Abstract
To assess energy expenditure of the diaphragm directly, a method was devised for percutaneous catheterization of the left inferior phrenic vein in dogs. Necropsy studies, including retrograde injection of india ink and measurement of radioactivity in diaphragmatic muscle strips, suggested that the territory drained by the inferior phrenic vein was uniformly perfused, and that there were no major anastomoses between this bed and adjacent ones.
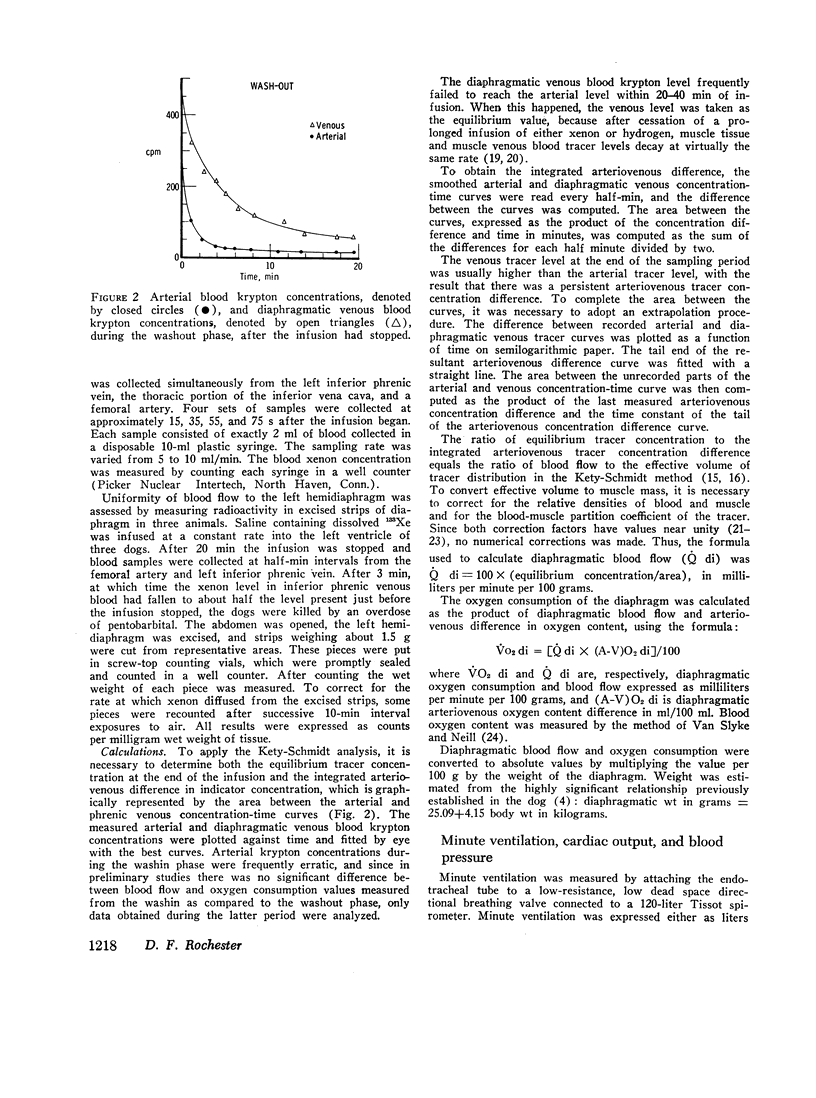
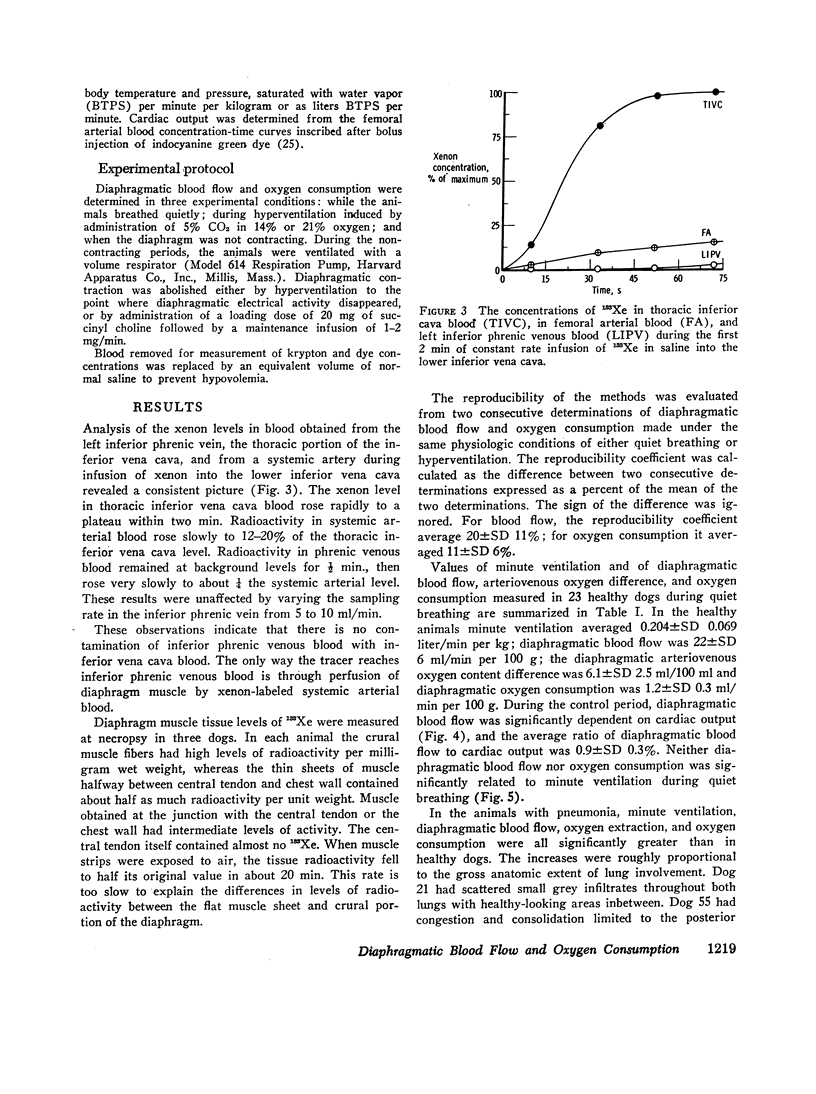
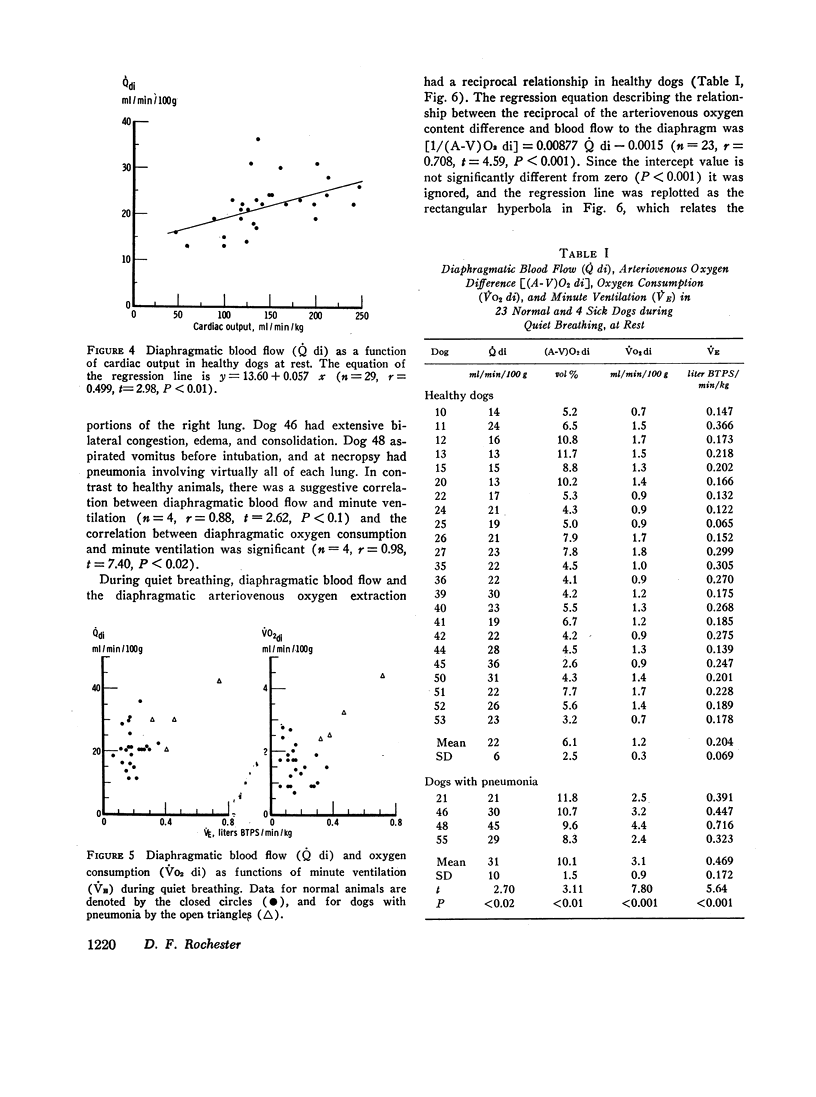
Diaphragmatic blood flow (˙Q di) was calculated from the integrated diaphragmatic arteriovenous difference of 85Kr by the Kety-Schmidt technique. Diaphragmatic oxygen consumption (˙Vo2 di) was determined as the product of ˙Q di and the diaphragmatic arteriovenous oxygen content difference [(A-V)O2 di]. When lightly anesthetized dogs breathed quietly, ˙Q di was 22±SD 6 ml/min/100 g, (A-V)O2 di was 6.1±SD 2.5 ml/100 ml, and ˙VO2 di averaged 1.2±SD 0.3 ml/min/100 g. This represented 1.0±SD 0.2% of total body oxygen consumption. ˙VO2 di remained relatively constant during quiet breathing, whereas ˙Q di varied directly with cardiac output and reciprocally with (A-V)O2 di. The oxygen consumption of the noncontracting diaphragm was 60±SD 20% of the level measured during quiet breathing.
