoto et al., isolated neurosphere forming GSC from human glioma sample and implanted them in the brain of immunodeficient mice where they formed highly invasive and vascular tumors. These immunodeficient mice with stem cell enriched intracerebral glioma xenograft were then treated with oncolytic herpes simplex virus oHSV G47Delta (ICP6(−), gamma34.5(−), alpha47(−)). Results showed that the viral vector not only killed GSCs but also inhibited their self-renewal as evidenced by the inability of viable cells to form secondary tumor spheres. Despite the highly invasive nature of the intracerebral tumors generated by GSCs, intratumoral injection of G47Delta significantly prolonged survival [97].
Radiation therapy has been shown to increase CD133+ cells in glioma cell cultures implicating their increased proliferative capacity following radiation. Survivin is a tumor specific radiation inducible promoter [98, 99], and an adenoviral vector carrying the survivin promoter and binding to heparan sulfate proteoglycans (CRAd-Survivin-pk7) has been shown to greatly enhance antitumor efficacy in experimental glioma model [100]. In a recent study, we treated glioma cell lines, primary tumor samples and nude mice with stem cell enriched glioma xenograft with CRAd-S-pk7 and found significant tumor growth inhibition in nude mice compared to the untreated group. Of note, the combination of CRAd-S-pk7 treatment with radiation therapy led to a 100-fold increase in viral replication compared to CRAd-S-pk7 treatment alone [101]. Same synergistic effect was reported when CRAd-S-pk7 was combined with chemotherapeutic agent temozolomide, which is known to induce glioma cell death via autophagy [102]. All these studies convincingly prove that even though tumor sustaining GSC successfully escape conventional therapy like chemo- and radiation, they can be successfully targeted with oncolytic viral vectors.
Future Challenges
Optimal strategy for specifically targeting glioma stem cells with a successful viral vector will involve transduction of viral vectors in only CD133+ GSCs and transcription of viral gene driven by glioma specific promoter. Some of the biggest challenges in the way of developing an ideal vector are:
Cancer stem cells show marked similarity with normal stem cells in terms of cellular and genetic architecture. Even though designing viral vectors that drive transgene expression using stem cell specific promoters represents an attractive proposition, special attention should be paid to the fact that the same promoter that will drive viral gene expression in CSC, if present in normal stem cell, will also drive same viral gene expression in normal stem cells leading to normal stem cell lysis. Thus, we not only have to identify promoters that are specific for stem cells but they have to be exclusively present in CSCs.
Modifying viral fiber knob to specifically bind to CSC surface receptor can optimize viral transduction. One such cell surface receptor is CD133, which is expressed in many CSC. Designing a viral vector that will specifically bind to CD133 will provide increased specificity in targeting these cells for virotherapy. However, one of the main challenges in using this technique is the fact that CD133 is expressed not only in glioma stem cells but also in other parts of the body. Human AC133 gene has at least 9 distinctive 5′-untranslated region (UTR) exons, resulting in the formation of at least 7 alternatively spliced 5′-UTR isoforms of AC133 mRNA, which are expressed in a tissue-dependent manner. Transcription of these AC133 isoforms is controlled by five alternative promoters, P1-P5 depending on their location relative to different exons, and in vitro methylation of P1 and P2 completely suppresses their activity, suggesting that methylation plays a role in their regulation [103]. Thus, finding the brain specific isoform of AC133 and directing vectors to that specific isoform will increase the specificity of these vectors.
Given the heterogeneous and evolving nature of malignant gliomas, the optimal treatment strategy will encompass a well planned interplay of several distinct treatment modalities. Hence, the synergistic effect between virotherapy and other currently established therapies like chemo- and radiation needs to be further evaluated to attain the optimal outcome.
Acknowledgements/Conflict of Interest
This work was supported by the National Cancer Institute (R01-CA122930, R01-CA138587, R21-CA135728), the National Institute of Neurological Disorders and Stroke (K08-NS046430), The Alliance for Cancer Gene Therapy Young Investigator Award, and the American Cancer Society (RSG-07-276-01-MGO).