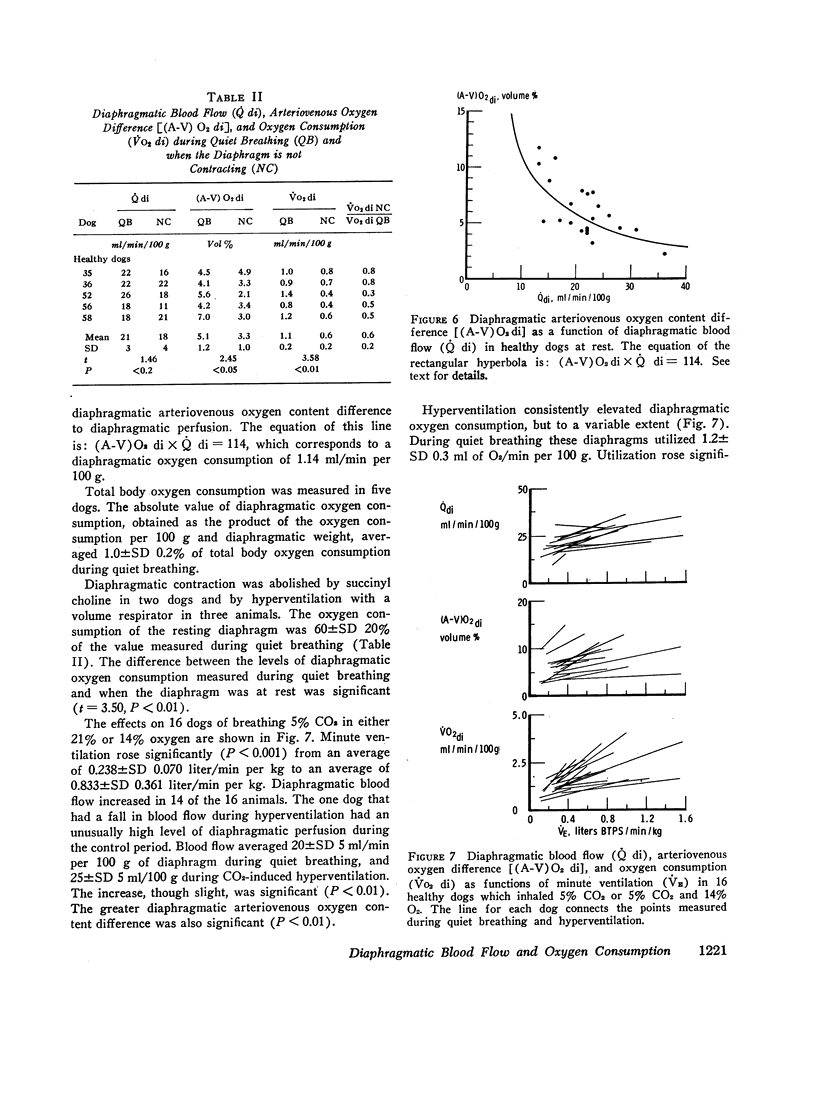
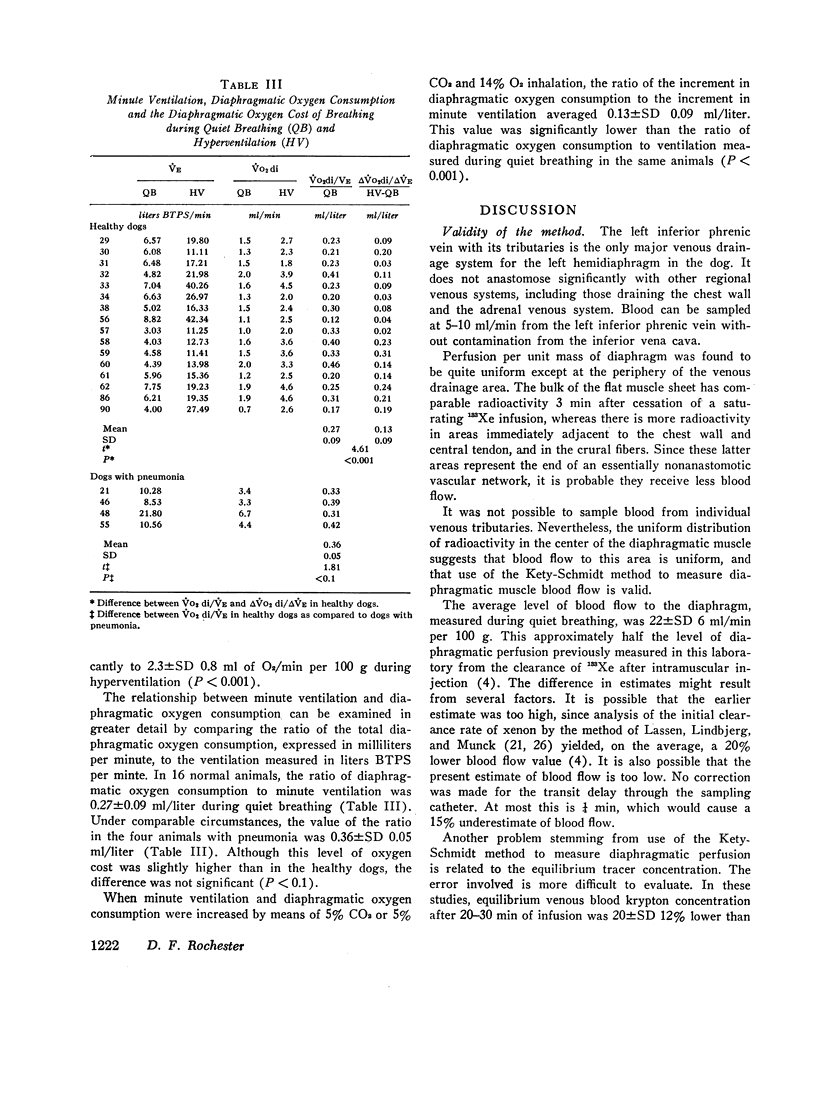
The energy expended by the diaphragm to support simple hyperventilation was small. A 100% increase in minute ventilation, induced by inhalation of 5% CO2 in 21% or 14% O2, increased ˙Q di 13%, (A-V)O2 di 19%, and ˙VO2 di 40%. The diaphragm consumed 0.13±SD 0.09 ml O2 for each additional liter of ventilation. In four dogs, pneumonia appeared to increase ˙VO2 both by increasing minute ventilation and by increasing the energy cost per liter of ventilation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUKLAND K., BOWER B. F., BERLINER R. W. MEASUREMENT OF LOCAL BLOOD FLOW WITH HYDROGEN GAS. Circ Res. 1964 Feb;14:164–187. doi: 10.1161/01.res.14.2.164. [DOI] [PubMed] [Google Scholar]
- BARTLETT R. G., Jr, BRUBACH H. F., SPECHT H. Oxygen cost of breathing. J Appl Physiol. 1958 May;12(3):413–424. doi: 10.1152/jappl.1958.12.3.413. [DOI] [PubMed] [Google Scholar]
- CAMPBELL E. J., WESTLAKE E. K., CHERNIACK R. M. Simple methods of estimating oxygen consumption and efficiency of the muscles of breathing. J Appl Physiol. 1957 Sep;11(2):303–308. doi: 10.1152/jappl.1957.11.2.303. [DOI] [PubMed] [Google Scholar]
- CHERNIACK R. M. The oxygen consumption and efficiency of the respiratory muscles in health and emphysema. J Clin Invest. 1959 Mar;38(3):494–499. doi: 10.1172/JCI103826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COURNAND A., RICHARDS D. W., Jr, BADER R. A., BADER M. E., FISHMAN A. P. The oxygen cost of breathing. Trans Assoc Am Physicians. 1954;67:162–173. [PubMed] [Google Scholar]
- Caldwell P. R., Echeverri U., Kilcoyne M. M., Fritts H. W., Jr Observations on a model of proliferative lung disease. II. Description of pulmonary gas exchange and comparison of Fick and dye cardiac outputs. J Clin Invest. 1970 Jul;49(7):1311–1315. doi: 10.1172/JCI106346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRITTS H. W., Jr, FILLER J., FISHMAN A. P., COURNAND A. The efficiency of ventilation during voluntary hyperpnea: studies in normal subjects and in dyspneic patients with either chronic pulmonary emphysema or obesity. J Clin Invest. 1959 Aug;38(8):1339–1348. doi: 10.1172/JCI103909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth R. P., Hoffbrand B. I., Melmon K. L. Redistribution of cardiac output during hemorrhage in the unanesthetized monkey. Circ Res. 1970 Sep;27(3):311–320. doi: 10.1161/01.res.27.3.311. [DOI] [PubMed] [Google Scholar]