References
- 1.Nowell PC. Foundations in cancer research. Chromosomes and cancer: the evolution of an idea. Advances in Cancer Research. 1993;62:1–17. doi: 10.1016/s0065-230x(08)60313-9. [DOI] [PubMed] [Google Scholar]
- 2.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Hegi ME. Targeting brain-tumor stem cells. Nature Biotechnology. 2007;25:193–194. doi: 10.1038/nbt0207-193. [DOI] [PubMed] [Google Scholar]
- 6.Till JE, Mc CE. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation Research. 1961;14:213–222. [PubMed] [Google Scholar]
- 7.Bruce WR, Van Der Gaag H. A quantitative assay for the number of murine lymphoma cells capable of proliferation in vivo. Nature. 1963;199:79–80. doi: 10.1038/199079a0. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Research. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 11.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan KS, Espinosa I, Chao M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Research. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 14.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Research. 2005;65:3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 15.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature Reviews Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 16.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 17.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death and Differention. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 19.Tirino V, Desiderio V, d'Aquino R, et al. Detection and characterization of cd133+ cancer stem cells in human solid tumours. Public Library of Science ONE. 2008;3:e3469. doi: 10.1371/journal.pone.0003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Research. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 21.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 22.Dahlstrand J, Collins VP, Lendahl U. Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Research. 1992;52:5334–5341. [PubMed] [Google Scholar]
- 23.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 24.Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miraglia S, Godfrey W, Yin AH, et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 26.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 27.Eramo A, Ricci-Vitiani L, Zeuner A, et al. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differention. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 28.Kang MK, Kang SK. Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells and Development. 2007;16:837–847. doi: 10.1089/scd.2007.0006. [DOI] [PubMed] [Google Scholar]
- 29.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of cd133+ cancer stem cells in glioblastoma. Molecular Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hegi ME, Diserens AC, Gorlia T, et al. Mgmt gene silencing and benefit from temozolomide in glioblastoma. New England Journal of Medicine. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 31.Bi CL, Fang JS, Chen FH, Wang YJ, Wu J. chemoresistance of cd133(+) tumor stem cells from human brain glioma. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007;32:568–573. [PubMed] [Google Scholar]
- 32.Salmaggi A, Boiardi A, Gelati M, et al. Glioblastoma-derived tumorospheres identify a population of tumor stem-like cells with angiogenic potential and enhanced multidrug resistance phenotype. Glia. 2006;54:850–860. doi: 10.1002/glia.20414. [DOI] [PubMed] [Google Scholar]
- 33.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 34.Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing il-12 in the treatment of experimental murine brain tumors. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulci G, Breymann L, Gianni D, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous ndv-huj oncolytic virus in recurrent glioblastoma multiforme. Molecular Therapy. 2006;13:221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Bar-Eli N, Giloh H, Schlesinger M, Zakay-Rones Z. Preferential cytotoxic effect of newcastle disease virus on lymphoma cells. Journal of Cancer Research and Clinical Oncology. 1996;122:409–415. doi: 10.1007/BF01212880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassel WA, Garrett RE. Newcastle disease virus as an antineoplastic agent. Cancer. 1965;18:863–868. doi: 10.1002/1097-0142(196507)18:7<863::aid-cncr2820180714>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 39.Guha A, Dashner K, Black PM, Wagner JA, Stiles CD. Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. International Journal of Cancer. 1995;60:168–173. doi: 10.1002/ijc.2910600206. [DOI] [PubMed] [Google Scholar]