- GREGG D. E., LONGINO F. H., GREEN P. A., CZERWONKA L. J. A comparison of coronary flow determined by the nitrous oxide method and by a direct method using the rotameter. Circulation. 1951 Jan;3(1):89–94. doi: 10.1161/01.cir.3.1.89. [DOI] [PubMed] [Google Scholar]
- HANSEN A. T., HAXHOLDT B. F., HUSFELDT E., LASSEN N. A., MUNCK O., SORENSEN H. R., WINKLER K. Measurement of coronary blood flow and cardiac efficiency in hypothermia by use of radioactive krypton 85. Scand J Clin Lab Invest. 1956;8(3):182–188. doi: 10.3109/00365515609049269. [DOI] [PubMed] [Google Scholar]
- HARDEWIG A., ROCHESTER D. F., BRISCOE W. A. Measurement of solubility coefficients of krypton in water, plasma and human blood, using radioactive Kr85. J Appl Physiol. 1960 Jul;15:723–725. doi: 10.1152/jappl.1960.15.4.723. [DOI] [PubMed] [Google Scholar]
- HOLZMAN G. B., WAGNER H. N., Jr, IIO M., RABINOWITZ D., ZIERLER K. L. MEASUREMENT OF MUSCLE BLOOD FLOW IN THE HUMAN FOREARM WITH RADIOACTIVE KRYPTON AND XENON. Circulation. 1964 Jul;30:27–34. doi: 10.1161/01.cir.30.1.27. [DOI] [PubMed] [Google Scholar]
- Hedworth-Whitty R. B., Housley E., Abraham A. S. An improved technique for measuring changes in myocardial perfusion by the nitrous oxide method. Cardiovasc Res. 1969 Oct;3(4):496–502. doi: 10.1093/cvr/3.4.496. [DOI] [PubMed] [Google Scholar]
- Jorgensen C. R., Kitamura K., Gobel F. L., Taylor H. L., Wang Y. Long-term precision of the N2O method for coronary flow during heavy upright exercise. J Appl Physiol. 1971 Mar;30(3):338–344. doi: 10.1152/jappl.1971.30.3.338. [DOI] [PubMed] [Google Scholar]
- Kety S. S., Schmidt C. F. THE NITROUS OXIDE METHOD FOR THE QUANTITATIVE DETERMINATION OF CEREBRAL BLOOD FLOW IN MAN: THEORY, PROCEDURE AND NORMAL VALUES. J Clin Invest. 1948 Jul;27(4):476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocke F. J., Rosing D. R., Pittman D. E. Inert gas measurements of coronary blood flow. Am J Cardiol. 1969 Apr;23(4):548–555. doi: 10.1016/0002-9149(69)90008-3. [DOI] [PubMed] [Google Scholar]
- LASSEN N. A., LINDBJERG J., MUNCK O. MEASUREMENT OF BLOOD-FLOW THROUGH SKELETAL MUSCLE BY INTRAMUSCULAR INJECTION OF XENON-133. Lancet. 1964 Mar 28;1(7335):686–689. doi: 10.1016/s0140-6736(64)91518-1. [DOI] [PubMed] [Google Scholar]
- LASSEN N. A. MUSCLE BLOOD FLOW IN NORMAL MAN AND IN PATIENTS WITH INTERMITTENT CLAUDICATION EVALUATED BY SIMULTANEOUS XE-133 AND NA24 CLEARANCES. J Clin Invest. 1964 Sep;43:1805–1812. doi: 10.1172/JCI105054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKERROW C. B., OTIS A. B. Oxygen cost of hyperventilation. J Appl Physiol. 1956 Nov;9(3):375–379. doi: 10.1152/jappl.1956.9.3.375. [DOI] [PubMed] [Google Scholar]