- 40.Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 41.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 42.Forsyth P, Roldan G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Molecular Therapy. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 43.Markovitz NS, Baunoch D, Roizman B. The range and distribution of murine central nervous system cells infected with the gamma(1)34.5- mutant of herpes simplex virus 1. The Journal of Virology. 1997;71:5560–5569. doi: 10.1128/jvi.71.7.5560-5569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nature Medicine. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 45.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, g207 for the treatment of malignant glioma: results of a phase I trial. Gene Therapy. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 46.Markert JM, Liechty PG, Wang W, et al. Phase ib trial of mutant herpes simplex virus g207 inoculated pre-and post-tumor resection for recurrent GBM. Molecular Therapy. 2008;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung RY, Saeki Y, Chiocca EA. B-myb promoter retargeting of herpes simplex virus gamma34.5 gene-mediated virulence toward tumor and cycling cells. The Journal of Virology. 1999;73:7556–7564. doi: 10.1128/jvi.73.9.7556-7564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in treatment of diseases of the nervous system. Annual Review of Microbiology. 2004;58:253–271. doi: 10.1146/annurev.micro.58.030603.123709. [DOI] [PubMed] [Google Scholar]
- 49.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Human Gene Therapy. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 50.Phuong LK, Allen C, Peng KW, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Research. 2003;63:2462–2469. [PubMed] [Google Scholar]
- 51.Puumalainen AM, Vapalahti M, Agrawal RS, et al. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Human Gene Therapy. 1998;9:1769–1774. doi: 10.1089/hum.1998.9.12-1769. [DOI] [PubMed] [Google Scholar]
- 52.Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. Journal of Clinical Oncology. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 53.Heise C, Hermiston T, Johnson L, et al. An adenovirus e1a mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nature Medicine. 6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 54.Alemany R, Balague C, Curiel DT. Replicative adenoviruses for cancer therapy. Nature Biotechnology. 2000;18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 55.Jiang H, Conrad C, Fueyo J, Gomez-Manzano C, Liu TJ. Oncolytic adenoviruses for malignant glioma therapy. Frontiers in Bioscience. 8:d577–588. doi: 10.2741/923. [DOI] [PubMed] [Google Scholar]
- 56.Lin E, Nemunaitis J. Oncolytic viral therapies. Cancer Gene Therapy. 2004;11:643–664. doi: 10.1038/sj.cgt.7700733. [DOI] [PubMed] [Google Scholar]
- 57.Lamfers ML, Grill J, Dirven CM, et al. Potential of the conditionally replicative adenovirus ad5-delta24rgd in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Research. 62:5736–5742. [PubMed] [Google Scholar]
- 58.Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clinical Cancer Research. 2001;7:120–126. [PubMed] [Google Scholar]
- 59.Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the RB pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 60.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 61.Khuri FR, Nemunaitis J, Ganly I, et al. A controlled trial of intratumoral onyx-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nature Medicine. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 62.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. Onyx-015, an e1b gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nature Medicine. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 63.Fults D, Brockmeyer D, Tullous MW, Pedone CA, Cawthon RM. P53 mutation and loss of heterozygosity on chromosomes 17 and 10 during human astrocytoma progression. Cancer Research. 1992;52:674–679. [PubMed] [Google Scholar]
- 64.Ueki K, Ono Y, Henson JW, Efird JT, von Deimling A, Louis DN. Cdkn2/p16 or rb alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Research. 1996;56:150–153. [PubMed] [Google Scholar]
- 65.Chiocca EA, Abbed KM, Tatter S, et al. A phase i open-label, dose-escalation, multi-institutional trial of injection with an e1b-attenuated adenovirus, onyx-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Molecular Therapy. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 66.van Beusechem VW, Grill J, Mastenbroek DC, et al. Efficient and selective gene transfer into primary human brain tumors by using single-chain antibody-targeted adenoviral vectors with native tropism abolished. The Journal of Virology. 2002;76:2753–2762. doi: 10.1128/JVI.76.6.2753-2762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller CR, Buchsbaum DJ, Reynolds PN, et al. Differential susceptibility of primary and established human glioma cells to adenovirus infection: Targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Research. 1998;58:5738–5748. [PubMed] [Google Scholar]
- 68.Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. Journal of the National Cancer Institute. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 69.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for coxsackie b viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 70.Tomko RP, Xu R, Philipson L. Hcar and mcar: The human and mouse cellular receptors for subgroup c adenoviruses and group b coxsackieviruses. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asaoka K, Tada M, Sawamura Y, Ikeda J, Abe H. Dependence of efficient adenoviral gene delivery in malignant glioma cells on the expression levels of the coxsackievirus and adenovirus receptor. Journal of Neurosurgery. 2000;92:1002–1008. doi: 10.3171/jns.2000.92.6.1002. [DOI] [PubMed] [Google Scholar]
- 72.Grill J, Van Beusechem VW, Van Der Valk P, et al. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clinical Cancer Research. 2001;7:641–650. [PubMed] [Google Scholar]
- 73.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Research. 1991;51:2164–2172. [PubMed] [Google Scholar]
- 75.Hu P, Margolis B, Skolnik EY, Lammers R, Ullrich A, Schlessinger J. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Molecular and Cellular Biology. 1992;12:981–990. doi: 10.1128/mcb.12.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li E, Stupack D, Klemke R, Cheresh DA, Nemerow GR. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-oh kinase. The Journal of Virology. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang W, Zhu NL, Chua J, et al. Retargeting of adenoviral vector using basic fibroblast growth factor ligand for malignant glioma gene therapy. Journal of Neurosurgery. 2005;103:1058–1066. doi: 10.3171/jns.2005.103.6.1058. [DOI] [PubMed] [Google Scholar]
- 78.Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic hsv-1 mutant expressing icp34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Research. 2005;65:2832–2839. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- 79.Vandier D, Rixe O, Besnard F, et al. Inhibition of glioma cells in vitro and in vivo using a recombinant adenoviral vector containing an astrocyte-specific promoter. Cancer Gene Therapy. 2000;7:1120–1126. doi: 10.1038/sj.cgt.7700211. [DOI] [PubMed] [Google Scholar]
- 80.Shinoura N, Saito K, Yoshida Y, et al. Adenovirus-mediated transfer of bax with caspase-8 controlled by myelin basic protein promoter exerts an enhanced cytotoxic effect in gliomas. Cancer Gene Therapy. 2000;7:739–748. doi: 10.1038/sj.cgt.7700158. [DOI] [PubMed] [Google Scholar]
- 81.Kohno S, Nakagawa K, Hamada K, et al. Midkine promoter-based conditionally replicative adenovirus for malignant glioma therapy. Oncology Reports. 2004;12:73–78. [PubMed] [Google Scholar]
- 82.Parr MJ, Manome Y, Tanaka T, et al. Tumor-selective transgene expression in vivo mediated by an e2f-responsive adenoviral vector. Nature Medicine. 1997;3:1145–1149. doi: 10.1038/nm1097-1145. [DOI] [PubMed] [Google Scholar]
- 83.Wilcox ME, Yang W, Senger D, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. Journal of the National Cancer Institute. 2001;293:903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 84.Komata T, Kondo Y, Kanzawa T, et al. Treatment of malignant glioma cells with the transfer of constitutively active caspase-6 using the human telomerase catalytic subunit (human telomerase reverse transcriptase) gene promoter. Cancer Research. 2001;61:5796–5802. [PubMed] [Google Scholar]