- Mognoni P., Saibene F., Sant'ambrogio G. Contribution of the diaphragm and the other inspiratory muscles to different levels of tidal volume and static inspiratory effort in the rabbit. J Physiol. 1969 Jun;202(3):517–534. doi: 10.1113/jphysiol.1969.sp008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester D. F., Pradel-Guena M. Measurement of diaphragmatic blood flow in dogs from xenon 133 clearance. J Appl Physiol. 1973 Jan;34(1):68–74. doi: 10.1152/jappl.1973.34.1.68. [DOI] [PubMed] [Google Scholar]
- Sejrsen P., Tonnesen K. H. Inert gas diffusion method for measurement of blood flow using saturation techniques. Comparison with directly measured blood flow in isolated gastrocnemius muscle of the cat. Circ Res. 1968 May;22(5):679–693. doi: 10.1161/01.res.22.5.679. [DOI] [PubMed] [Google Scholar]
- Spaich P., Usinger W., Albers C. Oxygen cost of panting in anaesthetized dogs. Respir Physiol. 1968 Sep;5(2):302–314. doi: 10.1016/0034-5687(68)90068-6. [DOI] [PubMed] [Google Scholar]
- Yeh S. Y., Peterson R. E. Solubility of krypton and xenon in blood, protein solutions, and tissue homogenates. J Appl Physiol. 1965 Sep;20(5):1041–1047. doi: 10.1152/jappl.1965.20.5.1041. [DOI] [PubMed] [Google Scholar]