- 85.Zhou Y, Larsen PH, Hao C, Yong VW. Cxcr4 is a major chemokine receptor on glioma cells and mediates their survival. The Journal of Biological Chemistry. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 86.Oh JW, Drabik K, Kutsch O, Choi C, Tousson A, Benveniste EN. Cxc chemokine receptor 4 expression and function in human astroglioma cells. The Journal of Immunology. 2001;166:2695–2704. doi: 10.4049/jimmunol.166.4.2695. [DOI] [PubMed] [Google Scholar]
- 87.Mishima K, Asai A, Kadomatsu K, et al. Increased expression of midkine during the progression of human astrocytomas. Neuroscience Letter. 1997;233:29–32. doi: 10.1016/s0304-3940(97)00619-8. [DOI] [PubMed] [Google Scholar]
- 88.Yang L, Cao Z, Li F, et al. Tumor-specific gene expression using the survivin promoter is further increased by hypoxia. Gene Therapy. 2004;11:1215–1223. doi: 10.1038/sj.gt.3302280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Post DE, Van Meir EG. A novel hypoxia-inducible factor (hif) activated oncolytic adenovirus for cancer therapy. Oncogene. 2003;22:2065–2072. doi: 10.1038/sj.onc.1206464. [DOI] [PubMed] [Google Scholar]
- 90.Harada K, Kurisu K, Tahara H, Tahara E, Ide T. Telomerase activity in primary and secondary glioblastomas multiforme as a novel molecular tumor marker. Journal of Neurosurgery. 2000;93:618–625. doi: 10.3171/jns.2000.93.4.0618. [DOI] [PubMed] [Google Scholar]
- 91.Alonso MM, Cascallo M, Gomez-Manzano C, et al. Icovir-5 shows e2f1 addiction and potent antiglioma effect in vivo. Cancer Research. 2007;67:8255–8263. doi: 10.1158/0008-5472.CAN-06-4675. [DOI] [PubMed] [Google Scholar]
- 92.Yazaki T, Manz HJ, Rabkin SD, Martuza RL. Treatment of human malignant meningiomas by g207, a replication-competent multimutated herpes simplex virus 1. Cancer Research. 1995;55:4752–4756. [PubMed] [Google Scholar]
- 93.Marcato P, Dean CA, Giacomantonio CA, Lee PW. Oncolytic reovirus effectively targets breast cancer stem cells. Molecular Therapy. 2009;17:972–979. doi: 10.1038/mt.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang X, Komaki R, Wang L, Fang B, Chang JY. Treatment of radioresistant stem-like esophageal cancer cells by an apoptotic gene-armed, telomerase-specific oncolytic adenovirus. Clinical Cancer Research. 2008;14:2813–2823. doi: 10.1158/1078-0432.CCR-07-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang H, Gomez-Manzano C, Aoki H, et al. Examination of the therapeutic potential of delta-24-rgd in brain tumor stem cells: role of autophagic cell death. Journal of the National Cancer Institute. 2007;99:1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- 96.Bao S, Wu Q, Li Z, et al. Targeting cancer stem cells through l1cam suppresses glioma growth. Cancer Research. 68:6043–6048. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wakimoto H, Kesari S, Farrell CJ, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Research. 2009;69:3472–3481. doi: 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Houdt WJ, Haviv YS, Lu B, et al. A novel transcriptional targeting strategy for treatment of glioma. Journal of Neurosurgery. 2006;104:583–592. doi: 10.3171/jns.2006.104.4.583. [DOI] [PubMed] [Google Scholar]
- 99.Ulasov IV, Rivera AA, Sonabend AM, et al. Comparative evaluation of survivin, midkine and cxcr4 promoters for transcriptional targeting of glioma gene therapy. Cancer Biology and Therapy. 2007;6:679–685. doi: 10.4161/cbt.6.5.3957. [DOI] [PubMed] [Google Scholar]
- 100.Ulasov IV, Zhu ZB, Tyler MA, et al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Human Gene Therapy. 2007;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 101.Nandi S, Ulasov IV, Tyler MA, et al. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Research. 2008;68:5778–5784. doi: 10.1158/0008-5472.CAN-07-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death and Differention. 2004;11:448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 103.Shmelkov SV, Jun L, St Clair R, et al. Alternative promoters regulate transcription of the gene that encodes stem cell surface protein ac133. Blood. 2004;103:2055–2061. doi: 10.1182/blood-2003-06-1881. [DOI] [PubMed] [Google Scholar]
- 104.Bleehen NM, Stenning SP. A medical research council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The medical research council brain tumour working party. British Journal of Cancer. 1991;64:769–774. doi: 10.1038/bjc.1991.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 106.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (bcnu) wafers (gliadel wafers) in patients with primary malignant glioma. Neuro Oncology. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


